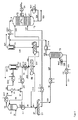
JP5951618B2 - 気相水素化からアニリンを精製するための方法 - Google Patents
気相水素化からアニリンを精製するための方法 Download PDFInfo
- Publication number
- JP5951618B2 JP5951618B2 JP2013534280A JP2013534280A JP5951618B2 JP 5951618 B2 JP5951618 B2 JP 5951618B2 JP 2013534280 A JP2013534280 A JP 2013534280A JP 2013534280 A JP2013534280 A JP 2013534280A JP 5951618 B2 JP5951618 B2 JP 5951618B2
- Authority
- JP
- Japan
- Prior art keywords
- stream
- aniline
- phase
- water
- aqueous
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 title claims description 182
- 238000000034 method Methods 0.000 title claims description 119
- 238000005984 hydrogenation reaction Methods 0.000 title claims description 10
- 239000000047 product Substances 0.000 claims description 98
- 238000004821 distillation Methods 0.000 claims description 65
- 239000012071 phase Substances 0.000 claims description 36
- 239000012074 organic phase Substances 0.000 claims description 33
- 239000008346 aqueous phase Substances 0.000 claims description 31
- 238000009833 condensation Methods 0.000 claims description 26
- 230000005494 condensation Effects 0.000 claims description 26
- 238000005191 phase separation Methods 0.000 claims description 26
- LQNUZADURLCDLV-UHFFFAOYSA-N nitrobenzene Chemical compound [O-][N+](=O)C1=CC=CC=C1 LQNUZADURLCDLV-UHFFFAOYSA-N 0.000 claims description 24
- 238000002156 mixing Methods 0.000 claims description 19
- 230000015572 biosynthetic process Effects 0.000 claims description 15
- 239000012535 impurity Substances 0.000 claims description 9
- 239000007795 chemical reaction product Substances 0.000 claims description 8
- 238000000746 purification Methods 0.000 claims description 7
- 230000002378 acidificating effect Effects 0.000 claims description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 66
- 239000002585 base Substances 0.000 description 56
- 239000007789 gas Substances 0.000 description 41
- 239000000243 solution Substances 0.000 description 28
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 21
- 239000007788 liquid Substances 0.000 description 19
- 150000001875 compounds Chemical class 0.000 description 14
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 10
- 238000004519 manufacturing process Methods 0.000 description 8
- 239000000203 mixture Substances 0.000 description 8
- 238000005406 washing Methods 0.000 description 8
- 150000003839 salts Chemical class 0.000 description 7
- 238000004088 simulation Methods 0.000 description 7
- 150000008044 alkali metal hydroxides Chemical class 0.000 description 5
- 238000009835 boiling Methods 0.000 description 5
- 238000000926 separation method Methods 0.000 description 5
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 4
- 241000183024 Populus tremula Species 0.000 description 4
- CYGKLLHTPPFPHH-UHFFFAOYSA-N aniline;hydrate Chemical compound O.NC1=CC=CC=C1 CYGKLLHTPPFPHH-UHFFFAOYSA-N 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 238000002485 combustion reaction Methods 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- DMBHHRLKUKUOEG-UHFFFAOYSA-N diphenylamine Chemical compound C=1C=CC=CC=1NC1=CC=CC=C1 DMBHHRLKUKUOEG-UHFFFAOYSA-N 0.000 description 4
- 238000005265 energy consumption Methods 0.000 description 4
- 230000008929 regeneration Effects 0.000 description 4
- 238000011069 regeneration method Methods 0.000 description 4
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- 150000004982 aromatic amines Chemical class 0.000 description 3
- 239000006227 byproduct Substances 0.000 description 3
- 239000001257 hydrogen Substances 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 239000007791 liquid phase Substances 0.000 description 3
- 150000004707 phenolate Chemical class 0.000 description 3
- 239000002351 wastewater Substances 0.000 description 3
- 238000004065 wastewater treatment Methods 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- 229910001860 alkaline earth metal hydroxide Inorganic materials 0.000 description 2
- 229910021529 ammonia Inorganic materials 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- 239000012043 crude product Substances 0.000 description 2
- 238000006481 deamination reaction Methods 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 238000000605 extraction Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 239000011261 inert gas Substances 0.000 description 2
- 239000000543 intermediate Substances 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-M phenolate Chemical compound [O-]C1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-M 0.000 description 2
- 229940031826 phenolate Drugs 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 230000008646 thermal stress Effects 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- CDAWCLOXVUBKRW-UHFFFAOYSA-N 2-aminophenol Chemical class NC1=CC=CC=C1O CDAWCLOXVUBKRW-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 230000004931 aggregating effect Effects 0.000 description 1
- 229910001854 alkali hydroxide Inorganic materials 0.000 description 1
- 229910000272 alkali metal oxide Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 239000012223 aqueous fraction Substances 0.000 description 1
- -1 aromatic nitro compound Chemical class 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 229910002090 carbon oxide Inorganic materials 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical class OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 238000009903 catalytic hydrogenation reaction Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- CZZYITDELCSZES-UHFFFAOYSA-N diphenylmethane Chemical class C=1C=CC=CC=1CC1=CC=CC=C1 CZZYITDELCSZES-UHFFFAOYSA-N 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- 229920001228 polyisocyanate Polymers 0.000 description 1
- 239000005056 polyisocyanate Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 239000012808 vapor phase Substances 0.000 description 1
- 239000003039 volatile agent Substances 0.000 description 1
- 239000002912 waste gas Substances 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C209/00—Preparation of compounds containing amino groups bound to a carbon skeleton
- C07C209/82—Purification; Separation; Stabilisation; Use of additives
- C07C209/86—Separation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C209/00—Preparation of compounds containing amino groups bound to a carbon skeleton
- C07C209/82—Purification; Separation; Stabilisation; Use of additives
- C07C209/84—Purification
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C211/00—Compounds containing amino groups bound to a carbon skeleton
- C07C211/43—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton
- C07C211/44—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton having amino groups bound to only one six-membered aromatic ring
- C07C211/45—Monoamines
- C07C211/46—Aniline
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102010042731A DE102010042731A1 (de) | 2010-10-21 | 2010-10-21 | Verfahren zur Reinigung von Anilin aus Gasphasenhydrierungen |
| DE102010042731.4 | 2010-10-21 | ||
| PCT/EP2011/068122 WO2012052407A1 (de) | 2010-10-21 | 2011-10-17 | Verfahren zur reinigung von anilin aus gasphasenhydrierungen |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2013543517A JP2013543517A (ja) | 2013-12-05 |
| JP2013543517A5 JP2013543517A5 (enExample) | 2014-12-04 |
| JP5951618B2 true JP5951618B2 (ja) | 2016-07-13 |
Family
ID=44860336
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013534280A Active JP5951618B2 (ja) | 2010-10-21 | 2011-10-17 | 気相水素化からアニリンを精製するための方法 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US9233910B2 (enExample) |
| EP (1) | EP2630117B1 (enExample) |
| JP (1) | JP5951618B2 (enExample) |
| KR (1) | KR101869996B1 (enExample) |
| CN (1) | CN103201253B (enExample) |
| DE (1) | DE102010042731A1 (enExample) |
| PL (1) | PL2630117T3 (enExample) |
| PT (1) | PT2630117E (enExample) |
| WO (1) | WO2012052407A1 (enExample) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2641892A1 (de) * | 2012-03-23 | 2013-09-25 | Bayer MaterialScience AG | Verfahren zur Reinigung von Anilin aus Gasphasenhydrierungen |
| WO2014057053A1 (de) * | 2012-10-11 | 2014-04-17 | Basf Se | Verfahren zur gewinnung von rein-anilin |
| US9115049B2 (en) * | 2012-10-11 | 2015-08-25 | Basf Se | Process for obtaining pure aniline |
| CN110627651B (zh) * | 2018-06-25 | 2022-06-14 | 中国石油化工股份有限公司 | 一种降低苯胺中苯酚含量的方法 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2174008A (en) * | 1938-01-29 | 1939-09-26 | Nat Aniline & Chem Co Inc | Distillation of amines from reduction masses |
| JPS4935341A (enExample) | 1972-07-31 | 1974-04-01 | ||
| DE4428017A1 (de) | 1994-08-08 | 1996-02-15 | Bayer Ag | Verfahren zur Herstellung von aromatischen Aminen |
| DE4428018A1 (de) * | 1994-08-08 | 1996-02-15 | Bayer Ag | Verfahren zur Herstellung von aromatischen Aminen |
| JP3804082B2 (ja) | 1995-04-27 | 2006-08-02 | 住友化学株式会社 | アニリンの精製方法 |
| JPH0935341A (ja) | 1995-07-18 | 1997-02-07 | Masayuki Matsuda | X線による情報の記録・読み出し方法 |
| JP2001226320A (ja) * | 2000-02-14 | 2001-08-21 | Nippon Shokubai Co Ltd | アクリル酸の捕集方法およびアクリル酸の精製方法 |
| US7049471B2 (en) | 2003-10-10 | 2006-05-23 | E. I. Du Pont De Nemours And Company | Separation of amine from a phenolic compound |
| DE102004026626A1 (de) | 2004-06-01 | 2005-12-29 | Bayer Materialscience Ag | Verfahren zur destillativen Trennung von wässrigen Aminlösungen |
| JP2005350388A (ja) | 2004-06-10 | 2005-12-22 | Sumitomo Chemical Co Ltd | アニリンの製造方法 |
| JP4552941B2 (ja) | 2006-01-17 | 2010-09-29 | 住友化学株式会社 | アニリンの精製方法および製造方法 |
| DE102006007619A1 (de) | 2006-02-18 | 2007-08-23 | Bayer Materialscience Ag | Verfahren zur Herstellung von Anilin |
| DE102006007620A1 (de) | 2006-02-18 | 2007-08-23 | Bayer Materialscience Ag | Verfahren zur Herstellung von Anilin |
| DE102006035203A1 (de) | 2006-07-29 | 2008-01-31 | Bayer Materialscience Ag | Verfahren zur Herstellung von aromatischen Aminen |
| JP2008184409A (ja) * | 2007-01-29 | 2008-08-14 | Mitsubishi Rayon Co Ltd | アルキルアミン含有混合物の蒸留法及びアルキルアミン類の製造方法 |
| DE102007039091A1 (de) | 2007-08-18 | 2009-02-19 | Bayer Materialscience Ag | Verfahren zur Reinigung von aromatischen Aminen |
| WO2012052411A1 (de) * | 2010-10-21 | 2012-04-26 | Bayer Technology Services Gmbh | Verfahren zur vereinfachten abtrennung eines reaktionsprodukts aus reaktionsgasgemischen mittels mindestens zweimaliger teilweiser kondensation |
-
2010
- 2010-10-21 DE DE102010042731A patent/DE102010042731A1/de not_active Withdrawn
-
2011
- 2011-10-17 EP EP11773236.2A patent/EP2630117B1/de active Active
- 2011-10-17 CN CN201180050920.9A patent/CN103201253B/zh active Active
- 2011-10-17 US US13/878,190 patent/US9233910B2/en active Active
- 2011-10-17 PT PT117732362T patent/PT2630117E/pt unknown
- 2011-10-17 PL PL11773236T patent/PL2630117T3/pl unknown
- 2011-10-17 KR KR1020137012854A patent/KR101869996B1/ko not_active Expired - Fee Related
- 2011-10-17 JP JP2013534280A patent/JP5951618B2/ja active Active
- 2011-10-17 WO PCT/EP2011/068122 patent/WO2012052407A1/de not_active Ceased
Also Published As
| Publication number | Publication date |
|---|---|
| PL2630117T3 (pl) | 2015-03-31 |
| CN103201253B (zh) | 2014-12-03 |
| PT2630117E (pt) | 2015-02-17 |
| EP2630117B1 (de) | 2014-10-15 |
| US20140046096A1 (en) | 2014-02-13 |
| US9233910B2 (en) | 2016-01-12 |
| WO2012052407A1 (de) | 2012-04-26 |
| EP2630117A1 (de) | 2013-08-28 |
| DE102010042731A1 (de) | 2012-04-26 |
| CN103201253A (zh) | 2013-07-10 |
| KR101869996B1 (ko) | 2018-06-22 |
| KR20130127983A (ko) | 2013-11-25 |
| JP2013543517A (ja) | 2013-12-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5204413B2 (ja) | アニリンの製造方法 | |
| JP2018016628A (ja) | 低沸点成分の分離、ならびに部分凝縮によるイソホロンジアミンにおけるアンモニア含分の低下 | |
| KR20070082895A (ko) | 아닐린의 제조 방법 | |
| JP2013535478A (ja) | ニトロベンゼンの連続製造方法 | |
| JP4773138B2 (ja) | アミン水溶液の蒸留分離法 | |
| JP5951618B2 (ja) | 気相水素化からアニリンを精製するための方法 | |
| JP2015522636A (ja) | 断熱的ニトロ化によりニトロベンゼンを製造する方法 | |
| JP6165844B2 (ja) | 芳香族アミンを含む物質混合物、特に粗製アニリンの物質混合物の処理方法 | |
| JP6062527B2 (ja) | 気相水素化からのアニリンの精製方法 | |
| JP2016522803A (ja) | ニトロベンゼン製造からの廃水の後処理方法 | |
| JP7663521B2 (ja) | アニリンの精製方法 | |
| JP5530516B2 (ja) | 化学装置 | |
| JP2008184409A (ja) | アルキルアミン含有混合物の蒸留法及びアルキルアミン類の製造方法 | |
| RU2612799C1 (ru) | Способ выделения осушенного анилина из продукта газофазного каталитического гидрирования нитробензола | |
| JP2007063159A (ja) | メチルアミン類の製造方法 | |
| KR101200218B1 (ko) | 아민 수용액 증류적 분리 방법 | |
| RU2167145C1 (ru) | Способ очистки технического нитробензола от серосодержащих, азот- и кислородсодержащих побочных продуктов | |
| JP2003221366A (ja) | メチルアミン類の製造方法 | |
| TW202237560A (zh) | 乙腈的分離方法 | |
| HK1106496A (en) | Process for preparing aniline |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20141016 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20141016 |
|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20150217 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20150513 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20150601 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150619 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150918 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20160115 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160408 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20160513 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20160608 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5951618 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |