JP2005537282A - セファロタキシンアルカロイドによる血管新生抑制、並びにその誘導体、組成物、および使用方法 - Google Patents
セファロタキシンアルカロイドによる血管新生抑制、並びにその誘導体、組成物、および使用方法 Download PDFInfo
- Publication number
- JP2005537282A JP2005537282A JP2004523305A JP2004523305A JP2005537282A JP 2005537282 A JP2005537282 A JP 2005537282A JP 2004523305 A JP2004523305 A JP 2004523305A JP 2004523305 A JP2004523305 A JP 2004523305A JP 2005537282 A JP2005537282 A JP 2005537282A
- Authority
- JP
- Japan
- Prior art keywords
- cephalotaxin
- host
- vascular disease
- angiogenesis
- homoharringtonine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
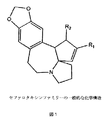
- DSRNKUZOWRFQFO-UHFFFAOYSA-N cephalotaxine Natural products COC1=CC23CCCN2CCc4cc5OCOc5cc4C3=C1O DSRNKUZOWRFQFO-UHFFFAOYSA-N 0.000 title claims abstract description 65
- 238000000034 method Methods 0.000 title claims abstract description 32
- 239000000203 mixture Substances 0.000 title abstract description 32
- 229930013930 alkaloid Natural products 0.000 title abstract description 4
- 230000014399 negative regulation of angiogenesis Effects 0.000 title 1
- 208000019553 vascular disease Diseases 0.000 claims abstract description 40
- 238000011321 prophylaxis Methods 0.000 claims abstract description 12
- 206010028980 Neoplasm Diseases 0.000 claims description 42
- HYFHYPWGAURHIV-JFIAXGOJSA-N omacetaxine mepesuccinate Chemical compound C1=C2CCN3CCC[C@]43C=C(OC)[C@@H](OC(=O)[C@@](O)(CCCC(C)(C)O)CC(=O)OC)[C@H]4C2=CC2=C1OCO2 HYFHYPWGAURHIV-JFIAXGOJSA-N 0.000 claims description 27
- 230000033115 angiogenesis Effects 0.000 claims description 26
- HYFHYPWGAURHIV-UHFFFAOYSA-N homoharringtonine Natural products C1=C2CCN3CCCC43C=C(OC)C(OC(=O)C(O)(CCCC(C)(C)O)CC(=O)OC)C4C2=CC2=C1OCO2 HYFHYPWGAURHIV-UHFFFAOYSA-N 0.000 claims description 23
- 229960002230 omacetaxine mepesuccinate Drugs 0.000 claims description 23
- 208000027866 inflammatory disease Diseases 0.000 claims description 15
- 201000011510 cancer Diseases 0.000 claims description 14
- 206010012689 Diabetic retinopathy Diseases 0.000 claims description 10
- 208000006673 asthma Diseases 0.000 claims description 9
- 201000008482 osteoarthritis Diseases 0.000 claims description 9
- 208000005069 pulmonary fibrosis Diseases 0.000 claims description 9
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 9
- 208000002780 macular degeneration Diseases 0.000 claims description 8
- 208000003788 Neoplasm Micrometastasis Diseases 0.000 claims 1
- 238000011282 treatment Methods 0.000 abstract description 11
- 239000004037 angiogenesis inhibitor Substances 0.000 abstract description 10
- 150000003797 alkaloid derivatives Chemical class 0.000 abstract description 3
- 210000003711 chorioallantoic membrane Anatomy 0.000 description 25
- 238000009472 formulation Methods 0.000 description 18
- 239000004480 active ingredient Substances 0.000 description 17
- 150000001875 compounds Chemical class 0.000 description 16
- 210000004204 blood vessel Anatomy 0.000 description 15
- 201000010099 disease Diseases 0.000 description 13
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 13
- 210000004027 cell Anatomy 0.000 description 12
- 230000012010 growth Effects 0.000 description 9
- 206010027476 Metastases Diseases 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 230000009401 metastasis Effects 0.000 description 7
- 229940121369 angiogenesis inhibitor Drugs 0.000 description 6
- 238000011161 development Methods 0.000 description 6
- 239000004615 ingredient Substances 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 230000004614 tumor growth Effects 0.000 description 6
- 206010012601 diabetes mellitus Diseases 0.000 description 5
- 230000002401 inhibitory effect Effects 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- -1 retinoids Chemical class 0.000 description 5
- 239000003826 tablet Substances 0.000 description 5
- 210000003462 vein Anatomy 0.000 description 5
- VEEGZPWAAPPXRB-BJMVGYQFSA-N (3e)-3-(1h-imidazol-5-ylmethylidene)-1h-indol-2-one Chemical compound O=C1NC2=CC=CC=C2\C1=C/C1=CN=CN1 VEEGZPWAAPPXRB-BJMVGYQFSA-N 0.000 description 4
- 241001433879 Camarea Species 0.000 description 4
- 230000001772 anti-angiogenic effect Effects 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 235000013601 eggs Nutrition 0.000 description 4
- 210000002257 embryonic structure Anatomy 0.000 description 4
- 210000002889 endothelial cell Anatomy 0.000 description 4
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 4
- 230000035755 proliferation Effects 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- 108010079709 Angiostatins Proteins 0.000 description 3
- 102000012936 Angiostatins Human genes 0.000 description 3
- 229930012538 Paclitaxel Natural products 0.000 description 3
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 3
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 3
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 3
- 230000002491 angiogenic effect Effects 0.000 description 3
- FZCSTZYAHCUGEM-UHFFFAOYSA-N aspergillomarasmine B Natural products OC(=O)CNC(C(O)=O)CNC(C(O)=O)CC(O)=O FZCSTZYAHCUGEM-UHFFFAOYSA-N 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 230000017531 blood circulation Effects 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- YMNCVRSYJBNGLD-KURKYZTESA-N cephalotaxine Chemical compound C([C@@]12C=C([C@H]([C@H]2C2=C3)O)OC)CCN1CCC2=CC1=C3OCO1 YMNCVRSYJBNGLD-KURKYZTESA-N 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 210000003743 erythrocyte Anatomy 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- 210000001161 mammalian embryo Anatomy 0.000 description 3
- 230000008520 organization Effects 0.000 description 3
- 229960001592 paclitaxel Drugs 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 3
- 230000002792 vascular Effects 0.000 description 3
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 description 2
- LGZKGOGODCLQHG-CYBMUJFWSA-N 5-[(2r)-2-hydroxy-2-(3,4,5-trimethoxyphenyl)ethyl]-2-methoxyphenol Chemical compound C1=C(O)C(OC)=CC=C1C[C@@H](O)C1=CC(OC)=C(OC)C(OC)=C1 LGZKGOGODCLQHG-CYBMUJFWSA-N 0.000 description 2
- 208000003120 Angiofibroma Diseases 0.000 description 2
- 208000022211 Arteriovenous Malformations Diseases 0.000 description 2
- 208000037260 Atherosclerotic Plaque Diseases 0.000 description 2
- 201000004569 Blindness Diseases 0.000 description 2
- 241000931913 Cephalotaxus fortunei Species 0.000 description 2
- 102400001047 Endostatin Human genes 0.000 description 2
- 108010079505 Endostatins Proteins 0.000 description 2
- 241000287828 Gallus gallus Species 0.000 description 2
- 208000010412 Glaucoma Diseases 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 206010018691 Granuloma Diseases 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 102000004877 Insulin Human genes 0.000 description 2
- 108090001061 Insulin Proteins 0.000 description 2
- 102000014150 Interferons Human genes 0.000 description 2
- 108010050904 Interferons Proteins 0.000 description 2
- 240000007472 Leucaena leucocephala Species 0.000 description 2
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 101000605401 Mus musculus Plasminogen Proteins 0.000 description 2
- 102000007079 Peptide Fragments Human genes 0.000 description 2
- 108010033276 Peptide Fragments Proteins 0.000 description 2
- 201000004681 Psoriasis Diseases 0.000 description 2
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 description 2
- 206010039710 Scleroderma Diseases 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- 208000013058 Weber syndrome Diseases 0.000 description 2
- 230000000692 anti-sense effect Effects 0.000 description 2
- 230000005744 arteriovenous malformation Effects 0.000 description 2
- 210000001367 artery Anatomy 0.000 description 2
- 206010003246 arthritis Diseases 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 239000004621 biodegradable polymer Substances 0.000 description 2
- 229920002988 biodegradable polymer Polymers 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 230000036770 blood supply Effects 0.000 description 2
- 210000000988 bone and bone Anatomy 0.000 description 2
- 201000007293 brain stem infarction Diseases 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- LGZKGOGODCLQHG-UHFFFAOYSA-N combretastatin Natural products C1=C(O)C(OC)=CC=C1CC(O)C1=CC(OC)=C(OC)C(OC)=C1 LGZKGOGODCLQHG-UHFFFAOYSA-N 0.000 description 2
- 208000018631 connective tissue disease Diseases 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 239000006071 cream Substances 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 230000009368 gene silencing by RNA Effects 0.000 description 2
- 238000005469 granulation Methods 0.000 description 2
- 230000003179 granulation Effects 0.000 description 2
- 201000011066 hemangioma Diseases 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 201000001371 inclusion conjunctivitis Diseases 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 229940125396 insulin Drugs 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 229940047124 interferons Drugs 0.000 description 2
- 206010025135 lupus erythematosus Diseases 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 239000006072 paste Substances 0.000 description 2
- 230000008506 pathogenesis Effects 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 230000036454 renin-angiotensin system Effects 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 125000001424 substituent group Chemical group 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 238000011200 topical administration Methods 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 206010044325 trachoma Diseases 0.000 description 2
- 230000005747 tumor angiogenesis Effects 0.000 description 2
- CQOQDQWUFQDJMK-SSTWWWIQSA-N 2-methoxy-17beta-estradiol Chemical compound C([C@@H]12)C[C@]3(C)[C@@H](O)CC[C@H]3[C@@H]1CCC1=C2C=C(OC)C(O)=C1 CQOQDQWUFQDJMK-SSTWWWIQSA-N 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 240000002559 Cephalotaxus drupacea Species 0.000 description 1
- 241000488902 Cephalotaxus wilsoniana Species 0.000 description 1
- WRCBXHDQHPUVHW-UHFFFAOYSA-N Deoxyharringtonine Natural products C1=C2CCN3CCCC43C=C(OC)C(OC(=O)C(O)(CCC(C)C)CC(=O)OC)C4C2=CC2=C1OCO2 WRCBXHDQHPUVHW-UHFFFAOYSA-N 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 101000605403 Homo sapiens Plasminogen Proteins 0.000 description 1
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 208000012659 Joint disease Diseases 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 206010027146 Melanoderma Diseases 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 206010029113 Neovascularisation Diseases 0.000 description 1
- WZFZRXGNVSHCOI-UHFFFAOYSA-N O-acetyl-cephalotaxine Natural products C1=C2C3C(OC(C)=O)C(OC)=CC43CCCN4CCC2=CC2=C1OCO2 WZFZRXGNVSHCOI-UHFFFAOYSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 102000013566 Plasminogen Human genes 0.000 description 1
- 108010051456 Plasminogen Proteins 0.000 description 1
- 208000007660 Residual Neoplasm Diseases 0.000 description 1
- 208000007135 Retinal Neovascularization Diseases 0.000 description 1
- 208000017442 Retinal disease Diseases 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 229940123237 Taxane Drugs 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 239000002870 angiogenesis inducing agent Substances 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 229940124346 antiarthritic agent Drugs 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000003435 antirheumatic agent Substances 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 230000002917 arthritic effect Effects 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 239000000022 bacteriostatic agent Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 210000001043 capillary endothelial cell Anatomy 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 208000037976 chronic inflammation Diseases 0.000 description 1
- 208000037893 chronic inflammatory disorder Diseases 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 229960001338 colchicine Drugs 0.000 description 1
- 239000007891 compressed tablet Substances 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 239000002872 contrast media Substances 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 229940111134 coxibs Drugs 0.000 description 1
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 1
- WRCBXHDQHPUVHW-QKBZBAIHSA-N deoxyharringtonine Chemical compound C1=C2CCN3CCC[C@]43C=C(OC)[C@@H](OC(=O)[C@@](O)(CCC(C)C)CC(=O)OC)[C@H]4C2=CC2=C1OCO2 WRCBXHDQHPUVHW-QKBZBAIHSA-N 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000000328 estrogen antagonist Substances 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 208000030533 eye disease Diseases 0.000 description 1
- 230000009969 flowable effect Effects 0.000 description 1
- 239000004088 foaming agent Substances 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 201000001421 hyperglycemia Diseases 0.000 description 1
- 229960001680 ibuprofen Drugs 0.000 description 1
- 238000010191 image analysis Methods 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- CAOHZEUEVKYHPF-XWHOPEMDSA-N isoharringtonine Chemical compound C1=C2CCN3CCC[C@]43C=C(OC)[C@@H](OC(=O)[C@@](O)(CCC(C)C)[C@H](O)C(=O)OC)[C@H]4C2=CC2=C1OCO2 CAOHZEUEVKYHPF-XWHOPEMDSA-N 0.000 description 1
- CAOHZEUEVKYHPF-UHFFFAOYSA-N isoharringtonine Natural products C1=C2CCN3CCCC43C=C(OC)C(OC(=O)C(O)(CCC(C)C)C(O)C(=O)OC)C4C2=CC2=C1OCO2 CAOHZEUEVKYHPF-UHFFFAOYSA-N 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000007932 molded tablet Substances 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 239000007923 nasal drop Substances 0.000 description 1
- 229940100662 nasal drops Drugs 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 201000003142 neovascular glaucoma Diseases 0.000 description 1
- 239000007764 o/w emulsion Substances 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 235000010603 pastilles Nutrition 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 230000037081 physical activity Effects 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 239000002985 plastic film Substances 0.000 description 1
- 229920006255 plastic film Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 210000001525 retina Anatomy 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 208000011581 secondary neoplasm Diseases 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 235000020183 skimmed milk Nutrition 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 244000041740 spotted evergreen Species 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 229960005137 succinic acid Drugs 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- FIAFUQMPZJWCLV-UHFFFAOYSA-N suramin Chemical compound OS(=O)(=O)C1=CC(S(O)(=O)=O)=C2C(NC(=O)C3=CC=C(C(=C3)NC(=O)C=3C=C(NC(=O)NC=4C=C(C=CC=4)C(=O)NC=4C(=CC=C(C=4)C(=O)NC=4C5=C(C=C(C=C5C(=CC=4)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C)C=CC=3)C)=CC=C(S(O)(=O)=O)C2=C1 FIAFUQMPZJWCLV-UHFFFAOYSA-N 0.000 description 1
- 229960005314 suramin Drugs 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 231100000057 systemic toxicity Toxicity 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 230000007306 turnover Effects 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 239000002525 vasculotropin inhibitor Substances 0.000 description 1
- 239000007762 w/o emulsion Substances 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
- 230000037314 wound repair Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/06—Antiglaucoma agents or miotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/12—Ophthalmic agents for cataracts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Ophthalmology & Optometry (AREA)
- Diabetes (AREA)
- Pulmonology (AREA)
- Epidemiology (AREA)
- Rheumatology (AREA)
- Endocrinology (AREA)
- Obesity (AREA)
- Pain & Pain Management (AREA)
- Hematology (AREA)
- Immunology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Emergency Medicine (AREA)
- Oncology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Medicines Containing Plant Substances (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US39765702P | 2002-07-22 | 2002-07-22 | |
| PCT/US2003/022926 WO2004009030A2 (en) | 2002-07-22 | 2003-07-22 | Angiogenesis inhibition by cephalotaxine alkaloids, derivatives, compositions and uses thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2005537282A true JP2005537282A (ja) | 2005-12-08 |
| JP2005537282A5 JP2005537282A5 (enExample) | 2006-09-07 |
Family
ID=30771096
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2004523305A Pending JP2005537282A (ja) | 2002-07-22 | 2003-07-22 | セファロタキシンアルカロイドによる血管新生抑制、並びにその誘導体、組成物、および使用方法 |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US7642252B2 (enExample) |
| EP (1) | EP1539179B1 (enExample) |
| JP (1) | JP2005537282A (enExample) |
| AU (1) | AU2003254120B2 (enExample) |
| CA (1) | CA2493544A1 (enExample) |
| ES (1) | ES2572145T3 (enExample) |
| WO (1) | WO2004009030A2 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102209386B1 (ko) * | 2020-01-09 | 2021-02-01 | 영남대학교 산학협력단 | 호모해링토닌을 유효성분으로 함유하는 대사질환 예방 또는 치료용 약학조성물 |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060211648A1 (en) * | 2000-04-12 | 2006-09-21 | Chemgenex Pharmaceuticals, Inc. | Naphthalimide compositions and uses thereof |
| US7135481B2 (en) * | 2000-04-12 | 2006-11-14 | Chemgenex Pharmaceuticals, Inc. | Naphthalimide compositions and uses thereof |
| US20050170015A1 (en) * | 2000-10-31 | 2005-08-04 | Brown Dennis M. | Antiproliferative colchicine compositions and uses thereof |
| WO2005087221A1 (en) * | 2004-03-15 | 2005-09-22 | Christine Allen | Biodegradable biocompatible implant and method of manufacturing same |
| EP1765341A4 (en) * | 2004-06-04 | 2010-04-14 | Chemgenex Pharmaceuticals Inc | PROCESS FOR TREATING PROLIFERATIVE CELL DISEASE USING NAPHTHALIMIDE AND PARP-1 INHIBITORS |
| EP1848391A2 (en) * | 2005-02-10 | 2007-10-31 | ChemGenex Pharmaceuticals, Inc. | Medical devices |
| CN103285015B (zh) * | 2007-04-13 | 2016-04-27 | 化学基因制药公司 | 三尖杉碱口服剂型 |
| US8466142B2 (en) | 2008-03-03 | 2013-06-18 | Sloan-Kettering Institute For Cancer Research | Cephalotaxus esters, methods of synthesis, and uses thereof |
| US20120283306A1 (en) * | 2011-04-27 | 2012-11-08 | Georgia Health Sciences University | Mouse model for avm |
| KR102066684B1 (ko) * | 2018-02-19 | 2020-01-15 | 가천대학교 산학협력단 | 개비자나무 추출물 또는 이로부터 분리한 화합물을 유효성분으로 함유하는 자가면역질환의 예방 또는 치료용 조성물 |
| KR102174166B1 (ko) * | 2018-12-13 | 2020-11-04 | 영남대학교 산학협력단 | 호모해링토닌을 유효성분으로 함유하는 세포노화 관련 질환 예방 또는 치료용 조성물 |
| KR102066683B1 (ko) * | 2019-09-16 | 2020-01-15 | 가천대학교 산학협력단 | 개비자나무 추출물 또는 이로부터 분리한 화합물을 유효성분으로 함유하는 자가면역질환의 예방 또는 치료용 조성물 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2000048595A1 (en) * | 1999-02-17 | 2000-08-24 | Bristol-Myers Squibb Co. | A novel anti-angiogenic agent comprising at least one kringle region and an upstream pre-activation domain of plasminogen |
| WO2001034174A2 (en) * | 1999-11-12 | 2001-05-17 | Entremed, Inc. | Methods for administration of therapeutic agents on an antiangiogenic schedule |
| WO2001068098A2 (en) * | 2000-03-15 | 2001-09-20 | Chemgenex Therapeutics, Inc. | Cephalotaxine alkaloid combination compositions and uses thereof |
| WO2002032904A1 (en) * | 2000-10-17 | 2002-04-25 | Oncopharm Corporation | New cephalotaxanes, their method of preparation and their use in treatment of cancers, leukemias, parasites including thus resistant to usual chemotherapeutic agents and as reversal agents |
| WO2002041882A2 (en) * | 2000-11-22 | 2002-05-30 | Novartis Ag | Combination comprising an agent decreasing vegf activity and an agent decreasing egf activity |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3793454A (en) * | 1971-11-12 | 1974-02-19 | Us Agriculture | Harringtonine and isopharringtonine for treating l1210 or p388 leukemic tumors in mice |
| US4152214A (en) * | 1977-10-07 | 1979-05-01 | The United States Of America As Represented By The Secretary Of Agriculture | Production of homodeoxyharringtonine and other cephalotaxine esters by tissue culture |
| JPS5835146A (ja) | 1981-08-28 | 1983-03-01 | Isukura Sangyo Kk | 2−ケト−6−メチル−5−ヘプテン酸エチルエステルの製造方法 |
| US5380747A (en) * | 1992-10-30 | 1995-01-10 | Emory University | Treatment for atherosclerosis and other cardiovascular and inflammatory diseases |
| US5629327A (en) * | 1993-03-01 | 1997-05-13 | Childrens Hospital Medical Center Corp. | Methods and compositions for inhibition of angiogenesis |
| CA2188813C (en) | 1994-04-26 | 2010-08-03 | Michael S. O'reilly | Angiostatin protein, nucleic acids encoding the same and methods of detection |
| CA2294247C (en) * | 1997-07-01 | 2004-10-26 | Atherogenics, Inc. | Antioxidant enhancement of therapy for hyperproliferative conditions |
| US6025353A (en) * | 1997-11-19 | 2000-02-15 | G.D. Searle & Co. | Method of using cyclooxygenase-2 inhibitors as anti-angiogenic agents |
| JP4979157B2 (ja) * | 1998-09-09 | 2012-07-18 | バイオリポックス アーべー | 置換されたγ−フェニル−Δ−ラクトンおよびそのアナログならびにこれらに関する使用 |
| EP1274458B1 (en) * | 2000-04-12 | 2005-09-28 | ChemGenex Pharmaceuticals, Inc. | Compositions containing a naphthalmide and an antiproliferative agent |
| WO2002003904A1 (en) | 2000-06-21 | 2002-01-17 | Shigeo Takizawa | Lift device capable of performing activating motion |
| CN100396286C (zh) * | 2002-12-30 | 2008-06-25 | 北京大学第一医院 | 高三尖杉酯碱和三尖杉酯碱在制备抑制血管生成药物中的应用 |
-
2003
- 2003-07-22 US US10/625,866 patent/US7642252B2/en not_active Expired - Fee Related
- 2003-07-22 AU AU2003254120A patent/AU2003254120B2/en not_active Ceased
- 2003-07-22 CA CA002493544A patent/CA2493544A1/en not_active Abandoned
- 2003-07-22 JP JP2004523305A patent/JP2005537282A/ja active Pending
- 2003-07-22 WO PCT/US2003/022926 patent/WO2004009030A2/en not_active Ceased
- 2003-07-22 ES ES03765931T patent/ES2572145T3/es not_active Expired - Lifetime
- 2003-07-22 EP EP03765931.5A patent/EP1539179B1/en not_active Expired - Lifetime
-
2009
- 2009-07-07 US US12/498,815 patent/US20090270368A1/en not_active Abandoned
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2000048595A1 (en) * | 1999-02-17 | 2000-08-24 | Bristol-Myers Squibb Co. | A novel anti-angiogenic agent comprising at least one kringle region and an upstream pre-activation domain of plasminogen |
| WO2001034174A2 (en) * | 1999-11-12 | 2001-05-17 | Entremed, Inc. | Methods for administration of therapeutic agents on an antiangiogenic schedule |
| WO2001068098A2 (en) * | 2000-03-15 | 2001-09-20 | Chemgenex Therapeutics, Inc. | Cephalotaxine alkaloid combination compositions and uses thereof |
| WO2002032904A1 (en) * | 2000-10-17 | 2002-04-25 | Oncopharm Corporation | New cephalotaxanes, their method of preparation and their use in treatment of cancers, leukemias, parasites including thus resistant to usual chemotherapeutic agents and as reversal agents |
| WO2002041882A2 (en) * | 2000-11-22 | 2002-05-30 | Novartis Ag | Combination comprising an agent decreasing vegf activity and an agent decreasing egf activity |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102209386B1 (ko) * | 2020-01-09 | 2021-02-01 | 영남대학교 산학협력단 | 호모해링토닌을 유효성분으로 함유하는 대사질환 예방 또는 치료용 약학조성물 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1539179B1 (en) | 2016-03-02 |
| US7642252B2 (en) | 2010-01-05 |
| WO2004009030A2 (en) | 2004-01-29 |
| AU2003254120B2 (en) | 2009-09-10 |
| AU2003254120A1 (en) | 2004-02-09 |
| CA2493544A1 (en) | 2004-01-29 |
| WO2004009030A3 (en) | 2004-09-10 |
| EP1539179A2 (en) | 2005-06-15 |
| US20040077629A1 (en) | 2004-04-22 |
| ES2572145T3 (es) | 2016-05-30 |
| US20090270368A1 (en) | 2009-10-29 |
| EP1539179A4 (en) | 2007-01-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20090270368A1 (en) | Angiogenesis inhibition by cephalotaxine alkaloids, derivatives, compositions and uses thereof | |
| AU738516B2 (en) | Quinazolinone-containing pharmaceutical compositions for prevention of neovascularization and for treating malignancies | |
| JP6868014B2 (ja) | 翼状片を治療するための組成物及び方法 | |
| TWI532480B (zh) | 以斑馬魚模組進行藥物篩選之方法及篩選所得藥物 | |
| JP2001278789A (ja) | サリドマイドを有効成分とする医薬品製剤 | |
| JPH09505809A (ja) | ヒドロキシカルバゾール化合物類による平滑筋移動および増殖の阻害 | |
| CN108883096A (zh) | 脉络膜新生血管抑制剂或玻璃膜疣抑制剂及其评价或筛选方法 | |
| Ma et al. | Platelet-activating factor (PAF) induces corneal neovascularization and upregulates VEGF expression in endothelial cells | |
| WO2014206056A1 (zh) | 柚皮素在制备预防和/或治疗腹主动脉瘤的药物中的应用 | |
| US20140161720A1 (en) | Treatment of angiogenic- or vascular-associated diseases | |
| KR101644440B1 (ko) | 개선된 혈관신생 억제용 펩티드와 이를 유효성분으로 함유하는 혈관신생과 관련된 질환의 예방 및 치료용 조성물 | |
| CN106692150B (zh) | 尼达尼布在制备预防和治疗肝纤维化与肝硬化的药物中的用途 | |
| WO2006092741A2 (en) | Treatment of inflammatory disorders with praziquantel | |
| JP2022504184A (ja) | ブドウ膜黒色腫の治療のための併用療法 | |
| CN111419800B (zh) | 用于治疗红斑狼疮的药物制剂及其制备方法 | |
| CN100577170C (zh) | 吡唑并嘧啶类Src家族酪氨酸激酶抑制剂在制备治疗心肌梗死的药物中的应用 | |
| JP5120848B2 (ja) | 血管新生阻害剤及び血管新生を伴う疾病に対する予防又は治療剤並びに食品 | |
| AU705955B2 (en) | Quinazolinone-containing pharmaceutical compositions for prevention of neovascularization and for treating human malignancies | |
| JP7486202B2 (ja) | 化合物の抗pcsk9(抗プロタンパク質変換酵素スブチリシン ケキシン9型)ナノ製剤および心血管疾患の治療および/または予防におけるその使用方法 | |
| JP2023030112A (ja) | 肥満細胞疾患の処置のための方法及び医薬組成物 | |
| US20210386754A1 (en) | Compositions and methods for treating atherosclerotic vascular disease | |
| BENELLI et al. | Trapidil inhibits endothelial cell proliferation and angiogenesis in the chick chorioallantoic membrane and in the rat cornea | |
| KR20110009084A (ko) | 눈-관련된 장애의 치료방법 | |
| TW200407119A (en) | Methods and compositions to treat conditions associated with neovascularization | |
| BRPI0713647A2 (pt) | formulações farmacêuticas e composições de um antagonista seletivo cxcr2 ou cxcr1 e métodos para o uso do mesmo visando o tratamento de distúrbios inflamatórios |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20060719 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20060719 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20090917 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100202 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20100428 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20100511 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20100526 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20100602 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20101019 |