JP2013525306A - 前駆細胞動員を伴う疾患の治療および予防のための多糖組成物および使用方法 - Google Patents
前駆細胞動員を伴う疾患の治療および予防のための多糖組成物および使用方法 Download PDFInfo
- Publication number
- JP2013525306A JP2013525306A JP2013505159A JP2013505159A JP2013525306A JP 2013525306 A JP2013525306 A JP 2013525306A JP 2013505159 A JP2013505159 A JP 2013505159A JP 2013505159 A JP2013505159 A JP 2013505159A JP 2013525306 A JP2013525306 A JP 2013525306A
- Authority
- JP
- Japan
- Prior art keywords
- formulation
- polysaccharide
- composition
- cancer
- activity
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 300
- 150000004676 glycans Chemical class 0.000 title claims abstract description 217
- 229920001282 polysaccharide Polymers 0.000 title claims abstract description 194
- 239000005017 polysaccharide Substances 0.000 title claims abstract description 194
- 238000000034 method Methods 0.000 title claims abstract description 59
- 238000011282 treatment Methods 0.000 title description 93
- 210000000130 stem cell Anatomy 0.000 title description 45
- 230000006806 disease prevention Effects 0.000 title 1
- 238000009472 formulation Methods 0.000 claims abstract description 249
- 230000014508 negative regulation of coagulation Effects 0.000 claims abstract description 24
- 206010028980 Neoplasm Diseases 0.000 claims description 130
- 230000000694 effects Effects 0.000 claims description 118
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 claims description 95
- 229920000669 heparin Polymers 0.000 claims description 77
- 201000011510 cancer Diseases 0.000 claims description 70
- 229940127089 cytotoxic agent Drugs 0.000 claims description 61
- 239000002246 antineoplastic agent Substances 0.000 claims description 58
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 claims description 50
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 46
- 239000002253 acid Substances 0.000 claims description 43
- 238000002360 preparation method Methods 0.000 claims description 43
- 201000010099 disease Diseases 0.000 claims description 42
- 206010027476 Metastases Diseases 0.000 claims description 39
- 230000009401 metastasis Effects 0.000 claims description 32
- 230000001858 anti-Xa Effects 0.000 claims description 30
- 206010006187 Breast cancer Diseases 0.000 claims description 29
- 239000003055 low molecular weight heparin Substances 0.000 claims description 29
- 208000026310 Breast neoplasm Diseases 0.000 claims description 27
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 claims description 25
- 150000004804 polysaccharides Polymers 0.000 claims description 23
- 230000002829 reductive effect Effects 0.000 claims description 23
- 206010061289 metastatic neoplasm Diseases 0.000 claims description 20
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 claims description 18
- 239000005483 tyrosine kinase inhibitor Substances 0.000 claims description 18
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 claims description 17
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 claims description 17
- 208000020816 lung neoplasm Diseases 0.000 claims description 16
- 239000008194 pharmaceutical composition Substances 0.000 claims description 13
- 101150021185 FGF gene Proteins 0.000 claims description 12
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 12
- IOVCWXUNBOPUCH-UHFFFAOYSA-N Nitrous acid Chemical compound ON=O IOVCWXUNBOPUCH-UHFFFAOYSA-N 0.000 claims description 12
- 201000005202 lung cancer Diseases 0.000 claims description 12
- KHIWWQKSHDUIBK-UHFFFAOYSA-N periodic acid Chemical compound OI(=O)(=O)=O KHIWWQKSHDUIBK-UHFFFAOYSA-N 0.000 claims description 12
- 150000004917 tyrosine kinase inhibitor derivatives Chemical class 0.000 claims description 12
- 150000003839 salts Chemical class 0.000 claims description 11
- 239000012279 sodium borohydride Substances 0.000 claims description 11
- 229910000033 sodium borohydride Inorganic materials 0.000 claims description 11
- 238000006243 chemical reaction Methods 0.000 claims description 9
- 230000001590 oxidative effect Effects 0.000 claims description 9
- 206010009944 Colon cancer Diseases 0.000 claims description 8
- 206010016654 Fibrosis Diseases 0.000 claims description 8
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 8
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 8
- 201000002528 pancreatic cancer Diseases 0.000 claims description 8
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 8
- 238000001356 surgical procedure Methods 0.000 claims description 8
- 208000023275 Autoimmune disease Diseases 0.000 claims description 7
- 108010008951 Chemokine CXCL12 Proteins 0.000 claims description 7
- 102000006573 Chemokine CXCL12 Human genes 0.000 claims description 7
- 206010033128 Ovarian cancer Diseases 0.000 claims description 7
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 7
- 208000027866 inflammatory disease Diseases 0.000 claims description 7
- 206010060862 Prostate cancer Diseases 0.000 claims description 6
- 201000001441 melanoma Diseases 0.000 claims description 6
- 229920001542 oligosaccharide Polymers 0.000 claims description 6
- 150000002482 oligosaccharides Chemical class 0.000 claims description 6
- MCHWWJLLPNDHGL-KVTDHHQDSA-N (2r,3s,4s,5r)-2,5-bis(hydroxymethyl)oxolane-3,4-diol Chemical group OC[C@H]1O[C@H](CO)[C@@H](O)[C@@H]1O MCHWWJLLPNDHGL-KVTDHHQDSA-N 0.000 claims description 5
- 230000004761 fibrosis Effects 0.000 claims description 5
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 4
- 230000033115 angiogenesis Effects 0.000 claims description 4
- 108010022901 Heparin Lyase Proteins 0.000 claims description 3
- 230000001404 mediated effect Effects 0.000 claims description 3
- 238000001959 radiotherapy Methods 0.000 claims description 3
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims description 2
- 238000007068 beta-elimination reaction Methods 0.000 claims description 2
- 208000029742 colonic neoplasm Diseases 0.000 claims description 2
- 208000015181 infectious disease Diseases 0.000 claims description 2
- 230000002401 inhibitory effect Effects 0.000 claims description 2
- 150000008273 hexosamines Chemical group 0.000 claims 3
- 125000000600 disaccharide group Chemical group 0.000 claims 2
- UXFQFBNBSPQBJW-UHFFFAOYSA-N 2-amino-2-methylpropane-1,3-diol Chemical compound OCC(N)(C)CO UXFQFBNBSPQBJW-UHFFFAOYSA-N 0.000 claims 1
- 208000003174 Brain Neoplasms Diseases 0.000 claims 1
- 208000035473 Communicable disease Diseases 0.000 claims 1
- 102000003800 Selectins Human genes 0.000 claims 1
- 108090000184 Selectins Proteins 0.000 claims 1
- 230000029087 digestion Effects 0.000 claims 1
- 230000032050 esterification Effects 0.000 claims 1
- 238000005886 esterification reaction Methods 0.000 claims 1
- AAEVYOVXGOFMJO-UHFFFAOYSA-N prometryn Chemical compound CSC1=NC(NC(C)C)=NC(NC(C)C)=N1 AAEVYOVXGOFMJO-UHFFFAOYSA-N 0.000 claims 1
- 241000699670 Mus sp. Species 0.000 description 55
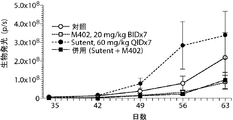
- 239000002147 L01XE04 - Sunitinib Substances 0.000 description 53
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 45
- 229960003668 docetaxel Drugs 0.000 description 45
- 210000001185 bone marrow Anatomy 0.000 description 41
- 229960001796 sunitinib Drugs 0.000 description 40
- WINHZLLDWRZWRT-ATVHPVEESA-N sunitinib Chemical compound CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C WINHZLLDWRZWRT-ATVHPVEESA-N 0.000 description 40
- 210000004027 cell Anatomy 0.000 description 39
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 38
- 229960002897 heparin Drugs 0.000 description 34
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 32
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 32
- 229960004316 cisplatin Drugs 0.000 description 32
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 32
- 239000003112 inhibitor Substances 0.000 description 31
- 239000011780 sodium chloride Substances 0.000 description 31
- 239000003795 chemical substances by application Substances 0.000 description 30
- 238000009792 diffusion process Methods 0.000 description 30
- 239000000243 solution Substances 0.000 description 24
- 229940127215 low-molecular weight heparin Drugs 0.000 description 23
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 23
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 22
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 22
- 229930012538 Paclitaxel Natural products 0.000 description 22
- 229960001592 paclitaxel Drugs 0.000 description 22
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 21
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 21
- 210000004072 lung Anatomy 0.000 description 21
- 229940123237 Taxane Drugs 0.000 description 20
- 230000008901 benefit Effects 0.000 description 17
- 238000002512 chemotherapy Methods 0.000 description 17
- 230000001394 metastastic effect Effects 0.000 description 17
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 16
- 229960002949 fluorouracil Drugs 0.000 description 16
- 229910052697 platinum Inorganic materials 0.000 description 16
- 239000004037 angiogenesis inhibitor Substances 0.000 description 15
- 229940121369 angiogenesis inhibitor Drugs 0.000 description 15
- 229940045799 anthracyclines and related substance Drugs 0.000 description 15
- 210000004369 blood Anatomy 0.000 description 15
- 239000008280 blood Substances 0.000 description 15
- 150000001875 compounds Chemical class 0.000 description 15
- 238000004519 manufacturing process Methods 0.000 description 15
- 229960004679 doxorubicin Drugs 0.000 description 14
- 239000003102 growth factor Substances 0.000 description 14
- 230000037361 pathway Effects 0.000 description 14
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 13
- -1 SDF-1-α Proteins 0.000 description 13
- 229940100198 alkylating agent Drugs 0.000 description 13
- 239000002168 alkylating agent Substances 0.000 description 13
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 13
- LBWFXVZLPYTWQI-IPOVEDGCSA-N n-[2-(diethylamino)ethyl]-5-[(z)-(5-fluoro-2-oxo-1h-indol-3-ylidene)methyl]-2,4-dimethyl-1h-pyrrole-3-carboxamide;(2s)-2-hydroxybutanedioic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O.CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C LBWFXVZLPYTWQI-IPOVEDGCSA-N 0.000 description 13
- 229940034785 sutent Drugs 0.000 description 13
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 12
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 12
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 12
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 12
- 229960004397 cyclophosphamide Drugs 0.000 description 12
- 229960003901 dacarbazine Drugs 0.000 description 12
- 229960000975 daunorubicin Drugs 0.000 description 12
- 238000009826 distribution Methods 0.000 description 12
- 239000003814 drug Substances 0.000 description 12
- 230000003511 endothelial effect Effects 0.000 description 12
- 229960001904 epirubicin Drugs 0.000 description 12
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 12
- 229960001101 ifosfamide Drugs 0.000 description 12
- 238000007920 subcutaneous administration Methods 0.000 description 12
- VEEGZPWAAPPXRB-BJMVGYQFSA-N (3e)-3-(1h-imidazol-5-ylmethylidene)-1h-indol-2-one Chemical compound O=C1NC2=CC=CC=C2\C1=C/C1=CN=CN1 VEEGZPWAAPPXRB-BJMVGYQFSA-N 0.000 description 11
- 229920002971 Heparan sulfate Polymers 0.000 description 11
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 11
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 11
- BPEGJWRSRHCHSN-UHFFFAOYSA-N Temozolomide Chemical compound O=C1N(C)N=NC2=C(C(N)=O)N=CN21 BPEGJWRSRHCHSN-UHFFFAOYSA-N 0.000 description 11
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 11
- 229960004562 carboplatin Drugs 0.000 description 11
- 238000002474 experimental method Methods 0.000 description 11
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 11
- 229960000908 idarubicin Drugs 0.000 description 11
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 11
- 229960001924 melphalan Drugs 0.000 description 11
- 229960001756 oxaliplatin Drugs 0.000 description 11
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 11
- 229960004964 temozolomide Drugs 0.000 description 11
- 229960000653 valrubicin Drugs 0.000 description 11
- ZOCKGBMQLCSHFP-KQRAQHLDSA-N valrubicin Chemical compound O([C@H]1C[C@](CC2=C(O)C=3C(=O)C4=CC=CC(OC)=C4C(=O)C=3C(O)=C21)(O)C(=O)COC(=O)CCCC)[C@H]1C[C@H](NC(=O)C(F)(F)F)[C@H](O)[C@H](C)O1 ZOCKGBMQLCSHFP-KQRAQHLDSA-N 0.000 description 11
- 229920002683 Glycosaminoglycan Polymers 0.000 description 10
- 241001465754 Metazoa Species 0.000 description 10
- 108010035766 P-Selectin Proteins 0.000 description 10
- 102100023472 P-selectin Human genes 0.000 description 10
- 238000002648 combination therapy Methods 0.000 description 10
- ATHLLZUXVPNPAW-UHFFFAOYSA-N lamellarin d Chemical compound C1=C(O)C(OC)=CC(C2=C3C4=CC(OC)=C(O)C=C4C=CN3C3=C2C=2C=C(OC)C(O)=CC=2OC3=O)=C1 ATHLLZUXVPNPAW-UHFFFAOYSA-N 0.000 description 10
- 230000009467 reduction Effects 0.000 description 10
- 238000009097 single-agent therapy Methods 0.000 description 10
- DKPFODGZWDEEBT-QFIAKTPHSA-N taxane Chemical class C([C@]1(C)CCC[C@@H](C)[C@H]1C1)C[C@H]2[C@H](C)CC[C@@H]1C2(C)C DKPFODGZWDEEBT-QFIAKTPHSA-N 0.000 description 10
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 9
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 9
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 9
- 241000699666 Mus <mouse, genus> Species 0.000 description 9
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 9
- 230000002001 anti-metastasis Effects 0.000 description 9
- 229960000397 bevacizumab Drugs 0.000 description 9
- 150000002016 disaccharides Chemical group 0.000 description 9
- 230000001225 therapeutic effect Effects 0.000 description 9
- VVIAGPKUTFNRDU-UHFFFAOYSA-N 6S-folinic acid Natural products C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-UHFFFAOYSA-N 0.000 description 8
- MLDQJTXFUGDVEO-UHFFFAOYSA-N BAY-43-9006 Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 MLDQJTXFUGDVEO-UHFFFAOYSA-N 0.000 description 8
- 201000009030 Carcinoma Diseases 0.000 description 8
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 8
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 8
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 8
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 8
- 239000005511 L01XE05 - Sorafenib Substances 0.000 description 8
- 208000006673 asthma Diseases 0.000 description 8
- VXNQMUVMEIGUJW-XNOMRPDFSA-L disodium;[2-methoxy-5-[(z)-2-(3,4,5-trimethoxyphenyl)ethenyl]phenyl] phosphate Chemical compound [Na+].[Na+].C1=C(OP([O-])([O-])=O)C(OC)=CC=C1\C=C/C1=CC(OC)=C(OC)C(OC)=C1 VXNQMUVMEIGUJW-XNOMRPDFSA-L 0.000 description 8
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 8
- 235000008191 folinic acid Nutrition 0.000 description 8
- 239000011672 folinic acid Substances 0.000 description 8
- VVIAGPKUTFNRDU-ABLWVSNPSA-N folinic acid Chemical compound C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-ABLWVSNPSA-N 0.000 description 8
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 8
- 238000001802 infusion Methods 0.000 description 8
- 238000002347 injection Methods 0.000 description 8
- 239000007924 injection Substances 0.000 description 8
- DXOJIXGRFSHVKA-BZVZGCBYSA-N larotaxel Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@@]23[C@H]1[C@@]1(CO[C@@H]1C[C@@H]2C3)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 DXOJIXGRFSHVKA-BZVZGCBYSA-N 0.000 description 8
- 229950005692 larotaxel Drugs 0.000 description 8
- 230000003902 lesion Effects 0.000 description 8
- 229960001691 leucovorin Drugs 0.000 description 8
- 239000002244 precipitate Substances 0.000 description 8
- 150000003230 pyrimidines Chemical class 0.000 description 8
- 208000024891 symptom Diseases 0.000 description 8
- 210000001519 tissue Anatomy 0.000 description 8
- 229960004528 vincristine Drugs 0.000 description 8
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 8
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 8
- MSWZFWKMSRAUBD-UHFFFAOYSA-N 2-Amino-2-Deoxy-Hexose Chemical group NC1C(O)OC(CO)C(O)C1O MSWZFWKMSRAUBD-UHFFFAOYSA-N 0.000 description 7
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 7
- 108010074860 Factor Xa Proteins 0.000 description 7
- 201000004681 Psoriasis Diseases 0.000 description 7
- 206010039491 Sarcoma Diseases 0.000 description 7
- 229940120638 avastin Drugs 0.000 description 7
- 229960004969 dalteparin Drugs 0.000 description 7
- 238000007912 intraperitoneal administration Methods 0.000 description 7
- 210000004185 liver Anatomy 0.000 description 7
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 7
- 229960001972 panitumumab Drugs 0.000 description 7
- 239000011541 reaction mixture Substances 0.000 description 7
- 208000037803 restenosis Diseases 0.000 description 7
- 206010039073 rheumatoid arthritis Diseases 0.000 description 7
- 210000002966 serum Anatomy 0.000 description 7
- 208000000587 small cell lung carcinoma Diseases 0.000 description 7
- 239000004066 vascular targeting agent Substances 0.000 description 7
- 239000003981 vehicle Substances 0.000 description 7
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 6
- 102000007644 Colony-Stimulating Factors Human genes 0.000 description 6
- 108010071942 Colony-Stimulating Factors Proteins 0.000 description 6
- ZBNZXTGUTAYRHI-UHFFFAOYSA-N Dasatinib Chemical compound C=1C(N2CCN(CCO)CC2)=NC(C)=NC=1NC(S1)=NC=C1C(=O)NC1=C(C)C=CC=C1Cl ZBNZXTGUTAYRHI-UHFFFAOYSA-N 0.000 description 6
- 102100024025 Heparanase Human genes 0.000 description 6
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 6
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 6
- 239000003798 L01XE11 - Pazopanib Substances 0.000 description 6
- 206010041067 Small cell lung cancer Diseases 0.000 description 6
- 238000003556 assay Methods 0.000 description 6
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 6
- 229960005420 etoposide Drugs 0.000 description 6
- 230000003176 fibrotic effect Effects 0.000 description 6
- 229960002584 gefitinib Drugs 0.000 description 6
- 108010037536 heparanase Proteins 0.000 description 6
- 229960004768 irinotecan Drugs 0.000 description 6
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 6
- 201000006417 multiple sclerosis Diseases 0.000 description 6
- 210000000056 organ Anatomy 0.000 description 6
- 238000007254 oxidation reaction Methods 0.000 description 6
- CUIHSIWYWATEQL-UHFFFAOYSA-N pazopanib Chemical compound C1=CC2=C(C)N(C)N=C2C=C1N(C)C(N=1)=CC=NC=1NC1=CC=C(C)C(S(N)(=O)=O)=C1 CUIHSIWYWATEQL-UHFFFAOYSA-N 0.000 description 6
- 229920000447 polyanionic polymer Polymers 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- 239000002243 precursor Substances 0.000 description 6
- 201000007282 progesterone-receptor negative breast cancer Diseases 0.000 description 6
- 230000007115 recruitment Effects 0.000 description 6
- 230000004083 survival effect Effects 0.000 description 6
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 6
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 6
- 229960000303 topotecan Drugs 0.000 description 6
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 102000004127 Cytokines Human genes 0.000 description 5
- 108090000695 Cytokines Proteins 0.000 description 5
- 102000001301 EGF receptor Human genes 0.000 description 5
- 108060006698 EGF receptor Proteins 0.000 description 5
- 239000002067 L01XE06 - Dasatinib Substances 0.000 description 5
- 239000002136 L01XE07 - Lapatinib Substances 0.000 description 5
- 108010038512 Platelet-Derived Growth Factor Proteins 0.000 description 5
- 102000010780 Platelet-Derived Growth Factor Human genes 0.000 description 5
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 5
- 108091008605 VEGF receptors Proteins 0.000 description 5
- 102000009484 Vascular Endothelial Growth Factor Receptors Human genes 0.000 description 5
- 238000011374 additional therapy Methods 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 230000027455 binding Effects 0.000 description 5
- BMQGVNUXMIRLCK-OAGWZNDDSA-N cabazitaxel Chemical compound O([C@H]1[C@@H]2[C@]3(OC(C)=O)CO[C@@H]3C[C@@H]([C@]2(C(=O)[C@H](OC)C2=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=3C=CC=CC=3)C[C@]1(O)C2(C)C)C)OC)C(=O)C1=CC=CC=C1 BMQGVNUXMIRLCK-OAGWZNDDSA-N 0.000 description 5
- 229960001573 cabazitaxel Drugs 0.000 description 5
- 208000019425 cirrhosis of liver Diseases 0.000 description 5
- 239000002254 cytotoxic agent Substances 0.000 description 5
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 5
- 229940079593 drug Drugs 0.000 description 5
- 239000003937 drug carrier Substances 0.000 description 5
- 229960001433 erlotinib Drugs 0.000 description 5
- 238000000684 flow cytometry Methods 0.000 description 5
- 229960002411 imatinib Drugs 0.000 description 5
- 238000001990 intravenous administration Methods 0.000 description 5
- 229940084651 iressa Drugs 0.000 description 5
- BCFGMOOMADDAQU-UHFFFAOYSA-N lapatinib Chemical compound O1C(CNCCS(=O)(=O)C)=CC=C1C1=CC=C(N=CN=C2NC=3C=C(Cl)C(OCC=4C=C(F)C=CC=4)=CC=3)C2=C1 BCFGMOOMADDAQU-UHFFFAOYSA-N 0.000 description 5
- 208000014018 liver neoplasm Diseases 0.000 description 5
- 208000037841 lung tumor Diseases 0.000 description 5
- 229960001156 mitoxantrone Drugs 0.000 description 5
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 5
- 230000003647 oxidation Effects 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- 230000000306 recurrent effect Effects 0.000 description 5
- 229960003787 sorafenib Drugs 0.000 description 5
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 5
- 229960001278 teniposide Drugs 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 229940124597 therapeutic agent Drugs 0.000 description 5
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 5
- 206010044412 transitional cell carcinoma Diseases 0.000 description 5
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical group C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 5
- 229940124676 vascular endothelial growth factor receptor Drugs 0.000 description 5
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 4
- XXJWYDDUDKYVKI-UHFFFAOYSA-N 4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-[3-(1-pyrrolidinyl)propoxy]quinazoline Chemical compound COC1=CC2=C(OC=3C(=C4C=C(C)NC4=CC=3)F)N=CN=C2C=C1OCCCN1CCCC1 XXJWYDDUDKYVKI-UHFFFAOYSA-N 0.000 description 4
- 201000001320 Atherosclerosis Diseases 0.000 description 4
- 208000012657 Atopic disease Diseases 0.000 description 4
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 4
- 208000024172 Cardiovascular disease Diseases 0.000 description 4
- 201000003883 Cystic fibrosis Diseases 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 4
- 208000017891 HER2 positive breast carcinoma Diseases 0.000 description 4
- 102000018710 Heparin-binding EGF-like Growth Factor Human genes 0.000 description 4
- 101800001649 Heparin-binding EGF-like growth factor Proteins 0.000 description 4
- 206010019695 Hepatic neoplasm Diseases 0.000 description 4
- 101000617130 Homo sapiens Stromal cell-derived factor 1 Proteins 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- 206010061309 Neoplasm progression Diseases 0.000 description 4
- 208000009905 Neurofibromatoses Diseases 0.000 description 4
- 208000035327 Oestrogen receptor positive breast cancer Diseases 0.000 description 4
- 206010038111 Recurrent cancer Diseases 0.000 description 4
- 206010039710 Scleroderma Diseases 0.000 description 4
- 102100021669 Stromal cell-derived factor 1 Human genes 0.000 description 4
- 208000003721 Triple Negative Breast Neoplasms Diseases 0.000 description 4
- 229940122803 Vinca alkaloid Drugs 0.000 description 4
- 150000001299 aldehydes Chemical group 0.000 description 4
- IAJILQKETJEXLJ-QTBDOELSSA-N aldehydo-D-glucuronic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-QTBDOELSSA-N 0.000 description 4
- 230000000340 anti-metabolite Effects 0.000 description 4
- 239000003146 anticoagulant agent Substances 0.000 description 4
- 229940100197 antimetabolite Drugs 0.000 description 4
- 239000002256 antimetabolite Substances 0.000 description 4
- 210000000601 blood cell Anatomy 0.000 description 4
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 4
- 229940127093 camptothecin Drugs 0.000 description 4
- 230000010261 cell growth Effects 0.000 description 4
- 229960005395 cetuximab Drugs 0.000 description 4
- 231100000599 cytotoxic agent Toxicity 0.000 description 4
- 229960002448 dasatinib Drugs 0.000 description 4
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 4
- 239000006185 dispersion Substances 0.000 description 4
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 4
- 239000002552 dosage form Substances 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 230000002255 enzymatic effect Effects 0.000 description 4
- 229940088598 enzyme Drugs 0.000 description 4
- 102000015694 estrogen receptors Human genes 0.000 description 4
- 108010038795 estrogen receptors Proteins 0.000 description 4
- 201000007280 estrogen-receptor negative breast cancer Diseases 0.000 description 4
- 201000007281 estrogen-receptor positive breast cancer Diseases 0.000 description 4
- 230000000893 fibroproliferative effect Effects 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- KANJSNBRCNMZMV-ABRZTLGGSA-N fondaparinux Chemical compound O[C@@H]1[C@@H](NS(O)(=O)=O)[C@@H](OC)O[C@H](COS(O)(=O)=O)[C@H]1O[C@H]1[C@H](OS(O)(=O)=O)[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](OS(O)(=O)=O)[C@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O[C@@H]4[C@@H]([C@@H](O)[C@H](O)[C@@H](COS(O)(=O)=O)O4)NS(O)(=O)=O)[C@H](O3)C(O)=O)O)[C@@H](COS(O)(=O)=O)O2)NS(O)(=O)=O)[C@H](C(O)=O)O1 KANJSNBRCNMZMV-ABRZTLGGSA-N 0.000 description 4
- 239000013022 formulation composition Substances 0.000 description 4
- 229940087051 fragmin Drugs 0.000 description 4
- 229940097043 glucuronic acid Drugs 0.000 description 4
- 238000011194 good manufacturing practice Methods 0.000 description 4
- 201000005787 hematologic cancer Diseases 0.000 description 4
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 4
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 4
- IAJILQKETJEXLJ-LECHCGJUSA-N iduronic acid Chemical compound O=C[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-LECHCGJUSA-N 0.000 description 4
- 238000003384 imaging method Methods 0.000 description 4
- 238000000338 in vitro Methods 0.000 description 4
- 230000003993 interaction Effects 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 201000010260 leiomyoma Diseases 0.000 description 4
- 208000032839 leukemia Diseases 0.000 description 4
- 208000002780 macular degeneration Diseases 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- 210000002901 mesenchymal stem cell Anatomy 0.000 description 4
- 201000004931 neurofibromatosis Diseases 0.000 description 4
- 230000003204 osmotic effect Effects 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- 229950011498 plinabulin Drugs 0.000 description 4
- UNRCMCRRFYFGFX-TYPNBTCFSA-N plinabulin Chemical compound N1C=NC(\C=C/2C(NC(=C\C=3C=CC=CC=3)/C(=O)N\2)=O)=C1C(C)(C)C UNRCMCRRFYFGFX-TYPNBTCFSA-N 0.000 description 4
- 239000013641 positive control Substances 0.000 description 4
- 201000007283 progesterone-receptor positive breast cancer Diseases 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 230000005855 radiation Effects 0.000 description 4
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 4
- JQWHASGSAFIOCM-UHFFFAOYSA-M sodium periodate Chemical compound [Na+].[O-]I(=O)(=O)=O JQWHASGSAFIOCM-UHFFFAOYSA-M 0.000 description 4
- 238000003756 stirring Methods 0.000 description 4
- 208000022679 triple-negative breast carcinoma Diseases 0.000 description 4
- 230000005751 tumor progression Effects 0.000 description 4
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 4
- XGOYIMQSIKSOBS-UHFFFAOYSA-N vadimezan Chemical compound C1=CC=C2C(=O)C3=CC=C(C)C(C)=C3OC2=C1CC(O)=O XGOYIMQSIKSOBS-UHFFFAOYSA-N 0.000 description 4
- LKAPTZKZHMOIRE-KVTDHHQDSA-N (2s,3s,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolane-2-carbaldehyde Chemical group OC[C@H]1O[C@H](C=O)[C@@H](O)[C@@H]1O LKAPTZKZHMOIRE-KVTDHHQDSA-N 0.000 description 3
- NDMPLJNOPCLANR-UHFFFAOYSA-N 3,4-dihydroxy-15-(4-hydroxy-18-methoxycarbonyl-5,18-seco-ibogamin-18-yl)-16-methoxy-1-methyl-6,7-didehydro-aspidospermidine-3-carboxylic acid methyl ester Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 NDMPLJNOPCLANR-UHFFFAOYSA-N 0.000 description 3
- HXHAJRMTJXHJJZ-UHFFFAOYSA-N 3-[(4-bromo-2,6-difluorophenyl)methoxy]-5-(4-pyrrolidin-1-ylbutylcarbamoylamino)-1,2-thiazole-4-carboxamide Chemical compound S1N=C(OCC=2C(=CC(Br)=CC=2F)F)C(C(=O)N)=C1NC(=O)NCCCCN1CCCC1 HXHAJRMTJXHJJZ-UHFFFAOYSA-N 0.000 description 3
- FJHBVJOVLFPMQE-QFIPXVFZSA-N 7-Ethyl-10-Hydroxy-Camptothecin Chemical compound C1=C(O)C=C2C(CC)=C(CN3C(C4=C([C@@](C(=O)OC4)(O)CC)C=C33)=O)C3=NC2=C1 FJHBVJOVLFPMQE-QFIPXVFZSA-N 0.000 description 3
- YXHLJMWYDTXDHS-IRFLANFNSA-N 7-aminoactinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=C(N)C=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 YXHLJMWYDTXDHS-IRFLANFNSA-N 0.000 description 3
- 108700012813 7-aminoactinomycin D Proteins 0.000 description 3
- 208000024827 Alzheimer disease Diseases 0.000 description 3
- 206010003827 Autoimmune hepatitis Diseases 0.000 description 3
- 208000023328 Basedow disease Diseases 0.000 description 3
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 3
- 206010055113 Breast cancer metastatic Diseases 0.000 description 3
- 206010009900 Colitis ulcerative Diseases 0.000 description 3
- 208000011231 Crohn disease Diseases 0.000 description 3
- 208000007342 Diabetic Nephropathies Diseases 0.000 description 3
- 206010012689 Diabetic retinopathy Diseases 0.000 description 3
- 238000002965 ELISA Methods 0.000 description 3
- 201000009273 Endometriosis Diseases 0.000 description 3
- 102000009024 Epidermal Growth Factor Human genes 0.000 description 3
- 101800003838 Epidermal growth factor Proteins 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 208000032612 Glial tumor Diseases 0.000 description 3
- 206010018338 Glioma Diseases 0.000 description 3
- 208000015023 Graves' disease Diseases 0.000 description 3
- 208000035895 Guillain-Barré syndrome Diseases 0.000 description 3
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 3
- 108090000031 Hedgehog Proteins Proteins 0.000 description 3
- 102000003693 Hedgehog Proteins Human genes 0.000 description 3
- 201000009794 Idiopathic Pulmonary Fibrosis Diseases 0.000 description 3
- 102000014150 Interferons Human genes 0.000 description 3
- 108010050904 Interferons Proteins 0.000 description 3
- 102000000588 Interleukin-2 Human genes 0.000 description 3
- 108010002350 Interleukin-2 Proteins 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- 239000005089 Luciferase Substances 0.000 description 3
- 206010050017 Lung cancer metastatic Diseases 0.000 description 3
- 108090000856 Lyases Proteins 0.000 description 3
- 102000004317 Lyases Human genes 0.000 description 3
- 208000001145 Metabolic Syndrome Diseases 0.000 description 3
- 206010049567 Miller Fisher syndrome Diseases 0.000 description 3
- IOVCWXUNBOPUCH-UHFFFAOYSA-M Nitrite anion Chemical compound [O-]N=O IOVCWXUNBOPUCH-UHFFFAOYSA-M 0.000 description 3
- 208000006265 Renal cell carcinoma Diseases 0.000 description 3
- 206010040070 Septic Shock Diseases 0.000 description 3
- 208000021386 Sjogren Syndrome Diseases 0.000 description 3
- 208000005718 Stomach Neoplasms Diseases 0.000 description 3
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 3
- 108090000190 Thrombin Proteins 0.000 description 3
- 201000006704 Ulcerative Colitis Diseases 0.000 description 3
- 206010047115 Vasculitis Diseases 0.000 description 3
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 3
- 208000000208 Wet Macular Degeneration Diseases 0.000 description 3
- GSOXMQLWUDQTNT-WAYWQWQTSA-N [3-methoxy-2-phosphonooxy-6-[(z)-2-(3,4,5-trimethoxyphenyl)ethenyl]phenyl] dihydrogen phosphate Chemical compound OP(=O)(O)OC1=C(OP(O)(O)=O)C(OC)=CC=C1\C=C/C1=CC(OC)=C(OC)C(OC)=C1 GSOXMQLWUDQTNT-WAYWQWQTSA-N 0.000 description 3
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 230000000996 additive effect Effects 0.000 description 3
- 206010064930 age-related macular degeneration Diseases 0.000 description 3
- 230000002491 angiogenic effect Effects 0.000 description 3
- 230000003110 anti-inflammatory effect Effects 0.000 description 3
- 230000000259 anti-tumor effect Effects 0.000 description 3
- 229940127219 anticoagulant drug Drugs 0.000 description 3
- 239000004019 antithrombin Substances 0.000 description 3
- 229910052796 boron Inorganic materials 0.000 description 3
- 238000012754 cardiac puncture Methods 0.000 description 3
- 229960002412 cediranib Drugs 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 230000007882 cirrhosis Effects 0.000 description 3
- 229940125782 compound 2 Drugs 0.000 description 3
- 208000033679 diabetic kidney disease Diseases 0.000 description 3
- 229940116977 epidermal growth factor Drugs 0.000 description 3
- 229930013356 epothilone Natural products 0.000 description 3
- 229940082789 erbitux Drugs 0.000 description 3
- 210000003743 erythrocyte Anatomy 0.000 description 3
- 206010017758 gastric cancer Diseases 0.000 description 3
- 229960005277 gemcitabine Drugs 0.000 description 3
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 3
- 229940080856 gleevec Drugs 0.000 description 3
- 208000005017 glioblastoma Diseases 0.000 description 3
- 150000002334 glycols Chemical class 0.000 description 3
- 210000003714 granulocyte Anatomy 0.000 description 3
- 201000010536 head and neck cancer Diseases 0.000 description 3
- 208000014829 head and neck neoplasm Diseases 0.000 description 3
- 201000011066 hemangioma Diseases 0.000 description 3
- 229960003685 imatinib mesylate Drugs 0.000 description 3
- YLMAHDNUQAMNNX-UHFFFAOYSA-N imatinib methanesulfonate Chemical compound CS(O)(=O)=O.C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 YLMAHDNUQAMNNX-UHFFFAOYSA-N 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 229940079322 interferon Drugs 0.000 description 3
- 208000036971 interstitial lung disease 2 Diseases 0.000 description 3
- 238000007918 intramuscular administration Methods 0.000 description 3
- 229960002014 ixabepilone Drugs 0.000 description 3
- FABUFPQFXZVHFB-CFWQTKTJSA-N ixabepilone Chemical compound C/C([C@@H]1C[C@@H]2O[C@]2(C)CCC[C@@H]([C@@H]([C@H](C)C(=O)C(C)(C)[C@H](O)CC(=O)N1)O)C)=C\C1=CSC(C)=N1 FABUFPQFXZVHFB-CFWQTKTJSA-N 0.000 description 3
- 229960004891 lapatinib Drugs 0.000 description 3
- 230000007774 longterm Effects 0.000 description 3
- 239000012139 lysis buffer Substances 0.000 description 3
- 229960000485 methotrexate Drugs 0.000 description 3
- 210000004088 microvessel Anatomy 0.000 description 3
- 238000012544 monitoring process Methods 0.000 description 3
- 206010028417 myasthenia gravis Diseases 0.000 description 3
- 210000000066 myeloid cell Anatomy 0.000 description 3
- 229940080607 nexavar Drugs 0.000 description 3
- HHZIURLSWUIHRB-UHFFFAOYSA-N nilotinib Chemical compound C1=NC(C)=CN1C1=CC(NC(=O)C=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)=CC(C(F)(F)F)=C1 HHZIURLSWUIHRB-UHFFFAOYSA-N 0.000 description 3
- 229950003600 ombrabulin Drugs 0.000 description 3
- IXWNTLSTOZFSCM-YVACAVLKSA-N ombrabulin Chemical compound C1=C(NC(=O)[C@@H](N)CO)C(OC)=CC=C1\C=C/C1=CC(OC)=C(OC)C(OC)=C1 IXWNTLSTOZFSCM-YVACAVLKSA-N 0.000 description 3
- 238000007911 parenteral administration Methods 0.000 description 3
- 229960000639 pazopanib Drugs 0.000 description 3
- 230000001766 physiological effect Effects 0.000 description 3
- 230000002980 postoperative effect Effects 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 201000000306 sarcoidosis Diseases 0.000 description 3
- 230000037390 scarring Effects 0.000 description 3
- 230000036303 septic shock Effects 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000007858 starting material Substances 0.000 description 3
- 238000007619 statistical method Methods 0.000 description 3
- 201000011549 stomach cancer Diseases 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 230000001629 suppression Effects 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 229940120982 tarceva Drugs 0.000 description 3
- 229960004072 thrombin Drugs 0.000 description 3
- 229950008737 vadimezan Drugs 0.000 description 3
- 229960000241 vandetanib Drugs 0.000 description 3
- UHTHHESEBZOYNR-UHFFFAOYSA-N vandetanib Chemical compound COC1=CC(C(/N=CN2)=N/C=3C(=CC(Br)=CC=3)F)=C2C=C1OCC1CCN(C)CC1 UHTHHESEBZOYNR-UHFFFAOYSA-N 0.000 description 3
- 229960003048 vinblastine Drugs 0.000 description 3
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 3
- 229960004355 vindesine Drugs 0.000 description 3
- UGGWPQSBPIFKDZ-KOTLKJBCSA-N vindesine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(N)=O)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1N=C1[C]2C=CC=C1 UGGWPQSBPIFKDZ-KOTLKJBCSA-N 0.000 description 3
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 3
- 229960002066 vinorelbine Drugs 0.000 description 3
- 229940069559 votrient Drugs 0.000 description 3
- 230000003442 weekly effect Effects 0.000 description 3
- SPMVMDHWKHCIDT-UHFFFAOYSA-N 1-[2-chloro-4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-3-(5-methyl-3-isoxazolyl)urea Chemical compound C=12C=C(OC)C(OC)=CC2=NC=CC=1OC(C=C1Cl)=CC=C1NC(=O)NC=1C=C(C)ON=1 SPMVMDHWKHCIDT-UHFFFAOYSA-N 0.000 description 2
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 2
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 2
- BFYIZQONLCFLEV-DAELLWKTSA-N Aromasine Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC(=C)C2=C1 BFYIZQONLCFLEV-DAELLWKTSA-N 0.000 description 2
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 2
- 0 C*=C[C@](C(CC*)CO)OC(CCN)CO Chemical compound C*=C[C@](C(CC*)CO)OC(CCN)CO 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 108010012236 Chemokines Proteins 0.000 description 2
- 102000019034 Chemokines Human genes 0.000 description 2
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 2
- 206010052358 Colorectal cancer metastatic Diseases 0.000 description 2
- 206010012438 Dermatitis atopic Diseases 0.000 description 2
- QXRSDHAAWVKZLJ-OXZHEXMSSA-N Epothilone B Natural products O=C1[C@H](C)[C@H](O)[C@@H](C)CCC[C@@]2(C)O[C@H]2C[C@@H](/C(=C\c2nc(C)sc2)/C)OC(=O)C[C@H](O)C1(C)C QXRSDHAAWVKZLJ-OXZHEXMSSA-N 0.000 description 2
- XOZIUKBZLSUILX-SDMHVBBESA-N Epothilone D Natural products O=C1[C@H](C)[C@@H](O)[C@@H](C)CCC/C(/C)=C/C[C@@H](/C(=C\c2nc(C)sc2)/C)OC(=O)C[C@H](O)C1(C)C XOZIUKBZLSUILX-SDMHVBBESA-N 0.000 description 2
- 102000003951 Erythropoietin Human genes 0.000 description 2
- 108090000394 Erythropoietin Proteins 0.000 description 2
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 2
- 102000009465 Growth Factor Receptors Human genes 0.000 description 2
- 108010009202 Growth Factor Receptors Proteins 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 208000007766 Kaposi sarcoma Diseases 0.000 description 2
- 108060001084 Luciferase Proteins 0.000 description 2
- 206010025323 Lymphomas Diseases 0.000 description 2
- 208000034578 Multiple myelomas Diseases 0.000 description 2
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 2
- 241000872931 Myoporum sandwicense Species 0.000 description 2
- 206010029260 Neuroblastoma Diseases 0.000 description 2
- 102000004264 Osteopontin Human genes 0.000 description 2
- 108010081689 Osteopontin Proteins 0.000 description 2
- 206010033296 Overdoses Diseases 0.000 description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 description 2
- 108010051742 Platelet-Derived Growth Factor beta Receptor Proteins 0.000 description 2
- 102100026547 Platelet-derived growth factor receptor beta Human genes 0.000 description 2
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 2
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 2
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 2
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 2
- 102100028286 Proto-oncogene tyrosine-protein kinase receptor Ret Human genes 0.000 description 2
- 102100033479 RAF proto-oncogene serine/threonine-protein kinase Human genes 0.000 description 2
- 101710141955 RAF proto-oncogene serine/threonine-protein kinase Proteins 0.000 description 2
- 206010039085 Rhinitis allergic Diseases 0.000 description 2
- 238000003639 Student–Newman–Keuls (SNK) method Methods 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 2
- 206010046798 Uterine leiomyoma Diseases 0.000 description 2
- 229940091171 VEGFR-2 tyrosine kinase inhibitor Drugs 0.000 description 2
- 229940127380 VEGFR1 Inhibitors Drugs 0.000 description 2
- 229940127432 VEGFR3 Inhibitors Drugs 0.000 description 2
- 241000021375 Xenogenes Species 0.000 description 2
- 210000003489 abdominal muscle Anatomy 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 210000000577 adipose tissue Anatomy 0.000 description 2
- 229940009456 adriamycin Drugs 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 201000010105 allergic rhinitis Diseases 0.000 description 2
- YBBLVLTVTVSKRW-UHFFFAOYSA-N anastrozole Chemical compound N#CC(C)(C)C1=CC(C(C)(C#N)C)=CC(CN2N=CN=C2)=C1 YBBLVLTVTVSKRW-UHFFFAOYSA-N 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 230000001772 anti-angiogenic effect Effects 0.000 description 2
- 230000003510 anti-fibrotic effect Effects 0.000 description 2
- 229940104697 arixtra Drugs 0.000 description 2
- 201000008937 atopic dermatitis Diseases 0.000 description 2
- 229960003005 axitinib Drugs 0.000 description 2
- RITAVMQDGBJQJZ-FMIVXFBMSA-N axitinib Chemical compound CNC(=O)C1=CC=CC=C1SC1=CC=C(C(\C=C\C=2N=CC=CC=2)=NN2)C2=C1 RITAVMQDGBJQJZ-FMIVXFBMSA-N 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000029918 bioluminescence Effects 0.000 description 2
- 238000005415 bioluminescence Methods 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 238000000423 cell based assay Methods 0.000 description 2
- 210000003169 central nervous system Anatomy 0.000 description 2
- 238000004182 chemical digestion Methods 0.000 description 2
- 239000012707 chemical precursor Substances 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 230000007012 clinical effect Effects 0.000 description 2
- 238000011260 co-administration Methods 0.000 description 2
- 230000015271 coagulation Effects 0.000 description 2
- 238000005345 coagulation Methods 0.000 description 2
- 239000000356 contaminant Substances 0.000 description 2
- SDZRWUKZFQQKKV-JHADDHBZSA-N cytochalasin D Chemical compound C([C@H]1[C@@H]2[C@@H](C([C@@H](O)[C@H]\3[C@]2([C@@H](/C=C/[C@@](C)(O)C(=O)[C@@H](C)C/C=C/3)OC(C)=O)C(=O)N1)=C)C)C1=CC=CC=C1 SDZRWUKZFQQKKV-JHADDHBZSA-N 0.000 description 2
- 230000000593 degrading effect Effects 0.000 description 2
- 239000008367 deionised water Substances 0.000 description 2
- 229910021641 deionized water Inorganic materials 0.000 description 2
- 230000001934 delay Effects 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- XOZIUKBZLSUILX-UHFFFAOYSA-N desoxyepothilone B Natural products O1C(=O)CC(O)C(C)(C)C(=O)C(C)C(O)C(C)CCCC(C)=CCC1C(C)=CC1=CSC(C)=N1 XOZIUKBZLSUILX-UHFFFAOYSA-N 0.000 description 2
- 239000000032 diagnostic agent Substances 0.000 description 2
- 229940039227 diagnostic agent Drugs 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 239000002612 dispersion medium Substances 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 238000010828 elution Methods 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 210000004696 endometrium Anatomy 0.000 description 2
- 229960000610 enoxaparin Drugs 0.000 description 2
- 230000006862 enzymatic digestion Effects 0.000 description 2
- HESCAJZNRMSMJG-KKQRBIROSA-N epothilone A Chemical class C/C([C@@H]1C[C@@H]2O[C@@H]2CCC[C@@H]([C@@H]([C@@H](C)C(=O)C(C)(C)[C@@H](O)CC(=O)O1)O)C)=C\C1=CSC(C)=N1 HESCAJZNRMSMJG-KKQRBIROSA-N 0.000 description 2
- HESCAJZNRMSMJG-HGYUPSKWSA-N epothilone A Natural products O=C1[C@H](C)[C@H](O)[C@H](C)CCC[C@H]2O[C@H]2C[C@@H](/C(=C\c2nc(C)sc2)/C)OC(=O)C[C@H](O)C1(C)C HESCAJZNRMSMJG-HGYUPSKWSA-N 0.000 description 2
- QXRSDHAAWVKZLJ-PVYNADRNSA-N epothilone B Chemical compound C/C([C@@H]1C[C@@H]2O[C@]2(C)CCC[C@@H]([C@@H]([C@@H](C)C(=O)C(C)(C)[C@@H](O)CC(=O)O1)O)C)=C\C1=CSC(C)=N1 QXRSDHAAWVKZLJ-PVYNADRNSA-N 0.000 description 2
- XOZIUKBZLSUILX-GIQCAXHBSA-N epothilone D Chemical compound O1C(=O)C[C@H](O)C(C)(C)C(=O)[C@H](C)[C@@H](O)[C@@H](C)CCC\C(C)=C/C[C@H]1C(\C)=C\C1=CSC(C)=N1 XOZIUKBZLSUILX-GIQCAXHBSA-N 0.000 description 2
- 229940105423 erythropoietin Drugs 0.000 description 2
- 208000024711 extrinsic asthma Diseases 0.000 description 2
- 238000000556 factor analysis Methods 0.000 description 2
- 210000002468 fat body Anatomy 0.000 description 2
- 238000002875 fluorescence polarization Methods 0.000 description 2
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- 229960001318 fondaparinux Drugs 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 229940095443 innohep Drugs 0.000 description 2
- 238000007913 intrathecal administration Methods 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- 208000028867 ischemia Diseases 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- HPJKCIUCZWXJDR-UHFFFAOYSA-N letrozole Chemical compound C1=CC(C#N)=CC=C1C(N1N=CN=C1)C1=CC=C(C#N)C=C1 HPJKCIUCZWXJDR-UHFFFAOYSA-N 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 229940118179 lovenox Drugs 0.000 description 2
- 229940076783 lucentis Drugs 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- 238000000569 multi-angle light scattering Methods 0.000 description 2
- 229960000899 nadroparin Drugs 0.000 description 2
- 239000013642 negative control Substances 0.000 description 2
- 238000001543 one-way ANOVA Methods 0.000 description 2
- 229960004762 parnaparin Drugs 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 210000003200 peritoneal cavity Anatomy 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 239000002953 phosphate buffered saline Substances 0.000 description 2
- OXCMYAYHXIHQOA-UHFFFAOYSA-N potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol Chemical compound [K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1 OXCMYAYHXIHQOA-UHFFFAOYSA-N 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- AQHHHDLHHXJYJD-UHFFFAOYSA-N propranolol Chemical compound C1=CC=C2C(OCC(O)CNC(C)C)=CC=CC2=C1 AQHHHDLHHXJYJD-UHFFFAOYSA-N 0.000 description 2
- 210000002307 prostate Anatomy 0.000 description 2
- 201000001514 prostate carcinoma Diseases 0.000 description 2
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 229960003876 ranibizumab Drugs 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 230000010410 reperfusion Effects 0.000 description 2
- 238000002271 resection Methods 0.000 description 2
- 238000007142 ring opening reaction Methods 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 235000010288 sodium nitrite Nutrition 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 230000002269 spontaneous effect Effects 0.000 description 2
- 238000010254 subcutaneous injection Methods 0.000 description 2
- 239000007929 subcutaneous injection Substances 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 239000011593 sulfur Substances 0.000 description 2
- 230000002195 synergetic effect Effects 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 230000001732 thrombotic effect Effects 0.000 description 2
- SYRHIZPPCHMRIT-UHFFFAOYSA-N tin(4+) Chemical compound [Sn+4] SYRHIZPPCHMRIT-UHFFFAOYSA-N 0.000 description 2
- 229960005062 tinzaparin Drugs 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 229960000575 trastuzumab Drugs 0.000 description 2
- 230000008733 trauma Effects 0.000 description 2
- 238000011277 treatment modality Methods 0.000 description 2
- 210000004881 tumor cell Anatomy 0.000 description 2
- 230000004614 tumor growth Effects 0.000 description 2
- 229940094060 tykerb Drugs 0.000 description 2
- 210000000689 upper leg Anatomy 0.000 description 2
- 210000003932 urinary bladder Anatomy 0.000 description 2
- 210000004291 uterus Anatomy 0.000 description 2
- 208000019553 vascular disease Diseases 0.000 description 2
- 239000002525 vasculotropin inhibitor Substances 0.000 description 2
- 210000003462 vein Anatomy 0.000 description 2
- 230000004580 weight loss Effects 0.000 description 2
- XAYAKDZVINDZGB-BMVMHAJPSA-N (4s,7r,8s,9s,10e,13z,16s)-4,8-dihydroxy-5,5,7,9,13-pentamethyl-16-[(e)-1-(2-methyl-1,3-thiazol-4-yl)prop-1-en-2-yl]-1-oxacyclohexadeca-10,13-diene-2,6-dione Chemical compound O1C(=O)C[C@H](O)C(C)(C)C(=O)[C@H](C)[C@@H](O)[C@@H](C)\C=C\C\C(C)=C/C[C@H]1C(\C)=C\C1=CSC(C)=N1 XAYAKDZVINDZGB-BMVMHAJPSA-N 0.000 description 1
- JRMGHBVACUJCRP-BTJKTKAUSA-N (z)-but-2-enedioic acid;4-[(4-fluoro-2-methyl-1h-indol-5-yl)oxy]-6-methoxy-7-(3-pyrrolidin-1-ylpropoxy)quinazoline Chemical compound OC(=O)\C=C/C(O)=O.COC1=CC2=C(OC=3C(=C4C=C(C)NC4=CC=3)F)N=CN=C2C=C1OCCCN1CCCC1 JRMGHBVACUJCRP-BTJKTKAUSA-N 0.000 description 1
- DDMOUSALMHHKOS-UHFFFAOYSA-N 1,2-dichloro-1,1,2,2-tetrafluoroethane Chemical compound FC(F)(Cl)C(F)(F)Cl DDMOUSALMHHKOS-UHFFFAOYSA-N 0.000 description 1
- VSNHCAURESNICA-NJFSPNSNSA-N 1-oxidanylurea Chemical compound N[14C](=O)NO VSNHCAURESNICA-NJFSPNSNSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- WEVYNIUIFUYDGI-UHFFFAOYSA-N 3-[6-[4-(trifluoromethoxy)anilino]-4-pyrimidinyl]benzamide Chemical compound NC(=O)C1=CC=CC(C=2N=CN=C(NC=3C=CC(OC(F)(F)F)=CC=3)C=2)=C1 WEVYNIUIFUYDGI-UHFFFAOYSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- HOZCHTFDGMSKNK-UHFFFAOYSA-N 6-methylspiro[4.5]dec-9-ene-10-carboxylic acid Chemical compound CC1CCC=C(C(O)=O)C11CCCC1 HOZCHTFDGMSKNK-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 208000030507 AIDS Diseases 0.000 description 1
- 108010012934 Albumin-Bound Paclitaxel Proteins 0.000 description 1
- 108091023037 Aptamer Proteins 0.000 description 1
- 229940122815 Aromatase inhibitor Drugs 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 102100030009 Azurocidin Human genes 0.000 description 1
- 101710154607 Azurocidin Proteins 0.000 description 1
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- 206010006002 Bone pain Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 1
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 1
- 102000008203 CTLA-4 Antigen Human genes 0.000 description 1
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 1
- 229940045513 CTLA4 antagonist Drugs 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 1
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 208000032544 Cicatrix Diseases 0.000 description 1
- 108050006400 Cyclin Proteins 0.000 description 1
- 102000016736 Cyclin Human genes 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 229940126190 DNA methyltransferase inhibitor Drugs 0.000 description 1
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 1
- 206010011906 Death Diseases 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 210000001956 EPC Anatomy 0.000 description 1
- MBYXEBXZARTUSS-QLWBXOBMSA-N Emetamine Natural products O(C)c1c(OC)cc2c(c(C[C@@H]3[C@H](CC)CN4[C@H](c5c(cc(OC)c(OC)c5)CC4)C3)ncc2)c1 MBYXEBXZARTUSS-QLWBXOBMSA-N 0.000 description 1
- 206010064212 Eosinophilic oesophagitis Diseases 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- HKVAMNSJSFKALM-GKUWKFKPSA-N Everolimus Chemical compound C1C[C@@H](OCCO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 HKVAMNSJSFKALM-GKUWKFKPSA-N 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 201000008808 Fibrosarcoma Diseases 0.000 description 1
- 238000011770 Fox Chase SCID mouse Methods 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 108010026389 Gramicidin Proteins 0.000 description 1
- 229940125497 HER2 kinase inhibitor Drugs 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- 108090000353 Histone deacetylase Proteins 0.000 description 1
- 102000003964 Histone deacetylase Human genes 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- 102100022338 Integrin alpha-M Human genes 0.000 description 1
- 208000002260 Keloid Diseases 0.000 description 1
- 206010023330 Keloid scar Diseases 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- 239000005536 L01XE08 - Nilotinib Substances 0.000 description 1
- 239000002118 L01XE12 - Vandetanib Substances 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 206010024291 Leukaemias acute myeloid Diseases 0.000 description 1
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 206010027458 Metastases to lung Diseases 0.000 description 1
- 206010063916 Metastatic gastric cancer Diseases 0.000 description 1
- 206010050513 Metastatic renal cell carcinoma Diseases 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 1
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 1
- 208000001132 Osteoporosis Diseases 0.000 description 1
- 208000018737 Parkinson disease Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 101100182935 Penicillium citrinum MSDC gene Proteins 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 229940079156 Proteasome inhibitor Drugs 0.000 description 1
- 206010056342 Pulmonary mass Diseases 0.000 description 1
- 239000012979 RPMI medium Substances 0.000 description 1
- 206010063837 Reperfusion injury Diseases 0.000 description 1
- 229940127395 Ribonucleotide Reductase Inhibitors Drugs 0.000 description 1
- 108010039491 Ricin Proteins 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 238000011579 SCID mouse model Methods 0.000 description 1
- AUVVAXYIELKVAI-UHFFFAOYSA-N SJ000285215 Natural products N1CCC2=CC(OC)=C(OC)C=C2C1CC1CC2C3=CC(OC)=C(OC)C=C3CCN2CC1CC AUVVAXYIELKVAI-UHFFFAOYSA-N 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 1
- BFZKMNSQCNVFGM-UCEYFQQTSA-N Sagopilone Chemical compound O1C(=O)C[C@H](O)C(C)(C)C(=O)[C@H](CC=C)[C@@H](O)[C@@H](C)CCC[C@@]2(C)O[C@H]2C[C@H]1C1=CC=C(SC(C)=N2)C2=C1 BFZKMNSQCNVFGM-UCEYFQQTSA-N 0.000 description 1
- 102100023085 Serine/threonine-protein kinase mTOR Human genes 0.000 description 1
- 108020004459 Small interfering RNA Proteins 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- 206010071051 Soft tissue mass Diseases 0.000 description 1
- 206010068771 Soft tissue neoplasm Diseases 0.000 description 1
- 208000021712 Soft tissue sarcoma Diseases 0.000 description 1
- 208000036765 Squamous cell carcinoma of the esophagus Diseases 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 108010065917 TOR Serine-Threonine Kinases Proteins 0.000 description 1
- CBPNZQVSJQDFBE-FUXHJELOSA-N Temsirolimus Chemical compound C1C[C@@H](OC(=O)C(C)(CO)CO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 CBPNZQVSJQDFBE-FUXHJELOSA-N 0.000 description 1
- 208000033781 Thyroid carcinoma Diseases 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 101710183280 Topoisomerase Proteins 0.000 description 1
- 102000009618 Transforming Growth Factors Human genes 0.000 description 1
- 108010009583 Transforming Growth Factors Proteins 0.000 description 1
- 206010064390 Tumour invasion Diseases 0.000 description 1
- 230000003187 abdominal effect Effects 0.000 description 1
- 229940028652 abraxane Drugs 0.000 description 1
- 238000005903 acid hydrolysis reaction Methods 0.000 description 1
- 238000010306 acid treatment Methods 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 208000009956 adenocarcinoma Diseases 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 125000003172 aldehyde group Chemical group 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 210000002255 anal canal Anatomy 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 229960002932 anastrozole Drugs 0.000 description 1
- 230000003527 anti-angiogenesis Effects 0.000 description 1
- 230000001093 anti-cancer Effects 0.000 description 1
- 230000002429 anti-coagulating effect Effects 0.000 description 1
- 230000002300 anti-fibrosis Effects 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 238000011319 anticancer therapy Methods 0.000 description 1
- 230000010100 anticoagulation Effects 0.000 description 1
- 229940045688 antineoplastic antimetabolites pyrimidine analogues Drugs 0.000 description 1
- 229960004676 antithrombotic agent Drugs 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 210000000576 arachnoid Anatomy 0.000 description 1
- 229940078010 arimidex Drugs 0.000 description 1
- 229940087620 aromasin Drugs 0.000 description 1
- 239000003886 aromatase inhibitor Substances 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 208000034158 bleeding Diseases 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 238000004159 blood analysis Methods 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- RSIHSRDYCUFFLA-DYKIIFRCSA-N boldenone Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 RSIHSRDYCUFFLA-DYKIIFRCSA-N 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 229960001467 bortezomib Drugs 0.000 description 1
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 201000008275 breast carcinoma Diseases 0.000 description 1
- 229940124630 bronchodilator Drugs 0.000 description 1
- 239000000168 bronchodilator agent Substances 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 230000005907 cancer growth Effects 0.000 description 1
- 230000009400 cancer invasion Effects 0.000 description 1
- 230000000711 cancerogenic effect Effects 0.000 description 1
- 229960004117 capecitabine Drugs 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 229960004424 carbon dioxide Drugs 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 231100000315 carcinogenic Toxicity 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 210000004720 cerebrum Anatomy 0.000 description 1
- 210000003679 cervix uteri Anatomy 0.000 description 1
- NDAYQJDHGXTBJL-MWWSRJDJSA-N chembl557217 Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC=3C4=CC=CC=C4NC=3)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC=3C4=CC=CC=C4NC=3)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC=3C4=CC=CC=C4NC=3)NC(=O)[C@@H](C(C)C)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](C(C)C)NC(=O)[C@H](C)NC(=O)[C@H](NC(=O)CNC(=O)[C@@H](NC=O)C(C)C)CC(C)C)C(=O)NCCO)=CNC2=C1 NDAYQJDHGXTBJL-MWWSRJDJSA-N 0.000 description 1
- 230000007073 chemical hydrolysis Effects 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 239000002576 chemokine receptor CXCR4 antagonist Substances 0.000 description 1
- LKAPTZKZHMOIRE-UHFFFAOYSA-N chitose Natural products OCC1OC(C=O)C(O)C1O LKAPTZKZHMOIRE-UHFFFAOYSA-N 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 238000011097 chromatography purification Methods 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 238000011278 co-treatment Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 230000005757 colony formation Effects 0.000 description 1
- 229940047120 colony stimulating factors Drugs 0.000 description 1
- 229940125904 compound 1 Drugs 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 210000004087 cornea Anatomy 0.000 description 1
- 229940121384 cxc chemokine receptor type 4 (cxcr4) antagonist Drugs 0.000 description 1
- 102000003675 cytokine receptors Human genes 0.000 description 1
- 108010057085 cytokine receptors Proteins 0.000 description 1
- 239000000824 cytostatic agent Substances 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- RSIHSRDYCUFFLA-UHFFFAOYSA-N dehydrotestosterone Natural products O=C1C=CC2(C)C3CCC(C)(C(CC4)O)C4C3CCC2=C1 RSIHSRDYCUFFLA-UHFFFAOYSA-N 0.000 description 1
- 239000003405 delayed action preparation Substances 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 1
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 1
- 229940042935 dichlorodifluoromethane Drugs 0.000 description 1
- 229940087091 dichlorotetrafluoroethane Drugs 0.000 description 1
- 210000002249 digestive system Anatomy 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000003968 dna methyltransferase inhibitor Substances 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- AUVVAXYIELKVAI-CKBKHPSWSA-N emetine Chemical compound N1CCC2=CC(OC)=C(OC)C=C2[C@H]1C[C@H]1C[C@H]2C3=CC(OC)=C(OC)C=C3CCN2C[C@@H]1CC AUVVAXYIELKVAI-CKBKHPSWSA-N 0.000 description 1
- 229960002694 emetine Drugs 0.000 description 1
- AUVVAXYIELKVAI-UWBTVBNJSA-N emetine Natural products N1CCC2=CC(OC)=C(OC)C=C2[C@H]1C[C@H]1C[C@H]2C3=CC(OC)=C(OC)C=C3CCN2C[C@H]1CC AUVVAXYIELKVAI-UWBTVBNJSA-N 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 201000000708 eosinophilic esophagitis Diseases 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 150000003883 epothilone derivatives Chemical class 0.000 description 1
- 208000007276 esophageal squamous cell carcinoma Diseases 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 description 1
- 229960005542 ethidium bromide Drugs 0.000 description 1
- 229960005167 everolimus Drugs 0.000 description 1
- 229960000255 exemestane Drugs 0.000 description 1
- 229940087476 femara Drugs 0.000 description 1
- 239000012894 fetal calf serum Substances 0.000 description 1
- 150000002224 folic acids Chemical class 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 238000007306 functionalization reaction Methods 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 238000003304 gavage Methods 0.000 description 1
- 238000001641 gel filtration chromatography Methods 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 230000014509 gene expression Effects 0.000 description 1
- 210000004392 genitalia Anatomy 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 150000002338 glycosides Chemical group 0.000 description 1
- 210000003128 head Anatomy 0.000 description 1
- 210000002216 heart Anatomy 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 229940121372 histone deacetylase inhibitor Drugs 0.000 description 1
- 239000003276 histone deacetylase inhibitor Substances 0.000 description 1
- 238000001794 hormone therapy Methods 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000003301 hydrolyzing effect Effects 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- 230000001969 hypertrophic effect Effects 0.000 description 1
- 229940127121 immunoconjugate Drugs 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 230000002584 immunomodulator Effects 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- 238000011503 in vivo imaging Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 238000002664 inhalation therapy Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 238000012482 interaction analysis Methods 0.000 description 1
- 239000000138 intercalating agent Substances 0.000 description 1
- 210000004347 intestinal mucosa Anatomy 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- PNDPGZBMCMUPRI-UHFFFAOYSA-N iodine Chemical compound II PNDPGZBMCMUPRI-UHFFFAOYSA-N 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 208000012947 ischemia reperfusion injury Diseases 0.000 description 1
- OWFXIOWLTKNBAP-UHFFFAOYSA-N isoamyl nitrite Chemical compound CC(C)CCON=O OWFXIOWLTKNBAP-UHFFFAOYSA-N 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 210000001117 keloid Anatomy 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 229960003881 letrozole Drugs 0.000 description 1
- 229940086263 leucovorin 25 mg Drugs 0.000 description 1
- 229960004194 lidocaine Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 210000005265 lung cell Anatomy 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 210000004324 lymphatic system Anatomy 0.000 description 1
- 229940124302 mTOR inhibitor Drugs 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 208000020984 malignant renal pelvis neoplasm Diseases 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 239000003628 mammalian target of rapamycin inhibitor Substances 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical class ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 102000006240 membrane receptors Human genes 0.000 description 1
- 108020004084 membrane receptors Proteins 0.000 description 1
- 208000010658 metastatic prostate carcinoma Diseases 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 231100000782 microtubule inhibitor Toxicity 0.000 description 1
- CFCUWKMKBJTWLW-BKHRDMLASA-N mithramycin Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1C)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](O[C@@H]2O[C@H](C)[C@H](O)[C@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@@](C)(O)C3)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@@H](O)[C@H](O)[C@@H](C)O1 CFCUWKMKBJTWLW-BKHRDMLASA-N 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- 210000003757 neuroblast Anatomy 0.000 description 1
- 210000004498 neuroglial cell Anatomy 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229960001346 nilotinib Drugs 0.000 description 1
- OSTGTTZJOCZWJG-UHFFFAOYSA-N nitrosourea Chemical compound NC(=O)N=NO OSTGTTZJOCZWJG-UHFFFAOYSA-N 0.000 description 1
- 231100000957 no side effect Toxicity 0.000 description 1
- 229940085033 nolvadex Drugs 0.000 description 1
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 1
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 1
- 239000003865 nucleic acid synthesis inhibitor Substances 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 150000003833 nucleoside derivatives Chemical class 0.000 description 1
- 238000003305 oral gavage Methods 0.000 description 1
- 210000004789 organ system Anatomy 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 238000007248 oxidative elimination reaction Methods 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- WVUNYSQLFKLYNI-AATRIKPKSA-N pelitinib Chemical compound C=12C=C(NC(=O)\C=C\CN(C)C)C(OCC)=CC2=NC=C(C#N)C=1NC1=CC=C(F)C(Cl)=C1 WVUNYSQLFKLYNI-AATRIKPKSA-N 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 210000003516 pericardium Anatomy 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 210000004303 peritoneum Anatomy 0.000 description 1
- 210000003800 pharynx Anatomy 0.000 description 1
- 210000004214 philadelphia chromosome Anatomy 0.000 description 1
- 229960003171 plicamycin Drugs 0.000 description 1
- 239000003495 polar organic solvent Substances 0.000 description 1
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000000186 progesterone Substances 0.000 description 1
- 229960003387 progesterone Drugs 0.000 description 1
- 102000003998 progesterone receptors Human genes 0.000 description 1
- 108090000468 progesterone receptors Proteins 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 229960003712 propranolol Drugs 0.000 description 1
- 239000003207 proteasome inhibitor Substances 0.000 description 1
- 238000001243 protein synthesis Methods 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 208000005069 pulmonary fibrosis Diseases 0.000 description 1
- 229950010131 puromycin Drugs 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 1
- 102000027426 receptor tyrosine kinases Human genes 0.000 description 1
- 108091008598 receptor tyrosine kinases Proteins 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 201000007444 renal pelvis carcinoma Diseases 0.000 description 1
- 229960004641 rituximab Drugs 0.000 description 1
- 229950008445 sagopilone Drugs 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 230000037387 scars Effects 0.000 description 1
- 239000008299 semisolid dosage form Substances 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 1
- 229960002930 sirolimus Drugs 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- IVDHYUQIDRJSTI-UHFFFAOYSA-N sorafenib tosylate Chemical compound [H+].CC1=CC=C(S([O-])(=O)=O)C=C1.C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 IVDHYUQIDRJSTI-UHFFFAOYSA-N 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 229940068117 sprycel Drugs 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000008174 sterile solution Substances 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 230000019635 sulfation Effects 0.000 description 1
- 238000005670 sulfation reaction Methods 0.000 description 1
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229940037128 systemic glucocorticoids Drugs 0.000 description 1
- 238000012353 t test Methods 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- 238000002626 targeted therapy Methods 0.000 description 1
- 229960000235 temsirolimus Drugs 0.000 description 1
- QFJCIRLUMZQUOT-UHFFFAOYSA-N temsirolimus Natural products C1CC(O)C(OC)CC1CC(C)C1OC(=O)C2CCCCN2C(=O)C(=O)C(O)(O2)C(C)CCC2CC(OC)C(C)=CC=CC=CC(C)CC(C)C(=O)C(OC)C(O)C(C)=CC(C)C(=O)C1 QFJCIRLUMZQUOT-UHFFFAOYSA-N 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- GKCBAIGFKIBETG-UHFFFAOYSA-N tetracaine Chemical compound CCCCNC1=CC=C(C(=O)OCCN(C)C)C=C1 GKCBAIGFKIBETG-UHFFFAOYSA-N 0.000 description 1
- 229960002372 tetracaine Drugs 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 210000000115 thoracic cavity Anatomy 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 210000001685 thyroid gland Anatomy 0.000 description 1
- 208000013077 thyroid gland carcinoma Diseases 0.000 description 1
- 208000019179 thyroid gland undifferentiated (anaplastic) carcinoma Diseases 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 229960005267 tositumomab Drugs 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 231100000167 toxic agent Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 239000003440 toxic substance Substances 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- AYNNSCRYTDRFCP-UHFFFAOYSA-N triazene Chemical compound NN=N AYNNSCRYTDRFCP-UHFFFAOYSA-N 0.000 description 1
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 1
- 229940029284 trichlorofluoromethane Drugs 0.000 description 1
- 210000003606 umbilical vein Anatomy 0.000 description 1
- 210000000626 ureter Anatomy 0.000 description 1
- 210000003708 urethra Anatomy 0.000 description 1
- 208000023747 urothelial carcinoma Diseases 0.000 description 1
- 210000003741 urothelium Anatomy 0.000 description 1
- 208000010579 uterine corpus leiomyoma Diseases 0.000 description 1
- 201000007954 uterine fibroid Diseases 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- WAEXFXRVDQXREF-UHFFFAOYSA-N vorinostat Chemical compound ONC(=O)CCCCCCC(=O)NC1=CC=CC=C1 WAEXFXRVDQXREF-UHFFFAOYSA-N 0.000 description 1
- 229960000237 vorinostat Drugs 0.000 description 1
- 238000003260 vortexing Methods 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08B—POLYSACCHARIDES; DERIVATIVES THEREOF
- C08B37/00—Preparation of polysaccharides not provided for in groups C08B1/00 - C08B35/00; Derivatives thereof
- C08B37/006—Heteroglycans, i.e. polysaccharides having more than one sugar residue in the main chain in either alternating or less regular sequence; Gellans; Succinoglycans; Arabinogalactans; Tragacanth or gum tragacanth or traganth from Astragalus; Gum Karaya from Sterculia urens; Gum Ghatti from Anogeissus latifolia; Derivatives thereof
- C08B37/0063—Glycosaminoglycans or mucopolysaccharides, e.g. keratan sulfate; Derivatives thereof, e.g. fucoidan
- C08B37/0075—Heparin; Heparan sulfate; Derivatives thereof, e.g. heparosan; Purification or extraction methods thereof
- C08B37/0078—Degradation products
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/715—Polysaccharides, i.e. having more than five saccharide radicals attached to each other by glycosidic linkages; Derivatives thereof, e.g. ethers, esters
- A61K31/726—Glycosaminoglycans, i.e. mucopolysaccharides
- A61K31/727—Heparin; Heparan
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/02—Nasal agents, e.g. decongestants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/04—Antipruritics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/04—Drugs for disorders of the muscular or neuromuscular system for myasthenia gravis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/14—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08B—POLYSACCHARIDES; DERIVATIVES THEREOF
- C08B37/00—Preparation of polysaccharides not provided for in groups C08B1/00 - C08B35/00; Derivatives thereof
- C08B37/006—Heteroglycans, i.e. polysaccharides having more than one sugar residue in the main chain in either alternating or less regular sequence; Gellans; Succinoglycans; Arabinogalactans; Tragacanth or gum tragacanth or traganth from Astragalus; Gum Karaya from Sterculia urens; Gum Ghatti from Anogeissus latifolia; Derivatives thereof
- C08B37/0063—Glycosaminoglycans or mucopolysaccharides, e.g. keratan sulfate; Derivatives thereof, e.g. fucoidan
- C08B37/0075—Heparin; Heparan sulfate; Derivatives thereof, e.g. heparosan; Purification or extraction methods thereof
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Molecular Biology (AREA)
- Diabetes (AREA)
- Biochemistry (AREA)
- Polymers & Plastics (AREA)
- Materials Engineering (AREA)
- Epidemiology (AREA)
- Dermatology (AREA)
- Neurology (AREA)
- Pulmonology (AREA)
- Biomedical Technology (AREA)
- Oncology (AREA)
- Neurosurgery (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Endocrinology (AREA)
- Immunology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Communicable Diseases (AREA)
- Rheumatology (AREA)
- Physical Education & Sports Medicine (AREA)
- Psychiatry (AREA)
- Urology & Nephrology (AREA)
- Hospice & Palliative Care (AREA)
- Pain & Pain Management (AREA)
- Ophthalmology & Optometry (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/762,268 | 2010-04-16 | ||
| US12/762,268 US8592393B2 (en) | 2007-11-02 | 2010-04-16 | Polysaccharide compositions and methods of use for the treatment and prevention of disorders associated with progenitor cell mobilization |
| PCT/US2011/032581 WO2011130572A1 (en) | 2010-04-16 | 2011-04-14 | Polysaccharide compositions and methods of use for the treatment and prevention of disorders associated with progenitor cell mobilization |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2013525306A true JP2013525306A (ja) | 2013-06-20 |
| JP2013525306A5 JP2013525306A5 (enExample) | 2014-05-29 |
Family
ID=44799388
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013505159A Pending JP2013525306A (ja) | 2010-04-16 | 2011-04-14 | 前駆細胞動員を伴う疾患の治療および予防のための多糖組成物および使用方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (3) | US8592393B2 (enExample) |
| EP (1) | EP2558124A4 (enExample) |
| JP (1) | JP2013525306A (enExample) |
| CN (1) | CN103096924A (enExample) |
| CA (1) | CA2796063A1 (enExample) |
| WO (1) | WO2011130572A1 (enExample) |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8569262B2 (en) | 2007-11-02 | 2013-10-29 | Momenta Pharmaceuticals, Inc. | Polysaccharide compositions and methods of use for the treatment and prevention of disorders associated with progenitor cell mobilization |
| ES2567079T3 (es) | 2007-11-02 | 2016-04-19 | Momenta Pharmaceuticals, Inc. | Composiciones de polisacáridos que no son anticoagulantes |
| US8592393B2 (en) | 2007-11-02 | 2013-11-26 | Momenta Pharmaceuticals, Inc. | Polysaccharide compositions and methods of use for the treatment and prevention of disorders associated with progenitor cell mobilization |
| CN102985443B (zh) | 2010-04-16 | 2017-05-10 | 动量制药公司 | 组织靶向 |
| WO2011159770A2 (en) | 2010-06-17 | 2011-12-22 | Momenta Pharmaceuticals, Inc. | Methods and compositions for modulating hair growth |
| US9351943B2 (en) * | 2010-07-01 | 2016-05-31 | Matthew T. McLeay | Anti-fibroblastic fluorochemical emulsion therapies |
| US20140351961A1 (en) * | 2011-08-31 | 2014-11-27 | Alexzander A. Asea | Compositions and methods for treatment of metastatic cancer |
| WO2013095215A1 (en) * | 2011-12-19 | 2013-06-27 | Dilaforette Ab | Low anticoagulant heparins |
| SMT201800143T1 (it) * | 2011-12-19 | 2018-05-02 | Dilafor Ab | Glicosaminoglicani non anticoagulanti comprendenti un'unita ripetitiva di disaccaridi e loro uso medico |
| CA2910837A1 (en) | 2013-05-28 | 2014-12-04 | Momenta Pharmaceuticals, Inc. | Pharmaceutical compositions |
| GB2515315A (en) | 2013-06-19 | 2014-12-24 | Dilafor Ab | New Processes |
| CA2975410A1 (en) * | 2015-04-07 | 2016-10-13 | Immunomedics, Inc. | Y-90-labeled anti-cd22 antibody (epratuzumab tetraxetan) in refractory/relapsed adult cd22+ b-cell acute lymphoblastic leukemia |
| US20190201556A1 (en) | 2016-05-16 | 2019-07-04 | Mtm Research, Llc | Fluorochemical targeted therapies |
| US10849995B2 (en) * | 2017-05-09 | 2020-12-01 | Crosby Innovations, LLC | Handheld sanitizing device |
| CA3104821A1 (en) | 2018-05-07 | 2019-11-14 | Mtm Research, Llc | Photodynamic compositions and methods of use |
| CN109248171A (zh) * | 2018-09-13 | 2019-01-22 | 黄泳华 | 含有伊马替尼与植物多糖的组合物 |
| CN109010354A (zh) * | 2018-09-15 | 2018-12-18 | 黄泳华 | 含有伊马替尼与植物多糖的组合物 |
| CN112949370A (zh) | 2019-12-10 | 2021-06-11 | 托比股份公司 | 眼睛事件检测 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009059283A1 (en) * | 2007-11-02 | 2009-05-07 | Momenta Pharmaceuticals, Inc. | Non-anticoagulant polysaccharide compositions |
Family Cites Families (73)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BE620906A (enExample) | ||||
| US3118816A (en) | 1962-04-03 | 1964-01-21 | Abbott Lab | N-succinyl heparin and process |
| US4235871A (en) | 1978-02-24 | 1980-11-25 | Papahadjopoulos Demetrios P | Method of encapsulating biologically active materials in lipid vesicles |
| CA1136620A (en) | 1979-01-08 | 1982-11-30 | Ulf P.F. Lindahl | Heparin fragments having selective anticoagulation activity |
| FR2538404B1 (enExample) | 1982-12-28 | 1985-08-23 | Anic Spa | |
| IT1195497B (it) | 1983-03-08 | 1988-10-19 | Opocrin Spa | Procedimento per la preparazione di frazioni oligosaccaridiche dotate di proprieta' farmacologiche per degradazione chimica di eparina |
| FR2553287B1 (fr) | 1983-10-18 | 1986-09-12 | Choay Sa | Compositions a base de mucopolysaccharides ou d'oligosaccharides, notamment a base de fractions ou fragments d'heparine, appropriees au traitement de desordres de la proliferation cellulaire |
| IT1169888B (it) | 1983-10-25 | 1987-06-03 | Italfarmaco Spa | Glicosaminoglicani modificati dotati di attivita' antitrombotica |
| US4847338A (en) | 1985-03-28 | 1989-07-11 | University Of Iowa Research Foundation | Low molecular weight heparin fragments as inhibitors of complement activation |
| US4916219A (en) | 1985-03-28 | 1990-04-10 | University Of Iowa Research Foundation | Oligosaccharide heparin fragments as inhibitors of complement cascade |
| US4868103A (en) | 1986-02-19 | 1989-09-19 | Enzo Biochem, Inc. | Analyte detection by means of energy transfer |
| US5262403A (en) | 1986-03-10 | 1993-11-16 | Board Of Regents, The University Of Texas System | Glycosaminoglycan derivatives and their use as inhibitors of tumor invasiveness of metastatic profusion-II |
| US5541166A (en) | 1987-01-23 | 1996-07-30 | The Australian National University | Sulphated polysaccharides having anti-metastatic and/or anti-inflammatory activity |
| US5668116A (en) | 1987-03-19 | 1997-09-16 | Anthropharm Pty. Limited | Anti-inflammatory compounds and compositions |
| FR2614026B1 (fr) | 1987-04-16 | 1992-04-17 | Sanofi Sa | Heparines de bas poids moleculaire, a structure reguliere, leur preparation et leurs applications biologiques |
| KR920700232A (ko) | 1988-06-03 | 1992-02-19 | 원본미기재 | 글리코사미노글리칸 염류, 그 제조방법 및 이를 함유하는 제약 조성물 |
| IL86753A0 (en) | 1988-06-15 | 1988-11-30 | Hadassah Med Org | Pharmaceutical compositions for treatment of lupus |
| DK191889D0 (da) | 1989-04-20 | 1989-04-20 | Bukh Meditec | Kosmetisk middel |
| SE9002550D0 (sv) | 1990-08-01 | 1990-08-01 | Kabivitrum Ab | Heparinfragment |
| IT1243987B (it) | 1990-10-29 | 1994-06-28 | Derivati Organici Lab | Procedimento per la preparazione di epossi-eparidi prodotti ottenuti ecomposizioni farmaceutiche che li contengono |
| US5250519A (en) | 1991-03-29 | 1993-10-05 | Glycomed Incorporated | Non-anticoagulant heparin derivatives |
| US5280016A (en) | 1991-03-29 | 1994-01-18 | Glycomed Incorporated | Non-anticoagulant heparin derivatives |
| SE9101155D0 (sv) | 1991-04-18 | 1991-04-18 | Kabi Pharmacia Ab | Novel heparin derivatives |
| IT1254216B (it) | 1992-02-25 | 1995-09-14 | Opocrin Spa | Derivati polisaccaridici di eparina, eparan solfato, loro frazioni e frammenti, procedimento per la loro preparazione e composizioni farmaceutiche che li contengono |
| US5668118A (en) | 1992-07-24 | 1997-09-16 | Cavalier Pharmaceuticals | Method of synthesis of 2-O-desulfated Heparin and use thereof for inhibition of elastase and Cathepspin G |
| US5296471A (en) | 1992-12-22 | 1994-03-22 | Glycomed Incorporated | Method for controlling o-desulfation of heparin and compositions produced thereby |
| US5696100A (en) | 1992-12-22 | 1997-12-09 | Glycomed Incorporated | Method for controlling O-desulfation of heparin and compositions produced thereby |
| PT690720E (pt) | 1993-03-12 | 2001-12-28 | Xoma Technology Ltd | Utilizacoes terapeuticas de produtos de proteina bactericida indutora de permeabilidade |
| IT1264709B1 (it) | 1993-07-12 | 1996-10-04 | Italfarmaco Spa | Derivati eparinici ad attivita' antimetastatica |
| US5583121A (en) | 1994-01-12 | 1996-12-10 | Michigan State University | Non-anticoagulant chemically modified heparinoids for treating hypovolemic shock and related shock syndromes |
| US6127347A (en) | 1994-01-12 | 2000-10-03 | Univ Michigan | Non-anticoagulant chemically modified heparinoids for treating hypovolemic shock and related shock syndromes |
| WO1995030424A1 (en) | 1994-05-06 | 1995-11-16 | Glycomed Incorporated | O-desulfated heparin derivatives, methods of making and uses thereof |
| EP1371666B1 (en) | 1994-07-01 | 2007-08-15 | Seikagaku Corporation | Use of desulfated heparin |
| US5733893A (en) | 1995-03-15 | 1998-03-31 | Washington University | Method of identifying molecules that regulate FGF activity |
| US6001820A (en) | 1995-03-31 | 1999-12-14 | Hamilton Civic Hospitals Research Development Inc. | Compositions and methods for inhibiting thrombogenesis |
| GB2299998B (en) * | 1995-03-31 | 1997-03-26 | Hamilton Civic Hospitals Res | Compositions for inhibiting thrombogenesis |
| US5744457A (en) | 1995-03-31 | 1998-04-28 | Hamilton Civic Hospitals Research Development Inc. | Compositions and methods for inhibiting thrombogenesis |
| US5690910A (en) | 1995-08-18 | 1997-11-25 | Baker Norton Pharmaceuticals, Inc. | Method for treating asthma |
| US5990097A (en) | 1996-07-29 | 1999-11-23 | Cavalier Pharmaceuticals | Methods of treating asthma with o-desulfated heparin |
| US5767269A (en) | 1996-10-01 | 1998-06-16 | Hamilton Civic Hospitals Research Development Inc. | Processes for the preparation of low-affinity, low molecular weight heparins useful as antithrombotics |
| AUPO573697A0 (en) | 1997-03-20 | 1997-04-10 | Prince Henry's Institute Of Medical Research | Diagnosis of endometrial cancer |
| US6596705B1 (en) | 1998-02-09 | 2003-07-22 | The Regents Of The University Of California | Inhibition of L-selectin and P-selection mediated binding using heparin |
| US7781416B2 (en) | 2000-01-25 | 2010-08-24 | Sigma-Tau Research Switzerland S.A. | Derivatives of partially desulphated glycosaminoglycans as heparanase inhibitors, endowed with antiangiogenic activity and devoid of anticoagulating effect |
| IT1316986B1 (it) | 2000-01-25 | 2003-05-26 | Sigma Tau Ind Farmaceuti | Derivati glicosamminoglicani parzialmente desolfatati nonanticoagulanti ad attivita' antiangiogenica. |
| CN1197587C (zh) | 2000-06-09 | 2005-04-20 | 中国科学院上海细胞生物学研究所 | N-位脱硫酸肝素在预防和治疗炎症中的应用 |
| US6514866B2 (en) | 2001-01-12 | 2003-02-04 | North Carolina State University | Chemically enhanced focused ion beam micro-machining of copper |
| BRPI0101486B1 (pt) | 2001-04-17 | 2017-09-19 | Cristália Produtos Químicos Farmacêuticos Ltda. | Pharmaceutical composition for topic use containing heparin for the treatment of skin or mucosal injuries caused by burns |
| CZ307433B6 (cs) | 2001-09-12 | 2018-08-22 | Leadiant Biosciences Sa | Deriváty částečně desulfátovaných glykosaminoglykanů jako inhibitory heparanázy mající antiangiogenní účinky a zabraňující antikoagulačnímu působení |
| WO2003078960A2 (en) | 2002-03-11 | 2003-09-25 | Momenta Pharmaceuticals, Inc. | Analysis of sulfated polysaccharides |
| JP2006501815A (ja) | 2002-04-25 | 2006-01-19 | モメンタ ファーマシューティカルズ インコーポレイテッド | 粘膜送達のための方法および製品 |
| US8071569B2 (en) | 2002-09-20 | 2011-12-06 | Mousa Shaker A | Oxidized heparin fractions and their use in inhibiting angiogenesis |
| US7964439B2 (en) | 2002-12-20 | 2011-06-21 | The Trustees Of Princeton University | Methods of fabricating devices by transfer of organic material |
| CA2525854C (en) | 2003-05-20 | 2009-12-15 | Genentech, Inc. | Acylsulfamide inhibitors of factor viia |
| WO2005032483A2 (en) | 2003-10-01 | 2005-04-14 | Momenta Pharmaceuticals, Inc. | Polysaccharides for pulmonary delivery of active agents |
| FR2866650B1 (fr) | 2004-02-24 | 2006-04-28 | Aventis Pharma Sa | Oligosaccharides, procede de preparation, utilisation et compositions pharmaceutiques les renfermant |
| US20050282775A1 (en) | 2004-06-16 | 2005-12-22 | Paringenix, Inc. | Method and medicament for sulfated polysaccharide treatment of inflammation without inducing platelet activation and heparin-induced thrombocytopenia syndrome |
| US20060040896A1 (en) | 2004-08-18 | 2006-02-23 | Paringenix, Inc. | Method and medicament for anticoagulation using a sulfated polysaccharide with enhanced anti-inflammatory activity |
| US7767420B2 (en) | 2005-11-03 | 2010-08-03 | Momenta Pharmaceuticals, Inc. | Heparan sulfate glycosaminoglycan lyase and uses thereof |
| WO2007059313A1 (en) | 2005-11-16 | 2007-05-24 | Children's Medical Center Corporation | Method to assess breast cancer risk |
| EP2447285A3 (en) | 2006-05-25 | 2013-01-16 | Momenta Pharmaceuticals, Inc. | Low molecular weight heparin composition and uses thereof |
| EP1867995A1 (en) | 2006-06-15 | 2007-12-19 | Cézanne S.A.S. | In vitro method for diagnosing and monitoring metastasized bladder cancer using the determination of MMP-7 in the circulation of patients |
| WO2007149938A2 (en) | 2006-06-21 | 2007-12-27 | The Salk Institute Biological Studies | Methods for promoting hair growth |
| JP4964577B2 (ja) | 2006-12-15 | 2012-07-04 | 有限会社応用糖質化学研究所 | 親脂質性ヘパリン修飾体、その製造方法及び毛髪成長促進剤 |
| FR2912409B1 (fr) | 2007-02-14 | 2012-08-24 | Sanofi Aventis | Heparines de bas poids moleculaire comprenant au moins une liaison covalente avec la biotine ou un derive de la biotine leur procede de preparation,leur utilisation |
| US8088753B2 (en) | 2007-06-29 | 2012-01-03 | Mediplex Corporation, Korea | Heparin conjugates and methods |
| US20090012165A1 (en) | 2007-07-03 | 2009-01-08 | Sucampo Ag | Pharmaceutical combination of nsaid and prostaglandin compound |
| WO2009007224A1 (en) | 2007-07-10 | 2009-01-15 | Sigma-Tau Industrie Farmaceutiche Riunite S.P.A. | Low molecular weight heparin derivatives having neuroprotective activity |
| US8569262B2 (en) | 2007-11-02 | 2013-10-29 | Momenta Pharmaceuticals, Inc. | Polysaccharide compositions and methods of use for the treatment and prevention of disorders associated with progenitor cell mobilization |
| US8592393B2 (en) | 2007-11-02 | 2013-11-26 | Momenta Pharmaceuticals, Inc. | Polysaccharide compositions and methods of use for the treatment and prevention of disorders associated with progenitor cell mobilization |
| WO2009105522A1 (en) | 2008-02-20 | 2009-08-27 | Momenta Pharmaceuticals, Inc. | Methods of making low molecular weight heparin compositions |
| CN102105133B (zh) | 2008-07-21 | 2015-06-17 | 奥德纳米有限公司 | 控制释放型耳结构调节和先天性免疫系统调节组合物以及治疗耳部病症的方法 |
| US20100331746A1 (en) | 2009-02-20 | 2010-12-30 | Aventis Pharma S.A. | Method of using enoxaparin for reducing the risk of death in acutely ill medical patients |
| US8435795B2 (en) | 2010-01-19 | 2013-05-07 | Momenta Pharmaceuticals, Inc. | Evaluating heparin preparations |
-
2010
- 2010-04-16 US US12/762,268 patent/US8592393B2/en active Active
-
2011
- 2011-04-14 CA CA2796063A patent/CA2796063A1/en not_active Abandoned
- 2011-04-14 CN CN2011800293971A patent/CN103096924A/zh active Pending
- 2011-04-14 JP JP2013505159A patent/JP2013525306A/ja active Pending
- 2011-04-14 WO PCT/US2011/032581 patent/WO2011130572A1/en not_active Ceased
- 2011-04-14 EP EP20110769624 patent/EP2558124A4/en not_active Withdrawn
-
2013
- 2013-09-27 US US14/039,801 patent/US9212233B2/en active Active
-
2015
- 2015-11-09 US US14/935,549 patent/US20160060366A1/en not_active Abandoned
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009059283A1 (en) * | 2007-11-02 | 2009-05-07 | Momenta Pharmaceuticals, Inc. | Non-anticoagulant polysaccharide compositions |
Non-Patent Citations (3)
| Title |
|---|
| JPN6015011247; Cancer Res Vol.70, No.8, Supp. SUPPL.1, 20100415, Abstract No.LB-43 * |
| JPN6015011248; Proceedings of the American Association for Cancer Research Annnal Meeting Vol.50, 2009, p.69 * |
| JPN6015011249; Proceedings of the American Association for Cancer Research Annnal Meeting Vol.50, 2009, p.1210 * |
Also Published As
| Publication number | Publication date |
|---|---|
| US20100324276A1 (en) | 2010-12-23 |
| US20140031315A1 (en) | 2014-01-30 |
| US9212233B2 (en) | 2015-12-15 |
| CN103096924A (zh) | 2013-05-08 |
| EP2558124A1 (en) | 2013-02-20 |
| CA2796063A1 (en) | 2011-10-20 |
| US8592393B2 (en) | 2013-11-26 |
| EP2558124A4 (en) | 2013-07-24 |
| WO2011130572A1 (en) | 2011-10-20 |
| US20160060366A1 (en) | 2016-03-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8569262B2 (en) | Polysaccharide compositions and methods of use for the treatment and prevention of disorders associated with progenitor cell mobilization | |
| US8592393B2 (en) | Polysaccharide compositions and methods of use for the treatment and prevention of disorders associated with progenitor cell mobilization | |
| JP5816342B2 (ja) | 非抗凝固性多糖組成物 | |
| EP2205642B1 (en) | Non-anticoagulant polysaccharide compositions | |
| EP3003324A1 (en) | Pharmaceutical compositions | |
| JP5721274B2 (ja) | 多糖組成物の活性を評価する方法 | |
| HK1146074B (en) | Non-anticoagulant polysaccharide compositions | |
| HK1051867B (en) | Derivatives of partially desulphated glycosaminoglycans endowed with antiangiogenic activity and devoid of anticoagulating effect | |
| HK1051867A1 (zh) | 具有抗血管生成活性且沒有抗凝作用的部分脫硫酸化糖胺聚糖的衍生物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140410 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20140410 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20150317 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150325 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20150623 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20151216 |