JP2009544969A - 検体を捕捉することが可能なキャピラリー用コーティング - Google Patents
検体を捕捉することが可能なキャピラリー用コーティング Download PDFInfo
- Publication number
- JP2009544969A JP2009544969A JP2009521800A JP2009521800A JP2009544969A JP 2009544969 A JP2009544969 A JP 2009544969A JP 2009521800 A JP2009521800 A JP 2009521800A JP 2009521800 A JP2009521800 A JP 2009521800A JP 2009544969 A JP2009544969 A JP 2009544969A
- Authority
- JP
- Japan
- Prior art keywords
- polymer
- analyte
- group
- induced
- coating
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000576 coating method Methods 0.000 title claims abstract description 66
- 239000011248 coating agent Substances 0.000 title claims abstract description 53
- 239000012491 analyte Substances 0.000 title claims abstract description 38
- 229920000642 polymer Polymers 0.000 claims abstract description 171
- 238000000926 separation method Methods 0.000 claims abstract description 36
- 238000001962 electrophoresis Methods 0.000 claims abstract description 12
- 230000001960 triggered effect Effects 0.000 claims abstract description 8
- 238000000034 method Methods 0.000 claims description 51
- 239000011521 glass Substances 0.000 claims description 34
- 125000000524 functional group Chemical group 0.000 claims description 29
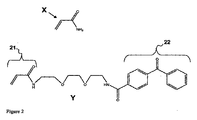
- -1 N-substituted acrylamide Chemical class 0.000 claims description 18
- 239000000203 mixture Substances 0.000 claims description 15
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical group C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 claims description 11
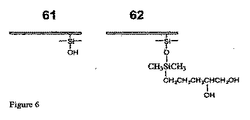
- 229910000077 silane Inorganic materials 0.000 claims description 11
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 claims description 10
- 239000003153 chemical reaction reagent Substances 0.000 claims description 10
- 238000001179 sorption measurement Methods 0.000 claims description 10
- 239000004372 Polyvinyl alcohol Substances 0.000 claims description 9
- 239000012965 benzophenone Substances 0.000 claims description 9
- 229920002451 polyvinyl alcohol Polymers 0.000 claims description 9
- 238000007306 functionalization reaction Methods 0.000 claims description 7
- 238000004519 manufacturing process Methods 0.000 claims description 7
- 102000004169 proteins and genes Human genes 0.000 claims description 7
- 108090000623 proteins and genes Proteins 0.000 claims description 7
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 claims description 7
- 230000004913 activation Effects 0.000 claims description 6
- 150000001502 aryl halides Chemical class 0.000 claims description 6
- 150000008366 benzophenones Chemical class 0.000 claims description 6
- 150000008062 acetophenones Chemical class 0.000 claims description 5
- 125000000751 azo group Chemical group [*]N=N[*] 0.000 claims description 5
- 229920001400 block copolymer Polymers 0.000 claims description 5
- 229920003023 plastic Polymers 0.000 claims description 5
- 239000004033 plastic Substances 0.000 claims description 5
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 5
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 5
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 5
- 150000001350 alkyl halides Chemical class 0.000 claims description 4
- 150000001343 alkyl silanes Chemical class 0.000 claims description 4
- IVRMZWNICZWHMI-UHFFFAOYSA-N azide group Chemical group [N-]=[N+]=[N-] IVRMZWNICZWHMI-UHFFFAOYSA-N 0.000 claims description 4
- 229920000578 graft copolymer Polymers 0.000 claims description 4
- 125000000864 peroxy group Chemical group O(O*)* 0.000 claims description 4
- YXHKONLOYHBTNS-UHFFFAOYSA-N Diazomethane Chemical compound C=[N+]=[N-] YXHKONLOYHBTNS-UHFFFAOYSA-N 0.000 claims description 3
- 239000003054 catalyst Substances 0.000 claims description 3
- 229920001282 polysaccharide Polymers 0.000 claims description 3
- RZVHIXYEVGDQDX-UHFFFAOYSA-N 9,10-anthraquinone Chemical compound C1=CC=C2C(=O)C3=CC=CC=C3C(=O)C2=C1 RZVHIXYEVGDQDX-UHFFFAOYSA-N 0.000 claims description 2
- 125000001749 primary amide group Chemical group 0.000 claims description 2
- 150000001282 organosilanes Chemical class 0.000 claims 4
- UXSJUHVTCKVKIR-UHFFFAOYSA-N 1-azido-2,3,4,5-tetrafluorobenzene Chemical group FC1=CC(N=[N+]=[N-])=C(F)C(F)=C1F UXSJUHVTCKVKIR-UHFFFAOYSA-N 0.000 claims 2
- 102000004190 Enzymes Human genes 0.000 claims 2
- 108090000790 Enzymes Proteins 0.000 claims 2
- 229920005604 random copolymer Polymers 0.000 claims 2
- KCXMKQUNVWSEMD-UHFFFAOYSA-N benzyl chloride Chemical compound ClCC1=CC=CC=C1 KCXMKQUNVWSEMD-UHFFFAOYSA-N 0.000 claims 1
- 229940073608 benzyl chloride Drugs 0.000 claims 1
- 150000002902 organometallic compounds Chemical class 0.000 claims 1
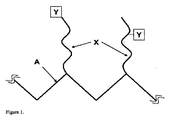
- 230000001939 inductive effect Effects 0.000 abstract description 5
- 239000000178 monomer Substances 0.000 description 29
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 24
- 239000000243 solution Substances 0.000 description 24
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 18
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 14
- 229920001002 functional polymer Polymers 0.000 description 14
- 230000003993 interaction Effects 0.000 description 14
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 13
- 238000006243 chemical reaction Methods 0.000 description 13
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 11
- 238000005251 capillar electrophoresis Methods 0.000 description 10
- 230000002209 hydrophobic effect Effects 0.000 description 10
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 10
- 239000000463 material Substances 0.000 description 9
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 9
- 238000006116 polymerization reaction Methods 0.000 description 9
- 239000000758 substrate Substances 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- BLRPTPMANUNPDV-UHFFFAOYSA-N Silane Chemical compound [SiH4] BLRPTPMANUNPDV-UHFFFAOYSA-N 0.000 description 8
- 150000001412 amines Chemical group 0.000 description 8
- 230000015572 biosynthetic process Effects 0.000 description 8
- 239000012634 fragment Substances 0.000 description 8
- 238000004381 surface treatment Methods 0.000 description 8
- 229910052786 argon Inorganic materials 0.000 description 7
- 238000005370 electroosmosis Methods 0.000 description 7
- 239000007787 solid Substances 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 6
- 125000005647 linker group Chemical group 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 5
- 241000233805 Phoenix Species 0.000 description 5
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 5
- 238000001994 activation Methods 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- 238000005516 engineering process Methods 0.000 description 5
- 239000005350 fused silica glass Substances 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 150000004756 silanes Chemical class 0.000 description 5
- 229910052710 silicon Inorganic materials 0.000 description 5
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical compound OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- 230000000274 adsorptive effect Effects 0.000 description 4
- 229910052799 carbon Inorganic materials 0.000 description 4
- 229920001577 copolymer Polymers 0.000 description 4
- 238000000151 deposition Methods 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- 230000007246 mechanism Effects 0.000 description 4
- 229920001223 polyethylene glycol Polymers 0.000 description 4
- 125000000467 secondary amino group Chemical class [H]N([*:1])[*:2] 0.000 description 4
- 125000005372 silanol group Chemical group 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 150000003573 thiols Chemical class 0.000 description 4
- 229920002554 vinyl polymer Polymers 0.000 description 4
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 3
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 3
- 239000003463 adsorbent Substances 0.000 description 3
- 125000000217 alkyl group Chemical group 0.000 description 3
- 125000003277 amino group Chemical group 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- PYKYMHQGRFAEBM-UHFFFAOYSA-N anthraquinone Chemical class CCC(=O)c1c(O)c2C(=O)C3C(C=CC=C3O)C(=O)c2cc1CC(=O)OC PYKYMHQGRFAEBM-UHFFFAOYSA-N 0.000 description 3
- 150000004056 anthraquinones Chemical class 0.000 description 3
- 125000003118 aryl group Chemical group 0.000 description 3
- 125000004429 atom Chemical group 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 3
- 238000012512 characterization method Methods 0.000 description 3
- 125000003636 chemical group Chemical group 0.000 description 3
- 229920001688 coating polymer Polymers 0.000 description 3
- 230000008021 deposition Effects 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 125000003700 epoxy group Chemical group 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 125000001165 hydrophobic group Chemical group 0.000 description 3
- 238000010348 incorporation Methods 0.000 description 3
- 125000003010 ionic group Chemical group 0.000 description 3
- 238000001155 isoelectric focusing Methods 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 235000019198 oils Nutrition 0.000 description 3
- 229920001451 polypropylene glycol Polymers 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- 229910002027 silica gel Inorganic materials 0.000 description 3
- 239000000741 silica gel Substances 0.000 description 3
- 239000010703 silicon Substances 0.000 description 3
- WHNPOQXWAMXPTA-UHFFFAOYSA-N 3-methylbut-2-enamide Chemical compound CC(C)=CC(N)=O WHNPOQXWAMXPTA-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 2
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- 229920001661 Chitosan Polymers 0.000 description 2
- 239000004593 Epoxy Substances 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- 235000019502 Orange oil Nutrition 0.000 description 2
- 239000005062 Polybutadiene Substances 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 238000005804 alkylation reaction Methods 0.000 description 2
- 150000001408 amides Chemical class 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- 238000001818 capillary gel electrophoresis Methods 0.000 description 2
- 238000005515 capillary zone electrophoresis Methods 0.000 description 2
- 239000004202 carbamide Substances 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- 230000003197 catalytic effect Effects 0.000 description 2
- XMPZTFVPEKAKFH-UHFFFAOYSA-P ceric ammonium nitrate Chemical compound [NH4+].[NH4+].[Ce+4].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O XMPZTFVPEKAKFH-UHFFFAOYSA-P 0.000 description 2
- 230000009920 chelation Effects 0.000 description 2
- 125000001309 chloro group Chemical group Cl* 0.000 description 2
- 238000006482 condensation reaction Methods 0.000 description 2
- 238000007334 copolymerization reaction Methods 0.000 description 2
- 229920006037 cross link polymer Polymers 0.000 description 2
- QDWLLVUFTWGZRZ-UHFFFAOYSA-N diphenylmethanone;prop-2-enamide Chemical class NC(=O)C=C.C=1C=CC=CC=1C(=O)C1=CC=CC=C1 QDWLLVUFTWGZRZ-UHFFFAOYSA-N 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 230000009881 electrostatic interaction Effects 0.000 description 2
- 230000002255 enzymatic effect Effects 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 238000000605 extraction Methods 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 238000003818 flash chromatography Methods 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 229910021397 glassy carbon Inorganic materials 0.000 description 2
- 150000002430 hydrocarbons Chemical group 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 238000002218 isotachophoresis Methods 0.000 description 2
- 125000005641 methacryl group Chemical group 0.000 description 2
- 125000005395 methacrylic acid group Chemical group 0.000 description 2
- 238000001012 micellar electrokinetic chromatography Methods 0.000 description 2
- 238000002493 microarray Methods 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 239000010502 orange oil Substances 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 150000002989 phenols Chemical class 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 229920002401 polyacrylamide Polymers 0.000 description 2
- 229920002857 polybutadiene Polymers 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 229940124530 sulfonamide Drugs 0.000 description 2
- 150000003456 sulfonamides Chemical class 0.000 description 2
- 238000009281 ultraviolet germicidal irradiation Methods 0.000 description 2
- MVQNJLJLEGZFGP-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-benzoylbenzoate Chemical compound C=1C=C(C(=O)C=2C=CC=CC=2)C=CC=1C(=O)ON1C(=O)CCC1=O MVQNJLJLEGZFGP-UHFFFAOYSA-N 0.000 description 1
- ZJFRBVORNIMGFU-UHFFFAOYSA-N 2-[2-(2-aminoethoxy)ethoxy]ethylcarbamic acid Chemical compound NCCOCCOCCNC(O)=O ZJFRBVORNIMGFU-UHFFFAOYSA-N 0.000 description 1
- 241001659321 ANME-2 cluster Species 0.000 description 1
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical group NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 229920002101 Chitin Polymers 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- 238000001712 DNA sequencing Methods 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- KWYHDKDOAIKMQN-UHFFFAOYSA-N N,N,N',N'-tetramethylethylenediamine Chemical compound CN(C)CCN(C)C KWYHDKDOAIKMQN-UHFFFAOYSA-N 0.000 description 1
- 238000005481 NMR spectroscopy Methods 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 229920002873 Polyethylenimine Polymers 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 229910002808 Si–O–Si Inorganic materials 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 229960000583 acetic acid Drugs 0.000 description 1
- 239000003929 acidic solution Substances 0.000 description 1
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 1
- HFBMWMNUJJDEQZ-UHFFFAOYSA-N acryloyl chloride Chemical compound ClC(=O)C=C HFBMWMNUJJDEQZ-UHFFFAOYSA-N 0.000 description 1
- 150000001263 acyl chlorides Chemical class 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 125000003172 aldehyde group Chemical group 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 239000012670 alkaline solution Substances 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 125000003368 amide group Chemical group 0.000 description 1
- DGWSVDZYLUCQRK-UHFFFAOYSA-N amino N-diazocarbamate Chemical compound NOC(=O)N=[N+]=[N-] DGWSVDZYLUCQRK-UHFFFAOYSA-N 0.000 description 1
- ROOXNKNUYICQNP-UHFFFAOYSA-N ammonium peroxydisulfate Substances [NH4+].[NH4+].[O-]S(=O)(=O)OOS([O-])(=O)=O ROOXNKNUYICQNP-UHFFFAOYSA-N 0.000 description 1
- VAZSKTXWXKYQJF-UHFFFAOYSA-N ammonium persulfate Chemical compound [NH4+].[NH4+].[O-]S(=O)OOS([O-])=O VAZSKTXWXKYQJF-UHFFFAOYSA-N 0.000 description 1
- 229910001870 ammonium persulfate Inorganic materials 0.000 description 1
- 238000005349 anion exchange Methods 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 238000010420 art technique Methods 0.000 description 1
- 150000001540 azides Chemical class 0.000 description 1
- 125000000852 azido group Chemical group *N=[N+]=[N-] 0.000 description 1
- AGEZXYOZHKGVCM-UHFFFAOYSA-N benzyl bromide Chemical group BrCC1=CC=CC=C1 AGEZXYOZHKGVCM-UHFFFAOYSA-N 0.000 description 1
- 230000008275 binding mechanism Effects 0.000 description 1
- 238000012742 biochemical analysis Methods 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000005341 cation exchange Methods 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 229910021419 crystalline silicon Inorganic materials 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 239000000412 dendrimer Substances 0.000 description 1
- 229920000736 dendritic polymer Polymers 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 150000004845 diazirines Chemical class 0.000 description 1
- 150000001993 dienes Chemical class 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 150000002085 enols Chemical class 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 125000003827 glycol group Chemical group 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 125000004356 hydroxy functional group Chemical group O* 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 239000003999 initiator Substances 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 150000002734 metacrylic acid derivatives Chemical class 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- 210000003463 organelle Anatomy 0.000 description 1
- 125000002524 organometallic group Chemical group 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- 125000004437 phosphorous atom Chemical group 0.000 description 1
- 229920002239 polyacrylonitrile Polymers 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 239000002157 polynucleotide Substances 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000010526 radical polymerization reaction Methods 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000007151 ring opening polymerisation reaction Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- FZHAPNGMFPVSLP-UHFFFAOYSA-N silanamine Chemical group [SiH3]N FZHAPNGMFPVSLP-UHFFFAOYSA-N 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 229910052814 silicon oxide Inorganic materials 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 125000003011 styrenyl group Chemical group [H]\C(*)=C(/[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 150000003460 sulfonic acids Chemical class 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 238000006557 surface reaction Methods 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 125000001302 tertiary amino group Chemical group 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- DAHWFTWPSFSFMS-UHFFFAOYSA-N trihydroxysilane Chemical group O[SiH](O)O DAHWFTWPSFSFMS-UHFFFAOYSA-N 0.000 description 1
- 238000003260 vortexing Methods 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 229910001928 zirconium oxide Inorganic materials 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/416—Systems
- G01N27/447—Systems using electrophoresis
- G01N27/44704—Details; Accessories
- G01N27/44717—Arrangements for investigating the separated zones, e.g. localising zones
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/52—Amides or imides
- C08F220/54—Amides, e.g. N,N-dimethylacrylamide or N-isopropylacrylamide
- C08F220/56—Acrylamide; Methacrylamide
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/416—Systems
- G01N27/447—Systems using electrophoresis
- G01N27/44756—Apparatus specially adapted therefor
- G01N27/44791—Microapparatus
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Molecular Biology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Electrochemistry (AREA)
- Dispersion Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US83306006P | 2006-07-24 | 2006-07-24 | |
| US83876606P | 2006-08-17 | 2006-08-17 | |
| US11/654,143 US20080017512A1 (en) | 2006-07-24 | 2007-01-16 | Coatings for capillaries capable of capturing analytes |
| PCT/US2007/016626 WO2008013801A2 (en) | 2006-07-24 | 2007-07-23 | Coatings for capillaries capable of capturing analytes |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009544969A true JP2009544969A (ja) | 2009-12-17 |
| JP2009544969A5 JP2009544969A5 (enExample) | 2010-09-09 |
Family
ID=38970409
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009521800A Pending JP2009544969A (ja) | 2006-07-24 | 2007-07-23 | 検体を捕捉することが可能なキャピラリー用コーティング |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US20080017512A1 (enExample) |
| EP (2) | EP2713158A1 (enExample) |
| JP (1) | JP2009544969A (enExample) |
| WO (1) | WO2008013801A2 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016517509A (ja) * | 2013-03-07 | 2016-06-16 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | 電気泳動分離装置およびそれを使用するための方法 |
| JP2021123712A (ja) * | 2020-01-31 | 2021-08-30 | 国立研究開発法人物質・材料研究機構 | 組成物、硬化膜、積層体、船舶、水中構造物、医療デバイス、及び、マイクロ流路チップ |
Families Citing this family (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7846676B2 (en) * | 2004-07-19 | 2010-12-07 | Cell Biosciences, Inc. | Methods and devices for analyte detection |
| US7935479B2 (en) | 2004-07-19 | 2011-05-03 | Cell Biosciences, Inc. | Methods and devices for analyte detection |
| US8945361B2 (en) | 2005-09-20 | 2015-02-03 | ProteinSimple | Electrophoresis standards, methods and kits |
| US10107782B2 (en) | 2008-01-25 | 2018-10-23 | ProteinSimple | Method to perform limited two dimensional separation of proteins and other biologicals |
| US9182411B2 (en) * | 2009-07-14 | 2015-11-10 | Colen Innovations, L.L.C. | Methods and apparatus for the detection and differentiation of non-sialated proteins from sialated proteins in a fluid sample |
| DE102009047802B4 (de) * | 2009-09-30 | 2012-07-05 | Siemens Aktiengesellschaft | Vorrichtung zum Filtern eines oder mehrerer nachzuweisender Partikel aus einem Fluid |
| US20110143378A1 (en) * | 2009-11-12 | 2011-06-16 | CyVek LLC. | Microfluidic method and apparatus for high performance biological assays |
| US10022696B2 (en) | 2009-11-23 | 2018-07-17 | Cyvek, Inc. | Microfluidic assay systems employing micro-particles and methods of manufacture |
| US10065403B2 (en) | 2009-11-23 | 2018-09-04 | Cyvek, Inc. | Microfluidic assay assemblies and methods of manufacture |
| JP5701894B2 (ja) | 2009-11-23 | 2015-04-15 | サイヴェク・インコーポレイテッド | アッセイを行う方法及び装置 |
| US9700889B2 (en) | 2009-11-23 | 2017-07-11 | Cyvek, Inc. | Methods and systems for manufacture of microarray assay systems, conducting microfluidic assays, and monitoring and scanning to obtain microfluidic assay results |
| WO2013134742A2 (en) | 2012-03-08 | 2013-09-12 | Cyvek, Inc | Micro-tube particles for microfluidic assays and methods of manufacture |
| US9759718B2 (en) | 2009-11-23 | 2017-09-12 | Cyvek, Inc. | PDMS membrane-confined nucleic acid and antibody/antigen-functionalized microlength tube capture elements, and systems employing them, and methods of their use |
| US9855735B2 (en) | 2009-11-23 | 2018-01-02 | Cyvek, Inc. | Portable microfluidic assay devices and methods of manufacture and use |
| US9500645B2 (en) | 2009-11-23 | 2016-11-22 | Cyvek, Inc. | Micro-tube particles for microfluidic assays and methods of manufacture |
| US9216412B2 (en) | 2009-11-23 | 2015-12-22 | Cyvek, Inc. | Microfluidic devices and methods of manufacture and use |
| EP3223015B1 (en) * | 2011-06-24 | 2020-12-16 | The Regents of The University of California | Microfluidic devices and methods for separating and detecting constituents in a fluid sample |
| US9804079B2 (en) | 2012-04-19 | 2017-10-31 | ProteinSimple | Dual wavelength isoelectric focusing for determining drug load in antibody drug conjugates |
| MX2015010853A (es) | 2013-02-21 | 2016-07-20 | Univ California | Modificaciones universales de superficie escalables y rentables. |
| US9303239B2 (en) | 2013-02-27 | 2016-04-05 | Shimadzu Corporation | Method for cleaning microfluidic device and cleaning liquid |
| US9766206B2 (en) | 2013-09-27 | 2017-09-19 | ProteinSimple | Apparatus, systems, and methods for capillary electrophoresis |
| CN105358236B (zh) | 2014-03-11 | 2019-11-29 | 生物辐射实验室股份有限公司 | 浸染条蛋白质印迹法 |
| CN104297491B (zh) * | 2014-10-11 | 2017-02-15 | 南京山诺生物科技有限公司 | 蛋白质层析电泳及其原位化学印迹和免疫成像装置和方法 |
| US10228367B2 (en) | 2015-12-01 | 2019-03-12 | ProteinSimple | Segmented multi-use automated assay cartridge |
| KR20230026540A (ko) | 2016-12-01 | 2023-02-24 | 더 리전트 오브 더 유니버시티 오브 캘리포니아 | 에너지 제공 장치 및 이의 용도 |
| AU2018304711B2 (en) | 2017-07-20 | 2025-04-10 | Cytomx Therapeutics, Inc. | Methods of qualitatively and/or quantitatively analyzing properties of activatable antibodies and uses thereof |
| AU2018374291A1 (en) | 2017-12-01 | 2020-06-11 | Hydrophilix, Inc. | Biofouling resistant coatings and methods of making and using the same |
| WO2020247629A1 (en) | 2019-06-05 | 2020-12-10 | The Regents Of The University Of California | Biofouling resistant coatings and methods of making and using the same |
| US11143650B1 (en) | 2020-04-15 | 2021-10-12 | ProteinSimple | Method for measurement of total protein content and detection of protein via immunoassay in a microfluidic device |
| WO2022109008A1 (en) * | 2020-11-18 | 2022-05-27 | Cercacor Laboratories, Inc. | Glucose sensors and methods of manufacturing |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003523499A (ja) * | 1999-01-12 | 2003-08-05 | スペクトラメディックス コーポレイション | キャピラリーゲル電気泳動のためのコポリマー |
| JP2004501994A (ja) * | 2000-06-30 | 2004-01-22 | アンスティテュ キュリィ | 吸着及び/又は電気浸透現象を最小限化する表面処理溶液 |
| WO2005033158A2 (en) * | 2003-10-01 | 2005-04-14 | Surmodics, Inc. | Attachment of molecules to surfaces |
| JP2005534749A (ja) * | 2002-07-29 | 2005-11-17 | アプレラ コーポレイション | グラフト共重合体、それらの調製、およびキャピラリー電気泳動での使用 |
| WO2006014680A1 (en) * | 2004-07-19 | 2006-02-09 | Cell Biosciences, Inc. | Methods and devices for analyte detection |
Family Cites Families (66)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US514753A (en) | 1894-02-13 | Horseshoe | ||
| US309593A (en) * | 1884-12-23 | Refrigerator | ||
| CH594444A5 (enExample) * | 1972-12-04 | 1978-01-13 | Gerd Birrenbach | |
| US4843010A (en) * | 1983-11-10 | 1989-06-27 | Genetic Systems Corporation | Polymerization-induced separation immunoassays |
| US4921790A (en) * | 1987-04-24 | 1990-05-01 | Research Corporation | Tumor specific assay for CA125 ovarian cancer antigen |
| US4788138A (en) * | 1987-04-30 | 1988-11-29 | Beckman Instruments, Inc. | Method to achieve a linear standard curve in a sandwich immunoassay |
| US4870003A (en) * | 1987-06-15 | 1989-09-26 | Coulter Corporation | Simultaneous enzyme immunoassay for detecting antigen and/or antibody in humans |
| US6300101B1 (en) * | 1988-10-24 | 2001-10-09 | Board Of Regents, The University Of Texas System | Methods and compositions including a 13kD B. burgdorferi protein |
| US5252743A (en) * | 1989-11-13 | 1993-10-12 | Affymax Technologies N.V. | Spatially-addressable immobilization of anti-ligands on surfaces |
| US5858188A (en) * | 1990-02-28 | 1999-01-12 | Aclara Biosciences, Inc. | Acrylic microchannels and their use in electrophoretic applications |
| US5143753A (en) | 1990-10-26 | 1992-09-01 | Indiana University Foundation | Suppression of electroosmosis with hydrolytically stable coatings |
| US5074982A (en) | 1990-10-26 | 1991-12-24 | Indiana University Foundation | Suppression of electroosmosis with hydrolytically stable coatings |
| US5110434A (en) * | 1990-12-20 | 1992-05-05 | Bio-Rad Laboratories, Inc. | Use of zwitterions to mobilize isoelectrically focused ampholyte zones |
| US5244813A (en) * | 1991-01-25 | 1993-09-14 | Trustees Of Tufts College | Fiber optic sensor, apparatus, and methods for detecting an organic analyte in a fluid or vapor sample |
| AU658257B2 (en) * | 1991-10-31 | 1995-04-06 | Matritech, Inc. | Nuclear matrix protein fluid assay |
| US5213669A (en) | 1992-01-31 | 1993-05-25 | Beckman Instruments, Inc. | Capillary column containing a dynamically cross-linked composition and method of use |
| US5759369A (en) | 1992-09-24 | 1998-06-02 | The Perkin-Elmer Corporation | Viscous electrophoresis polymer medium and method |
| US5290418A (en) | 1992-09-24 | 1994-03-01 | Applied Biosystems, Inc. | Viscous electrophoresis polymer medium and method |
| JP3277045B2 (ja) * | 1992-10-06 | 2002-04-22 | デイド・ベーリング・マルブルク・ゲゼルシヤフト・ミツト・ベシユレンクテル・ハフツング | Hiv−グループのレトロウイルスおよびその使用 |
| US5376249A (en) * | 1992-11-25 | 1994-12-27 | Perseptive Biosystems, Inc. | Analysis utilizing isoelectric focusing |
| AU1254295A (en) | 1993-12-17 | 1995-07-03 | Perkin-Elmer Corporation, The | Uncharged polymers for separation of biomolecules by capillary electrophoresis |
| US6358385B1 (en) * | 1993-12-17 | 2002-03-19 | The Perkin-Elmer Corporation | Polymers for separation of biomolecules by capillary electrophoresis |
| US6355709B1 (en) | 1993-12-17 | 2002-03-12 | The Perkin-Elmer Corporation | Polymers for separation of biomolecules by capillary electrophoresis |
| US5976896A (en) * | 1994-06-06 | 1999-11-02 | Idexx Laboratories, Inc. | Immunoassays in capillary tubes |
| JP4026724B2 (ja) | 1995-01-27 | 2007-12-26 | ノースイースタン ユニバーシティー | 毛管電気泳動のためのポリビニルアルコール(pva)をベースとする共有結合の安定した親水性コーティング |
| US5916427A (en) * | 1995-11-08 | 1999-06-29 | Kirkpatrick; Francis H | Methods and reagents for gel electrophoresis |
| US5830539A (en) * | 1995-11-17 | 1998-11-03 | The State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of The University Of Oregon | Methods for functionalizing and coating substrates and devices made according to the methods |
| US5798035A (en) * | 1996-10-03 | 1998-08-25 | Pharmacopeia, Inc. | High throughput solid phase chemical synthesis utilizing thin cylindrical reaction vessels useable for biological assay |
| US5804384A (en) * | 1996-12-06 | 1998-09-08 | Vysis, Inc. | Devices and methods for detecting multiple analytes in samples |
| AU5301598A (en) * | 1997-02-10 | 1998-08-13 | Gist-Brocades B.V. | Detection of analytes using electrochemistry |
| US6139797A (en) * | 1997-08-20 | 2000-10-31 | Suzuki Motor Corporation | Immunoassay apparatus |
| US6054032A (en) * | 1998-01-27 | 2000-04-25 | 3M Innovative Properties Company | Capillary electrophoresis array |
| DE69933850T2 (de) * | 1998-08-14 | 2007-05-24 | Dainichiseika Color & Chemicals Mfg. Co., Ltd. | Chemilumineszenzmittel und chemilumineszenzanalyseverfahren mit deren verwendung |
| EP1006355A3 (en) * | 1998-11-30 | 2000-11-08 | The Institute of Physical and Chemical Research | Capillary electrophoretic apparatus |
| US6475364B1 (en) * | 1999-02-02 | 2002-11-05 | Caliper Technologies Corp. | Methods, devices and systems for characterizing proteins |
| ATE469918T1 (de) * | 1999-02-12 | 2010-06-15 | Univ Louisville Res Found | Dihydroouabain-ähnlicher faktor, diagnostische und therapeutische verbindungen und methoden |
| US6326083B1 (en) * | 1999-03-08 | 2001-12-04 | Calipher Technologies Corp. | Surface coating for microfluidic devices that incorporate a biopolymer resistant moiety |
| US6375817B1 (en) * | 1999-04-16 | 2002-04-23 | Perseptive Biosystems, Inc. | Apparatus and methods for sample analysis |
| US6423536B1 (en) * | 1999-08-02 | 2002-07-23 | Molecular Dynamics, Inc. | Low volume chemical and biochemical reaction system |
| JP4361271B2 (ja) * | 2000-10-10 | 2009-11-11 | バイオトローブ・インコーポレイテツド | アッセイ、合成、および保存用の器具、ならびに、その作製、使用、および操作の方法 |
| WO2002052045A1 (en) * | 2000-12-26 | 2002-07-04 | Aviva Biosciences | Active and biocompatible platforms prepared by polymerization of surface coating films |
| AU2002247097A1 (en) * | 2001-02-05 | 2002-08-19 | Activx Biosciences, Inc. | Activity based probe analysis |
| US6878256B2 (en) * | 2001-04-02 | 2005-04-12 | Hitachi, Ltd. | Capillary array device |
| CA2443067A1 (en) * | 2001-04-05 | 2002-10-17 | Nextgen Sciences Ltd. | Protein analysis by means of immobilized arrays of antigens or antibodies |
| WO2002089972A1 (en) | 2001-05-03 | 2002-11-14 | Commissariat A L'energie Atomique | Microfluidic device for analyzing nucleic acids and/or proteins, methods of preparation and uses thereof |
| US7374724B2 (en) * | 2001-05-29 | 2008-05-20 | Tecan Trading Ag | Device for processing samples, use of the device, and method for producing the device |
| CA2652991A1 (en) * | 2001-07-16 | 2003-11-13 | Caprotec Bioanalytics Gmbh | Capture compounds, collections thereof and methods for analyzing the proteome and complex compositions |
| US20030171697A1 (en) * | 2001-12-04 | 2003-09-11 | Dave Smith | Tester for automated identification of analytes in bodily fluids |
| US6864480B2 (en) * | 2001-12-19 | 2005-03-08 | Sau Lan Tang Staats | Interface members and holders for microfluidic array devices |
| EP1487568A4 (en) * | 2002-03-12 | 2005-10-05 | Syngenta Participations Ag | Microcapillaries Hybridization Chamber |
| US7010964B2 (en) * | 2002-10-31 | 2006-03-14 | Nanostream, Inc. | Pressurized microfluidic devices with optical detection regions |
| US20060051879A9 (en) * | 2003-01-16 | 2006-03-09 | Hubert Koster | Capture compounds, collections thereof and methods for analyzing the proteome and complex compositions |
| US20040181443A1 (en) * | 2003-03-10 | 2004-09-16 | Horton Carl A. | Method and apparatus for the management of infrastructure assets, work orders,service requests, and work flows, utilizing an integrated call center, database, GIS system, and wireless handheld device |
| US7635734B2 (en) * | 2004-02-17 | 2009-12-22 | The Children's Hospital Of Philadelphia | Photochemical activation of surfaces for attaching biomaterial |
| US7270784B2 (en) * | 2003-04-30 | 2007-09-18 | Aurora Discovery, Incorporated | Automated laboratory for high-throughput biological assays and RNA interference |
| DE10343900A1 (de) * | 2003-09-19 | 2005-04-21 | Basf Ag | Verwendung von N-Vinyllactam enthaltenden Copolymeren zur Herstellung von funktionalisierten Membranen |
| AU2005220150A1 (en) * | 2004-02-13 | 2005-09-15 | The University Of North Carolina At Chapel Hill | Functional materials and novel methods for the fabrication of microfluidic devices |
| US7382258B2 (en) * | 2004-03-19 | 2008-06-03 | Applera Corporation | Sample carrier device incorporating radio frequency identification, and method |
| US20060292558A1 (en) * | 2004-07-19 | 2006-12-28 | Cell Biosciences Inc. | Methods and apparatus for protein assay diagnostics |
| US20060292649A1 (en) * | 2004-07-19 | 2006-12-28 | Cell Biosciences Inc. | Methods and apparatus for reference lab diagnostics |
| US7846676B2 (en) * | 2004-07-19 | 2010-12-07 | Cell Biosciences, Inc. | Methods and devices for analyte detection |
| US7807750B2 (en) * | 2004-08-06 | 2010-10-05 | Surmodics, Inc. | Thermally-reactive polymers |
| US20080206752A1 (en) * | 2005-02-10 | 2008-08-28 | Commissariat A L'energie Atomidque | Method For the Photochemical Attachment of Biomolecules to a Substrate |
| US8945361B2 (en) * | 2005-09-20 | 2015-02-03 | ProteinSimple | Electrophoresis standards, methods and kits |
| US7640078B2 (en) * | 2006-07-05 | 2009-12-29 | Advanced Energy Industries, Inc. | Multi-mode control algorithm |
| US20090023156A1 (en) * | 2007-07-20 | 2009-01-22 | Voss Karl O | Methods and reagents for quantifying analytes |
-
2007
- 2007-01-16 US US11/654,143 patent/US20080017512A1/en not_active Abandoned
- 2007-07-23 WO PCT/US2007/016626 patent/WO2008013801A2/en not_active Ceased
- 2007-07-23 JP JP2009521800A patent/JP2009544969A/ja active Pending
- 2007-07-23 EP EP13183395.6A patent/EP2713158A1/en not_active Withdrawn
- 2007-07-23 EP EP07836214A patent/EP2049652A4/en not_active Withdrawn
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003523499A (ja) * | 1999-01-12 | 2003-08-05 | スペクトラメディックス コーポレイション | キャピラリーゲル電気泳動のためのコポリマー |
| JP2004501994A (ja) * | 2000-06-30 | 2004-01-22 | アンスティテュ キュリィ | 吸着及び/又は電気浸透現象を最小限化する表面処理溶液 |
| JP2005534749A (ja) * | 2002-07-29 | 2005-11-17 | アプレラ コーポレイション | グラフト共重合体、それらの調製、およびキャピラリー電気泳動での使用 |
| WO2005033158A2 (en) * | 2003-10-01 | 2005-04-14 | Surmodics, Inc. | Attachment of molecules to surfaces |
| WO2006014680A1 (en) * | 2004-07-19 | 2006-02-09 | Cell Biosciences, Inc. | Methods and devices for analyte detection |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016517509A (ja) * | 2013-03-07 | 2016-06-16 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | 電気泳動分離装置およびそれを使用するための方法 |
| JP2021123712A (ja) * | 2020-01-31 | 2021-08-30 | 国立研究開発法人物質・材料研究機構 | 組成物、硬化膜、積層体、船舶、水中構造物、医療デバイス、及び、マイクロ流路チップ |
| JP7656895B2 (ja) | 2020-01-31 | 2025-04-04 | 国立研究開発法人物質・材料研究機構 | 組成物、硬化膜、積層体、船舶、水中構造物、医療デバイス、及び、マイクロ流路チップ |
Also Published As
| Publication number | Publication date |
|---|---|
| US20080017512A1 (en) | 2008-01-24 |
| WO2008013801A2 (en) | 2008-01-31 |
| WO2008013801A3 (en) | 2008-06-26 |
| EP2049652A2 (en) | 2009-04-22 |
| EP2713158A1 (en) | 2014-04-02 |
| EP2049652A4 (en) | 2010-07-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2009544969A (ja) | 検体を捕捉することが可能なキャピラリー用コーティング | |
| US5840388A (en) | Polyvinyl alcohol (PVA) based covalently bonded stable hydrophilic coating for capillary electrophoresis | |
| Albarghouthi et al. | Poly‐N‐hydroxyethylacrylamide as a novel, adsorbed coating for protein separation by capillary electrophoresis | |
| Horvath et al. | Polymer wall coatings for capillary electrophoresis | |
| US5792331A (en) | Preformed polymer coating process and product | |
| Muck et al. | Chemical modification of polymeric microchip devices | |
| US8945361B2 (en) | Electrophoresis standards, methods and kits | |
| US6074542A (en) | Compounds for molecular separations | |
| WO1996023220A9 (en) | Polyvinyl alcohol (pva) based covalently bonded stable hydrophilic coating for capillary electrophoresis | |
| EP0671000B1 (en) | Coated capillary columns and electrophoretic separation methods for their use | |
| KR20160122174A (ko) | 부착된 인식 화합물을 갖는 모놀리스, 이의 어레이 및 이의 용도 | |
| WO1995009360A9 (en) | Coated capillary columns and electrophoretic separation methods for their use | |
| US7303821B1 (en) | Material and process for precisely controlled polymeric coatings | |
| JP3811188B2 (ja) | 電気泳動及びクロマトグラフィー技術でのポリアクリルアミドマトリックスのための、新規アクリルアミド誘導体及び新規な配合物 | |
| US7815783B2 (en) | Multi-compartment filter and method of filtering using same | |
| US20100032296A1 (en) | Systems and methods for quantitative analyte transfer | |
| US20060111517A1 (en) | Recognition layers made of hydrogel based on polyacrylamide for use in biosensor technology | |
| JP2005538363A (ja) | コーティングされた毛管電気泳動用チューブおよびシステム | |
| US7833625B2 (en) | Materials, methods and systems for separating and identifying proteins from mixtures | |
| JPH05133947A (ja) | 液体クロマトグラフイー用担体およびそれを用いた液体クロマトグラフイー法 | |
| KR20040063655A (ko) | 광분해성 연결기가 도입된 생체 분자 분리용 고분자지지체 및 이를 이용한 생체분자의 검출 및 분석방법 | |
| CN109675344A (zh) | 固化pH梯度的毛细管等电聚焦亲水性整体柱及制备方法 | |
| US7488516B2 (en) | Method for modification of glass-based microchannel | |
| Sola et al. | Poly (N, N-Dimethylacrylamide)-Based Coatings to Modulate Electroosmotic Flow and Capillary Surface Properties for Protein Analysis | |
| KAJJOUT et al. | Surface chemistry for in situ sample desalting and dynamic MALDI targets |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100720 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100720 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20111209 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120105 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120330 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120406 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120507 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120514 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120604 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120611 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20120904 |