JP2018521663A - 治療薬 - Google Patents
治療薬 Download PDFInfo
- Publication number
- JP2018521663A JP2018521663A JP2018503642A JP2018503642A JP2018521663A JP 2018521663 A JP2018521663 A JP 2018521663A JP 2018503642 A JP2018503642 A JP 2018503642A JP 2018503642 A JP2018503642 A JP 2018503642A JP 2018521663 A JP2018521663 A JP 2018521663A
- Authority
- JP
- Japan
- Prior art keywords
- immune response
- cells
- receptor
- cell according
- response cell
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/7051—T-cell receptor (TcR)-CD3 complex
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/30—Cellular immunotherapy characterised by the recombinant expression of specific molecules in the cells of the immune system
- A61K40/31—Chimeric antigen receptors [CAR]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4202—Receptors, cell surface antigens or cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4202—Receptors, cell surface antigens or cell surface determinants
- A61K40/4203—Receptors for growth factors
- A61K40/4205—Her-2/neu/ErbB2, Her-3/ErbB3 or Her 4/ ErbB4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4202—Receptors, cell surface antigens or cell surface determinants
- A61K40/4214—Receptors for cytokines
- A61K40/4215—Receptors for tumor necrosis factors [TNF], e.g. lymphotoxin receptor [LTR], CD30
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4202—Receptors, cell surface antigens or cell surface determinants
- A61K40/4214—Receptors for cytokines
- A61K40/4216—Receptors for colony stimulating factors [CSF]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4231—Cytokines
- A61K40/4234—Interleukins [IL]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/52—Cytokines; Lymphokines; Interferons
- C07K14/53—Colony-stimulating factor [CSF]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/52—Cytokines; Lymphokines; Interferons
- C07K14/54—Interleukins [IL]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70517—CD8
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70521—CD28, CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70578—NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2121/00—Preparations for use in therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/27—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterized by targeting or presenting multiple antigens
- A61K2239/29—Multispecific CARs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/31—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterized by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/38—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the cancer treated
- A61K2239/48—Blood cells, e.g. leukemia or lymphoma
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/02—Fusion polypeptide containing a localisation/targetting motif containing a signal sequence
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/33—Fusion polypeptide fusions for targeting to specific cell types, e.g. tissue specific targeting, targeting of a bacterial subspecies
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Zoology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Toxicology (AREA)
- Gastroenterology & Hepatology (AREA)
- Biophysics (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Cell Biology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- Hematology (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Virology (AREA)
- Developmental Biology & Embryology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Oncology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
【選択図】なし
Description
(i)第二世代キメラ抗原受容体であって、
(a)シグナル伝達領域;
(b)共刺激シグナル伝達領域;
(c)膜貫通ドメイン;および
(d)標的抗原上の第1のエピトープと特異的に相互作用する結合エレメント
を含む第二世代キメラ抗原受容体と、
(ii)キメラ共刺激受容体であって、
(e)(b)の共刺激シグナル伝達領域と異なる共刺激シグナル伝達領域;
(f)膜貫通ドメイン;および
(g)標的抗原上の第2のエピトープと特異的に相互作用する結合エレメント
を含むキメラ共刺激受容体と
を発現する免疫応答細胞を提供する。
の220アミノ酸のタンパク質であり、膜貫通ドメインを太字で示す。
EQKLISEEDL(配列番号5)
のタグである。
RGDLX5X6L(配列番号7)、または
RGDLX5X6I(配列番号8)
を含み、LX5X6LまたはLX5X6Iは、αヘリックス構造内に含まれ、X5およびX6は、[α]−ヘリックスの中央に見られるために1.0より大きい配座優先性を有するヘリックス促進残基である(Creighton,1993 and Pace C.N.and Scholtz J.M.(1998),Biophysical Journal,Vol.75,pages 422−427から)。特に、そうした残基は、独立に、Glu、Ala、Leu、Met、Gln、Lys、Arg、Val、Ile、Trp、PheおよびAspからなる群から選択される。
YTASARGDLAHLTTTHARHL(配列番号9)
GFTTGRRGDLATIHGMNRPF(配列番号10)、もしくは
NAVPNLRGDLQVLAQKVART(配列番号11)
またはそれらの変異体が挙げられる。
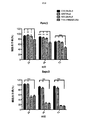
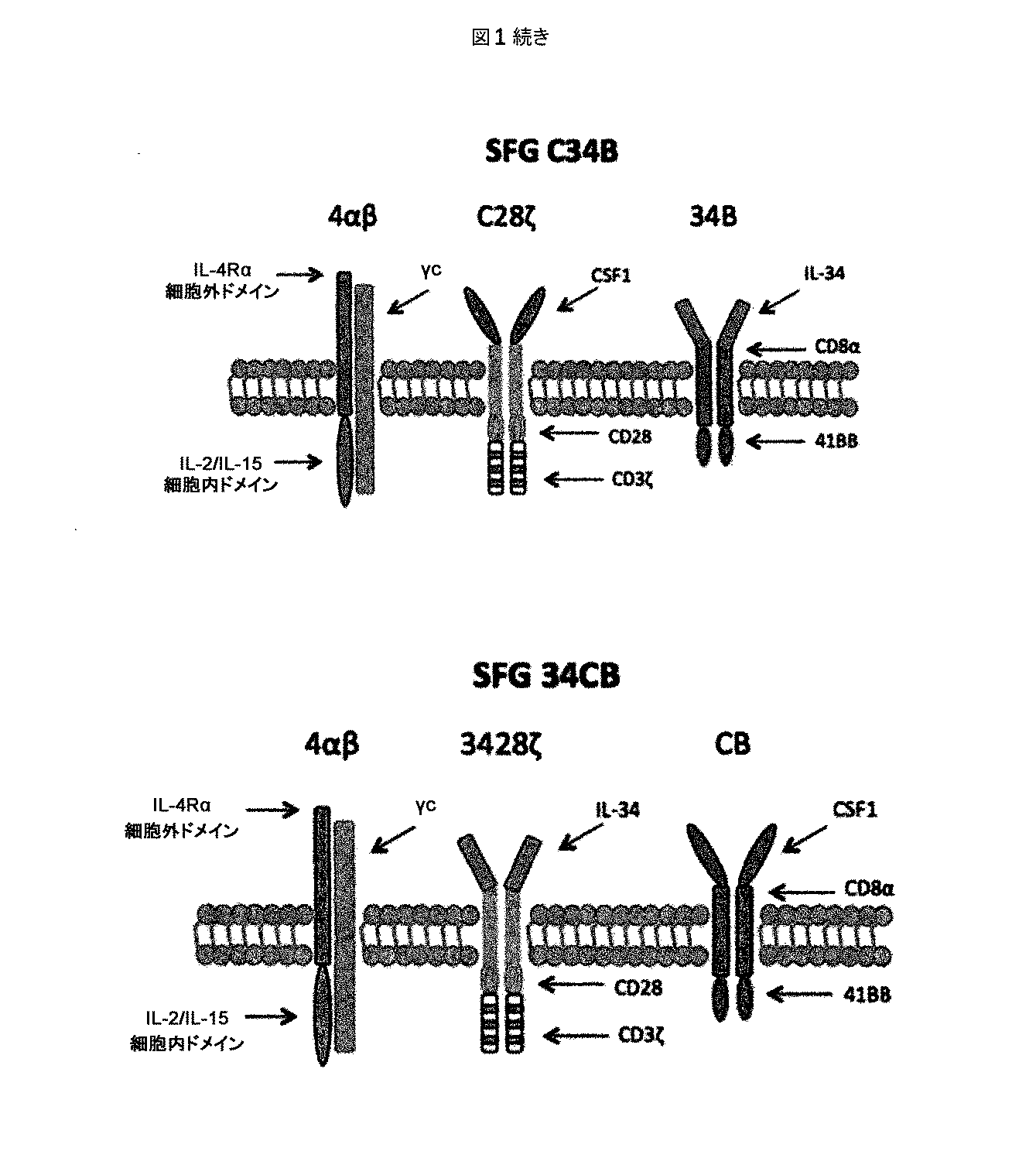
ホジキンリンパ腫、未分化大細胞リンパ腫、およびたとえばトリプルネガティブ乳癌などの一部の固形腫瘍に過剰発現するCSF−1受容体(c−FMSによりコードされる)を標的とする一連のCARを調製した。これらを概略的に図1に示す。これらの一連のCARは、標的化部分として2つの天然リガンドCSF−1またはIL−34の何れかを有する第二世代および第三世代CARの両方を含んでいた。CSF−1およびIL−34の両方がCSF−1受容体に結合するが、IL−34の方がかなり高い親和性(CSF−1より34倍高い)で結合する。
in vivoでの作用の解析
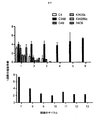
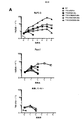
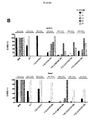
上記の実施例1に使用した一連のCARについて、CSF−1受容体標的を低レベルで発現し、かつ疾患がリンパ節全体に散在する、高悪性度in vivo異種移植モデルを用いて抗腫瘍活性を試験した(図7)。腫瘍細胞にホタルルシフェラーゼタグを付加し、疾患負荷の非侵襲的モニタリングを可能にした。
・C4B群:20×106個のC4B T細胞 IV
・C34B群:20×106個のC34B T細胞 IV
・43428Bz:20×106個の43428Bz T細胞 IV
・34CB群:20×106個の34CB T細胞 IV
・UT(形質導入していない)群:20×106個の形質導入していないT細胞 IV
・NT(無処置)群:200μL PBS IV
の1つで処置した。
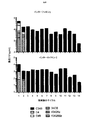
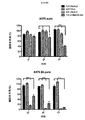
αvβ6依存性にT細胞活性化を誘導するpCARを操作するための標的化部分の選択
αvβ6インテグリンを単独または伸長(extended)ErbBファミリーと一緒に標的とする一連のCARを調製した。これらを図14に概略的に示す。この事例に使用した結合エレメントは、口蹄疫ウイルス(血清型01 BFS)由来のカプシドタンパク質VP1のGHループに由来するA20ペプチド(配列番号11)であった(米国特許第8,927,501号明細書)。これをCD124シグナルペプチドの下流に置き、CD28およびCD3ζ細胞内ドメインと融合して第二世代CAR、A20−28ζを形成した。同様のコンストラクトを含むが、重要なRGDLモチーフをAAAAで置き換えてスクランブルした標的化ペプチド(C20と命名される)を用いて対照(C20−28ζ)を調製した。第2の対照は、CD28切断細胞内ドメインと融合したA20(A20−Tr)を含んでいた。
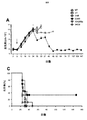
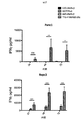
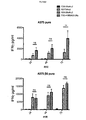
機能的pCARを操作するための代替のTNF受容体ファミリーメンバーCD27の使用
A20−28z/T1E−41BB pCARを出発材料として使用して、41BBモジュールをTNF受容体ファミリーの代替メンバー、すなわちCD27またはCD40で置き換えた別のpCARを操作した。細胞内ドメインを切断した(tr)対照pCARを操作した。αvβ6を発現する(Bxpc3)または欠いている(Panc1)標的細胞を24ウェルプレートの1ウェルあたり5×104細胞の密度で蒔いた。24時間後、5×104個のpCAR T細胞を、外来性サイトカイン添加物を用いずに標的細胞または空ウェル(「刺激していない」)に加えた。さらに72時間後、T細胞をウェルから回収し、カウントした(図19A)。MTTアッセイを行って残存標的細胞の生存割合を判定し、T細胞を添加せずに蒔いてあった対照標的細胞と比較した(図19B)。各刺激サイクル後にT細胞が増殖した場合、上記の通りに新鮮な標的細胞上で再刺激した。pCAR T細胞の増殖(図19A)およびMTTアッセイ(図19B)は、前の場合と同様に72時間後に行った。pCAR T細胞の反復再刺激および標的細胞殺傷の評価は、このように72時間の各サイクル期間にわたりT細胞がもはや増殖しなくなるまで継続した。
Claims (21)
- 免疫応答細胞において、
(i)第二世代キメラ抗原受容体であって、
(a)シグナル伝達領域;
(b)共刺激シグナル伝達領域;
(c)膜貫通ドメイン;および
(d)標的抗原上の第1のエピトープと特異的に相互作用する結合エレメント
を含む第二世代キメラ抗原受容体と、
(ii)キメラ共刺激受容体であって、
(e)(b)の共刺激シグナル伝達領域と異なる共刺激シグナル伝達領域;
(f)膜貫通ドメイン;および
(g)標的抗原上の第2のエピトープと特異的に相互作用する結合エレメント
を含むキメラ共刺激受容体と
を発現することを特徴とする免疫応答細胞。 - 請求項1に記載の免疫応答細胞において、T細胞であることを特徴とする免疫応答細胞。
- 請求項1または2に記載の免疫応答細胞において、前記シグナル伝達領域(a)は、ヒトCD3[ゼータ]鎖の細胞内ドメインまたはその変異体を含むことを特徴とする免疫応答細胞。
- 請求項1乃至3の何れか1項に記載の免疫応答細胞において、(b)および(e)の共刺激シグナル伝達領域は、CD28、CD27、ICOS、4−1BB、OX40、CD30、GITR、HVEM、DR3またはCD40から選択されることを特徴とする免疫応答細胞。
- 請求項4に記載の免疫応答細胞において、(b)または(e)の一方はCD28であり、および他方は4−1BBまたはOX40であることを特徴とする免疫応答細胞。
- 請求項5に記載の免疫応答細胞において、(b)はCD28であることを特徴とする免疫応答細胞。
- 請求項5または6に記載の免疫応答細胞において、(e)は4−1BBまたはCD27であることを特徴とする免疫応答細胞。
- 請求項1乃至7の何れか1項に記載の免疫応答細胞において、(c)および(f)の前記膜貫通ドメインは、CD8α膜貫通ドメインおよびCD28膜貫通ドメインから選択されることを特徴とする免疫応答細胞。
- 請求項1乃至8の何れか1項に記載の免疫応答細胞において、前記第1および第2のエピトープは、同一の受容体または抗原と関連付けられていることを特徴とする免疫応答細胞。
- 請求項1乃至9の何れか1項に記載の免疫応答細胞において、キメラサイトカイン受容体を共発現することを特徴とする免疫応答細胞。
- 請求項10に記載の免疫応答細胞において、前記キメラサイトカイン受容体は4αβであることを特徴とする免疫応答細胞。
- 請求項1乃至11の何れか1項に記載の免疫応答細胞において、結合エレメント(d)または結合エレメント(g)の少なくとも1つは、ErbB2量体のリガンド、コロニー刺激因子−1の受容体(CSF−1R)またはαvβ6インテグリン特異的結合剤であることを特徴とする免疫応答細胞。
- 請求項1乃至12の何れか1項に記載の免疫応答細胞において、結合エレメント(d)はCSF−1を含み、および結合エレメント(g)はIL−34を含むことを特徴とする免疫応答細胞。
- 請求項1乃至12の何れか1項に記載の免疫応答細胞において、結合エレメント(d)は、αvβ6インテグリン特異的結合剤であって、配列モチーフ
RGDLX5X6L(配列番号7)、または
RGDLX5X6I(配列番号8)
を含むペプチドであり、LX5X6LまたはLX5X6Iは、αヘリックス構造内に含まれ、X5およびX6はヘリックス促進残基である、αvβ6インテグリン特異的結合剤であり、および結合エレメント(g)はTIEペプチドであることを特徴とする免疫応答細胞。 - 請求項1乃至14の何れか1項に記載の免疫応答細胞において、結合エレメント(d)の結合親和性は結合エレメント(g)の結合親和性より低いことを特徴とする免疫応答細胞。
- 請求項1乃至15の何れか1項に記載の免疫応答細胞を調製するための方法において、請求項1に定義された構造(i)のCARをコードする第1の核酸およびまた請求項1に定義された構造(ii)のCCRをコードする第2の核酸を細胞に形質導入するステップを含むことを特徴とする方法。
- 請求項16に記載の方法において、前記免疫応答細胞はキメラサイトカイン受容体を含み、増殖ステップは、サイトカインの存在下で行われることを特徴とする方法。
- 組み合わせにおいて、請求項1に定義された(i)のCARをコードする第1の核酸と、請求項1に定義された(ii)のCCRをコードする第2の核酸との組み合わせであることを特徴とする組み合わせ。
- ベクターまたはベクターの組み合わせにおいて、請求項18に記載の組み合わせを含むことを特徴とするベクターまたはベクターの組み合わせ。
- キットにおいて、請求項18または19に記載の組み合わせを含むことを特徴とするキット。
- 標的細胞集団に対するT細胞性免疫応答の刺激を、それを必要とする患者において行うための方法において、上記のような請求項1乃至15の何れか1項に記載の免疫応答細胞を前記患者に投与するステップを含み、前記結合エレメント(d)および(g)は標的細胞に特異的であることを特徴とする方法。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2021114022A JP2021168675A (ja) | 2015-07-31 | 2021-07-09 | 治療薬 |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB1513540.3A GB201513540D0 (en) | 2015-07-31 | 2015-07-31 | Therapeutic agents |
| GB1513540.3 | 2015-07-31 | ||
| PCT/GB2016/052324 WO2017021701A1 (en) | 2015-07-31 | 2016-07-28 | Therapeutic agents |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2021114022A Division JP2021168675A (ja) | 2015-07-31 | 2021-07-09 | 治療薬 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2018521663A true JP2018521663A (ja) | 2018-08-09 |
| JP2018521663A5 JP2018521663A5 (ja) | 2019-08-29 |
| JP7053037B2 JP7053037B2 (ja) | 2022-04-12 |
Family
ID=54062973
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018503642A Active JP7053037B2 (ja) | 2015-07-31 | 2016-07-28 | 治療薬 |
| JP2021114022A Pending JP2021168675A (ja) | 2015-07-31 | 2021-07-09 | 治療薬 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2021114022A Pending JP2021168675A (ja) | 2015-07-31 | 2021-07-09 | 治療薬 |
Country Status (15)
| Country | Link |
|---|---|
| US (6) | US10703794B2 (ja) |
| EP (2) | EP3328880B1 (ja) |
| JP (2) | JP7053037B2 (ja) |
| KR (1) | KR102411571B1 (ja) |
| CN (1) | CN107735407B (ja) |
| AU (1) | AU2016303355B2 (ja) |
| CA (1) | CA2993746A1 (ja) |
| DK (1) | DK3328880T3 (ja) |
| ES (1) | ES2883633T3 (ja) |
| GB (1) | GB201513540D0 (ja) |
| IL (1) | IL256511B (ja) |
| MX (1) | MX2018001009A (ja) |
| RU (1) | RU2747733C1 (ja) |
| SG (1) | SG10201912416QA (ja) |
| WO (1) | WO2017021701A1 (ja) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2022545643A (ja) * | 2019-08-13 | 2022-10-28 | キングス・カレッジ・ロンドン | Il-1スーパーファミリーのサイトカインの空間時間的に制限された活性が武装化された免疫応答性細胞 |
| JP2022547416A (ja) * | 2019-08-28 | 2022-11-14 | キングス・カレッジ・ロンドン | B細胞標的化並列CAR(pCAR)治療的薬剤 |
| JP2023522038A (ja) * | 2020-04-17 | 2023-05-26 | 2セブンティ バイオ インコーポレイテッド | 修飾ccrポリペプチド及びその使用 |
Families Citing this family (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9511092B2 (en) | 2013-01-28 | 2016-12-06 | St. Jude Children's Research Hospital, Inc. | Chimeric receptor with NKG2D specificity for use in cell therapy against cancer and infectious disease |
| KR20210014210A (ko) | 2014-05-15 | 2021-02-08 | 내셔널 유니버시티 오브 싱가포르 | 변형된 천연 살해 세포 및 그의 용도 |
| GB201513540D0 (en) | 2015-07-31 | 2015-09-16 | King S College London | Therapeutic agents |
| GB201514874D0 (en) | 2015-08-20 | 2015-10-07 | Autolus Ltd | Cell |
| JP2020511462A (ja) | 2016-12-03 | 2020-04-16 | ジュノー セラピューティクス インコーポレイテッド | キナーゼ阻害剤との組み合わせで治療用t細胞を使用するための方法および組成物 |
| EP3336107A1 (en) * | 2016-12-15 | 2018-06-20 | Miltenyi Biotec GmbH | Immune cells expressing an antigen binding receptor and a chimeric costimulatory receptor |
| KR102312222B1 (ko) | 2016-12-22 | 2021-10-12 | 우니베르시따 델리 스투디 만냐 그레챠 카탄차로 | Cd43의 독특한 시알로글리코실화된 암-연관 에피토프를 표적으로 하는 모노클로날 항체 |
| BR112019020001A2 (pt) | 2017-03-27 | 2020-04-28 | Nat Univ Singapore | linhagens celulares estimuladoras para expansão ex vivo e ativação de células exterminadoras naturais |
| IL269553B2 (en) | 2017-03-27 | 2025-10-01 | Nat Univ Singapore | Truncated NKG2D chimeric receptors and their uses in immunotherapy with natural killer cells |
| CN111225675B (zh) | 2017-06-02 | 2024-05-03 | 朱诺治疗学股份有限公司 | 使用过继细胞疗法治疗的制品和方法 |
| EP3658578A1 (en) * | 2017-07-26 | 2020-06-03 | Cellectis | Methods of antigen-dependent chimeric antigen receptor (car) immune cell selection |
| CN109971724B (zh) * | 2017-12-28 | 2023-10-31 | 上海细胞治疗研究院 | 靶向ErbB受体家族且自表达PD-1抗体的CAR-T细胞及其用途 |
| JP7360174B2 (ja) | 2018-02-09 | 2023-10-12 | ナショナル ユニヴァーシティー オブ シンガポール | ナチュラルキラー細胞免疫療法における活性化キメラ受容体及びその使用 |
| JP7334985B2 (ja) | 2018-04-02 | 2023-08-29 | ナショナル ユニヴァーシティー オブ シンガポール | 免疫細胞で発現される膜結合抗サイトカイン非シグナル伝達バインダーによるヒトサイトカインの中和 |
| CN110615842B (zh) * | 2018-06-20 | 2023-05-09 | 上海隆耀生物科技有限公司 | 一种包含共刺激受体的嵌合抗原受体及应用 |
| EP3587454A1 (en) * | 2018-06-27 | 2020-01-01 | Albert-Ludwigs-Universität Freiburg | Chimeric antigen receptors that bind to prostate specific membrane antigen |
| AU2019330347B2 (en) | 2018-08-29 | 2025-07-24 | National University Of Singapore | A method to specifically stimulate survival and expansion of genetically-modified immune cells |
| GB201900858D0 (en) * | 2019-01-22 | 2019-03-13 | Price Nicola Kaye | Receptors providing targeted costimulation for adoptive cell therapy |
| GB201902277D0 (en) | 2019-02-19 | 2019-04-03 | King S College London | Therapeutic agents |
| WO2020180882A1 (en) | 2019-03-05 | 2020-09-10 | Nkarta, Inc. | Cd19-directed chimeric antigen receptors and uses thereof in immunotherapy |
| US20220152103A1 (en) | 2019-03-11 | 2022-05-19 | Leucid Bio Ltd | MUC1 PARALLEL CAR (pCAR) THERAPEUTIC AGENTS |
| TW202110873A (zh) | 2019-04-30 | 2021-03-16 | 美商聖堤生物科技股份有限公司 | 嵌合受體及其使用方法 |
| GB202003277D0 (en) | 2020-03-06 | 2020-04-22 | King S College London | Therapeutic agents |
| WO2022020456A2 (en) * | 2020-07-21 | 2022-01-27 | Allogene Therapeutics, Inc. | Chimeric antigen receptors with enhanced signaling and activities and uses thereof |
| WO2022036495A1 (en) | 2020-08-17 | 2022-02-24 | Utc Therapeutics Inc. | Lymphocytes-antigen presenting cells co-stimulators and uses thereof |
| WO2022083668A1 (en) * | 2020-10-21 | 2022-04-28 | Nanjing Legend Biotech Co., Ltd. | Use of a chimeric co-stimulatory receptor for cell therapy |
| WO2023017159A1 (en) * | 2021-08-13 | 2023-02-16 | Ludwig-Maximilians-Universität München | Anti-csf1r car expressing lymphocytes for targeted tumor therapy |
| EP4392463A4 (en) * | 2021-08-18 | 2025-09-17 | Legend Biotech Ireland Ltd | MODIFIED IMMUNE CELLS EXPRESSING TLR RECEPTORS |
| JP7732843B2 (ja) * | 2021-10-14 | 2025-09-02 | 株式会社三共 | 遊技機 |
| JP7732842B2 (ja) * | 2021-10-14 | 2025-09-02 | 株式会社三共 | 遊技機 |
| JP7732840B2 (ja) * | 2021-10-14 | 2025-09-02 | 株式会社三共 | 遊技機 |
| JP7732839B2 (ja) * | 2021-10-14 | 2025-09-02 | 株式会社三共 | 遊技機 |
| JP7732841B2 (ja) * | 2021-10-14 | 2025-09-02 | 株式会社三共 | 遊技機 |
| AU2022387845A1 (en) | 2021-11-15 | 2024-05-23 | Neogene Therapeutics B.V. | Engineered t cells with reduced tgf-beta receptor signaling |
| EP4456927A1 (en) | 2021-12-30 | 2024-11-06 | TR1X, Inc. | Cd4t cells expressing il-10 and chimeric antigen receptors and uses thereof |
| CN118510814A (zh) * | 2022-05-10 | 2024-08-16 | 星尘生物科技(上海)有限公司 | 一种嵌合抗原受体及其应用 |
| CN121311239A (zh) | 2023-04-05 | 2026-01-09 | 伦敦国王学院 | 包含替代信号传导结构域的平行嵌合抗原受体(pCAR)及衔接子嵌合抗原受体及其使用方法 |
| GB202403718D0 (en) | 2024-03-14 | 2024-05-01 | King S College London | Therapeutic agents |
| WO2026015450A1 (en) | 2024-07-06 | 2026-01-15 | Tr1X, Inc. | Cd4+ t cells expressing il-10 and chimeric antigen receptors and uses thereof |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009509562A (ja) * | 2005-10-03 | 2009-03-12 | キャンサー・リサーチ・テクノロジー・リミテッド | αvβ6ペプチドリガンド及びその活用 |
| WO2014055668A1 (en) * | 2012-10-02 | 2014-04-10 | Memorial Sloan-Kettering Cancer Center | Compositions and methods for immunotherapy |
| WO2016011210A2 (en) * | 2014-07-15 | 2016-01-21 | Juno Therapeutics, Inc. | Engineered cells for adoptive cell therapy |
| JP2017502251A (ja) * | 2013-12-26 | 2017-01-19 | バーダス テクノロジーズ プライベート リミテッドVerdus Technologies Pte.Ltd. | 流体処理装置および流体流れを加熱または冷却する方法 |
Family Cites Families (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| HU225688B1 (en) * | 1995-02-24 | 2007-06-28 | Gen Hospital Corp | Redirection of cellular immunity by receptor chimeras |
| US7446190B2 (en) | 2002-05-28 | 2008-11-04 | Sloan-Kettering Institute For Cancer Research | Nucleic acids encoding chimeric T cell receptors |
| EP2537416B1 (en) | 2007-03-30 | 2014-11-12 | Memorial Sloan-Kettering Cancer Center | Constitutive expression of costimulatory ligands on adoptively transferred T lymphocytes |
| US9206440B2 (en) | 2009-01-23 | 2015-12-08 | Roger Williams Hospital | Viral vectors encoding multiple highly homologus non-viral polypeptides and the use of same |
| WO2011041093A1 (en) | 2009-10-01 | 2011-04-07 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Anti-vascular endothelial growth factor receptor-2 chimeric antigen receptors and use of same for the treatment of cancer |
| BR112013024395B1 (pt) | 2011-03-23 | 2021-10-26 | Fred Hutchinson Cancer Research Center | Composições adotivas de imunoterapia celular e método para fabricação da dita composição |
| ES2888651T3 (es) | 2011-07-29 | 2022-01-05 | Univ Pennsylvania | Receptores de conmutación coestimulantes |
| WO2013033626A2 (en) | 2011-08-31 | 2013-03-07 | Trustees Of Dartmouth College | Nkp30 receptor targeted therapeutics |
| CN104136458A (zh) | 2012-02-22 | 2014-11-05 | 宾夕法尼亚大学董事会 | 在第二代嵌合抗原受体中cd2信号传导结构域的使用 |
| AU2013222288A1 (en) | 2012-02-22 | 2014-08-14 | The Trustees Of The University Of Pennsylvania | Use of ICOS-based CARs to enhance antitumor activity and CAR persistence |
| CN103483452B (zh) | 2012-06-12 | 2021-08-13 | 上海细胞治疗集团有限公司 | 双信号独立的嵌合抗原受体及其用途 |
| CN104769103B (zh) | 2012-09-04 | 2018-06-08 | 塞勒克提斯公司 | 多链嵌合抗原受体和其用途 |
| TW201414837A (zh) | 2012-10-01 | 2014-04-16 | Univ Pennsylvania | 標定基質細胞以治療癌症之組合物和方法 |
| WO2014055657A1 (en) | 2012-10-05 | 2014-04-10 | The Trustees Of The University Of Pennsylvania | Use of a trans-signaling approach in chimeric antigen receptors |
| CN105101803A (zh) | 2013-02-06 | 2015-11-25 | 人类起源公司 | 具有改进特异性的修饰的t淋巴细胞 |
| PT3300745T (pt) | 2013-02-15 | 2019-11-27 | Univ California | Recetor de antigénio quimérico e métodos de utilização do mesmo |
| US20160017286A1 (en) | 2013-03-06 | 2016-01-21 | The Trustees Of The University Of Pennsylvania | Ikaros inhibition to augment adoptive t cell transfer |
| HK1219980A1 (zh) | 2013-03-09 | 2017-04-21 | Baylor College Of Medicine | 靶向血管的t细胞疗法 |
| HK1217000A1 (zh) | 2013-03-14 | 2016-12-16 | Bellicum Pharmaceuticals, Inc. | 控制t细胞增殖的方法 |
| EP2986636B1 (en) | 2013-04-17 | 2018-11-14 | Baylor College Of Medicine | Immunosuppressive tgf-beta signal converter |
| CA2929087A1 (en) | 2013-10-31 | 2015-05-07 | Fred Hutchinson Cancer Research Center | Modified hematopoietic stem/progenitor and non-t effector cells, and uses thereof |
| PL3071222T3 (pl) | 2013-11-21 | 2021-04-19 | Ucl Business Ltd | Komórka |
| EP3087101B1 (en) | 2013-12-20 | 2024-06-05 | Novartis AG | Regulatable chimeric antigen receptor |
| WO2015164627A1 (en) | 2014-04-23 | 2015-10-29 | Discovery Genomics, Inc. | Chimeric antigen receptors specific to avb6 integrin and methods of use thereof to treat cancer |
| KR102612313B1 (ko) | 2014-07-21 | 2023-12-12 | 노파르티스 아게 | 인간화 항-bcma 키메라 항원 수용체를 사용한 암의 치료 |
| JP7170394B2 (ja) | 2015-01-31 | 2022-11-14 | ザ トラスティーズ オブ ザ ユニバーシティ オブ ペンシルバニア | 治療用分子のt細胞送達のための組成物および方法 |
| WO2016196388A1 (en) | 2015-05-29 | 2016-12-08 | Juno Therapeutics, Inc. | Composition and methods for regulating inhibitory interactions in genetically engineered cells |
| GB201513540D0 (en) * | 2015-07-31 | 2015-09-16 | King S College London | Therapeutic agents |
| US20220152103A1 (en) * | 2019-03-11 | 2022-05-19 | Leucid Bio Ltd | MUC1 PARALLEL CAR (pCAR) THERAPEUTIC AGENTS |
-
2015
- 2015-07-31 GB GBGB1513540.3A patent/GB201513540D0/en not_active Ceased
-
2016
- 2016-07-28 CA CA2993746A patent/CA2993746A1/en active Pending
- 2016-07-28 JP JP2018503642A patent/JP7053037B2/ja active Active
- 2016-07-28 KR KR1020187005607A patent/KR102411571B1/ko active Active
- 2016-07-28 ES ES16750211T patent/ES2883633T3/es active Active
- 2016-07-28 CN CN201680039617.1A patent/CN107735407B/zh active Active
- 2016-07-28 WO PCT/GB2016/052324 patent/WO2017021701A1/en not_active Ceased
- 2016-07-28 EP EP16750211.1A patent/EP3328880B1/en active Active
- 2016-07-28 SG SG10201912416QA patent/SG10201912416QA/en unknown
- 2016-07-28 MX MX2018001009A patent/MX2018001009A/es unknown
- 2016-07-28 DK DK16750211.1T patent/DK3328880T3/da active
- 2016-07-28 EP EP21177321.3A patent/EP3939992A1/en not_active Withdrawn
- 2016-07-28 RU RU2018105137A patent/RU2747733C1/ru active
- 2016-07-28 AU AU2016303355A patent/AU2016303355B2/en active Active
- 2016-07-28 US US15/749,016 patent/US10703794B2/en active Active
-
2017
- 2017-12-24 IL IL256511A patent/IL256511B/en unknown
-
2020
- 2020-05-18 US US16/877,008 patent/US10899818B2/en active Active
- 2020-05-18 US US16/877,035 patent/US10865231B2/en active Active
- 2020-12-10 US US17/118,045 patent/US11802143B2/en active Active
-
2021
- 2021-07-09 JP JP2021114022A patent/JP2021168675A/ja active Pending
-
2023
- 2023-09-22 US US18/472,441 patent/US20240076348A1/en active Pending
-
2024
- 2024-01-22 US US18/418,864 patent/US12509499B2/en active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009509562A (ja) * | 2005-10-03 | 2009-03-12 | キャンサー・リサーチ・テクノロジー・リミテッド | αvβ6ペプチドリガンド及びその活用 |
| WO2014055668A1 (en) * | 2012-10-02 | 2014-04-10 | Memorial Sloan-Kettering Cancer Center | Compositions and methods for immunotherapy |
| JP2015535689A (ja) * | 2012-10-02 | 2015-12-17 | メモリアル スローン−ケタリング キャンサー センター | 免疫療法のための組成物および方法 |
| JP2017502251A (ja) * | 2013-12-26 | 2017-01-19 | バーダス テクノロジーズ プライベート リミテッドVerdus Technologies Pte.Ltd. | 流体処理装置および流体流れを加熱または冷却する方法 |
| WO2016011210A2 (en) * | 2014-07-15 | 2016-01-21 | Juno Therapeutics, Inc. | Engineered cells for adoptive cell therapy |
Non-Patent Citations (2)
| Title |
|---|
| J.IMMUNOL.(2013)VOL.191, P.4589-4598, JPN6020016774, ISSN: 0004656335 * |
| MOL MED.(2012)VOL.18, P.565-576, JPN6020016773, ISSN: 0004656336 * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2022545643A (ja) * | 2019-08-13 | 2022-10-28 | キングス・カレッジ・ロンドン | Il-1スーパーファミリーのサイトカインの空間時間的に制限された活性が武装化された免疫応答性細胞 |
| JP2022547416A (ja) * | 2019-08-28 | 2022-11-14 | キングス・カレッジ・ロンドン | B細胞標的化並列CAR(pCAR)治療的薬剤 |
| JP7723420B2 (ja) | 2019-08-28 | 2025-08-14 | キングス・カレッジ・ロンドン | B細胞標的化並列CAR(pCAR)治療的薬剤 |
| JP2023522038A (ja) * | 2020-04-17 | 2023-05-26 | 2セブンティ バイオ インコーポレイテッド | 修飾ccrポリペプチド及びその使用 |
| JP7802684B2 (ja) | 2020-04-17 | 2026-01-20 | リジェネロン・ファーマシューティカルズ・インコーポレイテッド | 修飾ccrポリペプチド及びその使用 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12509499B2 (en) | Therapeutic agents | |
| JP7633937B2 (ja) | Pd-1外部ドメインを担持するキメラサイトカイン受容体 | |
| CN111247242B (zh) | 嵌合抗原受体(CARs)、组合物及其使用方法 | |
| KR102483822B1 (ko) | 태그된 키메라 이펙터 분자 및 그의 리셉터 | |
| CN113811327A (zh) | MUC1平行CAR(pCAR)治疗剂 | |
| KR20180002604A (ko) | 혈액 악성종양을 표적으로 하는 키메라 항원 수용체(car), 조성물 및 이의 용도 | |
| JP7723420B2 (ja) | B細胞標的化並列CAR(pCAR)治療的薬剤 | |
| JP2012501180A (ja) | T細胞の抗腫瘍エフェクター機能増進のための方法および組成物 | |
| JP2025511872A (ja) | 多部位受容体及びシグナル伝達複合体 | |
| CN111479918A (zh) | 细胞 | |
| HK1256383B (en) | Therapeutic agents | |
| JP2022001021A (ja) | Cd26特異的キメラ抗原受容体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190712 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20190712 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20200417 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20200602 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200902 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20200903 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20210309 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210709 |
|
| C60 | Trial request (containing other claim documents, opposition documents) |
Free format text: JAPANESE INTERMEDIATE CODE: C60 Effective date: 20210709 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20210709 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20210906 |
|
| C21 | Notice of transfer of a case for reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C21 Effective date: 20210907 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20211207 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20220302 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20220315 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20220324 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7053037 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |