EP0440620B2 - Halbzeug für elektrische kontakte aus einem verbundwerkstoff auf silber-zinnoxid-basis und pulvermetallurgisches verfahren zu seiner herstellung - Google Patents
Halbzeug für elektrische kontakte aus einem verbundwerkstoff auf silber-zinnoxid-basis und pulvermetallurgisches verfahren zu seiner herstellung Download PDFInfo
- Publication number
- EP0440620B2 EP0440620B2 EP89903734A EP89903734A EP0440620B2 EP 0440620 B2 EP0440620 B2 EP 0440620B2 EP 89903734 A EP89903734 A EP 89903734A EP 89903734 A EP89903734 A EP 89903734A EP 0440620 B2 EP0440620 B2 EP 0440620B2
- Authority
- EP
- European Patent Office
- Prior art keywords
- component
- oxide
- weight
- semi
- finished product
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000000843 powder Substances 0.000 title claims abstract description 72
- 239000002131 composite material Substances 0.000 title claims abstract description 70
- 239000011265 semifinished product Substances 0.000 title claims abstract description 36
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 title claims abstract description 31
- 229910001887 tin oxide Inorganic materials 0.000 title claims abstract description 31
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 title claims abstract description 21
- 239000004332 silver Substances 0.000 title claims abstract description 18
- 238000010310 metallurgical process Methods 0.000 title claims abstract description 4
- 229910001923 silver oxide Inorganic materials 0.000 title claims abstract 4
- 229910044991 metal oxide Inorganic materials 0.000 claims abstract description 46
- 150000004706 metal oxides Chemical class 0.000 claims description 44
- 239000000463 material Substances 0.000 claims description 23
- 238000000034 method Methods 0.000 claims description 18
- 229910052751 metal Inorganic materials 0.000 claims description 17
- 239000002184 metal Substances 0.000 claims description 17
- 229910052709 silver Inorganic materials 0.000 claims description 14
- 150000002739 metals Chemical class 0.000 claims description 12
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 11
- 239000000203 mixture Substances 0.000 claims description 9
- 238000009826 distribution Methods 0.000 claims description 8
- QGLKJKCYBOYXKC-UHFFFAOYSA-N nonaoxidotritungsten Chemical compound O=[W]1(=O)O[W](=O)(=O)O[W](=O)(=O)O1 QGLKJKCYBOYXKC-UHFFFAOYSA-N 0.000 claims description 8
- 229910001930 tungsten oxide Inorganic materials 0.000 claims description 8
- 238000004519 manufacturing process Methods 0.000 claims description 7
- 150000003839 salts Chemical class 0.000 claims description 7
- 229910001316 Ag alloy Inorganic materials 0.000 claims description 6
- 239000011159 matrix material Substances 0.000 claims description 6
- UONOETXJSWQNOL-UHFFFAOYSA-N tungsten carbide Chemical compound [W+]#[C-] UONOETXJSWQNOL-UHFFFAOYSA-N 0.000 claims description 6
- 150000001247 metal acetylides Chemical class 0.000 claims description 5
- 229910052759 nickel Inorganic materials 0.000 claims description 5
- QIJNJJZPYXGIQM-UHFFFAOYSA-N 1lambda4,2lambda4-dimolybdacyclopropa-1,2,3-triene Chemical compound [Mo]=C=[Mo] QIJNJJZPYXGIQM-UHFFFAOYSA-N 0.000 claims description 4
- 229910039444 MoC Inorganic materials 0.000 claims description 4
- 229910045601 alloy Inorganic materials 0.000 claims description 4
- 239000000956 alloy Substances 0.000 claims description 4
- 229910000476 molybdenum oxide Inorganic materials 0.000 claims description 4
- PQQKPALAQIIWST-UHFFFAOYSA-N oxomolybdenum Chemical compound [Mo]=O PQQKPALAQIIWST-UHFFFAOYSA-N 0.000 claims description 4
- 238000005096 rolling process Methods 0.000 claims description 4
- 238000003466 welding Methods 0.000 claims description 4
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 3
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 claims description 3
- 229910052802 copper Inorganic materials 0.000 claims description 3
- 239000010949 copper Substances 0.000 claims description 3
- 230000001590 oxidative effect Effects 0.000 claims description 3
- 229910052718 tin Inorganic materials 0.000 claims description 3
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 claims description 3
- 229910052721 tungsten Inorganic materials 0.000 claims description 3
- 239000010937 tungsten Substances 0.000 claims description 3
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 claims description 2
- 229910002115 bismuth titanate Inorganic materials 0.000 claims description 2
- 230000007423 decrease Effects 0.000 claims description 2
- 229910052750 molybdenum Inorganic materials 0.000 claims description 2
- 239000011733 molybdenum Substances 0.000 claims description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 claims 2
- 239000003870 refractory metal Substances 0.000 claims 2
- QPLDLSVMHZLSFG-UHFFFAOYSA-N Copper oxide Chemical compound [Cu]=O QPLDLSVMHZLSFG-UHFFFAOYSA-N 0.000 claims 1
- 239000005751 Copper oxide Substances 0.000 claims 1
- XHCLAFWTIXFWPH-UHFFFAOYSA-N [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] Chemical compound [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] XHCLAFWTIXFWPH-UHFFFAOYSA-N 0.000 claims 1
- 229910000416 bismuth oxide Inorganic materials 0.000 claims 1
- 229910000431 copper oxide Inorganic materials 0.000 claims 1
- TYIXMATWDRGMPF-UHFFFAOYSA-N dibismuth;oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[Bi+3].[Bi+3] TYIXMATWDRGMPF-UHFFFAOYSA-N 0.000 claims 1
- 229910052763 palladium Inorganic materials 0.000 claims 1
- 238000009827 uniform distribution Methods 0.000 claims 1
- 229910001935 vanadium oxide Inorganic materials 0.000 claims 1
- 239000000047 product Substances 0.000 abstract 1
- IVQODXYTQYNJFI-UHFFFAOYSA-N oxotin;silver Chemical compound [Ag].[Sn]=O IVQODXYTQYNJFI-UHFFFAOYSA-N 0.000 description 21
- 239000002245 particle Substances 0.000 description 21
- ASMQPJTXPYCZBL-UHFFFAOYSA-N [O-2].[Cd+2].[Ag+] Chemical compound [O-2].[Cd+2].[Ag+] ASMQPJTXPYCZBL-UHFFFAOYSA-N 0.000 description 8
- SQGYOTSLMSWVJD-UHFFFAOYSA-N silver(1+) nitrate Chemical compound [Ag+].[O-]N(=O)=O SQGYOTSLMSWVJD-UHFFFAOYSA-N 0.000 description 6
- 238000001125 extrusion Methods 0.000 description 5
- 238000010438 heat treatment Methods 0.000 description 5
- MOFOBJHOKRNACT-UHFFFAOYSA-N nickel silver Chemical compound [Ni].[Ag] MOFOBJHOKRNACT-UHFFFAOYSA-N 0.000 description 5
- 239000007864 aqueous solution Substances 0.000 description 4
- 230000003647 oxidation Effects 0.000 description 4
- 238000007254 oxidation reaction Methods 0.000 description 4
- 238000004663 powder metallurgy Methods 0.000 description 4
- 238000005118 spray pyrolysis Methods 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 230000002349 favourable effect Effects 0.000 description 3
- UXVJMYXIXYVFDD-UHFFFAOYSA-N oxotin oxotungsten silver Chemical compound [W]=O.[Sn]=O.[Ag] UXVJMYXIXYVFDD-UHFFFAOYSA-N 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 238000003825 pressing Methods 0.000 description 3
- 229910001961 silver nitrate Inorganic materials 0.000 description 3
- 238000005245 sintering Methods 0.000 description 3
- 238000005507 spraying Methods 0.000 description 3
- 238000010792 warming Methods 0.000 description 3
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229910052797 bismuth Inorganic materials 0.000 description 2
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 239000010410 layer Substances 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- -1 silver-tin oxide Silver-cadmium oxide Chemical compound 0.000 description 2
- 235000012431 wafers Nutrition 0.000 description 2
- 229910000925 Cd alloy Inorganic materials 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 229910000881 Cu alloy Inorganic materials 0.000 description 1
- 229910001111 Fine metal Inorganic materials 0.000 description 1
- 241000282320 Panthera leo Species 0.000 description 1
- 229910001128 Sn alloy Inorganic materials 0.000 description 1
- QCEUXSAXTBNJGO-UHFFFAOYSA-N [Ag].[Sn] Chemical compound [Ag].[Sn] QCEUXSAXTBNJGO-UHFFFAOYSA-N 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 238000000889 atomisation Methods 0.000 description 1
- 229910052793 cadmium Inorganic materials 0.000 description 1
- BDOSMKKIYDKNTQ-UHFFFAOYSA-N cadmium atom Chemical compound [Cd] BDOSMKKIYDKNTQ-UHFFFAOYSA-N 0.000 description 1
- NSAODVHAXBZWGW-UHFFFAOYSA-N cadmium silver Chemical compound [Ag].[Cd] NSAODVHAXBZWGW-UHFFFAOYSA-N 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 239000010946 fine silver Substances 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000004482 other powder Substances 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 235000014483 powder concentrate Nutrition 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- 210000004243 sweat Anatomy 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000008207 working material Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C32/00—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ
- C22C32/001—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ with only oxides
- C22C32/0015—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ with only oxides with only single oxides as main non-metallic constituents
- C22C32/0021—Matrix based on noble metals, Cu or alloys thereof
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/12—Metallic powder containing non-metallic particles
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01H—ELECTRIC SWITCHES; RELAYS; SELECTORS; EMERGENCY PROTECTIVE DEVICES
- H01H1/00—Contacts
- H01H1/02—Contacts characterised by the material thereof
- H01H1/021—Composite material
- H01H1/023—Composite material having a noble metal as the basic material
- H01H1/0237—Composite material having a noble metal as the basic material and containing oxides
- H01H1/02372—Composite material having a noble metal as the basic material and containing oxides containing as major components one or more oxides of the following elements only: Cd, Sn, Zn, In, Bi, Sb or Te
- H01H1/02376—Composite material having a noble metal as the basic material and containing oxides containing as major components one or more oxides of the following elements only: Cd, Sn, Zn, In, Bi, Sb or Te containing as major component SnO2
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01H—ELECTRIC SWITCHES; RELAYS; SELECTORS; EMERGENCY PROTECTIVE DEVICES
- H01H11/00—Apparatus or processes specially adapted for the manufacture of electric switches
- H01H11/04—Apparatus or processes specially adapted for the manufacture of electric switches of switch contacts
- H01H11/048—Apparatus or processes specially adapted for the manufacture of electric switches of switch contacts by powder-metallurgical processes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2998/00—Supplementary information concerning processes or compositions relating to powder metallurgy
- B22F2998/10—Processes characterised by the sequence of their steps
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12014—All metal or with adjacent metals having metal particles
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12014—All metal or with adjacent metals having metal particles
- Y10T428/12021—All metal or with adjacent metals having metal particles having composition or density gradient or differential porosity
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12014—All metal or with adjacent metals having metal particles
- Y10T428/12028—Composite; i.e., plural, adjacent, spatially distinct metal components [e.g., layers, etc.]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12014—All metal or with adjacent metals having metal particles
- Y10T428/1216—Continuous interengaged phases of plural metals, or oriented fiber containing
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12014—All metal or with adjacent metals having metal particles
- Y10T428/1216—Continuous interengaged phases of plural metals, or oriented fiber containing
- Y10T428/12167—Nonmetal containing
Definitions
- the invention relates to a semi-finished product for electrical Contacts made of a composite material based on silver-tin oxide and a powder metallurgical Process for its manufacture.
- the invention is based on an entanglement with those specified in the preamble of claim 1 Characteristics or of a semi-finished product with the specified in the preamble of claim 12 Characteristics.
- Contact pieces formed from such materials can contact pieces made of silver-cadmium oxide in service life under AC3 and AC4 test conditions (specified in IEC standard 158-1) be, however, show a stronger contact warming in the switchgear. what the lifespan of the Switchgear can impair. Moreover can the internally oxidized contact pieces subsequently no longer be deformed.
- DE-B-26 59 012 is a powder metallurgy Process for the production of a contact material made of silver with two different ones Metal oxides known in which two silver-metal oxide composite powders mixed, pressed and sintered, one of which is Compound powder just one metal oxide and that other composite powder only the other metal oxide contains.
- the object of the present invention is to achieve this based on a semi-finished product for electrical contacts to provide the base silver-tin oxide which is despite a very small content Tin oxide particles work well by extrusion and rolling can be processed and at the same time regarding Lifetime, tendency to weld and contact heating is as good or better than semi-finished products are based on silver-cadmium oxide.
- the semifinished product produced according to the invention consists of a composite material, which is through a special rough structure in combination with a special fine structure.
- the Coarse structure is given by the fact that in the composite material oxide rich areas where everything Metal oxide or the vast majority of Metal oxide component is concentrated, alternate with low-oxide areas. the only a small one Contain or even share of the metal oxide component are oxide free. Contain the low-oxide areas at most a small amount of metal oxide finely distributed in one from the material of the first component formed matrix.
- the oxide rich areas contain the lion's share of the metal oxide component (in a concentration that is far greater is higher than the usual average metal oxide concentration in a contact material based on silver-tin oxide) and the rest of the material of the first component like a penetration or a storage composite finely divided into one another. These areas have emerged from low-oxide and high-oxide powders, which were mixed, pressed and, if necessary, sintered. The size of the low and high oxide areas, which is the rough structure of the composite determine therefore depends on the size of the powder particles from.
- the fine structure of the composite is given by a finely dispersed oxide distribution in the oxide-rich forming the rough structure Areas of the composite, if necessary also in low-oxide areas, so far are present in these metal oxides.
- the majority of the metal oxide component must come concentrated in the composite powder and be involved. Only the relatively small one Metal oxide content, which may still be in the contain low-oxide areas of the composite material can be e.g. in the form of a pure Oxide powder with the powder from the first component of the material are mixed. Doing so it prefers that in the low-oxide areas the same oxides are present as in the oxide-rich ones Areas.
- any metal carbides still present especially tungsten carbide and / or molybdenum carbide
- those not solved in the first component Metals can separate the powder mixture in the form Powders are added; they are suitable in switching operation the wetting of the tin oxide with To promote silver and thereby the contact resistance to humiliate.
- the resulting Solid particles react with the oxygen in the oxidizing atmosphere in the flame or in the reactor at a temperature below that Melting temperature of the dissolved metals, whereby Powder particles arise in which the metals of the first component, i.e. the silver or the silver alloy, and the metal oxide component, so essentially tin oxide, in a very fine distribution tied together.
- the metal oxide particles mostly in sizes between 0.1 ⁇ m and 1 ⁇ m (diameter) before what for the inventive method is advantageous.
- spray pyrolysis Compound powder is also advantageous because by spray pyrolysis, in particular powder particles arise that are spherical or potato-shaped Have shape, the emergence of a deformable Semi-finished products favored because the spherical or potato-shaped particles of a plastic Deformation of the contact material less oppose as irregularly jagged powder particles.
- oxidic and carbidic components sometimes lower the contact point temperature in switching operation and partly an extension of the life of the contact pieces not only with small and medium current loads, but also in the heavy load area.
- Molybdenum carbide and tungsten carbide already work in small amounts.
- the additional carbides and Oxides should make up 6% by weight of the contact material not exceed so this does not gets too hard.
- nickel can also be advantageous to the composite material, which is not in silver is soluble and either as a very fine powder that formed from silver or a silver alloy Powder is mixed or also as spray-pyrolysized silver-nickel powder is introduced.
- a silver-tin oxide composite powder with 32% by weight of tin oxide is produced by spraying an aqueous solution of silver nitrate and tin-II-chloride in a reactor heated to approx. 950 ° C. with an oxygen-containing atmosphere, whereby a silver-tin oxide composite powder fails , in the powder particles of which the tin oxide is present in a very fine distribution. Then 75 parts by weight of a silver powder with a particle size smaller than 40 microns with 25 parts by weight of the silver-tin oxide, composite powder are dry mixed for one hour, then isostatically pressed to blocks of about 50 kg in weight and then at a temperature sintered at 830 ° C for 1.5 hours.
- the block thus formed is placed in the recipient of an extrusion press and hot-extruded, reducing the cross-section to a strand with a cross-section of 10 x 75 mm 2 , at a temperature of approx. 850 ° C., and then hot-rolled plated with a 1.5 mm thick fine silver sheet , hot rolled down to its final thickness of 2mm and processed into contact wafers according to the usual methods.
- the silver-tin oxide composite in the contact wafers has a tin oxide content of 8% by weight.
- the second example is modified that the powder mixture still contains 0.5% by weight Tungsten oxide (particle size smaller than 10 ⁇ m) and 0.3 wt% tungsten carbide (particle size smaller than 2.5 ⁇ m) are added. Furthermore is operated as in the first example. The addition of tungsten oxide and tungsten carbide leads to one Lowering the contact point temperature and to an extended lifespan from the Semi-finished electrical contact pieces.
- a silver-tin oxide-tungsten oxide composite powder with 20% by weight of tin oxide and 0.5% by weight of tungsten oxide is made by spraying an aqueous Solution of silver nitrate, tin-II-chloride and Tungsten ll chloride in a heated to approx. 950 ° C Reactor with an oxygen-containing atmosphere, being a silver-tin oxide-tungsten oxide composite powder precipitates, in the powder particles of the tin oxide and the tungsten oxide is in a very fine distribution. Then 50 parts by weight of a Silver powder with a particle size smaller than 40 ⁇ m with 50 parts by weight of the silver-tin oxide-tungsten oxide composite powder dry for an hour mixed and as in the 1st example for contact plates processed further.
- a silver-tin oxide composite powder with 30 % By weight of tin oxide is produced as in the second example.
- a silver-nickel composite powder with 2 wt .-% Nickel is made by spraying one aqueous solution of silver nitrate and nickel-II-chloride in a reactor heated to approx. 950 ° C with a protective gas atmosphere (e.g. argon), whereby a silver-nickel composite powder fails, in the Powder particles the nickel in a very fine distribution is present.
- a protective gas atmosphere e.g. argon
- the fourth example can be modified accordingly be that instead of a silver-nickel composite powder a silver powder and a carbonyl nickel powder mixed with the silver-tin oxide composite powder will. Otherwise the same as in the 4th example method.
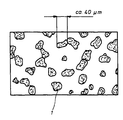
- the attached figure shows schematically the Structure of one according to the second example manufactured composite, in which silver-tin oxide areas 1, mostly less than 50 ⁇ m, are in a silver matrix that consists of the oxide-free silver powder particles has emerged.
- Semi-finished products produced according to the invention are suitable are particularly suitable for contact pieces in low-voltage switching devices, e.g. in motor contactors.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Composite Materials (AREA)
- Manufacturing & Machinery (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Contacts (AREA)
- Powder Metallurgy (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Manufacture Of Switches (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT89903734T ATE102387T1 (de) | 1988-03-26 | 1989-03-22 | Halbzeug fuer elektrische kontakte aus einem verbundwerkstoff auf silber-zinnoxid-basis und pulvermetallurgisches verfahren zu seiner herstellung. |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3810311 | 1988-03-26 | ||
| DE3810311 | 1988-03-26 | ||
| PCT/EP1989/000316 WO1989009478A1 (fr) | 1988-03-26 | 1989-03-22 | Produit semi-fini pour contacts electriques, constitue d'un materiau composite a base d'argent et d'oxyde d'etain, et procede de metallurgie des poudres pour sa fabrication |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0440620A1 EP0440620A1 (de) | 1991-08-14 |
| EP0440620B1 EP0440620B1 (de) | 1994-03-02 |
| EP0440620B2 true EP0440620B2 (de) | 1998-06-03 |
Family
ID=6350773
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP89903734A Expired - Lifetime EP0440620B2 (de) | 1988-03-26 | 1989-03-22 | Halbzeug für elektrische kontakte aus einem verbundwerkstoff auf silber-zinnoxid-basis und pulvermetallurgisches verfahren zu seiner herstellung |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US5360673A (es) |
| EP (1) | EP0440620B2 (es) |
| JP (1) | JPH03504615A (es) |
| CN (1) | CN1022934C (es) |
| CA (1) | CA1339713C (es) |
| DD (1) | DD283571A5 (es) |
| DE (2) | DE3909384A1 (es) |
| ES (1) | ES2012293A6 (es) |
| WO (1) | WO1989009478A1 (es) |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ATE136394T1 (de) * | 1992-06-10 | 1996-04-15 | Duerrwaechter E Dr Doduco | Werkstoff für elektrische kontakte auf der basis von silber-zinnoxid oder silber-zinkoxid |
| DE4220925C2 (de) * | 1992-06-25 | 1996-05-02 | Siemens Ag | Verfahren zur Herstellung von mit elektrischen Kontakten versehenen Formkörpern aus hochtemperatur-supraleitendem Material (HTSL) und nach diesem Verfahren hergestellter Formkörper |
| ES2091633T5 (es) * | 1992-09-16 | 2003-09-01 | Ami Doduco Gmbh | Material para contactos electricos a base de plata-oxido de estaño o de plata-oxido zinc y procedimiento para su fabricacion. |
| US5608766A (en) * | 1993-10-29 | 1997-03-04 | General Electric Company | Co-deposition of palladium during oxide film growth in high-temperature water to mitigate stress corrosion cracking |
| US5846288A (en) * | 1995-11-27 | 1998-12-08 | Chemet Corporation | Electrically conductive material and method for making |
| FR2916082B1 (fr) * | 2007-05-11 | 2009-06-12 | Schneider Electric Ind Sas | Procede de fabrication d'un materiau pour pastille de contact electrique, pastille de contact realise par un tel procede |
| CN100552845C (zh) * | 2007-09-27 | 2009-10-21 | 天津大学 | 银基氧化锡梯度电触头材料及制备方法 |
| CN101911219B (zh) * | 2008-01-17 | 2015-12-16 | 日亚化学工业株式会社 | 导电性材料及其制造方法、电子设备、发光装置及其制造方法 |
| DE102008056263A1 (de) * | 2008-11-06 | 2010-05-27 | Ami Doduco Gmbh | Verfahren zur Herstellung eines Halbzeugs und Halbzeug für elektrische Kontakte sowie Kontaktstück |
| DE102008056264A1 (de) * | 2008-11-06 | 2010-05-27 | Ami Doduco Gmbh | Verfahren zur Herstellung eines Halbzeugs und Halbzeug für elektrische Kontakte sowie Kontaktstück |
| CN102074278B (zh) * | 2010-12-09 | 2011-12-28 | 温州宏丰电工合金股份有限公司 | 颗粒定向排列增强银基电触头材料的制备方法 |
| CN102142325B (zh) * | 2010-12-30 | 2013-04-03 | 温州宏丰电工合金股份有限公司 | 颗粒定向排列增强银基氧化物电触头材料及其制备方法 |
| CN105374598A (zh) * | 2015-11-05 | 2016-03-02 | 福达合金材料股份有限公司 | 一种粗氧化物颗粒银基电接触材料的制备方法 |
| DE102016105437A1 (de) * | 2016-03-23 | 2017-09-28 | Doduco Gmbh | Verfahren zur Herstellung eines Kontaktwerkstoffes auf Basis von Silber-Zinnoxid oder Silber-Sinkoxid sowie Kontaktwerkstoff |
| CN106350692B (zh) * | 2016-09-23 | 2018-04-03 | 佛山市诺普材料科技有限公司 | 一种利用银镍合金废料制备银氧化镍的方法 |
| JP7084730B2 (ja) * | 2017-02-01 | 2022-06-15 | Dowaエレクトロニクス株式会社 | 銀合金粉末およびその製造方法 |
| CN111961910B (zh) * | 2020-07-24 | 2022-07-12 | 浙江耐迩合金科技有限公司 | 一种银氧化锡电接触材料的制备方法 |
| CN111961911B (zh) * | 2020-07-24 | 2022-04-26 | 浙江耐迩合金科技有限公司 | 高抗熔焊性能银基电接触材料的制备方法 |
| EP4563547A1 (en) | 2023-11-29 | 2025-06-04 | Any Color International Limited. | Far-infrared emitting material and preparation method thereof |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2486341A (en) * | 1945-06-30 | 1949-10-25 | Baker & Co Inc | Electrical contact element containing tin oxide |
| US3148981A (en) * | 1961-04-21 | 1964-09-15 | Nat Beryllia Corp | Metal-oxide gradient ceramic bodies |
| DE1564713A1 (de) * | 1966-09-20 | 1970-10-22 | Siemens Ag | Mehrschichtiger Sinterkontaktkoerper |
| US3827883A (en) * | 1972-10-24 | 1974-08-06 | Mallory & Co Inc P R | Electrical contact material |
| CH588152A5 (es) * | 1972-12-11 | 1977-05-31 | Siemens Ag | |
| GB1461176A (en) * | 1974-04-11 | 1977-01-13 | Plessey Inc | Method of producing powdered materials |
| DE2659012C3 (de) * | 1976-12-27 | 1980-01-24 | Siemens Ag, 1000 Berlin Und 8000 Muenchen | Verfahren zum Herstellen eines Sinterkontaktwerkstoffes aus Silber und eingelagerten Metalloxiden |
| JPS5553017A (en) * | 1978-10-16 | 1980-04-18 | Nippon Mining Co | Method of manufacturing multiple coating composite powder |
| DE2952128C2 (de) * | 1979-12-22 | 1984-10-11 | Degussa Ag, 6000 Frankfurt | Verfahren zur Vorbehandlung des Pulvers für gesintertes und stranggepreßtes Halbzeug aus Silber-Zinnoxid für elektrische Kontakte |
| DE3146972A1 (de) * | 1981-11-26 | 1983-06-01 | Siemens AG, 1000 Berlin und 8000 München | Verfahren zum herstellen von formteilen aus cadmiumfreien silber-metalloxid-verbundwerkstoffen fuer elektrische kontaktstuecke |
| DE3212005C2 (de) * | 1982-03-31 | 1986-05-28 | Siemens AG, 1000 Berlin und 8000 München | Verfahren zum Herstellen eines Zweischicht-Sinter-Kontaktstückes auf der Basis von Silber und Kupfer |
| US4426356A (en) * | 1982-09-30 | 1984-01-17 | E. I. Du Pont De Nemours And Company | Method for making capacitors with noble metal electrodes |
| DE3304637A1 (de) * | 1983-02-10 | 1984-08-16 | Siemens AG, 1000 Berlin und 8000 München | Sinterkontaktwerkstoff fuer niederspannungsschaltgeraete |
| DE3305270A1 (de) * | 1983-02-16 | 1984-08-16 | Siemens AG, 1000 Berlin und 8000 München | Sinterverbundwerkstoff fuer elektrische kontakte und verfahren zu seiner herstellung |
| US4479892A (en) * | 1983-05-16 | 1984-10-30 | Chugai Denki Kogyo K.K. | Ag-Metal oxides electrical contact materials |
| DE3421758A1 (de) * | 1984-06-12 | 1985-12-12 | Siemens AG, 1000 Berlin und 8000 München | Sinterkontaktwerkstoff fuer niederspannungsschaltgeraete der energietechnik und verfahren zu dessen herstellung |
| US4680162A (en) * | 1984-12-11 | 1987-07-14 | Chugai Denki Kogyo K.K. | Method for preparing Ag-SnO system alloy electrical contact material |
| IN165226B (es) * | 1985-08-30 | 1989-09-02 | Chugai Electric Ind Co Ltd | |
| US4622269A (en) * | 1985-12-30 | 1986-11-11 | Gte Products Corporation | Electrical contact and process for making the same |
| US4954170A (en) * | 1989-06-30 | 1990-09-04 | Westinghouse Electric Corp. | Methods of making high performance compacts and products |
-
1989
- 1989-03-22 DE DE3909384A patent/DE3909384A1/de not_active Withdrawn
- 1989-03-22 DE DE89903734T patent/DE58907140D1/de not_active Expired - Lifetime
- 1989-03-22 US US07/549,015 patent/US5360673A/en not_active Expired - Lifetime
- 1989-03-22 JP JP1503432A patent/JPH03504615A/ja active Pending
- 1989-03-22 EP EP89903734A patent/EP0440620B2/de not_active Expired - Lifetime
- 1989-03-22 WO PCT/EP1989/000316 patent/WO1989009478A1/de not_active Ceased
- 1989-03-22 ES ES8901059A patent/ES2012293A6/es not_active Expired - Lifetime
- 1989-03-23 CA CA000594639A patent/CA1339713C/en not_active Expired - Fee Related
- 1989-03-23 DD DD89326856A patent/DD283571A5/de not_active IP Right Cessation
- 1989-03-27 CN CN89101699A patent/CN1022934C/zh not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| CN1036991A (zh) | 1989-11-08 |
| DE58907140D1 (de) | 1994-04-07 |
| CA1339713C (en) | 1998-03-17 |
| EP0440620A1 (de) | 1991-08-14 |
| US5360673A (en) | 1994-11-01 |
| ES2012293A6 (es) | 1990-03-01 |
| CN1022934C (zh) | 1993-12-01 |
| WO1989009478A1 (fr) | 1989-10-05 |
| DE3909384A1 (de) | 1989-10-19 |
| EP0440620B1 (de) | 1994-03-02 |
| DD283571A5 (de) | 1990-10-17 |
| JPH03504615A (ja) | 1991-10-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0440620B2 (de) | Halbzeug für elektrische kontakte aus einem verbundwerkstoff auf silber-zinnoxid-basis und pulvermetallurgisches verfahren zu seiner herstellung | |
| DE69032065T2 (de) | Verbundwerkstoff von Silber und Metalloxyd und Verfahren zur Herstellung desselben | |
| DE69123183T2 (de) | Verbundmaterial aus Silber- oder Silber-Kupferlegierung mit Metalloxyden und Verfahren zu seiner Herstellung | |
| DE1125459C2 (de) | Verfahren zum Erzeugen von legiertem Pulver auf Eisenbasis fuer pulvermetallurgische Zwecke | |
| DE2822956C2 (de) | Verfahren zur Herstellung von Schaltkontakten für einen Vakuumschalter | |
| DE19535814C2 (de) | Material zur Herstellung elektrischer Kontakte auf Silberbasis | |
| DE2011002C3 (de) | Schmelzmetallurgisch hergestellter innenoxidierter Kontaktwerkstoff auf Silber-Kadmiumoxid-Basis | |
| EP0725154B1 (de) | Sinterwerkstoff auf der Basis Silberzinnoxid für elektrische Kontakte und Verfahren zu dessen Herstellung | |
| DE2446698A1 (de) | Zweischichten-sinterkontaktstueck fuer elektrische schaltgeraete | |
| DE3911904A1 (de) | Pulvermetallurgisches verfahren zum herstellen eines halbzeugs fuer elektrische kontakte aus einem verbundwerkstoff auf silberbasis mit eisen | |
| DE10010723B4 (de) | Verfahren zum Herstellen eines Kontaktwerkstoff-Halbzeuges für Kontaktstücke für Vakuumschaltgeräte sowie Kontaktwerkstoff-Halbzeuge und Kontaktstücke für Vakuumschaltgeräte | |
| EP0660964B2 (de) | Werkstoff für elektrische kontakte auf der basis von silber-zinnoxid oder silber-zinkoxid und verfahren zu seiner herstellung | |
| DE2824117A1 (de) | Verfahren zum herstellen eines anisotropen sinterverbundwerkstoffes mit richtgefuege | |
| DE807416C (de) | Elektrischer Kontaktwerkstoff und Verfahren zu Seiner Herstellung | |
| EP0338401B1 (de) | Pulvermetallurgisches Verfahren zum Herstellen eines Halbzeugs für elektrische Kontakte aus einem Verbundwerkstoff auf Silberbasis mit Eisen | |
| DE19916082C2 (de) | Pulvermetallurgisch hergestellter Verbundwerkstoff, Verfahren zu dessen Herstellung sowie dessen Verwendung | |
| DE2102996A1 (de) | Verfahren zum Herstellen eines Zweischichten-Sinterkontaktstückes | |
| DE4319137A1 (de) | Werkstoff für elektrische Kontakte auf der Basis von Silber-Zinnoxid oder Siler-Zinkoxid | |
| DE1938548A1 (de) | Elektroden zum Press- und Druckschweissen,insbesondere fuer das Widerstandsschweissen von Eisenwerkstoffen | |
| DE3232627C2 (es) | ||
| EP0876670B1 (de) | Verfahren zur herstellung eines formstücks aus einem kontaktwerkstoff auf silberbasis | |
| DE19523922A1 (de) | Verfahren zum pulvermetallurgischen Herstellen von Werkstoffen für elektrische Kontakte, welche Silber und ein oder mehrere Metalloxide enthalten | |
| EP0311134B1 (de) | Pulvermetallurgisch hergestellter Werkstoff für elektrische Kontakte aus Silber mit Graphit und Verfahren zu seiner Herstellung | |
| CH618044A5 (en) | Body of composite material in web or sheet form and production process for it | |
| DE2260559C3 (de) | Verfahren zum Herstellen eines Verbundwerkstoffes für elektrische Kontakte insbesondere der Starkstromtechnik |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19901212 |
|
| 17Q | First examination report despatched |
Effective date: 19930726 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19940302 Ref country code: SE Free format text: THE PATENT HAS BEEN ANNULLED BY A DECISION OF A NATIONAL AUTHORITY Effective date: 19940302 Ref country code: NL Effective date: 19940302 |
|
| REF | Corresponds to: |
Ref document number: 102387 Country of ref document: AT Date of ref document: 19940315 Kind code of ref document: T |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Effective date: 19940322 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19940331 |
|
| REF | Corresponds to: |
Ref document number: 58907140 Country of ref document: DE Date of ref document: 19940407 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19940408 |
|
| ITF | It: translation for a ep patent filed | ||
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PFA Free format text: DODUCO GMBH + CO. DR. EUGEN DUERRWAECHTER |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: DODUCO GMBH + CO DR. EUGEN DUERRWAECHTER |
|
| ET | Fr: translation filed | ||
| NLV1 | Nl: lapsed or annulled due to failure to fulfill the requirements of art. 29p and 29m of the patents act | ||
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| 26 | Opposition filed |
Opponent name: DEGUSSA AG, FRANKFURT - ZWEIGNIEDERLASSUNG WOLFGAN Effective date: 19941130 |
|
| PLAW | Interlocutory decision in opposition |
Free format text: ORIGINAL CODE: EPIDOS IDOP |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: DODUCO GMBH + CO DR. EUGEN DUERRWAECHTER I.K. |
|
| PLAW | Interlocutory decision in opposition |
Free format text: ORIGINAL CODE: EPIDOS IDOP |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19980226 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19980320 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19980331 Year of fee payment: 10 |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: DODUCO GMBH |
|
| 27A | Patent maintained in amended form |
Effective date: 19980603 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: AEN Free format text: AUFRECHTERHALTUNG DES PATENTES IN GEAENDERTER FORM |
|
| ET3 | Fr: translation filed ** decision concerning opposition | ||
| GBTA | Gb: translation of amended ep patent filed (gb section 77(6)(b)/1977) | ||
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990322 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990331 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990331 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19990322 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19991130 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20050322 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20080220 Year of fee payment: 20 |