JP2010534551A - 全バリアエラストマーゲル充填型乳房プロテーゼ - Google Patents
全バリアエラストマーゲル充填型乳房プロテーゼ Download PDFInfo
- Publication number
- JP2010534551A JP2010534551A JP2010520079A JP2010520079A JP2010534551A JP 2010534551 A JP2010534551 A JP 2010534551A JP 2010520079 A JP2010520079 A JP 2010520079A JP 2010520079 A JP2010520079 A JP 2010520079A JP 2010534551 A JP2010534551 A JP 2010534551A
- Authority
- JP
- Japan
- Prior art keywords
- shell
- implant
- silicone
- gel
- silicone elastomer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 230000004888 barrier function Effects 0.000 title abstract description 39
- 210000000481 breast Anatomy 0.000 title abstract description 31
- 229920001971 elastomer Polymers 0.000 title abstract description 7
- 239000000806 elastomer Substances 0.000 title abstract description 7
- 239000007943 implant Substances 0.000 claims abstract description 145
- 239000000463 material Substances 0.000 claims abstract description 105
- 229920001296 polysiloxane Polymers 0.000 claims abstract description 103
- 229920002379 silicone rubber Polymers 0.000 claims abstract description 96
- 125000006267 biphenyl group Chemical group 0.000 claims abstract description 50
- -1 polydimethylsiloxane Polymers 0.000 claims abstract description 47
- 239000004205 dimethyl polysiloxane Substances 0.000 claims abstract description 19
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 claims abstract description 19
- 125000003636 chemical group Chemical group 0.000 claims abstract description 17
- 238000000465 moulding Methods 0.000 claims abstract description 15
- 238000000034 method Methods 0.000 claims description 30
- 239000004305 biphenyl Substances 0.000 claims description 26
- 229920000642 polymer Polymers 0.000 claims description 25
- 239000006185 dispersion Substances 0.000 claims description 22
- 235000010290 biphenyl Nutrition 0.000 claims description 20
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N phenylbenzene Natural products C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 claims description 20
- 239000002904 solvent Substances 0.000 claims description 20
- 229920006395 saturated elastomer Polymers 0.000 claims description 17
- 239000011248 coating agent Substances 0.000 claims description 13
- 238000000576 coating method Methods 0.000 claims description 13
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 11
- 239000002243 precursor Substances 0.000 claims description 10
- 238000004519 manufacturing process Methods 0.000 claims description 8
- 239000000203 mixture Substances 0.000 claims description 7
- 239000002131 composite material Substances 0.000 claims description 4
- 125000000725 trifluoropropyl group Chemical group [H]C([H])(*)C([H])([H])C(F)(F)F 0.000 claims description 4
- 238000001704 evaporation Methods 0.000 claims description 3
- 238000009738 saturating Methods 0.000 claims description 2
- 125000003944 tolyl group Chemical group 0.000 claims description 2
- NYMPGSQKHIOWIO-UHFFFAOYSA-N hydroxy(diphenyl)silicon Chemical class C=1C=CC=CC=1[Si](O)C1=CC=CC=C1 NYMPGSQKHIOWIO-UHFFFAOYSA-N 0.000 claims 1
- 238000001175 rotational moulding Methods 0.000 abstract description 19
- 230000003416 augmentation Effects 0.000 abstract description 2
- 230000035515 penetration Effects 0.000 abstract 1
- 239000007921 spray Substances 0.000 abstract 1
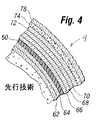
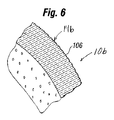
- 239000011257 shell material Substances 0.000 description 163
- 239000010410 layer Substances 0.000 description 103
- 208000015943 Coeliac disease Diseases 0.000 description 22
- 239000002356 single layer Substances 0.000 description 15
- 235000013870 dimethyl polysiloxane Nutrition 0.000 description 11
- 125000001424 substituent group Chemical group 0.000 description 11
- 230000000740 bleeding effect Effects 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- 238000005266 casting Methods 0.000 description 6
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 5
- 239000000945 filler Substances 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- 239000007789 gas Substances 0.000 description 4
- 230000008961 swelling Effects 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- 238000007598 dipping method Methods 0.000 description 3
- 125000001153 fluoro group Chemical group F* 0.000 description 3
- 239000012778 molding material Substances 0.000 description 3
- 229920001225 polyester resin Polymers 0.000 description 3
- 239000004645 polyester resin Substances 0.000 description 3
- 229920002635 polyurethane Polymers 0.000 description 3
- 239000004814 polyurethane Substances 0.000 description 3
- 239000004593 Epoxy Substances 0.000 description 2
- XQFRJNBWHJMXHO-RRKCRQDMSA-N IDUR Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 XQFRJNBWHJMXHO-RRKCRQDMSA-N 0.000 description 2
- 239000004677 Nylon Substances 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 239000004809 Teflon Substances 0.000 description 2
- 229920006362 Teflon® Polymers 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 210000001217 buttock Anatomy 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 230000006837 decompression Effects 0.000 description 2
- 230000032798 delamination Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 229920000840 ethylene tetrafluoroethylene copolymer Polymers 0.000 description 2
- 230000009969 flowable effect Effects 0.000 description 2
- 229920002313 fluoropolymer Polymers 0.000 description 2
- 239000004811 fluoropolymer Substances 0.000 description 2
- 229960004716 idoxuridine Drugs 0.000 description 2
- 238000002513 implantation Methods 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 229920001778 nylon Polymers 0.000 description 2
- 230000000704 physical effect Effects 0.000 description 2
- 229920000573 polyethylene Polymers 0.000 description 2
- 229920001155 polypropylene Polymers 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 229920005573 silicon-containing polymer Polymers 0.000 description 2
- 229920002545 silicone oil Polymers 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 229920006051 Capron® Polymers 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 238000004061 bleaching Methods 0.000 description 1
- 210000000746 body region Anatomy 0.000 description 1
- 244000309466 calf Species 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- SZPIKCAXBLKNNK-UHFFFAOYSA-N dimethyl-phenoxy-phenylsilane Chemical group C=1C=CC=CC=1[Si](C)(C)OC1=CC=CC=C1 SZPIKCAXBLKNNK-UHFFFAOYSA-N 0.000 description 1
- KPUWHANPEXNPJT-UHFFFAOYSA-N disiloxane Chemical class [SiH3]O[SiH3] KPUWHANPEXNPJT-UHFFFAOYSA-N 0.000 description 1
- 239000013536 elastomeric material Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- JBKVHLHDHHXQEQ-UHFFFAOYSA-N epsilon-caprolactam Chemical compound O=C1CCCCCN1 JBKVHLHDHHXQEQ-UHFFFAOYSA-N 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 210000001847 jaw Anatomy 0.000 description 1
- 238000003754 machining Methods 0.000 description 1
- 210000005075 mammary gland Anatomy 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 125000000962 organic group Chemical group 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 230000004800 psychological effect Effects 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 210000004872 soft tissue Anatomy 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- 229920005992 thermoplastic resin Polymers 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/12—Mammary prostheses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2210/0076—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof multilayered, e.g. laminated structures
Landscapes
- Health & Medical Sciences (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
- Materials For Medical Uses (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US95230407P | 2007-07-27 | 2007-07-27 | |
| PCT/US2008/071068 WO2009018105A1 (en) | 2007-07-27 | 2008-07-24 | All-barrier elastomeric gel-filled breast prothesis |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2010534551A true JP2010534551A (ja) | 2010-11-11 |
| JP2010534551A5 JP2010534551A5 (enExample) | 2011-09-08 |
Family
ID=39745102
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010520079A Pending JP2010534551A (ja) | 2007-07-27 | 2008-07-24 | 全バリアエラストマーゲル充填型乳房プロテーゼ |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US8043373B2 (enExample) |
| EP (4) | EP2180848B1 (enExample) |
| JP (1) | JP2010534551A (enExample) |
| AU (1) | AU2008282479B2 (enExample) |
| BR (1) | BRPI0814135A2 (enExample) |
| CA (1) | CA2694917C (enExample) |
| ES (2) | ES2787705T3 (enExample) |
| PT (1) | PT2180848T (enExample) |
| WO (1) | WO2009018105A1 (enExample) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012176982A3 (ko) * | 2011-06-23 | 2013-02-14 | Yu Won-Seok | 응력집중을 최소화한 실리콘 인공유방 보형물 및 그 제조방법 |
| KR101966979B1 (ko) * | 2018-04-25 | 2019-08-27 | 김한조 | 인체 주입 필러를 이용한 가슴보형물의 제조방법 |
| KR102324101B1 (ko) | 2021-02-21 | 2021-11-10 | 김재홍 | 압력 센서를 갖춘 보형물용 패치, 이를 포함하는 보형물, 및 보형물 이상 감지 장치 |
| WO2023055218A1 (ko) | 2021-10-03 | 2023-04-06 | 김재홍 | 보형물 이상 감지 장치 및 보형물 이상 감지 시스템 |
| US12329541B2 (en) | 2021-02-21 | 2025-06-17 | W. Ai Co., Ltd. | Breast implant and apparatus for sensing abnormality of the same |
Families Citing this family (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
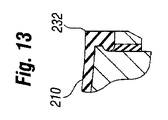
| US20110029077A1 (en) * | 2008-03-12 | 2011-02-03 | Jong-Soo Choi | Medical implant |
| US8377128B2 (en) | 2008-04-28 | 2013-02-19 | Allergan, Inc. | Flush patch for elastomeric implant shell |
| EP2303189A1 (en) * | 2008-04-28 | 2011-04-06 | Allergan, Inc. | Flush patch for elastomeric implant shell |
| CA2734252A1 (en) | 2008-08-20 | 2010-02-25 | Allergan, Inc. | Self-sealing shell for inflatable prostheses |
| US20100114311A1 (en) * | 2008-11-05 | 2010-05-06 | Hilton Becker | Multi-Lumen Breast Prothesis and Improved Valve Assembly Therefor |
| WO2011022235A2 (en) | 2009-08-18 | 2011-02-24 | Allergan, Inc. | Reinforced prosthetic implant with flexible shell |
| BR112012008774A2 (pt) * | 2009-10-16 | 2020-08-18 | Won-Seok Yu | protese de silicone artificial em silicone que minimiza a concentração da pressão e o método de produção |
| US9138308B2 (en) | 2010-02-03 | 2015-09-22 | Apollo Endosurgery, Inc. | Mucosal tissue adhesion via textured surface |
| WO2011097292A1 (en) | 2010-02-05 | 2011-08-11 | Allergan, Inc. | Inflatable prostheses and methods of making same |
| JP2013526932A (ja) | 2010-05-10 | 2013-06-27 | アラーガン、インコーポレイテッド | 多孔質材料、作製方法および使用 |
| EP2569021B1 (en) | 2010-05-11 | 2017-01-04 | Allergan, Inc. | Porogen compositions, methods of making and uses |
| JP2013528067A (ja) | 2010-05-11 | 2013-07-08 | アラーガン、インコーポレイテッド | ポロゲン材料、製造方法、および使用 |
| EP2582412A1 (en) | 2010-06-16 | 2013-04-24 | Allergan, Inc. | Open-cell surface foam materials |
| KR20120032392A (ko) * | 2010-09-28 | 2012-04-05 | 유원석 | 내구성이 향상된 쉘을 갖는 실리콘 보형물 제조방법 |
| US20120232652A1 (en) * | 2011-03-07 | 2012-09-13 | Rolando Mora | Implant with a visual indicator of a barrier layer |
| DK2581193T3 (en) | 2011-10-14 | 2016-02-29 | Polytech Health&Aesthetics Gmbh | A process for the production of implants or intermediates for such implants and implants as well as intermediates which are obtained by such a method |
| US8556968B2 (en) * | 2011-11-09 | 2013-10-15 | Ideal Implant Incorporated | Breast implant with low coefficient of friction between internal shells in an aqueous fluid environment |
| US9144489B2 (en) | 2012-06-13 | 2015-09-29 | Elwha Llc | Breast implant with covering, analyte sensors and internal power source |
| US8790400B2 (en) | 2012-06-13 | 2014-07-29 | Elwha Llc | Breast implant with covering and analyte sensors responsive to external power source |
| US8795359B2 (en) | 2012-06-13 | 2014-08-05 | Elwha Llc | Breast implant with regionalized analyte sensors and internal power source |
| US8808373B2 (en) | 2012-06-13 | 2014-08-19 | Elwha Llc | Breast implant with regionalized analyte sensors responsive to external power source |
| US9144488B2 (en) | 2012-06-13 | 2015-09-29 | Elwha Llc | Breast implant with analyte sensors responsive to external power source |
| US9211185B2 (en) | 2012-06-13 | 2015-12-15 | Elwha Llc | Breast implant with analyte sensors and internal power source |
| WO2014022657A1 (en) | 2012-08-02 | 2014-02-06 | Allergan, Inc. | Mucosal tissue adhesion via textured surface |
| WO2014047617A1 (en) | 2012-09-24 | 2014-03-27 | Allergan, Inc. | Porous materials, methods of making and uses |
| EP2900289A1 (en) | 2012-09-28 | 2015-08-05 | Allergan, Inc. | Porogen compositions, methods of making and uses |
| WO2014210605A2 (en) * | 2013-06-28 | 2014-12-31 | Autonomic Technologies, Inc. | Implantable medical device and method for laser processing |
| EP3622972A1 (en) | 2013-07-11 | 2020-03-18 | Tepha, Inc. | Absorbable implants for plastic surgery |
| WO2015176014A1 (en) * | 2014-05-16 | 2015-11-19 | Allergan, Inc. | Soft filled prosthesis shell with variable texture |
| USD736372S1 (en) * | 2014-12-07 | 2015-08-11 | Robert G. Anderson | Implant insertion device |
| RU2727976C2 (ru) | 2015-03-12 | 2020-07-28 | Джи энд Джи БАЙОТЕКНОЛОДЖИ ЛТД. | Композитный материал для имплантатов |
| US9737395B2 (en) | 2015-06-26 | 2017-08-22 | Phi Nguyen | Systems and methods for reducing scarring |
| US20180140410A1 (en) * | 2016-11-21 | 2018-05-24 | William A. Brennan | Cosmetic implant |
| EP3749251B1 (en) | 2018-02-09 | 2023-04-26 | Tepha, Inc. | Full contour breast implant |
| USD889654S1 (en) | 2018-02-09 | 2020-07-07 | Tepha, Inc. | Three dimensional mastopexy implant |
| CR20200419A (es) | 2018-02-18 | 2020-10-23 | G & G Biotechnology Ltd | Implantes con adhesión mejorada a la cubierta |
| USD892329S1 (en) | 2018-07-03 | 2020-08-04 | Tepha, Inc. | Three dimensional mastopexy implant |
| KR102754318B1 (ko) | 2018-07-25 | 2025-01-14 | 이스타블리쉬먼트 렙스 에스.에이. | 대칭 형상을 갖는 임플란트 |
| US11160630B2 (en) | 2018-09-13 | 2021-11-02 | Allergan, Inc. | Tissue expansion device |
| USD896383S1 (en) | 2018-09-13 | 2020-09-15 | Allergan, Inc. | Tissue expansion device |
| EP3860506A4 (en) | 2018-10-02 | 2022-06-29 | Tepha, Inc. | Medical devices to limit movement of breast implants |
| US11324585B2 (en) * | 2018-10-12 | 2022-05-10 | Biosense Webster (Israel) Ltd. | Method for producing shell and foam filler for a breast implant |
| KR20220106798A (ko) | 2019-11-25 | 2022-07-29 | 테파 인크. | 유방 보형물의 움직임을 제한하기 위한 유방 보형물 랩 및 관련 방법 |
| US12059341B2 (en) * | 2021-03-05 | 2024-08-13 | Mentor Worldwide Llc | Systems, devices and methods of making prosthetic implants having self-sealing membranes for closing punctures and preventing leaks |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS56501711A (enExample) * | 1979-12-17 | 1981-11-26 | ||
| US4455691A (en) * | 1979-10-03 | 1984-06-26 | Minnesota Mining And Manufacturing Company | Silicone gel filled prosthesis |
| JPS6238151A (ja) * | 1985-06-11 | 1987-02-19 | エシル コーポレーション | 生体内移植物として使用するのに適した製造物 |
| US4773909A (en) * | 1981-10-06 | 1988-09-27 | Memorial Hospital For Cancer And Allied Diseases | Multi-lumen high profile mammary implant |
| JP2004536236A (ja) * | 2001-07-18 | 2004-12-02 | マックガーン、メディカル、コーポレイション | 医療用物品の回転成形 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4650487A (en) * | 1980-10-27 | 1987-03-17 | Memorial Hospital For Cancer And Allied Diseases | Multi-lumen high profile mammary implant |
| US5534609A (en) * | 1995-02-03 | 1996-07-09 | Osi Specialties, Inc. | Polysiloxane compositions |
| US6099565A (en) * | 1995-06-07 | 2000-08-08 | Sakura, Jr.; Chester Y. | Prosthetic tissue implant and filler therefor |
| EP0872221B1 (en) | 1997-04-05 | 1999-02-24 | MediSyn Technologies Limited | Seamless breast prosthesis |
| US20030163197A1 (en) * | 2002-02-28 | 2003-08-28 | David Chen | Detachable multi-chamber breast form with permanently grown adhesive |
| CA2734252A1 (en) * | 2008-08-20 | 2010-02-25 | Allergan, Inc. | Self-sealing shell for inflatable prostheses |
-
2008
- 2008-07-24 JP JP2010520079A patent/JP2010534551A/ja active Pending
- 2008-07-24 EP EP08782335.7A patent/EP2180848B1/en not_active Not-in-force
- 2008-07-24 PT PT87823357T patent/PT2180848T/pt unknown
- 2008-07-24 WO PCT/US2008/071068 patent/WO2009018105A1/en not_active Ceased
- 2008-07-24 EP EP20156117.2A patent/EP3677222A1/en not_active Withdrawn
- 2008-07-24 CA CA2694917A patent/CA2694917C/en not_active Expired - Fee Related
- 2008-07-24 ES ES16190096T patent/ES2787705T3/es active Active
- 2008-07-24 AU AU2008282479A patent/AU2008282479B2/en not_active Ceased
- 2008-07-24 US US12/179,340 patent/US8043373B2/en active Active
- 2008-07-24 ES ES08782335.7T patent/ES2622833T3/es active Active
- 2008-07-24 BR BRPI0814135-5A2A patent/BRPI0814135A2/pt not_active IP Right Cessation
- 2008-07-24 EP EP16190096.4A patent/EP3150168B1/en active Active
- 2008-07-24 EP EP14155732.2A patent/EP2735285A1/en not_active Withdrawn
-
2011
- 2011-07-13 US US13/181,893 patent/US20110270392A1/en not_active Abandoned
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4455691A (en) * | 1979-10-03 | 1984-06-26 | Minnesota Mining And Manufacturing Company | Silicone gel filled prosthesis |
| JPS56501711A (enExample) * | 1979-12-17 | 1981-11-26 | ||
| US4773909A (en) * | 1981-10-06 | 1988-09-27 | Memorial Hospital For Cancer And Allied Diseases | Multi-lumen high profile mammary implant |
| JPS6238151A (ja) * | 1985-06-11 | 1987-02-19 | エシル コーポレーション | 生体内移植物として使用するのに適した製造物 |
| JP2004536236A (ja) * | 2001-07-18 | 2004-12-02 | マックガーン、メディカル、コーポレイション | 医療用物品の回転成形 |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012176982A3 (ko) * | 2011-06-23 | 2013-02-14 | Yu Won-Seok | 응력집중을 최소화한 실리콘 인공유방 보형물 및 그 제조방법 |
| KR101235284B1 (ko) * | 2011-06-23 | 2013-02-21 | 유원석 | 응력집중을 최소화한 실리콘 인공유방 보형물 및 그 제조방법 |
| US9132004B2 (en) | 2011-06-23 | 2015-09-15 | Won Seok Yu | Silicon breast implant which minimizes stress concentration and method for manufacturing same |
| KR101966979B1 (ko) * | 2018-04-25 | 2019-08-27 | 김한조 | 인체 주입 필러를 이용한 가슴보형물의 제조방법 |
| KR102324101B1 (ko) | 2021-02-21 | 2021-11-10 | 김재홍 | 압력 센서를 갖춘 보형물용 패치, 이를 포함하는 보형물, 및 보형물 이상 감지 장치 |
| WO2022177093A1 (ko) | 2021-02-21 | 2022-08-25 | 김재홍 | 가슴 보형물 및 가슴 보형물 이상 감지 장치 |
| US12329541B2 (en) | 2021-02-21 | 2025-06-17 | W. Ai Co., Ltd. | Breast implant and apparatus for sensing abnormality of the same |
| WO2023055218A1 (ko) | 2021-10-03 | 2023-04-06 | 김재홍 | 보형물 이상 감지 장치 및 보형물 이상 감지 시스템 |
| KR20230048473A (ko) | 2021-10-03 | 2023-04-11 | 김재홍 | 보형물 이상 감지 장치 및 보형물 이상 감지 시스템 |
| KR102639503B1 (ko) | 2021-10-03 | 2024-02-22 | 주식회사 더블유닷에이아이 | 보형물 이상 감지 장치 및 보형물 이상 감지 시스템 |
| KR20240025581A (ko) | 2021-10-03 | 2024-02-27 | 주식회사 더블유닷에이아이 | 보형물 이상 감지 장치 및 보형물 이상 감지 시스템 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2008282479A1 (en) | 2009-02-05 |
| ES2622833T3 (es) | 2017-07-07 |
| AU2008282479B2 (en) | 2013-05-02 |
| EP2180848B1 (en) | 2017-02-01 |
| PT2180848T (pt) | 2017-05-03 |
| US20090030515A1 (en) | 2009-01-29 |
| EP2180848A1 (en) | 2010-05-05 |
| EP3677222A1 (en) | 2020-07-08 |
| ES2787705T3 (es) | 2020-10-16 |
| US8043373B2 (en) | 2011-10-25 |
| CA2694917C (en) | 2016-02-16 |
| BRPI0814135A2 (pt) | 2015-02-03 |
| WO2009018105A1 (en) | 2009-02-05 |
| EP3150168A1 (en) | 2017-04-05 |
| EP3150168B1 (en) | 2020-02-12 |
| CA2694917A1 (en) | 2009-02-05 |
| EP2735285A1 (en) | 2014-05-28 |
| US20110270392A1 (en) | 2011-11-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2010534551A (ja) | 全バリアエラストマーゲル充填型乳房プロテーゼ | |
| EP2227175B1 (en) | Soft prosthesis shell texturing method | |
| CA2761902C (en) | Implants and methods for manufacturing same | |
| CA2610896C (en) | Vascular graft with kink resistance after clamping | |
| US5061276A (en) | Multi-layered poly(tetrafluoroethylene)/elastomer materials useful for in vivo implantation | |
| ES2433686T3 (es) | Moldeo por rotación de artículos médicos | |
| KR20140074327A (ko) | 관통가능하고 재밀봉가능한 이식편 | |
| AU2013211533A1 (en) | All-barrier elastomeric gel-filled breast prothesis | |
| KR20100111190A (ko) | 응력 집중을 최소화한 인공 유방 보형물 제조 방법 | |
| HK1168747B (en) | Implants and methods for manufacturing same | |
| HK1168747A (en) | Implants and methods for manufacturing same | |
| MX2007002161A (en) | Self-sealing ptfe graft with kink resistance |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110721 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110721 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130507 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130805 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130812 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130905 |
|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20130905 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20140507 |