EP0451437B1 - Galvanisiertes Stahlblech mit hohen Pressumformungseigenschaften und Verfahren zu seiner Herstellung - Google Patents
Galvanisiertes Stahlblech mit hohen Pressumformungseigenschaften und Verfahren zu seiner Herstellung Download PDFInfo
- Publication number
- EP0451437B1 EP0451437B1 EP91100089A EP91100089A EP0451437B1 EP 0451437 B1 EP0451437 B1 EP 0451437B1 EP 91100089 A EP91100089 A EP 91100089A EP 91100089 A EP91100089 A EP 91100089A EP 0451437 B1 EP0451437 B1 EP 0451437B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- steel sheet
- galvanized steel
- galvanized
- inorganic compound
- press formability
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 229910001335 Galvanized steel Inorganic materials 0.000 title claims description 46
- 239000008397 galvanized steel Substances 0.000 title claims description 46
- 238000000034 method Methods 0.000 title claims description 28
- 238000004519 manufacturing process Methods 0.000 title claims description 17
- 229910000831 Steel Inorganic materials 0.000 claims description 63
- 239000010959 steel Substances 0.000 claims description 63
- 150000002484 inorganic compounds Chemical class 0.000 claims description 30
- 229910010272 inorganic material Inorganic materials 0.000 claims description 30
- 229910052751 metal Inorganic materials 0.000 claims description 24
- 239000002184 metal Substances 0.000 claims description 24
- 238000007747 plating Methods 0.000 claims description 23
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 19
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 claims description 18
- 238000005238 degreasing Methods 0.000 claims description 16
- 238000010438 heat treatment Methods 0.000 claims description 12
- 239000003513 alkali Substances 0.000 claims description 10
- 238000001035 drying Methods 0.000 claims description 7
- 238000005244 galvannealing Methods 0.000 claims description 7
- 238000005406 washing Methods 0.000 claims description 5
- 238000005246 galvanizing Methods 0.000 claims description 3
- 239000011248 coating agent Substances 0.000 description 35
- 238000000576 coating method Methods 0.000 description 35
- 239000010408 film Substances 0.000 description 23
- 239000007864 aqueous solution Substances 0.000 description 14
- 235000010339 sodium tetraborate Nutrition 0.000 description 13
- 238000012360 testing method Methods 0.000 description 12
- 230000000052 comparative effect Effects 0.000 description 11
- 239000000243 solution Substances 0.000 description 11
- 229910045601 alloy Inorganic materials 0.000 description 10
- 239000000956 alloy Substances 0.000 description 10
- 229910021538 borax Inorganic materials 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- 239000004328 sodium tetraborate Substances 0.000 description 9
- 239000011701 zinc Substances 0.000 description 9
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 8
- 239000013078 crystal Substances 0.000 description 8
- UQGFMSUEHSUPRD-UHFFFAOYSA-N disodium;3,7-dioxido-2,4,6,8,9-pentaoxa-1,3,5,7-tetraborabicyclo[3.3.1]nonane Chemical compound [Na+].[Na+].O1B([O-])OB2OB([O-])OB1O2 UQGFMSUEHSUPRD-UHFFFAOYSA-N 0.000 description 8
- 238000005507 spraying Methods 0.000 description 8
- 229910052725 zinc Inorganic materials 0.000 description 8
- 150000002739 metals Chemical class 0.000 description 7
- 239000010410 layer Substances 0.000 description 6
- 229910007567 Zn-Ni Inorganic materials 0.000 description 5
- 229910007614 Zn—Ni Inorganic materials 0.000 description 5
- -1 alkali metal salts Chemical class 0.000 description 5
- 229910019142 PO4 Inorganic materials 0.000 description 4
- 239000011324 bead Substances 0.000 description 4
- 150000001642 boronic acid derivatives Chemical class 0.000 description 4
- 239000010960 cold rolled steel Substances 0.000 description 4
- 238000005260 corrosion Methods 0.000 description 4
- 230000007797 corrosion Effects 0.000 description 4
- 238000000605 extraction Methods 0.000 description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- 239000003921 oil Substances 0.000 description 4
- 235000021317 phosphate Nutrition 0.000 description 4
- 150000003839 salts Chemical class 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 229910052752 metalloid Inorganic materials 0.000 description 3
- 150000002738 metalloids Chemical class 0.000 description 3
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 3
- 239000010452 phosphate Substances 0.000 description 3
- 239000010409 thin film Substances 0.000 description 3
- 229910000640 Fe alloy Inorganic materials 0.000 description 2
- 229910004835 Na2B4O7 Inorganic materials 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 238000002441 X-ray diffraction Methods 0.000 description 2
- FZQSLXQPHPOTHG-UHFFFAOYSA-N [K+].[K+].O1B([O-])OB2OB([O-])OB1O2 Chemical compound [K+].[K+].O1B([O-])OB2OB([O-])OB1O2 FZQSLXQPHPOTHG-UHFFFAOYSA-N 0.000 description 2
- 229910052784 alkaline earth metal Chemical class 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- ZPWVASYFFYYZEW-UHFFFAOYSA-L dipotassium hydrogen phosphate Chemical compound [K+].[K+].OP([O-])([O-])=O ZPWVASYFFYYZEW-UHFFFAOYSA-L 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 235000019799 monosodium phosphate Nutrition 0.000 description 2
- 239000002120 nanofilm Substances 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- 238000007127 saponification reaction Methods 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000000344 soap Substances 0.000 description 2
- AJPJDKMHJJGVTQ-UHFFFAOYSA-M sodium dihydrogen phosphate Chemical compound [Na+].OP(O)([O-])=O AJPJDKMHJJGVTQ-UHFFFAOYSA-M 0.000 description 2
- NVIFVTYDZMXWGX-UHFFFAOYSA-N sodium metaborate Chemical compound [Na+].[O-]B=O NVIFVTYDZMXWGX-UHFFFAOYSA-N 0.000 description 2
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 229910001018 Cast iron Inorganic materials 0.000 description 1
- 229910003252 NaBO2 Inorganic materials 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- 229910001297 Zn alloy Inorganic materials 0.000 description 1
- 239000002390 adhesive tape Substances 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 238000005275 alloying Methods 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229910052787 antimony Inorganic materials 0.000 description 1
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 150000003841 chloride salts Chemical class 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- UFMZWBIQTDUYBN-UHFFFAOYSA-N cobalt dinitrate Chemical compound [Co+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O UFMZWBIQTDUYBN-UHFFFAOYSA-N 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 229910000396 dipotassium phosphate Inorganic materials 0.000 description 1
- 235000019797 dipotassium phosphate Nutrition 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- 238000004070 electrodeposition Methods 0.000 description 1
- 238000000921 elemental analysis Methods 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000005002 finish coating Substances 0.000 description 1
- XLYOFNOQVPJJNP-ZSJDYOACSA-N heavy water Substances [2H]O[2H] XLYOFNOQVPJJNP-ZSJDYOACSA-N 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000003595 mist Substances 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- CWQXQMHSOZUFJS-UHFFFAOYSA-N molybdenum disulfide Chemical compound S=[Mo]=S CWQXQMHSOZUFJS-UHFFFAOYSA-N 0.000 description 1
- 229910000403 monosodium phosphate Inorganic materials 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- 238000010422 painting Methods 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- 229910052714 tellurium Inorganic materials 0.000 description 1
- PORWMNRCUJJQNO-UHFFFAOYSA-N tellurium atom Chemical compound [Te] PORWMNRCUJJQNO-UHFFFAOYSA-N 0.000 description 1
- 238000004260 weight control Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C22/00—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C22/78—Pretreatment of the material to be coated
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M103/00—Lubricating compositions characterised by the base-material being an inorganic material
- C10M103/06—Metal compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M173/00—Lubricating compositions containing more than 10% water
- C10M173/02—Lubricating compositions containing more than 10% water not containing mineral or fatty oils
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/02—Water
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/0603—Metal compounds used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/061—Carbides; Hydrides; Nitrides
- C10M2201/0613—Carbides; Hydrides; Nitrides used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/062—Oxides; Hydroxides; Carbonates or bicarbonates
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/062—Oxides; Hydroxides; Carbonates or bicarbonates
- C10M2201/0623—Oxides; Hydroxides; Carbonates or bicarbonates used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/063—Peroxides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/065—Sulfides; Selenides; Tellurides
- C10M2201/0653—Sulfides; Selenides; Tellurides used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/065—Sulfides; Selenides; Tellurides
- C10M2201/066—Molybdenum sulfide
- C10M2201/0663—Molybdenum sulfide used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/08—Inorganic acids or salts thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/08—Inorganic acids or salts thereof
- C10M2201/0803—Inorganic acids or salts thereof used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/08—Inorganic acids or salts thereof
- C10M2201/081—Inorganic acids or salts thereof containing halogen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/08—Inorganic acids or salts thereof
- C10M2201/082—Inorganic acids or salts thereof containing nitrogen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/08—Inorganic acids or salts thereof
- C10M2201/084—Inorganic acids or salts thereof containing sulfur, selenium or tellurium
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/085—Phosphorus oxides, acids or salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/085—Phosphorus oxides, acids or salts
- C10M2201/0853—Phosphorus oxides, acids or salts used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/086—Chromium oxides, acids or salts
- C10M2201/0863—Chromium oxides, acids or salts used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/087—Boron oxides, acids or salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/087—Boron oxides, acids or salts
- C10M2201/0873—Boron oxides, acids or salts used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
- C10M2201/1006—Compounds containing silicon used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
- C10M2201/102—Silicates
- C10M2201/1023—Silicates used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
- C10M2201/102—Silicates
- C10M2201/103—Clays; Mica; Zeolites
- C10M2201/1033—Clays; Mica; Zeolites used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
- C10M2201/105—Silica
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
- C10M2201/105—Silica
- C10M2201/1053—Silica used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/12—Glass
- C10M2201/123—Glass used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/02—Groups 1 or 11
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/04—Groups 2 or 12
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2050/00—Form in which the lubricant is applied to the material being lubricated
- C10N2050/015—Dispersions of solid lubricants
- C10N2050/02—Dispersions of solid lubricants dissolved or suspended in a carrier which subsequently evaporates to leave a lubricant coating
Definitions
- the present invention relates to a galvanized steel sheet with a high press formability characteristic adapted for use as an automotive non-corrosion steel sheet and a method for manufacturing the same.
- FR-A-262 9 103 discloses a steel sheet which is provided on at least one side thereof with a thin film of a saponification agent which forms soap through reaction with oil.
- the oil is usually used in a press working station.
- the saponification agent are alkali metal salts and alkali earth metal salts.
- alloy electroplated steel sheets such as hot dip galvanized steel sheets, galvannealed steel sheets, electrogalvanized steel sheets, steel sheets electrogalvanized with Zn-Ni, etc., have been developed and placed into use.
- the inventors hereof examined the press formability of those galvanized steel sheets. Thereupon, it was revealed that the galvanized steel sheets, in contrast with the conventionally used cold-rolled steel sheets, are subjected to so great a frictional resistance against a mold during press forming operation that their press formability is relatively low.
- the steel sheet is prevented from being smoothly introduced into those portions which are subjected to hard sliding motion, such as a bead portion of a press mold for fixing the steel sheet at the time of press forming.
- the steel sheet may be broken. Since the proper cushion pressure range (range of cushion pressure within which the steel sheet cannot be wrinkled or broken) of the galvanized steel sheets for the press forming is much narrower than that of the cold-rolled steel sheets, the galvanized sheets are poorer in productivity. Therefore, an improvement of the frictional characteristics of the galvanized steel sheets is urgently needed.
- the frictional characteristics experienced during a press forming operation are greatly influenced by the properties of the plating surface which is directly in contact with the mold. Accordingly, the frictional characteristics are tentatively improved by coating the plating surface with some material other than a zinc plating, e.g. an organic high molecular film, so that the plating surface is kept from direct contact with the mold and is lubricated.
- some material other than a zinc plating e.g. an organic high molecular film
- a method for improving the formability of a steel sheet by improving the frictional characteristics of the sheet surface is disclosed in Published Examined Japanese Patent Application No. 61-26600, for example. According to this method, a specific organic high molecular film is formed on the steel sheet surface. Further, a lubricated-surface steel sheet is proposed which is coated with a film composed mainly of metallic soap or higher fatty acid wax, for example.
- galvanized steel sheets When galvanized steel sheets are used for automotive purposes, they are usually phosphated before being coated after the press forming operation. In doing this, however, the organic film partially remains on the steel sheet without being thoroughly removed by alkali degreasing as a pretreatment for the phosphating process. Therefore, a normal crystal of a phosphate on galvanized layer cannot be produced by the phosphating process. As a result, the adhesion of the coating film is lowered, so that the corrosion resistance of the coated steel sheet is deteriorated.
- the object of the present invention is to provide a galvanized steel sheet having better frictional characteristics and considerably improved in press formability without lowering the phosphatability thereof.
- a galvanized steel sheet with high press formability which has an inorganic compound on a metallic galvanized layer thereof, wherein said inorganic compound is a hydrous borate of alkaline metal, wherein 10 to 1000 mg/m2 of a film of said hydrous borate is applied on the plating surface of said galvanized steel sheet, the friction coefficient of said galvanized sheet being 0.15 or less and the amount of insoluble slimes of said inorganic compound left after water washing or alkali degreasing being less than 1 mg/m2.
- a method for manufacturing a galvanized steel sheet with high press formability comprising steps of bringing the plating surface of the galvanized steel sheet into contact with a water solution containing 0.1 wt% or more of a borate of alkaline metal, and drying the steel sheet by heating within a temperature range of 60 to 400°C.
- the inventors hereof examined the relationship between the coefficient of friction and press formability of a galvanized steel sheet. Thereupon, it was observed that the friction coefficient of a galvanized steel sheet, subjected to galvanizing such as electrogalvanizing, galvannealing, etc., is 0.15 or more, compared with about 0.10 for that of a conventional cold-rolled steel sheet with high press formability.
- the friction coefficient is a value which is obtained by oiled-state measurement in a draw-bead extraction test.
- the galvanized steel sheet has such a high friction coefficient because zinc and zinc alloy have so low a melting point and so great an affinity for other metals, especially for cast iron and the like frequently used for press molds, that they can easily stick to a mold. Since the frictional characteristics during press forming are influenced by the properties of the plating surface which is brought directly into contact with the mold, it was supposed that the frictional characteristics can be improved by coating the plating surface with some other material than a zinc plating so that the un-coated plating surface is kept from direct contact with the mold and is lubricated.
- the inventors continued the examination to find that the aforementioned problem on the press forming can be solved by providing a specific inorganic compound on the plating surface so that the resulting friction coefficient is 80% or less of the friction coefficient obtained without any specific substance on the plating surface, preferably 0.15 or less.
- a galvanized steel sheet according to the invention has an inorganic compound on its metallic galvanized layer.
- Such galvanized steel sheets include galvannealed steel sheets, hot dip galvanized steel sheets, electrogalvanized steel sheets, steel sheets electrogalvanized with alloyed zinc, such as Zn-Ni, Zn-Fe, etc., and steel sheets flashed with Fe-P, Fe-Zn, etc., for example.
- the inorganic compound should be one whose friction coefficient can be lowered when it exists on the metallic galvanized layer of the galvanized steel sheet, and the greater part of which can be removed by dissolution in water or by alkali degreasing. Any inorganic compounds may be used for the purpose provided they fulfill these requirements.
- Inorganic compounds suitably used according to the present invention include borates, carbonates, phosphates, sulfates, nitrates, chlorides, hydroxides, and oxides of alkaline metals such as Na and K, alkaline earth metals such as Ca and Mg, and metals or metalloids such as Fe, Ni, Co, Al, Ti, Si, etc., for example.
- the galvanized steel sheet according to the present invention can be easily manufactured by bringing the steel sheet into contact with a water solution of the inorganic compound and then drying the resulting sheet, as mentioned later.
- the inorganic compound is expected to be water-soluble.
- the inorganic compound should preferably be low-priced, in view of costs, and be highly soluble in water or basic aqueous solutions, since it is to be dissolved and removed by water washing or alkali degreasing.
- salts of alkaline metals are particularly suited for the purpose.
- hydrous borates of alkaline metals are very effective for the improvement of the frictional characteristics, and they typically include borax (sodium tetraborate: Na2B4O7 ⁇ 10H2O) which can be industrially mass-produced and is low-priced.
- hydrous borates of alkaline metals greatly surpasses an anhydrous one in solubility in water for washing or alkali for alkali degreasing.
- film is thoroughly dissolved by the alkali degreasing preceding the phosphating, and never remains on the steel sheet at all. This is the reason why hydrous borates of alkaline metals are preferably used.
- the inorganic compound on the zinc-based plating is not limited in form.
- a filmy or particulate compound is used for the purpose.
- the coating weight of the inorganic compound on the surface of the galvanized steel sheet has to be adjusted so that the friction coefficient of the galvanized steel sheet with the inorganic compound on plated layer is 80% or less of that of a galvanized steel sheet with no inorganic compound thereon, preferably 0.15 or less at the absolute value of coefficient of friction.
- the amount of insoluble slimes of the inorganic compound should be less than 1 mg/m2 at the end of the degreasing process before the phosphating process, lest the production of a satisfactory phosphate crystal be hindered during the phosphating process.
- the coating weight of a hydrous borate of alkaline metal is restricted to a range of 10 to 1,000 mg/m2 for the following reasons. If the coating weight is less than 10 mg/m2, a satisfactory improvement of the frictional characteristics cannot be achieved. If the coating weight exceeds 1,000 mg/m2, on the other hand, the effect of the improvement of the frictional characteristics is saturated, and besides, the film partially remains on the plated layer without being thoroughly removed in the degreasing process before the phosphating process, thus exerting a bad influence on the subsequent phosphating process.
- the coating weight ranges from 100 to 1,000 mg/m2.
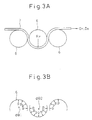
- Fig. 1 shows the relationships between the coating weight of borax (sodium tetraborate: Na2B4O7 ⁇ 5H2O) on a galvannealed steel sheet, the coefficient of friction obtained in a frictional characteristic test, and the water-resistant secondary adhesion. These individual tests are conducted in the manner described later in connection with various examples. As shown in Fig. 1, the frictional characteristics can be greatly improved without affecting satisfactory phosphatability in the case where the coating weight of borax ranges from 10 to 1,000 mg/m2.
- a galvanized steel sheet having the aforesaid film of the hydrous borate of alkaline metal thereon may be manufactured in the following manner.
- the galvanized steel sheet is brought into contact with a water solution containing 0.1 wt% or more of the borate of alkaline metal by dipping, spraying, or coating by means of a roll coater or the like, and is then dried by heating within a temperature range of 60 to 400°C.
- the borate content of the alkaline metal is adjusted to 0.1 wt% or more for the following reason.
- the present method in which the galvanized steel sheet is dried by heating after being brought into contact with the aqueous solution, entails some operational disadvantages, such as lowering of line speed.
- the lower limit of the heating temperature range for the drying is adjusted to 60°C because if the heating temperature is lower than 60°C, a solid film effective for the improvement of the frictional characteristics as aforesaid cannot be formed on the plating.
- the upper limit is set at 400°C because if the sheet is heated to a temperature exceeding 400°C, the hydrous crystal of the borate of alkaline metal turns into an anhydrous crystal, so that borate film turns into one, which is difficult to dissolve.
- Fig. 2 shows a process for manufacturing the galvanized steel sheet.
- numeral 1 denotes the steel sheet; 2, a hot zinc pot; 3, coating weight control means; 4, a galvannealing furnace; and 5, a cooling zone.
- a aqueous solution containing the aforesaid borate of alkaline metal is sprayed so that the steel sheet is brought into contact with a mist of the solution.
- the solution containing the borate of alkaline metal may be sprayed at any positions kept at a temperature within the temperature range for the steel sheet.
- the spraying can be effected at positions A, B and C of Fig. 2, which will be described later in connection with the examples.
- a aqueous solution containing 5 wt% of potassium tetraborate (K2B4O7 ⁇ 4H2O) was sprayed on the steel sheet at a position (position A of Fig. 2) reached immediately after the steel sheet is wiped over the melt plating pot.
- the sheet temperature immediately before the spraying was 380°C.
- a aqueous solution containing 0.7 wt% of sodium metaborate (NaBO2 ⁇ 4H2O) was sprayed on the steel sheet at a position (position B of Fig. 2) reached immediately after the steel sheet gets out of the alloying furnace.
- the sheet temperature immediately before the spraying was 250°C.
- An electrogalvanized steel sheet with the coating weight of 60/60 g/m2 (equivalent to the green material of Example 1).
- a hot dip galvanized steel sheet with the coating weight of 90/90 g/m2 (equivalent to the green material of Example 4).
- a galvannealed steel sheet with the coating weight of 45/45 g/m2 and alloy content of 9/9% (equivalent to the green material of Example 5).
- Rust preventing oil containing 10 wt% of molybdenum sulfide (MoS2) was applied to a steel sheet electroplated with Zn-Ni alloy having the coating weight of 30/30 g/m2 and Ni content of 11/11%.
- Phosphatability Phosphating was effected using a treatment solution, PALBOND L3020 (produced by Nihon Parkerizing Co., Ltd.), and phosphate crystals were observed by means of a 1,500-power scanning electron microscope (SEM). The phosphatability was evaluated in accordance with the following criteria based on the crystalline grain size.
- a galvanized steel sheet whose press formability is considerably improved without lowering the phosphatability thereof.
- the galvanized steel sheet according to the present invention has the inorganic compound thereon which serves to improve the frictional characteristics of the steel sheet and can be substantially thoroughly removed by the alkali degreasing or water washing preceding the phosphating process, so that it can reconcile the press formability and phosphatability.
- Use of the galvanized steel sheet of the invention greatly improves the productivity for the press forming.
- the steel sheet can be easily manufactured at low cost by bringing the galvanized steel sheet into contact with the aqueous solution containing 0.1 wt% or more of the borate of alkaline metal and then drying the sheet by heating within the temperature range of 60 to 400°C, or by only spraying the aqueous solution on the steel sheet after the galvanizing or galvannealing process in the case of the hot dip galvanized steel sheet.
- the steel sheet of the present invention is easy to manufacture.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Coating With Molten Metal (AREA)
Claims (3)
- Verzinktes Stahlblech mit hoher Pressformbarkeit, welches eine anorganische Verbindung auf einer metallischen Zinkschicht darauf aufweist, wobei die anorganische Verbindung ein wäßriges Alkalimetallborat ist, wobei 10 bis 1000 mg/m² eines Filmes aus dem wäßrigen Borat auf die Plattieroberfläche des verzinkten Stahlblechs aufgebracht wird, der Reibungskoeffizient des verzinkten Blechs 0,15 oder weniger beträgt und die Menge an unlöslichen Schlämmen der anorganischen Verbindung, die nach dem Waschen mit Wasser oder dem alkalischen Entfetten zurückbleibt, weniger als 1 mg/m² beträgt.
- Verfahren zur Herstellung eines verzinkten Stahlblechs mit großer Pressformbarkeit gemäß Anspruch 1, umfassend die Schritte des Inkontaktbringens der Plattieroberfläche des verzinkten Stahlblechs mit einer Wasserlösung, enthaltend 0,1 Gew.-% oder mehr eines Alkalimetallborats, und Trocknen des Stahlblechs durch Erwärmen innerhalb eines Temperaturbereichs von 60 bis 400 °C.
- Verfahren zur Herstellung eines verzinkten Stahlblechs mit großer Pressformbarkeit gemäß Anspruch 2, wobei, wenn die Temperatur des Stahlblechs innerhalb des Bereichs von 60 bis 400°C nach dem Schmelzverzinken oder nach dem darauf folgendem Feuerverzinken mit nachträglicher Wärmebehandlung des Überzugs liegt, die 0,1 Gew.-% oder mehr eines Alkaliborats enthaltende Wasserlösung auf die Plattieroberfläche in einem Herstellungsverfahren, bei dem das Stahlblech einem Schmelztauchverzinken unterworfen wird, aufgesprüht wird.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP9334890 | 1990-04-09 | ||
| JP93348/90 | 1990-04-09 | ||
| JP334296/90 | 1990-11-30 | ||
| JP33429690 | 1990-11-30 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0451437A2 EP0451437A2 (de) | 1991-10-16 |
| EP0451437A3 EP0451437A3 (en) | 1992-01-22 |
| EP0451437B1 true EP0451437B1 (de) | 1995-04-05 |
Family
ID=26434742
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP91100089A Expired - Lifetime EP0451437B1 (de) | 1990-04-09 | 1991-01-02 | Galvanisiertes Stahlblech mit hohen Pressumformungseigenschaften und Verfahren zu seiner Herstellung |
Country Status (3)
| Country | Link |
|---|---|
| EP (1) | EP0451437B1 (de) |
| CA (1) | CA2033862C (de) |
| DE (1) | DE69108594T2 (de) |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1347427A (fr) * | 1962-02-02 | 1963-12-27 | Paroxite London Ltd | Perfectionnements apportés aux produits d'enrobage utilisés pour le formage à froid des métaux |
| US3364081A (en) * | 1965-01-15 | 1968-01-16 | Lubrizol Corp | Aqueous phosphating solutions |
| FR2339446A1 (fr) * | 1976-01-28 | 1977-08-26 | Servimetal | Procede de filage a tres grande vitesse de metaux et alliages legers |
| CH629845A5 (en) * | 1977-10-26 | 1982-05-14 | Bbc Brown Boveri & Cie | High-temperature lubricant |
| FR2629103B1 (fr) * | 1988-03-23 | 1993-01-08 | Lorraine Laminage | Tole metallique pour emboutissage; procede et dispositif de traitement de surface pour sa fabrication |
-
1991
- 1991-01-02 EP EP91100089A patent/EP0451437B1/de not_active Expired - Lifetime
- 1991-01-02 DE DE69108594T patent/DE69108594T2/de not_active Expired - Fee Related
- 1991-01-09 CA CA002033862A patent/CA2033862C/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| EP0451437A3 (en) | 1992-01-22 |
| EP0451437A2 (de) | 1991-10-16 |
| CA2033862C (en) | 1999-05-11 |
| CA2033862A1 (en) | 1991-10-10 |
| DE69108594T2 (de) | 1995-08-17 |
| DE69108594D1 (de) | 1995-05-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0763608B1 (de) | Korrosionsbeständiges stahlblech für treibstofftank und verfahren zur herstellung des bleches | |
| US6555249B1 (en) | Surface treated steel sheet and method for production thereof | |
| JP3346338B2 (ja) | 亜鉛系めっき鋼板およびその製造方法 | |
| KR100234452B1 (ko) | 아연계 도금 강판 및 그 제조방법 | |
| KR20020035775A (ko) | 아연 함유 도금된 고장력 강판 | |
| EP0451437B1 (de) | Galvanisiertes Stahlblech mit hohen Pressumformungseigenschaften und Verfahren zu seiner Herstellung | |
| US5487919A (en) | Method of manufacturing of galvanized steel sheet having high press formability | |
| JP3445683B2 (ja) | プレス性、化成処理性、接着剤適合性に優れた亜鉛系めっき鋼板の製造方法 | |
| CN1846011B (zh) | 热镀锌钢板及其制造方法 | |
| EP1080246B1 (de) | Oberflächenbehandeltes stahlblech und verfahren zu seiner herstellung | |
| US20250206962A1 (en) | Surface treated steel sheet | |
| JP3132979B2 (ja) | 潤滑性、化成処理性、接着剤適合性に優れた亜鉛系めっき鋼板 | |
| JP4848737B2 (ja) | 脱脂性に優れる合金化溶融亜鉛めっき鋼板 | |
| JP2713002B2 (ja) | 亜鉛系めっき鋼板の製造方法 | |
| JP2819429B2 (ja) | プレス成形性、化成処理性に優れた亜鉛系めっき鋼板 | |
| JP2965090B2 (ja) | プレス加工性およびリン酸塩処理性に優れた亜鉛系めっき鋼板の製造方法 | |
| JP3199980B2 (ja) | 潤滑性、化成処理性、接着剤適合性に優れた亜鉛系めっき鋼板 | |
| JPH0713306B2 (ja) | プレス加工性に優れた亜鉛系めっき鋼板及びその製造方法 | |
| US5322741A (en) | Aluminum alloy sheet with improved formability and method of production | |
| JP3111888B2 (ja) | 亜鉛系メッキ鋼板の製造方法 | |
| JPH05148606A (ja) | プレス成形性およびスポツト溶接性に優れた合金化溶融亜鉛メツキ鋼板の製造方法 | |
| JPH08120431A (ja) | プレス成形性に優れた合金化溶融亜鉛メッキ鋼板およびその製造方法 | |
| CA2010898C (en) | Galvannealed steel sheet with distinguished anti-powdering and anti-flaking properties and process for producing the same | |
| JP3153097B2 (ja) | 潤滑性、化成処理性、接着剤適合性、溶接性に優れた亜鉛系めっき鋼板 | |
| JP3111880B2 (ja) | 亜鉛系メッキ鋼板の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): BE DE ES FR GB IT NL |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): BE DE ES FR GB IT NL |
|
| 17P | Request for examination filed |
Effective date: 19920227 |
|
| 17Q | First examination report despatched |
Effective date: 19920921 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE DE ES FR GB IT NL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRE;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED.SCRIBED TIME-LIMIT Effective date: 19950405 Ref country code: BE Effective date: 19950405 Ref country code: ES Free format text: THE PATENT HAS BEEN ANNULLED BY A DECISION OF A NATIONAL AUTHORITY Effective date: 19950405 |
|
| REF | Corresponds to: |
Ref document number: 69108594 Country of ref document: DE Date of ref document: 19950511 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20001227 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20001228 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20010125 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20010131 Year of fee payment: 11 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020801 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020801 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20020102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020930 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20020801 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |