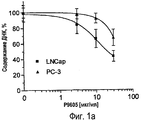
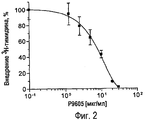
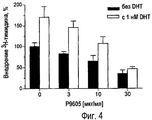
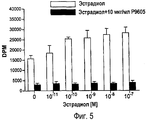
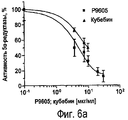
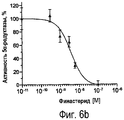
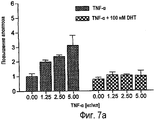
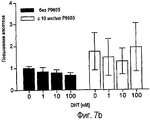
RU2470657C2 - ПРИМЕНЕНИЕ ЭКСТРАКТОВ ИЛИ ЭКСТРАКТИВНЫХ ВЕЩЕСТВ ИЗ Piper cubeba L. В КАЧЕСТВЕ АКТИВНЫХ ИНГРЕДИЕНТОВ В ЛЕКАРСТВЕННОМ СРЕДСТВЕ ДЛЯ ЛЕЧЕНИЯ РАКОВЫХ ЗАБОЛЕВАНИЙ - Google Patents
ПРИМЕНЕНИЕ ЭКСТРАКТОВ ИЛИ ЭКСТРАКТИВНЫХ ВЕЩЕСТВ ИЗ Piper cubeba L. В КАЧЕСТВЕ АКТИВНЫХ ИНГРЕДИЕНТОВ В ЛЕКАРСТВЕННОМ СРЕДСТВЕ ДЛЯ ЛЕЧЕНИЯ РАКОВЫХ ЗАБОЛЕВАНИЙ Download PDFInfo
- Publication number
- RU2470657C2 RU2470657C2 RU2010109750/15A RU2010109750A RU2470657C2 RU 2470657 C2 RU2470657 C2 RU 2470657C2 RU 2010109750/15 A RU2010109750/15 A RU 2010109750/15A RU 2010109750 A RU2010109750 A RU 2010109750A RU 2470657 C2 RU2470657 C2 RU 2470657C2
- Authority
- RU
- Russia
- Prior art keywords
- extract
- alcohol
- stage
- cancer
- piper cubeba
- Prior art date
Links
- 239000000284 extract Substances 0.000 title claims abstract description 123
- 239000001086 piper cubeba l. Substances 0.000 title claims abstract description 16
- 239000003814 drug Substances 0.000 title claims abstract description 15
- 239000004480 active ingredient Substances 0.000 title claims abstract description 12
- 239000000126 substance Substances 0.000 title claims abstract description 12
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 title claims abstract description 10
- 201000010099 disease Diseases 0.000 title claims abstract description 9
- 229940079593 drug Drugs 0.000 title claims abstract description 7
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims abstract description 31
- 235000013399 edible fruits Nutrition 0.000 claims abstract description 25
- 238000000034 method Methods 0.000 claims abstract description 23
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 10
- 206010004446 Benign prostatic hyperplasia Diseases 0.000 claims abstract description 7
- XUEHVOLRMXNRKQ-KHMAMNHCSA-N alpha-cubebene Chemical compound CC(C)[C@@H]([C@H]12)CC[C@@H](C)[C@]32[C@@H]1C(C)=CC3 XUEHVOLRMXNRKQ-KHMAMNHCSA-N 0.000 claims description 24
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 claims description 12
- 239000000341 volatile oil Substances 0.000 claims description 12
- 206010006187 Breast cancer Diseases 0.000 claims description 9
- 208000026310 Breast neoplasm Diseases 0.000 claims description 9
- 206010060862 Prostate cancer Diseases 0.000 claims description 9
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 9
- 230000002280 anti-androgenic effect Effects 0.000 claims description 9
- 239000000203 mixture Substances 0.000 claims description 9
- NVKAWKQGWWIWPM-ABEVXSGRSA-N 17-β-hydroxy-5-α-Androstan-3-one Chemical compound C1C(=O)CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@H]21 NVKAWKQGWWIWPM-ABEVXSGRSA-N 0.000 claims description 7
- 230000009471 action Effects 0.000 claims description 7
- 229960003473 androstanolone Drugs 0.000 claims description 7
- 238000000605 extraction Methods 0.000 claims description 7
- 208000004403 Prostatic Hyperplasia Diseases 0.000 claims description 6
- 230000001833 anti-estrogenic effect Effects 0.000 claims description 6
- 239000003163 gonadal steroid hormone Substances 0.000 claims description 6
- 206010028980 Neoplasm Diseases 0.000 claims description 5
- 230000002424 anti-apoptotic effect Effects 0.000 claims description 5
- 230000035755 proliferation Effects 0.000 claims description 5
- 201000011510 cancer Diseases 0.000 claims description 4
- 230000002708 enhancing effect Effects 0.000 claims description 2
- 206010027476 Metastases Diseases 0.000 claims 5
- 208000024313 Testicular Neoplasms Diseases 0.000 claims 3
- 206010057644 Testis cancer Diseases 0.000 claims 3
- 208000002495 Uterine Neoplasms Diseases 0.000 claims 3
- 201000003120 testicular cancer Diseases 0.000 claims 3
- 206010046766 uterine cancer Diseases 0.000 claims 3
- NBZANZVJRKXVBH-GYDPHNCVSA-N alpha-Cryptoxanthin Natural products O[C@H]1CC(C)(C)C(/C=C/C(=C\C=C\C(=C/C=C/C=C(\C=C\C=C(/C=C/[C@H]2C(C)=CCCC2(C)C)\C)/C)\C)/C)=C(C)C1 NBZANZVJRKXVBH-GYDPHNCVSA-N 0.000 claims 2
- FSRZGYRCMPZNJF-UHFFFAOYSA-N beta-Cubebene Natural products C12C(C(C)C)CCC(C)C32C1C(=C)CC3 FSRZGYRCMPZNJF-UHFFFAOYSA-N 0.000 claims 2
- FSRZGYRCMPZNJF-KHMAMNHCSA-N beta-cubebene Chemical compound CC(C)[C@@H]([C@H]12)CC[C@@H](C)[C@]32[C@@H]1C(=C)CC3 FSRZGYRCMPZNJF-KHMAMNHCSA-N 0.000 claims 2
- 125000004432 carbon atom Chemical group C* 0.000 claims 2
- 238000001035 drying Methods 0.000 claims 2
- QWTDNUCVQCZILF-UHFFFAOYSA-N isopentane Chemical compound CCC(C)C QWTDNUCVQCZILF-UHFFFAOYSA-N 0.000 claims 2
- 239000004215 Carbon black (E152) Substances 0.000 claims 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 claims 1
- 229930195725 Mannitol Natural products 0.000 claims 1
- 229910052799 carbon Inorganic materials 0.000 claims 1
- AFABGHUZZDYHJO-UHFFFAOYSA-N dimethyl butane Natural products CCCC(C)C AFABGHUZZDYHJO-UHFFFAOYSA-N 0.000 claims 1
- 210000004907 gland Anatomy 0.000 claims 1
- 229930195733 hydrocarbon Natural products 0.000 claims 1
- 150000002430 hydrocarbons Chemical class 0.000 claims 1
- 235000010355 mannitol Nutrition 0.000 claims 1
- 239000000594 mannitol Substances 0.000 claims 1
- 239000007921 spray Substances 0.000 claims 1
- 230000000694 effects Effects 0.000 abstract description 16
- 239000000546 pharmaceutical excipient Substances 0.000 abstract description 2
- 240000003731 Piper cubeba Species 0.000 abstract 1
- 235000002711 Piper cubeba Nutrition 0.000 abstract 1
- 239000000686 essence Substances 0.000 abstract 1
- 239000007787 solid Substances 0.000 abstract 1
- 210000004027 cell Anatomy 0.000 description 43
- 238000002474 experimental method Methods 0.000 description 20
- DIYWRNLYKJKHAM-MDOVXXIYSA-N (-)-cubebin Chemical compound C1=C2OCOC2=CC(C[C@@H]2[C@@H](CC=3C=C4OCOC4=CC=3)CO[C@@H]2O)=C1 DIYWRNLYKJKHAM-MDOVXXIYSA-N 0.000 description 19
- 239000007788 liquid Substances 0.000 description 19
- 210000004881 tumor cell Anatomy 0.000 description 16
- 102000007066 Prostate-Specific Antigen Human genes 0.000 description 15
- 108010072866 Prostate-Specific Antigen Proteins 0.000 description 15
- 230000006820 DNA synthesis Effects 0.000 description 14
- 230000005764 inhibitory process Effects 0.000 description 12
- 108020004414 DNA Proteins 0.000 description 8
- 239000001963 growth medium Substances 0.000 description 8
- 108010080146 androgen receptors Proteins 0.000 description 6
- 230000004663 cell proliferation Effects 0.000 description 6
- 230000002401 inhibitory effect Effects 0.000 description 6
- 239000002904 solvent Substances 0.000 description 6
- 231100000673 dose–response relationship Toxicity 0.000 description 5
- 102100033875 3-oxo-5-alpha-steroid 4-dehydrogenase 2 Human genes 0.000 description 4
- 108010066551 Cholestenone 5 alpha-Reductase Proteins 0.000 description 4
- 101000640851 Homo sapiens 3-oxo-5-alpha-steroid 4-dehydrogenase 2 Proteins 0.000 description 4
- 102000001307 androgen receptors Human genes 0.000 description 4
- 230000006907 apoptotic process Effects 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 230000001419 dependent effect Effects 0.000 description 4
- 230000012010 growth Effects 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 239000002994 raw material Substances 0.000 description 4
- 230000028327 secretion Effects 0.000 description 4
- 108010029908 3-oxo-5-alpha-steroid 4-dehydrogenase Proteins 0.000 description 3
- 102000001779 3-oxo-5-alpha-steroid 4-dehydrogenase Human genes 0.000 description 3
- 229940113178 5 Alpha reductase inhibitor Drugs 0.000 description 3
- 239000002677 5-alpha reductase inhibitor Substances 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 3
- 102100040247 Tumor necrosis factor Human genes 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 230000000996 additive effect Effects 0.000 description 3
- 230000001028 anti-proliverative effect Effects 0.000 description 3
- 230000034994 death Effects 0.000 description 3
- 238000004821 distillation Methods 0.000 description 3
- DBEPLOCGEIEOCV-WSBQPABSSA-N finasteride Chemical compound N([C@@H]1CC2)C(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](C(=O)NC(C)(C)C)[C@@]2(C)CC1 DBEPLOCGEIEOCV-WSBQPABSSA-N 0.000 description 3
- 229960004039 finasteride Drugs 0.000 description 3
- 230000001900 immune effect Effects 0.000 description 3
- 230000006882 induction of apoptosis Effects 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 229940104230 thymidine Drugs 0.000 description 3
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 2
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 2
- 244000215068 Acacia senegal Species 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- 102100032187 Androgen receptor Human genes 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 229920000084 Gum arabic Polymers 0.000 description 2
- 235000010489 acacia gum Nutrition 0.000 description 2
- 239000000205 acacia gum Substances 0.000 description 2
- 239000003098 androgen Substances 0.000 description 2
- 230000000259 anti-tumor effect Effects 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 229960005309 estradiol Drugs 0.000 description 2
- 229930182833 estradiol Natural products 0.000 description 2
- 235000013861 fat-free Nutrition 0.000 description 2
- 235000012432 gingerbread Nutrition 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 229930013686 lignan Natural products 0.000 description 2
- 235000009408 lignans Nutrition 0.000 description 2
- 230000035407 negative regulation of cell proliferation Effects 0.000 description 2
- 230000024717 negative regulation of secretion Effects 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 239000008347 soybean phospholipid Substances 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 230000001629 suppression Effects 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 238000001291 vacuum drying Methods 0.000 description 2
- 206010019233 Headaches Diseases 0.000 description 1
- 102000006947 Histones Human genes 0.000 description 1
- 108010033040 Histones Proteins 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 210000000270 basal cell Anatomy 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- -1 cubebin lignan Chemical class 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 239000000645 desinfectant Substances 0.000 description 1
- 230000001079 digestive effect Effects 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 230000001882 diuretic effect Effects 0.000 description 1
- 235000011869 dried fruits Nutrition 0.000 description 1
- 230000003149 estradiol stimulation Effects 0.000 description 1
- 239000000469 ethanolic extract Substances 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000019634 flavors Nutrition 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 235000012907 honey Nutrition 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 150000005692 lignans Chemical class 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229940057917 medium chain triglycerides Drugs 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000003774 positive effect on hair Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000005258 radioactive decay Effects 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 235000013599 spices Nutrition 0.000 description 1
- 229960003604 testosterone Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/67—Piperaceae (Pepper family), e.g. Jamaican pepper or kava
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
- A61P5/28—Antiandrogens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
- A61P5/32—Antioestrogens
Landscapes
- Health & Medical Sciences (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Endocrinology (AREA)
- Mycology (AREA)
- Alternative & Traditional Medicine (AREA)
- Biotechnology (AREA)
- Botany (AREA)
- Medical Informatics (AREA)
- Microbiology (AREA)
- Epidemiology (AREA)
- Diabetes (AREA)
- Reproductive Health (AREA)
- Medicines Containing Plant Substances (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH1289/07 | 2007-08-16 | ||
| CH01289/07A CH698627B1 (de) | 2007-08-16 | 2007-08-16 | Herstellung und Verwendung von Extrakten oder Extraktivstoffen aus Piper cubeba L. als wirksame Bestandteile in einem Medikament zur Behandlung von Krebserkrankungen. |
| PCT/CH2008/000350 WO2009021347A1 (de) | 2007-08-16 | 2008-08-15 | Verwendung von extrakten oder extraktivstoffen aus piper cubeba l. als wirksame bestandteile in einem medikament zur behandlung von krebserkrankungen |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2010109750A RU2010109750A (ru) | 2011-09-27 |
| RU2470657C2 true RU2470657C2 (ru) | 2012-12-27 |
Family
ID=38668948
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2010109750/15A RU2470657C2 (ru) | 2007-08-16 | 2008-08-15 | ПРИМЕНЕНИЕ ЭКСТРАКТОВ ИЛИ ЭКСТРАКТИВНЫХ ВЕЩЕСТВ ИЗ Piper cubeba L. В КАЧЕСТВЕ АКТИВНЫХ ИНГРЕДИЕНТОВ В ЛЕКАРСТВЕННОМ СРЕДСТВЕ ДЛЯ ЛЕЧЕНИЯ РАКОВЫХ ЗАБОЛЕВАНИЙ |
Country Status (14)
| Country | Link |
|---|---|
| US (3) | US8404286B2 (enExample) |
| EP (1) | EP2182966B1 (enExample) |
| JP (1) | JP5378379B2 (enExample) |
| KR (1) | KR20100089815A (enExample) |
| CN (1) | CN102065875B (enExample) |
| AU (1) | AU2008286636B2 (enExample) |
| BR (1) | BRPI0815188A2 (enExample) |
| CA (1) | CA2696499A1 (enExample) |
| CH (1) | CH698627B1 (enExample) |
| ES (1) | ES2392310T3 (enExample) |
| PL (1) | PL2182966T3 (enExample) |
| RU (1) | RU2470657C2 (enExample) |
| WO (1) | WO2009021347A1 (enExample) |
| ZA (1) | ZA201000959B (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2773173C2 (ru) * | 2017-03-20 | 2022-05-31 | Пьер Фабр Медикамент | Применение олеорезинов копаиферы при патологиях простаты |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI472333B (zh) * | 2008-06-26 | 2015-02-11 | Piramal Entpr Ltd | 治療口腔念珠菌病之口服草本組成物 |
| JP5806231B2 (ja) * | 2009-12-21 | 2015-11-10 | アセフ エス.アー. | 勃起障害の治療における血管拡張剤としてのクベビン、ジベンジルブチルロラクトリンリグナン、その半合成誘導体および合成誘導体、ならびに他のリグナンおよびネオリグナン |
| WO2014027857A1 (ko) * | 2012-08-16 | 2014-02-20 | 부산대학교 산학협력단 | 알파-이소-쿠베벤 또는 알파-이소-쿠베베놀을 유효성분으로 포함하는 감염성 및 염증성 질환의 예방 또는 치료용 조성물 |
| WO2014086379A1 (en) | 2012-12-03 | 2014-06-12 | Alpinia Laudanum Institute Of Phytopharmaceutical Sciences Ag | Lignan compositions |
| FR3085846B1 (fr) * | 2018-09-13 | 2022-01-21 | Robertet Sa | Utilisation d’extrait de p.nigrum, p. cubeba et/ou s. therebentifolius dans des preparations cosmetiques pour favoriser la pousse des poils |
| JP7197908B2 (ja) * | 2019-03-04 | 2022-12-28 | 株式会社ナチュファルマ琉球 | 骨減少性疾患の予防及び/又は治療用組成物 |
| CN114574286B (zh) * | 2022-02-18 | 2024-03-22 | 中国热带农业科学院香料饮料研究所 | 一种具有抗炎活性的胡椒精油及其制备方法 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1094813B1 (en) * | 1998-07-13 | 2003-09-10 | Btg International Limited | Use of piperine and derivatives thereof for treating skin pigmentation disorders and skin cancer |
| US20050154215A1 (en) * | 2002-03-25 | 2005-07-14 | Fundacao De Amparo A Pesquisa Do Estado De Sao Pau | Process to obtain dibenzylbutyrolactonic lignans process to obtain synthetic derivatives from lignanes bearing anti-chagas chemoprophylatic and therapeutical activies |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3091962B2 (ja) * | 1998-09-25 | 2000-09-25 | アルプス薬品工業株式会社 | テストステロン−5α−レダクターゼ阻害剤 |
| WO2003047551A1 (en) * | 2001-11-29 | 2003-06-12 | Penwest Pharmaceutical Company | Agglomerated particles including an active agent coprocessed with silicified microcrystalline cellulose |
| US20030228383A1 (en) * | 2002-06-06 | 2003-12-11 | J.B. Chemicals & Pharmaceuticals, Ltd. | Herbal cough formulations and process for the preparation thereof |
| JP2004160165A (ja) * | 2002-09-17 | 2004-06-10 | Pauretsuku:Kk | 生薬成分製剤の造粒方法 |
| EP1581237B1 (en) * | 2002-12-30 | 2007-06-27 | Council of Scientific and Industrial Research | Development of an anti-cough, anti-tussive and throat soothing herbal formulation |
| JP4319417B2 (ja) * | 2003-01-30 | 2009-08-26 | 学校法人近畿大学 | メラニン産生不全症治療剤 |
| US20070248693A1 (en) * | 2003-08-02 | 2007-10-25 | Elizabeth Mazzio | Nutraceutical composition and method of use for treatment / prevention of cancer |
-
2007
- 2007-08-16 CH CH01289/07A patent/CH698627B1/de not_active IP Right Cessation
-
2008
- 2008-08-15 JP JP2010520393A patent/JP5378379B2/ja not_active Expired - Fee Related
- 2008-08-15 CN CN200880111798XA patent/CN102065875B/zh not_active Expired - Fee Related
- 2008-08-15 PL PL08783450T patent/PL2182966T3/pl unknown
- 2008-08-15 AU AU2008286636A patent/AU2008286636B2/en not_active Ceased
- 2008-08-15 WO PCT/CH2008/000350 patent/WO2009021347A1/de not_active Ceased
- 2008-08-15 CA CA2696499A patent/CA2696499A1/en not_active Abandoned
- 2008-08-15 US US12/673,656 patent/US8404286B2/en not_active Expired - Fee Related
- 2008-08-15 ES ES08783450T patent/ES2392310T3/es active Active
- 2008-08-15 KR KR1020107005684A patent/KR20100089815A/ko not_active Ceased
- 2008-08-15 RU RU2010109750/15A patent/RU2470657C2/ru not_active IP Right Cessation
- 2008-08-15 BR BRPI0815188 patent/BRPI0815188A2/pt not_active IP Right Cessation
- 2008-08-15 EP EP08783450A patent/EP2182966B1/de active Active
-
2010
- 2010-02-10 ZA ZA201000959A patent/ZA201000959B/xx unknown
-
2013
- 2013-02-28 US US13/780,129 patent/US9248156B2/en not_active Expired - Fee Related
-
2016
- 2016-02-02 US US15/013,654 patent/US20160193272A1/en not_active Abandoned
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1094813B1 (en) * | 1998-07-13 | 2003-09-10 | Btg International Limited | Use of piperine and derivatives thereof for treating skin pigmentation disorders and skin cancer |
| US20050154215A1 (en) * | 2002-03-25 | 2005-07-14 | Fundacao De Amparo A Pesquisa Do Estado De Sao Pau | Process to obtain dibenzylbutyrolactonic lignans process to obtain synthetic derivatives from lignanes bearing anti-chagas chemoprophylatic and therapeutical activies |
Non-Patent Citations (1)
| Title |
|---|
| A. Chatterjee et al. Spectral properties of Cubebin // Jour. Indian Chem. Soc, Vol.45, no 8, 1968, p.423-725. * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2773173C2 (ru) * | 2017-03-20 | 2022-05-31 | Пьер Фабр Медикамент | Применение олеорезинов копаиферы при патологиях простаты |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20100089815A (ko) | 2010-08-12 |
| EP2182966B1 (de) | 2012-08-08 |
| AU2008286636A1 (en) | 2009-02-19 |
| WO2009021347A1 (de) | 2009-02-19 |
| AU2008286636B2 (en) | 2013-10-24 |
| US20110135760A1 (en) | 2011-06-09 |
| JP5378379B2 (ja) | 2013-12-25 |
| CH698627B1 (de) | 2009-09-15 |
| ZA201000959B (en) | 2010-10-27 |
| CN102065875B (zh) | 2013-07-17 |
| RU2010109750A (ru) | 2011-09-27 |
| US9248156B2 (en) | 2016-02-02 |
| BRPI0815188A2 (pt) | 2015-03-31 |
| US20160193272A1 (en) | 2016-07-07 |
| CN102065875A (zh) | 2011-05-18 |
| PL2182966T3 (pl) | 2013-01-31 |
| CA2696499A1 (en) | 2009-02-19 |
| US20130251826A1 (en) | 2013-09-26 |
| ES2392310T3 (es) | 2012-12-07 |
| US8404286B2 (en) | 2013-03-26 |
| JP2010536718A (ja) | 2010-12-02 |
| EP2182966A1 (de) | 2010-05-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2470657C2 (ru) | ПРИМЕНЕНИЕ ЭКСТРАКТОВ ИЛИ ЭКСТРАКТИВНЫХ ВЕЩЕСТВ ИЗ Piper cubeba L. В КАЧЕСТВЕ АКТИВНЫХ ИНГРЕДИЕНТОВ В ЛЕКАРСТВЕННОМ СРЕДСТВЕ ДЛЯ ЛЕЧЕНИЯ РАКОВЫХ ЗАБОЛЕВАНИЙ | |
| AU2004281707B2 (en) | Composition comprising xanthoceras sorbifolia extracts, compounds isolated from same, methods for preparing same and uses thereof | |
| US20100021574A1 (en) | Pharmaceutical Composition and Procedure to Treat and Prevent Prostatic Hyperplasia and Prostatitis from the Royal Palm (Roystonea regia) Fruits | |
| CN102281888B (zh) | Gip升高抑制剂 | |
| Kim et al. | Lipase inhibitory activity of ethyl acetate fraction from Ecklonia cava extracts | |
| US10377902B2 (en) | Crystallized red pigment, method of producing a recrystallized red pigment and a method for improving the appearance of or preventing wrinkles | |
| JP7305546B2 (ja) | 前立腺の病変におけるCopaifera属オレオレジンの使用 | |
| CN106999530A (zh) | 用于治疗乳腺癌的方法和组合物 | |
| CH698610B1 (de) | Verwendung von Extrakten oder Extraktivstoffen aus Piper cubeba L. als wirksame Bestandteile in einem Medikament zur Behandlung von Krebserkrankungen. | |
| HK1084059B (en) | Pharmaceutical composition and method for the treatment and prevention of prostatic hyperplasia and prostatitis using roystonea regia (royal palm) fruits |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20150816 |