KR20100089815A - 피퍼 쿠베바 엘.로부터의 추출물 또는 추출된 물질의 암 질환의 치료를 위한 약물에서 효과적인 성분으로서의 용도 - Google Patents
피퍼 쿠베바 엘.로부터의 추출물 또는 추출된 물질의 암 질환의 치료를 위한 약물에서 효과적인 성분으로서의 용도 Download PDFInfo
- Publication number
- KR20100089815A KR20100089815A KR1020107005684A KR20107005684A KR20100089815A KR 20100089815 A KR20100089815 A KR 20100089815A KR 1020107005684 A KR1020107005684 A KR 1020107005684A KR 20107005684 A KR20107005684 A KR 20107005684A KR 20100089815 A KR20100089815 A KR 20100089815A
- Authority
- KR
- South Korea
- Prior art keywords
- extract
- alcohol
- cancer
- fruit
- prostate cancer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000000284 extract Substances 0.000 title claims abstract description 110
- 239000003814 drug Substances 0.000 title claims description 15
- 238000011282 treatment Methods 0.000 title claims description 14
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 title claims description 8
- 206010028980 Neoplasm Diseases 0.000 title claims description 5
- 201000011510 cancer Diseases 0.000 title claims description 5
- 239000001086 piper cubeba l. Substances 0.000 title claims description 3
- 229940079593 drug Drugs 0.000 title description 6
- 239000000463 material Substances 0.000 title description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims abstract description 32
- 235000013399 edible fruits Nutrition 0.000 claims abstract description 22
- 239000000341 volatile oil Substances 0.000 claims abstract description 14
- 239000000203 mixture Substances 0.000 claims abstract description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 9
- 238000000605 extraction Methods 0.000 claims abstract description 8
- 238000004519 manufacturing process Methods 0.000 claims abstract description 4
- 238000001035 drying Methods 0.000 claims abstract 5
- 230000000694 effects Effects 0.000 claims description 31
- 238000000034 method Methods 0.000 claims description 15
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 claims description 12
- 206010060862 Prostate cancer Diseases 0.000 claims description 11
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 11
- 230000002280 anti-androgenic effect Effects 0.000 claims description 9
- 239000000051 antiandrogen Substances 0.000 claims description 9
- 206010006187 Breast cancer Diseases 0.000 claims description 8
- 208000026310 Breast neoplasm Diseases 0.000 claims description 8
- 150000001875 compounds Chemical class 0.000 claims description 8
- 239000004480 active ingredient Substances 0.000 claims description 7
- 229940046836 anti-estrogen Drugs 0.000 claims description 6
- 230000001833 anti-estrogenic effect Effects 0.000 claims description 6
- 239000000328 estrogen antagonist Substances 0.000 claims description 6
- 239000003163 gonadal steroid hormone Substances 0.000 claims description 6
- 235000021028 berry Nutrition 0.000 claims description 5
- 206010004446 Benign prostatic hyperplasia Diseases 0.000 claims description 4
- 208000004403 Prostatic Hyperplasia Diseases 0.000 claims description 4
- 230000002424 anti-apoptotic effect Effects 0.000 claims description 4
- 201000010099 disease Diseases 0.000 claims description 4
- 239000012752 auxiliary agent Substances 0.000 claims description 3
- RSIHSRDYCUFFLA-DYKIIFRCSA-N boldenone Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 RSIHSRDYCUFFLA-DYKIIFRCSA-N 0.000 claims description 3
- RSIHSRDYCUFFLA-UHFFFAOYSA-N dehydrotestosterone Natural products O=C1C=CC2(C)C3CCC(C)(C(CC4)O)C4C3CCC2=C1 RSIHSRDYCUFFLA-UHFFFAOYSA-N 0.000 claims description 3
- 230000035755 proliferation Effects 0.000 claims description 3
- 238000000227 grinding Methods 0.000 claims description 2
- 206010020718 hyperplasia Diseases 0.000 claims description 2
- 210000002307 prostate Anatomy 0.000 claims description 2
- 206010027476 Metastases Diseases 0.000 claims 5
- 208000024313 Testicular Neoplasms Diseases 0.000 claims 3
- 206010057644 Testis cancer Diseases 0.000 claims 3
- 208000002495 Uterine Neoplasms Diseases 0.000 claims 3
- 201000003120 testicular cancer Diseases 0.000 claims 3
- 206010046766 uterine cancer Diseases 0.000 claims 3
- 150000001298 alcohols Chemical class 0.000 claims 2
- 125000004432 carbon atom Chemical group C* 0.000 claims 2
- QWTDNUCVQCZILF-UHFFFAOYSA-N isopentane Chemical compound CCC(C)C QWTDNUCVQCZILF-UHFFFAOYSA-N 0.000 claims 2
- 230000009401 metastasis Effects 0.000 claims 2
- 239000004215 Carbon black (E152) Substances 0.000 claims 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 claims 1
- 229930195725 Mannitol Natural products 0.000 claims 1
- NBZANZVJRKXVBH-GYDPHNCVSA-N alpha-Cryptoxanthin Natural products O[C@H]1CC(C)(C)C(/C=C/C(=C\C=C\C(=C/C=C/C=C(\C=C\C=C(/C=C/[C@H]2C(C)=CCCC2(C)C)\C)/C)\C)/C)=C(C)C1 NBZANZVJRKXVBH-GYDPHNCVSA-N 0.000 claims 1
- 230000003042 antagnostic effect Effects 0.000 claims 1
- FSRZGYRCMPZNJF-UHFFFAOYSA-N beta-Cubebene Natural products C12C(C(C)C)CCC(C)C32C1C(=C)CC3 FSRZGYRCMPZNJF-UHFFFAOYSA-N 0.000 claims 1
- FSRZGYRCMPZNJF-KHMAMNHCSA-N beta-cubebene Chemical compound CC(C)[C@@H]([C@H]12)CC[C@@H](C)[C@]32[C@@H]1C(=C)CC3 FSRZGYRCMPZNJF-KHMAMNHCSA-N 0.000 claims 1
- 239000003795 chemical substances by application Substances 0.000 claims 1
- AFABGHUZZDYHJO-UHFFFAOYSA-N dimethyl butane Natural products CCCC(C)C AFABGHUZZDYHJO-UHFFFAOYSA-N 0.000 claims 1
- 229930195733 hydrocarbon Natural products 0.000 claims 1
- 150000002430 hydrocarbons Chemical class 0.000 claims 1
- 235000010355 mannitol Nutrition 0.000 claims 1
- 239000000594 mannitol Substances 0.000 claims 1
- 238000001694 spray drying Methods 0.000 claims 1
- 230000008719 thickening Effects 0.000 abstract description 7
- 210000004027 cell Anatomy 0.000 description 36
- 239000000126 substance Substances 0.000 description 17
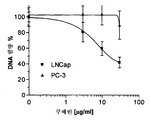
- 210000004881 tumor cell Anatomy 0.000 description 17
- 102000007066 Prostate-Specific Antigen Human genes 0.000 description 15
- 108010072866 Prostate-Specific Antigen Proteins 0.000 description 15
- 230000005764 inhibitory process Effects 0.000 description 13
- 239000007788 liquid Substances 0.000 description 13
- 239000001963 growth medium Substances 0.000 description 10
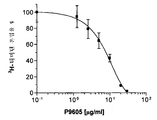
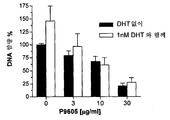
- 230000006820 DNA synthesis Effects 0.000 description 9
- 108010066551 Cholestenone 5 alpha-Reductase Proteins 0.000 description 8
- 238000002474 experimental method Methods 0.000 description 8
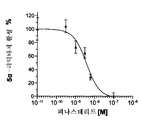
- 102100032187 Androgen receptor Human genes 0.000 description 6
- 108020004414 DNA Proteins 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 108010080146 androgen receptors Proteins 0.000 description 6
- 230000004663 cell proliferation Effects 0.000 description 6
- 230000002401 inhibitory effect Effects 0.000 description 6
- 230000028327 secretion Effects 0.000 description 5
- NVKAWKQGWWIWPM-ABEVXSGRSA-N 17-β-hydroxy-5-α-Androstan-3-one Chemical compound C1C(=O)CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@H]21 NVKAWKQGWWIWPM-ABEVXSGRSA-N 0.000 description 4
- 239000002677 5-alpha reductase inhibitor Substances 0.000 description 4
- 229960003473 androstanolone Drugs 0.000 description 4
- 230000006907 apoptotic process Effects 0.000 description 4
- 230000001419 dependent effect Effects 0.000 description 4
- 239000012530 fluid Substances 0.000 description 4
- 229940113178 5 Alpha reductase inhibitor Drugs 0.000 description 3
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 3
- 102100040247 Tumor necrosis factor Human genes 0.000 description 3
- 230000001028 anti-proliverative effect Effects 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 230000001909 effect on DNA Effects 0.000 description 3
- 230000003389 potentiating effect Effects 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 230000002062 proliferating effect Effects 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- 229940104230 thymidine Drugs 0.000 description 3
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 2
- PZNPLUBHRSSFHT-RRHRGVEJSA-N 1-hexadecanoyl-2-octadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[C@@H](COP([O-])(=O)OCC[N+](C)(C)C)COC(=O)CCCCCCCCCCCCCCC PZNPLUBHRSSFHT-RRHRGVEJSA-N 0.000 description 2
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 2
- 108010029908 3-oxo-5-alpha-steroid 4-dehydrogenase Proteins 0.000 description 2
- 102000001779 3-oxo-5-alpha-steroid 4-dehydrogenase Human genes 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000003098 androgen Substances 0.000 description 2
- 230000000259 anti-tumor effect Effects 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 238000004821 distillation Methods 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- 235000011869 dried fruits Nutrition 0.000 description 2
- 229960005309 estradiol Drugs 0.000 description 2
- 229930182833 estradiol Natural products 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 230000006882 induction of apoptosis Effects 0.000 description 2
- 229930013686 lignan Natural products 0.000 description 2
- 150000005692 lignans Chemical class 0.000 description 2
- 235000009408 lignans Nutrition 0.000 description 2
- 230000035407 negative regulation of cell proliferation Effects 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 239000012053 oil suspension Substances 0.000 description 2
- 239000008347 soybean phospholipid Substances 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 150000003626 triacylglycerols Chemical class 0.000 description 2
- DIYWRNLYKJKHAM-MDOVXXIYSA-N (-)-cubebin Chemical compound C1=C2OCOC2=CC(C[C@@H]2[C@@H](CC=3C=C4OCOC4=CC=3)CO[C@@H]2O)=C1 DIYWRNLYKJKHAM-MDOVXXIYSA-N 0.000 description 1
- 206010018612 Gonorrhoea Diseases 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- 108010033040 Histones Proteins 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- XUEHVOLRMXNRKQ-KHMAMNHCSA-N alpha cubebene Natural products CC(C)[C@@H]([C@H]12)CC[C@@H](C)[C@]32[C@@H]1C(C)=CC3 XUEHVOLRMXNRKQ-KHMAMNHCSA-N 0.000 description 1
- 230000006909 anti-apoptosis Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 230000009674 basal proliferation Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 201000003146 cystitis Diseases 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 229940030606 diuretics Drugs 0.000 description 1
- 230000002900 effect on cell Effects 0.000 description 1
- 239000000469 ethanolic extract Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000000417 fungicide Substances 0.000 description 1
- 239000004083 gastrointestinal agent Substances 0.000 description 1
- 235000012432 gingerbread Nutrition 0.000 description 1
- 208000001786 gonorrhea Diseases 0.000 description 1
- 230000003779 hair growth Effects 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 235000020094 liqueur Nutrition 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 235000013599 spices Nutrition 0.000 description 1
- 238000001256 steam distillation Methods 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 229960003604 testosterone Drugs 0.000 description 1
- 229940095585 testosterone-5-alpha reductase inhibitors for benign prostatic hypertrophy Drugs 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/67—Piperaceae (Pepper family), e.g. Jamaican pepper or kava
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
- A61P5/28—Antiandrogens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
- A61P5/32—Antioestrogens
Landscapes
- Health & Medical Sciences (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Endocrinology (AREA)
- Alternative & Traditional Medicine (AREA)
- Biotechnology (AREA)
- Botany (AREA)
- Medical Informatics (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Diabetes (AREA)
- Reproductive Health (AREA)
- Medicines Containing Plant Substances (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH1289/07 | 2007-08-16 | ||
| CH01289/07A CH698627B1 (de) | 2007-08-16 | 2007-08-16 | Herstellung und Verwendung von Extrakten oder Extraktivstoffen aus Piper cubeba L. als wirksame Bestandteile in einem Medikament zur Behandlung von Krebserkrankungen. |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20100089815A true KR20100089815A (ko) | 2010-08-12 |
Family
ID=38668948
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020107005684A Ceased KR20100089815A (ko) | 2007-08-16 | 2008-08-15 | 피퍼 쿠베바 엘.로부터의 추출물 또는 추출된 물질의 암 질환의 치료를 위한 약물에서 효과적인 성분으로서의 용도 |
Country Status (14)
| Country | Link |
|---|---|
| US (3) | US8404286B2 (enExample) |
| EP (1) | EP2182966B1 (enExample) |
| JP (1) | JP5378379B2 (enExample) |
| KR (1) | KR20100089815A (enExample) |
| CN (1) | CN102065875B (enExample) |
| AU (1) | AU2008286636B2 (enExample) |
| BR (1) | BRPI0815188A2 (enExample) |
| CA (1) | CA2696499A1 (enExample) |
| CH (1) | CH698627B1 (enExample) |
| ES (1) | ES2392310T3 (enExample) |
| PL (1) | PL2182966T3 (enExample) |
| RU (1) | RU2470657C2 (enExample) |
| WO (1) | WO2009021347A1 (enExample) |
| ZA (1) | ZA201000959B (enExample) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI472333B (zh) * | 2008-06-26 | 2015-02-11 | Piramal Entpr Ltd | 治療口腔念珠菌病之口服草本組成物 |
| EP2517705B1 (en) * | 2009-12-21 | 2016-10-19 | Acef S/A | Cubebin as vasodilating agent in the therapy of erectile dysfunction |
| WO2014027857A1 (ko) * | 2012-08-16 | 2014-02-20 | 부산대학교 산학협력단 | 알파-이소-쿠베벤 또는 알파-이소-쿠베베놀을 유효성분으로 포함하는 감염성 및 염증성 질환의 예방 또는 치료용 조성물 |
| WO2014086379A1 (en) * | 2012-12-03 | 2014-06-12 | Alpinia Laudanum Institute Of Phytopharmaceutical Sciences Ag | Lignan compositions |
| FR3085846B1 (fr) * | 2018-09-13 | 2022-01-21 | Robertet Sa | Utilisation d’extrait de p.nigrum, p. cubeba et/ou s. therebentifolius dans des preparations cosmetiques pour favoriser la pousse des poils |
| JP7197908B2 (ja) * | 2019-03-04 | 2022-12-28 | 株式会社ナチュファルマ琉球 | 骨減少性疾患の予防及び/又は治療用組成物 |
| CN114574286B (zh) * | 2022-02-18 | 2024-03-22 | 中国热带农业科学院香料饮料研究所 | 一种具有抗炎活性的胡椒精油及其制备方法 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9815177D0 (en) | 1998-07-13 | 1998-09-09 | King S College London | Treatment of skin disorders |
| JP3091962B2 (ja) * | 1998-09-25 | 2000-09-25 | アルプス薬品工業株式会社 | テストステロン−5α−レダクターゼ阻害剤 |
| AU2002219964A1 (en) * | 2001-11-29 | 2003-06-17 | Penwest Pharmaceutical Company | Agglomerated particles including an active agent coprocessed with silicified microcrystalline cellulose |
| BR0201237A (pt) * | 2002-03-25 | 2003-12-02 | Fundacao De Amparo A Pesquisa | Processo de obtenção de lignanas dibenzilbutirolactÈnicas, processo de obtenção de derivados sintéticos de lignanas ostentando atividades quimioprofilática e terapêutica antichagásica |
| US20030228383A1 (en) * | 2002-06-06 | 2003-12-11 | J.B. Chemicals & Pharmaceuticals, Ltd. | Herbal cough formulations and process for the preparation thereof |
| JP2004160165A (ja) * | 2002-09-17 | 2004-06-10 | Pauretsuku:Kk | 生薬成分製剤の造粒方法 |
| ATE365559T1 (de) * | 2002-12-30 | 2007-07-15 | Council Scient Ind Res | Entwicklung einer hustenstillenden und rachenschmerzlindernden formulierung aus kräutern |
| JP4319417B2 (ja) * | 2003-01-30 | 2009-08-26 | 学校法人近畿大学 | メラニン産生不全症治療剤 |
| US20070248693A1 (en) * | 2003-08-02 | 2007-10-25 | Elizabeth Mazzio | Nutraceutical composition and method of use for treatment / prevention of cancer |
-
2007
- 2007-08-16 CH CH01289/07A patent/CH698627B1/de not_active IP Right Cessation
-
2008
- 2008-08-15 KR KR1020107005684A patent/KR20100089815A/ko not_active Ceased
- 2008-08-15 BR BRPI0815188 patent/BRPI0815188A2/pt not_active IP Right Cessation
- 2008-08-15 ES ES08783450T patent/ES2392310T3/es active Active
- 2008-08-15 AU AU2008286636A patent/AU2008286636B2/en not_active Ceased
- 2008-08-15 JP JP2010520393A patent/JP5378379B2/ja not_active Expired - Fee Related
- 2008-08-15 PL PL08783450T patent/PL2182966T3/pl unknown
- 2008-08-15 EP EP08783450A patent/EP2182966B1/de active Active
- 2008-08-15 CN CN200880111798XA patent/CN102065875B/zh not_active Expired - Fee Related
- 2008-08-15 CA CA2696499A patent/CA2696499A1/en not_active Abandoned
- 2008-08-15 WO PCT/CH2008/000350 patent/WO2009021347A1/de not_active Ceased
- 2008-08-15 US US12/673,656 patent/US8404286B2/en not_active Expired - Fee Related
- 2008-08-15 RU RU2010109750/15A patent/RU2470657C2/ru not_active IP Right Cessation
-
2010
- 2010-02-10 ZA ZA201000959A patent/ZA201000959B/xx unknown
-
2013
- 2013-02-28 US US13/780,129 patent/US9248156B2/en not_active Expired - Fee Related
-
2016
- 2016-02-02 US US15/013,654 patent/US20160193272A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| WO2009021347A1 (de) | 2009-02-19 |
| US20160193272A1 (en) | 2016-07-07 |
| US9248156B2 (en) | 2016-02-02 |
| RU2470657C2 (ru) | 2012-12-27 |
| PL2182966T3 (pl) | 2013-01-31 |
| CH698627B1 (de) | 2009-09-15 |
| US20110135760A1 (en) | 2011-06-09 |
| BRPI0815188A2 (pt) | 2015-03-31 |
| US20130251826A1 (en) | 2013-09-26 |
| CA2696499A1 (en) | 2009-02-19 |
| RU2010109750A (ru) | 2011-09-27 |
| ZA201000959B (en) | 2010-10-27 |
| CN102065875A (zh) | 2011-05-18 |
| EP2182966A1 (de) | 2010-05-12 |
| JP5378379B2 (ja) | 2013-12-25 |
| US8404286B2 (en) | 2013-03-26 |
| CN102065875B (zh) | 2013-07-17 |
| EP2182966B1 (de) | 2012-08-08 |
| ES2392310T3 (es) | 2012-12-07 |
| AU2008286636B2 (en) | 2013-10-24 |
| AU2008286636A1 (en) | 2009-02-19 |
| JP2010536718A (ja) | 2010-12-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20100089815A (ko) | 피퍼 쿠베바 엘.로부터의 추출물 또는 추출된 물질의 암 질환의 치료를 위한 약물에서 효과적인 성분으로서의 용도 | |
| EP1670491B1 (en) | Composition comprising xanthoceras sorbifolia extracts, compounds isolated from same, methods for preparing same and uses thereof | |
| Pongprayoon et al. | Antispasmodic activity of β-damascenone and E-phytol isolated from Ipomoea pes-caprae | |
| JP5756264B2 (ja) | キサンチンオキシダーゼ阻害剤、キサンチンオキシダーゼ及び5α−レダクターゼ阻害剤、並びに該阻害剤を含有する医薬組成物 | |
| JP2010536718A5 (enExample) | ||
| JP2007513941A (ja) | メラニン生合成阻害活性を有するテレイン(terrein)化合物及びその製造方法 | |
| KR102598042B1 (ko) | 중대가리풀 추출물을 포함하는 항염 및 자가면역 질환 예방 및 치료용 조성물 및 이의 제조 방법 | |
| KR102057091B1 (ko) | Nrf2 인덕션 리바이탈라이징 제제용 피이지프리 가용화제, 이를 포함하는 Nrf2 인덕션 리바이탈라이징 제제, 이를 포함하는 화장품 및 이의 제조방법 | |
| Wang et al. | Antioxidant activity and inhibition effect on the growth of human colon carcinoma (HT-29) cells of esculetin from Cortex Fraxini | |
| KR102843355B1 (ko) | 쑥, 쌀겨, 울금 및 유황의 4종 복합물을 유효성분으로 포함하는 항염증, 항산화 및 혈액순환 개선용 조성물 | |
| JP5052026B2 (ja) | 新規化合物およびその製造方法、用途 | |
| CN104582713A (zh) | 格雷伊拉德科菲耶提取物及其应用 | |
| Heo et al. | Effect of UV irradiation on the antioxidant and anti-adipogenic activities of quercetin | |
| JP4557582B2 (ja) | 抗かゆみ剤 | |
| WO2012026641A1 (ko) | 치매의 예방 또는 치료용 조성물 | |
| JP4261413B2 (ja) | メラニン産生抑制剤 | |
| Khat-Udomkiri et al. | Polyol-based ultrasound-assisted extraction: Unlocking the anti-tyrosinase and anti-melanogenesis potential of Vitex trifolia Linn. Leaves | |
| JP4731266B2 (ja) | チロシナーゼ活性阻害剤、美白剤及び皮膚化粧料 | |
| JP2018123091A (ja) | エストロゲン様作用剤 | |
| KR100502072B1 (ko) | 라쓰란계 다이터르펜류를 함유하는 5-알파 리덕테이즈억제제 및 이를 함유한 피부 외용제 조성물 | |
| Kim et al. | Machilus Thunbergii Water Extract Induces Cytotoxic Effect against Human Acute Jurkat T Lymphoma | |
| JP2010111645A (ja) | 美白剤及び皮膚化粧料 | |
| CH698610B1 (de) | Verwendung von Extrakten oder Extraktivstoffen aus Piper cubeba L. als wirksame Bestandteile in einem Medikament zur Behandlung von Krebserkrankungen. | |
| KR20170054156A (ko) | 골다공증 예방 및 개선용 약학적 조성물 및 건강기능식품 | |
| JP2014210753A (ja) | アスナロエキスの製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20100315 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20130802 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20141219 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20150401 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20141219 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |