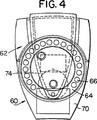
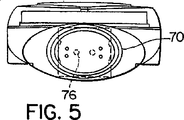
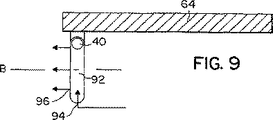
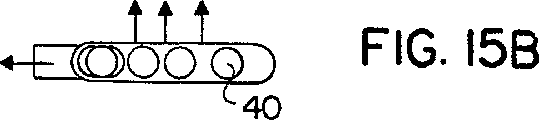JP4886143B2 - 乾燥粉末吸入器 - Google Patents
乾燥粉末吸入器 Download PDFInfo
- Publication number
- JP4886143B2 JP4886143B2 JP2001556536A JP2001556536A JP4886143B2 JP 4886143 B2 JP4886143 B2 JP 4886143B2 JP 2001556536 A JP2001556536 A JP 2001556536A JP 2001556536 A JP2001556536 A JP 2001556536A JP 4886143 B2 JP4886143 B2 JP 4886143B2
- Authority
- JP
- Japan
- Prior art keywords
- bead
- chamber
- inhaler
- dispersion chamber
- inhaler according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0086—Inhalation chambers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0005—Details of inhalators; Constructional features thereof with means for agitating the medicament
- A61M15/0006—Details of inhalators; Constructional features thereof with means for agitating the medicament using rotating means
- A61M15/0008—Details of inhalators; Constructional features thereof with means for agitating the medicament using rotating means rotating by airflow
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0031—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up by bursting or breaking the package, i.e. without cutting or piercing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0043—Non-destructive separation of the package, e.g. peeling
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0046—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier
- A61M15/0048—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier the dosages being arranged in a plane, e.g. on diskettes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/02—Inhalators with activated or ionised fluids, e.g. electrohydrodynamic [EHD] or electrostatic devices; Ozone-inhalators with radioactive tagged particles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/43—General characteristics of the apparatus making noise when used correctly
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2206/00—Characteristics of a physical parameter; associated device therefor
- A61M2206/10—Flow characteristics
- A61M2206/16—Rotating swirling helical flow, e.g. by tangential inflows
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2206/00—Characteristics of a physical parameter; associated device therefor
- A61M2206/10—Flow characteristics
- A61M2206/18—Coaxial flows, e.g. one flow within another
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pulmonology (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/495,494 US6427688B1 (en) | 2000-02-01 | 2000-02-01 | Dry powder inhaler |
| US09/495,494 | 2000-02-01 | ||
| PCT/US2001/003248 WO2001056640A1 (en) | 2000-02-01 | 2001-01-31 | Dry powder inhaler |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2004502472A JP2004502472A (ja) | 2004-01-29 |
| JP2004502472A5 JP2004502472A5 (cg-RX-API-DMAC7.html) | 2005-01-20 |
| JP4886143B2 true JP4886143B2 (ja) | 2012-02-29 |
Family
ID=23968860
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2001556536A Expired - Fee Related JP4886143B2 (ja) | 2000-02-01 | 2001-01-31 | 乾燥粉末吸入器 |
Country Status (7)
| Country | Link |
|---|---|
| US (5) | US6427688B1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP1307256B1 (cg-RX-API-DMAC7.html) |
| JP (1) | JP4886143B2 (cg-RX-API-DMAC7.html) |
| AU (1) | AU2001233201A1 (cg-RX-API-DMAC7.html) |
| CA (1) | CA2398815C (cg-RX-API-DMAC7.html) |
| ES (1) | ES2432353T3 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2001056640A1 (cg-RX-API-DMAC7.html) |
Families Citing this family (127)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9006175B2 (en) | 1999-06-29 | 2015-04-14 | Mannkind Corporation | Potentiation of glucose elimination |
| US7464706B2 (en) | 1999-07-23 | 2008-12-16 | Mannkind Corporation | Unit dose cartridge and dry powder inhaler |
| US7305986B1 (en) | 1999-07-23 | 2007-12-11 | Mannkind Corporation | Unit dose capsules for use in a dry powder inhaler |
| US6511676B1 (en) * | 1999-11-05 | 2003-01-28 | Teni Boulikas | Therapy for human cancers using cisplatin and other drugs or genes encapsulated into liposomes |
| US6651655B1 (en) | 2000-01-18 | 2003-11-25 | Quadrant Technologies Limited | Inhaled vaccines |
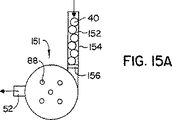
| US6427688B1 (en) * | 2000-02-01 | 2002-08-06 | Dura Pharmaceuticals, Icn. | Dry powder inhaler |
| US7171965B2 (en) * | 2000-02-01 | 2007-02-06 | Valois S.A.S. | Breath actuated dry powder inhaler and tape dose strip |
| US7069929B2 (en) * | 2000-02-01 | 2006-07-04 | Quadrant Technologies Limited | Dry powder inhaler |
| US6595210B2 (en) * | 2000-11-27 | 2003-07-22 | Unisia Jecs Corporation | Inhalator for administering powder composition |
| FI20010538A0 (fi) * | 2001-03-16 | 2001-03-16 | Orion Corp | Jauheinhalaattori |
| GB2375308A (en) * | 2001-05-10 | 2002-11-13 | Cambridge Consultants | Inhalers |
| EP1392383A2 (en) * | 2001-05-10 | 2004-03-03 | Vectura Delivery Devices Limited | Inhalers |
| ATE385193T1 (de) | 2002-03-20 | 2008-02-15 | Mannkind Corp | Inhalationsgerät |
| JP2004195191A (ja) * | 2002-10-22 | 2004-07-15 | Akihiko Miyamoto | 呼子付喘息治療薬吸入器 |
| AU2002952683A0 (en) * | 2002-11-15 | 2002-11-28 | Commonwealth Scientific And Industrial Research Organisation | Apparatus for delivering dry aerosols to the respiratory tract |
| BR0316924A (pt) * | 2002-12-02 | 2005-10-18 | Univ Alberta | Dispositivo e método para desaglomeração de pó para inalação |
| BR0316603B1 (pt) * | 2002-12-13 | 2014-03-11 | Otsuka Pharma Co Ltd | Dispositivo de inalação para administração transpulmonar |
| US6941947B2 (en) * | 2002-12-18 | 2005-09-13 | Quadrant Technologies Limited | Unit dose dry powder inhaler |
| US20040206350A1 (en) * | 2002-12-19 | 2004-10-21 | Nektar Therapeutics | Aerosolization apparatus with non-circular aerosolization chamber |
| US8869794B1 (en) | 2003-04-09 | 2014-10-28 | Novartis Pharma Ag | Aerosolization apparatus with capsule puncturing member |
| KR101066788B1 (ko) | 2003-04-09 | 2011-09-21 | 노바르티스 아게 | 공기 입구 차폐 부재를 갖춘 에어로졸화 장치 |
| GB0313604D0 (en) * | 2003-06-12 | 2003-07-16 | Britannia Pharmaceuticals Ltd | Delivery device for powdered medicament |
| EP1635762B1 (en) * | 2003-06-13 | 2021-03-03 | Civitas Therapeutics, Inc. | Low dose pharmaceutical powders for inhalation |
| US20050263153A1 (en) * | 2004-05-28 | 2005-12-01 | Quadrant Technologies Limited | Unit dose dry powder inhaler |
| US7556035B2 (en) * | 2004-05-28 | 2009-07-07 | Quadrant Technologies Limited | Unit dose dry powder inhaler |
| DE602005024413D1 (de) | 2004-08-20 | 2010-12-09 | Mannkind Corp | Katalyse der diketopiperazinsynthese |
| CN104436170B (zh) | 2004-08-23 | 2018-02-23 | 曼金德公司 | 用于药物输送的二酮哌嗪盐 |
| EP1802669B1 (en) * | 2004-10-21 | 2011-03-09 | Basell Polyolefine GmbH | 1-butene polymer and process for the preparation thereof |
| BRPI0516701B1 (pt) * | 2004-11-10 | 2017-11-28 | Cipla Ltd. | Inhaler device for inhalation of a medication of a perforable capsule |
| US20070020330A1 (en) | 2004-11-24 | 2007-01-25 | Medpointe Healthcare Inc. | Compositions comprising azelastine and methods of use thereof |
| US8758816B2 (en) * | 2004-11-24 | 2014-06-24 | Meda Pharmaceuticals Inc. | Compositions comprising azelastine and methods of use thereof |
| NZ555501A (en) | 2004-11-24 | 2010-01-29 | Medpointe Healthcare Inc | Compositions comprising azelastine and methods of use thereof |
| FR2881117B1 (fr) * | 2005-01-25 | 2010-07-30 | Valois Sas | Dispositif de distribution de produit fluide. |
| FR2881118B1 (fr) * | 2005-01-25 | 2007-04-20 | Valois Sas | Dispositif de distribution de produit fluide. |
| US7407156B2 (en) * | 2005-03-22 | 2008-08-05 | Toshiba Tec Kabushiki Kaisha | Sheet finishing apparatus |
| US8763605B2 (en) | 2005-07-20 | 2014-07-01 | Manta Devices, Llc | Inhalation device |
| JP5167133B2 (ja) | 2005-09-14 | 2013-03-21 | マンカインド コーポレイション | 結晶性微粒子表面に対する活性薬剤の親和性の増大に基づく薬物処方の方法 |
| GB0520794D0 (en) * | 2005-10-12 | 2005-11-23 | Innovata Biomed Ltd | Inhaler |
| DE102005057685A1 (de) * | 2005-12-01 | 2007-06-06 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Inhalator und Speicher für eine trockene Arzneimittelformulierung sowie diesbezügliche Verfahren und Verwendung |
| US7832397B2 (en) * | 2005-12-28 | 2010-11-16 | Philip Morris Usa Inc. | Aerosol powder delivery device |
| GB2433207B (en) * | 2006-02-21 | 2009-01-07 | Jianhe Li | Active suction actuated inhalers with timing devices |
| MX2008010721A (es) | 2006-02-22 | 2008-09-01 | Mannkind Corp | Un metodo para mejorar las propiedades farmaceuticas de microparticulas que contienen dicetopiperazina y un agente activo. |
| JP5188991B2 (ja) * | 2006-03-03 | 2013-04-24 | エスティーシー.ユーエヌエム | 空力弾性分配メカニズムを有するドライパウダー吸入器 |
| GB0611659D0 (en) * | 2006-06-13 | 2006-07-19 | Cambridge Consultants | Dry powder inhalers |
| AP2007000277S (en) * | 2007-03-28 | 2007-10-04 | Xerxes Rao | Cartridge for multidose inhaler |
| CA2683724A1 (en) * | 2007-04-30 | 2009-01-15 | Sun Pharma Advanced Research Company Limited | Inhalation device |
| EP1992376A1 (en) * | 2007-05-16 | 2008-11-19 | Boehringer Ingelheim Pharma GmbH & Co. KG | Dispensing device |
| US11224704B2 (en) | 2007-07-06 | 2022-01-18 | Manta Devices, Llc | Dose delivery device for inhalation |
| EP2898914B1 (en) * | 2007-07-06 | 2018-06-20 | Manta Devices, LLC | Inhalation devices for storing and delivering medicament |
| EP2224983B1 (en) | 2007-12-20 | 2017-05-10 | AstraZeneca AB | Device and method for deaggregating powder 854 |
| EP2082762A1 (en) * | 2008-01-24 | 2009-07-29 | Boehringer Ingelheim International Gmbh | Inhaler |
| EP2082764A1 (en) * | 2008-01-24 | 2009-07-29 | Boehringer Ingelheim International GmbH | Inhaler |
| PT2254629E (pt) * | 2008-03-27 | 2016-03-02 | Mannkind Corp | Um sistema de inalação de pó seco |
| GB2459257B (en) * | 2008-04-14 | 2012-12-19 | Alchemy Healthcare Ltd | Particulate dispenser |
| JP5421362B2 (ja) | 2008-06-13 | 2014-02-19 | マンカインド コーポレイション | 薬物送達用の乾燥粉末吸入器およびシステム |
| US8485180B2 (en) | 2008-06-13 | 2013-07-16 | Mannkind Corporation | Dry powder drug delivery system |
| RU2470681C2 (ru) | 2008-06-20 | 2012-12-27 | Маннкайнд Корпорейшн | Интерактивное устройство и способ профилирования усилий при ингаляции в реальном масштабе времени |
| FR2933619B1 (fr) * | 2008-07-11 | 2012-03-30 | Valois Sas | Dispositif d'inhalation de poudre |
| FR2933620B1 (fr) * | 2008-07-11 | 2010-09-03 | Valois Sa | Dispositif d'inhalation de poudre. |
| WO2010011329A2 (en) * | 2008-07-23 | 2010-01-28 | Map Pharmaceuticals, Inc. | The delivery of powdered drug via inhalation |
| TWI614024B (zh) | 2008-08-11 | 2018-02-11 | 曼凱公司 | 超快起作用胰島素之用途 |
| FR2936424B1 (fr) * | 2008-09-30 | 2012-01-20 | Valois Sas | Dispositif d'inhalation de poudre. |
| FR2936425B1 (fr) * | 2008-09-30 | 2012-02-17 | Valois Sas | Dispositif d'inhalation de poudre. |
| GB0818476D0 (en) * | 2008-10-09 | 2008-11-12 | Vectura Delivery Device Ltd | Inhaler |
| BRPI0922255A2 (pt) * | 2008-12-15 | 2016-01-12 | Profibrix Bv | dispositivo para fornecimento de soluções em pó |
| US8314106B2 (en) | 2008-12-29 | 2012-11-20 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| US8550074B2 (en) | 2009-01-15 | 2013-10-08 | Manta Devices, Llc | Delivery device and related methods |
| EP2405963B1 (en) | 2009-03-11 | 2013-11-06 | MannKind Corporation | Apparatus, system and method for measuring resistance of an inhaler |
| FR2946537B1 (fr) * | 2009-06-11 | 2012-08-03 | Valois Sas | Dispositif d'inhalation de poudre |
| FR2946538B1 (fr) * | 2009-06-11 | 2012-08-03 | Valois Sas | Dispositif d'inhalation de poudre |
| US8551528B2 (en) | 2009-06-12 | 2013-10-08 | Mannkind Corporation | Diketopiperazine microparticles with defined specific surface areas |
| CA2765497A1 (en) | 2009-07-01 | 2011-01-06 | Astrazeneca Ab | Dispenser and method for entraining powder in an airflow |
| US9138167B1 (en) * | 2009-09-25 | 2015-09-22 | Krispin Johan Leydon | Means for rendering key respiratory measurements accessible to mobile digital devices |
| US10869638B2 (en) | 2009-09-25 | 2020-12-22 | Krispin Johan Leydon | Systems, devices and methods for rendering key respiratory measurements accessible to mobile digital devices |
| EP2496295A1 (en) | 2009-11-03 | 2012-09-12 | MannKind Corporation | An apparatus and method for simulating inhalation efforts |
| US9492625B2 (en) | 2009-11-12 | 2016-11-15 | Stc.Unm | Dry powder inhaler with flutter dispersion member |
| BR112012011249A2 (pt) * | 2009-11-13 | 2016-04-12 | Schering Corp | dispensador de pó, droga, e, produto de droga |
| GB2489383A (en) | 2009-12-30 | 2012-09-26 | Vijayan Thirumalai Anandampillai | An improved dry powder inhaler |
| WO2011116293A2 (en) | 2010-03-19 | 2011-09-22 | Manta Devices, Llc | Delivery device and related methods |
| USD635246S1 (en) * | 2010-03-26 | 2011-03-29 | Oriel Therapeutics, Inc. | Dose disk for dry powder inhalers |
| JP6385673B2 (ja) | 2010-06-21 | 2018-09-05 | マンカインド コーポレイション | 乾燥粉末薬物送達システム |
| AU2016222336B2 (en) * | 2010-06-21 | 2018-09-13 | Mannkind Corporation | Dry powder drug delivery system and methods |
| ES2535979T3 (es) * | 2010-07-21 | 2015-05-19 | Astrazeneca Ab | Inhalador |
| US9427533B2 (en) * | 2010-11-29 | 2016-08-30 | Sanofi-Aventis Deutschland Gmbh | Medicated module for an inhaler |
| WO2012078804A1 (en) | 2010-12-07 | 2012-06-14 | Respira Therapeutics, Inc. | Dry powder inhaler |
| CN102553038B (zh) * | 2010-12-17 | 2014-07-02 | 陈庆堂 | 药粉吸嘴放置盒 |
| GB201021881D0 (en) | 2010-12-23 | 2011-02-02 | Profibrix Bv | Powder delivery device |
| WO2012135765A2 (en) | 2011-04-01 | 2012-10-04 | Mannkind Corporation | Blister package for pharmaceutical cartridges |
| WO2012174472A1 (en) | 2011-06-17 | 2012-12-20 | Mannkind Corporation | High capacity diketopiperazine microparticles |
| US11103659B2 (en) | 2011-07-06 | 2021-08-31 | Manta Devices, Llc | Delivery device and related methods |
| EP2731572B1 (en) * | 2011-07-13 | 2021-04-07 | Pharmaxis Ltd. | Improvements relating to delivery devices |
| EP2731573B1 (en) * | 2011-07-13 | 2019-10-30 | Pharmaxis Ltd | Improvements relating to delivery devices |
| DK2731571T3 (da) | 2011-07-13 | 2020-02-17 | Pharmaxis Ltd | Forbedringer vedrørende leveringsanordninger |
| FR2979057B1 (fr) * | 2011-08-19 | 2013-08-30 | Valois Sas | Dispositif d'inhalation de poudre. |
| FR2979058B1 (fr) * | 2011-08-19 | 2013-08-30 | Valois Sas | Dispositif d'inhalation de poudre. |
| CN106730186B (zh) | 2011-09-07 | 2020-06-09 | 康森特里克斯药物公司 | 干粉吸入装置 |
| IN2014DN03093A (cg-RX-API-DMAC7.html) | 2011-10-24 | 2015-05-15 | Mannkind Corp | |
| US10463815B2 (en) * | 2012-02-21 | 2019-11-05 | Respira Therapeutics, Inc. | Inhaler to deliver substances for prophylaxis or prevention of disease or injury caused by the inhalation of biological or chemical agents |
| CA2865876A1 (en) | 2012-03-07 | 2013-09-12 | Advanced Inhalation Therapies (Ait) Ltd. | Inhalation of nitric oxide for treating respiratory diseases |
| US9649454B2 (en) | 2012-05-03 | 2017-05-16 | Manta Devices, Llc | Delivery device and related methods |
| AU2013289957B2 (en) | 2012-07-12 | 2017-02-23 | Mannkind Corporation | Dry powder drug delivery systems and methods |
| WO2014066856A1 (en) | 2012-10-26 | 2014-05-01 | Mannkind Corporation | Inhalable influenza vaccine compositions and methods |
| US9839756B2 (en) | 2012-11-27 | 2017-12-12 | Resmed Limited | Methods and apparatus for ionization therapy |
| ES2754388T3 (es) | 2013-03-15 | 2020-04-17 | Mannkind Corp | Composiciones y métodos de dicetopiperazina microcristalina |
| AU2014290438B2 (en) | 2013-07-18 | 2019-11-07 | Mannkind Corporation | Heat-stable dry powder pharmaceutical compositions and methods |
| US20150020806A1 (en) * | 2013-07-19 | 2015-01-22 | Merck Sharp & Dohme Corp. | Dry powder inhaler for delivering multipe agglomerate formulations |
| JP2016530930A (ja) | 2013-08-05 | 2016-10-06 | マンカインド コーポレイション | 通気装置及び方法 |
| EP3110484A4 (en) * | 2014-02-21 | 2018-02-14 | Respira Therapeutics, Inc. | Powder inhaler, system and methods |
| NO2709641T3 (cg-RX-API-DMAC7.html) | 2014-03-10 | 2018-05-12 | ||
| WO2015148905A1 (en) | 2014-03-28 | 2015-10-01 | Mannkind Corporation | Use of ultrarapid acting insulin |
| US11147936B2 (en) | 2014-05-02 | 2021-10-19 | Manta Devices, Llc | Dose delivery device with cover connected to dose chamber seal |
| PT3151893T (pt) * | 2014-06-06 | 2018-05-18 | Univ Groningen | Um inalador de pó seco acionado por respiração |
| FR3025110B1 (fr) * | 2014-09-02 | 2016-12-23 | Univ Francois-Rabelais De Tours | Dispositif de pulverisation nasale de produit fluide |
| US10561806B2 (en) | 2014-10-02 | 2020-02-18 | Mannkind Corporation | Mouthpiece cover for an inhaler |
| EP3244952A4 (en) * | 2015-01-14 | 2018-11-14 | Respira Therapeutics, Inc. | Powder dispersion methods and devices |
| EP3061501A1 (en) | 2015-02-27 | 2016-08-31 | Rottapharm Ltd. | Composition for the treatment of acne |
| WO2016159889A1 (en) | 2015-04-02 | 2016-10-06 | Hill-Rom Services Pte. Ltd. | Manifold for respiratory device |
| EP3117825A1 (en) | 2015-07-16 | 2017-01-18 | Rottapharm S.p.A. | Oral formulation comprising berberine and morus alba extract |
| US20180164134A1 (en) * | 2015-07-28 | 2018-06-14 | Nazhiyuan Technology (Tangshan), LLC. | Pneumatic sensor in electronic cigarette, device for processing airflow, and electronic cigarette |
| US10238821B2 (en) * | 2016-10-11 | 2019-03-26 | Microdose Therapeutx, Inc. | Inhaler and methods of use thereof |
| EP3600504B1 (en) | 2017-03-28 | 2023-07-12 | Concentrx Pharmaceuticals, Inc. | Device for delivering dry powder medicaments |
| EP3840803A4 (en) * | 2018-09-10 | 2022-05-18 | Cipla Limited | Single blister-strip based dispenser |
| DE102019219277A1 (de) * | 2019-12-10 | 2021-06-10 | Christian-Albrechts-Universität Zu Kiel | Pulverförmige Formulierungen zur Inhalation |
| US11289063B1 (en) * | 2020-07-17 | 2022-03-29 | Whistle Shield LLC | Hygienic whistle with enhanced sound-generating chamber |
| CN115105695B (zh) * | 2022-06-02 | 2023-05-16 | 宁波职业技术学院 | 一种哮喘呼吸器及哮喘呼吸器的使用方法 |
| WO2025252974A1 (en) * | 2024-06-07 | 2025-12-11 | Vectura Delivery Devices Limited | Dry powder inhaler with a mixing element |
| CN118491823B (zh) * | 2024-07-17 | 2024-09-13 | 安徽墙煌科技股份有限公司 | 一种铝单板喷涂工艺 |
Family Cites Families (52)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BE483136A (cg-RX-API-DMAC7.html) | 1947-09-04 | 1942-06-30 | ||
| US2470296A (en) * | 1948-04-30 | 1949-05-17 | Abbott Lab | Inhalator |
| US2534636A (en) | 1949-02-12 | 1950-12-19 | American Cyanamid Co | Powder dispenser |
| US2816549A (en) | 1956-11-16 | 1957-12-17 | Isaac E Webster | Dispensing device |
| US3362405A (en) * | 1964-04-06 | 1968-01-09 | Hamilton O. Hazel | Method and apparatus for admixing gas with solid particles |
| US3809084A (en) | 1970-02-16 | 1974-05-07 | American Cyanamid Co | Pressurized portable dispenser |
| SE367249B (cg-RX-API-DMAC7.html) * | 1971-11-10 | 1974-05-20 | T Griverus | |
| YU41046B (en) * | 1974-08-22 | 1986-10-31 | Schering Ag | Medicine inholating device |
| US3998226A (en) * | 1975-09-22 | 1976-12-21 | Edward G. Gomez | Inhalation device for encapsulated concentrates |
| FR2352556A1 (fr) | 1976-05-26 | 1977-12-23 | Pasteur Institut | Inhalateur de poudre |
| US4452239A (en) * | 1980-03-25 | 1984-06-05 | Hilal Malem | Medical nebulizing apparatus |
| ES8206980A1 (es) * | 1980-10-30 | 1982-09-01 | Riker Laboratories Inc | Un dispositivo para facilitar la inhalacion oral de medica- mentos en forma de polvo |
| US4509515A (en) | 1982-02-23 | 1985-04-09 | Fisons Plc | Inhalation device |
| AT396333B (de) * | 1982-10-08 | 1993-08-25 | Glaxo Group Ltd | Vorrichtung zur verabreichung von medikamenten an patienten, einrichtung mit mehreren solchen vorrichtungen und traeger mit medikamentbehaeltern hiefuer |
| AT384552B (de) | 1985-08-01 | 1987-12-10 | Hurka Wilhelm | Inhalationsgeraet zur dosierung und verteilung von festkoerpern in die atemluft |
| US4653494A (en) * | 1985-12-20 | 1987-03-31 | Ruderian Max J | Nasal inhalation system |
| US4790305A (en) * | 1986-06-23 | 1988-12-13 | The Johns Hopkins University | Medication delivery system |
| GB8909891D0 (en) | 1989-04-28 | 1989-06-14 | Riker Laboratories Inc | Device |
| US5176132A (en) * | 1989-05-31 | 1993-01-05 | Fisons Plc | Medicament inhalation device and formulation |
| DE407028T1 (de) | 1989-05-31 | 1994-03-17 | Fisons Plc | Medikament und Inhalationsvorrichtung dafür. |
| FI84698C (fi) | 1989-06-16 | 1992-01-10 | Huhtamaeki Oy | Anordning foer finfoerdelning av agglomerat av en enkeldos av ett laekemedelpreparat i pulverform. |
| IT1230313B (it) * | 1989-07-07 | 1991-10-18 | Somova Spa | Inalatore per medicamenti in capsule. |
| US5042467A (en) * | 1990-03-28 | 1991-08-27 | Trudell Medical | Medication inhaler with fitting having a sonic signalling device |
| IL111254A (en) * | 1990-06-14 | 1998-10-30 | Rhone Poulenc Rorer Ltd | Powder inhalers |
| US5042472A (en) | 1990-10-15 | 1991-08-27 | Merck & Co., Inc. | Powder inhaler device |
| EP0504459B1 (de) | 1991-03-21 | 1996-06-05 | PAUL RITZAU PARI-WERK GmbH | Vernebler insbesondere zur Anwendung in Geräten für die Inhalationstherapie |
| US5797391A (en) | 1991-03-28 | 1998-08-25 | Rhone-Poulenc Rorer Limited | Inhaler |
| US5492112A (en) | 1991-05-20 | 1996-02-20 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| DE4211475A1 (de) * | 1991-12-14 | 1993-06-17 | Asta Medica Ag | Pulverinhalator |
| US5476093A (en) * | 1992-02-14 | 1995-12-19 | Huhtamaki Oy | Device for more effective pulverization of a powdered inhalation medicament |
| EP0558879B1 (en) | 1992-03-04 | 1997-05-14 | Astra Aktiebolag | Disposable inhaler |
| SE9302550D0 (sv) * | 1993-07-30 | 1993-07-30 | Ernst Hoerlin | Powder inhaler |
| ES2122319T3 (es) * | 1993-08-18 | 1998-12-16 | Fisons Plc | Inhalador con regulacion del caudal de aire. |
| DE4340768A1 (de) * | 1993-11-30 | 1995-06-01 | Bayer Ag | Vorrichtung zum Inhalieren |
| DE59409856D1 (de) * | 1994-05-19 | 2001-10-11 | Pari Gmbh | Vorrichtung zur Trocknung und Pufferung von Aerosolen |
| US5714007A (en) | 1995-06-06 | 1998-02-03 | David Sarnoff Research Center, Inc. | Apparatus for electrostatically depositing a medicament powder upon predefined regions of a substrate |
| SE504458C2 (sv) | 1995-06-21 | 1997-02-17 | Lars Gunnar Nilsson | Inhalator för elektrisk dosering av substanser |
| US5642727A (en) | 1995-07-25 | 1997-07-01 | David Sarnoff Research Center, Inc. | Inhaler apparatus using a tribo-electric charging technique |
| US5669378A (en) * | 1995-12-21 | 1997-09-23 | Pera; Ivo | Inhaling device |
| US6470884B2 (en) * | 1996-01-29 | 2002-10-29 | Aventis Pharma Limited | Capsule opening arrangement for use in a powder inhaler |
| SE9600306D0 (sv) * | 1996-01-29 | 1996-01-29 | Ernst Hoerlin | Capsule opening arrangement for use in a powder inhaler |
| GB9626233D0 (en) * | 1996-12-18 | 1997-02-05 | Chawla Brinda P S | Medicament packaging and deliveery device |
| GB9720283D0 (en) | 1997-09-25 | 1997-11-26 | Norton Healthcare Ltd | Inhaler spacer |
| US6073629A (en) * | 1997-09-25 | 2000-06-13 | Norton Healthcare Ltd. | Inhaler spacer |
| US6125998A (en) * | 1997-12-17 | 2000-10-03 | Batista; Christina | Whistle case |
| US6096368A (en) | 1998-02-19 | 2000-08-01 | Delsys Pharmaceutical Corporation | Bead transporter chucks using repulsive field guidance and method |
| US6063194A (en) | 1998-06-10 | 2000-05-16 | Delsys Pharmaceutical Corporation | Dry powder deposition apparatus |
| US6615826B1 (en) * | 1999-02-26 | 2003-09-09 | 3M Innovative Properties Company | Slow spray metered dose inhaler |
| GB9905538D0 (en) | 1999-03-10 | 1999-05-05 | Glaxo Group Ltd | A device |
| US6427688B1 (en) * | 2000-02-01 | 2002-08-06 | Dura Pharmaceuticals, Icn. | Dry powder inhaler |
| AU2001283546A1 (en) | 2000-08-14 | 2002-02-25 | Advanced Inhalation Research, Inc. | Inhalation device and method |
| US6722364B2 (en) * | 2001-01-12 | 2004-04-20 | Becton, Dickinson And Company | Medicament inhalation delivery devices and methods for using the same |
-
2000
- 2000-02-01 US US09/495,494 patent/US6427688B1/en not_active Expired - Lifetime
-
2001
- 2001-01-31 AU AU2001233201A patent/AU2001233201A1/en not_active Abandoned
- 2001-01-31 JP JP2001556536A patent/JP4886143B2/ja not_active Expired - Fee Related
- 2001-01-31 ES ES01905307T patent/ES2432353T3/es not_active Expired - Lifetime
- 2001-01-31 CA CA2398815A patent/CA2398815C/en not_active Expired - Fee Related
- 2001-01-31 WO PCT/US2001/003248 patent/WO2001056640A1/en not_active Ceased
- 2001-01-31 EP EP01905307.3A patent/EP1307256B1/en not_active Expired - Lifetime
- 2001-01-31 US US09/773,261 patent/US6715486B2/en not_active Expired - Lifetime
-
2004
- 2004-02-19 US US10/782,449 patent/US6971384B2/en not_active Expired - Lifetime
-
2005
- 2005-09-12 US US11/224,406 patent/US20060005833A1/en not_active Abandoned
-
2008
- 2008-12-04 US US12/328,657 patent/US7958890B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| US7958890B2 (en) | 2011-06-14 |
| US20010027790A1 (en) | 2001-10-11 |
| EP1307256B1 (en) | 2013-07-24 |
| US20090084380A1 (en) | 2009-04-02 |
| US6715486B2 (en) | 2004-04-06 |
| CA2398815A1 (en) | 2001-08-09 |
| US20060005833A1 (en) | 2006-01-12 |
| AU2001233201A1 (en) | 2001-08-14 |
| WO2001056640A1 (en) | 2001-08-09 |
| JP2004502472A (ja) | 2004-01-29 |
| CA2398815C (en) | 2012-04-17 |
| EP1307256A4 (en) | 2006-04-12 |
| US20040163644A1 (en) | 2004-08-26 |
| US6427688B1 (en) | 2002-08-06 |
| WO2001056640A9 (en) | 2002-10-24 |
| ES2432353T3 (es) | 2013-12-02 |
| EP1307256A1 (en) | 2003-05-07 |
| US6971384B2 (en) | 2005-12-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4886143B2 (ja) | 乾燥粉末吸入器 | |
| US11471623B2 (en) | Powder dispersion methods and devices | |
| US20040211419A1 (en) | Inhalers | |
| US20070125375A1 (en) | Device and method for deagglomeration of powder for inhalation | |
| US20040159321A1 (en) | Inhaler | |
| KR20090074284A (ko) | 단위 투약 카트리지 및 드라이파우더 흡입기 | |
| CN106794325B (zh) | 粉末吸入器、系统和方法 | |
| JP2005522277A (ja) | 乾燥薬剤粉末の解凝集および空気中への分散 | |
| US20250108180A1 (en) | Powder dispersion methods and devices |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20051027 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20051027 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080129 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20100723 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100817 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20101117 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20101125 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110209 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110802 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111018 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20111115 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20111209 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20141216 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4886143 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |