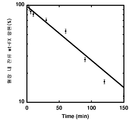
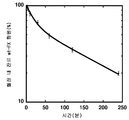
KR20110114587A - 세린 프로테아제 유도체 및 혈액응고 장애의 치료 또는 예방의 용도 - Google Patents
세린 프로테아제 유도체 및 혈액응고 장애의 치료 또는 예방의 용도 Download PDFInfo
- Publication number
- KR20110114587A KR20110114587A KR1020117016908A KR20117016908A KR20110114587A KR 20110114587 A KR20110114587 A KR 20110114587A KR 1020117016908 A KR1020117016908 A KR 1020117016908A KR 20117016908 A KR20117016908 A KR 20117016908A KR 20110114587 A KR20110114587 A KR 20110114587A
- Authority
- KR
- South Korea
- Prior art keywords
- seq
- factor
- protein
- chimeric
- substituted
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 108010022999 Serine Proteases Proteins 0.000 title claims abstract description 39
- 102000012479 Serine Proteases Human genes 0.000 title claims abstract description 39
- 238000011282 treatment Methods 0.000 title claims abstract description 39
- 230000002265 prevention Effects 0.000 title claims abstract description 25
- 208000015294 blood coagulation disease Diseases 0.000 title claims abstract description 13
- 206010053567 Coagulopathies Diseases 0.000 title abstract description 9
- 230000009852 coagulant defect Effects 0.000 title abstract description 5
- 108090000765 processed proteins & peptides Proteins 0.000 claims abstract description 221
- 108010014173 Factor X Proteins 0.000 claims abstract description 163
- 229960000856 protein c Drugs 0.000 claims abstract description 113
- 101800004937 Protein C Proteins 0.000 claims abstract description 111
- 101800001700 Saposin-D Proteins 0.000 claims abstract description 110
- 230000003213 activating effect Effects 0.000 claims abstract description 73
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 163
- 229920001184 polypeptide Polymers 0.000 claims description 145
- 108090000623 proteins and genes Proteins 0.000 claims description 85
- 235000018102 proteins Nutrition 0.000 claims description 76
- 102000004169 proteins and genes Human genes 0.000 claims description 76
- 150000001413 amino acids Chemical group 0.000 claims description 56
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 55
- 102000037865 fusion proteins Human genes 0.000 claims description 53
- 108020001507 fusion proteins Proteins 0.000 claims description 53
- 238000000034 method Methods 0.000 claims description 39
- JWICNZAGYSIBAR-LEEGLKINSA-N (4s)-4-[[2-[[(2s)-2-[[(2s)-2-[[(2s)-2-aminopropanoyl]amino]-3-carboxypropanoyl]amino]-3-hydroxypropanoyl]amino]acetyl]amino]-5-[[2-[[(2s)-3-carboxy-1-[[(2s)-1-[[1-[[(2s)-1-[[(2s)-4-carboxy-1-[[2-[[2-[[2-[[(2s)-1-[[(1s)-1-carboxy-4-(diaminomethylideneamino Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)CNC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)C(CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)N)CC1=CC=CC=C1 JWICNZAGYSIBAR-LEEGLKINSA-N 0.000 claims description 38
- 101800000974 Fibrinopeptide A Proteins 0.000 claims description 36
- 102400000525 Fibrinopeptide A Human genes 0.000 claims description 36
- 102100022641 Coagulation factor IX Human genes 0.000 claims description 29
- 235000004279 alanine Nutrition 0.000 claims description 27
- 230000015271 coagulation Effects 0.000 claims description 27
- 238000005345 coagulation Methods 0.000 claims description 27
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 25
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 claims description 24
- 239000004474 valine Substances 0.000 claims description 24
- 230000007170 pathology Effects 0.000 claims description 23
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 claims description 18
- COLNVLDHVKWLRT-QMMMGPOBSA-N phenylalanine group Chemical group N[C@@H](CC1=CC=CC=C1)C(=O)O COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 18
- 108010074860 Factor Xa Proteins 0.000 claims description 17
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical group C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 claims description 15
- 229960001153 serine Drugs 0.000 claims description 15
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 claims description 14
- 239000003146 anticoagulant agent Substances 0.000 claims description 14
- 108020004707 nucleic acids Proteins 0.000 claims description 13
- 150000007523 nucleic acids Chemical class 0.000 claims description 13
- 102000039446 nucleic acids Human genes 0.000 claims description 13
- 238000004321 preservation Methods 0.000 claims description 13
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Chemical group OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 claims description 12
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 claims description 12
- 229940127219 anticoagulant drug Drugs 0.000 claims description 12
- 239000004471 Glycine Substances 0.000 claims description 11
- 208000032843 Hemorrhage Diseases 0.000 claims description 11
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 claims description 11
- 230000000740 bleeding effect Effects 0.000 claims description 11
- 208000009429 hemophilia B Diseases 0.000 claims description 11
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical group OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 claims description 10
- 208000009292 Hemophilia A Diseases 0.000 claims description 10
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 claims description 10
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 10
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 claims description 9
- 208000007536 Thrombosis Diseases 0.000 claims description 9
- 229960001230 asparagine Drugs 0.000 claims description 9
- 235000009582 asparagine Nutrition 0.000 claims description 9
- 102100026735 Coagulation factor VIII Human genes 0.000 claims description 8
- 201000003542 Factor VIII deficiency Diseases 0.000 claims description 8
- 101000911390 Homo sapiens Coagulation factor VIII Proteins 0.000 claims description 8
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 claims description 8
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical group CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 8
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Chemical group CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 claims description 8
- 229960000310 isoleucine Drugs 0.000 claims description 8
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 claims description 8
- 239000003055 low molecular weight heparin Substances 0.000 claims description 8
- 229940127215 low-molecular weight heparin Drugs 0.000 claims description 8
- 239000008194 pharmaceutical composition Substances 0.000 claims description 8
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 claims description 6
- 230000002008 hemorrhagic effect Effects 0.000 claims description 6
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 claims description 4
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims description 4
- 229940009098 aspartate Drugs 0.000 claims description 4
- 239000004475 Arginine Substances 0.000 claims description 3
- 239000004472 Lysine Substances 0.000 claims description 3
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 claims description 3
- 102400000827 Saposin-D Human genes 0.000 claims 12
- 230000002708 enhancing effect Effects 0.000 claims 1
- 102000017975 Protein C Human genes 0.000 abstract description 98
- 239000012634 fragment Substances 0.000 abstract description 8
- 102000010911 Enzyme Precursors Human genes 0.000 abstract description 5
- 108010062466 Enzyme Precursors Proteins 0.000 abstract description 5
- 229940012426 factor x Drugs 0.000 description 144
- 125000003275 alpha amino acid group Chemical group 0.000 description 84
- 235000001014 amino acid Nutrition 0.000 description 46
- 229940024606 amino acid Drugs 0.000 description 43
- 210000002381 plasma Anatomy 0.000 description 42
- 241000699670 Mus sp. Species 0.000 description 29
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 29
- 210000004027 cell Anatomy 0.000 description 27
- 238000002347 injection Methods 0.000 description 26
- 239000007924 injection Substances 0.000 description 26
- 241000282414 Homo sapiens Species 0.000 description 24
- 108090000190 Thrombin Proteins 0.000 description 24
- 229960004072 thrombin Drugs 0.000 description 24
- 108010037716 aspartyl-phenylalanyl-leucyl-alanyl-glutamate Proteins 0.000 description 23
- PAIHPOGPJVUFJY-WDSKDSINSA-N Ala-Glu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O PAIHPOGPJVUFJY-WDSKDSINSA-N 0.000 description 21
- 239000000427 antigen Substances 0.000 description 21
- 108091007433 antigens Proteins 0.000 description 21
- 102000036639 antigens Human genes 0.000 description 21
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 21
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 21
- OLPPXYMMIARYAL-QMMMGPOBSA-N Gly-Gly-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)CNC(=O)CN OLPPXYMMIARYAL-QMMMGPOBSA-N 0.000 description 19
- 239000000203 mixture Substances 0.000 description 19
- 108010076282 Factor IX Proteins 0.000 description 18
- 229960004222 factor ix Drugs 0.000 description 18
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 17
- 210000004369 blood Anatomy 0.000 description 16
- 239000008280 blood Substances 0.000 description 16
- 230000000694 effects Effects 0.000 description 16
- 241000699666 Mus <mouse, genus> Species 0.000 description 15
- 102000004877 Insulin Human genes 0.000 description 14
- 108090001061 Insulin Proteins 0.000 description 14
- 229940125396 insulin Drugs 0.000 description 14
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 13
- 235000002639 sodium chloride Nutrition 0.000 description 13
- 108020004414 DNA Proteins 0.000 description 12
- 238000002965 ELISA Methods 0.000 description 12
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 12
- 208000035475 disorder Diseases 0.000 description 12
- 238000002474 experimental method Methods 0.000 description 12
- 238000011084 recovery Methods 0.000 description 12
- 230000004988 N-glycosylation Effects 0.000 description 11
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 11
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 11
- 150000001875 compounds Chemical class 0.000 description 11
- 239000000243 solution Substances 0.000 description 11
- 208000034158 bleeding Diseases 0.000 description 10
- 239000002299 complementary DNA Substances 0.000 description 10
- 238000004519 manufacturing process Methods 0.000 description 10
- 108010054218 Factor VIII Proteins 0.000 description 8
- 102000001690 Factor VIII Human genes 0.000 description 8
- 229960000301 factor viii Drugs 0.000 description 8
- 230000004048 modification Effects 0.000 description 8
- 238000012986 modification Methods 0.000 description 8
- YBYRMVIVWMBXKQ-UHFFFAOYSA-N phenylmethanesulfonyl fluoride Chemical compound FS(=O)(=O)CC1=CC=CC=C1 YBYRMVIVWMBXKQ-UHFFFAOYSA-N 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 8
- 150000003839 salts Chemical class 0.000 description 8
- 239000013598 vector Substances 0.000 description 8
- 102000004190 Enzymes Human genes 0.000 description 7
- 108090000790 Enzymes Proteins 0.000 description 7
- 239000004480 active ingredient Substances 0.000 description 7
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 description 7
- 238000003776 cleavage reaction Methods 0.000 description 7
- 229940088598 enzyme Drugs 0.000 description 7
- 239000013604 expression vector Substances 0.000 description 7
- 239000002609 medium Substances 0.000 description 7
- 230000037361 pathway Effects 0.000 description 7
- 239000013612 plasmid Substances 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- 230000007017 scission Effects 0.000 description 7
- 102100021277 Beta-secretase 2 Human genes 0.000 description 6
- 101710150190 Beta-secretase 2 Proteins 0.000 description 6
- 102000015081 Blood Coagulation Factors Human genes 0.000 description 6
- 108010039209 Blood Coagulation Factors Proteins 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- 125000000539 amino acid group Chemical group 0.000 description 6
- 239000003114 blood coagulation factor Substances 0.000 description 6
- 239000002552 dosage form Substances 0.000 description 6
- 230000013595 glycosylation Effects 0.000 description 6
- 238000006206 glycosylation reaction Methods 0.000 description 6
- 239000011780 sodium chloride Substances 0.000 description 6
- 238000002560 therapeutic procedure Methods 0.000 description 6
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 108091028043 Nucleic acid sequence Proteins 0.000 description 5
- 241000700605 Viruses Species 0.000 description 5
- 210000004899 c-terminal region Anatomy 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 230000007812 deficiency Effects 0.000 description 5
- 238000012217 deletion Methods 0.000 description 5
- 230000037430 deletion Effects 0.000 description 5
- 201000010099 disease Diseases 0.000 description 5
- 239000006185 dispersion Substances 0.000 description 5
- 239000003623 enhancer Substances 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 230000007246 mechanism Effects 0.000 description 5
- 150000003904 phospholipids Chemical class 0.000 description 5
- 150000003354 serine derivatives Chemical class 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 230000014616 translation Effects 0.000 description 5
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 4
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- 206010028980 Neoplasm Diseases 0.000 description 4
- 108010076504 Protein Sorting Signals Proteins 0.000 description 4
- 208000010378 Pulmonary Embolism Diseases 0.000 description 4
- 239000007983 Tris buffer Substances 0.000 description 4
- 206010047249 Venous thrombosis Diseases 0.000 description 4
- 230000002159 abnormal effect Effects 0.000 description 4
- 230000005856 abnormality Effects 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- PXXJHWLDUBFPOL-UHFFFAOYSA-N benzamidine Chemical compound NC(=N)C1=CC=CC=C1 PXXJHWLDUBFPOL-UHFFFAOYSA-N 0.000 description 4
- 230000023555 blood coagulation Effects 0.000 description 4
- 230000037396 body weight Effects 0.000 description 4
- 201000011510 cancer Diseases 0.000 description 4
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 4
- 230000035602 clotting Effects 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 208000010125 myocardial infarction Diseases 0.000 description 4
- SHUZOJHMOBOZST-UHFFFAOYSA-N phylloquinone Natural products CC(C)CCCCC(C)CCC(C)CCCC(=CCC1=C(C)C(=O)c2ccccc2C1=O)C SHUZOJHMOBOZST-UHFFFAOYSA-N 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 4
- 239000000758 substrate Substances 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 230000009261 transgenic effect Effects 0.000 description 4
- 238000013519 translation Methods 0.000 description 4
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 4
- 210000003462 vein Anatomy 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- BUDNAJYVCUHLSV-ZLUOBGJFSA-N Ala-Asp-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O BUDNAJYVCUHLSV-ZLUOBGJFSA-N 0.000 description 3
- JPAWCMXVNZPJLO-IHRRRGAJSA-N Arg-Ser-Phe Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O JPAWCMXVNZPJLO-IHRRRGAJSA-N 0.000 description 3
- 206010003178 Arterial thrombosis Diseases 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- VAIWPXWHWAPYDF-FXQIFTODSA-N Glu-Asp-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O VAIWPXWHWAPYDF-FXQIFTODSA-N 0.000 description 3
- 102000003886 Glycoproteins Human genes 0.000 description 3
- 108090000288 Glycoproteins Proteins 0.000 description 3
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 3
- 101000976075 Homo sapiens Insulin Proteins 0.000 description 3
- 101500025568 Homo sapiens Saposin-D Proteins 0.000 description 3
- 206010020608 Hypercoagulation Diseases 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 230000002424 anti-apoptotic effect Effects 0.000 description 3
- 230000002429 anti-coagulating effect Effects 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 210000004204 blood vessel Anatomy 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 230000003197 catalytic effect Effects 0.000 description 3
- 230000004087 circulation Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 230000003176 fibrotic effect Effects 0.000 description 3
- 238000001476 gene delivery Methods 0.000 description 3
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 3
- 230000023597 hemostasis Effects 0.000 description 3
- 229960002897 heparin Drugs 0.000 description 3
- 229920000669 heparin Polymers 0.000 description 3
- 229940088597 hormone Drugs 0.000 description 3
- 239000005556 hormone Substances 0.000 description 3
- 229940100689 human protein c Drugs 0.000 description 3
- 230000003027 hypercoagulation Effects 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- PBGKTOXHQIOBKM-FHFVDXKLSA-N insulin (human) Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3NC=NC=3)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)=O)CSSC[C@@H](C(N2)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1=CN=CN1 PBGKTOXHQIOBKM-FHFVDXKLSA-N 0.000 description 3
- 238000007918 intramuscular administration Methods 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 229910052740 iodine Inorganic materials 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 229960000485 methotrexate Drugs 0.000 description 3
- 244000005700 microbiome Species 0.000 description 3
- 238000002703 mutagenesis Methods 0.000 description 3
- 231100000350 mutagenesis Toxicity 0.000 description 3
- 239000002953 phosphate buffered saline Substances 0.000 description 3
- 230000001323 posttranslational effect Effects 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- NTQVODZUQIATFS-WAUHAFJUSA-N (2s)-2-[[(2s)-6-amino-2-[[2-[[(2s,3s)-2-[[(2s)-2-[[(2s)-2-amino-3-hydroxypropanoyl]amino]-4-methylpentanoyl]amino]-3-methylpentanoyl]amino]acetyl]amino]hexanoyl]amino]-3-methylbutanoic acid Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O NTQVODZUQIATFS-WAUHAFJUSA-N 0.000 description 2
- 101800001401 Activation peptide Proteins 0.000 description 2
- 102400000069 Activation peptide Human genes 0.000 description 2
- DKJPOZOEBONHFS-ZLUOBGJFSA-N Ala-Ala-Asp Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC(O)=O DKJPOZOEBONHFS-ZLUOBGJFSA-N 0.000 description 2
- DHBKYZYFEXXUAK-ONGXEEELSA-N Ala-Phe-Gly Chemical compound OC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)C)CC1=CC=CC=C1 DHBKYZYFEXXUAK-ONGXEEELSA-N 0.000 description 2
- LLUGJARLJCGLAR-CYDGBPFRSA-N Arg-Ile-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N LLUGJARLJCGLAR-CYDGBPFRSA-N 0.000 description 2
- DNUKXVMPARLPFN-XUXIUFHCSA-N Arg-Leu-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O DNUKXVMPARLPFN-XUXIUFHCSA-N 0.000 description 2
- URAUIUGLHBRPMF-NAKRPEOUSA-N Arg-Ser-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O URAUIUGLHBRPMF-NAKRPEOUSA-N 0.000 description 2
- OKZOABJQOMAYEC-NUMRIWBASA-N Asn-Gln-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OKZOABJQOMAYEC-NUMRIWBASA-N 0.000 description 2
- FHETWELNCBMRMG-HJGDQZAQSA-N Asn-Leu-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O FHETWELNCBMRMG-HJGDQZAQSA-N 0.000 description 2
- 208000023275 Autoimmune disease Diseases 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- 108020004705 Codon Proteins 0.000 description 2
- 241000699800 Cricetinae Species 0.000 description 2
- 108010074864 Factor XI Proteins 0.000 description 2
- 108010073385 Fibrin Proteins 0.000 description 2
- 102000009123 Fibrin Human genes 0.000 description 2
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 2
- XXLBHPPXDUWYAG-XQXXSGGOSA-N Gln-Ala-Thr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XXLBHPPXDUWYAG-XQXXSGGOSA-N 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- KKBWDNZXYLGJEY-UHFFFAOYSA-N Gly-Arg-Pro Natural products NCC(=O)NC(CCNC(=N)N)C(=O)N1CCCC1C(=O)O KKBWDNZXYLGJEY-UHFFFAOYSA-N 0.000 description 2
- XTQFHTHIAKKCTM-YFKPBYRVSA-N Gly-Glu-Gly Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O XTQFHTHIAKKCTM-YFKPBYRVSA-N 0.000 description 2
- CUYLIWAAAYJKJH-RYUDHWBXSA-N Gly-Glu-Tyr Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 CUYLIWAAAYJKJH-RYUDHWBXSA-N 0.000 description 2
- GJHWILMUOANXTG-WPRPVWTQSA-N Gly-Val-Arg Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O GJHWILMUOANXTG-WPRPVWTQSA-N 0.000 description 2
- JBCLFWXMTIKCCB-UHFFFAOYSA-N H-Gly-Phe-OH Natural products NCC(=O)NC(C(O)=O)CC1=CC=CC=C1 JBCLFWXMTIKCCB-UHFFFAOYSA-N 0.000 description 2
- RVKIPWVMZANZLI-UHFFFAOYSA-N H-Lys-Trp-OH Natural products C1=CC=C2C(CC(NC(=O)C(N)CCCCN)C(O)=O)=CNC2=C1 RVKIPWVMZANZLI-UHFFFAOYSA-N 0.000 description 2
- 208000031220 Hemophilia Diseases 0.000 description 2
- 101500025440 Homo sapiens Fibrinopeptide A Proteins 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 108010065920 Insulin Lispro Proteins 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- RCFDOSNHHZGBOY-UHFFFAOYSA-N L-isoleucyl-L-alanine Natural products CCC(C)C(N)C(=O)NC(C)C(O)=O RCFDOSNHHZGBOY-UHFFFAOYSA-N 0.000 description 2
- JQSXWJXBASFONF-KKUMJFAQSA-N Leu-Asp-Phe Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O JQSXWJXBASFONF-KKUMJFAQSA-N 0.000 description 2
- PVMPDMIKUVNOBD-CIUDSAMLSA-N Leu-Asp-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O PVMPDMIKUVNOBD-CIUDSAMLSA-N 0.000 description 2
- RVVBWTWPNFDYBE-SRVKXCTJSA-N Leu-Glu-Arg Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O RVVBWTWPNFDYBE-SRVKXCTJSA-N 0.000 description 2
- KOSWSHVQIVTVQF-ZPFDUUQYSA-N Leu-Ile-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O KOSWSHVQIVTVQF-ZPFDUUQYSA-N 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- ABSPRNADVQNDOU-UHFFFAOYSA-N Menaquinone 1 Natural products C1=CC=C2C(=O)C(CC=C(C)C)=C(C)C(=O)C2=C1 ABSPRNADVQNDOU-UHFFFAOYSA-N 0.000 description 2
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 2
- OVRNDRQMDRJTHS-UHFFFAOYSA-N N-acelyl-D-glucosamine Natural products CC(=O)NC1C(O)OC(CO)C(O)C1O OVRNDRQMDRJTHS-UHFFFAOYSA-N 0.000 description 2
- OVRNDRQMDRJTHS-FMDGEEDCSA-N N-acetyl-beta-D-glucosamine Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-FMDGEEDCSA-N 0.000 description 2
- MBLBDJOUHNCFQT-LXGUWJNJSA-N N-acetylglucosamine Natural products CC(=O)N[C@@H](C=O)[C@@H](O)[C@H](O)[C@H](O)CO MBLBDJOUHNCFQT-LXGUWJNJSA-N 0.000 description 2
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 2
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 2
- 229930182555 Penicillin Natural products 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- 102000035195 Peptidases Human genes 0.000 description 2
- 108091005804 Peptidases Proteins 0.000 description 2
- RIYZXJVARWJLKS-KKUMJFAQSA-N Phe-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC1=CC=CC=C1 RIYZXJVARWJLKS-KKUMJFAQSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 239000004353 Polyethylene glycol 8000 Substances 0.000 description 2
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 2
- ZCXQTRXYZOSGJR-FXQIFTODSA-N Pro-Asp-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O ZCXQTRXYZOSGJR-FXQIFTODSA-N 0.000 description 2
- CWZUFLWPEFHWEI-IHRRRGAJSA-N Pro-Tyr-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O CWZUFLWPEFHWEI-IHRRRGAJSA-N 0.000 description 2
- 102000007327 Protamines Human genes 0.000 description 2
- 108010007568 Protamines Proteins 0.000 description 2
- 239000004365 Protease Substances 0.000 description 2
- 102100037132 Proteinase-activated receptor 2 Human genes 0.000 description 2
- 101710121435 Proteinase-activated receptor 2 Proteins 0.000 description 2
- 108010094028 Prothrombin Proteins 0.000 description 2
- 102100027378 Prothrombin Human genes 0.000 description 2
- 108010054530 RGDN peptide Proteins 0.000 description 2
- 229920005654 Sephadex Polymers 0.000 description 2
- 239000012507 Sephadex™ Substances 0.000 description 2
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 2
- PMTWIUBUQRGCSB-FXQIFTODSA-N Ser-Val-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O PMTWIUBUQRGCSB-FXQIFTODSA-N 0.000 description 2
- MYNYCUXMIIWUNW-IEGACIPQSA-N Thr-Trp-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CCCCN)C(=O)O)N)O MYNYCUXMIIWUNW-IEGACIPQSA-N 0.000 description 2
- 239000004473 Threonine Substances 0.000 description 2
- OTJMMKPMLUNTQT-AVGNSLFASA-N Val-Leu-Arg Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](C(C)C)N OTJMMKPMLUNTQT-AVGNSLFASA-N 0.000 description 2
- 108700005077 Viral Genes Proteins 0.000 description 2
- 229930003448 Vitamin K Natural products 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 238000001042 affinity chromatography Methods 0.000 description 2
- 108010044940 alanylglutamine Proteins 0.000 description 2
- 108010087924 alanylproline Proteins 0.000 description 2
- 238000002399 angioplasty Methods 0.000 description 2
- 210000004102 animal cell Anatomy 0.000 description 2
- 230000003110 anti-inflammatory effect Effects 0.000 description 2
- 230000002785 anti-thrombosis Effects 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 108010013835 arginine glutamate Proteins 0.000 description 2
- 108010047857 aspartylglycine Proteins 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 230000017531 blood circulation Effects 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 239000013599 cloning vector Substances 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 210000004351 coronary vessel Anatomy 0.000 description 2
- 108010016616 cysteinylglycine Proteins 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- 230000003831 deregulation Effects 0.000 description 2
- 239000002612 dispersion medium Substances 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 230000002255 enzymatic effect Effects 0.000 description 2
- 229950003499 fibrin Drugs 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 230000006251 gamma-carboxylation Effects 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 238000001415 gene therapy Methods 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 description 2
- 108010015792 glycyllysine Proteins 0.000 description 2
- 108010081551 glycylphenylalanine Proteins 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 108010085325 histidylproline Proteins 0.000 description 2
- 238000005805 hydroxylation reaction Methods 0.000 description 2
- 208000027866 inflammatory disease Diseases 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 239000007972 injectable composition Substances 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 108010034529 leucyl-lysine Proteins 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 108010003700 lysyl aspartic acid Proteins 0.000 description 2
- 229940126619 mouse monoclonal antibody Drugs 0.000 description 2
- 229950006780 n-acetylglucosamine Drugs 0.000 description 2
- 230000014508 negative regulation of coagulation Effects 0.000 description 2
- 239000002773 nucleotide Substances 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- 239000006186 oral dosage form Substances 0.000 description 2
- 238000004806 packaging method and process Methods 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 229940049954 penicillin Drugs 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 239000011772 phylloquinone Substances 0.000 description 2
- 235000019175 phylloquinone Nutrition 0.000 description 2
- MBWXNTAXLNYFJB-NKFFZRIASA-N phylloquinone Chemical compound C1=CC=C2C(=O)C(C/C=C(C)/CCC[C@H](C)CCC[C@H](C)CCCC(C)C)=C(C)C(=O)C2=C1 MBWXNTAXLNYFJB-NKFFZRIASA-N 0.000 description 2
- 230000004962 physiological condition Effects 0.000 description 2
- 229960001898 phytomenadione Drugs 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229940085678 polyethylene glycol 8000 Drugs 0.000 description 2
- 235000019446 polyethylene glycol 8000 Nutrition 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 230000002947 procoagulating effect Effects 0.000 description 2
- 229940048914 protamine Drugs 0.000 description 2
- 108020001580 protein domains Proteins 0.000 description 2
- 229940039716 prothrombin Drugs 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 208000023504 respiratory system disease Diseases 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 108010048818 seryl-histidine Proteins 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 229940124547 specific antidotes Drugs 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 230000007838 tissue remodeling Effects 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- 230000032258 transport Effects 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- 108010038745 tryptophylglycine Proteins 0.000 description 2
- 108010087967 type I signal peptidase Proteins 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 241001430294 unidentified retrovirus Species 0.000 description 2
- 239000013603 viral vector Substances 0.000 description 2
- 235000019168 vitamin K Nutrition 0.000 description 2
- 239000011712 vitamin K Substances 0.000 description 2
- 150000003721 vitamin K derivatives Chemical class 0.000 description 2
- 229940046010 vitamin k Drugs 0.000 description 2
- 229960005080 warfarin Drugs 0.000 description 2
- PJVWKTKQMONHTI-UHFFFAOYSA-N warfarin Chemical compound OC=1C2=CC=CC=C2OC(=O)C=1C(CC(=O)C)C1=CC=CC=C1 PJVWKTKQMONHTI-UHFFFAOYSA-N 0.000 description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- ZXJZGWOMAFPSJH-DCAQKATOSA-N (2S)-1-[2-[[2-[[(2S)-2-[[(2S)-2-[(2-aminoacetyl)amino]-3-carboxypropanoyl]amino]-3-hydroxypropanoyl]amino]acetyl]amino]acetyl]pyrrolidine-2-carboxylic acid Chemical compound [H]NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)NCC(=O)NCC(=O)N1CCC[C@H]1C(O)=O ZXJZGWOMAFPSJH-DCAQKATOSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- 125000000979 2-amino-2-oxoethyl group Chemical group [H]C([*])([H])C(=O)N([H])[H] 0.000 description 1
- 108700025721 3K3A-APC Proteins 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 239000013607 AAV vector Substances 0.000 description 1
- 206010067484 Adverse reaction Diseases 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- WQVFQXXBNHHPLX-ZKWXMUAHSA-N Ala-Ala-His Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O WQVFQXXBNHHPLX-ZKWXMUAHSA-N 0.000 description 1
- PJNSIUPOXFBHDM-GUBZILKMSA-N Ala-Arg-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O PJNSIUPOXFBHDM-GUBZILKMSA-N 0.000 description 1
- GSCLWXDNIMNIJE-ZLUOBGJFSA-N Ala-Asp-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O GSCLWXDNIMNIJE-ZLUOBGJFSA-N 0.000 description 1
- WCBVQNZTOKJWJS-ACZMJKKPSA-N Ala-Cys-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(O)=O WCBVQNZTOKJWJS-ACZMJKKPSA-N 0.000 description 1
- PUBLUECXJRHTBK-ACZMJKKPSA-N Ala-Glu-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O PUBLUECXJRHTBK-ACZMJKKPSA-N 0.000 description 1
- WMYJZJRILUVVRG-WDSKDSINSA-N Ala-Gly-Gln Chemical compound C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(N)=O WMYJZJRILUVVRG-WDSKDSINSA-N 0.000 description 1
- LMFXXZPPZDCPTA-ZKWXMUAHSA-N Ala-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@H](C)N LMFXXZPPZDCPTA-ZKWXMUAHSA-N 0.000 description 1
- LTSBJNNXPBBNDT-HGNGGELXSA-N Ala-His-Gln Chemical compound N[C@@H](C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(N)=O)C(=O)O LTSBJNNXPBBNDT-HGNGGELXSA-N 0.000 description 1
- DVJSJDDYCYSMFR-ZKWXMUAHSA-N Ala-Ile-Gly Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O DVJSJDDYCYSMFR-ZKWXMUAHSA-N 0.000 description 1
- RGDKRCPIFODMHK-HJWJTTGWSA-N Ala-Leu-Leu-His Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 RGDKRCPIFODMHK-HJWJTTGWSA-N 0.000 description 1
- NINQYGGNRIBFSC-CIUDSAMLSA-N Ala-Lys-Ser Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)C)C(=O)N[C@@H](CO)C(O)=O NINQYGGNRIBFSC-CIUDSAMLSA-N 0.000 description 1
- SGFBVLBKDSXGAP-GKCIPKSASA-N Ala-Phe-Trp Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N SGFBVLBKDSXGAP-GKCIPKSASA-N 0.000 description 1
- HOVPGJUNRLMIOZ-CIUDSAMLSA-N Ala-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)N HOVPGJUNRLMIOZ-CIUDSAMLSA-N 0.000 description 1
- VHAQSYHSDKERBS-XPUUQOCRSA-N Ala-Val-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O VHAQSYHSDKERBS-XPUUQOCRSA-N 0.000 description 1
- CLOMBHBBUKAUBP-LSJOCFKGSA-N Ala-Val-His Chemical compound C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N CLOMBHBBUKAUBP-LSJOCFKGSA-N 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- DFCIPNHFKOQAME-FXQIFTODSA-N Arg-Ala-Asn Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(O)=O DFCIPNHFKOQAME-FXQIFTODSA-N 0.000 description 1
- PEFFAAKJGBZBKL-NAKRPEOUSA-N Arg-Ala-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O PEFFAAKJGBZBKL-NAKRPEOUSA-N 0.000 description 1
- SBVJJNJLFWSJOV-UBHSHLNASA-N Arg-Ala-Phe Chemical compound NC(=N)NCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 SBVJJNJLFWSJOV-UBHSHLNASA-N 0.000 description 1
- YSUVMPICYVWRBX-VEVYYDQMSA-N Arg-Asp-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YSUVMPICYVWRBX-VEVYYDQMSA-N 0.000 description 1
- ASQYTJJWAMDISW-BPUTZDHNSA-N Arg-Asp-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCCN=C(N)N)N ASQYTJJWAMDISW-BPUTZDHNSA-N 0.000 description 1
- PTVGLOCPAVYPFG-CIUDSAMLSA-N Arg-Gln-Asp Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O PTVGLOCPAVYPFG-CIUDSAMLSA-N 0.000 description 1
- BEXGZLUHRXTZCC-CIUDSAMLSA-N Arg-Gln-Ser Chemical compound C(C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CO)C(=O)O)N)CN=C(N)N BEXGZLUHRXTZCC-CIUDSAMLSA-N 0.000 description 1
- NYZGVTGOMPHSJW-CIUDSAMLSA-N Arg-Glu-Cys Chemical compound C(C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CS)C(=O)O)N)CN=C(N)N NYZGVTGOMPHSJW-CIUDSAMLSA-N 0.000 description 1
- MZRBYBIQTIKERR-GUBZILKMSA-N Arg-Glu-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O MZRBYBIQTIKERR-GUBZILKMSA-N 0.000 description 1
- GOWZVQXTHUCNSQ-NHCYSSNCSA-N Arg-Glu-Val Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O GOWZVQXTHUCNSQ-NHCYSSNCSA-N 0.000 description 1
- OFIYLHVAAJYRBC-HJWJTTGWSA-N Arg-Ile-Phe Chemical compound CC[C@H](C)[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(O)=O OFIYLHVAAJYRBC-HJWJTTGWSA-N 0.000 description 1
- IIAXFBUTKIDDIP-ULQDDVLXSA-N Arg-Leu-Phe Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O IIAXFBUTKIDDIP-ULQDDVLXSA-N 0.000 description 1
- FSNVAJOPUDVQAR-AVGNSLFASA-N Arg-Lys-Arg Chemical compound NC(=N)NCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O FSNVAJOPUDVQAR-AVGNSLFASA-N 0.000 description 1
- NGTYEHIRESTSRX-UWVGGRQHSA-N Arg-Lys-Gly Chemical compound NCCCC[C@@H](C(=O)NCC(O)=O)NC(=O)[C@@H](N)CCCN=C(N)N NGTYEHIRESTSRX-UWVGGRQHSA-N 0.000 description 1
- VIINVRPKMUZYOI-DCAQKATOSA-N Arg-Met-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(O)=O VIINVRPKMUZYOI-DCAQKATOSA-N 0.000 description 1
- BSGSDLYGGHGMND-IHRRRGAJSA-N Arg-Phe-Cys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N BSGSDLYGGHGMND-IHRRRGAJSA-N 0.000 description 1
- KZXPVYVSHUJCEO-ULQDDVLXSA-N Arg-Phe-Lys Chemical compound NC(=N)NCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CCCCN)C(O)=O)CC1=CC=CC=C1 KZXPVYVSHUJCEO-ULQDDVLXSA-N 0.000 description 1
- OQPAZKMGCWPERI-GUBZILKMSA-N Arg-Ser-Val Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O OQPAZKMGCWPERI-GUBZILKMSA-N 0.000 description 1
- MOGMYRUNTKYZFB-UNQGMJICSA-N Arg-Thr-Phe Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MOGMYRUNTKYZFB-UNQGMJICSA-N 0.000 description 1
- ZWASIOHRQWRWAS-UGYAYLCHSA-N Asn-Asp-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ZWASIOHRQWRWAS-UGYAYLCHSA-N 0.000 description 1
- VJTWLBMESLDOMK-WDSKDSINSA-N Asn-Gln-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O VJTWLBMESLDOMK-WDSKDSINSA-N 0.000 description 1
- GNKVBRYFXYWXAB-WDSKDSINSA-N Asn-Glu-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O GNKVBRYFXYWXAB-WDSKDSINSA-N 0.000 description 1
- GQRDIVQPSMPQME-ZPFDUUQYSA-N Asn-Ile-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O GQRDIVQPSMPQME-ZPFDUUQYSA-N 0.000 description 1
- RLHANKIRBONJBK-IHRRRGAJSA-N Asn-Met-Phe Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CC(=O)N)N RLHANKIRBONJBK-IHRRRGAJSA-N 0.000 description 1
- KYQJHBWHRASMKG-ZLUOBGJFSA-N Asn-Ser-Cys Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(O)=O KYQJHBWHRASMKG-ZLUOBGJFSA-N 0.000 description 1
- VLDRQOHCMKCXLY-SRVKXCTJSA-N Asn-Ser-Phe Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O VLDRQOHCMKCXLY-SRVKXCTJSA-N 0.000 description 1
- NCXTYSVDWLAQGZ-ZKWXMUAHSA-N Asn-Ser-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O NCXTYSVDWLAQGZ-ZKWXMUAHSA-N 0.000 description 1
- ZUFPUBYQYWCMDB-NUMRIWBASA-N Asn-Thr-Glu Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZUFPUBYQYWCMDB-NUMRIWBASA-N 0.000 description 1
- CBWCQCANJSGUOH-ZKWXMUAHSA-N Asn-Val-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O CBWCQCANJSGUOH-ZKWXMUAHSA-N 0.000 description 1
- GBAWQWASNGUNQF-ZLUOBGJFSA-N Asp-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)O)N GBAWQWASNGUNQF-ZLUOBGJFSA-N 0.000 description 1
- GWTLRDMPMJCNMH-WHFBIAKZSA-N Asp-Asn-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O GWTLRDMPMJCNMH-WHFBIAKZSA-N 0.000 description 1
- SBHUBSDEZQFJHJ-CIUDSAMLSA-N Asp-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(O)=O SBHUBSDEZQFJHJ-CIUDSAMLSA-N 0.000 description 1
- BFOYULZBKYOKAN-OLHMAJIHSA-N Asp-Asp-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O BFOYULZBKYOKAN-OLHMAJIHSA-N 0.000 description 1
- MJKBOVWWADWLHV-ZLUOBGJFSA-N Asp-Cys-Asp Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)O)C(=O)O)N)C(=O)O MJKBOVWWADWLHV-ZLUOBGJFSA-N 0.000 description 1
- SMZCLQGDQMGESY-ACZMJKKPSA-N Asp-Gln-Cys Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)O)N SMZCLQGDQMGESY-ACZMJKKPSA-N 0.000 description 1
- PSLSTUMPZILTAH-BYULHYEWSA-N Asp-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(O)=O PSLSTUMPZILTAH-BYULHYEWSA-N 0.000 description 1
- QCVXMEHGFUMKCO-YUMQZZPRSA-N Asp-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(O)=O QCVXMEHGFUMKCO-YUMQZZPRSA-N 0.000 description 1
- PZXPWHFYZXTFBI-YUMQZZPRSA-N Asp-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(O)=O PZXPWHFYZXTFBI-YUMQZZPRSA-N 0.000 description 1
- PAYPSKIBMDHZPI-CIUDSAMLSA-N Asp-Leu-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O PAYPSKIBMDHZPI-CIUDSAMLSA-N 0.000 description 1
- GWIJZUVQVDJHDI-AVGNSLFASA-N Asp-Phe-Glu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O GWIJZUVQVDJHDI-AVGNSLFASA-N 0.000 description 1
- ZKAOJVJQGVUIIU-GUBZILKMSA-N Asp-Pro-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O ZKAOJVJQGVUIIU-GUBZILKMSA-N 0.000 description 1
- RVMXMLSYBTXCAV-VEVYYDQMSA-N Asp-Pro-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O RVMXMLSYBTXCAV-VEVYYDQMSA-N 0.000 description 1
- KGHLGJAXYSVNJP-WHFBIAKZSA-N Asp-Ser-Gly Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O KGHLGJAXYSVNJP-WHFBIAKZSA-N 0.000 description 1
- DRCOAZZDQRCGGP-GHCJXIJMSA-N Asp-Ser-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O DRCOAZZDQRCGGP-GHCJXIJMSA-N 0.000 description 1
- HRVQDZOWMLFAOD-BIIVOSGPSA-N Asp-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CC(=O)O)N)C(=O)O HRVQDZOWMLFAOD-BIIVOSGPSA-N 0.000 description 1
- JDDYEZGPYBBPBN-JRQIVUDYSA-N Asp-Thr-Tyr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O JDDYEZGPYBBPBN-JRQIVUDYSA-N 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 101710117545 C protein Proteins 0.000 description 1
- 125000001433 C-terminal amino-acid group Chemical group 0.000 description 1
- 101100512078 Caenorhabditis elegans lys-1 gene Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 108020004635 Complementary DNA Proteins 0.000 description 1
- 206010011086 Coronary artery occlusion Diseases 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- AMRLSQGGERHDHJ-FXQIFTODSA-N Cys-Ala-Arg Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AMRLSQGGERHDHJ-FXQIFTODSA-N 0.000 description 1
- AEJSNWMRPXAKCW-WHFBIAKZSA-N Cys-Ala-Gly Chemical compound SC[C@H](N)C(=O)N[C@@H](C)C(=O)NCC(O)=O AEJSNWMRPXAKCW-WHFBIAKZSA-N 0.000 description 1
- GEEXORWTBTUOHC-FXQIFTODSA-N Cys-Arg-Ser Chemical compound C(C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CS)N)CN=C(N)N GEEXORWTBTUOHC-FXQIFTODSA-N 0.000 description 1
- BDWIZLQVVWQMTB-XKBZYTNZSA-N Cys-Glu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CS)N)O BDWIZLQVVWQMTB-XKBZYTNZSA-N 0.000 description 1
- HQZGVYJBRSISDT-BQBZGAKWSA-N Cys-Gly-Arg Chemical compound [H]N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O HQZGVYJBRSISDT-BQBZGAKWSA-N 0.000 description 1
- RWAZRMXTVSIVJR-YUMQZZPRSA-N Cys-Gly-His Chemical compound [H]N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CC1=CNC=N1)C(O)=O RWAZRMXTVSIVJR-YUMQZZPRSA-N 0.000 description 1
- LBOLGUYQEPZSKM-YUMQZZPRSA-N Cys-Gly-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CS)N LBOLGUYQEPZSKM-YUMQZZPRSA-N 0.000 description 1
- ODDOYXKAHLKKQY-MMWGEVLESA-N Cys-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CS)N ODDOYXKAHLKKQY-MMWGEVLESA-N 0.000 description 1
- XLLSMEFANRROJE-GUBZILKMSA-N Cys-Leu-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CS)N XLLSMEFANRROJE-GUBZILKMSA-N 0.000 description 1
- VXLXATVURDNDCG-CIUDSAMLSA-N Cys-Lys-Asp Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CS)N VXLXATVURDNDCG-CIUDSAMLSA-N 0.000 description 1
- LWYKPOCGGTYAIH-FXQIFTODSA-N Cys-Met-Asp Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CS)N LWYKPOCGGTYAIH-FXQIFTODSA-N 0.000 description 1
- GGRDJANMZPGMNS-CIUDSAMLSA-N Cys-Ser-Leu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O GGRDJANMZPGMNS-CIUDSAMLSA-N 0.000 description 1
- SHZGCJCMOBCMKK-UHFFFAOYSA-N D-mannomethylose Natural products CC1OC(O)C(O)C(O)C1O SHZGCJCMOBCMKK-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 1
- 206010051055 Deep vein thrombosis Diseases 0.000 description 1
- 241000702421 Dependoparvovirus Species 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- 101100352762 Drosophila melanogaster pnut gene Proteins 0.000 description 1
- 101100117236 Drosophila melanogaster speck gene Proteins 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 102000002322 Egg Proteins Human genes 0.000 description 1
- 108010000912 Egg Proteins Proteins 0.000 description 1
- 208000005189 Embolism Diseases 0.000 description 1
- 108090000394 Erythropoietin Proteins 0.000 description 1
- 102000003951 Erythropoietin Human genes 0.000 description 1
- 108010014172 Factor V Proteins 0.000 description 1
- 108010061932 Factor VIIIa Proteins 0.000 description 1
- 108010054265 Factor VIIa Proteins 0.000 description 1
- 108010071289 Factor XIII Proteins 0.000 description 1
- 229940123583 Factor Xa inhibitor Drugs 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- PNNNRSAQSRJVSB-SLPGGIOYSA-N Fucose Natural products C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C=O PNNNRSAQSRJVSB-SLPGGIOYSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- LZRMPXRYLLTAJX-GUBZILKMSA-N Gln-Arg-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O LZRMPXRYLLTAJX-GUBZILKMSA-N 0.000 description 1
- LMPBBFWHCRURJD-LAEOZQHASA-N Gln-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCC(=O)N)N LMPBBFWHCRURJD-LAEOZQHASA-N 0.000 description 1
- SNLOOPZHAQDMJG-CIUDSAMLSA-N Gln-Glu-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O SNLOOPZHAQDMJG-CIUDSAMLSA-N 0.000 description 1
- CELXWPDNIGWCJN-WDCWCFNPSA-N Gln-Lys-Thr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CELXWPDNIGWCJN-WDCWCFNPSA-N 0.000 description 1
- DSRVQBZAMPGEKU-AVGNSLFASA-N Gln-Phe-Cys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)N)N DSRVQBZAMPGEKU-AVGNSLFASA-N 0.000 description 1
- HMIXCETWRYDVMO-GUBZILKMSA-N Gln-Pro-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O HMIXCETWRYDVMO-GUBZILKMSA-N 0.000 description 1
- JJKKWYQVHRUSDG-GUBZILKMSA-N Glu-Ala-Lys Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O JJKKWYQVHRUSDG-GUBZILKMSA-N 0.000 description 1
- IRDASPPCLZIERZ-XHNCKOQMSA-N Glu-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)O)N IRDASPPCLZIERZ-XHNCKOQMSA-N 0.000 description 1
- MPZWMIIOPAPAKE-BQBZGAKWSA-N Glu-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(O)=O)CCCNC(N)=N MPZWMIIOPAPAKE-BQBZGAKWSA-N 0.000 description 1
- CGYDXNKRIMJMLV-GUBZILKMSA-N Glu-Arg-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O CGYDXNKRIMJMLV-GUBZILKMSA-N 0.000 description 1
- LXAUHIRMWXQRKI-XHNCKOQMSA-N Glu-Asn-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCC(=O)O)N)C(=O)O LXAUHIRMWXQRKI-XHNCKOQMSA-N 0.000 description 1
- XKPOCESCRTVRPL-KBIXCLLPSA-N Glu-Cys-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O XKPOCESCRTVRPL-KBIXCLLPSA-N 0.000 description 1
- KVBPDJIFRQUQFY-ACZMJKKPSA-N Glu-Cys-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O KVBPDJIFRQUQFY-ACZMJKKPSA-N 0.000 description 1
- CGOHAEBMDSEKFB-FXQIFTODSA-N Glu-Glu-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O CGOHAEBMDSEKFB-FXQIFTODSA-N 0.000 description 1
- AUTNXSQEVVHSJK-YVNDNENWSA-N Glu-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O AUTNXSQEVVHSJK-YVNDNENWSA-N 0.000 description 1
- WTMZXOPHTIVFCP-QEWYBTABSA-N Glu-Ile-Phe Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 WTMZXOPHTIVFCP-QEWYBTABSA-N 0.000 description 1
- VSRCAOIHMGCIJK-SRVKXCTJSA-N Glu-Leu-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O VSRCAOIHMGCIJK-SRVKXCTJSA-N 0.000 description 1
- WNRZUESNGGDCJX-JYJNAYRXSA-N Glu-Leu-Phe Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O WNRZUESNGGDCJX-JYJNAYRXSA-N 0.000 description 1
- BCYGDJXHAGZNPQ-DCAQKATOSA-N Glu-Lys-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O BCYGDJXHAGZNPQ-DCAQKATOSA-N 0.000 description 1
- OCJRHJZKGGSPRW-IUCAKERBSA-N Glu-Lys-Gly Chemical compound NCCCC[C@@H](C(=O)NCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O OCJRHJZKGGSPRW-IUCAKERBSA-N 0.000 description 1
- IDEODOAVGCMUQV-GUBZILKMSA-N Glu-Ser-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O IDEODOAVGCMUQV-GUBZILKMSA-N 0.000 description 1
- QOXDAWODGSIDDI-GUBZILKMSA-N Glu-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)O)N QOXDAWODGSIDDI-GUBZILKMSA-N 0.000 description 1
- VHPVBPCCWVDGJL-IRIUXVKKSA-N Glu-Thr-Tyr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O VHPVBPCCWVDGJL-IRIUXVKKSA-N 0.000 description 1
- HQTDNEZTGZUWSY-XVKPBYJWSA-N Glu-Val-Gly Chemical compound CC(C)[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)NCC(O)=O HQTDNEZTGZUWSY-XVKPBYJWSA-N 0.000 description 1
- NTNUEBVGKMVANB-NHCYSSNCSA-N Glu-Val-Met Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(O)=O NTNUEBVGKMVANB-NHCYSSNCSA-N 0.000 description 1
- RMWAOBGCZZSJHE-UMNHJUIQSA-N Glu-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)O)N RMWAOBGCZZSJHE-UMNHJUIQSA-N 0.000 description 1
- DTPOVRRYXPJJAZ-FJXKBIBVSA-N Gly-Arg-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CCCN=C(N)N DTPOVRRYXPJJAZ-FJXKBIBVSA-N 0.000 description 1
- IWAXHBCACVWNHT-BQBZGAKWSA-N Gly-Asp-Arg Chemical compound NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N IWAXHBCACVWNHT-BQBZGAKWSA-N 0.000 description 1
- XEJTYSCIXKYSHR-WDSKDSINSA-N Gly-Asp-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)CN XEJTYSCIXKYSHR-WDSKDSINSA-N 0.000 description 1
- GVVKYKCOFMMTKZ-WHFBIAKZSA-N Gly-Cys-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)CN GVVKYKCOFMMTKZ-WHFBIAKZSA-N 0.000 description 1
- MOJKRXIRAZPZLW-WDSKDSINSA-N Gly-Glu-Ala Chemical compound [H]NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O MOJKRXIRAZPZLW-WDSKDSINSA-N 0.000 description 1
- BIRKKBCSAIHDDF-WDSKDSINSA-N Gly-Glu-Cys Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(O)=O BIRKKBCSAIHDDF-WDSKDSINSA-N 0.000 description 1
- IDOGEHIWMJMAHT-BYPYZUCNSA-N Gly-Gly-Cys Chemical compound NCC(=O)NCC(=O)N[C@@H](CS)C(O)=O IDOGEHIWMJMAHT-BYPYZUCNSA-N 0.000 description 1
- QPTNELDXWKRIFX-YFKPBYRVSA-N Gly-Gly-Gln Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CCC(N)=O QPTNELDXWKRIFX-YFKPBYRVSA-N 0.000 description 1
- GDOZQTNZPCUARW-YFKPBYRVSA-N Gly-Gly-Glu Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CCC(O)=O GDOZQTNZPCUARW-YFKPBYRVSA-N 0.000 description 1
- COVXELOAORHTND-LSJOCFKGSA-N Gly-Ile-Val Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(O)=O COVXELOAORHTND-LSJOCFKGSA-N 0.000 description 1
- PAWIVEIWWYGBAM-YUMQZZPRSA-N Gly-Leu-Ala Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O PAWIVEIWWYGBAM-YUMQZZPRSA-N 0.000 description 1
- VBOBNHSVQKKTOT-YUMQZZPRSA-N Gly-Lys-Ala Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O VBOBNHSVQKKTOT-YUMQZZPRSA-N 0.000 description 1
- FHQRLHFYVZAQHU-IUCAKERBSA-N Gly-Lys-Gln Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O FHQRLHFYVZAQHU-IUCAKERBSA-N 0.000 description 1
- PCPOYRCAHPJXII-UWVGGRQHSA-N Gly-Lys-Met Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(O)=O PCPOYRCAHPJXII-UWVGGRQHSA-N 0.000 description 1
- OCPPBNKYGYSLOE-IUCAKERBSA-N Gly-Pro-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CN OCPPBNKYGYSLOE-IUCAKERBSA-N 0.000 description 1
- JSLVAHYTAJJEQH-QWRGUYRKSA-N Gly-Ser-Phe Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 JSLVAHYTAJJEQH-QWRGUYRKSA-N 0.000 description 1
- ZKJZBRHRWKLVSJ-ZDLURKLDSA-N Gly-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)CN)O ZKJZBRHRWKLVSJ-ZDLURKLDSA-N 0.000 description 1
- HUFUVTYGPOUCBN-MBLNEYKQSA-N Gly-Thr-Ile Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HUFUVTYGPOUCBN-MBLNEYKQSA-N 0.000 description 1
- NIOPEYHPOBWLQO-KBPBESRZSA-N Gly-Trp-Glu Chemical compound NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(O)=O NIOPEYHPOBWLQO-KBPBESRZSA-N 0.000 description 1
- KBBFOULZCHWGJX-KBPBESRZSA-N Gly-Tyr-His Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)NC(=O)CN)O KBBFOULZCHWGJX-KBPBESRZSA-N 0.000 description 1
- GBYYQVBXFVDJPJ-WLTAIBSBSA-N Gly-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)CN)O GBYYQVBXFVDJPJ-WLTAIBSBSA-N 0.000 description 1
- IZVICCORZOSGPT-JSGCOSHPSA-N Gly-Val-Tyr Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O IZVICCORZOSGPT-JSGCOSHPSA-N 0.000 description 1
- 102000007625 Hirudins Human genes 0.000 description 1
- 108010007267 Hirudins Proteins 0.000 description 1
- HTZKFIYQMHJWSQ-INTQDDNPSA-N His-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CN=CN2)N HTZKFIYQMHJWSQ-INTQDDNPSA-N 0.000 description 1
- QZAFGJNKLMNDEM-DCAQKATOSA-N His-Asn-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC1=CN=CN1 QZAFGJNKLMNDEM-DCAQKATOSA-N 0.000 description 1
- VLPMGIJPAWENQB-SRVKXCTJSA-N His-Cys-Leu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O VLPMGIJPAWENQB-SRVKXCTJSA-N 0.000 description 1
- TXLQHACKRLWYCM-DCAQKATOSA-N His-Glu-Glu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O TXLQHACKRLWYCM-DCAQKATOSA-N 0.000 description 1
- OQDLKDUVMTUPPG-AVGNSLFASA-N His-Leu-Glu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O OQDLKDUVMTUPPG-AVGNSLFASA-N 0.000 description 1
- RNMNYMDTESKEAJ-KKUMJFAQSA-N His-Leu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CN=CN1 RNMNYMDTESKEAJ-KKUMJFAQSA-N 0.000 description 1
- VIJMRAIWYWRXSR-CIUDSAMLSA-N His-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CN=CN1 VIJMRAIWYWRXSR-CIUDSAMLSA-N 0.000 description 1
- GBMSSORHVHAYLU-QTKMDUPCSA-N His-Val-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC1=CN=CN1)N)O GBMSSORHVHAYLU-QTKMDUPCSA-N 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000635804 Homo sapiens Tissue factor Proteins 0.000 description 1
- 108090000144 Human Proteins Proteins 0.000 description 1
- 102000003839 Human Proteins Human genes 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- SACHLUOUHCVIKI-GMOBBJLQSA-N Ile-Arg-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N SACHLUOUHCVIKI-GMOBBJLQSA-N 0.000 description 1
- YOTNPRLPIPHQSB-XUXIUFHCSA-N Ile-Arg-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)O)N YOTNPRLPIPHQSB-XUXIUFHCSA-N 0.000 description 1
- UASTVUQJMLZWGG-PEXQALLHSA-N Ile-His-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)NCC(=O)O)N UASTVUQJMLZWGG-PEXQALLHSA-N 0.000 description 1
- AXNGDPAKKCEKGY-QPHKQPEJSA-N Ile-Ile-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N AXNGDPAKKCEKGY-QPHKQPEJSA-N 0.000 description 1
- ADDYYRVQQZFIMW-MNXVOIDGSA-N Ile-Lys-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N ADDYYRVQQZFIMW-MNXVOIDGSA-N 0.000 description 1
- MLSUZXHSNRBDCI-CYDGBPFRSA-N Ile-Pro-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)O)N MLSUZXHSNRBDCI-CYDGBPFRSA-N 0.000 description 1
- KXUKTDGKLAOCQK-LSJOCFKGSA-N Ile-Val-Gly Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O KXUKTDGKLAOCQK-LSJOCFKGSA-N 0.000 description 1
- JZBVBOKASHNXAD-NAKRPEOUSA-N Ile-Val-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)O)N JZBVBOKASHNXAD-NAKRPEOUSA-N 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 108020005350 Initiator Codon Proteins 0.000 description 1
- 108010090444 Innovin Proteins 0.000 description 1
- 241000581650 Ivesia Species 0.000 description 1
- 241000242362 Kordia Species 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- SHZGCJCMOBCMKK-DHVFOXMCSA-N L-fucopyranose Chemical compound C[C@@H]1OC(O)[C@@H](O)[C@H](O)[C@@H]1O SHZGCJCMOBCMKK-DHVFOXMCSA-N 0.000 description 1
- KFKWRHQBZQICHA-STQMWFEESA-N L-leucyl-L-phenylalanine Natural products CC(C)C[C@H](N)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 KFKWRHQBZQICHA-STQMWFEESA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- 101710173438 Late L2 mu core protein Proteins 0.000 description 1
- 241000713666 Lentivirus Species 0.000 description 1
- 241000880493 Leptailurus serval Species 0.000 description 1
- KVRKAGGMEWNURO-CIUDSAMLSA-N Leu-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N KVRKAGGMEWNURO-CIUDSAMLSA-N 0.000 description 1
- MJOZZTKJZQFKDK-GUBZILKMSA-N Leu-Ala-Gln Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(N)=O MJOZZTKJZQFKDK-GUBZILKMSA-N 0.000 description 1
- BAJIJEGGUYXZGC-CIUDSAMLSA-N Leu-Asn-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CS)C(=O)O)N BAJIJEGGUYXZGC-CIUDSAMLSA-N 0.000 description 1
- RFUBXQQFJFGJFV-GUBZILKMSA-N Leu-Asn-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O RFUBXQQFJFGJFV-GUBZILKMSA-N 0.000 description 1
- MMEDVBWCMGRKKC-GARJFASQSA-N Leu-Asp-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N MMEDVBWCMGRKKC-GARJFASQSA-N 0.000 description 1
- GBDMISNMNXVTNV-XIRDDKMYSA-N Leu-Asp-Trp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O GBDMISNMNXVTNV-XIRDDKMYSA-N 0.000 description 1
- DXYBNWJZJVSZAE-GUBZILKMSA-N Leu-Gln-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CS)C(=O)O)N DXYBNWJZJVSZAE-GUBZILKMSA-N 0.000 description 1
- WIDZHJTYKYBLSR-DCAQKATOSA-N Leu-Glu-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WIDZHJTYKYBLSR-DCAQKATOSA-N 0.000 description 1
- NEEOBPIXKWSBRF-IUCAKERBSA-N Leu-Glu-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O NEEOBPIXKWSBRF-IUCAKERBSA-N 0.000 description 1
- IWTBYNQNAPECCS-AVGNSLFASA-N Leu-Glu-His Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 IWTBYNQNAPECCS-AVGNSLFASA-N 0.000 description 1
- LAPSXOAUPNOINL-YUMQZZPRSA-N Leu-Gly-Asp Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(O)=O LAPSXOAUPNOINL-YUMQZZPRSA-N 0.000 description 1
- BTNXKBVLWJBTNR-SRVKXCTJSA-N Leu-His-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(N)=O)C(O)=O BTNXKBVLWJBTNR-SRVKXCTJSA-N 0.000 description 1
- USLNHQZCDQJBOV-ZPFDUUQYSA-N Leu-Ile-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O USLNHQZCDQJBOV-ZPFDUUQYSA-N 0.000 description 1
- QJXHMYMRGDOHRU-NHCYSSNCSA-N Leu-Ile-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O QJXHMYMRGDOHRU-NHCYSSNCSA-N 0.000 description 1
- JFSGIJSCJFQGSZ-MXAVVETBSA-N Leu-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC(C)C)N JFSGIJSCJFQGSZ-MXAVVETBSA-N 0.000 description 1
- YOKVEHGYYQEQOP-QWRGUYRKSA-N Leu-Leu-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O YOKVEHGYYQEQOP-QWRGUYRKSA-N 0.000 description 1
- UBZGNBKMIJHOHL-BZSNNMDCSA-N Leu-Leu-Phe Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C([O-])=O)CC1=CC=CC=C1 UBZGNBKMIJHOHL-BZSNNMDCSA-N 0.000 description 1
- RXGLHDWAZQECBI-SRVKXCTJSA-N Leu-Leu-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O RXGLHDWAZQECBI-SRVKXCTJSA-N 0.000 description 1
- OVZLLFONXILPDZ-VOAKCMCISA-N Leu-Lys-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OVZLLFONXILPDZ-VOAKCMCISA-N 0.000 description 1
- JVTYXRRFZCEPPK-RHYQMDGZSA-N Leu-Met-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(C)C)N)O JVTYXRRFZCEPPK-RHYQMDGZSA-N 0.000 description 1
- INCJJHQRZGQLFC-KBPBESRZSA-N Leu-Phe-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)NCC(O)=O INCJJHQRZGQLFC-KBPBESRZSA-N 0.000 description 1
- VULJUQZPSOASBZ-SRVKXCTJSA-N Leu-Pro-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O VULJUQZPSOASBZ-SRVKXCTJSA-N 0.000 description 1
- YRRCOJOXAJNSAX-IHRRRGAJSA-N Leu-Pro-Lys Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)O)N YRRCOJOXAJNSAX-IHRRRGAJSA-N 0.000 description 1
- AKVBOOKXVAMKSS-GUBZILKMSA-N Leu-Ser-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O AKVBOOKXVAMKSS-GUBZILKMSA-N 0.000 description 1
- JIHDFWWRYHSAQB-GUBZILKMSA-N Leu-Ser-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O JIHDFWWRYHSAQB-GUBZILKMSA-N 0.000 description 1
- ILDSIMPXNFWKLH-KATARQTJSA-N Leu-Thr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O ILDSIMPXNFWKLH-KATARQTJSA-N 0.000 description 1
- FBNPMTNBFFAMMH-UHFFFAOYSA-N Leu-Val-Arg Natural products CC(C)CC(N)C(=O)NC(C(C)C)C(=O)NC(C(O)=O)CCCN=C(N)N FBNPMTNBFFAMMH-UHFFFAOYSA-N 0.000 description 1
- ALSRJRIWBNENFY-DCAQKATOSA-N Lys-Arg-Asn Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(O)=O ALSRJRIWBNENFY-DCAQKATOSA-N 0.000 description 1
- NQCJGQHHYZNUDK-DCAQKATOSA-N Lys-Arg-Ser Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CCCN=C(N)N NQCJGQHHYZNUDK-DCAQKATOSA-N 0.000 description 1
- WLCYCADOWRMSAJ-CIUDSAMLSA-N Lys-Asn-Cys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(O)=O WLCYCADOWRMSAJ-CIUDSAMLSA-N 0.000 description 1
- MWVUEPNEPWMFBD-SRVKXCTJSA-N Lys-Cys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCCCN MWVUEPNEPWMFBD-SRVKXCTJSA-N 0.000 description 1
- HWMZUBUEOYAQSC-DCAQKATOSA-N Lys-Gln-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O HWMZUBUEOYAQSC-DCAQKATOSA-N 0.000 description 1
- GJJQCBVRWDGLMQ-GUBZILKMSA-N Lys-Glu-Ala Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O GJJQCBVRWDGLMQ-GUBZILKMSA-N 0.000 description 1
- VMTYLUGCXIEDMV-QWRGUYRKSA-N Lys-Leu-Gly Chemical compound OC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCCCN VMTYLUGCXIEDMV-QWRGUYRKSA-N 0.000 description 1
- AIRZWUMAHCDDHR-KKUMJFAQSA-N Lys-Leu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O AIRZWUMAHCDDHR-KKUMJFAQSA-N 0.000 description 1
- WRODMZBHNNPRLN-SRVKXCTJSA-N Lys-Leu-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O WRODMZBHNNPRLN-SRVKXCTJSA-N 0.000 description 1
- YUAXTFMFMOIMAM-QWRGUYRKSA-N Lys-Lys-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O YUAXTFMFMOIMAM-QWRGUYRKSA-N 0.000 description 1
- WBSCNDJQPKSPII-KKUMJFAQSA-N Lys-Lys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O WBSCNDJQPKSPII-KKUMJFAQSA-N 0.000 description 1
- VSTNAUBHKQPVJX-IHRRRGAJSA-N Lys-Met-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(O)=O VSTNAUBHKQPVJX-IHRRRGAJSA-N 0.000 description 1
- AEIIJFBQVGYVEV-YESZJQIVSA-N Lys-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CCCCN)N)C(=O)O AEIIJFBQVGYVEV-YESZJQIVSA-N 0.000 description 1
- OHXUUQDOBQKSNB-AVGNSLFASA-N Lys-Val-Arg Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O OHXUUQDOBQKSNB-AVGNSLFASA-N 0.000 description 1
- GPAHWYRSHCKICP-GUBZILKMSA-N Met-Glu-Glu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O GPAHWYRSHCKICP-GUBZILKMSA-N 0.000 description 1
- FYRUJIJAUPHUNB-IUCAKERBSA-N Met-Gly-Arg Chemical compound CSCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCNC(N)=N FYRUJIJAUPHUNB-IUCAKERBSA-N 0.000 description 1
- RBGLBUDVQVPTEG-DCAQKATOSA-N Met-Leu-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCSC)N RBGLBUDVQVPTEG-DCAQKATOSA-N 0.000 description 1
- RKRFGIBULDYDPF-XIRDDKMYSA-N Met-Trp-Gln Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N RKRFGIBULDYDPF-XIRDDKMYSA-N 0.000 description 1
- OVRNDRQMDRJTHS-CBQIKETKSA-N N-Acetyl-D-Galactosamine Chemical compound CC(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@H](O)[C@@H]1O OVRNDRQMDRJTHS-CBQIKETKSA-N 0.000 description 1
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 1
- WYBVBIHNJWOLCJ-UHFFFAOYSA-N N-L-arginyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCCN=C(N)N WYBVBIHNJWOLCJ-UHFFFAOYSA-N 0.000 description 1
- MBLBDJOUHNCFQT-UHFFFAOYSA-N N-acetyl-D-galactosamine Natural products CC(=O)NC(C=O)C(O)C(O)C(O)CO MBLBDJOUHNCFQT-UHFFFAOYSA-N 0.000 description 1
- AJHCSUXXECOXOY-UHFFFAOYSA-N N-glycyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)CN)C(O)=O)=CNC2=C1 AJHCSUXXECOXOY-UHFFFAOYSA-N 0.000 description 1
- 125000000729 N-terminal amino-acid group Chemical group 0.000 description 1
- 108010087066 N2-tryptophyllysine Proteins 0.000 description 1
- 206010056677 Nerve degeneration Diseases 0.000 description 1
- 108010035766 P-Selectin Proteins 0.000 description 1
- 108010070519 PAR-1 Receptor Proteins 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 101150005926 Pc gene Proteins 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- QGMRQYFBGABWDR-UHFFFAOYSA-M Pentobarbital sodium Chemical compound [Na+].CCCC(C)C1(CC)C(=O)NC(=O)[N-]C1=O QGMRQYFBGABWDR-UHFFFAOYSA-M 0.000 description 1
- PLNHHOXNVSYKOB-JYJNAYRXSA-N Phe-Arg-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC1=CC=CC=C1)N PLNHHOXNVSYKOB-JYJNAYRXSA-N 0.000 description 1
- IUVYJBMTHARMIP-PCBIJLKTSA-N Phe-Asp-Ile Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O IUVYJBMTHARMIP-PCBIJLKTSA-N 0.000 description 1
- ALHULIGNEXGFRM-QWRGUYRKSA-N Phe-Cys-Gly Chemical compound OC(=O)CNC(=O)[C@H](CS)NC(=O)[C@@H](N)CC1=CC=CC=C1 ALHULIGNEXGFRM-QWRGUYRKSA-N 0.000 description 1
- MPFGIYLYWUCSJG-AVGNSLFASA-N Phe-Glu-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC1=CC=CC=C1 MPFGIYLYWUCSJG-AVGNSLFASA-N 0.000 description 1
- FIRWJEJVFFGXSH-RYUDHWBXSA-N Phe-Glu-Gly Chemical compound OC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC1=CC=CC=C1 FIRWJEJVFFGXSH-RYUDHWBXSA-N 0.000 description 1
- SFKOEHXABNPLRT-KBPBESRZSA-N Phe-His-Gly Chemical compound N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)NCC(O)=O SFKOEHXABNPLRT-KBPBESRZSA-N 0.000 description 1
- FXPZZKBHNOMLGA-HJWJTTGWSA-N Phe-Ile-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N FXPZZKBHNOMLGA-HJWJTTGWSA-N 0.000 description 1
- WEMYTDDMDBLPMI-DKIMLUQUSA-N Phe-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N WEMYTDDMDBLPMI-DKIMLUQUSA-N 0.000 description 1
- LRBSWBVUCLLRLU-BZSNNMDCSA-N Phe-Leu-Lys Chemical compound CC(C)C[C@H](NC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(O)=O LRBSWBVUCLLRLU-BZSNNMDCSA-N 0.000 description 1
- PTDAGKJHZBGDKD-OEAJRASXSA-N Phe-Thr-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N)O PTDAGKJHZBGDKD-OEAJRASXSA-N 0.000 description 1
- MMPBPRXOFJNCCN-ZEWNOJEFSA-N Phe-Tyr-Ile Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O MMPBPRXOFJNCCN-ZEWNOJEFSA-N 0.000 description 1
- 229920002594 Polyethylene Glycol 8000 Polymers 0.000 description 1
- 229920002873 Polyethylenimine Polymers 0.000 description 1
- ALJGSKMBIUEJOB-FXQIFTODSA-N Pro-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@@H]1CCCN1 ALJGSKMBIUEJOB-FXQIFTODSA-N 0.000 description 1
- CGBYDGAJHSOGFQ-LPEHRKFASA-N Pro-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 CGBYDGAJHSOGFQ-LPEHRKFASA-N 0.000 description 1
- HFZNNDWPHBRNPV-KZVJFYERSA-N Pro-Ala-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O HFZNNDWPHBRNPV-KZVJFYERSA-N 0.000 description 1
- OOLOTUZJUBOMAX-GUBZILKMSA-N Pro-Ala-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O OOLOTUZJUBOMAX-GUBZILKMSA-N 0.000 description 1
- MLQVJYMFASXBGZ-IHRRRGAJSA-N Pro-Asn-Tyr Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O MLQVJYMFASXBGZ-IHRRRGAJSA-N 0.000 description 1
- FKKHDBFNOLCYQM-FXQIFTODSA-N Pro-Cys-Ala Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(O)=O FKKHDBFNOLCYQM-FXQIFTODSA-N 0.000 description 1
- LANQLYHLMYDWJP-SRVKXCTJSA-N Pro-Gln-Lys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCCN)C(=O)O LANQLYHLMYDWJP-SRVKXCTJSA-N 0.000 description 1
- PULPZRAHVFBVTO-DCAQKATOSA-N Pro-Glu-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O PULPZRAHVFBVTO-DCAQKATOSA-N 0.000 description 1
- QEWBZBLXDKIQPS-STQMWFEESA-N Pro-Gly-Tyr Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O QEWBZBLXDKIQPS-STQMWFEESA-N 0.000 description 1
- FDINZVJXLPILKV-DCAQKATOSA-N Pro-His-Asn Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(N)=O)C(O)=O FDINZVJXLPILKV-DCAQKATOSA-N 0.000 description 1
- TYMBHHITTMGGPI-NAKRPEOUSA-N Pro-Ile-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@@H]1CCCN1 TYMBHHITTMGGPI-NAKRPEOUSA-N 0.000 description 1
- KLSOMAFWRISSNI-OSUNSFLBSA-N Pro-Ile-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]1CCCN1 KLSOMAFWRISSNI-OSUNSFLBSA-N 0.000 description 1
- FYPGHGXAOZTOBO-IHRRRGAJSA-N Pro-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@@H]2CCCN2 FYPGHGXAOZTOBO-IHRRRGAJSA-N 0.000 description 1
- XSXABUHLKPUVLX-JYJNAYRXSA-N Pro-Ser-Trp Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O XSXABUHLKPUVLX-JYJNAYRXSA-N 0.000 description 1
- GNFHQWNCSSPOBT-ULQDDVLXSA-N Pro-Trp-Gln Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)N[C@@H](CCC(=O)N)C(=O)O GNFHQWNCSSPOBT-ULQDDVLXSA-N 0.000 description 1
- FYXCBXDAMPEHIQ-FHWLQOOXSA-N Pro-Trp-Lys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)N[C@@H](CCCCN)C(=O)O FYXCBXDAMPEHIQ-FHWLQOOXSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-N Propionic acid Substances CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 1
- 102000002020 Protease-activated receptors Human genes 0.000 description 1
- 108050009310 Protease-activated receptors Proteins 0.000 description 1
- 201000005660 Protein C Deficiency Diseases 0.000 description 1
- 102000014961 Protein Precursors Human genes 0.000 description 1
- 108010078762 Protein Precursors Proteins 0.000 description 1
- 229940096437 Protein S Drugs 0.000 description 1
- 102000029301 Protein S Human genes 0.000 description 1
- 108010066124 Protein S Proteins 0.000 description 1
- 101710188315 Protein X Proteins 0.000 description 1
- 102100037136 Proteinase-activated receptor 1 Human genes 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 101710140994 Secreted protein C Proteins 0.000 description 1
- WXUBSIDKNMFAGS-IHRRRGAJSA-N Ser-Arg-Tyr Chemical compound NC(N)=NCCC[C@H](NC(=O)[C@H](CO)N)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 WXUBSIDKNMFAGS-IHRRRGAJSA-N 0.000 description 1
- COAHUSQNSVFYBW-FXQIFTODSA-N Ser-Asn-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O COAHUSQNSVFYBW-FXQIFTODSA-N 0.000 description 1
- BYIROAKULFFTEK-CIUDSAMLSA-N Ser-Asp-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO BYIROAKULFFTEK-CIUDSAMLSA-N 0.000 description 1
- KCFKKAQKRZBWJB-ZLUOBGJFSA-N Ser-Cys-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(O)=O KCFKKAQKRZBWJB-ZLUOBGJFSA-N 0.000 description 1
- KNCJWSPMTFFJII-ZLUOBGJFSA-N Ser-Cys-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(O)=O)C(O)=O KNCJWSPMTFFJII-ZLUOBGJFSA-N 0.000 description 1
- JFWDJFULOLKQFY-QWRGUYRKSA-N Ser-Gly-Phe Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O JFWDJFULOLKQFY-QWRGUYRKSA-N 0.000 description 1
- SFTZWNJFZYOLBD-ZDLURKLDSA-N Ser-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO SFTZWNJFZYOLBD-ZDLURKLDSA-N 0.000 description 1
- NLOAIFSWUUFQFR-CIUDSAMLSA-N Ser-Leu-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O NLOAIFSWUUFQFR-CIUDSAMLSA-N 0.000 description 1
- IAORETPTUDBBGV-CIUDSAMLSA-N Ser-Leu-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CO)N IAORETPTUDBBGV-CIUDSAMLSA-N 0.000 description 1
- SRKMDKACHDVPMD-SRVKXCTJSA-N Ser-Lys-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)N SRKMDKACHDVPMD-SRVKXCTJSA-N 0.000 description 1
- FPCGZYMRFFIYIH-CIUDSAMLSA-N Ser-Lys-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O FPCGZYMRFFIYIH-CIUDSAMLSA-N 0.000 description 1
- IFLVBVIYADZIQO-DCAQKATOSA-N Ser-Met-Lys Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N IFLVBVIYADZIQO-DCAQKATOSA-N 0.000 description 1
- UPLYXVPQLJVWMM-KKUMJFAQSA-N Ser-Phe-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(O)=O UPLYXVPQLJVWMM-KKUMJFAQSA-N 0.000 description 1
- XQAPEISNMXNKGE-FXQIFTODSA-N Ser-Pro-Cys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CO)N)C(=O)N[C@@H](CS)C(=O)O XQAPEISNMXNKGE-FXQIFTODSA-N 0.000 description 1
- KQNDIKOYWZTZIX-FXQIFTODSA-N Ser-Ser-Arg Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCNC(N)=N KQNDIKOYWZTZIX-FXQIFTODSA-N 0.000 description 1
- ILZAUMFXKSIUEF-SRVKXCTJSA-N Ser-Ser-Phe Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 ILZAUMFXKSIUEF-SRVKXCTJSA-N 0.000 description 1
- CUXJENOFJXOSOZ-BIIVOSGPSA-N Ser-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CO)N)C(=O)O CUXJENOFJXOSOZ-BIIVOSGPSA-N 0.000 description 1
- STIAINRLUUKYKM-WFBYXXMGSA-N Ser-Trp-Ala Chemical compound C1=CC=C2C(C[C@@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)[C@@H](N)CO)=CNC2=C1 STIAINRLUUKYKM-WFBYXXMGSA-N 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 108020005038 Terminator Codon Proteins 0.000 description 1
- IGROJMCBGRFRGI-YTLHQDLWSA-N Thr-Ala-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O IGROJMCBGRFRGI-YTLHQDLWSA-N 0.000 description 1
- CAGTXGDOIFXLPC-KZVJFYERSA-N Thr-Arg-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C)C(O)=O)CCCN=C(N)N CAGTXGDOIFXLPC-KZVJFYERSA-N 0.000 description 1
- XYEXCEPTALHNEV-RCWTZXSCSA-N Thr-Arg-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O XYEXCEPTALHNEV-RCWTZXSCSA-N 0.000 description 1
- TWLMXDWFVNEFFK-FJXKBIBVSA-N Thr-Arg-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O TWLMXDWFVNEFFK-FJXKBIBVSA-N 0.000 description 1
- VFEHSAJCWWHDBH-RHYQMDGZSA-N Thr-Arg-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O VFEHSAJCWWHDBH-RHYQMDGZSA-N 0.000 description 1
- NAXBBCLCEOTAIG-RHYQMDGZSA-N Thr-Arg-Lys Chemical compound NC(N)=NCCC[C@H](NC(=O)[C@@H](N)[C@H](O)C)C(=O)N[C@@H](CCCCN)C(O)=O NAXBBCLCEOTAIG-RHYQMDGZSA-N 0.000 description 1
- QGXCWPNQVCYJEL-NUMRIWBASA-N Thr-Asn-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O QGXCWPNQVCYJEL-NUMRIWBASA-N 0.000 description 1
- MMTOHPRBJKEZHT-BWBBJGPYSA-N Thr-Cys-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O MMTOHPRBJKEZHT-BWBBJGPYSA-N 0.000 description 1
- UZJDBCHMIQXLOQ-HEIBUPTGSA-N Thr-Cys-Thr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N)O UZJDBCHMIQXLOQ-HEIBUPTGSA-N 0.000 description 1
- CQNFRKAKGDSJFR-NUMRIWBASA-N Thr-Glu-Asn Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)O CQNFRKAKGDSJFR-NUMRIWBASA-N 0.000 description 1
- MSIYNSBKKVMGFO-BHNWBGBOSA-N Thr-Gly-Pro Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)N1CCC[C@@H]1C(=O)O)N)O MSIYNSBKKVMGFO-BHNWBGBOSA-N 0.000 description 1
- YSXYEJWDHBCTDJ-DVJZZOLTSA-N Thr-Gly-Trp Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N)O YSXYEJWDHBCTDJ-DVJZZOLTSA-N 0.000 description 1
- YDWLCDQXLCILCZ-BWAGICSOSA-N Thr-His-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O YDWLCDQXLCILCZ-BWAGICSOSA-N 0.000 description 1
- XYFISNXATOERFZ-OSUNSFLBSA-N Thr-Ile-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N XYFISNXATOERFZ-OSUNSFLBSA-N 0.000 description 1
- KZSYAEWQMJEGRZ-RHYQMDGZSA-N Thr-Leu-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O KZSYAEWQMJEGRZ-RHYQMDGZSA-N 0.000 description 1
- DXPURPNJDFCKKO-RHYQMDGZSA-N Thr-Lys-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)[C@@H](C)O)C(O)=O DXPURPNJDFCKKO-RHYQMDGZSA-N 0.000 description 1
- YRJOLUDFVAUXLI-GSSVUCPTSA-N Thr-Thr-Asp Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(O)=O YRJOLUDFVAUXLI-GSSVUCPTSA-N 0.000 description 1
- UMFLBPIPAJMNIM-LYARXQMPSA-N Thr-Trp-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC3=CC=CC=C3)C(=O)O)N)O UMFLBPIPAJMNIM-LYARXQMPSA-N 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 208000001435 Thromboembolism Diseases 0.000 description 1
- 102100026966 Thrombomodulin Human genes 0.000 description 1
- 108010079274 Thrombomodulin Proteins 0.000 description 1
- 108010000499 Thromboplastin Proteins 0.000 description 1
- 102000002262 Thromboplastin Human genes 0.000 description 1
- 108700019146 Transgenes Proteins 0.000 description 1
- 108060008539 Transglutaminase Proteins 0.000 description 1
- QNMIVTOQXUSGLN-SZMVWBNQSA-N Trp-Arg-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 QNMIVTOQXUSGLN-SZMVWBNQSA-N 0.000 description 1
- IUFQHOCOKQIOMC-XIRDDKMYSA-N Trp-Asn-Lys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCCCN)C(=O)O)N IUFQHOCOKQIOMC-XIRDDKMYSA-N 0.000 description 1
- JLTQXEOXIJMCLZ-ZVZYQTTQSA-N Trp-Gln-Val Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O)=CNC2=C1 JLTQXEOXIJMCLZ-ZVZYQTTQSA-N 0.000 description 1
- YXONONCLMLHWJX-SZMVWBNQSA-N Trp-Glu-Leu Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O)=CNC2=C1 YXONONCLMLHWJX-SZMVWBNQSA-N 0.000 description 1
- OBAMASZCXDIXSS-SZMVWBNQSA-N Trp-Glu-Lys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N OBAMASZCXDIXSS-SZMVWBNQSA-N 0.000 description 1
- DZIKVMCFXIIETR-JSGCOSHPSA-N Trp-Gly-Glu Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O DZIKVMCFXIIETR-JSGCOSHPSA-N 0.000 description 1
- DNUJCLUFRGGSDJ-YLVFBTJISA-N Trp-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC1=CNC2=CC=CC=C21)N DNUJCLUFRGGSDJ-YLVFBTJISA-N 0.000 description 1
- HLDFBNPSURDYEN-VHWLVUOQSA-N Trp-Ile-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N HLDFBNPSURDYEN-VHWLVUOQSA-N 0.000 description 1
- MNMYOSZWCKYEDI-JRQIVUDYSA-N Tyr-Asp-Thr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MNMYOSZWCKYEDI-JRQIVUDYSA-N 0.000 description 1
- QHEGAOPHISYNDF-XDTLVQLUSA-N Tyr-Gln-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC1=CC=C(C=C1)O)N QHEGAOPHISYNDF-XDTLVQLUSA-N 0.000 description 1
- FNWGDMZVYBVAGJ-XEGUGMAKSA-N Tyr-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC1=CC=C(C=C1)O)N FNWGDMZVYBVAGJ-XEGUGMAKSA-N 0.000 description 1
- HSBZWINKRYZCSQ-KKUMJFAQSA-N Tyr-Lys-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O HSBZWINKRYZCSQ-KKUMJFAQSA-N 0.000 description 1
- VYTUETMEZZLJFU-IHRRRGAJSA-N Tyr-Pro-Cys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)N)C(=O)N[C@@H](CS)C(=O)O VYTUETMEZZLJFU-IHRRRGAJSA-N 0.000 description 1
- CLEGSEJVGBYZBJ-MEYUZBJRSA-N Tyr-Thr-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H]([C@H](O)C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 CLEGSEJVGBYZBJ-MEYUZBJRSA-N 0.000 description 1
- 108010064997 VPY tripeptide Proteins 0.000 description 1
- ZLFHAAGHGQBQQN-GUBZILKMSA-N Val-Ala-Pro Natural products CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O ZLFHAAGHGQBQQN-GUBZILKMSA-N 0.000 description 1
- AZSHAZJLOZQYAY-FXQIFTODSA-N Val-Ala-Ser Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O AZSHAZJLOZQYAY-FXQIFTODSA-N 0.000 description 1
- SLLKXDSRVAOREO-KZVJFYERSA-N Val-Ala-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C)NC(=O)[C@H](C(C)C)N)O SLLKXDSRVAOREO-KZVJFYERSA-N 0.000 description 1
- JYVKKBDANPZIAW-AVGNSLFASA-N Val-Arg-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C(C)C)N JYVKKBDANPZIAW-AVGNSLFASA-N 0.000 description 1
- XQVRMLRMTAGSFJ-QXEWZRGKSA-N Val-Asp-Arg Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N XQVRMLRMTAGSFJ-QXEWZRGKSA-N 0.000 description 1
- QHDXUYOYTPWCSK-RCOVLWMOSA-N Val-Asp-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)NCC(=O)O)N QHDXUYOYTPWCSK-RCOVLWMOSA-N 0.000 description 1
- DDNIHOWRDOXXPF-NGZCFLSTSA-N Val-Asp-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N DDNIHOWRDOXXPF-NGZCFLSTSA-N 0.000 description 1
- HIZMLPKDJAXDRG-FXQIFTODSA-N Val-Cys-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O)N HIZMLPKDJAXDRG-FXQIFTODSA-N 0.000 description 1
- UEHRGZCNLSWGHK-DLOVCJGASA-N Val-Glu-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UEHRGZCNLSWGHK-DLOVCJGASA-N 0.000 description 1
- URIRWLJVWHYLET-ONGXEEELSA-N Val-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)C(C)C URIRWLJVWHYLET-ONGXEEELSA-N 0.000 description 1
- SDUBQHUJJWQTEU-XUXIUFHCSA-N Val-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](C(C)C)N SDUBQHUJJWQTEU-XUXIUFHCSA-N 0.000 description 1
- APQIVBCUIUDSMB-OSUNSFLBSA-N Val-Ile-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](C(C)C)N APQIVBCUIUDSMB-OSUNSFLBSA-N 0.000 description 1
- FEXILLGKGGTLRI-NHCYSSNCSA-N Val-Leu-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N FEXILLGKGGTLRI-NHCYSSNCSA-N 0.000 description 1
- ZHQWPWQNVRCXAX-XQQFMLRXSA-N Val-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](C(C)C)N ZHQWPWQNVRCXAX-XQQFMLRXSA-N 0.000 description 1
- SYSWVVCYSXBVJG-RHYQMDGZSA-N Val-Leu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N)O SYSWVVCYSXBVJG-RHYQMDGZSA-N 0.000 description 1
- KISFXYYRKKNLOP-IHRRRGAJSA-N Val-Phe-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)O)N KISFXYYRKKNLOP-IHRRRGAJSA-N 0.000 description 1
- MJOUSKQHAIARKI-JYJNAYRXSA-N Val-Phe-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C(C)C)C(O)=O)CC1=CC=CC=C1 MJOUSKQHAIARKI-JYJNAYRXSA-N 0.000 description 1
- VIKZGAUAKQZDOF-NRPADANISA-N Val-Ser-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O VIKZGAUAKQZDOF-NRPADANISA-N 0.000 description 1
- QZKVWWIUSQGWMY-IHRRRGAJSA-N Val-Ser-Phe Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 QZKVWWIUSQGWMY-IHRRRGAJSA-N 0.000 description 1
- SDHZOOIGIUEPDY-JYJNAYRXSA-N Val-Ser-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 SDHZOOIGIUEPDY-JYJNAYRXSA-N 0.000 description 1
- CEKSLIVSNNGOKH-KZVJFYERSA-N Val-Thr-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](C(C)C)N)O CEKSLIVSNNGOKH-KZVJFYERSA-N 0.000 description 1
- YQYFYUSYEDNLSD-YEPSODPASA-N Val-Thr-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O YQYFYUSYEDNLSD-YEPSODPASA-N 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 230000001594 aberrant effect Effects 0.000 description 1
- QPMSXSBEVQLBIL-CZRHPSIPSA-N ac1mix0p Chemical compound C1=CC=C2N(C[C@H](C)CN(C)C)C3=CC(OC)=CC=C3SC2=C1.O([C@H]1[C@]2(OC)C=CC34C[C@@H]2[C@](C)(O)CCC)C2=C5[C@]41CCN(C)[C@@H]3CC5=CC=C2O QPMSXSBEVQLBIL-CZRHPSIPSA-N 0.000 description 1
- 235000011054 acetic acid Nutrition 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 208000013633 acquired hemophilia Diseases 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 230000006838 adverse reaction Effects 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 1
- 108010005233 alanylglutamic acid Proteins 0.000 description 1
- 108010047495 alanylglycine Proteins 0.000 description 1
- 108010070944 alanylhistidine Proteins 0.000 description 1
- 108010011559 alanylphenylalanine Proteins 0.000 description 1
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 239000000908 ammonium hydroxide Substances 0.000 description 1
- 238000012870 ammonium sulfate precipitation Methods 0.000 description 1
- APKFDSVGJQXUKY-INPOYWNPSA-N amphotericin B Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-INPOYWNPSA-N 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000002555 anti-neurodegenerative effect Effects 0.000 description 1
- 230000000840 anti-viral effect Effects 0.000 description 1
- 239000000729 antidote Substances 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 101150010487 are gene Proteins 0.000 description 1
- 108010043240 arginyl-leucyl-glycine Proteins 0.000 description 1
- 108010059459 arginyl-threonyl-phenylalanine Proteins 0.000 description 1
- 108010068380 arginylarginine Proteins 0.000 description 1
- 229940104697 arixtra Drugs 0.000 description 1
- 108010077245 asparaginyl-proline Proteins 0.000 description 1
- 108010092854 aspartyllysine Proteins 0.000 description 1
- IDIPWEYIBKUDNY-UHFFFAOYSA-N benzenesulfonyl fluoride Chemical compound FS(=O)(=O)C1=CC=CC=C1 IDIPWEYIBKUDNY-UHFFFAOYSA-N 0.000 description 1
- SXTGIAYWYXVNLT-NRFANRHFSA-N benzyl n-[2-[[2-[[(2s)-5-(diaminomethylideneamino)-1-[(4-methyl-2-oxochromen-7-yl)amino]-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-2-oxoethyl]carbamate Chemical compound N([C@@H](CCCNC(N)=N)C(=O)NC1=CC=2OC(=O)C=C(C=2C=C1)C)C(=O)CNC(=O)CNC(=O)OCC1=CC=CC=C1 SXTGIAYWYXVNLT-NRFANRHFSA-N 0.000 description 1
- 108010079115 benzyloxycarbonyl-glycyl-glycyl-arginine-4-methylcoumaryl-7-amide Proteins 0.000 description 1
- SQVRNKJHWKZAKO-UHFFFAOYSA-N beta-N-Acetyl-D-neuraminic acid Natural products CC(=O)NC1C(O)CC(O)(C(O)=O)OC1C(O)C(O)CO SQVRNKJHWKZAKO-UHFFFAOYSA-N 0.000 description 1
- 229950011103 betrixaban Drugs 0.000 description 1
- XHOLNRLADUSQLD-UHFFFAOYSA-N betrixaban Chemical compound C=1C=C(Cl)C=NC=1NC(=O)C1=CC(OC)=CC=C1NC(=O)C1=CC=C(C(=N)N(C)C)C=C1 XHOLNRLADUSQLD-UHFFFAOYSA-N 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000008033 biological extinction Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000002051 biphasic effect Effects 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 230000000981 bystander Effects 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 235000011148 calcium chloride Nutrition 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 210000001638 cerebellum Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 230000010405 clearance mechanism Effects 0.000 description 1
- 239000000701 coagulant Substances 0.000 description 1
- 239000003636 conditioned culture medium Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- NKLPQNGYXWVELD-UHFFFAOYSA-M coomassie brilliant blue Chemical compound [Na+].C1=CC(OCC)=CC=C1NC1=CC=C(C(=C2C=CC(C=C2)=[N+](CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=2C=CC(=CC=2)N(CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=C1 NKLPQNGYXWVELD-UHFFFAOYSA-M 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 229940097362 cyclodextrins Drugs 0.000 description 1
- 108010004073 cysteinylcysteine Proteins 0.000 description 1
- 108010060199 cysteinylproline Proteins 0.000 description 1
- 230000001120 cytoprotective effect Effects 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- XEKSTYNIJLDDAZ-JASSWCPGSA-D decasodium;(2s,3s,4r,5r,6r)-6-[(2r,3r,4r,5r,6r)-6-[(2r,3s,4s,5r,6r)-2-carboxylato-4-hydroxy-6-[(2r,3s,4r,5r,6s)-4-hydroxy-6-methoxy-5-(sulfonatoamino)-2-(sulfonatooxymethyl)oxan-3-yl]oxy-5-sulfonatooxyoxan-3-yl]oxy-5-(sulfonatoamino)-4-sulfonatooxy-2-(sul Chemical compound [Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].O[C@@H]1[C@@H](NS([O-])(=O)=O)[C@@H](OC)O[C@H](COS([O-])(=O)=O)[C@H]1O[C@H]1[C@H](OS([O-])(=O)=O)[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O[C@@H]4[C@@H]([C@@H](O)[C@H](O)[C@@H](COS([O-])(=O)=O)O4)NS([O-])(=O)=O)[C@H](O3)C([O-])=O)O)[C@@H](COS([O-])(=O)=O)O2)NS([O-])(=O)=O)[C@H](C([O-])=O)O1 XEKSTYNIJLDDAZ-JASSWCPGSA-D 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- UGMCXQCYOVCMTB-UHFFFAOYSA-K dihydroxy(stearato)aluminium Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Al](O)O UGMCXQCYOVCMTB-UHFFFAOYSA-K 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- 229910000397 disodium phosphate Inorganic materials 0.000 description 1
- 235000019800 disodium phosphate Nutrition 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 235000013345 egg yolk Nutrition 0.000 description 1
- 210000002969 egg yolk Anatomy 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 230000007368 endocrine function Effects 0.000 description 1
- 229940105423 erythropoietin Drugs 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 239000013613 expression plasmid Substances 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 108010094072 factor X antigen Proteins 0.000 description 1
- 229940012444 factor xiii Drugs 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 230000000893 fibroproliferative effect Effects 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 229930182830 galactose Natural products 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 108010080575 glutamyl-aspartyl-alanine Proteins 0.000 description 1
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 1
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 1
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 1
- 108010084264 glycyl-glycyl-cysteine Proteins 0.000 description 1
- 108010051307 glycyl-glycyl-proline Proteins 0.000 description 1
- 108010033719 glycyl-histidyl-glycine Proteins 0.000 description 1
- 108010077435 glycyl-phenylalanyl-glycine Proteins 0.000 description 1
- 108010074027 glycyl-seryl-phenylalanine Proteins 0.000 description 1
- 108010089804 glycyl-threonine Proteins 0.000 description 1
- 108010020688 glycylhistidine Proteins 0.000 description 1
- 108010050848 glycylleucine Proteins 0.000 description 1
- 108010084389 glycyltryptophan Proteins 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 208000031169 hemorrhagic disease Diseases 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 208000013746 hereditary thrombophilia due to congenital protein C deficiency Diseases 0.000 description 1
- 229940006607 hirudin Drugs 0.000 description 1
- WQPDUTSPKFMPDP-OUMQNGNKSA-N hirudin Chemical compound C([C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(OS(O)(=O)=O)=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]2CSSC[C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@H](C(NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N2)=O)CSSC1)C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=2C=CC(O)=CC=2)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)[C@@H](C)O)CSSC1)C(C)C)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 WQPDUTSPKFMPDP-OUMQNGNKSA-N 0.000 description 1
- 108010025306 histidylleucine Proteins 0.000 description 1
- 108010018006 histidylserine Proteins 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 208000036260 idiopathic disease Diseases 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 239000003547 immunosorbent Substances 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 238000012528 insulin ELISA Methods 0.000 description 1
- 239000004026 insulin derivative Substances 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 235000014413 iron hydroxide Nutrition 0.000 description 1
- NCNCGGDMXMBVIA-UHFFFAOYSA-L iron(ii) hydroxide Chemical class [OH-].[OH-].[Fe+2] NCNCGGDMXMBVIA-UHFFFAOYSA-L 0.000 description 1
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 1
- 108010027338 isoleucylcysteine Proteins 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 108010044056 leucyl-phenylalanine Proteins 0.000 description 1
- 108010030617 leucyl-phenylalanyl-valine Proteins 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 201000001268 lymphoproliferative syndrome Diseases 0.000 description 1
- 108010053062 lysyl-arginyl-phenylalanyl-lysine Proteins 0.000 description 1
- 108010045397 lysyl-tyrosyl-lysine Proteins 0.000 description 1
- 108010009298 lysylglutamic acid Proteins 0.000 description 1
- 108010054155 lysyllysine Proteins 0.000 description 1
- 108010017391 lysylvaline Proteins 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 235000011147 magnesium chloride Nutrition 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 239000002808 molecular sieve Substances 0.000 description 1
- 150000004712 monophosphates Chemical class 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- UPSFMJHZUCSEHU-JYGUBCOQSA-N n-[(2s,3r,4r,5s,6r)-2-[(2r,3s,4r,5r,6s)-5-acetamido-4-hydroxy-2-(hydroxymethyl)-6-(4-methyl-2-oxochromen-7-yl)oxyoxan-3-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide Chemical compound CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@H]1[C@H](O)[C@@H](NC(C)=O)[C@H](OC=2C=C3OC(=O)C=C(C)C3=CC=2)O[C@@H]1CO UPSFMJHZUCSEHU-JYGUBCOQSA-N 0.000 description 1
- 238000007857 nested PCR Methods 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 229940127066 new oral anticoagluant drug Drugs 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000002482 oligosaccharides Chemical class 0.000 description 1
- 229940127216 oral anticoagulant drug Drugs 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 229960002275 pentobarbital sodium Drugs 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 230000004983 pleiotropic effect Effects 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 239000001103 potassium chloride Substances 0.000 description 1
- 235000011164 potassium chloride Nutrition 0.000 description 1
- OXCMYAYHXIHQOA-UHFFFAOYSA-N potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol Chemical compound [K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1 OXCMYAYHXIHQOA-UHFFFAOYSA-N 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000009862 primary prevention Effects 0.000 description 1
- 230000000019 pro-fibrinolytic effect Effects 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 239000003805 procoagulant Substances 0.000 description 1
- 108010079317 prolyl-tyrosine Proteins 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 235000019419 proteases Nutrition 0.000 description 1
- 230000004224 protection Effects 0.000 description 1
- 235000004252 protein component Nutrition 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 108010014806 prothrombinase complex Proteins 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 230000003362 replicative effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 239000012313 reversal agent Substances 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 230000009863 secondary prevention Effects 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- SQVRNKJHWKZAKO-OQPLDHBCSA-N sialic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@](O)(C(O)=O)OC1[C@H](O)[C@H](O)CO SQVRNKJHWKZAKO-OQPLDHBCSA-N 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 108010061238 threonyl-glycine Proteins 0.000 description 1
- 108010031491 threonyl-lysyl-glutamic acid Proteins 0.000 description 1
- 230000009424 thromboembolic effect Effects 0.000 description 1
- 230000002537 thrombolytic effect Effects 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 102000003601 transglutaminase Human genes 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- YFDSDPIBEUFTMI-UHFFFAOYSA-N tribromoethanol Chemical compound OCC(Br)(Br)Br YFDSDPIBEUFTMI-UHFFFAOYSA-N 0.000 description 1
- 229950004616 tribromoethanol Drugs 0.000 description 1
- YNJBWRMUSHSURL-UHFFFAOYSA-N trichloroacetic acid Chemical compound OC(=O)C(Cl)(Cl)Cl YNJBWRMUSHSURL-UHFFFAOYSA-N 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- HRXKRNGNAMMEHJ-UHFFFAOYSA-K trisodium citrate Chemical compound [Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O HRXKRNGNAMMEHJ-UHFFFAOYSA-K 0.000 description 1
- 229940038773 trisodium citrate Drugs 0.000 description 1
- SOBHUZYZLFQYFK-UHFFFAOYSA-K trisodium;hydroxy-[[phosphonatomethyl(phosphonomethyl)amino]methyl]phosphinate Chemical compound [Na+].[Na+].[Na+].OP(O)(=O)CN(CP(O)([O-])=O)CP([O-])([O-])=O SOBHUZYZLFQYFK-UHFFFAOYSA-K 0.000 description 1
- 108010051110 tyrosyl-lysine Proteins 0.000 description 1
- 108010078580 tyrosylleucine Proteins 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 108010073969 valyllysine Proteins 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/745—Blood coagulation or fibrinolysis factors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/04—Antihaemorrhagics; Procoagulants; Haemostatic agents; Antifibrinolytic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K19/00—Hybrid peptides, i.e. peptides covalently bound to nucleic acids, or non-covalently bound protein-protein complexes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/48—Hydrolases (3) acting on peptide bonds (3.4)
- C12N9/50—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25)
- C12N9/64—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/48—Hydrolases (3) acting on peptide bonds (3.4)
- C12N9/50—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25)
- C12N9/64—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue
- C12N9/6421—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue from mammals
- C12N9/6424—Serine endopeptidases (3.4.21)
- C12N9/6429—Thrombin (3.4.21.5)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/48—Hydrolases (3) acting on peptide bonds (3.4)
- C12N9/50—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25)
- C12N9/64—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue
- C12N9/6421—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue from mammals
- C12N9/6424—Serine endopeptidases (3.4.21)
- C12N9/6432—Coagulation factor Xa (3.4.21.6)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/48—Hydrolases (3) acting on peptide bonds (3.4)
- C12N9/50—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25)
- C12N9/64—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue
- C12N9/6421—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue from mammals
- C12N9/6424—Serine endopeptidases (3.4.21)
- C12N9/6464—Protein C (3.4.21.69)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y304/00—Hydrolases acting on peptide bonds, i.e. peptidases (3.4)
- C12Y304/21—Serine endopeptidases (3.4.21)
- C12Y304/21005—Thrombin (3.4.21.5)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y304/00—Hydrolases acting on peptide bonds, i.e. peptidases (3.4)
- C12Y304/21—Serine endopeptidases (3.4.21)
- C12Y304/21006—Coagulation factor Xa (3.4.21.6)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y304/00—Hydrolases acting on peptide bonds, i.e. peptidases (3.4)
- C12Y304/21—Serine endopeptidases (3.4.21)
- C12Y304/21069—Protein C activated (3.4.21.69)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Biomedical Technology (AREA)
- Medicinal Chemistry (AREA)
- General Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Hematology (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- General Chemical & Material Sciences (AREA)
- Diabetes (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP08305990A EP2199387A1 (en) | 2008-12-19 | 2008-12-19 | Serine protease derivatives and uses for the prevention and/or the treatment of blood coagulation disorders |
| EP08305990.7 | 2008-12-19 | ||
| EP09305349 | 2009-04-23 | ||
| EP09305349.4 | 2009-04-23 | ||
| US22296009P | 2009-07-03 | 2009-07-03 | |
| US61/222,960 | 2009-07-03 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20110114587A true KR20110114587A (ko) | 2011-10-19 |
Family
ID=41718355
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117016908A Ceased KR20110114587A (ko) | 2008-12-19 | 2009-12-21 | 세린 프로테아제 유도체 및 혈액응고 장애의 치료 또는 예방의 용도 |
Country Status (9)
| Country | Link |
|---|---|
| US (3) | US8436144B2 (enExample) |
| EP (1) | EP2358874B1 (enExample) |
| JP (1) | JP5833448B2 (enExample) |
| KR (1) | KR20110114587A (enExample) |
| CN (2) | CN104262479A (enExample) |
| AU (1) | AU2009329493B2 (enExample) |
| CA (1) | CA2747065A1 (enExample) |
| ES (1) | ES2629099T3 (enExample) |
| WO (1) | WO2010070137A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20150113205A (ko) * | 2013-02-04 | 2015-10-07 | 라보라토이레 프란카이즈 듀 프락티온네먼트 에트 데스 바이오테크놀로지스 | 인자 x 돌연변이체 |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MX2008006313A (es) * | 2005-11-15 | 2008-11-06 | Philadelphia Children Hospital | Metodos y composiciones para modular la hemostasia. |
| DK2915564T3 (da) | 2007-09-28 | 2021-02-08 | Alexion Pharma Inc | Antidoter mod faktor XA-inhibitorer og fremgangsmåder til anvendelse deraf |
| US8455439B2 (en) | 2008-11-14 | 2013-06-04 | Portola Pharmaceuticals, Inc. | Antidotes for factor Xa inhibitors and methods of using the same in combination with blood coagulating agents |
| CN104262479A (zh) * | 2008-12-19 | 2015-01-07 | 国家健康与医学研究院 | 丝氨酸蛋白酶衍生物及其在凝血功能障碍的预防或治疗中的用途 |
| PL3604510T3 (pl) | 2009-03-30 | 2025-07-28 | Alexion Pharmaceuticals, Inc. | Antidota na inhibitory czynnika xa i sposoby ich stosowania |
| CN102625712B (zh) | 2009-07-15 | 2017-07-25 | 博尔托拉制药公司 | 用于因子xa抑制剂的解毒剂的单位剂量制剂及其使用方法 |
| CA2850603C (en) * | 2011-09-30 | 2021-11-16 | The Children's Hospital Of Philadelphia | Compositions and methods for modulating hemostasis |
| WO2014018120A1 (en) | 2012-07-25 | 2014-01-30 | Catalyst Biosciences, Inc. | Modified factor x polypeptides and uses thereof |
| HK1215196A1 (zh) | 2013-01-31 | 2016-08-19 | 辉瑞公司 | 用於抵消因子xa抑制的组合物和方法 |
| EP2970933A2 (en) * | 2013-03-12 | 2016-01-20 | Novo Nordisk A/S | Thrombin sensitive coagulation factor x molecules |
| CN112195169A (zh) | 2013-09-24 | 2021-01-08 | 辉瑞大药厂 | 包含重组人凝血因子Xa蛋白的异质性群体的组合物 |
| EP3242936A1 (en) | 2015-01-07 | 2017-11-15 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Mutated factor x polypeptides and uses thereof for the treatment of haemophilia |
| WO2017017109A1 (en) * | 2015-07-27 | 2017-02-02 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Mutated factor x polypeptides and uses thereof for the treatment of haemophilia |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4861719A (en) | 1986-04-25 | 1989-08-29 | Fred Hutchinson Cancer Research Center | DNA constructs for retrovirus packaging cell lines |
| US4992373A (en) | 1987-12-04 | 1991-02-12 | Eli Lilly And Company | Vectors and compounds for direct expression of activated human protein C |
| US5278056A (en) | 1988-02-05 | 1994-01-11 | The Trustees Of Columbia University In The City Of New York | Retroviral packaging cell lines and process of using same |
| US4981952A (en) | 1988-10-04 | 1991-01-01 | Eli Lilly And Company | Method for the purification of vitamin K-dependent proteins |
| US5670488A (en) | 1992-12-03 | 1997-09-23 | Genzyme Corporation | Adenovirus vector for gene therapy |
| MY110664A (en) | 1992-05-21 | 1999-01-30 | Lilly Co Eli | Protein c derivatives |
| DE69434860T2 (de) | 1993-02-22 | 2007-03-15 | The Rockefeller University | Herstellung von helfer-freien retroviren mit hohem titer mittels transienter transfektion |
| FR2712812B1 (fr) | 1993-11-23 | 1996-02-09 | Centre Nat Rech Scient | Composition pour la production de produits thérapeutiques in vivo. |
| IL116816A (en) | 1995-01-20 | 2003-05-29 | Rhone Poulenc Rorer Sa | Cell for the production of a defective recombinant adenovirus or an adeno-associated virus and the various uses thereof |
| US6013516A (en) | 1995-10-06 | 2000-01-11 | The Salk Institute For Biological Studies | Vector and method of use for nucleic acid delivery to non-dividing cells |
| FR2831170B1 (fr) | 2001-10-19 | 2004-03-19 | Inst Nat Sante Rech Med | Proteines c modifiees activables directement par la thrombine |
| FR2841904B1 (fr) | 2002-07-03 | 2004-08-20 | Inst Nat Sante Rech Med | Analogues de facteurs x clivables par la thrombine |
| WO2005007820A2 (en) | 2003-07-08 | 2005-01-27 | The Scripps Research Institute | Activated protein c variants with normal cytoprotective activity but reduced anticoagulant activity |
| WO2006000020A1 (en) | 2004-06-29 | 2006-01-05 | European Nickel Plc | Improved leaching of base metals |
| MX2007001294A (es) * | 2004-08-17 | 2008-03-04 | Zlb Behring Gmbh | Polipeptidos modificados que dependen de vitamina k. |
| EP1820508A1 (en) * | 2006-02-21 | 2007-08-22 | CSL Behring GmbH | Coagulation factor X polypeptides with modified activation properties |
| US8246856B2 (en) | 2007-08-01 | 2012-08-21 | Dow Global Technologies Llc | Highly efficient process for manufacture of exfoliated graphene |
| CN104262479A (zh) * | 2008-12-19 | 2015-01-07 | 国家健康与医学研究院 | 丝氨酸蛋白酶衍生物及其在凝血功能障碍的预防或治疗中的用途 |
-
2009
- 2009-12-21 CN CN201410496015.1A patent/CN104262479A/zh active Pending
- 2009-12-21 WO PCT/EP2009/067632 patent/WO2010070137A1/en not_active Ceased
- 2009-12-21 KR KR1020117016908A patent/KR20110114587A/ko not_active Ceased
- 2009-12-21 JP JP2011541497A patent/JP5833448B2/ja not_active Expired - Fee Related
- 2009-12-21 ES ES09796378.9T patent/ES2629099T3/es active Active
- 2009-12-21 AU AU2009329493A patent/AU2009329493B2/en not_active Ceased
- 2009-12-21 US US13/139,367 patent/US8436144B2/en not_active Expired - Fee Related
- 2009-12-21 CN CN200980157388.3A patent/CN102325880B/zh not_active Expired - Fee Related
- 2009-12-21 CA CA2747065A patent/CA2747065A1/en not_active Abandoned
- 2009-12-21 EP EP09796378.9A patent/EP2358874B1/en not_active Not-in-force
-
2013
- 2013-01-02 US US13/732,465 patent/US8518400B2/en not_active Expired - Fee Related
- 2013-06-04 US US13/909,323 patent/US8741286B2/en not_active Expired - Fee Related
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20150113205A (ko) * | 2013-02-04 | 2015-10-07 | 라보라토이레 프란카이즈 듀 프락티온네먼트 에트 데스 바이오테크놀로지스 | 인자 x 돌연변이체 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN102325880B (zh) | 2014-10-01 |
| US20130261060A1 (en) | 2013-10-03 |
| US8436144B2 (en) | 2013-05-07 |
| ES2629099T3 (es) | 2017-08-07 |
| CA2747065A1 (en) | 2010-06-24 |
| US20130101570A1 (en) | 2013-04-25 |
| US8741286B2 (en) | 2014-06-03 |
| CN102325880A (zh) | 2012-01-18 |
| EP2358874B1 (en) | 2017-03-29 |
| JP2012512645A (ja) | 2012-06-07 |
| WO2010070137A1 (en) | 2010-06-24 |
| AU2009329493A1 (en) | 2011-07-21 |
| CN104262479A (zh) | 2015-01-07 |
| AU2009329493B2 (en) | 2014-09-18 |
| US8518400B2 (en) | 2013-08-27 |
| US20110293597A1 (en) | 2011-12-01 |
| EP2358874A1 (en) | 2011-08-24 |
| JP5833448B2 (ja) | 2015-12-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20110114587A (ko) | 세린 프로테아제 유도체 및 혈액응고 장애의 치료 또는 예방의 용도 | |
| JP3894795B2 (ja) | ヒト細胞株における組換え血液凝固因子の製造 | |
| JP3051995B2 (ja) | 酸化抵抗性トロンボモジュリン類縁体 | |
| US10106786B2 (en) | Compositions and methods for modulating hemostasis | |
| AU2001254715A1 (en) | Production of recombinant blood clotting factors in human cell lines | |
| JP3517236B2 (ja) | プロテアーゼ−耐性トロンボモジュリン・アナログ | |
| JP4855627B2 (ja) | 医薬用途のためのトロンボモジュリン | |
| EP0458903A1 (en) | Soluble analogs of thrombomodulin | |
| AU2020242945B2 (en) | Factor IX variants and uses thereof in therapy | |
| CZ140298A3 (cs) | Hybridní faktor VIII s modifikovanou aktivitou | |
| JP4505416B2 (ja) | 新規キメラプラスミノゲンアクチベータおよびその薬学的用途 | |
| JPH08511948A (ja) | C4bp結合活性が不足しているがapc補因子活性を有するプロテインs欠失変異体、組成物及び治療法 | |
| JP6629744B2 (ja) | 第Xa因子の半減期を延ばす組成物および方法 | |
| Class et al. | Patent application title: Serene Protease Derivatives and Uses in the Prevention or the Treatment of Blood Coagulation Disorders Inventors: Olivier Christophe (Le Kremlin Bicetre Cedex, FR) Cecile Denis (Le Kremlin Bicetre Cedex, FR) Ghislaine Cherel (Le Kremlin Bicetre Cedex, FR) Paul Gueguen (Brest Cedex, FR) Assignees: INSTITUT NATIONAL DE LA SANTE ET DE LA RECHERCHE MEDICALE (INSERM) | |
| EP2199387A1 (en) | Serine protease derivatives and uses for the prevention and/or the treatment of blood coagulation disorders |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20110719 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20141119 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20151113 Patent event code: PE09021S01D |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20160927 Patent event code: PE09021S01D |
|
| PE0601 | Decision on rejection of patent |
Patent event date: 20161130 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20160927 Comment text: Notification of reason for refusal Patent event code: PE06011S01I Patent event date: 20151113 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |