EP2394762B1 - Reaktionsgefäß zur Herstellung phlegmatisierter Metallpulver oder Legierungspulver - Google Patents
Reaktionsgefäß zur Herstellung phlegmatisierter Metallpulver oder Legierungspulver Download PDFInfo
- Publication number
- EP2394762B1 EP2394762B1 EP11180240.1A EP11180240A EP2394762B1 EP 2394762 B1 EP2394762 B1 EP 2394762B1 EP 11180240 A EP11180240 A EP 11180240A EP 2394762 B1 EP2394762 B1 EP 2394762B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- retort
- reaction vessel
- metal
- crucible
- cooling
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Not-in-force
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/16—Making metallic powder or suspensions thereof using chemical processes
- B22F9/18—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds
- B22F9/20—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds starting from solid metal compounds
- B22F9/22—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds starting from solid metal compounds using gaseous reductors
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F27—FURNACES; KILNS; OVENS; RETORTS
- F27B—FURNACES, KILNS, OVENS OR RETORTS IN GENERAL; OPEN SINTERING OR LIKE APPARATUS
- F27B19/00—Combinations of different kinds of furnaces that are not all covered by any single one of main groups F27B1/00 - F27B17/00
- F27B19/02—Combinations of different kinds of furnaces that are not all covered by any single one of main groups F27B1/00 - F27B17/00 combined in one structure
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/16—Making metallic powder or suspensions thereof using chemical processes
- B22F9/18—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/16—Making metallic powder or suspensions thereof using chemical processes
- B22F9/18—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds
- B22F9/20—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds starting from solid metal compounds
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B34/00—Obtaining refractory metals
- C22B34/10—Obtaining titanium, zirconium or hafnium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B34/00—Obtaining refractory metals
- C22B34/10—Obtaining titanium, zirconium or hafnium
- C22B34/12—Obtaining titanium or titanium compounds from ores or scrap by metallurgical processing; preparation of titanium compounds from other titanium compounds see C01G23/00 - C01G23/08
- C22B34/1263—Obtaining titanium or titanium compounds from ores or scrap by metallurgical processing; preparation of titanium compounds from other titanium compounds see C01G23/00 - C01G23/08 obtaining metallic titanium from titanium compounds, e.g. by reduction
- C22B34/1268—Obtaining titanium or titanium compounds from ores or scrap by metallurgical processing; preparation of titanium compounds from other titanium compounds see C01G23/00 - C01G23/08 obtaining metallic titanium from titanium compounds, e.g. by reduction using alkali or alkaline-earth metals or amalgams
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B34/00—Obtaining refractory metals
- C22B34/10—Obtaining titanium, zirconium or hafnium
- C22B34/12—Obtaining titanium or titanium compounds from ores or scrap by metallurgical processing; preparation of titanium compounds from other titanium compounds see C01G23/00 - C01G23/08
- C22B34/1263—Obtaining titanium or titanium compounds from ores or scrap by metallurgical processing; preparation of titanium compounds from other titanium compounds see C01G23/00 - C01G23/08 obtaining metallic titanium from titanium compounds, e.g. by reduction
- C22B34/1277—Obtaining titanium or titanium compounds from ores or scrap by metallurgical processing; preparation of titanium compounds from other titanium compounds see C01G23/00 - C01G23/08 obtaining metallic titanium from titanium compounds, e.g. by reduction using other metals, e.g. Al, Si, Mn
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B34/00—Obtaining refractory metals
- C22B34/10—Obtaining titanium, zirconium or hafnium
- C22B34/14—Obtaining zirconium or hafnium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B5/00—General methods of reducing to metals
- C22B5/02—Dry methods smelting of sulfides or formation of mattes
- C22B5/04—Dry methods smelting of sulfides or formation of mattes by aluminium, other metals or silicon
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2999/00—Aspects linked to processes or compositions used in powder metallurgy
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F27—FURNACES; KILNS; OVENS; RETORTS
- F27D—DETAILS OR ACCESSORIES OF FURNACES, KILNS, OVENS OR RETORTS, IN SO FAR AS THEY ARE OF KINDS OCCURRING IN MORE THAN ONE KIND OF FURNACE
- F27D5/00—Supports, screens or the like for the charge within the furnace
- F27D5/0068—Containers
- F27D2005/0075—Pots, e.g. slag pots, ladles
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/29—Coated or structually defined flake, particle, cell, strand, strand portion, rod, filament, macroscopic fiber or mass thereof
- Y10T428/2982—Particulate matter [e.g., sphere, flake, etc.]
Definitions
- the invention relates to a reaction vessel for the preparation of passivated, air-manageable finest metal powder of the elements zirconium, titanium and / or hafnium, having a mean particle size below 10 microns (measured by permeability methods such as the Blaine or Fisher method) by metallothermal reduction of their oxides by means of calcium and magnesium.
- This reaction vessel consists of retort crucible, retort lid and inner crucible and allows the addition of phlegmatizing acting gases and / or solids before, during and / or after the reduction reaction.
- hydrogen in an amount of at least 500 ppm and nitrogen in an amount of at least 1000 ppm are used as phlegmatizing additives, as phlegmatizing solid additives carbon, silicon, boron, nickel, chromium and aluminum in quantities of at least 2000 ppm.
- the oxides can be individually reduced to produce pure metal powders. However, they may also be reduced in admixture with each other or in admixture with metal powders and / or oxides of the elements nickel, chromium and aluminum to produce alloys of titanium, zirconium and hafnium with these elements.
- Metallothermic reductions using calcium and magnesium as reducing agents are used to recover rare metals from their oxides when they are otherwise treated, for example, electrochemically from aqueous solutions, from molten salts or by reducing their oxides with carbon or with gases such as hydrogen or Carbon monoxide are not or only in low purity to win.
- a typical industrial example of this is the production of rare earth metals such as yttrium, cerium, lanthanum and others and of the metal beryllium from their oxides or halides with magnesium, Calcium or aluminum [Römpps Chemie-Lexikon "Metallothermie"].
- the metal-thermal reduction is used to recover the rare metals in a defined fine-powdery form, for example for applications in powder metallurgy, pyrotechnics or as a getter in vacuum technology.
- the particle size of the metal powder to be obtained can be largely predetermined by the choice of the particle size of the corresponding metal oxide to be reduced [ Petrikeev, et al., Tsvetnye Met., No. 8 (1991) 71-72 ].
- the EP 1 644 544 B1 a process for producing metal powders or metal hydride powders, the elements Ti, Zr, Hf, V, Nb, Ta and Cr, in which an oxide of these elements is mixed with a reducing agent and this mixture is heated in a furnace, optionally under a hydrogen atmosphere, until the Reduction reaction begins, the reaction product is leached and then washed and dried, wherein the oxide used has an average particle size of 0.5 to 20 pm, a BET specific surface area of 0.5 to 20 m2 / g and a minimum content of 94Gew.- % having.
- the design of a suitable reaction vessel is not explained.
- the metal oxide of the reduction metal formed with the reduction should not form double oxides or other mixed oxides with the oxide to be reduced, because the yield is reduced by this parallel side reaction.
- the vapor pressure of the reducing metal at the expected reaction temperature usually 800 to 1400 ° C
- the reduction metal oxide formed in the reduction must be soluble in water or aqueous acids in order to be able to remove it from the reaction mass by leaching after completion of the reaction.
- the poor solubility of the oxides of silicon and aluminum as well as their tendency to form mixed oxides is the reason that these low-priced elements are often not used as a reducing agent.
- Metal-thermal reduction reactions are generally self-contained. This term refers to reactions that are initiated by an initial ignition and then automatically continue to run without external energy input.
- the initial ignition can be initiated chemically, electrically (by a filament or by induction) or simply by hot heating of a portion of the metal / metal oxide mixture [ DE PS 96317 ].
- a gas-fired crucible furnace has the advantage that the retort is heated quickly. At a temperature of about 100 to 450 ° C, depending on the grain sizes and the nature of the starting materials, sets an initial ignition, which starts at a hot spot, which is usually laterally in the lower third of the crucible in which the mixture to be reacted located.
- the temperature then rises within a few minutes to values between 900 ° C and 1200 ° C, depending on whether calcium or magnesium is used as the primary reducing metal.
- Calcium leads to higher than 1000 ° C, magnesium to slightly lower peak temperatures.
- the pressure inside the retort increases. When reaching an overpressure of about 50 to 100 mbar, therefore, a valve is opened and the pressure released, It is usually hydrogen, resulting from the moisture of the starting materials forms, magnesium metal vapor and alkali metal vapors from impurities of the starting materials. This can lead to a flame on the drain valve. Resulting vapors and dusts must be extracted at the place of their formation.
- the opening of the valve can be done manually, but also electromechanical or pneumatic, and it can be controlled for security reasons remotely, for example, under video observation.
- As relief valves for the overpressure primarily sealless plug valves or ball valves with a large cross section are used.
- Metal-thermal reductions continue to go on - when ignited - inexorably. Once initiated, the reaction can no longer be stopped using conventional processing techniques such as cooling or adding diluents.
- Metallic titanium, zirconium and hafnium, and alloys of these metals are only stable to air because they are surrounded by a dense, oxygen-impermeable, oxide or oxynitride envelope at room temperature, the so-called passive layer.
- the passivation is also known from many other metals, such as aluminum, zinc and chromium. Passivation occurs automatically on most metals. By contact of the metal surface with the oxygen and nitrogen of the air, with moisture and in air Carbon dioxide builds up the protective passive film without any special action. This is not so with the metals Ti, Zr and Hf and their alloys, when they are in fine powder form and produced in a protective atmosphere under argon, helium or in vacuum.
- the object of the present invention was therefore to provide a method and a reaction vessel for carrying out this process for the production of metal powders or alloy powders of the reactive metals zirconium, titanium or hafnium from the corresponding oxides or oxide mixtures, wherein the prepared reactive metal powders or alloy powders subsequently, For example, for the purpose of further processing, to be handled in the air.
- the reaction vessel according to the invention makes it possible to carry out the reduction reaction under protective gases, such as argon or helium, or in vacuo, in order to preclude uncontrolled access of air and moisture.
- protective gases such as argon or helium, or in vacuo
- the construction further allows in particular the targeted addition of a measured amount of gases during and / or after the reduction reaction in order to specifically phlegmatize the metals or alloys formed and to influence their chemical behavior.
- the construction further permits the reduction of the oxides or oxide mixtures under a reactive gas atmosphere, especially under hydrogen, when it is intended to produce hydrides of the metals Ti, Zr and Hf. It also allows the hydrogenation of alloys made by fusion metallurgy, e.g. an alloy of 70% Zr and 30% nickel or titanium sponge by heating and introducing hydrogen.
- Besides hydrogen, ammonia, methane, carbon monoxide, carbon dioxide and nitrogen may also be introduced into the retort to produce hydrides, subhydrides, carbides, nitrides, hydride-nitride mixtures or oxinitrides of the metals zirconium, titanium and hafnium.
- the design incorporates a special flange and lid cooling design to prevent unwanted ingress of cooling water into the retort space.
- a special spacer with support ring allows the retort to be inserted at different depths into the combustion chamber of the reduction furnace.
- the reduction metal used is preferably calcium and / or magnesium. Calcium and magnesium can therefore be used individually or together. In principle, further additives, such as carbon, silicon or silicon oxide and other substances, can be added in order to influence the properties of the reactive metal powder produced during the reduction
- a passivating gas preferably nitrogen and / or hydrogen is introduced.
- at least 500 ppm of hydrogen and 1000 ppm of nitrogen should be present in the metal powders to the above-described reactions to avoid.
- the amount of hydrogen should better be at least 1000 ppm (0.1%), preferably 1000 to 2000 ppm, and nitrogen at least 2000 ppm (0.2%), preferably 2000 - 3000 ppm.
- Nitrogen and hydrogen can also be introduced in the form of ammonia.
- At least 2,000 ppm (0.2% by weight) and at most 30,000 ppm (3% by weight) of carbon, silicon, boron, nickel, chromium and / or aluminum can be introduced as passivating solids.
- the passivating solid can also be introduced in the form of a fine oxide of the elements Ni, Cr, Al, Si and B having an average particle size of less than 20 ⁇ m and reduced together with the metal oxide.
- carbon can be introduced via the gas phase in the form of methane, carbon dioxide or carbon monoxide.
- the passivating gases and solids can also be introduced together.
- the ignitability of the phlegmatized metal powders or alloy powders can be further reduced by washing out submicroscopic particles of less than 0.2 ⁇ m grain size during leaching and / or washing.
- a hypothetical idea of the inventor is the following: by the inclusion of the gases in the metal grid, the total energy level of the free electrons in the metal is lowered so far that the spontaneous reaction with oxygen under combustion or the reaction with water is omitted.
- wet-chemical preparation of the metal powders in water and acid only the actual, oxidic passive layer on the particle surface is formed by a slow oxidation reaction with atmospheric oxygen or by slow reaction with water. Since the metal powder is heated to room temperature or at most to the boiling point of the water in the wet-chemical processing, all diffusion processes are slow and it can indeed form a dense, firmly adhering "passive layer" of metal oxide (and metal nitride), the metal permanently protects against further oxidation.
- the degree of passivation is difficult to quantify, it can best be deduced from the ignition point of the metal powder in air.
- z.T. also standardized methods available.
- the metals Ti, Zr and Hf the following simple test arrangement is suitable: in a copper or steel cylinder with a diameter and a height of 70 mm each, a hole of 15 mm diameter and 35 mm depth is drilled in the middle. At a distance of 4 mm, a hole 5 mm thick, also 35 mm deep, is drilled to accommodate a thermocouple.
- the block is preheated uniformly to about 140 - 150 ° C, then an amount of 1 - 2 g of the metal powder to be tested is filled into the larger bore and it is heated further until ignition.
- This can be recognized optically (e.g., by video camera).
- By evaluating the time / temperature curve of the thermocouple you can determine the ignition point quite accurately. If the ignition points are below 150 ° C, one can not assume a safe passivation or phlegmatization. Metal powders with such low ignition points should be destroyed by burning in a safe place.
- the firing time also indicates the degree of phlegmatization.
- the method is described in Example 1. References to this can also be taken from measurements of the minimum electrical ignition energy, which however is very difficult to determine. [ Berger, B., Gyseler, J., Method for Testing the Sensitivity of Explosives to Electrostatic Discharge, Techn. Of Energetic Metals, 18th Ann. Conf. of ICT, Düsseldorf 1987, pp. 55/1 to 55/14 ].
- the phlegmatization of the metal powders of Ti, Zr and Hf and of alloy powders of these metals with Ni, Cr and Al takes place during and / or after the reduction in the evacuable and gas-tight retort by addition of a measured amount of hydrogen and / or nitrogen , Part of these gases can also be present in the retort from the very beginning.
- the passively acting gases can be introduced into the reaction vessel (the retort) after reaching the peak temperature during cooling of the reacted mass.
- Ni, Cr and Al have a dual function, they can not only serve for the production of alloys of Ti, Zr and Hf, but act in small amounts between 2000 ppm to 3% as phlegmatizing solid additives in the pure metals.
- non-metallic additives such as carbon, silicon, boron or metallic additives such as iron, nickel, chromium, aluminum and others can influence the reactivity of zirconium, titanium and hafnium to water, air and oxidants.
- Addition of silicon or boron generally slows down the burning rate only slightly, but can increase the ignition temperature.
- a rather negative example is iron, additions of iron lead to spray sparks, lower the ignition temperature of the zirconium metal rather and usually increase the ignitability to friction.
- Carbon can be introduced into the retort according to the invention by adding measured amounts of carbon dioxide or methane. It generally leads to a phlegmatization.
- reaction vessel for the production of phlegmatized metal powder or alloy powder having an average particle size of less than 10 microns, consisting of or containing at least one of the reactive metals zirconium, titanium or hafnium, by metallothermal reduction of oxides or halides of said reactive metals by means of a reducing metal according to the described method is characterized in that the reaction vessel consists of a usable in a heatable reduction oven retort crucible with a double-coolable lid and an inner crucible, wherein in the annular cooling provided lid at least one nozzle for introducing a passivating acting gas or solid is incorporated and to the retort crucible for mounting the retort lid, a flange is welded to the underside a cooling for
- the cooling is congruent under a ring on the flange extending seal and this cooling has no connection to the actual retort crucible.
- any other cooling media can be used as an alternative to water.
- organic heat transfer media such as heat transfer oils, preferably silicone oils, or even air can be used.
- a suitable silicone oil can be obtained, for example, as Therminol® VP from Solutia GmbH.
- the cooling media circulate in a common or in independent suitable cooling circuits.
- the lid may have the following connections: a nozzle with a heat-resistant, sealless ball valve or cock tap for releasing excess pressure, a nozzle for connecting a vacuum pump for evacuating the retort, a nozzle for introducing protective gas, such as argon, from a line, a Nozzle for introducing reactive gases, such as H 2 or N 2 , from a pipe, a nozzle for receiving a safety valve, a nozzle for connection to a vacuum and pressure gauge and a nozzle for passing one or several thermocouples (Pt / RhPt).
- a groove for receiving a sealing ring, preferably made of Viton, unless provided on the retort crucible, may be provided on the lid.
- the water cooling can be configured, for example, as running on the lid annular channel.
- the lid can preferably be connected via a screw with the flange.
- the cooling of the retort lid has no connection to the nozzle and passages of the cover plate.
- the cooling of the flange should have no connection to the retort crucible and the retort wall out.
- the wall thickness is at least 10 mm, preferably 15 mm.
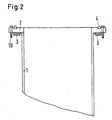
- a flange 2 is welded with a material thickness of 30 mm and a ring width of 150 mm, on the underside of a cooling 3 is welded for cooling water.
- the flange 2 is preferably also made of the heat-resistant steel 1.4841 or a comparable steel.
- Decisive design feature is that the cooling 3 is exactly below a ring 4 extending on the flange 2 seal and this cooling 3 has no connection to the actual retort crucible 1.
- the flange 2 allows the attachment of the lid 5, wherein between the cover 5 and the flange 2 of the sealing ring 4 made of Viton, Perbunan, Teflon or other common sealing material is used, which allows the gas and vacuum-tight connection between the lid and retort crucible.
- the sealing ring 4 may optionally be inserted into a groove milled into the flange.
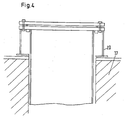
- a support ring is screwed to the crucible flange 2 with a spacer 20, which makes it possible to insert the retort at different depths into the furnace chamber or the combustion chamber 18 of the heatable reduction furnace 17.
- the heating of the reduction furnace 17 may preferably be effected by means of an electric heating 16.1 or alternatively a gas heating 16.2.
- a spacer 20 with support ring is provided between the flange 2 and the heatable reduction furnace 17, a spacer 20 with support ring.
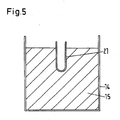
- the inner crucible 14 serves according to the Fig. 5 for receiving the batch mixture 15, that is, the mixture of the metal oxide and the reduction metal to be reduced.
- the inner crucible 14 is made of mild steel, heat-resistant steel or stainless steel, preferably St37 or VA, depending on the purity requirements in a thickness of 2 to 5 mm, preferably 2 to 4 mm.
- the reaction mass is kept away from the actual retort, which serves only as a "receptacle" during the duration of the reduction reaction.
- the inner crucible can be removed from the retort and optionally stored under protective gas in another vessel, eg a stainless steel drum, until the reduced mass contained in it is processed.
- a protective tube 21 can be inserted for receiving one or more thermocouples.
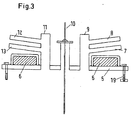
- a particular inventive feature consists in the execution of the cooling of the cover 5 and flange 2 of the reduction retort.
- Lid 5 and retort crucible 1 are gas-tight and vacuum-tight connected by a sealing ring 4 made of Viton, Perbunan, Teflon or other common sealing materials.
- the seals 4 may be designed as a flat ring or as an O-ring.
- the gaskets 4 must be cooled because they would be decomposed at the high reaction temperatures. The cooling takes place in this embodiment with water. It would be catastrophic if water entered the retort space through cracks or corrosion holes during the reduction reaction. This would lead to a violent evolution of hydrogen and an explosion of the retort.
- the design of the cooling is therefore a very important feature of the reaction vessel.
- the cooling 3 on the crucible flange is placed on the bottom of the flange 2 and has only one connection to the flange itself, but not to the retort wall. Thus, water can never penetrate into the retort from this area.
- the cover 5, the cooling is designed so that it cools only the surface of the lid 5, but has no connection to the nozzle and bushings. The cooling water would have to penetrate through the solid lid 5 to get into the retort, which is very unlikely with a wall thickness of at least 30 mm heat resistant steel.
- the cooling is as water cooling 6 in Fig. 3 shown in more detail.
- the retort crucible and retort lid are connected with a suitable number of screws and nuts 19.
- the retort lid 5 and retort crucible 1 existing retort can be used immediately after removal of the inner crucible 14 with the reacted and phlegmatized mass again for receiving another inner crucible with a new approach , Thus, several retorts can be reacted one after the other in an oven.
- An example of a metallothermal reduction using the above principles and for the present invention is the recovery of zirconium in powder form by reduction of zirconium oxide with calcium for use in gettering (lamps, vacuum components) and military pyrotechnics, e.g. for the production of thermal batteries.
- Zirconia with a mean grain size of 5 +/- 0.5 micron measured by the Blaine method or the Fisher Sub Sieve Sizer method, is mixed with calcium chips or granules of 0.5 to 5 mm size.
- Calcium metal is added in the theoretically necessary stoichiometric amount.
- a small amount, for example 2 to 10% by weight, of the theoretically necessary stoichiometric amount of magnesium chips of similar size to calcium is additionally added.
- further additives for example carbon, silicon or silicon oxide and other substances can be added in order to influence the properties of the zirconium powder formed during the reduction.
- the amount of gaseous additives is measured to be in the range of 500 to about 5000 ppm in the later isolated zirconium powder, and "impurity" for solids of at least 2,000 to 3%.
- a small amount of silicon oxide is used, which is known as Si impurity in the isolated zirconium powder reappears.
- the mixture of the starting materials is carried out under argon in a Rhönradmischer, a helical mixer or other comparable mixing device for solids. All feedstocks must be kept scrupulously dry.
- the addition of a small amount of the second reducing metal (magnesium) lowers the initial ignition threshold so that the reaction mixture is easier to ignite than using calcium alone. Since magnesium vaporizes earlier than calcium, the vaporization of magnesium removes heat from the reaction mass, thereby limiting the peak temperature of the reacting mass.
- the starting materials are weighed in a drum mixer under Ar atmosphere, intimately mixed, transferred to an inner crucible and stored dry until used in the reduction of the invention under argon atmosphere.
- the inner crucible with the mixture of feedstocks in the retort crucible according to the invention is used, the retort closed by placing the lid, the entire retort pumped out twice to a final pressure of less than 1 mbar to remove the air and any moisture, and with argon flooded.
- a thermocouple is used to control the temperature in the reaction chamber to eat. It is connected to a pressure gauge, which displays both negative pressure to 0.1 mbar as overpressure to +1000 mbar.
- Compounds are made to gas pressure bottles with argon, nitrogen and hydrogen.
- the gas pressure bottles are equipped with fine pressure reducers, which are designed for a max. Pressure of 100 mbar are set.
- the pressure cylinders for nitrogen and hydrogen are filled with measured quantities of these gases.
- the protective gas argon must always be available in sufficient excess quantity.
- the reaction starts at a temperature of about. 100 - 140 ° C and 1100 ° C are reached within 2 minutes. After exceeding the peak temperature, recognizable by the falling of the measured temperature in the reaction space by means of a thermocouple, the necessary for phlegmatization or for adjusting the burning and ignition properties of the zirconium metal powder gas quantities are introduced.
- 50 liters of nitrogen and 130 liters of hydrogen from the connected compressed gas cylinders are added in the course of the cooling phase. This corresponds to an amount of 500 ppm of hydrogen and 2500 ppm of nitrogen in the resulting zirconium metal powder. The gases are rapidly absorbed by the zirconium metal during the cooling phase.
- a metal sludge of fine zirconium metal powder whose grain size approximately corresponds to that of the zirconium oxide used, ie 5 +/- 1 ⁇ m measured according to Blaine or Fisher.
- the metal powder is washed out, wet sieved ( ⁇ 45 ⁇ m) and dried carefully ( ⁇ 80 ° C). Due to the added auxiliaries (here SiO 2 ) and the gases, here N 2 and hydrogen, the metal powder can be safely processed in water and acid without reacting with water, and it can later be handled without spontaneous spontaneous combustion in air.
- the yield is 25-26 kg of a fine, gray zirconium metal powder.
- the burning rate of the metal powder thus obtained is measured as follows: into a steel block of 60 cm in length, 1 cm in height and 4 cm in width, a rectangular gutter, which is 2 mm deep and 3 mm wide, is continuously milled.
- the trough is filled with 15 g of the metal powder to be tested, the powder filling is ignited at one end and the time taken for the burning front to go through a marked distance of 500 mm distance is measured.
- the burning time is 80 +/- 10 seconds / 50 cm.
- the ignition temperature is 240 +/- 20 ° C.
- the electrical energy for ignition is approx. 18 ⁇ J.
- In the final product can be found due to a further hydrogen uptake in the aqueous workup, a total of 2000 ppm of hydrogen. Also impurities of the reduction metals are found in the metal powders, but these amounts i.a. low. The found amount of 1800 ppm silicon, 2500 ppm nitrogen and 1000 ppm titanium corresponds quite well to the theoretical amount
- the retort is left in the reduction furnace after the reduction reaction with the reacted mass. Further heating from outside influences the grain size of the rare metal or its burning properties and chemical properties. By heating for several hours at about 900 ° C, a sintering effect can be achieved, which leads to a grain coarsening of the zirconium metal obtained. In the present example, by heating for 3-4 hours, the mean grain size of the zirconium metal can be increased from about 5 .mu.m to 6.-.7 .mu.m and the burning rate can be slowed from about 75 s / 50 cm to 100 to 120 s / 50 cm. The ignition point of the metal remains almost unchanged in this procedure and is at 250 ° C +/- 20 ° C.
- zirconium metal which is suitable for use in ignition systems of airbag detonators and militarily used ignition sets is proceeded as in Example (1), but the following starting materials are used: Zirconium oxide (average particle size 1.5 / -0.25 / + 0.5 ⁇ m) 36.0 kg Magnesium (chips, at least 99.8%, bulk density 0.45 g / cm 3 ) 17.1 kg silica 0.35 kg
- the burning rate in a channel (see Example (1) in air is 10 +/- 3 s / 50 cm.)
- the mean particle size of the metal powder is 1.7 +/- 0.3 ⁇ m. 10 ° C.
- the minimum electrical ignition energy was measured at about 2 ⁇ J.
- the content of silicon corresponds approximately to the use and is 5900 ppm (theor. 6530 ppm).
- the hydrogen content in the final product is 1400 ppm (theor. 900 ppm), due to a further hydrogen uptake in the acid leaching.
- the nitrogen content in the final product is 4000 ppm (theoretical 5000 ppm).
- the high degree of ignitability of the metal powder results from the high degree of fineness and the high sensitivity to electrostatic charging. These metal powders are generally not dried, but stored and traded in suspension under at least 30% by weight of water.
- Example (1) The procedure is as in Example (1), but without additions of SiO 2 and TiO 2.
- Zirconium oxide (average particle size 4.5 ⁇ m) 36.0 kg
- Calcium granules 26.5 kg
- the reaction is carried out as in Example (1), but the retort is filled after pumping not with argon, but with 100 l of nitrogen (99,995). By heating, the reaction is started, it starts in this case already at 80 to 100 ° C and reaches a peak of about 1050 ° C.
- reaction mass After complete cooling, the reaction mass is broken, but not crushed leached, but finely ground under Argon atmosphere and exclusion of moisture to a particle size below 150 microns.
- calcium oxide and magnesium oxide and excess magnesium and calcium 12 kg of nickel powder (average particle size after Fsss 5 microns) are added (attention, Ni powders are carcinogenic) and mixed under an argon atmosphere in a drum mixer.
- the mass is then filled into the inner crucible, used in the retort according to the invention, evacuated and heated slowly under argon atmosphere, the oven temperature is limited to 860 ° C.
- the oven temperature is reached after about 1 h, the internal temperature measured in the reaction mixture begins to rise after about 3 to 5 h, then it runs within 15 minutes from about 400 ° C to 880-900 ° C. The heating will be switched off as soon as the implementation starts.
- the nickel oxide always contained in the nickel powder is reduced to Ni by the reducing agent excess still contained in the Zr reducing mass, and at the same time, the Zr powder combines with the nickel to form a Zr-Ni alloy having a composition of 70 % By weight of Zr and 30% by weight of nickel.
- 200 l of hydrogen are added.
- the reaction mass is allowed to cool overnight in the retort in a cooling rack under argon supply. After opening, the mass is broken up, crushed and leached in acid to wash out calcium and magnesium oxide. In this case, the leaching must be carried out in a strongly acetate-buffered hydrochloric acid, since the ZrNi alloy would be attacked by pure hydrochloric acid. The remaining as a suspension Zr / Ni alloy is wet sieved ( ⁇ 45 microns) and dried.
- the obtained Zr-Ni alloy powder has a grain size of 4-6 ⁇ m measured according to Blaine or Fisher.
- the yield is about 36 kg.
- the one burning time is 200 +/- 30 s / 50 cm measured in the hearth described in Example (1).
- the ignition point is 260-280 ° C, the hydrogen content at 0.2% (2000 ppm) versus 500 ppm theoretically.
- the nitrogen content was not determined, theoretically it is 1%. (10,000 ppm).
- As electrical minimum ignition energy was determined about 100 ⁇ J.
- the alloy powder is suitable for the production of delay igniters according to US specification MIL-Z-114108.
- the zirconium metal powder produced in the examples described are phlegmatized according to the invention and are not spontaneously self-ignitable, ie manageable upon access of air.
- the aqueous workup itself also contributes to the passivation of the metal surface.
- the latter also leads to the fact that Zr, Ti and Hf metal powders are surrounded by a thin oxide film and can thus be charged electrostatically. It can then enter a spontaneous inflammation, which is not based on the "classic" Dientzündige but is due to an electrostatic discharge.
- Zr, Ti and hafnium - metal powders must therefore always be handled in grounded, preferably metallic vessels and processed as far as possible under argon.
- appropriate safety measures must be taken and professional advice should be obtained from trained safety experts.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Environmental & Geological Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- General Engineering & Computer Science (AREA)
- Manufacture And Refinement Of Metals (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Powder Metallurgy (AREA)
- Crucibles And Fluidized-Bed Furnaces (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PL11180240T PL2394762T3 (pl) | 2008-01-23 | 2009-01-08 | Naczynie reakcyjne do wytwarzania flegmatyzowanych proszków metalowych lub proszków stopowych |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102008005781A DE102008005781A1 (de) | 2008-01-23 | 2008-01-23 | Phlegmatisierte Metallpulver oder Legierungspulver und Verfahren bzw. Reaktionsgefäß zu deren Herstellung |
| EP09703271.8A EP2247398B1 (de) | 2008-01-23 | 2009-01-08 | Phlegmatisierte metallpulver oder legierungspulver und verfahren bzw. reaktionsgefäss zu deren herstellung |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP09703271.8 Division | 2009-01-08 | ||
| EP09703271.8A Division-Into EP2247398B1 (de) | 2008-01-23 | 2009-01-08 | Phlegmatisierte metallpulver oder legierungspulver und verfahren bzw. reaktionsgefäss zu deren herstellung |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2394762A1 EP2394762A1 (de) | 2011-12-14 |
| EP2394762B1 true EP2394762B1 (de) | 2013-11-27 |
Family
ID=40474642
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP11180240.1A Not-in-force EP2394762B1 (de) | 2008-01-23 | 2009-01-08 | Reaktionsgefäß zur Herstellung phlegmatisierter Metallpulver oder Legierungspulver |
| EP09703271.8A Not-in-force EP2247398B1 (de) | 2008-01-23 | 2009-01-08 | Phlegmatisierte metallpulver oder legierungspulver und verfahren bzw. reaktionsgefäss zu deren herstellung |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP09703271.8A Not-in-force EP2247398B1 (de) | 2008-01-23 | 2009-01-08 | Phlegmatisierte metallpulver oder legierungspulver und verfahren bzw. reaktionsgefäss zu deren herstellung |
Country Status (16)
| Country | Link |
|---|---|
| US (2) | US8821610B2 (pl) |
| EP (2) | EP2394762B1 (pl) |
| JP (2) | JP5876651B2 (pl) |
| KR (1) | KR101557174B1 (pl) |
| CN (1) | CN101925427B (pl) |
| AU (1) | AU2009207739B2 (pl) |
| BR (1) | BRPI0907383A2 (pl) |
| CA (1) | CA2712929C (pl) |
| DE (2) | DE102008005781A1 (pl) |
| IL (2) | IL206966A (pl) |
| MX (1) | MX2010007826A (pl) |
| MY (1) | MY152942A (pl) |
| PL (1) | PL2394762T3 (pl) |
| RU (1) | RU2492966C2 (pl) |
| UA (1) | UA102086C2 (pl) |
| WO (1) | WO2009092631A1 (pl) |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102008005781A1 (de) * | 2008-01-23 | 2009-07-30 | Tradium Gmbh | Phlegmatisierte Metallpulver oder Legierungspulver und Verfahren bzw. Reaktionsgefäß zu deren Herstellung |
| DE102008000433A1 (de) * | 2008-02-28 | 2009-09-03 | Chemetall Gmbh | Verfahren zur Herstellung von Legierungspulvern auf der Basis von Titan, Zirconium und Hafnium, legiert mit den Elementen Ni, Cu, Ta, W, Re, Os und Ir |
| RU2424085C1 (ru) * | 2010-03-29 | 2011-07-20 | Государственное образовательное учреждение высшего профессионального образования "Национальный исследовательский Томский политехнический университет" | Способ получения газопоглотителя из порошка титана |
| WO2013006256A1 (en) * | 2011-07-01 | 2013-01-10 | General Electric Company | Continuous process for the production of titanium alloy powders |
| GB201218675D0 (en) | 2012-10-17 | 2012-11-28 | Univ Bradford | Improved method for metal production |
| KR101494340B1 (ko) * | 2013-08-27 | 2015-03-04 | 주식회사 나노테크 | 타이타늄 카바이드 분말의 제조방법 |
| AT13691U1 (de) | 2013-09-02 | 2014-06-15 | Plansee Se | Chrommetallpulver |
| KR101691410B1 (ko) | 2014-08-13 | 2017-01-02 | 주식회사 나노테크 | 탄질화티타늄 분말의 제조 방법 |
| BR112017004592A2 (pt) * | 2014-09-09 | 2018-11-06 | The Arizona Board Of Regents On Behalf Of The University Of Arizona | sistema, equipamento e processo para lixiviar metal e armazenar energia térmica durante a extração do metal |
| CA2956451C (en) * | 2014-09-15 | 2017-07-11 | Materiaux Nieka Inc. | Method and apparatus for preparing an analytical sample by fusion |
| CN108367361A (zh) | 2015-10-29 | 2018-08-03 | Ap&C高端粉末涂料公司 | 金属粉末雾化制造方法 |
| US10455680B2 (en) * | 2016-02-29 | 2019-10-22 | Asml Netherlands B.V. | Method and apparatus for purifying target material for EUV light source |
| WO2017177315A1 (en) | 2016-04-11 | 2017-10-19 | Ap&C Advanced Powders & Coatings Inc. | Reactive metal powders in-flight heat treatment processes |
| RU2638869C1 (ru) * | 2016-10-11 | 2017-12-18 | федеральное государственное бюджетное образовательное учреждение высшего образования "Ульяновский государственный технический университет" | Способ получения защитной оксидной пленки на металлической поверхности |
| RU2634111C1 (ru) * | 2016-10-17 | 2017-10-23 | Российская Федерация, от имени которой выступает Государственная корпорация по атомной энергии "Росатом" (Госкорпорация "Росатом") | Способ дезагрегирования порошка натриетермического циркония |
| US20190160528A1 (en) * | 2017-11-27 | 2019-05-30 | Hamilton Sundstrand Corporation | Method and apparatus for improving powder flowability |
| CN107971500B (zh) * | 2017-12-01 | 2020-09-25 | 北京汽车集团越野车有限公司 | 一种镁合金制件的制作方法及镁合金制件 |
| CN110319690A (zh) * | 2019-07-03 | 2019-10-11 | 宁夏秦氏新材料有限公司 | 燃气加热金属氮化物合成设备 |
| CN110285672A (zh) * | 2019-07-03 | 2019-09-27 | 宁夏秦氏新材料有限公司 | 一种金属氮化物合成设备 |
| CN110319689A (zh) * | 2019-07-03 | 2019-10-11 | 宁夏秦氏新材料有限公司 | 一种燃气加热金属氮化物合成设备 |
| CN110255510A (zh) * | 2019-07-03 | 2019-09-20 | 宁夏秦氏新材料有限公司 | 燃气加热合成锰系氮化物的方法 |
| CN110319691A (zh) * | 2019-07-03 | 2019-10-11 | 宁夏秦氏新材料有限公司 | 一种燃气加热金属氮化物合成设备 |
| CN110907052A (zh) * | 2020-01-09 | 2020-03-24 | 遵义钛业股份有限公司 | 一种海绵钛还原生产反应点的测温装置 |
| CN112458316A (zh) * | 2020-11-13 | 2021-03-09 | 云南国钛金属股份有限公司 | 一种防止反应器大盖气体通道堵塞的装置及方法 |
| CN113149797A (zh) * | 2021-04-29 | 2021-07-23 | 江苏长积材料科技有限公司 | 一种本安型二氧化碳气化剂及其制备方法 |
| CN113996401B (zh) * | 2021-11-16 | 2022-09-23 | 湖南先导电子陶瓷科技产业园发展有限公司 | 一种钛酸盐陶瓷粉体高温快速合成设备 |
| DE102023200287A1 (de) * | 2023-01-16 | 2024-08-01 | Mahle International Gmbh | Ventil für eine Brennkraftmaschine und Herstellungsverfahren |
| CN117419566B (zh) * | 2023-12-18 | 2024-03-15 | 河北睿阳稀有金属制品有限公司 | 一种海绵铪生产用还原装置 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB771351A (en) * | 1954-01-19 | 1957-04-03 | Gen Electric Co Ltd | Improvements in or relating to the bright annealing of brass |
Family Cites Families (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE96317C (pl) | ||||
| US1602542A (en) | 1921-01-06 | 1926-10-12 | Westinghouse Lamp Co | Reduction of rare-metal oxides |
| US4062679A (en) * | 1973-03-29 | 1977-12-13 | Fansteel Inc. | Embrittlement-resistant tantalum wire |
| US3992192A (en) * | 1974-07-01 | 1976-11-16 | Haig Vartanian | Metal powder production |
| DE2523423C2 (de) * | 1975-02-03 | 1981-12-10 | PPG Industries, Inc., 15222 Pittsburgh, Pa. | Submikrones Titandiborid und Verfahren zu seiner Herstellung |
| JPS5452608A (en) * | 1977-10-04 | 1979-04-25 | Nippon Mining Co Ltd | Manufacture of zirconium |
| US4149876A (en) * | 1978-06-06 | 1979-04-17 | Fansteel Inc. | Process for producing tantalum and columbium powder |
| CA1202183A (en) * | 1982-05-31 | 1986-03-25 | Hiroshi Ishizuka | Apparatus and method for producing purified refractory metal from a chloride thereof |
| US4470847A (en) * | 1982-11-08 | 1984-09-11 | Occidental Research Corporation | Process for making titanium, zirconium and hafnium-based metal particles for powder metallurgy |
| JPH0317197Y2 (pl) * | 1985-07-17 | 1991-04-11 | ||
| CN1015640B (zh) * | 1988-05-28 | 1992-02-26 | 中国石油化工总公司石油化工科学研究院 | 负载型非贵金属加氢裂化催化剂 |
| CN1022579C (zh) * | 1988-06-24 | 1993-10-27 | 冶金工业部攀枝花钢铁公司钢铁研究院 | 还原钛铁矿粉的制取方法 |
| JPH02129314A (ja) * | 1988-11-08 | 1990-05-17 | Sumitomo Metal Ind Ltd | 真空精錬容器継手部の冷却方法 |
| JPH0623556Y2 (ja) * | 1989-02-17 | 1994-06-22 | 株式会社神戸製鋼所 | エレクトロスラグ再溶解炉 |
| US5073409A (en) | 1990-06-28 | 1991-12-17 | The United States Of America As Represented By The Secretary Of The Navy | Environmentally stable metal powders |
| JPH05163511A (ja) * | 1991-12-10 | 1993-06-29 | Mitsui Mining & Smelting Co Ltd | 合金粉末の製造方法 |
| JPH0688104A (ja) * | 1992-07-21 | 1994-03-29 | Nippon Steel Corp | チタン粉末の製造方法 |
| DE4226982C1 (de) | 1992-08-14 | 1993-12-09 | Elektro Thermit Gmbh | Metallothermisches Reaktionsgemisch |
| US5442978A (en) | 1994-05-19 | 1995-08-22 | H. C. Starck, Inc. | Tantalum production via a reduction of K2TAF7, with diluent salt, with reducing agent provided in a fast series of slug additions |
| WO1996004407A1 (en) * | 1994-08-01 | 1996-02-15 | Kroftt-Brakston International, Inc. | Method of making metals and other elements |
| JPH08269502A (ja) * | 1995-03-28 | 1996-10-15 | Mitsubishi Heavy Ind Ltd | 活性金属の活性能抑制法 |
| RU2185262C2 (ru) * | 1999-08-27 | 2002-07-20 | Российский Федеральный Ядерный Центр - Всероссийский Научно-Исследовательский Институт Экспериментальной Физики | Способ пассивации пирофорных металлических порошков |
| CN2515206Y (zh) * | 2001-12-15 | 2002-10-09 | 王艳 | 双罐式金属锆还原装置 |
| JP2004052003A (ja) * | 2002-07-16 | 2004-02-19 | Cabot Supermetal Kk | ニオブ粉末またはタンタル粉末の製造方法および製造装置 |
| JP2004052033A (ja) | 2002-07-18 | 2004-02-19 | Nippon Steel Corp | ボタンヘッドを付与した高強度圧延pc鋼棒およびその製造方法 |
| US6902601B2 (en) * | 2002-09-12 | 2005-06-07 | Millennium Inorganic Chemicals, Inc. | Method of making elemental materials and alloys |
| US7067197B2 (en) * | 2003-01-07 | 2006-06-27 | Cabot Corporation | Powder metallurgy sputtering targets and methods of producing same |
| JP2004247177A (ja) * | 2003-02-13 | 2004-09-02 | Jeol Ltd | 複合型プラズマ発生装置 |
| KR20040074828A (ko) * | 2003-02-19 | 2004-08-26 | 한국기계연구원 | 금속열환원법에 의한 티아이씨계 나노복합분말 합성방법 |
| DE10332033A1 (de) | 2003-07-15 | 2005-02-03 | Chemetall Gmbh | Verfahren zur Herstellung von Metallpulvern, bzw. von Metallhydridpulvern der Elemente Ti, Zr, Hf, V, Nb, Ta und Cr |
| US6939389B2 (en) * | 2003-08-08 | 2005-09-06 | Frank Mooney | Method and apparatus for manufacturing fine powders |
| JP5284094B2 (ja) * | 2005-09-16 | 2013-09-11 | ハー.ツェー.スタルク ゲゼルシャフト ミット ベシュレンクテル ハフツング | 還元法 |
| JP2007084847A (ja) * | 2005-09-20 | 2007-04-05 | Sumitomo Titanium Corp | Tiの製造方法および装置 |
| CN2934267Y (zh) * | 2006-08-07 | 2007-08-15 | 贵阳铝镁设计研究院 | 反应器顶盖 |
| US7753989B2 (en) * | 2006-12-22 | 2010-07-13 | Cristal Us, Inc. | Direct passivation of metal powder |
| DE102008005781A1 (de) * | 2008-01-23 | 2009-07-30 | Tradium Gmbh | Phlegmatisierte Metallpulver oder Legierungspulver und Verfahren bzw. Reaktionsgefäß zu deren Herstellung |
-
2008
- 2008-01-23 DE DE102008005781A patent/DE102008005781A1/de not_active Withdrawn
- 2008-01-23 DE DE102008064648A patent/DE102008064648A1/de not_active Withdrawn
-
2009
- 2009-01-08 KR KR1020107016614A patent/KR101557174B1/ko not_active Expired - Fee Related
- 2009-01-08 RU RU2010134800/02A patent/RU2492966C2/ru not_active IP Right Cessation
- 2009-01-08 MX MX2010007826A patent/MX2010007826A/es active IP Right Grant
- 2009-01-08 UA UAA201009299A patent/UA102086C2/ru unknown
- 2009-01-08 AU AU2009207739A patent/AU2009207739B2/en not_active Ceased
- 2009-01-08 CA CA2712929A patent/CA2712929C/en not_active Expired - Fee Related
- 2009-01-08 CN CN200980103057.1A patent/CN101925427B/zh not_active Expired - Fee Related
- 2009-01-08 EP EP11180240.1A patent/EP2394762B1/de not_active Not-in-force
- 2009-01-08 PL PL11180240T patent/PL2394762T3/pl unknown
- 2009-01-08 US US12/746,985 patent/US8821610B2/en not_active Expired - Fee Related
- 2009-01-08 BR BRPI0907383-3A patent/BRPI0907383A2/pt not_active IP Right Cessation
- 2009-01-08 EP EP09703271.8A patent/EP2247398B1/de not_active Not-in-force
- 2009-01-08 JP JP2010543454A patent/JP5876651B2/ja not_active Expired - Fee Related
- 2009-01-08 MY MYPI20103225 patent/MY152942A/en unknown
- 2009-01-08 WO PCT/EP2009/050163 patent/WO2009092631A1/de not_active Ceased
-
2010
- 2010-07-13 IL IL206966A patent/IL206966A/en not_active IP Right Cessation
-
2013
- 2013-11-14 US US14/079,768 patent/US9279617B2/en not_active Expired - Fee Related
-
2014
- 2014-02-05 JP JP2014020082A patent/JP2014129605A/ja active Pending
-
2015
- 2015-02-22 IL IL237346A patent/IL237346A/en not_active IP Right Cessation
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB771351A (en) * | 1954-01-19 | 1957-04-03 | Gen Electric Co Ltd | Improvements in or relating to the bright annealing of brass |
Also Published As
| Publication number | Publication date |
|---|---|
| RU2010134800A (ru) | 2012-02-27 |
| JP2014129605A (ja) | 2014-07-10 |
| US8821610B2 (en) | 2014-09-02 |
| BRPI0907383A2 (pt) | 2015-07-21 |
| CA2712929A1 (en) | 2009-07-30 |
| WO2009092631A1 (de) | 2009-07-30 |
| JP5876651B2 (ja) | 2016-03-02 |
| US20100272999A1 (en) | 2010-10-28 |
| RU2492966C2 (ru) | 2013-09-20 |
| IL237346A (en) | 2016-12-29 |
| MY152942A (en) | 2014-12-15 |
| IL237346A0 (en) | 2015-04-30 |
| EP2394762A1 (de) | 2011-12-14 |
| US20150130121A1 (en) | 2015-05-14 |
| EP2247398B1 (de) | 2014-08-20 |
| DE102008005781A1 (de) | 2009-07-30 |
| AU2009207739A1 (en) | 2009-07-30 |
| US9279617B2 (en) | 2016-03-08 |
| CN101925427B (zh) | 2014-06-18 |
| JP2011514435A (ja) | 2011-05-06 |
| KR20100113092A (ko) | 2010-10-20 |
| MX2010007826A (es) | 2010-09-22 |
| AU2009207739B2 (en) | 2013-03-07 |
| KR101557174B1 (ko) | 2015-10-02 |
| EP2247398A1 (de) | 2010-11-10 |
| IL206966A0 (en) | 2010-12-30 |
| UA102086C2 (ru) | 2013-06-10 |
| CN101925427A (zh) | 2010-12-22 |
| CA2712929C (en) | 2016-03-08 |
| DE102008064648A1 (de) | 2010-05-20 |
| IL206966A (en) | 2015-06-30 |
| PL2394762T3 (pl) | 2014-05-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2394762B1 (de) | Reaktionsgefäß zur Herstellung phlegmatisierter Metallpulver oder Legierungspulver | |
| EP2567766B1 (de) | Verfahren zur herstellung von legierungspulvern auf der basis von titan legiert mit kupfer | |
| US20180094336A1 (en) | Process for preparing metal powders and metal hydride powders of the elements ti, zr, hf, v, nb, ta and cr | |
| DE2802425C3 (de) | Verfahren zur Herstellung von Titankarbid | |
| KR101542607B1 (ko) | 자전연소반응을 이용한 티타늄 합금의 제조방법 | |
| EP0401374A1 (de) | Verfahren zur herstellung von verbundstoff | |
| Cao et al. | Sintering kinetics of disperse ultrafine equiaxed α-Al2O3 nanoparticles | |
| DE2303697C2 (de) | Verfahren zur Herstellung von Legierungspulvern aus Seltenen Erden und Kobalt | |
| GB2450750A (en) | Modified metallic powders for pyrotechnic use | |
| WO2019211404A1 (de) | Azotiertes silicium-haltiges pulver | |
| DD283962A5 (de) | Verfahren zur herstellung von verbundstoff |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AC | Divisional application: reference to earlier application |
Ref document number: 2247398 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK TR |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20120614 |
|
| 17Q | First examination report despatched |
Effective date: 20120907 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTG | Intention to grant announced |
Effective date: 20130909 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| INTG | Intention to grant announced |
Effective date: 20131009 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 2247398 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 642468 Country of ref document: AT Kind code of ref document: T Effective date: 20131215 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: GERMAN |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 502009008437 Country of ref document: DE Effective date: 20140123 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: VDEP Effective date: 20131127 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140227 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140327 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 |
|
| REG | Reference to a national code |
Ref country code: PL Ref legal event code: T3 |
|
| REG | Reference to a national code |
Ref country code: SK Ref legal event code: T3 Ref document number: E 15885 Country of ref document: SK |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140327 |
|
| BERE | Be: lapsed |
Owner name: TRADIUM G.M.B.H. Effective date: 20140131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 502009008437 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 Ref country code: LU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140108 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140131 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140131 |
|
| 26N | No opposition filed |
Effective date: 20140828 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 502009008437 Country of ref document: DE Effective date: 20140828 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140108 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20090108 Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SK Payment date: 20161228 Year of fee payment: 9 Ref country code: CZ Payment date: 20161227 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20170131 Year of fee payment: 9 Ref country code: FR Payment date: 20170124 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PL Payment date: 20170104 Year of fee payment: 9 Ref country code: AT Payment date: 20170120 Year of fee payment: 9 Ref country code: GB Payment date: 20170125 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20170130 Year of fee payment: 9 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20131127 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 502009008437 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 642468 Country of ref document: AT Kind code of ref document: T Effective date: 20180108 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20180108 |
|
| REG | Reference to a national code |
Ref country code: SK Ref legal event code: MM4A Ref document number: E 15885 Country of ref document: SK Effective date: 20180108 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180801 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180131 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20180928 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180108 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180108 Ref country code: CZ Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180108 Ref country code: SK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180108 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180108 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180108 |