EP0440620B2 - Semifinished product for electrical contacts, made of a composite material based on silver and tin oxide, and powder metallurgical process for producing it - Google Patents
Semifinished product for electrical contacts, made of a composite material based on silver and tin oxide, and powder metallurgical process for producing it Download PDFInfo
- Publication number
- EP0440620B2 EP0440620B2 EP89903734A EP89903734A EP0440620B2 EP 0440620 B2 EP0440620 B2 EP 0440620B2 EP 89903734 A EP89903734 A EP 89903734A EP 89903734 A EP89903734 A EP 89903734A EP 0440620 B2 EP0440620 B2 EP 0440620B2
- Authority
- EP
- European Patent Office
- Prior art keywords
- component
- oxide
- weight
- semi
- finished product
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000000843 powder Substances 0.000 title claims abstract description 72
- 239000002131 composite material Substances 0.000 title claims abstract description 70
- 239000011265 semifinished product Substances 0.000 title claims abstract description 36
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 title claims abstract description 31
- 229910001887 tin oxide Inorganic materials 0.000 title claims abstract description 31
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 title claims abstract description 21
- 239000004332 silver Substances 0.000 title claims abstract description 18
- 238000010310 metallurgical process Methods 0.000 title claims abstract description 4
- 229910001923 silver oxide Inorganic materials 0.000 title claims abstract 4
- 229910044991 metal oxide Inorganic materials 0.000 claims abstract description 46
- 150000004706 metal oxides Chemical class 0.000 claims description 44
- 239000000463 material Substances 0.000 claims description 23
- 238000000034 method Methods 0.000 claims description 18
- 229910052751 metal Inorganic materials 0.000 claims description 17
- 239000002184 metal Substances 0.000 claims description 17
- 229910052709 silver Inorganic materials 0.000 claims description 14
- 150000002739 metals Chemical class 0.000 claims description 12
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 11
- 239000000203 mixture Substances 0.000 claims description 9
- 238000009826 distribution Methods 0.000 claims description 8
- QGLKJKCYBOYXKC-UHFFFAOYSA-N nonaoxidotritungsten Chemical compound O=[W]1(=O)O[W](=O)(=O)O[W](=O)(=O)O1 QGLKJKCYBOYXKC-UHFFFAOYSA-N 0.000 claims description 8
- 229910001930 tungsten oxide Inorganic materials 0.000 claims description 8
- 238000004519 manufacturing process Methods 0.000 claims description 7
- 150000003839 salts Chemical class 0.000 claims description 7
- 229910001316 Ag alloy Inorganic materials 0.000 claims description 6
- 239000011159 matrix material Substances 0.000 claims description 6
- UONOETXJSWQNOL-UHFFFAOYSA-N tungsten carbide Chemical compound [W+]#[C-] UONOETXJSWQNOL-UHFFFAOYSA-N 0.000 claims description 6
- 150000001247 metal acetylides Chemical class 0.000 claims description 5
- 229910052759 nickel Inorganic materials 0.000 claims description 5
- QIJNJJZPYXGIQM-UHFFFAOYSA-N 1lambda4,2lambda4-dimolybdacyclopropa-1,2,3-triene Chemical compound [Mo]=C=[Mo] QIJNJJZPYXGIQM-UHFFFAOYSA-N 0.000 claims description 4
- 229910039444 MoC Inorganic materials 0.000 claims description 4
- 229910045601 alloy Inorganic materials 0.000 claims description 4
- 239000000956 alloy Substances 0.000 claims description 4
- 229910000476 molybdenum oxide Inorganic materials 0.000 claims description 4
- PQQKPALAQIIWST-UHFFFAOYSA-N oxomolybdenum Chemical compound [Mo]=O PQQKPALAQIIWST-UHFFFAOYSA-N 0.000 claims description 4
- 238000005096 rolling process Methods 0.000 claims description 4
- 238000003466 welding Methods 0.000 claims description 4
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 3
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 claims description 3
- 229910052802 copper Inorganic materials 0.000 claims description 3
- 239000010949 copper Substances 0.000 claims description 3
- 230000001590 oxidative effect Effects 0.000 claims description 3
- 229910052718 tin Inorganic materials 0.000 claims description 3
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 claims description 3
- 229910052721 tungsten Inorganic materials 0.000 claims description 3
- 239000010937 tungsten Substances 0.000 claims description 3
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 claims description 2
- 229910002115 bismuth titanate Inorganic materials 0.000 claims description 2
- 230000007423 decrease Effects 0.000 claims description 2
- 229910052750 molybdenum Inorganic materials 0.000 claims description 2
- 239000011733 molybdenum Substances 0.000 claims description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 claims 2
- 239000003870 refractory metal Substances 0.000 claims 2
- QPLDLSVMHZLSFG-UHFFFAOYSA-N Copper oxide Chemical compound [Cu]=O QPLDLSVMHZLSFG-UHFFFAOYSA-N 0.000 claims 1
- 239000005751 Copper oxide Substances 0.000 claims 1
- XHCLAFWTIXFWPH-UHFFFAOYSA-N [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] Chemical compound [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] XHCLAFWTIXFWPH-UHFFFAOYSA-N 0.000 claims 1
- 229910000416 bismuth oxide Inorganic materials 0.000 claims 1
- 229910000431 copper oxide Inorganic materials 0.000 claims 1
- TYIXMATWDRGMPF-UHFFFAOYSA-N dibismuth;oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[Bi+3].[Bi+3] TYIXMATWDRGMPF-UHFFFAOYSA-N 0.000 claims 1
- 229910052763 palladium Inorganic materials 0.000 claims 1
- 238000009827 uniform distribution Methods 0.000 claims 1
- 229910001935 vanadium oxide Inorganic materials 0.000 claims 1
- 239000000047 product Substances 0.000 abstract 1
- IVQODXYTQYNJFI-UHFFFAOYSA-N oxotin;silver Chemical compound [Ag].[Sn]=O IVQODXYTQYNJFI-UHFFFAOYSA-N 0.000 description 21
- 239000002245 particle Substances 0.000 description 21
- ASMQPJTXPYCZBL-UHFFFAOYSA-N [O-2].[Cd+2].[Ag+] Chemical compound [O-2].[Cd+2].[Ag+] ASMQPJTXPYCZBL-UHFFFAOYSA-N 0.000 description 8
- SQGYOTSLMSWVJD-UHFFFAOYSA-N silver(1+) nitrate Chemical compound [Ag+].[O-]N(=O)=O SQGYOTSLMSWVJD-UHFFFAOYSA-N 0.000 description 6
- 238000001125 extrusion Methods 0.000 description 5
- 238000010438 heat treatment Methods 0.000 description 5
- MOFOBJHOKRNACT-UHFFFAOYSA-N nickel silver Chemical compound [Ni].[Ag] MOFOBJHOKRNACT-UHFFFAOYSA-N 0.000 description 5
- 239000007864 aqueous solution Substances 0.000 description 4
- 230000003647 oxidation Effects 0.000 description 4
- 238000007254 oxidation reaction Methods 0.000 description 4
- 238000004663 powder metallurgy Methods 0.000 description 4
- 238000005118 spray pyrolysis Methods 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 230000002349 favourable effect Effects 0.000 description 3
- UXVJMYXIXYVFDD-UHFFFAOYSA-N oxotin oxotungsten silver Chemical compound [W]=O.[Sn]=O.[Ag] UXVJMYXIXYVFDD-UHFFFAOYSA-N 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 238000003825 pressing Methods 0.000 description 3
- 229910001961 silver nitrate Inorganic materials 0.000 description 3
- 238000005245 sintering Methods 0.000 description 3
- 238000005507 spraying Methods 0.000 description 3
- 238000010792 warming Methods 0.000 description 3
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229910052797 bismuth Inorganic materials 0.000 description 2
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 239000010410 layer Substances 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- -1 silver-tin oxide Silver-cadmium oxide Chemical compound 0.000 description 2
- 235000012431 wafers Nutrition 0.000 description 2
- 229910000925 Cd alloy Inorganic materials 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 229910000881 Cu alloy Inorganic materials 0.000 description 1
- 229910001111 Fine metal Inorganic materials 0.000 description 1
- 241000282320 Panthera leo Species 0.000 description 1
- 229910001128 Sn alloy Inorganic materials 0.000 description 1
- QCEUXSAXTBNJGO-UHFFFAOYSA-N [Ag].[Sn] Chemical compound [Ag].[Sn] QCEUXSAXTBNJGO-UHFFFAOYSA-N 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 238000000889 atomisation Methods 0.000 description 1
- 229910052793 cadmium Inorganic materials 0.000 description 1
- BDOSMKKIYDKNTQ-UHFFFAOYSA-N cadmium atom Chemical compound [Cd] BDOSMKKIYDKNTQ-UHFFFAOYSA-N 0.000 description 1
- NSAODVHAXBZWGW-UHFFFAOYSA-N cadmium silver Chemical compound [Ag].[Cd] NSAODVHAXBZWGW-UHFFFAOYSA-N 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 239000010946 fine silver Substances 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000004482 other powder Substances 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 235000014483 powder concentrate Nutrition 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- 210000004243 sweat Anatomy 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000008207 working material Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C32/00—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ
- C22C32/001—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ with only oxides
- C22C32/0015—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ with only oxides with only single oxides as main non-metallic constituents
- C22C32/0021—Matrix based on noble metals, Cu or alloys thereof
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/12—Metallic powder containing non-metallic particles
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01H—ELECTRIC SWITCHES; RELAYS; SELECTORS; EMERGENCY PROTECTIVE DEVICES
- H01H1/00—Contacts
- H01H1/02—Contacts characterised by the material thereof
- H01H1/021—Composite material
- H01H1/023—Composite material having a noble metal as the basic material
- H01H1/0237—Composite material having a noble metal as the basic material and containing oxides
- H01H1/02372—Composite material having a noble metal as the basic material and containing oxides containing as major components one or more oxides of the following elements only: Cd, Sn, Zn, In, Bi, Sb or Te
- H01H1/02376—Composite material having a noble metal as the basic material and containing oxides containing as major components one or more oxides of the following elements only: Cd, Sn, Zn, In, Bi, Sb or Te containing as major component SnO2
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01H—ELECTRIC SWITCHES; RELAYS; SELECTORS; EMERGENCY PROTECTIVE DEVICES
- H01H11/00—Apparatus or processes specially adapted for the manufacture of electric switches
- H01H11/04—Apparatus or processes specially adapted for the manufacture of electric switches of switch contacts
- H01H11/048—Apparatus or processes specially adapted for the manufacture of electric switches of switch contacts by powder-metallurgical processes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2998/00—Supplementary information concerning processes or compositions relating to powder metallurgy
- B22F2998/10—Processes characterised by the sequence of their steps
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12014—All metal or with adjacent metals having metal particles
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12014—All metal or with adjacent metals having metal particles
- Y10T428/12021—All metal or with adjacent metals having metal particles having composition or density gradient or differential porosity
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12014—All metal or with adjacent metals having metal particles
- Y10T428/12028—Composite; i.e., plural, adjacent, spatially distinct metal components [e.g., layers, etc.]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12014—All metal or with adjacent metals having metal particles
- Y10T428/1216—Continuous interengaged phases of plural metals, or oriented fiber containing
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12014—All metal or with adjacent metals having metal particles
- Y10T428/1216—Continuous interengaged phases of plural metals, or oriented fiber containing
- Y10T428/12167—Nonmetal containing
Definitions
- the invention relates to a semi-finished product for electrical Contacts made of a composite material based on silver-tin oxide and a powder metallurgical Process for its manufacture.
- the invention is based on an entanglement with those specified in the preamble of claim 1 Characteristics or of a semi-finished product with the specified in the preamble of claim 12 Characteristics.
- Contact pieces formed from such materials can contact pieces made of silver-cadmium oxide in service life under AC3 and AC4 test conditions (specified in IEC standard 158-1) be, however, show a stronger contact warming in the switchgear. what the lifespan of the Switchgear can impair. Moreover can the internally oxidized contact pieces subsequently no longer be deformed.
- DE-B-26 59 012 is a powder metallurgy Process for the production of a contact material made of silver with two different ones Metal oxides known in which two silver-metal oxide composite powders mixed, pressed and sintered, one of which is Compound powder just one metal oxide and that other composite powder only the other metal oxide contains.
- the object of the present invention is to achieve this based on a semi-finished product for electrical contacts to provide the base silver-tin oxide which is despite a very small content Tin oxide particles work well by extrusion and rolling can be processed and at the same time regarding Lifetime, tendency to weld and contact heating is as good or better than semi-finished products are based on silver-cadmium oxide.
- the semifinished product produced according to the invention consists of a composite material, which is through a special rough structure in combination with a special fine structure.
- the Coarse structure is given by the fact that in the composite material oxide rich areas where everything Metal oxide or the vast majority of Metal oxide component is concentrated, alternate with low-oxide areas. the only a small one Contain or even share of the metal oxide component are oxide free. Contain the low-oxide areas at most a small amount of metal oxide finely distributed in one from the material of the first component formed matrix.
- the oxide rich areas contain the lion's share of the metal oxide component (in a concentration that is far greater is higher than the usual average metal oxide concentration in a contact material based on silver-tin oxide) and the rest of the material of the first component like a penetration or a storage composite finely divided into one another. These areas have emerged from low-oxide and high-oxide powders, which were mixed, pressed and, if necessary, sintered. The size of the low and high oxide areas, which is the rough structure of the composite determine therefore depends on the size of the powder particles from.
- the fine structure of the composite is given by a finely dispersed oxide distribution in the oxide-rich forming the rough structure Areas of the composite, if necessary also in low-oxide areas, so far are present in these metal oxides.
- the majority of the metal oxide component must come concentrated in the composite powder and be involved. Only the relatively small one Metal oxide content, which may still be in the contain low-oxide areas of the composite material can be e.g. in the form of a pure Oxide powder with the powder from the first component of the material are mixed. Doing so it prefers that in the low-oxide areas the same oxides are present as in the oxide-rich ones Areas.
- any metal carbides still present especially tungsten carbide and / or molybdenum carbide
- those not solved in the first component Metals can separate the powder mixture in the form Powders are added; they are suitable in switching operation the wetting of the tin oxide with To promote silver and thereby the contact resistance to humiliate.
- the resulting Solid particles react with the oxygen in the oxidizing atmosphere in the flame or in the reactor at a temperature below that Melting temperature of the dissolved metals, whereby Powder particles arise in which the metals of the first component, i.e. the silver or the silver alloy, and the metal oxide component, so essentially tin oxide, in a very fine distribution tied together.
- the metal oxide particles mostly in sizes between 0.1 ⁇ m and 1 ⁇ m (diameter) before what for the inventive method is advantageous.
- spray pyrolysis Compound powder is also advantageous because by spray pyrolysis, in particular powder particles arise that are spherical or potato-shaped Have shape, the emergence of a deformable Semi-finished products favored because the spherical or potato-shaped particles of a plastic Deformation of the contact material less oppose as irregularly jagged powder particles.
- oxidic and carbidic components sometimes lower the contact point temperature in switching operation and partly an extension of the life of the contact pieces not only with small and medium current loads, but also in the heavy load area.
- Molybdenum carbide and tungsten carbide already work in small amounts.
- the additional carbides and Oxides should make up 6% by weight of the contact material not exceed so this does not gets too hard.
- nickel can also be advantageous to the composite material, which is not in silver is soluble and either as a very fine powder that formed from silver or a silver alloy Powder is mixed or also as spray-pyrolysized silver-nickel powder is introduced.
- a silver-tin oxide composite powder with 32% by weight of tin oxide is produced by spraying an aqueous solution of silver nitrate and tin-II-chloride in a reactor heated to approx. 950 ° C. with an oxygen-containing atmosphere, whereby a silver-tin oxide composite powder fails , in the powder particles of which the tin oxide is present in a very fine distribution. Then 75 parts by weight of a silver powder with a particle size smaller than 40 microns with 25 parts by weight of the silver-tin oxide, composite powder are dry mixed for one hour, then isostatically pressed to blocks of about 50 kg in weight and then at a temperature sintered at 830 ° C for 1.5 hours.
- the block thus formed is placed in the recipient of an extrusion press and hot-extruded, reducing the cross-section to a strand with a cross-section of 10 x 75 mm 2 , at a temperature of approx. 850 ° C., and then hot-rolled plated with a 1.5 mm thick fine silver sheet , hot rolled down to its final thickness of 2mm and processed into contact wafers according to the usual methods.
- the silver-tin oxide composite in the contact wafers has a tin oxide content of 8% by weight.
- the second example is modified that the powder mixture still contains 0.5% by weight Tungsten oxide (particle size smaller than 10 ⁇ m) and 0.3 wt% tungsten carbide (particle size smaller than 2.5 ⁇ m) are added. Furthermore is operated as in the first example. The addition of tungsten oxide and tungsten carbide leads to one Lowering the contact point temperature and to an extended lifespan from the Semi-finished electrical contact pieces.
- a silver-tin oxide-tungsten oxide composite powder with 20% by weight of tin oxide and 0.5% by weight of tungsten oxide is made by spraying an aqueous Solution of silver nitrate, tin-II-chloride and Tungsten ll chloride in a heated to approx. 950 ° C Reactor with an oxygen-containing atmosphere, being a silver-tin oxide-tungsten oxide composite powder precipitates, in the powder particles of the tin oxide and the tungsten oxide is in a very fine distribution. Then 50 parts by weight of a Silver powder with a particle size smaller than 40 ⁇ m with 50 parts by weight of the silver-tin oxide-tungsten oxide composite powder dry for an hour mixed and as in the 1st example for contact plates processed further.
- a silver-tin oxide composite powder with 30 % By weight of tin oxide is produced as in the second example.
- a silver-nickel composite powder with 2 wt .-% Nickel is made by spraying one aqueous solution of silver nitrate and nickel-II-chloride in a reactor heated to approx. 950 ° C with a protective gas atmosphere (e.g. argon), whereby a silver-nickel composite powder fails, in the Powder particles the nickel in a very fine distribution is present.
- a protective gas atmosphere e.g. argon
- the fourth example can be modified accordingly be that instead of a silver-nickel composite powder a silver powder and a carbonyl nickel powder mixed with the silver-tin oxide composite powder will. Otherwise the same as in the 4th example method.
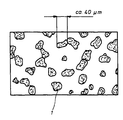
- the attached figure shows schematically the Structure of one according to the second example manufactured composite, in which silver-tin oxide areas 1, mostly less than 50 ⁇ m, are in a silver matrix that consists of the oxide-free silver powder particles has emerged.
- Semi-finished products produced according to the invention are suitable are particularly suitable for contact pieces in low-voltage switching devices, e.g. in motor contactors.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Composite Materials (AREA)
- Manufacturing & Machinery (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Contacts (AREA)
- Powder Metallurgy (AREA)
Abstract
Description
Die Erfindung betrifft ein Halbzeug für elektrische Kontakte aus einem Verbundwerkstoff auf Silber-Zinnoxid-Basis und ein pulvermetallurgisches Verfahren zu seiner Herstellung.The invention relates to a semi-finished product for electrical Contacts made of a composite material based on silver-tin oxide and a powder metallurgical Process for its manufacture.
Die Erfindung geht aus von einem Verfanren
mit den im Oberbegriff des Anspruchs 1 angegebenen
Merkmalen bzw. von einem Halbzeug mit den
im Oberbegriff des Anspruchs 12 angegebenen
Merkmalen.The invention is based on an entanglement
with those specified in the preamble of
Kontaktwerkstoffe auf der Basis Silber-Zinnoxid haben gegenwärtig die beste Aussicht, die bewährten, aber wegen der Giftigkeit des Kadmiums in Verruf geratenen Kontaktwerkstoffe auf der Basis von Silber-Kadmiumoxid zu ersetzen. Die große Bedeutung, die Kontaktstücke aus Silber-Kadmiumoxid in Niederspannungsschaltgeräten, insbesondere in Motorschützen erlangt haben, ist darauf zurückzuführen, dass sie hohe Lebensdauer, geringe Verschweißneigung, gleichbleibend niedrigen Kontaktübergangswiderstand (und damit eine geringe Kontakterwärmung), gute Lichtbogenlöschung und gute Verarbeitbarkeit optimal miteinander verbinden. Heute bekannte Kontaktstücke auf der Basis von Silber-Zinnoxid liegen den Kontaktstücken aus Silber-Kadmiumoxid in der Kombination ihrer Eigenschaften am nächsten, erreichen jedoch noch nicht solche günstigen Eigenschaften in allen vorstehend genannten Punkten zugleich.Contact materials based on silver-tin oxide currently have the best views, the proven ones but because of the toxicity of the cadmium in Contact materials based on bad reputation of silver-cadmium oxide to replace. The size Meaning, the contact pieces made of silver-cadmium oxide in low-voltage switchgear, in particular in motor contactors is due to that they have long life, low Welding tendency, consistently low contact resistance (and thus a small one Contact heating), good arc quenching and Optimally combine good workability. Today known contact pieces on the base The contact pieces are exposed from silver-tin oxide Silver-cadmium oxide in the combination of their properties closest, but still reach not such favorable properties in all of the above mentioned points at the same time.
Es ist bekannt (DE-26 59 012 B2), dass eine möglichst feine Verteilung der Metalloxide in der Silbermatrix zu günstigen Kontakteigenschaften führt. Silber-Kadmiumoxiawerkstoffe werden deshalb häufig durch innere Oxidation einer Silber-Kadmium-Legierung hergestellt. Halbzeuge aus Silber-Zinnoxid lassen sich jedoch im allgemeinen nicht durch innere Oxidation eines entsprechenden Werkstücks aus einer Silber-Zinn-Legierung herstellen, da eine vollständige Oxidation des im Innern des Werkstücks befindlichen Zinns durch die Ausbildung von Passivschichten behindert wird, so dass die Oxidation praktisch auf eine Oberflächenschicht beschränkt ist. Durch Zusatz weiterer oxidierbarer Metalle, namentlich Indium oder Wismut, kann die Ausbildung einer passivierenden Schicht weitgehend unterdrückt werden (DE-A 29 08 923). Aus derartigen Werkstoffen gebildete Kontaktstükke können Kontaktstücken aus Silber-Kadmiumoxid in der Lebensdauer unter AC3- und AC4-Prüfbedingungen (festgelegt in der IEC-Norm 158-1) überlegen sein, zeigen jedoch eine stärkere Kontakterwärmung im Schaltgerät. was die Lebensdauer der Schaltgeräte beeinträchtigen kann. Ausserdem können die innerlich oxidierten Kontaktstücke nachträglich nicht mehr verformt werden.It is known (DE-26 59 012 B2) that a distribution of the metal oxides as fine as possible in the Silver matrix with favorable contact properties leads. Silver cadmium oxide materials are therefore often by internal oxidation of a silver-cadmium alloy produced. Semi-finished products made of silver-tin oxide however, in general not by internal oxidation of a corresponding one Manufacture the workpiece from a silver-tin alloy, because a complete oxidation of the inside of the workpiece located by the Formation of passive layers is hindered, so that the oxidation is practically on a surface layer is limited. By adding more oxidizable Metals, namely indium or bismuth, can form a passivating layer are largely suppressed (DE-A 29 08 923). Contact pieces formed from such materials can contact pieces made of silver-cadmium oxide in service life under AC3 and AC4 test conditions (specified in IEC standard 158-1) be, however, show a stronger contact warming in the switchgear. what the lifespan of the Switchgear can impair. Moreover can the internally oxidized contact pieces subsequently no longer be deformed.
Es ist auch bekannt, Kontaktwerkstoffe aus Silber-Zinnoxid auf pulvermetallurgischem Wege herzustellen, nämlich durch Mischen eines Silberpulvers mit einem Zinnoxidpulver, Bilden von Silber-Zinnoxidrohlingen durch Pressen und Sintern der Pulvermischung, und Umformen der Rohlinge durch Strangpressen oder durch Strangpressen und Walzen. Verglichen mit einem Silber-Kadmiumoxid-Kontaktwerkstoff kann ein solcher pulvermetallurgisch hergestellter Werkstoff, wenn er zusätzlich noch kleine Mengen Wolframoxid oder Molybdänoxid enthält, in der Kontakterwärmung ungefähr gleich gut und in der AC4-Lebensdauerprüfung besser abschneiden, in der AC3-Lebensdauerprüfung schneidet er jedoch schlechter ab. Das Umformen der Rohlinge durch Walzen oder Strangpressen ist aber schwierig, weil die Zinnoxidteilchen im Silber-Zinnoxid-Verbundwerkstoff dessen plastische Verformung ausserordentlich behindern. Erschwerend kommt hinzu, dass die Verarbeitbarkeit des Silber-Zinnoxids um so schwieriger wird, je feiner das Zinnoxid im Werkstoff dispergiert ist, denn desto wirkungsvoller behindern die Zinnoxid-Teilchen beim mechanischen Umformen des Verbundwerkstoffs dessen plastische Verformung. Um der schlechten Verarbeitbarkeit zu begegnen, ist deshalb in der DE-A 29 52 128 vorgeschlagen, das Zinnoxidpulver vor dem Vermischen mit dem Silberpulver bei 900° C bis 1600° C zu glühen, wodurch die Zinnoxidpulverteilchen vergröbert werden und so die spätere mechanische Umformung des Verbundwerkstoffes weniger behindern. Die bessere Verarbeitbarkeit wird jedoch erkauft mit einer teilweisen Verschlechterung der Schalteigenschaften der Kontaktstücke, weil das Zinnoxid nicht mehr so fein im Verbundwerkstoff verteilt ist wie ehedem.It is also known to make contact materials made from silver-tin oxide to manufacture by powder metallurgy, namely by mixing a silver powder with a tin oxide powder, forming silver tin oxide blanks by pressing and sintering the Powder mixture, and forming of the blanks by extrusion or by extrusion and rollers. Compared to a silver-cadmium oxide contact material can be such a powder metallurgy manufactured material if it is additional small amounts of tungsten oxide or molybdenum oxide contains, in contact heating approximately equally good and in the AC4 durability test perform better in the AC3 endurance test however, it does worse. Forming the blanks by rolling or extrusion is difficult because the tin oxide particles in the Silver-tin oxide composite whose plastic Extremely hinder deformation. Aggravating In addition, the processability of the Silver-tin oxide becomes more difficult the finer the tin oxide is dispersed in the material, because the more effectively the tin oxide particles interfere during mechanical forming of the composite material its plastic deformation. To the Countering poor processability is therefore proposed in DE-A 29 52 128 that Tin oxide powder before mixing with the silver powder to glow at 900 ° C to 1600 ° C, whereby the tin oxide powder particles are coarsened and so the later mechanical transformation of the Hinder composite less. The better one However, workability is bought with a partial deterioration of the switching properties of the contact pieces because the tin oxide is not is distributed as finely as possible in the composite material formerly.
Halbzeuge für elektrische Kontakte, die aus einem pulvermetallurgisch hergestellten Verbundwerkstoff auf der Basis von Silber-Zinnoxid mit einem Zusatz wenigstens eines weiteren Metalloxids (Molybdänoxid, Wolframoxid, Wismuttitanat) und eines karbidischen Bestandteils (Wolframkarbid und/oder Molybdänkarbid) bestehen, sind aus der DE-32 32 627 C2 bekannt.Semi-finished products for electrical contacts made from a powder metallurgically manufactured composite based on silver-tin oxide an addition of at least one further metal oxide (Molybdenum oxide, tungsten oxide, bismuth titanate) and a carbide component (tungsten carbide and / or molybdenum carbide) consist of known from DE-32 32 627 C2.
Aus der EP 0 170 812 A2 ist es bekannt, aus Silber, Zinn, Wismut und Kupfer eine AgSnBiCu-Legierung zu erschmelzen, durch Druckverdüsen der Schmelze ein Legierungspulver herzustellen, dieses innerlich zu oxidieren und daraus durch Pressen und Sintern Kontaktstücke zu formen. Verglichen mit Kontaktstücken aus Silber-Kadmiumoxid zeigen diese Kontaktstücke ungefähr eine gleich starke Erwärmung und haben eine längere Lebensdauer in der AC3-Prüfung, jedoch eine kürzere Lebensdauer in der AC4-Prüfung.It is known from EP 0 170 812 A2 Silver, tin, bismuth and copper an AgSnBiCu alloy to melt, by pressure atomization to produce an alloy powder from the melt, to oxidize this internally and from it Pressing and sintering to form contact pieces. Compared with contacts made of silver-cadmium oxide these contact pieces show approximately one equally strong warming and have a longer one Service life in the AC3 test, but a shorter one Service life in the AC4 test.
Aus der DE-29 29 630 A1 ist es bekannt, ein Silber-Zinnoxid-Verbundpulver nach einem pyrolytischen Verfahren herzustellen und aus diesem Verbundpulver durch Pressen und Sintern Kontaktstükke zu formen. Verglichen mit Kontaktstücken aus Silber-Kadmiumoxid-Kontaktstücken zeigen diese Kontaktstücke zwar eine längere Lebensdauer, aber eine stärkere Kontakterwärmung und eine schlechtere Verarbeitbarkeit. Entsprechendes gilt für das aus der DE 32 12 005 A1 bekannte, pulvermetallurgisch aus einem Silberzinnoxidverbundpulver hergestellte Kontaktstück mit einem oxidfreien Rücken aus Kupfer oder aus einer Kupferlegierung. Aus derselben DE 29 29 630 A1 ist es ferner bekannt, im Verbundpulver zusätzlich Wolframoxid oder Molybdänoxid einzulagern. Dadurch kann zwar die Kontakterwärmung verringert werden, gleichzeitig sinkt jedoch die Lebensdauer in der AC3-Prüfung.From DE-29 29 630 A1 it is known a Silver-tin oxide composite powder after a pyrolytic Process to manufacture and from this composite powder by pressing and sintering contact pieces to shape. Compared to contact pieces Silver-cadmium oxide contact pieces show this Contacts have a longer lifespan, but more contact warming and one poorer processability. The same applies for powder metallurgy known from DE 32 12 005 A1 from a silver tin oxide composite powder manufactured contact piece with an oxide-free Back made of copper or a copper alloy. From the same DE 29 29 630 A1 it is also known, additionally tungsten oxide in the composite powder or store molybdenum oxide. This can contact heating is reduced, at the same time, however, the lifespan decreases AC3 exam.
Aus der DE-B-26 59 012 ist ein pulvermetallurgisches Verfahren zur Herstellung eines Kontaktwerkstoffs aus Silber mit zwei eingelagerten unterschiedlichen Metalloxiden bekannt, bei welchem zwei Silber-Metalloxid-Verbundpulver gemischt, gepreßt und gesintert werden, von denen das eine Verbundpulver nur das eine Metalloxid und das andere Verbundpulver nur das andere Metalloxid enthält.DE-B-26 59 012 is a powder metallurgy Process for the production of a contact material made of silver with two different ones Metal oxides known in which two silver-metal oxide composite powders mixed, pressed and sintered, one of which is Compound powder just one metal oxide and that other composite powder only the other metal oxide contains.
Der vorliegenden Erfindung liegt die Aufgabe zugrunde, ein Halbzeug für elektrische Kontakte auf der Basis Silber-Zinnoxid zur Verfügung zu stellen, welches sich trotz eines Gehalts an sehr kleinen Zinnoxidteilchen gut durch Strangpressen und Walzen verarbeiten läßt und gleichzeitig hinsichtlich Lebensdauer, Verschweißneigung und Kontakterwärmung gleich gut oder besser ist als es Halbzeuge auf Silber-Kadmiumoxidbasis sind.The object of the present invention is to achieve this based on a semi-finished product for electrical contacts to provide the base silver-tin oxide which is despite a very small content Tin oxide particles work well by extrusion and rolling can be processed and at the same time regarding Lifetime, tendency to weld and contact heating is as good or better than semi-finished products are based on silver-cadmium oxide.
Diese Aufgabe wird gelöst durch ein Verfahren
mit den im Ansprucn 1 angegebenen Merkmalen
sowie durch ein Halbzeug mit den im Anspruch 12
angegebenen Merkmalen. Vorteilhafte Weiterbildungen
der Erfindung sind Gegenstand der Unteransprüche.This problem is solved by a method
with the features specified in
Das erfindungsgemäß hergestellte Halbzeug besteht aus einem Verbundwerkstoff, welcher sich durch eine besondere Grobstruktur in Kombination mit einer besonderen Feinstruktur auszeichnet. Die Grobstruktur ist dadurch gegeben, dass im Verbundwerkstoff oxidreiche Bereiche, in denen alles Metalloxid oder der weit überwiegende Anteil der Metalloxidkomponente konzentriert ist, abwechseln mit oxidarmen Bereichen. die nur einen kleinen Anteil der Metalloxidkomponente enthalten oder sogar oxidfrei sind. Die oxidarmen Bereiche enthalten allenfalls einen geringen Metalloxidanteil fein verteilt in einer aus dem Material der ersten Komponente gebildeten Matrix. Die oxidreichen Bereiche enthalten den Löwenanteil der Metalloxidkomponente (und zwar in einer Konzentration, die weitaus höher liegt als die übliche durchschnittliche Metalloxidkonzentration in einem Kontaktwerkstoff auf Silber-Zinnoxid-Basis) und den Rest des Materials der ersten Komponente nach Art eines Durchdringungs- oder eines Einlagerungsverbundwerkstoffs fein ineinander verteilt. Diese Bereiche sind hervorgegangen aus oxidarmen und oxidreichen Pulvern, die gemischt, gepreßt und ggfs. gesintert wurden. Die Größe der oxidarmen und der oxidreichen Bereiche, die die Grobstruktur des Verbundwerkstoffs bestimmen, hängt deshalb von der Größe der Pulverteilchen ab. Die Feinstruktur des Verbundwerkstoffs ist gegeben durch eine feindisperse Oxidverteilung in den die Grobstruktur bildenden oxidreichen Bereichen des Verbundwerkstoffs, gegebenenfalls auch in den oxidarmen Bereichen, soweit in diesen Metalloxide vorliegen. Am besten ist die gesamte Metalloxidkomponente in dem verwendeten Verbundpulver konzentriert, so dass das andere Pulver, welches den überwiegenden Teil des Silbers oder der hauptsächlich Silber enthaltenden Legierung (erste Komponente) enthält, ganz oxidfrei ist. In diesem Fall wechseln im Verbundwerkstoff Bereiche, in denen die Metalloxidkomponente konzentriert ist, mit Bereichen ab, die völlig frei von der Metalloxidkomponente sind. Das hat den Vorteil, dass die Bereiche, die die Metalloxidkomponente, insbesondere das Zinnoxid, enthalten, voneinander weitgehend durch eine oxidfreie Matrix getrennt sind (sie "schwimmen" gleichsam in einer oxidfreien Matrix), so dass sie die plastische Verformung beim Walzen oder Strangpressen des Halbzeuges viel weniger behindern als im Falle von mehr oder weniger gleichmässig über den gesamten Werkstoff verteilten Metalloxiden. Demgegenüber zeichnet sich das erfindungsgemäße Halbzeug durch eine verbesserte Verformbarkeit aus. Diese wird jedoch nicht erkauftdurch eine erhöhte Verschweißneigung oder durch eine verringerte Lebensdauer oder durch einen erhöhten elektrischen Kontaktübergangswiderstand.The semifinished product produced according to the invention consists of a composite material, which is through a special rough structure in combination with a special fine structure. The Coarse structure is given by the fact that in the composite material oxide rich areas where everything Metal oxide or the vast majority of Metal oxide component is concentrated, alternate with low-oxide areas. the only a small one Contain or even share of the metal oxide component are oxide free. Contain the low-oxide areas at most a small amount of metal oxide finely distributed in one from the material of the first component formed matrix. The oxide rich areas contain the lion's share of the metal oxide component (in a concentration that is far greater is higher than the usual average metal oxide concentration in a contact material based on silver-tin oxide) and the rest of the material of the first component like a penetration or a storage composite finely divided into one another. These areas have emerged from low-oxide and high-oxide powders, which were mixed, pressed and, if necessary, sintered. The size of the low and high oxide areas, which is the rough structure of the composite determine therefore depends on the size of the powder particles from. The fine structure of the composite is given by a finely dispersed oxide distribution in the oxide-rich forming the rough structure Areas of the composite, if necessary also in low-oxide areas, so far are present in these metal oxides. It is best total metal oxide component used in the Composite powder concentrated, so the other Powder, which is the major part of the silver or mainly containing silver Alloy (first component) contains, completely oxide-free is. In this case, change in the composite material Areas where the metal oxide component is focused, with areas that are completely devoid of of the metal oxide component. That has the advantage, that the areas that the metal oxide component, in particular the tin oxide, from one another largely through an oxide-free matrix are separated (they "swim" as it were in one oxide-free matrix) so that they undergo plastic deformation when rolling or extruding the Semi-finished goods much less than in the case of more or less evenly over the whole Distributed metal oxides material. In contrast stands out the semi-finished product according to the invention through improved ductility. However, this is not bought by an increased one Tendency to sweat or due to a reduced service life or by an increased electrical Contact resistance.
Dieses überraschend günstige Verhalten des erfindungsgemäß hergestellten Kontaktwerkstoffs hat seine Ursache vermutlich darin, dass sich der Kontaktwerkstoff von bekannten Kontaktwerkstoffen auf Silber-Zinnoxid-Basis nicht durch einen veränderten Gesamtoxidgehalt auszeichnet, sondern dadurch, dass dieser Gesamtoxidgehalt auf neuartige Weise im Werkstoff verteilt worden ist, nämlich so, dass Bereiche mit honer Metalloxidkonzentration im Material der ersten Komponente abwechseln mit Bereichen geringer oder verschwindender Metalloxidkonzentration im Matenal der ersten Komponente, wobei wegen der pulvermetallurgischen Herstellung die Größe dieser Bereiche von der Größe der Pulverteilchen abhängt, aus denen der Verbundwerkstoff hergestellt wird. In den Bereichen des Verbundwerkstoffes, in denen die Metalloxidkomponente vorliegt, soll sie erfindungsgemäß in sehr feiner Verteilung vorliegen. Der Gesamtgehalt der Metalloxidkomponente im Halbzeug kann und soll im üblichen Rahmen zwischen 5 und 25 Gew.-% liegen.This surprisingly favorable behavior of the Contact material produced according to the invention is probably due to the fact that the Contact material from known contact materials on silver-tin oxide basis not by an altered Overall oxide content, but by that this total oxide content is due to novel Distributed in the material, namely, that areas with honer metal oxide concentration in Alternate material of the first component with Areas of low or vanishing metal oxide concentration in the material of the first component, being because of the powder metallurgical manufacture the size of these areas by the size depends on the powder particles that make up the composite will be produced. In the fields of of the composite in which the metal oxide component is present, it should according to the invention in very fine distribution. The total salary the metal oxide component in the semifinished product can and should be in the usual range between 5 and 25% by weight lie.
Wenn auch bevorzugt wird, die gesamte Metalloxidkomponente in dem einen Verbundpulver zu konzentrieren mit der Folge, dass man im Halbzeug Bereiche hat, die von Metalloxiden völlig frei sind und deshalb die Verformbarkeit des Halbzeugs besonders gut wird, ist es doch möglich, einen geringen Metalloxidanteil in dem zweiten Pulver unterzubringen, welches den größten Teil des Silbers bzw. der Silberlegierung enthält; in diesem zweiten Pulver, bei dem es sich um ein Verbundpulver oder um eine Pulvermischung handeln kann, sollte der Gehalt des Zinnoxids und der ggfs. vorgesehenen weiteren Oxide zusammengenommen 3 Gew.-% (bezogen auf das Gewicht dieses zweiten Pulvers) nicht überschreiten. Dieser Anteil könnte einzeln zugegeben werden oder auch als Verbundpulver.Whilst it is preferred, the entire metal oxide component in which a composite powder concentrate with the result that you are in the semi-finished product Has areas that are completely free of metal oxides and therefore the deformability of the semi-finished product becomes particularly good, it is possible a small amount of metal oxide in the second powder to accommodate most of the Contains silver or the silver alloy; in this second powder, which is a composite powder or can be a powder mixture, the content of the tin oxide and, if applicable, the further oxides taken together 3 % By weight (based on the weight of this second Powder). This share could can be added individually or as a composite powder.
Überraschenderweise hat sich gezeigt, dass aus erfindungsgemäßem Halbzeug hergestellte Kontaktstücke gegenüber auf herkömmliche Weise hergestellten Kontaktstücken gleicher Zusammensetzung einen geringeren elektrischen Kontaktübergangswiderstand aufweisen und damit eine geringere Kontakterwärmung zeigen, was ein weiterer wesentlicher Vorteil der Erfindung ist. Vermutlich hängt das damit zusammen, dass sich bei erfindungsgemäßen Kontaktstücken das Zinnoxid weniger stark an der kontaktgebenden Oberfläche anreichert, wobei der nur bereichsweise hohe, feindispers verteilte, Zinnoxidgehalt für das Schaltverhalten günstig ist, z.B. eine nur geringe Verschweißneigung zur Folge hat.Surprisingly, it has been shown that produced from the semi-finished product according to the invention Contact pieces opposite in a conventional way manufactured contact pieces of the same composition a lower electrical contact resistance have and thus a lower Contact heating show what another essential advantage of the invention is. Probably this is related to the fact that the invention The tin oxide contacts less accumulates strongly on the contact surface, whereby the only partially high, finely dispersed distributed, tin oxide content for switching behavior is cheap, e.g. only a slight tendency to weld has the consequence.
Ausserdem hat sich gezeigt, dass aus erfindungsgemäßem Halbzeug hergestellte Kontakte einen geringeren Abbrand erleiden als auf herkömmliche Weise hergestellte Kontaktstücke gleicher Zusammensetzung. Die Lebensdauer auf der Grundlage der AC3- und AC4-Prüfungen ist höher als bei vergleichbaren AgCdO-Kontakten.It has also been shown that from the inventive Semi-finished contacts one suffer less burnout than conventional ones Wise manufactured contact pieces of the same composition. The lifespan based the AC3 and AC4 tests is higher than at comparable AgCdO contacts.
Auch das ist ein Vorteil der Erfindung.This is also an advantage of the invention.
Um zu der erfindungsgemäßen Werktoffstruktur mit den oxidarmen und oxidreichen Bereichen zu kommen, muss der überwiegende Teil der Metalloxidkomponente in dem Verbundpulver konzentriert und eingebunden werden. Nur der relativ kleine Metalloxidanteil, der gegebenenfalls noch in den oxidarmen Bereichen des Verbundwerkstoffs enthalten sein kann, kann z.B. in Form eines reinen Oxidpulvers mit dem Pulver aus der ersten Komponente des Werkstofts vermischt werden. Dabei wird es bevorzugt, dass in den oxidarmen Bereichen dieselben Oxide vorliegen, wie in den oxidreichen Bereichen. Die im Rahmen der zweiten Komponente gegebenenfalls noch vorhandenen Metallkarbide (vor allem Wolframkarbid und/oder Molybdänkarbid) und die in der ersten Komponente nicht gelösten Metalle (vor allem Wolfram und/oder Molybdän) können der Pulvermischung in Form gesonderter Pulver zugegeben werden; sie sind geeignet, im Schaltbetrieb die Benetzung des Zinnoxids mit Silber zu fördern und dadurch den Kontaktüberangswiderstand zu erniedrigen.To the working material structure according to the invention with the low-oxide and high-oxide areas the majority of the metal oxide component must come concentrated in the composite powder and be involved. Only the relatively small one Metal oxide content, which may still be in the contain low-oxide areas of the composite material can be e.g. in the form of a pure Oxide powder with the powder from the first component of the material are mixed. Doing so it prefers that in the low-oxide areas the same oxides are present as in the oxide-rich ones Areas. The part of the second component any metal carbides still present (especially tungsten carbide and / or molybdenum carbide) and those not solved in the first component Metals (especially tungsten and / or molybdenum) can separate the powder mixture in the form Powders are added; they are suitable in switching operation the wetting of the tin oxide with To promote silver and thereby the contact resistance to humiliate.
Das Verbundpulver wird dadurch hergestellt, dass man eine wässrige Lösung von Salzen der Metalle der ersten Komponente und von Zinn in einer Heißen, oxidierenden Atmosphäre versprüht und so die Salze pyrolytisch zersetzt. Das auch als Sprühpyrolyse bezeichnete Verfahren ist z.B. in der US-A 3 510 291, in der EP-0 012 202 A1 sowie in der DE-29 29 630C2 beschrieben. Dabei werden für das Verbundpulver vorgesehene Metalle in einer Flüssigkeit gelöst und die Lösung in einem Heißen Reaktor oder in eine Flamme hinein zerstäubt, so dass das Lösungsmittel schlagartig verdampft. Die dabei entstehenden Feststoffpartikel reagieren mit dem Sauerstoff in der oxidierenden Atmosphäre in der Flamme bzw. im Reaktor bei einer Temperatur unterhalb der Schmelztemperatur der gelösten Metalle, wobei Pulverteilchen entstehen, in denen die Metalle der ersten Komponente, also das Silber oder die Silberlegierung, und die Metalloxidkomponente, also im wesentlichen das Zinnoxid, in sehr feiner Verteilung aneinander gebunden vorliegen. In dem durch die Sprühpyrolyse erzeugten Verbundpulver liegen die Metalloxidteilchen zumeist in Größen zwischen 0,1 µm und 1 µm (Durchmesser) vor, was für das erfindungsgemäße Verfahren vorteilhaft ist. Das Vorhandensein derartig feiner Metalloxidteilchen begünstigt das Ausbilden der erwünschten Eigenschaften der Kontaktstücke (niedriger Abbrand, geringe Verschweißneigung, gleichbleibend geringer Kontaktübergangswiderstand) insbesondere, wenn diese Oxidkomponente im Verbund mit einem elektrisch gut leitenden Material (erste Komponente) vorliegt, was erfindungsgemäß der Fall ist.The composite powder is produced by that you have an aqueous solution of salts of the metals of the first component and tin in a hot, oxidizing atmosphere sprayed and so the salts pyrolytic decomposes. This is also known as spray pyrolysis The procedure is e.g. in US-A 3,510,291, in EP-0 012 202 A1 and in DE-29 29 630C2 described. Doing so for the composite powder intended metals dissolved in a liquid and the solution in a hot reactor or in a Flame atomized into it, leaving the solvent evaporated suddenly. The resulting Solid particles react with the oxygen in the oxidizing atmosphere in the flame or in the reactor at a temperature below that Melting temperature of the dissolved metals, whereby Powder particles arise in which the metals of the first component, i.e. the silver or the silver alloy, and the metal oxide component, so essentially tin oxide, in a very fine distribution tied together. In the through the spray pyrolysis produced composite powder the metal oxide particles mostly in sizes between 0.1 µm and 1 µm (diameter) before what for the inventive method is advantageous. The Presence of such fine metal oxide particles favors the formation of the desired properties the contact pieces (low erosion, low Welding tendency, consistently lower Contact resistance) especially if this oxide component in combination with an electrical highly conductive material (first component) is present, which is the case according to the invention.
Die Verwendung sprühpyrolytisch hergestellter Verbundpulver ist auch deshalb vorteilhaft, weil durch die Sprühpyrolyse insbesondere Pulverteilchen entstehen, die eine kugelige oder kartoffelförmige Gestalt haben, die das Entstehen eines verformbaren Halbzeuges begünstigt, weil sich die kugeligen bzw. kartoffelförmigen Partikel einer plastischen Verformung des Kontaktwerkstoffs weniger widersetzen als unregelmässig gezackte Pulverteilchen.The use of spray pyrolysis Compound powder is also advantageous because by spray pyrolysis, in particular powder particles arise that are spherical or potato-shaped Have shape, the emergence of a deformable Semi-finished products favored because the spherical or potato-shaped particles of a plastic Deformation of the contact material less oppose as irregularly jagged powder particles.
Die gegebenenfalls zusätzlich zum Zinnoxid vorgesehenen oxidischen und karbidischen Bestandteile bewirken teils eine Erniedrigung der Kontaktstellentemperatur im Schaltbetrieb und teils eine Verlängerung der Lebensdauer der Kontaktstücke nicht nur bei kleiner und mittlerer Strombelastung, sondern auch im Schwerlastbereich. Molybdänkarbid und Wolframkarbid wirken schon in geringen Mengen. Die zusätzlichen Karbide und Oxide sollten einen Anteil von 6 Gew.-% am Kontaktwerkstoff nicht überschreiten, damit dieser nicht zu hart wird.The possibly in addition to the tin oxide provided oxidic and carbidic components sometimes lower the contact point temperature in switching operation and partly an extension of the life of the contact pieces not only with small and medium current loads, but also in the heavy load area. Molybdenum carbide and tungsten carbide already work in small amounts. The additional carbides and Oxides should make up 6% by weight of the contact material not exceed so this does not gets too hard.
Vorteilhaft kann auch ein Zusatz von Nickel zum Verbundwerkstoff sein, welches in Silber nicht löslich ist und entweder als sehr feines Pulver mit dem aus Silber bzw. einer Silberlegierung gebildeten Pulver vermischt wird oder aber ebenfalls als sprühpyrolytisch hergestelltes Silber-Nickelpulver eingebracht wird.The addition of nickel can also be advantageous to the composite material, which is not in silver is soluble and either as a very fine powder that formed from silver or a silver alloy Powder is mixed or also as spray-pyrolysized silver-nickel powder is introduced.
Ein Silber-Zinnoxid-Verbundpulver mit 32 Gew.-% Zinnoxid wird hergestellt durch Versprühen einer wässrigen Lösung von Silbernitrat und Zinn-ll-Chlorid in einem auf ca. 950°C aufgeheizten Reaktor mit sauerstoffhaltiger Atmosphäre, wobei ein Silber-Zinnoxid-Verbundpulver ausfällt, in dessen Pulverteilchen das Zinnoxid in sehr feiner Verteilung vorliegt. Anschließend werden 75 Gew.-Teile eines Silberpulvers mit einer Teilchengröße kleiner als 40 µm mit 25 Gew.-Teilen des Silber-Zinnoxid, Verbundpulvers eine Stunde lang trocken gemischt, anschließend isostatisch zu Blöcken von ca. 50 kg Gewicht gepreßt und danach bei einer Temperatur von 830° C 1,5 Stunden lang gesintert. Der so gebildete Block wird in den Rezipienten einer Strangpresse gelegt und unter Querschnittsverminderung zu einem Strang mit einem Querschnitt von 10 x 75 mm2 heiß, bei einer Temperatur von ca. 850° C, stranggepreßt, anschließend mit einem 1,5 mm dicken Feinsilberblech warmwalzplattiert, warm auf seine Enddicke von 2mm herabgewalzt und nach üblichen Verfahren zu Kontaktplättchen weiterverarbeitet. Der Silber-Zinnoxid-Verbundwerkstoff in den Kontaktplättchen hat einen Zinnoxidgehalt von 8 Gew.-%.A silver-tin oxide composite powder with 32% by weight of tin oxide is produced by spraying an aqueous solution of silver nitrate and tin-II-chloride in a reactor heated to approx. 950 ° C. with an oxygen-containing atmosphere, whereby a silver-tin oxide composite powder fails , in the powder particles of which the tin oxide is present in a very fine distribution. Then 75 parts by weight of a silver powder with a particle size smaller than 40 microns with 25 parts by weight of the silver-tin oxide, composite powder are dry mixed for one hour, then isostatically pressed to blocks of about 50 kg in weight and then at a temperature sintered at 830 ° C for 1.5 hours. The block thus formed is placed in the recipient of an extrusion press and hot-extruded, reducing the cross-section to a strand with a cross-section of 10 x 75 mm 2 , at a temperature of approx. 850 ° C., and then hot-rolled plated with a 1.5 mm thick fine silver sheet , hot rolled down to its final thickness of 2mm and processed into contact wafers according to the usual methods. The silver-tin oxide composite in the contact wafers has a tin oxide content of 8% by weight.
Das zweite Beispiel wird dahingehend abgewandelt, dass der Pulvermischung noch 0,5 Gew.-% Wolframoxid (Teilchengröße kleiner als 10 µm) und 0,3 Gew.-% Wolframkarbid (Teilchengröße kleiner als 2,5 µm) zugegeben werden. Im übrigen wird wie im ersten Beispiel verfahren. Die Zugabe des Wolframoxids und Wolframkarbids führt zu einer Absenkung der Kontaktstellentemperatur und zu einer verlängerten Lebensdauer von aus dem Halbzeug hergestellten elektrischen Kontaktstükken.The second example is modified that the powder mixture still contains 0.5% by weight Tungsten oxide (particle size smaller than 10 µm) and 0.3 wt% tungsten carbide (particle size smaller than 2.5 µm) are added. Furthermore is operated as in the first example. The addition of tungsten oxide and tungsten carbide leads to one Lowering the contact point temperature and to an extended lifespan from the Semi-finished electrical contact pieces.
Ein Silber-Zinnoxid-Wolframoxid-Verbundpulver mit 20 Gew.-% Zinnoxid und 0,5 Gew.-% Wolframoxid wird hergestellt durch Versprühen einer wässrigen Lösung von Silbernitrat, Zinn-II-Chlorid und Wolfram-ll-Chlorid in einem auf ca. 950 °C aufgeheizten Reaktor mit sauerstoffhaltiger Atmosphäre, wobei ein Silber-Zinnoxid-Wolframoxid-Verbundpulver ausfällt, in dessen Pulverteilchen das Zinnoxid und das Wolframoxid in sehr feiner Verteilung vorliegen. Anschließend werden 50 Gew.-Teile eines Silberpulvers mit einer Teilchengröße kleiner als 40 µm mit 50 Gew.-Teilen des Silber-Zinnoxid-Wolframoxid-Verbundpulvers eine Stunde lang trocken gemischt und wie im 1. Beispiel zu Kontaktplättchen weiterverarbeitet.A silver-tin oxide-tungsten oxide composite powder with 20% by weight of tin oxide and 0.5% by weight of tungsten oxide is made by spraying an aqueous Solution of silver nitrate, tin-II-chloride and Tungsten ll chloride in a heated to approx. 950 ° C Reactor with an oxygen-containing atmosphere, being a silver-tin oxide-tungsten oxide composite powder precipitates, in the powder particles of the tin oxide and the tungsten oxide is in a very fine distribution. Then 50 parts by weight of a Silver powder with a particle size smaller than 40 µm with 50 parts by weight of the silver-tin oxide-tungsten oxide composite powder dry for an hour mixed and as in the 1st example for contact plates processed further.
Ein Silber-Zinnoxid-Verbundpulver mit 30 Gew.-% Zinnoxid wird hergestellt wie im 2. Beispiel. Ein Silber-Nickel-Verbundpulver mit 2 Gew.-% Nickel wird hergestellt durch Versprühen einer wässrigen Lösung von Silbernitrat und Nickel-II-Chlorid in einem auf ca. 950 °C aufgeheizten Reaktor mit Schutzgasatmosphäre (z.B. Argon), wobei ein Silber-Nickel-Verbundpulver ausfällt, in dessen Pulverteilchen das Nickel in sehr feiner Verteilung vorliegt.A silver-tin oxide composite powder with 30 % By weight of tin oxide is produced as in the second example. A silver-nickel composite powder with 2 wt .-% Nickel is made by spraying one aqueous solution of silver nitrate and nickel-II-chloride in a reactor heated to approx. 950 ° C with a protective gas atmosphere (e.g. argon), whereby a silver-nickel composite powder fails, in the Powder particles the nickel in a very fine distribution is present.
Anschließend werden 50 Gew.-Teile des Silber-Zinnoxid-Verbundpulvers und 50 Gew.-Teile des Silber-Nickel-Verbundpulvers eine Stunde lang trocken gemischt und wie im ersten Beispiel zu Kontaktplättchen weiterverarbeitet.Then 50 parts by weight of the silver-tin oxide composite powder and 50 parts by weight of the Silver-nickel composite powder for one hour mixed dry and as in the first example too Contact plates processed further.
Das 4. Beispiel kann dahingehend abgewandelt werden, dass anstelle eines Silber-Nickel-Verbundpulvers ein Silber-Pulver und ein Carbonyl-Nickel-Pulver mit dem Silber-Zinnoxid-Verbundpulver gemischt werden. Im übrigen wird wie im 4. Beispiel verfahren.The fourth example can be modified accordingly be that instead of a silver-nickel composite powder a silver powder and a carbonyl nickel powder mixed with the silver-tin oxide composite powder will. Otherwise the same as in the 4th example method.
Die beigefügte Figur zeigt schematisch den
Gefügeaufbau eines gemäß dem zweiten Beispiel
hergestellten Verbundwerkstoffs, in welchem Silber-Zinnoxidbereiche
1, die zumeist kleiner als 50
µm sind, in einer Silbermatrix liegen, die aus den
oxidfreien Silberpulverteilchen hervorgegangen ist.The attached figure shows schematically the
Structure of one according to the second example
manufactured composite, in which silver-
Erfindungsgemäß hergestellte Halbzeuge eignen sich besonders für Kontaktstücke in Niederspannungsscnaltgeräten, z.B. in Motorschützen.Semi-finished products produced according to the invention are suitable are particularly suitable for contact pieces in low-voltage switching devices, e.g. in motor contactors.
Claims (28)
- A powder-metallurgical process of producing a semi-finished product made of silver and tin oxide for use in electric contacts, consisting of a composite material which consists of 60 to 95 % by weight of a first component having a high electric conductivity, namely, silver or an alloy mainly consisting of silver,whereas the remainder consists of a second component, which is insoluble in the first component and decreases the tendency to exhibit contact welding and the contact burn-off and consists based on the weight of the composite material of 3 to 25 % by weight tin oxide, 0 to 10 % by weight of one or more further metal oxides which together with the tin oxide will be described hereinafter as the metal oxide component, 0 to 10 % by weight of one or more metal carbides and 0 to 10 % by weight of one or more further metals, which are insoluble in the first component,wherein the tin oxide predominates in the second component and the average content of the metal oxide component is not in excess of 25 % by weight of the composite material, whereina composite powder which is produced in that a solution of salts of metals of the first component and of a salt of tin is sprayed into a hot oxidizing atmosphere, in which the salts are pyrolytically decomposed, and which contains less than one-half of the first component and 60 to 100 % based on the metal oxide component of the metal oxide componentis mixed with one or more powders which contain the remainder of the first component and of the second component andthe powder mixture is compacted to form shaped pieces consisting of the composite material.
- A process according to claim 1, characterized in that the shaped bodies are subsequently sintered.
- A process according to claim 1 or 2, characterized in that the shaped bodies are subsequently deformed by coining extruding or by extruding followed by rolling.
- A process according to claim 1, characterized in that the entire metal oxide component is incorporated in the composite powder.
- A process according to claim 4, characterized in that the entire second component is incorporated in the composite powder.
- A process according to claim 1 or 4, characterized in that the further metal oxides in pulverulent form are mixed with the powder of the first component and with the composite powder of the second component.
- A process according to claim 1 or 4, characterized in that the metal carbides in pulverulent form are mixed with the powder of the first component and with the composite powder of the second component.
- A process according to claim 1 or 4, characterized in that the further metals of the second component in pulverulent form are mixed with the powder of the first component and with the composite powder of the second component.
- A process according to claim 1, characterized in that the solution contains also salts of the further oxidizable metals.
- A process according to claim 9, characterized in that the solution contains salts of all oxidizable metals which are intended for the second component.
- A process according to any of the preceding claims, characterized in that the composite powder is not in excess of 45 % by volume of the powder mixture.
- A semi-finished product produced by a process according to claim 1,which is made of silver and tin oxide and intended for use in the manufacture of electric contacts, consisting of a composite material that consists of 60 to 95 % by weight of a first component having a high electrical conductivity, namely of silver or an alloy which mainly contains silver, and 40 to 5 % by weight of a second component, which is distributed but insoluble in the first component and reduces the tendency to exhibit contact welding and burn-off and which based on the weight of the composite material contains 3 to 25 % by weight of tin oxide, 0 to 10 % by weight of one or more further metal oxides which together with the tin oxide will be described hereinafter as the metal oxide component, 0 to 10 by weight of one or more metal carbides and 0 to 10 % by weight of one or more further metals, which are insoluble in the first component,
wherein the tin oxide predominates in the second component and the average content of the metal oxide component is not in excess of 25 % by weight of the composite material,
characterized in that the structure of the composite material comprises low-oxide regions, in which the content of the metal oxide component is 0 to 20 % of its average content and is present in a fine distribution in a matrix consisting of the material of the first component, in alternation with high-oxide regions comprising the metal oxide component in an amount of 1.5 to 6 times its average content as averaged over the semi-finished product and the remainder of the first component finely distributed one into another,
wherein the low-oxide regions and the high-oxide regions are present in the composite material in a statistically uniform distribution and a substantial part of the high-oxide regions is surrounded by the low-oxide regions. - A semi-finished product according to claim 12, characterized in that the low-oxide regions occupy at least 40 % by volume of the composite material and the high-oxide regions occupy the remainder of the volume of the composite material.
- A semi-finished product according to claim 13, characterized in that the low-oxide regions occupy at least 55 % by volume of the composite material.
- A semi-finished product according to any of claims 12 to 14, characterized in that the metal oxide component has the same composition in the low-oxide regions and in the high-oxide regions.
- A semi-finished product according to any of claims 12 to 14, characterized in that the entire metal oxide component is concentrated in the high-oxide regions.
- A semi-finished product according to claim 16, characterized in that the entire second component is concentrated in the high-oxide regions.
- A semi-finished product according to any of claims 12 to 17, characterized in that the high-oxide regions are smaller than 500 x 10-6 mm3.
- A semi-finished product according to claim 18, characterized in that the high-oxide regions are smaller than 35 x 10-6 mm3.
- A semi-finished product according to any of claims 12 to 19, characterized in that the first component consists of fine silve
- A semi-finished product according to any of claims 12 to 19, characterized in that the first component is an alloy of silver and 0.1 to 10 % by weight of copper.
- A semi-finished product according to any of claims 12 to 19, characterized in that the first component is an alloy of silver and 0.1 to 10 % by weight of palladium.
- A semi-finished product according to any of claims 12 to 21, charcterized in that the second component contains a refractory metal in an amount of 0.1 to 10 % by weight of the entire composite material.
- A semi-finished product according to claim 23, characterized in that the refractory metal is tungsten or molybdenum.
- A semi-finished product according to any of claims 12 to 24, characterized in that the further metal oxides contained in the second component are selected from the group consisting of tungsten oxide, molybdenum oxide, vanadium oxide, bismuth oxide, bismuth titanate, and copper oxide.
- A semi-finished product according to any of claims 12 to 25, characterized in that the metal carbide contained in the second component is selected from the group comprising tungsten carbide and molybdenum carbide.
- A semi-finished product according to any of claims 12 to 26, characterized in that the composite material contains up to 10 % by weight of nickel.
- A semi-finished product according to claim 27, characterized in that the composite material contains less than 1 % by weight of nickel.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT89903734T ATE102387T1 (en) | 1988-03-26 | 1989-03-22 | SEMI-FINISHED PRODUCT FOR ELECTRICAL CONTACTS MADE OF A COMPOSITE MATERIAL BASED ON SILVER-TIN OXIDE AND POWDER METALLURGICAL PROCESS FOR ITS PRODUCTION. |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3810311 | 1988-03-26 | ||
| DE3810311 | 1988-03-26 | ||
| PCT/EP1989/000316 WO1989009478A1 (en) | 1988-03-26 | 1989-03-22 | Semifinished product for electrical contacts, made of a composite material based on silver and tin oxide, and powder metallurgical process for producing it |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0440620A1 EP0440620A1 (en) | 1991-08-14 |
| EP0440620B1 EP0440620B1 (en) | 1994-03-02 |
| EP0440620B2 true EP0440620B2 (en) | 1998-06-03 |
Family
ID=6350773
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP89903734A Expired - Lifetime EP0440620B2 (en) | 1988-03-26 | 1989-03-22 | Semifinished product for electrical contacts, made of a composite material based on silver and tin oxide, and powder metallurgical process for producing it |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US5360673A (en) |
| EP (1) | EP0440620B2 (en) |
| JP (1) | JPH03504615A (en) |
| CN (1) | CN1022934C (en) |
| CA (1) | CA1339713C (en) |
| DD (1) | DD283571A5 (en) |
| DE (2) | DE58907140D1 (en) |
| ES (1) | ES2012293A6 (en) |
| WO (1) | WO1989009478A1 (en) |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ATE136394T1 (en) * | 1992-06-10 | 1996-04-15 | Duerrwaechter E Dr Doduco | MATERIAL FOR ELECTRICAL CONTACTS BASED ON SILVER-TIN OXIDE OR SILVER-ZINC OXIDE |
| DE4220925C2 (en) * | 1992-06-25 | 1996-05-02 | Siemens Ag | Process for the production of moldings provided with electrical contacts from high-temperature superconducting material (HTSL) and moldings produced by this process |
| JP3441074B2 (en) * | 1992-09-16 | 2003-08-25 | ドドウコ・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング・ウント・コンパニー・ドクトル・オイゲン・デュルベヒテル | Member for electrical contact based on silver-tin oxide or silver-zinc oxide and method for producing the same |
| US5608766A (en) * | 1993-10-29 | 1997-03-04 | General Electric Company | Co-deposition of palladium during oxide film growth in high-temperature water to mitigate stress corrosion cracking |
| US5846288A (en) * | 1995-11-27 | 1998-12-08 | Chemet Corporation | Electrically conductive material and method for making |
| FR2916082B1 (en) * | 2007-05-11 | 2009-06-12 | Schneider Electric Ind Sas | METHOD FOR MANUFACTURING MATERIAL FOR AN ELECTRICAL CONTACT PASTILLE, CONTACT PASTILLE PRODUCED BY SUCH A METHOD |
| CN100552845C (en) * | 2007-09-27 | 2009-10-21 | 天津大学 | Silver-based tin oxide gradient electric contact material and preparation method |
| US8968608B2 (en) * | 2008-01-17 | 2015-03-03 | Nichia Corporation | Method for producing conductive material, conductive material obtained by the method, electronic device containing the conductive material, light-emitting device, and method for producing light-emitting device |
| DE102008056264A1 (en) * | 2008-11-06 | 2010-05-27 | Ami Doduco Gmbh | Process for producing a semifinished product and semifinished product for electrical contacts and contact piece |
| DE102008056263A1 (en) * | 2008-11-06 | 2010-05-27 | Ami Doduco Gmbh | Process for producing a semifinished product and semifinished product for electrical contacts and contact piece |
| CN102074278B (en) * | 2010-12-09 | 2011-12-28 | 温州宏丰电工合金股份有限公司 | Preparation method of particle-aligned reinforced silver based contact material |
| CN102142325B (en) * | 2010-12-30 | 2013-04-03 | 温州宏丰电工合金股份有限公司 | Preparation method of particle direction-arrangement enhanced silver-based oxide electrical contact material |
| CN105374598A (en) * | 2015-11-05 | 2016-03-02 | 福达合金材料股份有限公司 | Manufacturing method for coarse oxide particle silver-based electric contact materials |
| CN106350692B (en) * | 2016-09-23 | 2018-04-03 | 佛山市诺普材料科技有限公司 | A kind of method that silver-colored nickel oxide is prepared using silver-nickel waste material |
| JP7084730B2 (en) * | 2017-02-01 | 2022-06-15 | Dowaエレクトロニクス株式会社 | Silver alloy powder and its manufacturing method |
| CN111961910B (en) * | 2020-07-24 | 2022-07-12 | 浙江耐迩合金科技有限公司 | Preparation method of silver tin oxide electric contact material |
| CN111961911B (en) * | 2020-07-24 | 2022-04-26 | 浙江耐迩合金科技有限公司 | Preparation method of silver-based electric contact material with high fusion welding resistance |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2486341A (en) * | 1945-06-30 | 1949-10-25 | Baker & Co Inc | Electrical contact element containing tin oxide |
| US3148981A (en) * | 1961-04-21 | 1964-09-15 | Nat Beryllia Corp | Metal-oxide gradient ceramic bodies |
| DE1564713A1 (en) * | 1966-09-20 | 1970-10-22 | Siemens Ag | Multi-layer sintered contact body |
| US3827883A (en) * | 1972-10-24 | 1974-08-06 | Mallory & Co Inc P R | Electrical contact material |
| CH588152A5 (en) * | 1972-12-11 | 1977-05-31 | Siemens Ag | |
| GB1461176A (en) * | 1974-04-11 | 1977-01-13 | Plessey Inc | Method of producing powdered materials |
| DE2659012C3 (en) * | 1976-12-27 | 1980-01-24 | Siemens Ag, 1000 Berlin Und 8000 Muenchen | Process for producing a sintered contact material from silver and embedded metal oxides |
| JPS5553017A (en) * | 1978-10-16 | 1980-04-18 | Nippon Mining Co | Method of manufacturing multiple coating composite powder |
| DE2952128C2 (en) * | 1979-12-22 | 1984-10-11 | Degussa Ag, 6000 Frankfurt | Process for the pretreatment of the powder for sintered and extruded semifinished products made of silver-tin oxide for electrical contacts |
| DE3146972A1 (en) * | 1981-11-26 | 1983-06-01 | Siemens AG, 1000 Berlin und 8000 München | METHOD FOR PRODUCING MOLDED PARTS FROM CADMIUM-FREE SILVER METAL OXIDE COMPOSITIONS FOR ELECTRICAL CONTACTS |
| DE3212005C2 (en) * | 1982-03-31 | 1986-05-28 | Siemens AG, 1000 Berlin und 8000 München | Process for the production of a two-layer sintered contact piece on the basis of silver and copper |
| US4426356A (en) * | 1982-09-30 | 1984-01-17 | E. I. Du Pont De Nemours And Company | Method for making capacitors with noble metal electrodes |
| DE3304637A1 (en) * | 1983-02-10 | 1984-08-16 | Siemens AG, 1000 Berlin und 8000 München | SINTER CONTACT MATERIAL FOR LOW VOLTAGE SWITCHGEAR |
| DE3305270A1 (en) * | 1983-02-16 | 1984-08-16 | Siemens AG, 1000 Berlin und 8000 München | SINTER COMPOSITE FOR ELECTRICAL CONTACTS AND METHOD FOR THE PRODUCTION THEREOF |
| US4479892A (en) * | 1983-05-16 | 1984-10-30 | Chugai Denki Kogyo K.K. | Ag-Metal oxides electrical contact materials |
| DE3421758A1 (en) * | 1984-06-12 | 1985-12-12 | Siemens AG, 1000 Berlin und 8000 München | SINTER CONTACT MATERIAL FOR LOW VOLTAGE SWITCHGEAR IN ENERGY TECHNOLOGY AND METHOD FOR THE PRODUCTION THEREOF |
| US4680162A (en) * | 1984-12-11 | 1987-07-14 | Chugai Denki Kogyo K.K. | Method for preparing Ag-SnO system alloy electrical contact material |
| IN165226B (en) * | 1985-08-30 | 1989-09-02 | Chugai Electric Ind Co Ltd | |
| US4622269A (en) * | 1985-12-30 | 1986-11-11 | Gte Products Corporation | Electrical contact and process for making the same |
| US4954170A (en) * | 1989-06-30 | 1990-09-04 | Westinghouse Electric Corp. | Methods of making high performance compacts and products |
-
1989
- 1989-03-22 DE DE89903734T patent/DE58907140D1/en not_active Expired - Lifetime
- 1989-03-22 EP EP89903734A patent/EP0440620B2/en not_active Expired - Lifetime
- 1989-03-22 WO PCT/EP1989/000316 patent/WO1989009478A1/en active IP Right Grant
- 1989-03-22 DE DE3909384A patent/DE3909384A1/en not_active Withdrawn
- 1989-03-22 US US07/549,015 patent/US5360673A/en not_active Expired - Lifetime
- 1989-03-22 JP JP1503432A patent/JPH03504615A/en active Pending
- 1989-03-22 ES ES8901059A patent/ES2012293A6/en not_active Expired - Lifetime
- 1989-03-23 DD DD89326856A patent/DD283571A5/en not_active IP Right Cessation
- 1989-03-23 CA CA000594639A patent/CA1339713C/en not_active Expired - Fee Related
- 1989-03-27 CN CN89101699A patent/CN1022934C/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| CN1036991A (en) | 1989-11-08 |
| DE3909384A1 (en) | 1989-10-19 |
| CA1339713C (en) | 1998-03-17 |
| ES2012293A6 (en) | 1990-03-01 |
| US5360673A (en) | 1994-11-01 |
| WO1989009478A1 (en) | 1989-10-05 |
| EP0440620B1 (en) | 1994-03-02 |
| CN1022934C (en) | 1993-12-01 |
| DD283571A5 (en) | 1990-10-17 |
| JPH03504615A (en) | 1991-10-09 |
| DE58907140D1 (en) | 1994-04-07 |
| EP0440620A1 (en) | 1991-08-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0440620B2 (en) | Semifinished product for electrical contacts, made of a composite material based on silver and tin oxide, and powder metallurgical process for producing it | |
| DE69032065T2 (en) | Composite of silver and metal oxide and method of manufacturing the same | |
| DE69123183T2 (en) | Composite material made of silver or silver-copper alloy with metal oxides and process for its production | |
| DE1125459C2 (en) | Process for producing alloyed iron-based powder for powder metallurgical purposes | |
| DE2822956C2 (en) | Process for the production of switching contacts for a vacuum switch | |
| DE19535814C2 (en) | Material for making electrical contacts based on silver | |
| DE2011002C3 (en) | Internally oxidized contact material on the basis of silver-cadmium oxide produced by melt metallurgy | |
| EP0725154B1 (en) | Sintered material based on silver-tinoxide for electrical contacts and process for its production | |
| DE2446698A1 (en) | TWO-LAYER SINTER CONTACT PIECE FOR ELECTRIC SWITCHGEAR | |
| DE3911904A1 (en) | Powder-metallurgical process for producing a semifinished product for electric contacts from a silver-based composite with iron | |
| EP0660964B2 (en) | Material for electric contacts based on silver-tin oxide or silver-zinc oxide and process for its production | |
| DE807416C (en) | Electrical contact material and process for its manufacture | |
| EP0338401B1 (en) | Powder-metallurgical process for the production of a semi-finished product for electrical contacts made from a composite material based on silver and iron | |
| DE2824117A1 (en) | PROCESS FOR MANUFACTURING ANISOTROPIC SINTER COMPOSITE MATERIAL WITH ORIENTATIONAL STRUCTURE | |
| DE19916082C2 (en) | Composite material produced by powder metallurgy, process for its production and its use | |
| DE4319137A1 (en) | Material for electrical contacts consisting of silver@ or silver@-alloy matrix - incorporate tin oxide and other oxide(s) and carbide(s), has longer service life but is less brittle than other materials | |
| DE2102996A1 (en) | Method for producing a two-layer sintered contact piece | |
| DE1938548A1 (en) | Electrodes for resistance welding or ferrous matls - of ferrous materials | |
| DE3232627C2 (en) | ||
| DE19523922A1 (en) | Electrical contact material prodn. for low and middle voltage applications | |
| EP0311134B1 (en) | Powder-metallurgically produced electrical contact material comprising silver and graphite, and process for producing it | |
| EP0876670B1 (en) | Method of producing a shaped part from a silver-based contact material | |
| CH618044A5 (en) | Body of composite material in web or sheet form and production process for it | |
| DE2260559C3 (en) | Method for producing a composite material for electrical contacts, in particular in high-voltage engineering | |
| DE1963860C (en) | Iron chromium sintered alloy and processes for the production of sintered molded parts from it |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19901212 |
|
| 17Q | First examination report despatched |
Effective date: 19930726 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19940302 Ref country code: SE Free format text: THE PATENT HAS BEEN ANNULLED BY A DECISION OF A NATIONAL AUTHORITY Effective date: 19940302 Ref country code: NL Effective date: 19940302 |
|
| REF | Corresponds to: |
Ref document number: 102387 Country of ref document: AT Date of ref document: 19940315 Kind code of ref document: T |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Effective date: 19940322 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19940331 |
|
| REF | Corresponds to: |
Ref document number: 58907140 Country of ref document: DE Date of ref document: 19940407 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19940408 |
|
| ITF | It: translation for a ep patent filed | ||
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PFA Free format text: DODUCO GMBH + CO. DR. EUGEN DUERRWAECHTER |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: DODUCO GMBH + CO DR. EUGEN DUERRWAECHTER |
|
| ET | Fr: translation filed | ||
| NLV1 | Nl: lapsed or annulled due to failure to fulfill the requirements of art. 29p and 29m of the patents act | ||
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| 26 | Opposition filed |
Opponent name: DEGUSSA AG, FRANKFURT - ZWEIGNIEDERLASSUNG WOLFGAN Effective date: 19941130 |
|
| PLAW | Interlocutory decision in opposition |
Free format text: ORIGINAL CODE: EPIDOS IDOP |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: DODUCO GMBH + CO DR. EUGEN DUERRWAECHTER I.K. |
|
| PLAW | Interlocutory decision in opposition |
Free format text: ORIGINAL CODE: EPIDOS IDOP |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19980226 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19980320 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19980331 Year of fee payment: 10 |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: DODUCO GMBH |
|
| 27A | Patent maintained in amended form |
Effective date: 19980603 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: AEN Free format text: AUFRECHTERHALTUNG DES PATENTES IN GEAENDERTER FORM |
|
| ET3 | Fr: translation filed ** decision concerning opposition | ||
| GBTA | Gb: translation of amended ep patent filed (gb section 77(6)(b)/1977) | ||
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990322 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990331 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990331 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19990322 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19991130 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20050322 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20080220 Year of fee payment: 20 |