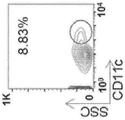
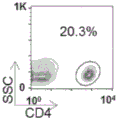
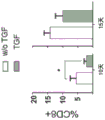
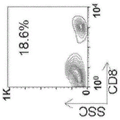
CN109069875B - 产生免疫耐受反应的组合物和方法 - Google Patents
产生免疫耐受反应的组合物和方法 Download PDFInfo
- Publication number
- CN109069875B CN109069875B CN201780015637.XA CN201780015637A CN109069875B CN 109069875 B CN109069875 B CN 109069875B CN 201780015637 A CN201780015637 A CN 201780015637A CN 109069875 B CN109069875 B CN 109069875B
- Authority
- CN
- China
- Prior art keywords
- tgf
- cells
- beta
- cell
- aftgf
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/51—Nanocapsules; Nanoparticles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
- A61K38/1841—Transforming growth factor [TGF]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/715—Polysaccharides, i.e. having more than five saccharide radicals attached to each other by glycosidic linkages; Derivatives thereof, e.g. ethers, esters
- A61K31/737—Sulfated polysaccharides, e.g. chondroitin sulfate, dermatan sulfate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
- A61K38/1858—Platelet-derived growth factor [PDGF]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
- A61K38/1858—Platelet-derived growth factor [PDGF]
- A61K38/1866—Vascular endothelial growth factor [VEGF]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/61—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule the organic macromolecular compound being a polysaccharide or a derivative thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/06—Ointments; Bases therefor; Other semi-solid forms, e.g. creams, sticks, gels
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0024—Solid, semi-solid or solidifying implants, which are implanted or injected in body tissue
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- General Chemical & Material Sciences (AREA)
- Diabetes (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Dermatology (AREA)
- Transplantation (AREA)
- Vascular Medicine (AREA)
- Endocrinology (AREA)
- Molecular Biology (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Emergency Medicine (AREA)
- Inorganic Chemistry (AREA)
- Biomedical Technology (AREA)
- Optics & Photonics (AREA)
- Nanotechnology (AREA)
- Physics & Mathematics (AREA)
- Neurosurgery (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662275827P | 2016-01-07 | 2016-01-07 | |
| US62/275,827 | 2016-01-07 | ||
| PCT/IL2017/050013 WO2017118979A1 (en) | 2016-01-07 | 2017-01-05 | Compositions and methods for generating immunotolerant responses |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN109069875A CN109069875A (zh) | 2018-12-21 |
| CN109069875B true CN109069875B (zh) | 2021-12-24 |
Family
ID=59273512
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201780015637.XA Expired - Fee Related CN109069875B (zh) | 2016-01-07 | 2017-01-05 | 产生免疫耐受反应的组合物和方法 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US11123404B2 (enExample) |
| EP (1) | EP3400074B1 (enExample) |
| JP (1) | JP7033066B2 (enExample) |
| KR (1) | KR20180104646A (enExample) |
| CN (1) | CN109069875B (enExample) |
| IL (1) | IL260436B2 (enExample) |
| WO (1) | WO2017118979A1 (enExample) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EA035413B1 (ru) | 2013-06-13 | 2020-06-10 | Ордженисис Лтд. | Популяции клеток, способы трансдифференцировки и способы их применения |
| MA41296A (fr) | 2014-12-30 | 2017-11-07 | Orgenesis Ltd | Procédés de transdifférenciation et procédés d'utilisation de ceux-ci |
| SG11201911256UA (en) | 2017-05-08 | 2020-01-30 | Orgenesis Ltd | Transdifferentiated cell populations and methods of use thereof |
| WO2019171377A1 (en) * | 2018-03-06 | 2019-09-12 | Orgenesis Inc | Three dimensional clusters of transdifferentiated cells, compositions and methods thereof |
| JP2022506154A (ja) * | 2018-11-01 | 2022-01-17 | バークレー ライツ,インコーポレイテッド | マイクロ流体環境において生体細胞をアッセイする方法 |
| CN109771638A (zh) * | 2018-12-28 | 2019-05-21 | 中国人民解放军陆军军医大学第一附属医院 | 一种免疫耐受剂 |
| WO2024080183A1 (ja) * | 2022-10-14 | 2024-04-18 | 国立大学法人東海国立大学機構 | 糖鎖複合体、薬物担体、及びドラッグデリバリーシステム |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007043050A2 (en) * | 2005-10-11 | 2007-04-19 | Ben-Gurion University Of The Negev Research And Development Authority | Bioconjugates comprising sulfated polysaccharides and their uses |
| CN103002915A (zh) * | 2010-04-13 | 2013-03-27 | 免疫创新治疗有限公司 | 抑制Treg细胞的方法和组合物 |
| US9125883B2 (en) * | 2004-06-14 | 2015-09-08 | Institut Francais De Recherche Pour L'exploitation De La Mer (Ifremer) | Sulfated depolymerized derivatives of exopolysaccharides (EPS) from mesophilic marine bacteria, method for preparing same, and uses thereof in tissue regeneration |
| CN105611937A (zh) * | 2013-06-18 | 2016-05-25 | 复制细胞生命科学公司 | 治疗皮肤的组合物和方法 |
Family Cites Families (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4178361A (en) | 1973-09-10 | 1979-12-11 | Union Corporation | Sustained release pharmaceutical composition |
| US5100668A (en) | 1988-06-14 | 1992-03-31 | Massachusetts Institute Of Technology | Controlled release systems containing heparin and growth factors |
| IL118376A0 (en) | 1996-05-22 | 1996-09-12 | Univ Ben Gurion | Polysaccharide sponges for cell culture and transplantation |
| GB9722604D0 (en) | 1997-10-28 | 1997-12-24 | Cancer Res Campaign Tech | Heparin-binding growth factor derivatives |
| US6388060B1 (en) | 1998-11-06 | 2002-05-14 | Vascular Therapeutics Inc. | Process for the sulfation of uronic acid-containing polysaccharides |
| JP2002542301A (ja) | 1999-04-22 | 2002-12-10 | アイトゲネシッシェ、テヒニッシェ、ホッホシューレ(エーテーハー) | ヘパリン含有マトリックスからの増殖因子の制御放出 |
| WO2001066164A1 (en) | 1999-04-22 | 2001-09-13 | Eidgenossisch Technische Hochschule Zurich | Modified protein matrices |
| US6479064B1 (en) | 1999-12-29 | 2002-11-12 | Children's Medical Center Corporation | Culturing different cell populations on a decellularized natural biostructure for organ reconstruction |
| US6773723B1 (en) | 2000-08-30 | 2004-08-10 | Depuy Acromed, Inc. | Collagen/polysaccharide bilayer matrix |
| GB0029847D0 (en) | 2000-12-07 | 2001-01-24 | Univ Bristol | Preparation of spheroids |
| DE60236974D1 (de) | 2001-07-26 | 2010-08-19 | Transparent Inc | Kultiviertes Zellkonstrukt mit Sphäroiden kultivierter Tierzellen und seine Verwendung |
| AU2003217700A1 (en) | 2002-02-26 | 2003-09-09 | Stelsys Llc | Hepatocyte bioreactor system for long term culture of functional hepatocyte spheroids |
| US20050069572A1 (en) | 2002-10-09 | 2005-03-31 | Jennifer Elisseeff | Multi-layered polymerizing hydrogels for tissue regeneration |
| CA2556169C (en) | 2004-01-29 | 2011-11-22 | Andrew Lees | Use of amino-oxy functional groups in the preparation of vaccines |
| CN101068574B (zh) | 2004-07-20 | 2012-07-18 | 伊索格尼斯股份有限公司 | 与自身抗原有关的自身免疫和疾病的特异性抑制 |
| US8187621B2 (en) | 2005-04-19 | 2012-05-29 | Advanced Cardiovascular Systems, Inc. | Methods and compositions for treating post-myocardial infarction damage |
| EP1886696A1 (en) | 2006-08-03 | 2008-02-13 | Endotis Pharma | Conjugates of antithrombin binding oligosaccharide derivatives and therapeutic proteins |
| US8275594B2 (en) | 2006-10-30 | 2012-09-25 | The Regents Of The University Of Michigan | Engineered scaffolds for intervertebral disc repair and regeneration and for articulating joint repair and regeneration |
| ES2322637B1 (es) | 2007-06-26 | 2010-03-05 | Universidad Del Pais Vasco | Microparticulas de alginato modificado con rgd como sistema de liberacion de farmacos. |
| IL187707A0 (en) | 2007-11-27 | 2008-11-03 | Univ Ben Gurion | Alginate scaffold in hepatectomy |
| WO2010030964A2 (en) | 2008-09-12 | 2010-03-18 | The Brigham And Women's Hospital, Inc. | 3-dimensional multi-layered hydrogels and methods of making the same |
| WO2010056378A2 (en) | 2008-11-14 | 2010-05-20 | Histogen, Inc. | Extracellular matrix compositions for the treatment of cancer |
| WO2011078990A1 (en) * | 2009-12-14 | 2011-06-30 | Benaroya Research Institute At Virginia Mason | Hydrogels comprising hyaluronan and at least one t cell induction agent |
| US20120321665A1 (en) * | 2009-12-14 | 2012-12-20 | Benaroya Research Institute At Virginia Mason | Compositions and methods for treating airway inflammatory diseases |
| WO2011163568A1 (en) | 2010-06-24 | 2011-12-29 | University Of Kansas | Conjugates comprising an n-oxime bond and associated methods |
| EP2678048B1 (en) | 2011-02-22 | 2018-11-21 | Institut National de la Sante et de la Recherche Medicale (INSERM) | Nano-reservoirs technology for use in bone and/or cartilage regeneration |
| EP2816964B1 (en) | 2012-02-21 | 2023-12-27 | Ben-Gurion University of The Negev Research and Development Authority | Hydrogel system comprising spatially separated bioactive polypeptides |
| WO2017175229A1 (en) * | 2016-04-07 | 2017-10-12 | B.G. Negev Technologies And Applications Ltd. | Polysaccharide compositions and uses thereof |
-
2017
- 2017-01-05 KR KR1020187022661A patent/KR20180104646A/ko not_active Ceased
- 2017-01-05 EP EP17735926.2A patent/EP3400074B1/en active Active
- 2017-01-05 WO PCT/IL2017/050013 patent/WO2017118979A1/en not_active Ceased
- 2017-01-05 US US16/068,133 patent/US11123404B2/en active Active
- 2017-01-05 CN CN201780015637.XA patent/CN109069875B/zh not_active Expired - Fee Related
- 2017-01-05 JP JP2018535329A patent/JP7033066B2/ja not_active Expired - Fee Related
-
2018
- 2018-07-05 IL IL260436A patent/IL260436B2/en unknown
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9125883B2 (en) * | 2004-06-14 | 2015-09-08 | Institut Francais De Recherche Pour L'exploitation De La Mer (Ifremer) | Sulfated depolymerized derivatives of exopolysaccharides (EPS) from mesophilic marine bacteria, method for preparing same, and uses thereof in tissue regeneration |
| WO2007043050A2 (en) * | 2005-10-11 | 2007-04-19 | Ben-Gurion University Of The Negev Research And Development Authority | Bioconjugates comprising sulfated polysaccharides and their uses |
| CN103002915A (zh) * | 2010-04-13 | 2013-03-27 | 免疫创新治疗有限公司 | 抑制Treg细胞的方法和组合物 |
| CN105611937A (zh) * | 2013-06-18 | 2016-05-25 | 复制细胞生命科学公司 | 治疗皮肤的组合物和方法 |
Non-Patent Citations (4)
| Title |
|---|
| Inhibition of Angiogenesis Is Associated With Reduced Islet;N. Zhang;《INHIBITION OF ANGIOGENESIS》;20051231;第37卷(第10期);第4452-4457页 * |
| The influence of the sequential delivery of angiogenic factors from affinitybinding;Inbar Freeman;《Biomaterials》;20090430;第30卷(第11期);第2122-2131页 * |
| Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets;Jeffrey M.H.Liu;《Biomaterials》;20151202;第80卷;第11-19页 * |
| VEGF-conjugated alginate hydrogel prompt angiogenesis and improve;Nina Yin;《Materials Science and Engineering C》;20151104;第59卷;第958-964页 * |
Also Published As
| Publication number | Publication date |
|---|---|
| IL260436B1 (en) | 2023-04-01 |
| WO2017118979A1 (en) | 2017-07-13 |
| EP3400074B1 (en) | 2021-10-20 |
| IL260436A (enExample) | 2018-08-30 |
| KR20180104646A (ko) | 2018-09-21 |
| EP3400074A4 (en) | 2019-07-10 |
| JP7033066B2 (ja) | 2022-03-09 |
| CN109069875A (zh) | 2018-12-21 |
| JP2019504832A (ja) | 2019-02-21 |
| US11123404B2 (en) | 2021-09-21 |
| US20190008924A1 (en) | 2019-01-10 |
| EP3400074A1 (en) | 2018-11-14 |
| IL260436B2 (en) | 2023-08-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109069875B (zh) | 产生免疫耐受反应的组合物和方法 | |
| US20220265779A1 (en) | Enhancement of skeletal muscle stem cell engraftment by dual delivery of vegf and igf-1 | |
| Kim et al. | Heparin functionalized injectable cryogel with rapid shape-recovery property for neovascularization | |
| Andorko et al. | Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine | |
| Passipieri et al. | Keratin hydrogel enhances in vivo skeletal muscle function in a rat model of volumetric muscle loss | |
| JP5496675B2 (ja) | 細胞移植療法に用いられる心疾患治療薬 | |
| TWI607760B (zh) | 含膠原蛋白與透明質酸的細胞組織膠體 | |
| JP2022514568A (ja) | 免疫系のモジュレーションのための生物工学的スキャフォールドおよびその使用 | |
| EP3065701B1 (en) | Eluting matrix and uses thereof | |
| Long et al. | Decellularized extracellular matrix (d-ECM): the key role of the inflammatory process in pre-regeneration after implantation | |
| Schackel et al. | Peptides and astroglia improve the regenerative capacity of alginate gels in the injured spinal cord | |
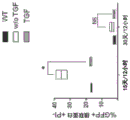
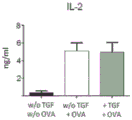
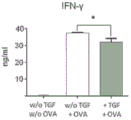
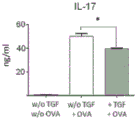
| Orr et al. | TGF-β affinity-bound to a macroporous alginate scaffold generates local and peripheral immunotolerant responses and improves allocell transplantation | |
| Ge et al. | The soluble and particulate form of alginates positively regulate immune response | |
| Liu et al. | Impact of marine-based biomaterials on the immunoregulatory properties of bone marrow-derived mesenchymal stem cells: potential use of fish collagen in bone tissue engineering | |
| US20170290954A1 (en) | Compositions and methods for treating spinal cord injury | |
| Safina et al. | Cell-Biomaterial constructs for wound healing and skin regeneration | |
| JP2018534353A (ja) | 感染の防御を提供する軟組織増大のための組成物 | |
| WO2017175229A1 (en) | Polysaccharide compositions and uses thereof | |
| US20200323784A1 (en) | Use of cxcl12 to promote survival, function, and immunoisolation of stem cell-derived beta cells | |
| Carnes | Tuning the Biophysical and Biochemical Cues of Fibrin Microthread Scaffolds Towards the Treatment of Volumetric Muscle Loss | |
| US20220016219A1 (en) | Means and Methods to Treat Diabetes | |
| Kwee | Biomaterial-based Helper T-cell Therapies for Ischemic Diseases | |
| Su | Design of Immunoactive Biomaterials for Therapeutic Applications | |
| JP6429237B2 (ja) | 免疫寛容部位形成剤及び免疫抑制性細胞の誘引剤 | |
| Chiu | Angiogenesis in Patches and Injectable Biomaterials for Cardiac Repair |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20211224 |