JP6861465B2 - 核酸増幅 - Google Patents
核酸増幅 Download PDFInfo
- Publication number
- JP6861465B2 JP6861465B2 JP2015516255A JP2015516255A JP6861465B2 JP 6861465 B2 JP6861465 B2 JP 6861465B2 JP 2015516255 A JP2015516255 A JP 2015516255A JP 2015516255 A JP2015516255 A JP 2015516255A JP 6861465 B2 JP6861465 B2 JP 6861465B2
- Authority
- JP
- Japan
- Prior art keywords
- temperature
- amplification
- chelating agent
- nucleic acid
- sensitive
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P19/00—Preparation of compounds containing saccharide radicals
- C12P19/26—Preparation of nitrogen-containing carbohydrates
- C12P19/28—N-glycosides
- C12P19/30—Nucleotides
- C12P19/34—Polynucleotides, e.g. nucleic acids, oligoribonucleotides
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6844—Nucleic acid amplification reactions
- C12Q1/6846—Common amplification features
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Immunology (AREA)
- General Chemical & Material Sciences (AREA)
- Analytical Chemistry (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Enzymes And Modification Thereof (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
Description
この開示は、核酸増幅に関する。
多くの酵素(核酸と相互作用するほとんど全ての酵素及びほとんどのプロテアーゼを包含する)は、二価イオン補因子を必要とする。例えば、核酸増幅反応に関わる酵素は、補因子として二価のマグネシウムイオン(Mg++)をしばしば必要とする。
この開示は、酵素反応の制御方法の開発に、少なくとも一部、基づく。
(a)標的を含む試料並びに(b)結合剤、結合剤によって結合したイオン、バッファー、及びイオンが結合剤によって結合しているときにイオンの存在下で第1の活性を有し、イオンが結合剤から遊離しているときにイオンの存在下で第2の異なる活性を有する少なくとも1つの構成成分を含む増幅試薬を含む試薬を含む混合物を形成するステップと、
第1の温度から第2の温度まで混合物の温度を増加させることによって、増幅試薬の少なくとも1つの構成成分の活性を第1の活性から第2の活性に変化させるのに十分な量のイオンを結合剤から放出するステップと
を含む方法を提供する。
この開示は、反応混合物中の二価イオンを可逆的に結合することによって、二価イオン補因子を必要とする酵素の活性を制御することができるという発見に少なくとも一部基づく。例示的な方法では、反応混合物は、酵素及び試薬混合物を組み合わせることによって調製され、反応混合物は溶液中で可逆的に結合した二価イオンを含む。次に、反応混合物のpHを調整して可逆的に結合した二価イオンを放出し、それによって酵素を活性化させる。
本開示を考慮すると、当業者は、用いる具体的な核酸増幅方法の特性に基づいて、第1の温度、第2の温度、温度感受性バッファーの条件、及びpH感受性キレート化剤を選択することができる。高い反応温度が必要とされる場合は、用いる酵素は好熱種(例えば、テルムス・アカティクス(Thermus aquaticus))から取り出すことができる。
pH依存性キレート化剤EGTAの有り無しで、NEAR増幅をホットスタート条件下で実施した。0又は100コピーの精製されたインフルエンザA型ウイルスRNA、及び150nMのフォワード鋳型、250nMのリバース鋳型、及び200nMの分子ビーコンプローブを用いてアッセイを設定した。鋳型及び分子ビーコンプローブの配列は、以下の通りであった:フォワード鋳型、5’−AGACTCCACACGGAGTCTACTGACAGCCAGACA−3’(配列番号1);リバース鋳型、5’−AGACTCCATATGGAGTCTTGATGGCCATCCGAA’(配列番号2);及び分子ビーコンプローブ、5’−6−Fam−CTGGTAGCCAGGCA GCGACCAG−BHQ1−3’(配列番号3)。以下の条件下で反応を実行した:100mMトリス−Cl(20℃でpH7.9)、15mM Na2SO4、15mM(NH4)2SO4、15mM MgSO4、14mM EGTA、1mM DTT、0.1% Triton X−100、0.3mMの各dNTP、19.2UのBst DNAポリメラーゼ及び15UのNt.BstNBIニッキング酵素。アッセイの構成成分を室温で組み合わせ、約20分の間室温に維持し、その後反応を56℃に置いた。リアルタイム蛍光を用いて反応を10分の間モニターした。EGTA及び100コピーのウイルスRNAを含む反応だけで、増幅を観察した(図1)。
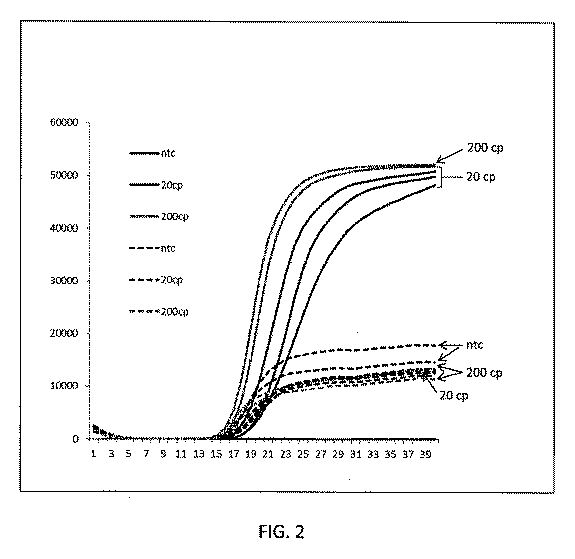
凍結乾燥された構成成分を用いて、ホットスタート条件下でNEAR反応を実施した。凍結乾燥された反応ペレットに、50mMトリス−HCl(20℃でpH7.75)、15mM (NH4)2SO4、15mM MgSO4及び15mM EGTAを含有する50μLの再構成バッファーを加えた。凍結乾燥されたペレットからの構成成分は、再構成後の50μLに、50nMフォワード鋳型、750nMリバース鋳型、300nM分子ビーコンプローブ、50mMトレハロース、225mMマンニトール、50mMトリス−HCl(20℃でpH8.5)、1mM DTT、5mM Na2SO4、0.1% Triton X−100、0.3mMの各dNTP、0.2×SYBR Green I、120UのManta DNAポリメラーゼ及び15UのNt.BstNBIニッキング酵素を含んでいた。鋳型及び分子ビーコンプローブの配列は、以下の通りであった:フォワード鋳型、5’−CGACTCCATATGGA GTCCTCGTCAGACCCAAAA−3’(配列番号4)、リバース鋳型、5’−TGACTCCATATGGAGTCTCATCTTTCCGTCCCC−3’(配列番号5)、及び分子ビーコン、5’−Rox−TCGGGGCAGACCCAAAACCCCGA−BHQ2−3’(配列番号6)。マイコバクテリウム・ボビス(Mycobacterium bovis)BCG(ATCC190115株)からの20又は200コピーのゲノムDNAを用いて増幅を実施した。混合物は、15分の間室温に保持した。室温でのインキュベーションの後、反応を56℃に移行し、リアルタイム蛍光を用いて反応を40分の間モニターした。EGTAが反応に存在したとき、鋳型なしの対照と比較して、20又は200コピーの鋳型DNAを用いて有意な増幅が観察された(図2)。
本発明のいくつかの実施形態が記載されている。それにもかかわらず、本発明の精神及び範囲から逸脱することなく、様々な改変を加えることができることが理解されよう。したがって、他の実施形態は以下の特許請求の範囲内である。
Claims (8)
- (a)ポリヌクレオチド及び増幅試薬混合物を第1の温度で組み合わせて、反応混合物を形成するステップであって、前記第1の温度が20℃〜30℃であり、(i)前記増幅試薬混合物がpH感受性キレート化剤、二価イオン、ニッキングエンドヌクレアーゼ、温度感受性バッファー、及び、DNA又はRNAポリメラーゼを含み、前記キレート化剤が、エチレングリコール−ビス(2−アミノエチルエーテル)四酢酸(EGTA)、EGTA誘導体、エチレンジアミン四酢酸(EDTA)及びEDTA誘導体からなる群から選択されており、前記温度感受性バッファーが、トリシン、グリシンアミド、ビシン、グリシルグリシン、トリス(ヒドロキシメチル)メチル−アミノエタンスルホン酸、N−2−アセトアミド−2−アミノエタンスルホン酸、トリス(ヒドロキシメチル)アミノメタン及びこれらの組み合わせからなる群から選択されており、キレート化剤濃度対二価イオン濃度の比が0.5〜2であり、(ii)前記二価イオンが、ポリヌクレオチドの増幅が進行しないように、第1の温度でキレート化剤と可逆的に結合している、ステップと、
(b)第1の温度から50℃〜60℃である第2の温度まで反応混合物の温度を増加するステップと、を含み、
前記二価イオンが溶液中に存在し、第2の温度で前記キレート化剤と結合していないことにより、前記ポリヌクレオチドの増幅が開始する、方法。 - 前記二価イオンが、マグネシウム、カルシウム、銅、亜鉛、マンガン、鉄、カドミウム及び鉛からなる群から選択される、請求項1に記載の方法。
- 前記温度感受性バッファーがトリス(ヒドロキシメチル)アミノメタンを含む、請求項1に記載の方法。
- 前記反応混合物のpHが、第1の温度から第2の温度に反応混合物の温度を変化させることによって調整される、請求項1に記載の方法。
- 前記温度感受性バッファーが−0.04℃−1〜−0.015℃−1のΔpKaを有する、請求項1に記載の方法。
- 前記増幅試薬混合物が、凍結乾燥形態の1つもしくは複数の構成成分を含み、及び/又は、前記増幅試薬混合物の1つもしくは複数の構成成分が、流体装置、カートリッジ又は側方流動装置での使用に適する容器で提供される、請求項1に記載の方法。
- 前記増幅試薬混合物が逆転写酵素を含む、請求項1に記載の方法。
- 前記キレート化剤が、エチレングリコール−ビス(2−アミノエチルエーテル)四酢酸(EGTA)及びEGTA誘導体からなる群から選択される、請求項1に記載の方法。
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261657227P | 2012-06-08 | 2012-06-08 | |
| US61/657,227 | 2012-06-08 | ||
| US201361782199P | 2013-03-14 | 2013-03-14 | |
| US61/782,199 | 2013-03-14 | ||
| PCT/US2013/044796 WO2013185081A1 (en) | 2012-06-08 | 2013-06-07 | Nucleic acid amplifications |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019017903A Division JP2019115344A (ja) | 2012-06-08 | 2019-02-04 | 核酸増幅 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2015518735A JP2015518735A (ja) | 2015-07-06 |
| JP2015518735A5 JP2015518735A5 (ja) | 2016-07-21 |
| JP6861465B2 true JP6861465B2 (ja) | 2021-04-21 |
Family
ID=49712703
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015516255A Active JP6861465B2 (ja) | 2012-06-08 | 2013-06-07 | 核酸増幅 |
| JP2019017903A Pending JP2019115344A (ja) | 2012-06-08 | 2019-02-04 | 核酸増幅 |
| JP2021012803A Pending JP2021072833A (ja) | 2012-06-08 | 2021-01-29 | 核酸増幅 |
| JP2024082357A Pending JP2024109739A (ja) | 2012-06-08 | 2024-05-21 | 核酸増幅 |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019017903A Pending JP2019115344A (ja) | 2012-06-08 | 2019-02-04 | 核酸増幅 |
| JP2021012803A Pending JP2021072833A (ja) | 2012-06-08 | 2021-01-29 | 核酸増幅 |
| JP2024082357A Pending JP2024109739A (ja) | 2012-06-08 | 2024-05-21 | 核酸増幅 |
Country Status (8)
| Country | Link |
|---|---|
| US (3) | US10927393B2 (ja) |
| EP (2) | EP3778915A1 (ja) |
| JP (4) | JP6861465B2 (ja) |
| CN (2) | CN114214394A (ja) |
| AU (4) | AU2013271404B2 (ja) |
| CA (2) | CA3096342C (ja) |
| ES (1) | ES2823551T3 (ja) |
| WO (1) | WO2013185081A1 (ja) |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7399590B2 (en) | 2002-02-21 | 2008-07-15 | Asm Scientific, Inc. | Recombinase polymerase amplification |
| AU2003215391B2 (en) | 2002-02-21 | 2007-05-24 | Abbott Diagnostics Scarborough, Inc. | Recombinase Polymerase Amplification |
| US8030000B2 (en) | 2002-02-21 | 2011-10-04 | Alere San Diego, Inc. | Recombinase polymerase amplification |
| CA2616241C (en) | 2005-07-25 | 2012-02-07 | Asm Scientific, Inc. | Methods for multiplexing recombinase polymerase amplification |
| CA2650993C (en) | 2006-05-04 | 2015-06-16 | Asm Scientific, Inc. | Recombinase polymerase amplification |
| WO2010135310A1 (en) | 2009-05-20 | 2010-11-25 | Biosite Incorporated | Dna glycosylase/lyase and ap endonuclease substrates |
| EP2438196B1 (en) | 2009-06-05 | 2016-12-21 | Alere San Diego, Inc. | Recombinase polymerase amplification reagents and kits |
| CN114214394A (zh) | 2012-06-08 | 2022-03-22 | 爱奥尼安技术公司 | 核苷酸扩增反应 |
| US9850525B2 (en) | 2014-01-29 | 2017-12-26 | Agilent Technologies, Inc. | CAS9-based isothermal method of detection of specific DNA sequence |
| EP3099810B1 (en) * | 2014-01-31 | 2019-05-01 | Qiagen GmbH | Cation chelator hot start |
| WO2016004333A2 (en) * | 2014-07-02 | 2016-01-07 | Promega Corporation | Reversible metal ion chelators |
| US11008604B2 (en) * | 2014-12-18 | 2021-05-18 | Global Life Sciences Solutions Operations UK Ltd | Analyte detection on a solid support by nucleic acid amplification coupled to an immunoassay |
| GB201515355D0 (en) * | 2015-08-28 | 2015-10-14 | Ge Healthcare Ltd | A method and kit for analyte detection |
| US10329601B2 (en) * | 2015-12-28 | 2019-06-25 | Ionian Technologies, Inc. | Nicking and extension amplification reaction (NEAR) of Streptococcus species |
| US11118206B2 (en) | 2016-01-13 | 2021-09-14 | The Trustees Of The University Of Pennsylvania | Multiple stage isothermal enzymatic amplification |
| WO2017141067A1 (en) | 2016-02-16 | 2017-08-24 | The University Of Tokyo | Method of eliminating background amplification of nucleic acid targets |
| US9617587B1 (en) | 2016-04-04 | 2017-04-11 | Nat Diagnostics, Inc. | Isothermal amplification components and processes |
| US11299777B2 (en) | 2016-04-04 | 2022-04-12 | Nat Diagnostics, Inc. | Isothermal amplification components and processes |
| CN106093415B (zh) * | 2016-04-29 | 2017-12-19 | 浙江大学 | 一种蛋白实时定量检测方法 |
| CN106053403B (zh) * | 2016-04-29 | 2019-05-03 | 浙江大学 | 一种蛋白定量检测方法 |
| GB201611469D0 (en) | 2016-06-30 | 2016-08-17 | Lumiradx Tech Ltd | Improvements in or relating to nucleic acid amplification processes |
| AU2018227436A1 (en) * | 2017-02-28 | 2019-09-12 | Abbott Diagnostics Scarborough, Inc. | Microfluidic devices and related methods |
| GB2569965A (en) | 2018-01-04 | 2019-07-10 | Lumiradx Uk Ltd | Improvements in or relating to amplification of nucleic acids |
| CN111979303A (zh) * | 2020-08-11 | 2020-11-24 | 上海奕谱生物科技有限公司 | 一种核酸检测试剂盒、方法及其应用 |
| WO2025171104A1 (en) * | 2024-02-08 | 2025-08-14 | Alere San Diego, Inc. | Universal hairpin probes |
Family Cites Families (53)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5693517A (en) * | 1987-06-17 | 1997-12-02 | Roche Molecular Systems, Inc. | Reagents and methods for coupled high temperature reverse transcription and polymerase chain reactions |
| WO1991009944A2 (en) * | 1989-12-22 | 1991-07-11 | F.Hoffmann-La Roche Ag | High temperature reverse transcriptases |
| US5455166A (en) | 1991-01-31 | 1995-10-03 | Becton, Dickinson And Company | Strand displacement amplification |
| US5556751A (en) | 1991-04-25 | 1996-09-17 | Amoco Corporation | Selective amplification system using Q-β replicase |
| US5834202A (en) | 1992-08-04 | 1998-11-10 | Replicon, Inc. | Methods for the isothermal amplification of nucleic acid molecules |
| WO1994003624A1 (en) | 1992-08-04 | 1994-02-17 | Auerbach Jeffrey I | Methods for the isothermal amplification of nucleic acid molecules |
| US5733733A (en) | 1992-08-04 | 1998-03-31 | Replicon, Inc. | Methods for the isothermal amplification of nucleic acid molecules |
| US5614389A (en) | 1992-08-04 | 1997-03-25 | Replicon, Inc. | Methods for the isothermal amplification of nucleic acid molecules |
| US5648211A (en) | 1994-04-18 | 1997-07-15 | Becton, Dickinson And Company | Strand displacement amplification using thermophilic enzymes |
| US5942391A (en) | 1994-06-22 | 1999-08-24 | Mount Sinai School Of Medicine | Nucleic acid amplification method: ramification-extension amplification method (RAM) |
| US5916779A (en) | 1995-09-21 | 1999-06-29 | Becton, Dickinson And Company | Strand displacement amplification of RNA targets |
| US5731150A (en) | 1995-11-01 | 1998-03-24 | Chiron Diagnostic Corporation | IS6110 based molecular detection of mycobacterium tuberculosis |
| US6197557B1 (en) * | 1997-03-05 | 2001-03-06 | The Regents Of The University Of Michigan | Compositions and methods for analysis of nucleic acids |
| WO1998045474A1 (en) | 1997-04-04 | 1998-10-15 | Innogenetics N.V. | Isothermal polymerase chain reaction by cycling the concentration of divalent metal ions |
| US6245506B1 (en) * | 1997-07-30 | 2001-06-12 | Bbi Bioseq, Inc. | Integrated sequencing device |
| CN1099464C (zh) * | 2000-11-28 | 2003-01-22 | 复旦大学 | 一种端粒酶活性检测方法 |
| US6448085B1 (en) * | 2001-04-13 | 2002-09-10 | Sysmex Corporation | Quality control material and calibrator for nucleated red blood cell tested on hematology analyzer |
| US20020172972A1 (en) * | 2001-05-15 | 2002-11-21 | President And Fellows Of Harvard College | Use of a selectively inactivatable enzyme to digest contaminating nucleic acid |
| US7112423B2 (en) | 2001-07-15 | 2006-09-26 | Keck Graduate Institute | Nucleic acid amplification using nicking agents |
| WO2004022701A2 (en) | 2001-07-15 | 2004-03-18 | Keck Graduate Institute | Exponential amplification of nucleic acids using nicking agents |
| WO2003012066A2 (en) | 2001-08-02 | 2003-02-13 | Barnes Wayne M | Magnesium precipitate hot start method for molecular manipulation of nucleic acids |
| US6403341B1 (en) | 2001-08-02 | 2002-06-11 | Wayne M. Barnes | Magnesium precipitate hot start method for PCR |
| AU2003215391B2 (en) | 2002-02-21 | 2007-05-24 | Abbott Diagnostics Scarborough, Inc. | Recombinase Polymerase Amplification |
| US7399590B2 (en) | 2002-02-21 | 2008-07-15 | Asm Scientific, Inc. | Recombinase polymerase amplification |
| US8030000B2 (en) * | 2002-02-21 | 2011-10-04 | Alere San Diego, Inc. | Recombinase polymerase amplification |
| US20040038213A1 (en) | 2002-08-06 | 2004-02-26 | Kwon Jai W. | Genotyping by in situ PCR amplification of a polynucleotide in a tissue biopsy |
| US7662594B2 (en) | 2002-09-20 | 2010-02-16 | New England Biolabs, Inc. | Helicase-dependent amplification of RNA |
| AU2003272438B2 (en) | 2002-09-20 | 2009-04-02 | New England Biolabs, Inc. | Helicase dependent amplification of nucleic acids |
| CA2533910A1 (en) * | 2003-07-29 | 2005-02-24 | Sigma-Aldrich Co. | Methods and compositions for amplification of dna |
| US7700281B2 (en) * | 2004-06-30 | 2010-04-20 | Usb Corporation | Hot start nucleic acid amplification |
| GB2416352B (en) * | 2004-07-21 | 2009-01-28 | Bioline Ltd | A method for performing the hot start of enzymatic reactions |
| CA2616241C (en) | 2005-07-25 | 2012-02-07 | Asm Scientific, Inc. | Methods for multiplexing recombinase polymerase amplification |
| US7939645B2 (en) * | 2006-01-06 | 2011-05-10 | Agilent Technologies, Inc | Reaction buffer composition for nucleic acid replication with packed DNA polymerases |
| CN103172700B (zh) * | 2006-02-27 | 2014-12-17 | 霍夫曼-拉罗奇有限公司 | 经由镁螯合的pcr热启动 |
| CA2650993C (en) | 2006-05-04 | 2015-06-16 | Asm Scientific, Inc. | Recombinase polymerase amplification |
| US9481912B2 (en) * | 2006-09-12 | 2016-11-01 | Longhorn Vaccines And Diagnostics, Llc | Compositions and methods for detecting and identifying nucleic acid sequences in biological samples |
| US8357488B2 (en) | 2007-05-18 | 2013-01-22 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services, Centers For Disease Control And Prevention | Primers and probes for the detection of streptococcus pneumoniae |
| US9689031B2 (en) | 2007-07-14 | 2017-06-27 | Ionian Technologies, Inc. | Nicking and extension amplification reaction for the exponential amplification of nucleic acids |
| KR101414713B1 (ko) * | 2007-10-11 | 2014-07-03 | 삼성전자주식회사 | 리가제 및 엔도뉴클레아제의 존재하에서 롤링서클 증폭에의하여 표적 핵산을 증폭하는 방법 |
| JP2009139487A (ja) | 2007-12-04 | 2009-06-25 | Nippon Sheet Glass Co Ltd | 正立等倍レンズアレイプレート |
| US8911948B2 (en) * | 2008-04-30 | 2014-12-16 | Integrated Dna Technologies, Inc. | RNase H-based assays utilizing modified RNA monomers |
| JP5572943B2 (ja) * | 2008-12-17 | 2014-08-20 | 株式会社島津製作所 | 血液検体からのdna増幅方法及びdna増幅キット |
| CN102369297B (zh) * | 2009-03-31 | 2016-07-06 | 凸版印刷株式会社 | 识别基因型的方法 |
| EP2438196B1 (en) | 2009-06-05 | 2016-12-21 | Alere San Diego, Inc. | Recombinase polymerase amplification reagents and kits |
| EP2464738A4 (en) * | 2009-08-12 | 2013-05-01 | Nugen Technologies Inc | METHODS, COMPOSITIONS, AND KITS FOR GENERATING NUCLEIC ACID PRODUCTS SUBSTANTIALLY FREE OF MATRIX NUCLEIC ACID |
| AU2011204313A1 (en) * | 2010-01-08 | 2012-07-26 | Qiagen Gaithersburg, Inc. | Materials and methods for isothermal nucleic acid amplification |
| EP2714931A1 (en) * | 2011-06-03 | 2014-04-09 | Genomic Vision | Assessment of cancer risk based on rnu2 cnv and interplay between rnu2 and brca1 |
| EP2769007B1 (en) * | 2011-10-19 | 2016-12-07 | Nugen Technologies, Inc. | Compositions and methods for directional nucleic acid amplification and sequencing |
| WO2013101783A2 (en) * | 2011-12-30 | 2013-07-04 | Bio-Rad Laboratories, Inc. | Methods and compositions for performing nucleic acid amplification reactions |
| KR101870311B1 (ko) | 2012-03-09 | 2018-06-25 | (주)바이오니아 | 핫스타트 역전사반응 또는 핫스타트 역전사 중합효소 연쇄반응용 조성물 |
| CN114214394A (zh) | 2012-06-08 | 2022-03-22 | 爱奥尼安技术公司 | 核苷酸扩增反应 |
| EP3099810B1 (en) | 2014-01-31 | 2019-05-01 | Qiagen GmbH | Cation chelator hot start |
| WO2016004333A2 (en) | 2014-07-02 | 2016-01-07 | Promega Corporation | Reversible metal ion chelators |
-
2013
- 2013-06-07 CN CN202111168266.3A patent/CN114214394A/zh active Pending
- 2013-06-07 CA CA3096342A patent/CA3096342C/en active Active
- 2013-06-07 AU AU2013271404A patent/AU2013271404B2/en active Active
- 2013-06-07 WO PCT/US2013/044796 patent/WO2013185081A1/en not_active Ceased
- 2013-06-07 JP JP2015516255A patent/JP6861465B2/ja active Active
- 2013-06-07 CA CA2876159A patent/CA2876159C/en active Active
- 2013-06-07 CN CN201380029231.9A patent/CN104662159B/zh active Active
- 2013-06-07 EP EP20184582.3A patent/EP3778915A1/en active Pending
- 2013-06-07 US US13/913,153 patent/US10927393B2/en active Active
- 2013-06-07 ES ES13799829T patent/ES2823551T3/es active Active
- 2013-06-07 EP EP13799829.0A patent/EP2859111B1/en active Active
-
2017
- 2017-09-15 AU AU2017228698A patent/AU2017228698B2/en active Active
-
2019
- 2019-02-04 JP JP2019017903A patent/JP2019115344A/ja active Pending
- 2019-09-27 AU AU2019236751A patent/AU2019236751A1/en not_active Abandoned
-
2020
- 2020-12-03 US US17/110,714 patent/US20210155965A1/en not_active Abandoned
-
2021
- 2021-01-29 JP JP2021012803A patent/JP2021072833A/ja active Pending
-
2022
- 2022-02-07 AU AU2022200787A patent/AU2022200787B2/en active Active
-
2024
- 2024-05-21 JP JP2024082357A patent/JP2024109739A/ja active Pending
-
2025
- 2025-01-07 US US19/012,325 patent/US20250277247A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| AU2022200787B2 (en) | 2024-03-28 |
| AU2013271404B2 (en) | 2017-10-12 |
| JP2024109739A (ja) | 2024-08-14 |
| EP2859111A4 (en) | 2016-04-27 |
| CA3096342C (en) | 2023-04-04 |
| AU2019236751A1 (en) | 2019-10-17 |
| JP2019115344A (ja) | 2019-07-18 |
| CN104662159A (zh) | 2015-05-27 |
| AU2017228698B2 (en) | 2019-06-27 |
| US20250277247A1 (en) | 2025-09-04 |
| WO2013185081A1 (en) | 2013-12-12 |
| JP2015518735A (ja) | 2015-07-06 |
| AU2017228698A1 (en) | 2017-10-05 |
| AU2013271404A1 (en) | 2014-12-18 |
| ES2823551T3 (es) | 2021-05-07 |
| US20210155965A1 (en) | 2021-05-27 |
| EP3778915A1 (en) | 2021-02-17 |
| CN114214394A (zh) | 2022-03-22 |
| AU2022200787A1 (en) | 2022-02-24 |
| JP2021072833A (ja) | 2021-05-13 |
| CA3096342A1 (en) | 2013-12-12 |
| US10927393B2 (en) | 2021-02-23 |
| CN104662159B (zh) | 2021-10-26 |
| CA2876159C (en) | 2022-09-06 |
| EP2859111B1 (en) | 2020-07-08 |
| US20130330777A1 (en) | 2013-12-12 |
| EP2859111A1 (en) | 2015-04-15 |
| CA2876159A1 (en) | 2013-12-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6861465B2 (ja) | 核酸増幅 | |
| EP3252172B1 (en) | Fast hybridization for next generation sequencing target enrichment | |
| AU2010298202B2 (en) | Detection of nucleic acids in crude matrices | |
| US10633697B2 (en) | Caution chelator hot start | |
| WO2005111209A1 (ja) | 高速dnaポリメラーゼを用いた高速pcr | |
| JP5798631B2 (ja) | Rt−pcr反応緩衝液中での細胞溶解のための方法 | |
| HK40070420A (en) | Nucleic acid amplifications | |
| US20250346887A1 (en) | Rapid pathogen identification and detection molecular diagnostics technology |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160530 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160530 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20170425 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20170710 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170925 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20180220 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180521 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20181002 |
|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7422 Effective date: 20190122 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190204 |
|
| C60 | Trial request (containing other claim documents, opposition documents) |
Free format text: JAPANESE INTERMEDIATE CODE: C60 Effective date: 20190204 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20190122 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20190123 |
|
| C11 | Written invitation by the commissioner to file amendments |
Free format text: JAPANESE INTERMEDIATE CODE: C11 Effective date: 20190402 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20190409 |
|
| C21 | Notice of transfer of a case for reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C21 Effective date: 20190416 |
|
| A912 | Re-examination (zenchi) completed and case transferred to appeal board |
Free format text: JAPANESE INTERMEDIATE CODE: A912 Effective date: 20190607 |
|
| C211 | Notice of termination of reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C211 Effective date: 20190611 |
|
| C22 | Notice of designation (change) of administrative judge |
Free format text: JAPANESE INTERMEDIATE CODE: C22 Effective date: 20191015 |
|
| C22 | Notice of designation (change) of administrative judge |
Free format text: JAPANESE INTERMEDIATE CODE: C22 Effective date: 20200414 |
|
| C13 | Notice of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: C13 Effective date: 20201006 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201223 |
|
| C23 | Notice of termination of proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C23 Effective date: 20210126 |
|
| C03 | Trial/appeal decision taken |
Free format text: JAPANESE INTERMEDIATE CODE: C03 Effective date: 20210302 |
|
| C30A | Notification sent |
Free format text: JAPANESE INTERMEDIATE CODE: C3012 Effective date: 20210302 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210330 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6861465 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| S533 | Written request for registration of change of name |
Free format text: JAPANESE INTERMEDIATE CODE: R313533 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |