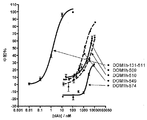
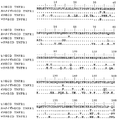
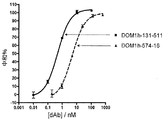
JP2012517818A - 改善された抗tnfr1ポリペプチド、抗体可変ドメインおよびアンタゴニスト - Google Patents
改善された抗tnfr1ポリペプチド、抗体可変ドメインおよびアンタゴニスト Download PDFInfo
- Publication number
- JP2012517818A JP2012517818A JP2011550552A JP2011550552A JP2012517818A JP 2012517818 A JP2012517818 A JP 2012517818A JP 2011550552 A JP2011550552 A JP 2011550552A JP 2011550552 A JP2011550552 A JP 2011550552A JP 2012517818 A JP2012517818 A JP 2012517818A
- Authority
- JP
- Japan
- Prior art keywords
- dom1h
- seq
- tnfr1
- variable domain
- single variable
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000005557 antagonist Substances 0.000 title claims abstract description 213
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 205
- 102000004196 processed proteins & peptides Human genes 0.000 title claims abstract description 175
- 229920001184 polypeptide Polymers 0.000 title claims abstract description 150
- 239000003446 ligand Substances 0.000 claims abstract description 225
- 238000000034 method Methods 0.000 claims abstract description 55
- 238000012384 transportation and delivery Methods 0.000 claims abstract description 34
- 206010003246 arthritis Diseases 0.000 claims abstract description 25
- 230000002685 pulmonary effect Effects 0.000 claims abstract description 17
- 210000004072 lung Anatomy 0.000 claims abstract description 11
- 230000002496 gastric effect Effects 0.000 claims abstract description 5
- 102100033732 Tumor necrosis factor receptor superfamily member 1A Human genes 0.000 claims description 405
- 101710187743 Tumor necrosis factor receptor superfamily member 1A Proteins 0.000 claims description 325
- 230000027455 binding Effects 0.000 claims description 199
- 238000009739 binding Methods 0.000 claims description 198
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 188
- 108060003951 Immunoglobulin Proteins 0.000 claims description 139
- 102000018358 immunoglobulin Human genes 0.000 claims description 139
- 108060008682 Tumor Necrosis Factor Proteins 0.000 claims description 95
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 claims description 95
- 102000005962 receptors Human genes 0.000 claims description 94
- 108020003175 receptors Proteins 0.000 claims description 94
- 150000007523 nucleic acids Chemical class 0.000 claims description 83
- 238000003556 assay Methods 0.000 claims description 81
- 102000039446 nucleic acids Human genes 0.000 claims description 79
- 108020004707 nucleic acids Proteins 0.000 claims description 79
- 102000007562 Serum Albumin Human genes 0.000 claims description 74
- 108010071390 Serum Albumin Proteins 0.000 claims description 74
- 241000282414 Homo sapiens Species 0.000 claims description 73
- 235000001014 amino acid Nutrition 0.000 claims description 73
- 101100425753 Homo sapiens TNFRSF1A gene Proteins 0.000 claims description 66
- 150000001413 amino acids Chemical class 0.000 claims description 62
- 101100112922 Candida albicans CDR3 gene Proteins 0.000 claims description 56
- 102100035361 Cerebellar degeneration-related protein 2 Human genes 0.000 claims description 43
- 101000737796 Homo sapiens Cerebellar degeneration-related protein 2 Proteins 0.000 claims description 43
- 108091005804 Peptidases Proteins 0.000 claims description 43
- 239000004365 Protease Substances 0.000 claims description 43
- 239000002773 nucleotide Substances 0.000 claims description 41
- 125000003729 nucleotide group Chemical group 0.000 claims description 41
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 claims description 38
- 102000004890 Interleukin-8 Human genes 0.000 claims description 35
- 108090001007 Interleukin-8 Proteins 0.000 claims description 35
- 229920001223 polyethylene glycol Polymers 0.000 claims description 31
- 239000013598 vector Substances 0.000 claims description 31
- 101100425754 Mus musculus Tnfrsf1a gene Proteins 0.000 claims description 30
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 30
- 201000010099 disease Diseases 0.000 claims description 26
- 238000011282 treatment Methods 0.000 claims description 26
- 241000282567 Macaca fascicularis Species 0.000 claims description 25
- 239000003814 drug Substances 0.000 claims description 23
- 230000005764 inhibitory process Effects 0.000 claims description 23
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 claims description 23
- 238000010494 dissociation reaction Methods 0.000 claims description 20
- 230000005593 dissociations Effects 0.000 claims description 20
- 230000035772 mutation Effects 0.000 claims description 18
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 17
- 206010035664 Pneumonia Diseases 0.000 claims description 17
- 230000028327 secretion Effects 0.000 claims description 16
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 15
- 238000004519 manufacturing process Methods 0.000 claims description 14
- 230000002265 prevention Effects 0.000 claims description 14
- 241000282465 Canis Species 0.000 claims description 11
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 claims description 11
- 125000003827 glycol group Chemical group 0.000 claims description 11
- 208000019693 Lung disease Diseases 0.000 claims description 9
- 239000002202 Polyethylene glycol Substances 0.000 claims description 8
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 claims description 8
- 206010006451 bronchitis Diseases 0.000 claims description 8
- 230000003013 cytotoxicity Effects 0.000 claims description 8
- 231100000135 cytotoxicity Toxicity 0.000 claims description 8
- 229910052739 hydrogen Inorganic materials 0.000 claims description 8
- 239000003112 inhibitor Substances 0.000 claims description 8
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 8
- 239000004474 valine Substances 0.000 claims description 8
- 108010085129 DMS5540 Proteins 0.000 claims description 7
- 208000006673 asthma Diseases 0.000 claims description 7
- 230000004968 inflammatory condition Effects 0.000 claims description 6
- 230000036963 noncompetitive effect Effects 0.000 claims description 6
- 229920001515 polyalkylene glycol Polymers 0.000 claims description 6
- 208000023504 respiratory system disease Diseases 0.000 claims description 6
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 6
- 206010006458 Bronchitis chronic Diseases 0.000 claims description 5
- 206010014561 Emphysema Diseases 0.000 claims description 5
- 208000007451 chronic bronchitis Diseases 0.000 claims description 5
- 238000012385 systemic delivery Methods 0.000 claims description 5
- 201000001178 Bacterial Pneumonia Diseases 0.000 claims description 4
- 208000011231 Crohn disease Diseases 0.000 claims description 4
- 201000003883 Cystic fibrosis Diseases 0.000 claims description 4
- 206010020751 Hypersensitivity Diseases 0.000 claims description 4
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 claims description 4
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 claims description 4
- 208000010378 Pulmonary Embolism Diseases 0.000 claims description 4
- 208000026935 allergic disease Diseases 0.000 claims description 4
- 201000009267 bronchiectasis Diseases 0.000 claims description 4
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 claims description 4
- 229910052757 nitrogen Inorganic materials 0.000 claims description 4
- 230000008685 targeting Effects 0.000 claims description 4
- 206010009900 Colitis ulcerative Diseases 0.000 claims description 3
- 208000003456 Juvenile Arthritis Diseases 0.000 claims description 3
- 206010059176 Juvenile idiopathic arthritis Diseases 0.000 claims description 3
- 206010035734 Pneumonia staphylococcal Diseases 0.000 claims description 3
- 201000006704 Ulcerative Colitis Diseases 0.000 claims description 3
- 208000019069 chronic childhood arthritis Diseases 0.000 claims description 3
- 230000001684 chronic effect Effects 0.000 claims description 3
- 201000002215 juvenile rheumatoid arthritis Diseases 0.000 claims description 3
- 201000006417 multiple sclerosis Diseases 0.000 claims description 3
- 208000004048 staphylococcal pneumonia Diseases 0.000 claims description 3
- 229910052717 sulfur Inorganic materials 0.000 claims description 3
- 206010001052 Acute respiratory distress syndrome Diseases 0.000 claims description 2
- 206010001881 Alveolar proteinosis Diseases 0.000 claims description 2
- 208000033116 Asbestos intoxication Diseases 0.000 claims description 2
- 206010003487 Aspergilloma Diseases 0.000 claims description 2
- 201000002909 Aspergillosis Diseases 0.000 claims description 2
- 208000036641 Aspergillus infections Diseases 0.000 claims description 2
- 208000018071 Diaphragmatic disease Diseases 0.000 claims description 2
- 206010014950 Eosinophilia Diseases 0.000 claims description 2
- 208000004248 Familial Primary Pulmonary Hypertension Diseases 0.000 claims description 2
- 208000009329 Graft vs Host Disease Diseases 0.000 claims description 2
- 206010072579 Granulomatosis with polyangiitis Diseases 0.000 claims description 2
- 206010021133 Hypoventilation Diseases 0.000 claims description 2
- 201000009794 Idiopathic Pulmonary Fibrosis Diseases 0.000 claims description 2
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 2
- 208000029001 Mediastinal disease Diseases 0.000 claims description 2
- 206010027406 Mesothelioma Diseases 0.000 claims description 2
- 206010029443 Nocardia Infections Diseases 0.000 claims description 2
- 208000002151 Pleural effusion Diseases 0.000 claims description 2
- 208000035109 Pneumococcal Infections Diseases 0.000 claims description 2
- 241000233870 Pneumocystis Species 0.000 claims description 2
- 206010035667 Pneumonia anthrax Diseases 0.000 claims description 2
- 206010064911 Pulmonary arterial hypertension Diseases 0.000 claims description 2
- 208000013616 Respiratory Distress Syndrome Diseases 0.000 claims description 2
- 208000025747 Rheumatic disease Diseases 0.000 claims description 2
- 206010039085 Rhinitis allergic Diseases 0.000 claims description 2
- 206010039491 Sarcoma Diseases 0.000 claims description 2
- 206010052779 Transplant rejections Diseases 0.000 claims description 2
- 201000007691 actinomycosis Diseases 0.000 claims description 2
- 201000000028 adult respiratory distress syndrome Diseases 0.000 claims description 2
- 201000010105 allergic rhinitis Diseases 0.000 claims description 2
- 230000007815 allergy Effects 0.000 claims description 2
- 206010003441 asbestosis Diseases 0.000 claims description 2
- 230000007613 environmental effect Effects 0.000 claims description 2
- 201000009580 eosinophilic pneumonia Diseases 0.000 claims description 2
- 208000024908 graft versus host disease Diseases 0.000 claims description 2
- 230000009610 hypersensitivity Effects 0.000 claims description 2
- 208000000122 hyperventilation Diseases 0.000 claims description 2
- 230000000870 hyperventilation Effects 0.000 claims description 2
- 208000009449 inhalation anthrax Diseases 0.000 claims description 2
- 208000023372 inhalational anthrax Diseases 0.000 claims description 2
- 208000036971 interstitial lung disease 2 Diseases 0.000 claims description 2
- 201000005202 lung cancer Diseases 0.000 claims description 2
- 208000020816 lung neoplasm Diseases 0.000 claims description 2
- 210000001161 mammalian embryo Anatomy 0.000 claims description 2
- 230000000414 obstructive effect Effects 0.000 claims description 2
- 208000024356 pleural disease Diseases 0.000 claims description 2
- 206010035653 pneumoconiosis Diseases 0.000 claims description 2
- 201000000317 pneumocystosis Diseases 0.000 claims description 2
- 201000008312 primary pulmonary hypertension Diseases 0.000 claims description 2
- 201000009732 pulmonary eosinophilia Diseases 0.000 claims description 2
- 208000002815 pulmonary hypertension Diseases 0.000 claims description 2
- 210000003492 pulmonary vein Anatomy 0.000 claims description 2
- 230000000552 rheumatic effect Effects 0.000 claims description 2
- 201000000306 sarcoidosis Diseases 0.000 claims description 2
- 201000002859 sleep apnea Diseases 0.000 claims description 2
- 102100035360 Cerebellar degeneration-related antigen 1 Human genes 0.000 claims 7
- 101000737793 Homo sapiens Cerebellar degeneration-related antigen 1 Proteins 0.000 claims 7
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 claims 4
- 208000008128 pulmonary tuberculosis Diseases 0.000 claims 1
- 208000027866 inflammatory disease Diseases 0.000 abstract description 3
- 210000004027 cell Anatomy 0.000 description 121
- 108090000623 proteins and genes Proteins 0.000 description 81
- 241000699666 Mus <mouse, genus> Species 0.000 description 70
- 102000004169 proteins and genes Human genes 0.000 description 66
- 235000018102 proteins Nutrition 0.000 description 59
- 108020003519 protein disulfide isomerase Proteins 0.000 description 49
- 239000000203 mixture Substances 0.000 description 46
- 102000035195 Peptidases Human genes 0.000 description 39
- 239000012634 fragment Substances 0.000 description 37
- 230000004927 fusion Effects 0.000 description 36
- 230000014509 gene expression Effects 0.000 description 35
- 102000009027 Albumins Human genes 0.000 description 34
- 108010088751 Albumins Proteins 0.000 description 34
- 229940096397 interleukin-8 Drugs 0.000 description 33
- XKTZWUACRZHVAN-VADRZIEHSA-N interleukin-8 Chemical compound C([C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@@H](NC(C)=O)CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N1[C@H](CCC1)C(=O)N1[C@H](CCC1)C(=O)N[C@@H](C)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC=1C=CC(O)=CC=1)C(=O)N[C@H](CO)C(=O)N1[C@H](CCC1)C(N)=O)C1=CC=CC=C1 XKTZWUACRZHVAN-VADRZIEHSA-N 0.000 description 33
- 241000699670 Mus sp. Species 0.000 description 31
- 239000000427 antigen Substances 0.000 description 31
- 230000000694 effects Effects 0.000 description 31
- 102000036639 antigens Human genes 0.000 description 30
- 108091007433 antigens Proteins 0.000 description 30
- 102000008100 Human Serum Albumin Human genes 0.000 description 29
- 108091006905 Human Serum Albumin Proteins 0.000 description 29
- 239000000178 monomer Substances 0.000 description 24
- 238000012512 characterization method Methods 0.000 description 22
- 238000001727 in vivo Methods 0.000 description 22
- 239000000243 solution Substances 0.000 description 21
- 108090000631 Trypsin Proteins 0.000 description 20
- 102000004142 Trypsin Human genes 0.000 description 20
- 239000012588 trypsin Substances 0.000 description 20
- 230000000875 corresponding effect Effects 0.000 description 19
- 210000002966 serum Anatomy 0.000 description 18
- 241000588724 Escherichia coli Species 0.000 description 17
- 230000009824 affinity maturation Effects 0.000 description 17
- 238000006386 neutralization reaction Methods 0.000 description 16
- 238000004458 analytical method Methods 0.000 description 15
- 230000001404 mediated effect Effects 0.000 description 15
- 239000000047 product Substances 0.000 description 15
- 238000012360 testing method Methods 0.000 description 15
- 241000124008 Mammalia Species 0.000 description 14
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 14
- 238000003752 polymerase chain reaction Methods 0.000 description 14
- 208000024891 symptom Diseases 0.000 description 14
- 230000006872 improvement Effects 0.000 description 13
- 238000002347 injection Methods 0.000 description 13
- 239000007924 injection Substances 0.000 description 13
- 238000002864 sequence alignment Methods 0.000 description 13
- 108020004414 DNA Proteins 0.000 description 12
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 description 12
- 230000006870 function Effects 0.000 description 12
- 108020001507 fusion proteins Proteins 0.000 description 12
- 102000037865 fusion proteins Human genes 0.000 description 12
- 238000011534 incubation Methods 0.000 description 12
- 230000003389 potentiating effect Effects 0.000 description 12
- 239000011780 sodium chloride Substances 0.000 description 12
- 238000001525 receptor binding assay Methods 0.000 description 11
- 230000002829 reductive effect Effects 0.000 description 11
- 238000002965 ELISA Methods 0.000 description 10
- 241001465754 Metazoa Species 0.000 description 10
- 238000006243 chemical reaction Methods 0.000 description 10
- 239000003795 chemical substances by application Substances 0.000 description 10
- 238000001514 detection method Methods 0.000 description 10
- 239000013604 expression vector Substances 0.000 description 10
- 230000005714 functional activity Effects 0.000 description 10
- 102200077165 rs118204021 Human genes 0.000 description 10
- 239000000126 substance Substances 0.000 description 10
- 241000283707 Capra Species 0.000 description 9
- 102000004190 Enzymes Human genes 0.000 description 9
- 108090000790 Enzymes Proteins 0.000 description 9
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 9
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 9
- 238000000423 cell based assay Methods 0.000 description 9
- 230000009260 cross reactivity Effects 0.000 description 9
- 229940088598 enzyme Drugs 0.000 description 9
- 238000002474 experimental method Methods 0.000 description 9
- 230000002068 genetic effect Effects 0.000 description 9
- 241000894007 species Species 0.000 description 9
- 230000001225 therapeutic effect Effects 0.000 description 9
- 241000282560 Macaca mulatta Species 0.000 description 8
- 239000011324 bead Substances 0.000 description 8
- 208000037976 chronic inflammation Diseases 0.000 description 8
- 229940079593 drug Drugs 0.000 description 8
- 102000057041 human TNF Human genes 0.000 description 8
- 238000000338 in vitro Methods 0.000 description 8
- 238000007912 intraperitoneal administration Methods 0.000 description 8
- 238000001990 intravenous administration Methods 0.000 description 8
- 108010026228 mRNA guanylyltransferase Proteins 0.000 description 8
- 238000013507 mapping Methods 0.000 description 8
- 230000011664 signaling Effects 0.000 description 8
- 102220471946 Axin interactor, dorsalization-associated protein_H56R_mutation Human genes 0.000 description 7
- 241001504519 Papio ursinus Species 0.000 description 7
- 241000700159 Rattus Species 0.000 description 7
- 108010090804 Streptavidin Proteins 0.000 description 7
- 102000007238 Transferrin Receptors Human genes 0.000 description 7
- 108010033576 Transferrin Receptors Proteins 0.000 description 7
- 230000008901 benefit Effects 0.000 description 7
- 210000004369 blood Anatomy 0.000 description 7
- 239000008280 blood Substances 0.000 description 7
- 239000000872 buffer Substances 0.000 description 7
- 210000004899 c-terminal region Anatomy 0.000 description 7
- 230000021615 conjugation Effects 0.000 description 7
- 230000017854 proteolysis Effects 0.000 description 7
- 231100000765 toxin Toxicity 0.000 description 7
- 239000003053 toxin Substances 0.000 description 7
- 102220470698 BUD13 homolog_V30P_mutation Human genes 0.000 description 6
- 102000002812 Heat-Shock Proteins Human genes 0.000 description 6
- 108010004889 Heat-Shock Proteins Proteins 0.000 description 6
- 241000282412 Homo Species 0.000 description 6
- -1 Kabat amino acid Chemical class 0.000 description 6
- 102000007079 Peptide Fragments Human genes 0.000 description 6
- 108010033276 Peptide Fragments Proteins 0.000 description 6
- 102000004338 Transferrin Human genes 0.000 description 6
- 108090000901 Transferrin Proteins 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 208000037893 chronic inflammatory disorder Diseases 0.000 description 6
- 239000000499 gel Substances 0.000 description 6
- 238000007918 intramuscular administration Methods 0.000 description 6
- 102200005465 rs121434284 Human genes 0.000 description 6
- 102220008832 rs193922279 Human genes 0.000 description 6
- 239000000523 sample Substances 0.000 description 6
- 239000006228 supernatant Substances 0.000 description 6
- 239000012581 transferrin Substances 0.000 description 6
- 208000009386 Experimental Arthritis Diseases 0.000 description 5
- 241000235058 Komagataella pastoris Species 0.000 description 5
- 241001529936 Murinae Species 0.000 description 5
- 206010028980 Neoplasm Diseases 0.000 description 5
- 108091034117 Oligonucleotide Proteins 0.000 description 5
- BLFLLBZGZJTVJG-UHFFFAOYSA-N benzocaine Chemical compound CCOC(=O)C1=CC=C(N)C=C1 BLFLLBZGZJTVJG-UHFFFAOYSA-N 0.000 description 5
- 239000011230 binding agent Substances 0.000 description 5
- 230000037396 body weight Effects 0.000 description 5
- 239000007795 chemical reaction product Substances 0.000 description 5
- 230000002860 competitive effect Effects 0.000 description 5
- 230000029087 digestion Effects 0.000 description 5
- 238000010348 incorporation Methods 0.000 description 5
- 230000035800 maturation Effects 0.000 description 5
- 238000002844 melting Methods 0.000 description 5
- 230000008018 melting Effects 0.000 description 5
- 239000006199 nebulizer Substances 0.000 description 5
- 239000013642 negative control Substances 0.000 description 5
- 238000000746 purification Methods 0.000 description 5
- 230000009870 specific binding Effects 0.000 description 5
- 238000007920 subcutaneous administration Methods 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical group N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- 102000014914 Carrier Proteins Human genes 0.000 description 4
- 102000008186 Collagen Human genes 0.000 description 4
- 108010035532 Collagen Proteins 0.000 description 4
- 108010092160 Dactinomycin Proteins 0.000 description 4
- 241001198387 Escherichia coli BL21(DE3) Species 0.000 description 4
- 108091028043 Nucleic acid sequence Proteins 0.000 description 4
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 4
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 4
- 102100040247 Tumor necrosis factor Human genes 0.000 description 4
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 4
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 4
- 230000002776 aggregation Effects 0.000 description 4
- 238000004220 aggregation Methods 0.000 description 4
- 230000002917 arthritic effect Effects 0.000 description 4
- 230000009286 beneficial effect Effects 0.000 description 4
- 229960002685 biotin Drugs 0.000 description 4
- 239000011616 biotin Substances 0.000 description 4
- 230000015556 catabolic process Effects 0.000 description 4
- 229920001436 collagen Polymers 0.000 description 4
- 239000012228 culture supernatant Substances 0.000 description 4
- 230000001472 cytotoxic effect Effects 0.000 description 4
- 238000006731 degradation reaction Methods 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 239000000539 dimer Substances 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 230000002147 killing effect Effects 0.000 description 4
- 238000012544 monitoring process Methods 0.000 description 4
- 239000008194 pharmaceutical composition Substances 0.000 description 4
- 239000013641 positive control Substances 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 230000000069 prophylactic effect Effects 0.000 description 4
- 238000001542 size-exclusion chromatography Methods 0.000 description 4
- 231100000617 superantigen Toxicity 0.000 description 4
- 238000011830 transgenic mouse model Methods 0.000 description 4
- 239000013638 trimer Substances 0.000 description 4
- 230000003442 weekly effect Effects 0.000 description 4
- 102000003966 Alpha-1-microglobulin Human genes 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 3
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 3
- 241000196324 Embryophyta Species 0.000 description 3
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 3
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 3
- 108010049003 Fibrinogen Proteins 0.000 description 3
- 102000008946 Fibrinogen Human genes 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- 101000801228 Homo sapiens Tumor necrosis factor receptor superfamily member 1A Proteins 0.000 description 3
- 101000930477 Mus musculus Albumin Proteins 0.000 description 3
- 108010067372 Pancreatic elastase Proteins 0.000 description 3
- 102000016387 Pancreatic elastase Human genes 0.000 description 3
- 108010019160 Pancreatin Proteins 0.000 description 3
- 241000009328 Perro Species 0.000 description 3
- 241000235648 Pichia Species 0.000 description 3
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 3
- 102100039037 Vascular endothelial growth factor A Human genes 0.000 description 3
- 239000000556 agonist Substances 0.000 description 3
- 125000000539 amino acid group Chemical group 0.000 description 3
- 108091008324 binding proteins Proteins 0.000 description 3
- 210000000988 bone and bone Anatomy 0.000 description 3
- 201000011510 cancer Diseases 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- 230000003833 cell viability Effects 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 230000000295 complement effect Effects 0.000 description 3
- 238000010276 construction Methods 0.000 description 3
- 231100000433 cytotoxic Toxicity 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 239000008121 dextrose Substances 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 238000012377 drug delivery Methods 0.000 description 3
- 230000009977 dual effect Effects 0.000 description 3
- 239000012636 effector Substances 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 210000003527 eukaryotic cell Anatomy 0.000 description 3
- 210000002744 extracellular matrix Anatomy 0.000 description 3
- 229940012952 fibrinogen Drugs 0.000 description 3
- 210000002950 fibroblast Anatomy 0.000 description 3
- 230000009454 functional inhibition Effects 0.000 description 3
- BRZYSWJRSDMWLG-CAXSIQPQSA-N geneticin Natural products O1C[C@@](O)(C)[C@H](NC)[C@@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](C(C)O)O2)N)[C@@H](N)C[C@H]1N BRZYSWJRSDMWLG-CAXSIQPQSA-N 0.000 description 3
- 210000004602 germ cell Anatomy 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 210000000987 immune system Anatomy 0.000 description 3
- 229940072221 immunoglobulins Drugs 0.000 description 3
- 230000006698 induction Effects 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 210000004185 liver Anatomy 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000003472 neutralizing effect Effects 0.000 description 3
- 230000002018 overexpression Effects 0.000 description 3
- 229940055695 pancreatin Drugs 0.000 description 3
- 239000013612 plasmid Substances 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 210000001236 prokaryotic cell Anatomy 0.000 description 3
- 238000003753 real-time PCR Methods 0.000 description 3
- 230000006798 recombination Effects 0.000 description 3
- 238000005215 recombination Methods 0.000 description 3
- 230000008929 regeneration Effects 0.000 description 3
- 238000011069 regeneration method Methods 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 108091000053 retinol binding Proteins 0.000 description 3
- 102000029752 retinol binding Human genes 0.000 description 3
- 238000012216 screening Methods 0.000 description 3
- 238000010187 selection method Methods 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 239000003981 vehicle Substances 0.000 description 3
- 239000012114 Alexa Fluor 647 Substances 0.000 description 2
- 101800001761 Alpha-1-microglobulin Proteins 0.000 description 2
- 102000005666 Apolipoprotein A-I Human genes 0.000 description 2
- 108010059886 Apolipoprotein A-I Proteins 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 108010081589 Becaplermin Proteins 0.000 description 2
- 102100022545 Bone morphogenetic protein 8B Human genes 0.000 description 2
- 108010074051 C-Reactive Protein Proteins 0.000 description 2
- 102100032752 C-reactive protein Human genes 0.000 description 2
- OBMZMSLWNNWEJA-XNCRXQDQSA-N C1=CC=2C(C[C@@H]3NC(=O)[C@@H](NC(=O)[C@H](NC(=O)N(CC#CCN(CCCC[C@H](NC(=O)[C@@H](CC4=CC=CC=C4)NC3=O)C(=O)N)CC=C)NC(=O)[C@@H](N)C)CC3=CNC4=C3C=CC=C4)C)=CNC=2C=C1 Chemical compound C1=CC=2C(C[C@@H]3NC(=O)[C@@H](NC(=O)[C@H](NC(=O)N(CC#CCN(CCCC[C@H](NC(=O)[C@@H](CC4=CC=CC=C4)NC3=O)C(=O)N)CC=C)NC(=O)[C@@H](N)C)CC3=CNC4=C3C=CC=C4)C)=CNC=2C=C1 OBMZMSLWNNWEJA-XNCRXQDQSA-N 0.000 description 2
- 108010078791 Carrier Proteins Proteins 0.000 description 2
- 102000005600 Cathepsins Human genes 0.000 description 2
- 108010084457 Cathepsins Proteins 0.000 description 2
- 102000000503 Collagen Type II Human genes 0.000 description 2
- 108010041390 Collagen Type II Proteins 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 241000255581 Drosophila <fruit fly, genus> Species 0.000 description 2
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 2
- 108010087819 Fc receptors Proteins 0.000 description 2
- 102000009109 Fc receptors Human genes 0.000 description 2
- 102000008857 Ferritin Human genes 0.000 description 2
- 108050000784 Ferritin Proteins 0.000 description 2
- 238000008416 Ferritin Methods 0.000 description 2
- 102000018233 Fibroblast Growth Factor Human genes 0.000 description 2
- 108050007372 Fibroblast Growth Factor Proteins 0.000 description 2
- 102100037362 Fibronectin Human genes 0.000 description 2
- 108010067306 Fibronectins Proteins 0.000 description 2
- 102000006395 Globulins Human genes 0.000 description 2
- 108010044091 Globulins Proteins 0.000 description 2
- 102100036683 Growth arrest-specific protein 1 Human genes 0.000 description 2
- 102000013271 Hemopexin Human genes 0.000 description 2
- 108010026027 Hemopexin Proteins 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- 101000899368 Homo sapiens Bone morphogenetic protein 8B Proteins 0.000 description 2
- 101001072723 Homo sapiens Growth arrest-specific protein 1 Proteins 0.000 description 2
- 101000599951 Homo sapiens Insulin-like growth factor I Proteins 0.000 description 2
- 101001076292 Homo sapiens Insulin-like growth factor II Proteins 0.000 description 2
- 101000595923 Homo sapiens Placenta growth factor Proteins 0.000 description 2
- 241000714260 Human T-lymphotropic virus 1 Species 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- 102100037852 Insulin-like growth factor I Human genes 0.000 description 2
- 102100025947 Insulin-like growth factor II Human genes 0.000 description 2
- 102100039064 Interleukin-3 Human genes 0.000 description 2
- 108010002386 Interleukin-3 Proteins 0.000 description 2
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 2
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- 108010001831 LDL receptors Proteins 0.000 description 2
- 102100024640 Low-density lipoprotein receptor Human genes 0.000 description 2
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 2
- 102000009112 Mannose-Binding Lectin Human genes 0.000 description 2
- 108010087870 Mannose-Binding Lectin Proteins 0.000 description 2
- 101000648740 Mus musculus Tumor necrosis factor Proteins 0.000 description 2
- 101710176384 Peptide 1 Proteins 0.000 description 2
- 102100035194 Placenta growth factor Human genes 0.000 description 2
- 102000007584 Prealbumin Human genes 0.000 description 2
- 108010071690 Prealbumin Proteins 0.000 description 2
- 241000288906 Primates Species 0.000 description 2
- 108010076504 Protein Sorting Signals Proteins 0.000 description 2
- 201000004681 Psoriasis Diseases 0.000 description 2
- 102220513579 Pulmonary surfactant-associated protein D_S12P_mutation Human genes 0.000 description 2
- 108010025832 RANK Ligand Proteins 0.000 description 2
- 102000014128 RANK Ligand Human genes 0.000 description 2
- 101100288143 Rattus norvegicus Klkb1 gene Proteins 0.000 description 2
- 108020004511 Recombinant DNA Proteins 0.000 description 2
- 238000012300 Sequence Analysis Methods 0.000 description 2
- 210000001744 T-lymphocyte Anatomy 0.000 description 2
- 102100031372 Thymidine phosphorylase Human genes 0.000 description 2
- 108700023160 Thymidine phosphorylases Proteins 0.000 description 2
- 101800004564 Transforming growth factor alpha Proteins 0.000 description 2
- 108060008683 Tumor Necrosis Factor Receptor Proteins 0.000 description 2
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 2
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 229930183665 actinomycin Natural products 0.000 description 2
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 208000038016 acute inflammation Diseases 0.000 description 2
- 230000006022 acute inflammation Effects 0.000 description 2
- 108010050122 alpha 1-Antitrypsin Proteins 0.000 description 2
- 102000015395 alpha 1-Antitrypsin Human genes 0.000 description 2
- 229940024142 alpha 1-antitrypsin Drugs 0.000 description 2
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 2
- 229960000723 ampicillin Drugs 0.000 description 2
- 230000003321 amplification Effects 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 238000012575 bio-layer interferometry Methods 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 230000008499 blood brain barrier function Effects 0.000 description 2
- 210000001218 blood-brain barrier Anatomy 0.000 description 2
- 230000030833 cell death Effects 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 230000006020 chronic inflammation Effects 0.000 description 2
- 230000001268 conjugating effect Effects 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 230000002596 correlated effect Effects 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 238000002784 cytotoxicity assay Methods 0.000 description 2
- 231100000263 cytotoxicity test Toxicity 0.000 description 2
- 229960000640 dactinomycin Drugs 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 238000000113 differential scanning calorimetry Methods 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 239000000975 dye Substances 0.000 description 2
- 229940126864 fibroblast growth factor Drugs 0.000 description 2
- 238000005194 fractionation Methods 0.000 description 2
- 238000004108 freeze drying Methods 0.000 description 2
- 238000002825 functional assay Methods 0.000 description 2
- 210000002216 heart Anatomy 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 230000036039 immunity Effects 0.000 description 2
- 230000002779 inactivation Effects 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 206010022000 influenza Diseases 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 229940068935 insulin-like growth factor 2 Drugs 0.000 description 2
- 229940076264 interleukin-3 Drugs 0.000 description 2
- 238000001361 intraarterial administration Methods 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 229960000485 methotrexate Drugs 0.000 description 2
- 206010028417 myasthenia gravis Diseases 0.000 description 2
- 238000003199 nucleic acid amplification method Methods 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 230000002797 proteolythic effect Effects 0.000 description 2
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 238000003127 radioimmunoassay Methods 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 238000013223 sprague-dawley female rat Methods 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 230000001629 suppression Effects 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 230000037317 transdermal delivery Effects 0.000 description 2
- 102000003298 tumor necrosis factor receptor Human genes 0.000 description 2
- 208000035408 type 1 diabetes mellitus 1 Diseases 0.000 description 2
- KUHSEZKIEJYEHN-BXRBKJIMSA-N (2s)-2-amino-3-hydroxypropanoic acid;(2s)-2-aminopropanoic acid Chemical compound C[C@H](N)C(O)=O.OC[C@H](N)C(O)=O KUHSEZKIEJYEHN-BXRBKJIMSA-N 0.000 description 1
- NMWKYTGJWUAZPZ-WWHBDHEGSA-N (4S)-4-[[(4R,7S,10S,16S,19S,25S,28S,31R)-31-[[(2S)-2-[[(1R,6R,9S,12S,18S,21S,24S,27S,30S,33S,36S,39S,42R,47R,53S,56S,59S,62S,65S,68S,71S,76S,79S,85S)-47-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-methylbutanoyl]amino]-3-methylbutanoyl]amino]-3-hydroxypropanoyl]amino]-3-(1H-imidazol-4-yl)propanoyl]amino]-3-phenylpropanoyl]amino]-4-oxobutanoyl]amino]-3-carboxypropanoyl]amino]-18-(4-aminobutyl)-27,68-bis(3-amino-3-oxopropyl)-36,71,76-tribenzyl-39-(3-carbamimidamidopropyl)-24-(2-carboxyethyl)-21,56-bis(carboxymethyl)-65,85-bis[(1R)-1-hydroxyethyl]-59-(hydroxymethyl)-62,79-bis(1H-imidazol-4-ylmethyl)-9-methyl-33-(2-methylpropyl)-8,11,17,20,23,26,29,32,35,38,41,48,54,57,60,63,66,69,72,74,77,80,83,86-tetracosaoxo-30-propan-2-yl-3,4,44,45-tetrathia-7,10,16,19,22,25,28,31,34,37,40,49,55,58,61,64,67,70,73,75,78,81,84,87-tetracosazatetracyclo[40.31.14.012,16.049,53]heptaoctacontane-6-carbonyl]amino]-3-methylbutanoyl]amino]-7-(3-carbamimidamidopropyl)-25-(hydroxymethyl)-19-[(4-hydroxyphenyl)methyl]-28-(1H-imidazol-4-ylmethyl)-10-methyl-6,9,12,15,18,21,24,27,30-nonaoxo-16-propan-2-yl-1,2-dithia-5,8,11,14,17,20,23,26,29-nonazacyclodotriacontane-4-carbonyl]amino]-5-[[(2S)-1-[[(2S)-1-[[(2S)-3-carboxy-1-[[(2S)-1-[[(2S)-1-[[(1S)-1-carboxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-5-oxopentanoic acid Chemical compound CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@@H]3NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]4CCCN4C(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](Cc4ccccc4)NC3=O)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N3CCC[C@H]3C(=O)N[C@@H](C)C(=O)N2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)C(=O)N[C@@H](C)C(O)=O NMWKYTGJWUAZPZ-WWHBDHEGSA-N 0.000 description 1
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- KISWVXRQTGLFGD-UHFFFAOYSA-N 2-[[2-[[6-amino-2-[[2-[[2-[[5-amino-2-[[2-[[1-[2-[[6-amino-2-[(2,5-diamino-5-oxopentanoyl)amino]hexanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxypropanoyl]amino]-5-oxopentanoyl]amino]-5-(diaminomethylideneamino)p Chemical compound C1CCN(C(=O)C(CCCN=C(N)N)NC(=O)C(CCCCN)NC(=O)C(N)CCC(N)=O)C1C(=O)NC(CO)C(=O)NC(CCC(N)=O)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(CCCCN)C(=O)NC(C(=O)NC(CC(C)C)C(O)=O)CC1=CC=C(O)C=C1 KISWVXRQTGLFGD-UHFFFAOYSA-N 0.000 description 1
- APRZHQXAAWPYHS-UHFFFAOYSA-N 4-[5-[3-(carboxymethoxy)phenyl]-3-(4,5-dimethyl-1,3-thiazol-2-yl)tetrazol-3-ium-2-yl]benzenesulfonate Chemical compound S1C(C)=C(C)N=C1[N+]1=NC(C=2C=C(OCC(O)=O)C=CC=2)=NN1C1=CC=C(S([O-])(=O)=O)C=C1 APRZHQXAAWPYHS-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- 102000007698 Alcohol dehydrogenase Human genes 0.000 description 1
- 108010021809 Alcohol dehydrogenase Proteins 0.000 description 1
- 102100022524 Alpha-1-antichymotrypsin Human genes 0.000 description 1
- 102100033312 Alpha-2-macroglobulin Human genes 0.000 description 1
- 102100022987 Angiogenin Human genes 0.000 description 1
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 1
- 235000002198 Annona diversifolia Nutrition 0.000 description 1
- 102000004411 Antithrombin III Human genes 0.000 description 1
- 108090000935 Antithrombin III Proteins 0.000 description 1
- 101710095342 Apolipoprotein B Proteins 0.000 description 1
- 102100040202 Apolipoprotein B-100 Human genes 0.000 description 1
- 102100040214 Apolipoprotein(a) Human genes 0.000 description 1
- 241000228212 Aspergillus Species 0.000 description 1
- 241000228257 Aspergillus sp. Species 0.000 description 1
- 244000063299 Bacillus subtilis Species 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- 102100037437 Beta-defensin 1 Human genes 0.000 description 1
- 101710125314 Beta-defensin 1 Proteins 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 108010049931 Bone Morphogenetic Protein 2 Proteins 0.000 description 1
- 108010049955 Bone Morphogenetic Protein 4 Proteins 0.000 description 1
- 108010049976 Bone Morphogenetic Protein 5 Proteins 0.000 description 1
- 108010049974 Bone Morphogenetic Protein 6 Proteins 0.000 description 1
- 108010049870 Bone Morphogenetic Protein 7 Proteins 0.000 description 1
- 102100024506 Bone morphogenetic protein 2 Human genes 0.000 description 1
- 102100024505 Bone morphogenetic protein 4 Human genes 0.000 description 1
- 102100022526 Bone morphogenetic protein 5 Human genes 0.000 description 1
- 102100022525 Bone morphogenetic protein 6 Human genes 0.000 description 1
- 102100022544 Bone morphogenetic protein 7 Human genes 0.000 description 1
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 1
- 102000008203 CTLA-4 Antigen Human genes 0.000 description 1
- 229940045513 CTLA4 antagonist Drugs 0.000 description 1
- 241000282832 Camelidae Species 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 108010075016 Ceruloplasmin Proteins 0.000 description 1
- 102100023321 Ceruloplasmin Human genes 0.000 description 1
- 108010078239 Chemokine CX3CL1 Proteins 0.000 description 1
- 241000251730 Chondrichthyes Species 0.000 description 1
- 108090000317 Chymotrypsin Proteins 0.000 description 1
- 101100007328 Cocos nucifera COS-1 gene Proteins 0.000 description 1
- 102000012422 Collagen Type I Human genes 0.000 description 1
- 108010022452 Collagen Type I Proteins 0.000 description 1
- 102000004266 Collagen Type IV Human genes 0.000 description 1
- 108010042086 Collagen Type IV Proteins 0.000 description 1
- 108700040183 Complement C1 Inhibitor Proteins 0.000 description 1
- 102000055157 Complement C1 Inhibitor Human genes 0.000 description 1
- 206010056370 Congestive cardiomyopathy Diseases 0.000 description 1
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 1
- 108010036949 Cyclosporine Proteins 0.000 description 1
- 108010061642 Cystatin C Proteins 0.000 description 1
- 102000012192 Cystatin C Human genes 0.000 description 1
- 241000701022 Cytomegalovirus Species 0.000 description 1
- 101710112752 Cytotoxin Proteins 0.000 description 1
- 230000004544 DNA amplification Effects 0.000 description 1
- 238000001712 DNA sequencing Methods 0.000 description 1
- 102000052510 DNA-Binding Proteins Human genes 0.000 description 1
- 101710096438 DNA-binding protein Proteins 0.000 description 1
- 101150082070 Dab gene Proteins 0.000 description 1
- 108010002069 Defensins Proteins 0.000 description 1
- 102000000541 Defensins Human genes 0.000 description 1
- 208000034423 Delivery Diseases 0.000 description 1
- 208000016192 Demyelinating disease Diseases 0.000 description 1
- 201000010046 Dilated cardiomyopathy Diseases 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- YQYJSBFKSSDGFO-UHFFFAOYSA-N Epihygromycin Natural products OC1C(O)C(C(=O)C)OC1OC(C(=C1)O)=CC=C1C=C(C)C(=O)NC1C(O)C(O)C2OCOC2C1O YQYJSBFKSSDGFO-UHFFFAOYSA-N 0.000 description 1
- 206010015866 Extravasation Diseases 0.000 description 1
- 108010014173 Factor X Proteins 0.000 description 1
- 102000009123 Fibrin Human genes 0.000 description 1
- 108010073385 Fibrin Proteins 0.000 description 1
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 1
- 108090000386 Fibroblast Growth Factor 1 Proteins 0.000 description 1
- 102000003971 Fibroblast Growth Factor 1 Human genes 0.000 description 1
- 102000003974 Fibroblast growth factor 2 Human genes 0.000 description 1
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 description 1
- 241000724791 Filamentous phage Species 0.000 description 1
- 102100020997 Fractalkine Human genes 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 108010053070 Glutathione Disulfide Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 101150009006 HIS3 gene Proteins 0.000 description 1
- 108010045100 HSP27 Heat-Shock Proteins Proteins 0.000 description 1
- 108050005077 Haptoglobin Proteins 0.000 description 1
- 102000014702 Haptoglobin Human genes 0.000 description 1
- 102100039165 Heat shock protein beta-1 Human genes 0.000 description 1
- 101000883685 Heliothis virescens 60 kDa chaperonin, mitochondrial Proteins 0.000 description 1
- 102100022057 Hepatocyte nuclear factor 1-alpha Human genes 0.000 description 1
- 101000780591 Homo sapiens AFG3-like protein 2 Proteins 0.000 description 1
- 101001045751 Homo sapiens Hepatocyte nuclear factor 1-alpha Proteins 0.000 description 1
- 101001082060 Homo sapiens Interferon-induced protein with tetratricopeptide repeats 3 Proteins 0.000 description 1
- 101001055222 Homo sapiens Interleukin-8 Proteins 0.000 description 1
- 101001013648 Homo sapiens Methionine synthase Proteins 0.000 description 1
- 101000797623 Homo sapiens Protein AMBP Proteins 0.000 description 1
- 101000841411 Homo sapiens Protein ecdysoneless homolog Proteins 0.000 description 1
- 101000752245 Homo sapiens Rho guanine nucleotide exchange factor 5 Proteins 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000003746 Insulin Receptor Human genes 0.000 description 1
- 108010001127 Insulin Receptor Proteins 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 208000029523 Interstitial Lung disease Diseases 0.000 description 1
- 108010076876 Keratins Proteins 0.000 description 1
- 102000011782 Keratins Human genes 0.000 description 1
- 241000282838 Lama Species 0.000 description 1
- 241000282852 Lama guanicoe Species 0.000 description 1
- 102000007547 Laminin Human genes 0.000 description 1
- 108010085895 Laminin Proteins 0.000 description 1
- 102000019298 Lipocalin Human genes 0.000 description 1
- 108050006654 Lipocalin Proteins 0.000 description 1
- 108010033266 Lipoprotein(a) Proteins 0.000 description 1
- 241000829100 Macaca mulatta polyomavirus 1 Species 0.000 description 1
- 102000004378 Melanocortin Receptors Human genes 0.000 description 1
- 108090000950 Melanocortin Receptors Proteins 0.000 description 1
- 102000005741 Metalloproteases Human genes 0.000 description 1
- 108010006035 Metalloproteases Proteins 0.000 description 1
- 102100030335 Midkine Human genes 0.000 description 1
- 108010092801 Midkine Proteins 0.000 description 1
- 101000686985 Mouse mammary tumor virus (strain C3H) Protein PR73 Proteins 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- 102000047918 Myelin Basic Human genes 0.000 description 1
- 102000006386 Myelin Proteins Human genes 0.000 description 1
- 108010083674 Myelin Proteins Proteins 0.000 description 1
- 101710107068 Myelin basic protein Proteins 0.000 description 1
- 102100030856 Myoglobin Human genes 0.000 description 1
- 108010062374 Myoglobin Proteins 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 description 1
- 206010028851 Necrosis Diseases 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- 102400001060 Neutrophil defensin 2 Human genes 0.000 description 1
- 101710117153 Neutrophil defensin 2 Proteins 0.000 description 1
- 102100024761 Neutrophil defensin 3 Human genes 0.000 description 1
- 101710117152 Neutrophil defensin 3 Proteins 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- 241001452677 Ogataea methanolica Species 0.000 description 1
- 102000007990 Organic Anion Transporters Human genes 0.000 description 1
- 108010089503 Organic Anion Transporters Proteins 0.000 description 1
- 108010061952 Orosomucoid Proteins 0.000 description 1
- 102000012404 Orosomucoid Human genes 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 101710126321 Pancreatic trypsin inhibitor Proteins 0.000 description 1
- 241000282520 Papio Species 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108010051456 Plasminogen Proteins 0.000 description 1
- 102000013566 Plasminogen Human genes 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 108010015078 Pregnancy-Associated alpha 2-Macroglobulins Proteins 0.000 description 1
- 102000011195 Profilin Human genes 0.000 description 1
- 108050001408 Profilin Proteins 0.000 description 1
- 201000001263 Psoriatic Arthritis Diseases 0.000 description 1
- 208000036824 Psoriatic arthropathy Diseases 0.000 description 1
- 206010037423 Pulmonary oedema Diseases 0.000 description 1
- 238000011529 RT qPCR Methods 0.000 description 1
- 101000930457 Rattus norvegicus Albumin Proteins 0.000 description 1
- 102100021688 Rho guanine nucleotide exchange factor 5 Human genes 0.000 description 1
- 101100394989 Rhodopseudomonas palustris (strain ATCC BAA-98 / CGA009) hisI gene Proteins 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 241000714474 Rous sarcoma virus Species 0.000 description 1
- 101000620888 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) Repressible acid phosphatase Proteins 0.000 description 1
- 241000235347 Schizosaccharomyces pombe Species 0.000 description 1
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 1
- 206010040070 Septic Shock Diseases 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 108700028909 Serum Amyloid A Proteins 0.000 description 1
- 102000054727 Serum Amyloid A Human genes 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- 108010085004 TGF-beta Superfamily Proteins Proteins 0.000 description 1
- 102000007453 TGF-beta Superfamily Proteins Human genes 0.000 description 1
- 108010004408 TRPP Cation Channels Proteins 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 108010022394 Threonine synthase Proteins 0.000 description 1
- 108010034949 Thyroglobulin Proteins 0.000 description 1
- 102000009843 Thyroglobulin Human genes 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- 102000006747 Transforming Growth Factor alpha Human genes 0.000 description 1
- 102400001320 Transforming growth factor alpha Human genes 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 description 1
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 description 1
- 206010054094 Tumour necrosis Diseases 0.000 description 1
- 102000044159 Ubiquitin Human genes 0.000 description 1
- 108090000848 Ubiquitin Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 241001416177 Vicugna pacos Species 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- CUJRVFIICFDLGR-UHFFFAOYSA-N acetylacetonate Chemical compound CC(=O)[CH-]C(C)=O CUJRVFIICFDLGR-UHFFFAOYSA-N 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000002730 additional effect Effects 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 239000004479 aerosol dispenser Substances 0.000 description 1
- 230000008484 agonism Effects 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 108010091628 alpha 1-Antichymotrypsin Proteins 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 230000002491 angiogenic effect Effects 0.000 description 1
- 108010072788 angiogenin Proteins 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 1
- 102000025171 antigen binding proteins Human genes 0.000 description 1
- 108091000831 antigen binding proteins Proteins 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 229960005348 antithrombin iii Drugs 0.000 description 1
- 229940072107 ascorbate Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 238000002819 bacterial display Methods 0.000 description 1
- 102000015736 beta 2-Microglobulin Human genes 0.000 description 1
- 108010081355 beta 2-Microglobulin Proteins 0.000 description 1
- 238000002306 biochemical method Methods 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 238000013378 biophysical characterization Methods 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 210000004781 brain capillary Anatomy 0.000 description 1
- 229940009550 c1 esterase inhibitor Drugs 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 108091092328 cellular RNA Proteins 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 229960002376 chymotrypsin Drugs 0.000 description 1
- 239000003541 chymotrypsin inhibitor Substances 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 230000024203 complement activation Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 210000004087 cornea Anatomy 0.000 description 1
- 230000037029 cross reaction Effects 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 239000004148 curcumin Substances 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 239000002619 cytotoxin Substances 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 239000000032 diagnostic agent Substances 0.000 description 1
- 229940039227 diagnostic agent Drugs 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 102000004419 dihydrofolate reductase Human genes 0.000 description 1
- 238000011143 downstream manufacturing Methods 0.000 description 1
- 238000007876 drug discovery Methods 0.000 description 1
- 238000003255 drug test Methods 0.000 description 1
- 229940112141 dry powder inhaler Drugs 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 230000002996 emotional effect Effects 0.000 description 1
- 230000008519 endogenous mechanism Effects 0.000 description 1
- 210000001163 endosome Anatomy 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 231100000655 enterotoxin Toxicity 0.000 description 1
- 239000002532 enzyme inhibitor Substances 0.000 description 1
- 102000015694 estrogen receptors Human genes 0.000 description 1
- 108010038795 estrogen receptors Proteins 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000013401 experimental design Methods 0.000 description 1
- 230000036251 extravasation Effects 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 101150054895 ftsH gene Proteins 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 238000001641 gel filtration chromatography Methods 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- YPZRWBKMTBYPTK-BJDJZHNGSA-N glutathione disulfide Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@H](C(=O)NCC(O)=O)CSSC[C@@H](C(=O)NCC(O)=O)NC(=O)CC[C@H](N)C(O)=O YPZRWBKMTBYPTK-BJDJZHNGSA-N 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- 239000000833 heterodimer Substances 0.000 description 1
- 239000000710 homodimer Substances 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 102000054037 human AFG3L2 Human genes 0.000 description 1
- 102000047791 human ECD Human genes 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 239000002596 immunotoxin Substances 0.000 description 1
- 231100000608 immunotoxin Toxicity 0.000 description 1
- 230000002637 immunotoxin Effects 0.000 description 1
- 229940051026 immunotoxin Drugs 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 102000006495 integrins Human genes 0.000 description 1
- 108010044426 integrins Proteins 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000003871 intestinal function Effects 0.000 description 1
- 230000006831 intrinsic signaling Effects 0.000 description 1
- 210000001503 joint Anatomy 0.000 description 1
- 208000018937 joint inflammation Diseases 0.000 description 1
- 210000003041 ligament Anatomy 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 230000003340 mental effect Effects 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229940071648 metered dose inhaler Drugs 0.000 description 1
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000004001 molecular interaction Effects 0.000 description 1
- 239000003068 molecular probe Substances 0.000 description 1
- 230000000921 morphogenic effect Effects 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 238000000569 multi-angle light scattering Methods 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- 210000005012 myelin Anatomy 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 230000011234 negative regulation of signal transduction Effects 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 210000000944 nerve tissue Anatomy 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 210000003458 notochord Anatomy 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 230000002188 osteogenic effect Effects 0.000 description 1
- YPZRWBKMTBYPTK-UHFFFAOYSA-N oxidized gamma-L-glutamyl-L-cysteinylglycine Natural products OC(=O)C(N)CCC(=O)NC(C(=O)NCC(O)=O)CSSCC(C(=O)NCC(O)=O)NC(=O)CCC(N)C(O)=O YPZRWBKMTBYPTK-UHFFFAOYSA-N 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000006320 pegylation Effects 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 210000001322 periplasm Anatomy 0.000 description 1
- 230000002688 persistence Effects 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 210000002826 placenta Anatomy 0.000 description 1
- 229920001748 polybutylene Polymers 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 210000004896 polypeptide structure Anatomy 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 238000007639 printing Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 238000002818 protein evolution Methods 0.000 description 1
- 208000005333 pulmonary edema Diseases 0.000 description 1
- 210000004879 pulmonary tissue Anatomy 0.000 description 1
- 229950010131 puromycin Drugs 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000001177 retroviral effect Effects 0.000 description 1
- 238000002702 ribosome display Methods 0.000 description 1
- 238000013391 scatchard analysis Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 230000036303 septic shock Effects 0.000 description 1
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 1
- 230000037432 silent mutation Effects 0.000 description 1
- 238000004513 sizing Methods 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 230000000391 smoking effect Effects 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 238000012289 standard assay Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 230000001839 systemic circulation Effects 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 210000002435 tendon Anatomy 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 229960002175 thyroglobulin Drugs 0.000 description 1
- 206010043778 thyroiditis Diseases 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 102000027257 transmembrane receptors Human genes 0.000 description 1
- 108091008578 transmembrane receptors Proteins 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 210000002993 trophoblast Anatomy 0.000 description 1
- 229960001322 trypsin Drugs 0.000 description 1
- 201000008827 tuberculosis Diseases 0.000 description 1
- 241000701161 unidentified adenovirus Species 0.000 description 1
- 241000701447 unidentified baculovirus Species 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- 230000009677 vaginal delivery Effects 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 210000001835 viscera Anatomy 0.000 description 1
- 210000004127 vitreous body Anatomy 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/02—Nasal agents, e.g. decongestants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/16—Antivirals for RNA viruses for influenza or rhinoviruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/40—Immunoglobulins specific features characterized by post-translational modification
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/569—Single domain, e.g. dAb, sdAb, VHH, VNAR or nanobody®
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/94—Stability, e.g. half-life, pH, temperature or enzyme-resistance
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/31—Fusion polypeptide fusions, other than Fc, for prolonged plasma life, e.g. albumin
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Pulmonology (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Virology (AREA)
- Rheumatology (AREA)
- Otolaryngology (AREA)
- Cardiology (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Heart & Thoracic Surgery (AREA)
- Transplantation (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Pain & Pain Management (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15374609P | 2009-02-19 | 2009-02-19 | |
| US61/153,746 | 2009-02-19 | ||
| US24119809P | 2009-09-10 | 2009-09-10 | |
| US61/241,198 | 2009-09-10 | ||
| PCT/EP2010/052005 WO2010094720A2 (en) | 2009-02-19 | 2010-02-17 | Improved anti-tnfr1 polypeptides, antibody variable domains & antagonists |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015151376A Division JP2016007218A (ja) | 2009-02-19 | 2015-07-31 | 改善された抗tnfr1ポリペプチド、抗体可変ドメインおよびアンタゴニスト |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012517818A true JP2012517818A (ja) | 2012-08-09 |
| JP2012517818A5 JP2012517818A5 (enExample) | 2013-03-07 |
Family
ID=42136168
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011550552A Pending JP2012517818A (ja) | 2009-02-19 | 2010-02-17 | 改善された抗tnfr1ポリペプチド、抗体可変ドメインおよびアンタゴニスト |
| JP2015151376A Pending JP2016007218A (ja) | 2009-02-19 | 2015-07-31 | 改善された抗tnfr1ポリペプチド、抗体可変ドメインおよびアンタゴニスト |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015151376A Pending JP2016007218A (ja) | 2009-02-19 | 2015-07-31 | 改善された抗tnfr1ポリペプチド、抗体可変ドメインおよびアンタゴニスト |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US20110301335A1 (enExample) |
| EP (1) | EP2398827A2 (enExample) |
| JP (2) | JP2012517818A (enExample) |
| KR (1) | KR20110119806A (enExample) |
| CN (1) | CN102405236A (enExample) |
| AU (1) | AU2010215479B2 (enExample) |
| BR (1) | BRPI1008014A2 (enExample) |
| CA (1) | CA2750477A1 (enExample) |
| EA (1) | EA022925B1 (enExample) |
| IL (1) | IL214648A0 (enExample) |
| MX (1) | MX2011008799A (enExample) |
| SG (1) | SG173173A1 (enExample) |
| WO (1) | WO2010094720A2 (enExample) |
| ZA (1) | ZA201105627B (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013541522A (ja) * | 2010-09-16 | 2013-11-14 | バリオファルム・アクチェンゲゼルシャフト | 抗huTNFR1抗体 |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9028822B2 (en) * | 2002-06-28 | 2015-05-12 | Domantis Limited | Antagonists against TNFR1 and methods of use therefor |
| KR101819754B1 (ko) | 2009-02-19 | 2018-01-18 | 글락소 그룹 리미티드 | 개선된 항-혈청 알부민 결합 변이체 |
| WO2011008814A2 (en) | 2009-07-14 | 2011-01-20 | Immune Tolerance Institute, Inc., A California Not-For-Profit Corporation | Multiplexed measurement of exogenous and endogenous dna |
| US20120107330A1 (en) * | 2009-07-16 | 2012-05-03 | Adriaan Allart Stoop | Antagonists, uses & methods for partially inhibiting tnfr1 |
| PH12012500826A1 (en) * | 2009-10-27 | 2013-01-07 | Glaxo Group Ltd | Stable anti-tnfr1 polypeptides, antibody variable domains & antagonists |
| AU2011254559B2 (en) * | 2010-05-20 | 2014-09-04 | Glaxo Group Limited | Improved anti-serum albumin binding variants |
| WO2012172070A1 (en) * | 2011-06-17 | 2012-12-20 | Glaxo Group Limited | Tumour necrosis factor receptor 1 antagonists |
| WO2015104322A1 (en) | 2014-01-09 | 2015-07-16 | Glaxosmithkline Intellectual Property Development Limited | Treatment of inflammatory diseases with non-competitive tnfr1 antagonists |
| US9107906B1 (en) | 2014-10-28 | 2015-08-18 | Adma Biologics, Inc. | Compositions and methods for the treatment of immunodeficiency |
| WO2016094309A1 (en) * | 2014-12-10 | 2016-06-16 | Myosotis | Inhibition of tnf signaling in cancer immunotherapy |
| NO2768984T3 (enExample) * | 2015-11-12 | 2018-06-09 | ||
| CN107043413A (zh) * | 2016-02-05 | 2017-08-15 | 华中农业大学 | 一种肺结核相关的尿液生物标志蛋白及其应用 |
| US10259865B2 (en) | 2017-03-15 | 2019-04-16 | Adma Biologics, Inc. | Anti-pneumococcal hyperimmune globulin for the treatment and prevention of pneumococcal infection |
| US12352766B2 (en) * | 2019-01-31 | 2025-07-08 | Sekisui Medical Co., Ltd. | Immunoassay method for free aim in biological sample, and method for detecting NASH in subject |
| US12472266B2 (en) | 2019-02-21 | 2025-11-18 | Enosi Therapeutics Corporation | Antibodies and enonomers |
| IL300930A (en) * | 2020-08-27 | 2023-04-01 | Enosi Therapeutics Corp | Methods and compositions for the treatment of autoimmune diseases and cancer |
| WO2022117569A1 (en) | 2020-12-02 | 2022-06-09 | Oncurious Nv | A ccr8 antagonist antibody in combination with a lymphotoxin beta receptor agonist antibody in therapy against cancer |
| WO2025021184A1 (zh) * | 2023-07-27 | 2025-01-30 | 上海复宏汉霖生物医药有限公司 | 编码尿酸氧化酶的多核苷酸及其相关组合物和方法 |
| WO2025049818A1 (en) | 2023-08-29 | 2025-03-06 | Enosi Therapeutics Corporation | Tnfr1 antagonists lacking agonist activity and uses thereof |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008515420A (ja) * | 2004-10-08 | 2008-05-15 | ドマンティス リミテッド | Tnfr1に対する単一ドメイン抗体およびその使用方法 |
| WO2008149148A2 (en) * | 2007-06-06 | 2008-12-11 | Domantis Limited | Polypeptides, antibody variable domains and antagonists |
| WO2008149144A2 (en) * | 2007-06-06 | 2008-12-11 | Domantis Limited | Polypeptides, antibody variable domains and antagonists |
| JP2012532620A (ja) * | 2009-07-16 | 2012-12-20 | グラクソ グループ リミテッド | 改良型抗血清アルブミン結合単一可変ドメイン |
| JP2012532619A (ja) * | 2009-07-16 | 2012-12-20 | グラクソ グループ リミテッド | Tnfr1を部分的に阻害するためのアンタゴニスト、用途および方法 |
| JP2013507978A (ja) * | 2009-10-27 | 2013-03-07 | グラクソ グループ リミテッド | 安定な抗tnfr1ポリペプチド、抗体可変ドメイン及びアンタゴニスト |
Family Cites Families (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3883899T3 (de) | 1987-03-18 | 1999-04-22 | Sb2, Inc., Danville, Calif. | Geänderte antikörper. |
| GB8725529D0 (en) | 1987-10-30 | 1987-12-02 | Delta Biotechnology Ltd | Polypeptides |
| WO1989007142A1 (en) | 1988-02-05 | 1989-08-10 | Morrison Sherie L | Domain-modified constant region antibodies |
| ATE151110T1 (de) | 1988-09-02 | 1997-04-15 | Protein Eng Corp | Herstellung und auswahl von rekombinantproteinen mit verschiedenen bindestellen |
| ATE92107T1 (de) | 1989-04-29 | 1993-08-15 | Delta Biotechnology Ltd | N-terminale fragmente von menschliches serumalbumin enthaltenden fusionsproteinen. |
| US5977307A (en) | 1989-09-07 | 1999-11-02 | Alkermes, Inc. | Transferrin receptor specific ligand-neuropharmaceutical agent fusion proteins |
| US6172197B1 (en) | 1991-07-10 | 2001-01-09 | Medical Research Council | Methods for producing members of specific binding pairs |
| WO1994026087A2 (en) | 1993-05-14 | 1994-11-24 | Connor Kim C O | Recombinant protein production and insect cell culture and process |
| JPH08511420A (ja) | 1993-06-16 | 1996-12-03 | セルテック・セラピューテイクス・リミテッド | 抗 体 |
| WO1999002671A1 (en) | 1997-07-07 | 1999-01-21 | Medical Research Council | In vitro sorting method |
| IL127127A0 (en) | 1998-11-18 | 1999-09-22 | Peptor Ltd | Small functional units of antibody heavy chain variable regions |
| AU2001238076A1 (en) | 2000-02-11 | 2001-08-20 | The Government Of The United States Of America, As Represented By The Secretary, Department Of Health And Human Services, National Institutes Of Health | Identification of a novel domain in the tumor necrosis factor receptor family that mediates pre-ligand receptor assembly and function |
| WO2004003019A2 (en) | 2002-06-28 | 2004-01-08 | Domantis Limited | Immunoglobin single variant antigen-binding domains and dual-specific constructs |
| JP4303105B2 (ja) | 2001-06-28 | 2009-07-29 | ドマンティス リミテッド | 二重特異性リガンドとその利用 |
| AU2003210806A1 (en) | 2002-03-05 | 2003-09-22 | Eli Lilly And Company | Heterologous g-csf fusion proteins |
| US7696320B2 (en) | 2004-08-24 | 2010-04-13 | Domantis Limited | Ligands that have binding specificity for VEGF and/or EGFR and methods of use therefor |
| US9321832B2 (en) * | 2002-06-28 | 2016-04-26 | Domantis Limited | Ligand |
| EP2284192A3 (en) | 2002-11-08 | 2011-07-20 | Ablynx N.V. | Camelidae antibodies for sublingual administration |
| GB0230203D0 (en) | 2002-12-27 | 2003-02-05 | Domantis Ltd | Fc fusion |
| AU2003290330A1 (en) | 2002-12-27 | 2004-07-22 | Domantis Limited | Dual specific single domain antibodies specific for a ligand and for the receptor of the ligand |
| JP4889493B2 (ja) | 2003-05-14 | 2012-03-07 | ドマンティス リミテッド | ポリペプチドレパートリーから可逆的にアンフォールドするポリペプチドを回収するための方法 |
| ES2315664T3 (es) | 2003-06-30 | 2009-04-01 | Domantis Limited | Anticuerpos de dominio unico (dab) pegilados. |
| PL1729795T3 (pl) | 2004-02-09 | 2016-08-31 | Human Genome Sciences Inc | Białka fuzyjne albuminy |
| EP2172553A1 (en) | 2004-03-24 | 2010-04-07 | Domantis Limited | Gas1 universal leader |
| GB0418651D0 (en) | 2004-08-20 | 2004-09-22 | Medical Res Council | Method |
| CN101084014A (zh) * | 2004-10-08 | 2007-12-05 | 杜门蒂斯有限公司 | 抗肿瘤坏死因子受体1的单域抗体及其使用方法 |
| GB0521621D0 (en) | 2005-10-24 | 2005-11-30 | Domantis Ltd | Tumor necrosis factor receptor 1 antagonists for treating respiratory diseases |
| JP2009519011A (ja) * | 2005-12-01 | 2009-05-14 | ドマンティス リミテッド | インターロイキン1受容体1型に結合する非競合ドメイン抗体フォーマット |
| US20100056439A1 (en) * | 2005-12-06 | 2010-03-04 | Domantis Limited | Ligands that have binding specificity for egfr and/or vegf and methods of use therefor |
| US7868885B2 (en) | 2007-06-22 | 2011-01-11 | Microsoft Corporation | Direct manipulation of subdivision surfaces using a graphics processing unit |
-
2010
- 2010-02-17 KR KR1020117021824A patent/KR20110119806A/ko not_active Ceased
- 2010-02-17 BR BRPI1008014A patent/BRPI1008014A2/pt not_active IP Right Cessation
- 2010-02-17 US US13/202,349 patent/US20110301335A1/en not_active Abandoned
- 2010-02-17 CN CN2010800173301A patent/CN102405236A/zh active Pending
- 2010-02-17 JP JP2011550552A patent/JP2012517818A/ja active Pending
- 2010-02-17 MX MX2011008799A patent/MX2011008799A/es not_active Application Discontinuation
- 2010-02-17 AU AU2010215479A patent/AU2010215479B2/en not_active Ceased
- 2010-02-17 EP EP10704151A patent/EP2398827A2/en not_active Withdrawn
- 2010-02-17 EA EA201190116A patent/EA022925B1/ru not_active IP Right Cessation
- 2010-02-17 SG SG2011054459A patent/SG173173A1/en unknown
- 2010-02-17 CA CA2750477A patent/CA2750477A1/en not_active Abandoned
- 2010-02-17 WO PCT/EP2010/052005 patent/WO2010094720A2/en not_active Ceased
-
2011
- 2011-07-29 ZA ZA2011/05627A patent/ZA201105627B/en unknown
- 2011-08-15 IL IL214648A patent/IL214648A0/en unknown
-
2015
- 2015-07-31 JP JP2015151376A patent/JP2016007218A/ja active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008515420A (ja) * | 2004-10-08 | 2008-05-15 | ドマンティス リミテッド | Tnfr1に対する単一ドメイン抗体およびその使用方法 |
| WO2008149148A2 (en) * | 2007-06-06 | 2008-12-11 | Domantis Limited | Polypeptides, antibody variable domains and antagonists |
| WO2008149144A2 (en) * | 2007-06-06 | 2008-12-11 | Domantis Limited | Polypeptides, antibody variable domains and antagonists |
| JP2012532620A (ja) * | 2009-07-16 | 2012-12-20 | グラクソ グループ リミテッド | 改良型抗血清アルブミン結合単一可変ドメイン |
| JP2012532619A (ja) * | 2009-07-16 | 2012-12-20 | グラクソ グループ リミテッド | Tnfr1を部分的に阻害するためのアンタゴニスト、用途および方法 |
| JP2013507978A (ja) * | 2009-10-27 | 2013-03-07 | グラクソ グループ リミテッド | 安定な抗tnfr1ポリペプチド、抗体可変ドメイン及びアンタゴニスト |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013541522A (ja) * | 2010-09-16 | 2013-11-14 | バリオファルム・アクチェンゲゼルシャフト | 抗huTNFR1抗体 |
Also Published As
| Publication number | Publication date |
|---|---|
| EA022925B1 (ru) | 2016-03-31 |
| AU2010215479B2 (en) | 2015-01-22 |
| BRPI1008014A2 (pt) | 2016-10-04 |
| MX2011008799A (es) | 2011-09-27 |
| WO2010094720A3 (en) | 2011-01-13 |
| KR20110119806A (ko) | 2011-11-02 |
| EA201190116A1 (ru) | 2012-06-29 |
| IL214648A0 (en) | 2011-09-27 |
| JP2016007218A (ja) | 2016-01-18 |
| EP2398827A2 (en) | 2011-12-28 |
| CN102405236A (zh) | 2012-04-04 |
| CA2750477A1 (en) | 2010-08-26 |
| ZA201105627B (en) | 2013-01-30 |
| WO2010094720A2 (en) | 2010-08-26 |
| US20110301335A1 (en) | 2011-12-08 |
| SG173173A1 (en) | 2011-08-29 |
| AU2010215479A1 (en) | 2011-08-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2010215479B2 (en) | Improved anti-TNFR1 polypeptides, antibody variable domains & antagonists | |
| US20120107330A1 (en) | Antagonists, uses & methods for partially inhibiting tnfr1 | |
| JP5325211B2 (ja) | ポリペプチド、抗体可変ドメインおよびアンタゴニスト | |
| US20090191217A1 (en) | Anti-IL-1R1 Single Domain Antibodies And Therapeutic Uses | |
| CN102686239B (zh) | 稳定的抗-tnfr1多肽、抗体可变结构域和拮抗剂 | |
| JP2010529840A (ja) | ポリペプチド、抗体可変ドメインおよびアンタゴニスト | |
| JP2010528647A (ja) | ポリペプチド、抗体可変ドメインおよびアンタゴニスト | |
| US9028817B2 (en) | Stable anti-TNFR1 polypeptides, antibody variable domains and antagonists | |
| US20110236380A1 (en) | Ligands that bind il-13 | |
| MX2008006882A (en) | Noncompetitive domain antibody formats that bind interleukin 1 receptor type 1 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130121 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130121 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140610 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140901 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140908 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20141208 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20150331 |