JP2013506304A - スーパーキャパシタ電極 - Google Patents
スーパーキャパシタ電極 Download PDFInfo
- Publication number
- JP2013506304A JP2013506304A JP2012531185A JP2012531185A JP2013506304A JP 2013506304 A JP2013506304 A JP 2013506304A JP 2012531185 A JP2012531185 A JP 2012531185A JP 2012531185 A JP2012531185 A JP 2012531185A JP 2013506304 A JP2013506304 A JP 2013506304A
- Authority
- JP
- Japan
- Prior art keywords
- electrode
- metal oxide
- electrolyte
- coated
- manganese dioxide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000003990 capacitor Substances 0.000 title description 9
- 229910044991 metal oxide Inorganic materials 0.000 claims abstract description 60
- 150000004706 metal oxides Chemical class 0.000 claims abstract description 60
- 238000004070 electrodeposition Methods 0.000 claims abstract description 43
- 238000000576 coating method Methods 0.000 claims abstract description 10
- 239000011248 coating agent Substances 0.000 claims abstract description 9
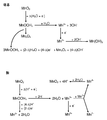
- NUJOXMJBOLGQSY-UHFFFAOYSA-N manganese dioxide Chemical group O=[Mn]=O NUJOXMJBOLGQSY-UHFFFAOYSA-N 0.000 claims description 176
- 239000003792 electrolyte Substances 0.000 claims description 92
- 238000000034 method Methods 0.000 claims description 75
- 239000011572 manganese Substances 0.000 claims description 67
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 64
- 229910052697 platinum Inorganic materials 0.000 claims description 32
- 238000000970 chrono-amperometry Methods 0.000 claims description 24
- 229910052751 metal Inorganic materials 0.000 claims description 22
- 239000002184 metal Substances 0.000 claims description 22
- 150000001768 cations Chemical class 0.000 claims description 19
- 239000002253 acid Substances 0.000 claims description 17
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 13
- 238000001075 voltammogram Methods 0.000 claims description 13
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 12
- 229910052799 carbon Inorganic materials 0.000 claims description 12
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 9
- 239000011521 glass Substances 0.000 claims description 9
- 229910052719 titanium Inorganic materials 0.000 claims description 9
- 239000010936 titanium Substances 0.000 claims description 9
- ZOMNIUBKTOKEHS-UHFFFAOYSA-L dimercury dichloride Chemical class Cl[Hg][Hg]Cl ZOMNIUBKTOKEHS-UHFFFAOYSA-L 0.000 claims description 8
- 239000000758 substrate Substances 0.000 claims description 8
- 238000009792 diffusion process Methods 0.000 claims description 7
- 238000001035 drying Methods 0.000 claims description 6
- 239000000243 solution Substances 0.000 claims description 6
- 229910001873 dinitrogen Inorganic materials 0.000 claims description 5
- 239000008151 electrolyte solution Substances 0.000 claims description 5
- 238000004146 energy storage Methods 0.000 claims description 5
- 229910052723 transition metal Inorganic materials 0.000 claims description 5
- 150000003624 transition metals Chemical class 0.000 claims description 5
- 229910052757 nitrogen Inorganic materials 0.000 claims description 4
- 238000004458 analytical method Methods 0.000 claims description 3
- 230000001143 conditioned effect Effects 0.000 claims description 3
- 230000001351 cycling effect Effects 0.000 claims description 3
- 238000007872 degassing Methods 0.000 claims description 3
- KJTLSVCANCCWHF-UHFFFAOYSA-N Ruthenium Chemical compound [Ru] KJTLSVCANCCWHF-UHFFFAOYSA-N 0.000 claims description 2
- 238000011067 equilibration Methods 0.000 claims description 2
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 claims description 2
- 229910052707 ruthenium Inorganic materials 0.000 claims description 2
- 238000005406 washing Methods 0.000 claims description 2
- 230000003750 conditioning effect Effects 0.000 claims 1
- 239000002244 precipitate Substances 0.000 abstract description 15
- 238000004519 manufacturing process Methods 0.000 abstract description 4
- 210000004027 cell Anatomy 0.000 description 30
- 239000000463 material Substances 0.000 description 24
- 230000008569 process Effects 0.000 description 18
- 239000013078 crystal Substances 0.000 description 15
- 230000002378 acidificating effect Effects 0.000 description 10
- 238000000151 deposition Methods 0.000 description 10
- AMWRITDGCCNYAT-UHFFFAOYSA-L hydroxy(oxo)manganese;manganese Chemical compound [Mn].O[Mn]=O.O[Mn]=O AMWRITDGCCNYAT-UHFFFAOYSA-L 0.000 description 10
- 230000007246 mechanism Effects 0.000 description 10
- 230000009467 reduction Effects 0.000 description 10
- 238000006722 reduction reaction Methods 0.000 description 10
- 230000008021 deposition Effects 0.000 description 9
- 238000002474 experimental method Methods 0.000 description 9
- 238000007254 oxidation reaction Methods 0.000 description 9
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 239000007772 electrode material Substances 0.000 description 8
- 229910052748 manganese Inorganic materials 0.000 description 8
- 230000003647 oxidation Effects 0.000 description 8
- 230000004913 activation Effects 0.000 description 7
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 7
- 239000001301 oxygen Substances 0.000 description 7
- 229910052760 oxygen Inorganic materials 0.000 description 7
- 230000037361 pathway Effects 0.000 description 7
- 239000011148 porous material Substances 0.000 description 7
- 241000894007 species Species 0.000 description 7
- 229910003174 MnOOH Inorganic materials 0.000 description 6
- 238000005063 solubilization Methods 0.000 description 6
- 230000007928 solubilization Effects 0.000 description 6
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 238000010586 diagram Methods 0.000 description 5
- 239000000446 fuel Substances 0.000 description 5
- 238000003780 insertion Methods 0.000 description 5
- 230000037431 insertion Effects 0.000 description 5
- 239000002245 particle Substances 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 4
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 4
- 238000004364 calculation method Methods 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 239000000543 intermediate Substances 0.000 description 4
- 150000002500 ions Chemical class 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 230000006911 nucleation Effects 0.000 description 4
- 238000010899 nucleation Methods 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- WOCIAKWEIIZHES-UHFFFAOYSA-N ruthenium(iv) oxide Chemical compound O=[Ru]=O WOCIAKWEIIZHES-UHFFFAOYSA-N 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- -1 supercapacitors) Substances 0.000 description 4
- 229910021642 ultra pure water Inorganic materials 0.000 description 4
- 239000012498 ultrapure water Substances 0.000 description 4
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 229920001940 conductive polymer Polymers 0.000 description 3
- 238000007323 disproportionation reaction Methods 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- 230000007062 hydrolysis Effects 0.000 description 3
- 238000006460 hydrolysis reaction Methods 0.000 description 3
- 229910021645 metal ion Inorganic materials 0.000 description 3
- 238000001556 precipitation Methods 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 238000006479 redox reaction Methods 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000010409 thin film Substances 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- 239000004593 Epoxy Substances 0.000 description 2
- 239000012901 Milli-Q water Substances 0.000 description 2
- 239000011149 active material Substances 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 239000004744 fabric Substances 0.000 description 2
- 239000002803 fossil fuel Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 239000005457 ice water Substances 0.000 description 2
- 238000004502 linear sweep voltammetry Methods 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical class C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 238000002161 passivation Methods 0.000 description 2
- 230000010287 polarization Effects 0.000 description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- NDVLTYZPCACLMA-UHFFFAOYSA-N silver oxide Chemical compound [O-2].[Ag+].[Ag+] NDVLTYZPCACLMA-UHFFFAOYSA-N 0.000 description 2
- 125000006850 spacer group Chemical group 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 239000002028 Biomass Substances 0.000 description 1
- MBMLMWLHJBBADN-UHFFFAOYSA-N Ferrous sulfide Chemical compound [Fe]=S MBMLMWLHJBBADN-UHFFFAOYSA-N 0.000 description 1
- GOPYZMJAIPBUGX-UHFFFAOYSA-N [O-2].[O-2].[Mn+4] Chemical group [O-2].[O-2].[Mn+4] GOPYZMJAIPBUGX-UHFFFAOYSA-N 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 229910001413 alkali metal ion Inorganic materials 0.000 description 1
- 229910001420 alkaline earth metal ion Inorganic materials 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 244000309464 bull Species 0.000 description 1
- 239000003575 carbonaceous material Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 239000002800 charge carrier Substances 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000000536 complexating effect Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000002659 electrodeposit Substances 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 239000005431 greenhouse gas Substances 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 239000012212 insulator Substances 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229910001437 manganese ion Inorganic materials 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- 239000002086 nanomaterial Substances 0.000 description 1
- VIKNJXKGJWUCNN-XGXHKTLJSA-N norethisterone Chemical compound O=C1CC[C@@H]2[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=C1 VIKNJXKGJWUCNN-XGXHKTLJSA-N 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000008521 reorganization Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 229910001923 silver oxide Inorganic materials 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000012916 structural analysis Methods 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 239000003115 supporting electrolyte Substances 0.000 description 1
- 238000010408 sweeping Methods 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 230000033772 system development Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 125000005207 tetraalkylammonium group Chemical group 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 238000004832 voltammetry Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D9/00—Electrolytic coating other than with metals
- C25D9/04—Electrolytic coating other than with metals with inorganic materials
- C25D9/06—Electrolytic coating other than with metals with inorganic materials by anodic processes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/22—Electrodes
- H01G11/26—Electrodes characterised by their structure, e.g. multi-layered, porosity or surface features
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/22—Electrodes
- H01G11/30—Electrodes characterised by their material
- H01G11/32—Carbon-based
- H01G11/42—Powders or particles, e.g. composition thereof
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/22—Electrodes
- H01G11/30—Electrodes characterised by their material
- H01G11/46—Metal oxides
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G9/00—Electrolytic capacitors, rectifiers, detectors, switching devices, light-sensitive or temperature-sensitive devices; Processes of their manufacture
- H01G9/0029—Processes of manufacture
- H01G9/0032—Processes of manufacture formation of the dielectric layer
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/13—Energy storage using capacitors
Landscapes
- Engineering & Computer Science (AREA)
- Power Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Materials Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Inorganic Chemistry (AREA)
- Electrochemistry (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Electric Double-Layer Capacitors Or The Like (AREA)
- Battery Electrode And Active Subsutance (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2009904798A AU2009904798A0 (en) | 2009-10-02 | Supercapacitor electrodes | |
| AU2009904798 | 2009-10-02 | ||
| PCT/AU2010/001288 WO2011038463A1 (en) | 2009-10-02 | 2010-09-30 | Supercapacitor electrodes |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2013506304A true JP2013506304A (ja) | 2013-02-21 |
| JP2013506304A5 JP2013506304A5 (enExample) | 2013-11-14 |
Family
ID=43825441
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012531185A Pending JP2013506304A (ja) | 2009-10-02 | 2010-09-30 | スーパーキャパシタ電極 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20120200308A1 (enExample) |
| EP (1) | EP2483453A1 (enExample) |
| JP (1) | JP2013506304A (enExample) |
| CN (1) | CN102639756B (enExample) |
| AU (1) | AU2010302957A1 (enExample) |
| CA (1) | CA2776388A1 (enExample) |
| WO (1) | WO2011038463A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2024102831A (ja) * | 2023-01-19 | 2024-07-31 | 台達電子企業管理(上海)有限公司 | 電源装置、その制御方法及び電源システム |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012087409A2 (en) | 2010-10-12 | 2012-06-28 | The Regents Of The University Of Michigan | High performance transition metal carbide and nitride and boride based asymmetric supercapacitors |
| US8780527B2 (en) * | 2010-10-12 | 2014-07-15 | The Regents Of The University Of Michigan | Transition metal carbide or nitride or boride based supercapacitors with metal foam electrode substrate |
| KR101389826B1 (ko) | 2012-07-23 | 2014-05-07 | 한밭대학교 산학협력단 | 고분자겔 전해질과 금속산화물 전극을 포함하는 수퍼캐패시터 |
| CN107889502A (zh) | 2015-03-31 | 2018-04-06 | 柯碧恩生物技术公司 | 适应于异养培养条件的微藻 |
| CN105244187A (zh) * | 2015-10-19 | 2016-01-13 | 济南大学 | 一种“锰氧化物烯”MnxOy薄膜电极材料的制备方法 |
| KR102635455B1 (ko) | 2016-05-20 | 2024-02-13 | 교세라 에이브이엑스 컴포넌츠 코포레이션 | 고온용 울트라커패시터 |
| EP3459097A4 (en) | 2016-05-20 | 2020-05-06 | AVX Corporation | NON-AQUEOUS ELECTROLYTE FOR SUPERCAPACITOR |
| KR20190000388A (ko) | 2016-05-20 | 2019-01-02 | 에이브이엑스 코포레이션 | 커패시터 충전 시스템 및 방법 |
| CN109155204B (zh) | 2016-05-20 | 2020-12-22 | 阿维科斯公司 | 用于超级电容器的电极构造体 |
| CN107937966B (zh) * | 2017-10-30 | 2020-01-07 | 燕山大学 | 一种铁基氢氧化物赝电容薄膜材料的原位制备方法 |
| CN108231432B (zh) * | 2017-12-29 | 2019-12-13 | 武汉艾特米克超能新材料科技有限公司 | 一种改善超级电容器自放电的方法 |
| CN113098321B (zh) * | 2021-03-24 | 2022-09-20 | 华中科技大学 | 一种纤维工作电极及海洋能量回收装置 |
| US12002631B2 (en) * | 2021-10-20 | 2024-06-04 | KYOCERA AVX Components Corporation | Electrodeposited dielectric for a solid electrolytic capacitor |
| CN114093684B (zh) * | 2021-11-19 | 2023-04-07 | 沈阳农业大学 | 一种β-Ni(OH)2/β-NiOOH聚苯胺复合电极及其制备方法和应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH02168559A (ja) * | 1988-12-21 | 1990-06-28 | Mitsui Mining & Smelting Co Ltd | リチウム一次電池およびその陽極活物質 |
| JPH0362456A (ja) * | 1989-07-28 | 1991-03-18 | Yuasa Battery Co Ltd | 電池 |
| JP2006076865A (ja) * | 2004-09-13 | 2006-03-23 | Yamaguchi Univ | 層状マンガン酸化物の製造方法 |
| JP2010103051A (ja) * | 2008-10-27 | 2010-05-06 | Nissan Motor Co Ltd | 蓄電デバイス用複合電極、その製造方法及び蓄電デバイス |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB977569A (en) * | 1961-10-05 | 1964-12-09 | Union Carbide Corp | Improvements in and relating to the electrolytic production of manganese dioxide |
| US3841978A (en) * | 1972-12-11 | 1974-10-15 | Kerr Mc Gee Chem Corp | Method of treating a titanium anode |
| US4295943A (en) * | 1980-04-17 | 1981-10-20 | Diamond Shamrock Technologies S.A. | Process for the electrolytic production of manganese dioxide |
| US7501208B2 (en) * | 2001-06-01 | 2009-03-10 | Eveready Battery Company, Inc. | Doped manganese dioxides |
| GR20030100208A (el) * | 2002-05-15 | 2004-02-02 | Mitsui Mining & Smelting Co., Ltd. | Ενεργο υλικο καθοδου συσσωρευτη, μεθοδος παραγωγης του και μπαταρια που το χρησιμοποιει |
| JP2004325441A (ja) * | 2003-04-25 | 2004-11-18 | Rohm & Haas Electronic Materials Llc | 分析方法 |
| CN1958856B (zh) * | 2006-10-13 | 2010-07-14 | 华东理工大学 | 一种用于有机电化学合成过程的纳米碳纤维电催化电极的制备 |
| US8435676B2 (en) * | 2008-01-09 | 2013-05-07 | Nanotek Instruments, Inc. | Mixed nano-filament electrode materials for lithium ion batteries |
| CN101286418A (zh) * | 2008-04-30 | 2008-10-15 | 清华大学深圳研究生院 | 一种二氧化锰电化学超级电容器 |
| CN101503805B (zh) * | 2009-01-24 | 2010-10-27 | 燕山大学 | 超级电容器和电池的复合正极材料的制备方法 |
-
2010
- 2010-09-30 CA CA2776388A patent/CA2776388A1/en not_active Abandoned
- 2010-09-30 JP JP2012531185A patent/JP2013506304A/ja active Pending
- 2010-09-30 AU AU2010302957A patent/AU2010302957A1/en not_active Abandoned
- 2010-09-30 US US13/499,910 patent/US20120200308A1/en not_active Abandoned
- 2010-09-30 CN CN201080054872.6A patent/CN102639756B/zh not_active Expired - Fee Related
- 2010-09-30 EP EP10819748A patent/EP2483453A1/en not_active Withdrawn
- 2010-09-30 WO PCT/AU2010/001288 patent/WO2011038463A1/en not_active Ceased
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH02168559A (ja) * | 1988-12-21 | 1990-06-28 | Mitsui Mining & Smelting Co Ltd | リチウム一次電池およびその陽極活物質 |
| JPH0362456A (ja) * | 1989-07-28 | 1991-03-18 | Yuasa Battery Co Ltd | 電池 |
| JP2006076865A (ja) * | 2004-09-13 | 2006-03-23 | Yamaguchi Univ | 層状マンガン酸化物の製造方法 |
| JP2010103051A (ja) * | 2008-10-27 | 2010-05-06 | Nissan Motor Co Ltd | 蓄電デバイス用複合電極、その製造方法及び蓄電デバイス |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2024102831A (ja) * | 2023-01-19 | 2024-07-31 | 台達電子企業管理(上海)有限公司 | 電源装置、その制御方法及び電源システム |
| JP7715851B2 (ja) | 2023-01-19 | 2025-07-30 | 台達電子企業管理(上海)有限公司 | 電源装置、その制御方法及び電源システム |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2776388A1 (en) | 2011-04-07 |
| CN102639756B (zh) | 2015-03-11 |
| US20120200308A1 (en) | 2012-08-09 |
| WO2011038463A1 (en) | 2011-04-07 |
| EP2483453A1 (en) | 2012-08-08 |
| AU2010302957A1 (en) | 2012-05-03 |
| CN102639756A (zh) | 2012-08-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2013506304A (ja) | スーパーキャパシタ電極 | |
| Cross et al. | Enhanced manganese dioxide supercapacitor electrodes produced by electrodeposition | |
| Islam et al. | Recent advancements in electrochemical deposition of metal‐based electrode materials for electrochemical supercapacitors | |
| Prasad et al. | Electrochemical synthesis and characterization of nanostructured tin oxide for electrochemical redox supercapacitors | |
| Srinivasan et al. | Studies on the capacitance of nickel oxide films: effect of heating temperature and electrolyte concentration | |
| Hu et al. | Cyclic voltammetric deposition of hydrous ruthenium oxide for electrochemical capacitors | |
| Gujar et al. | Electrochemically deposited nanograin ruthenium oxide as a pseudocapacitive electrode | |
| Kandalkar et al. | Preparation of cobalt oxide thin films and its use in supercapacitor application | |
| Prasad et al. | Electrochemically synthesized MnO2-based mixed oxides for high performance redox supercapacitors | |
| Khomenko et al. | Optimisation of an asymmetric manganese oxide/activated carbon capacitor working at 2 V in aqueous medium | |
| KR101749160B1 (ko) | 프렉서블한 3차원 꽃-형상의 NiCo2O4 나노구조가 증착된 3차원-니켈/니켈- 와이어 전류 집전체 제조방법 | |
| Kumbhar et al. | Supercapacitive evaluation of soft chemically deposited α-Sm2S3 thin films | |
| CN103361698A (zh) | 一种用共电沉积法制备超级电容器电极材料的方法 | |
| Wen et al. | Pseudocapacitance characterization of hydrous ruthenium oxide prepared via cyclic voltammetric deposition | |
| JP5207104B2 (ja) | 電極及びその製造方法、並びに色素増感型太陽電池 | |
| Chakraborty et al. | Structural influence of the anode materials towards efficient Zn deposition/dissolution in aqueous Zn-Iodide flow batteries | |
| Wang et al. | A sustainable light-chargeable two-electrode energy storage system based on aqueous sodium-ion photo-intercalation | |
| Kalkan et al. | Mn-Ni-based coating on flexible graphite fiber with high length capacitance for flexible supercapacitor applications | |
| Hegde | Effect of surfactant on high capacitance of galvanostatically deposited MnO2 | |
| CN105280397A (zh) | 一种水系电解液与超级电容器 | |
| Semente et al. | Electrochemical growth optimization of IrOx films on stainless steel as electrodes for high performance supercapacitors | |
| US9988732B2 (en) | Manufacturing method of titanium oxide electrode, active oxygen species production system including same, chlorine production system, dye-sensitised solar cell and electric double-layer capacitor | |
| CN104299787B (zh) | 采用循环伏安法在石墨基体上电沉积MnO2工艺方法 | |
| Joshi et al. | Mn doped ruthenium oxide permeable structures and their electrochemical properties | |
| Yu et al. | Electrochemical deposited nanoflakes Co (OH) 2 porous films for electrochemical capacitors |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130930 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130930 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20140528 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140624 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140917 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140925 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20141121 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20141201 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20141219 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150630 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20150930 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20160301 |