WO2022014525A1 - 開繊剤 - Google Patents
開繊剤 Download PDFInfo
- Publication number
- WO2022014525A1 WO2022014525A1 PCT/JP2021/026099 JP2021026099W WO2022014525A1 WO 2022014525 A1 WO2022014525 A1 WO 2022014525A1 JP 2021026099 W JP2021026099 W JP 2021026099W WO 2022014525 A1 WO2022014525 A1 WO 2022014525A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- amino acid
- seq
- fiber
- sequence
- acid sequence
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/04—Reinforcing macromolecular compounds with loose or coherent fibrous material
- C08J5/10—Reinforcing macromolecular compounds with loose or coherent fibrous material characterised by the additives used in the polymer mixture
-
- D—TEXTILES; PAPER
- D02—YARNS; MECHANICAL FINISHING OF YARNS OR ROPES; WARPING OR BEAMING
- D02J—FINISHING OR DRESSING OF FILAMENTS, YARNS, THREADS, CORDS, ROPES OR THE LIKE
- D02J1/00—Modifying the structure or properties resulting from a particular structure; Modifying, retaining, or restoring the physical form or cross-sectional shape, e.g. by use of dies or squeeze rollers
- D02J1/18—Separating or spreading
Definitions
- the present invention relates to a fiber-spreading agent, which is blended into a fiber-reinforced composite material containing a fiber bundle in which a plurality of reinforcing fibers are bundled and a matrix resin to promote the opening of the fiber bundle.
- Patent Document 1 describes a fiber-reinforced resin material molded product containing a fiber bundle of reinforcing fibers and a matrix resin.
- Patent Document 1 proposes a method of producing a fiber-reinforced resin material having a small variation in fiber content by opening and cutting a long fiber bundle.
- Patent Document 1 In the method described in Patent Document 1, it is necessary to open and cut the fiber bundle in advance, and there is a problem that the work efficiency is low.
- the present invention is a fiber-spreading agent blended in a fiber-reinforced composite material together with a fiber bundle of reinforcing fibers and a matrix resin, and is capable of promoting the opening of fiber bundles of reinforcing fibers in a compounding step.
- the purpose is to provide.
- One aspect of the present invention is a fiber-spreading agent that is blended into a fiber-reinforced composite material containing a fiber bundle in which a plurality of reinforcing fibers are bundled and a matrix resin to promote the opening of the fiber bundle, and has a structure.
- a fiber-spreading agent containing protein is a fiber-spreading agent that is blended into a fiber-reinforced composite material containing a fiber bundle in which a plurality of reinforcing fibers are bundled and a matrix resin to promote the opening of the fiber bundle, and has a structure.
- a fiber-spreading agent containing protein is containing protein.
- the fiber-spreading agent may contain the above-mentioned structural protein as a staple fiber.
- the reinforcing fiber may be a short fiber.
- the reinforcing fiber may be carbon fiber.
- the structural protein may contain modified fibroin.
- the structural protein may contain spider silk fibroin.
- a fiber-spreading agent blended in a fiber-reinforced composite material together with a fiber bundle of reinforcing fibers and a matrix resin which can promote the opening of fiber bundles of reinforcing fibers in a compounding step. Fibers are provided.
- FIG. 5A is a diagram showing the distribution of the angle of the fiber bundle of the carbon fiber with respect to the reference line in the fiber reinforced composite material of Example 2-1 by a radar chart in increments of 30 °.
- FIG. 5B is a diagram showing the distribution of the angle of the fiber bundle of the carbon fiber with respect to the reference line in the fiber reinforced composite material of Comparative Example 2-1 by a radar chart in increments of 30 °.
- FIG. 5A is a diagram showing the distribution of the angle of the fiber bundle of the carbon fiber with respect to the reference line in the fiber reinforced composite material of Comparative Example 2-1 by a radar chart in increments of 30 °.
- FIG. 5B is a diagram showing the distribution of the angle of the fiber bundle of the carbon fiber with respect to the reference line in the fiber reinforced composite material of Comparative Example 2-1 by a radar chart in increments of 30 °.
- FIG. 6A is a diagram showing the distribution of the angle of the fiber bundle of the carbon fiber with respect to the reference line in the fiber reinforced composite material of Example 2-2 by a radar chart in increments of 30 °.
- FIG. 6B is a diagram showing the distribution of the angle of the fiber bundle of the carbon fiber with respect to the reference line in the fiber reinforced composite material of Comparative Example 2-2 by a radar chart in increments of 30 °.
- the fiber-spreading agent of the present embodiment is a fiber-spreading agent blended in a fiber-reinforced composite material containing a fiber bundle in which a plurality of reinforcing fibers are bundled and a matrix resin, and is used to open the fiber bundle of the reinforcing fiber. It is a fiber-spreading agent that promotes.
- the spreading agent of this embodiment contains a structural protein.
- the structural protein examples include spider silk fibroin, silk fibroin, collagen, resilin, elastin and keratin, and proteins derived from these.
- the structural protein may be of natural origin or may be an artificially produced artificial protein. Further, the amino acid sequence of the artificial protein may be the same as or different from the amino acid sequence of the naturally occurring protein.
- the structural protein is preferably fibroin, more preferably spider silk fibroin.
- spider fibroin examples include spider fibroin produced by spiders such as large spider tube bookmark thread protein, weft protein, and vial-shaped gland protein. Since the large spit tube bookmark yarn has a repeating region consisting of a crystalline region and an amorphous region (also referred to as an amorphous region), it has both high stress and elasticity.
- the warp and weft of the spider silk has a characteristic that it does not have a crystalline region but has a repeating region consisting of an amorphous region. The weft is inferior in stress to the large discharge tube bookmark thread, but has high elasticity.
- Large spider tube bookmark thread protein is produced in the large bottle-shaped gland of spiders and has the characteristic of being excellent in toughness.
- Examples of the large spider thread protein include large bottle-shaped gland spiders MaSp1 and MaSp2 derived from Nephila clavipes, and ADF3 and ADF4 derived from Araneus diadematus.
- ADF3 is one of the two major bookmarking thread proteins of the European garden spider.
- the spider silk protein derived from ADF3 is relatively easy to synthesize, and has excellent properties in terms of strength and elongation and toughness.
- the warp and weft protein is produced in the flagelliform gland of the spider.
- Examples of the warp and weft protein include flagellar silk protein derived from Nephila clavipes.
- spider silk fibroins produced by spiders include spiders belonging to the genus Araneus, such as spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders,
- Spiders belonging to the genus (Neoscona genus), spiders belonging to the genus Kooni spider (Pronus genus) such as Kooni spider, spiders belonging to the genus Torinofundamashi (genus Cyrtarachne) such as Torinofundamashi and Otorinofundamashi, and spiders Spiders belonging to the spider genus (Gasteracantha genus) such as Chibusatoge spider, spiders belonging to the spider genus (Ordgarius genus) such as Mameitai seki spider and Mutsutogei seki spider, Koganegumo, Kogatakoganegmo and Nagakoganegmo Spiders belonging to the genus Arachunura such as spiders, spiders belonging to the genus Achsilas, spiders belonging to the genus Acusilas, spiders belonging to the genus Cytophora, spiders belonging to the genus Cytophora Spider
- Proteins, spiders belonging to the genus Tetragnatha such as spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spiders, spider
- spider silk protein produced by spiders for example, fibroin-3 (aff-3) [derived from Araneus diadematus] (GenBank accession number AAC47010 (amino acid sequence), U47855 (base sequence)), fibroin-4 (aff-4) [derived from Araneus diadematus] (GenBank accession number AAC47011 (nucleotide sequence), U47856 (base sequence)), dragline silk protein spidroin 1 [derived from Nephila clavipes] (GenBank sequence AAC47011) ), U37520 (base sequence)), major amplified protein 1 [derived from Latrodictus hesperus] (GenBank accession number ABR68856 (nucleotide sequence), EF595246 (base sequence)), dragline silk protein-derived from fibroin Numbers AAL32472 (amino acid sequence), AF441245 (base sequence)), major protein spidroin 1 [derived from Europe protein
- Fibroin may be modified fibroin.
- the modified fibroin is, for example, one in which the amino acid sequence is modified based on the amino acid sequence of the naturally occurring fibroin (for example, the amino acid sequence is modified by modifying the gene sequence of the cloned naturally occurring fibroin). It may be artificially designed and synthesized without depending on naturally occurring fibroin (for example, one having a desired amino acid sequence by chemically synthesizing a nucleic acid encoding the designed amino acid sequence). ..
- the modified fibroin is, for example, modifying the amino acid sequence corresponding to the substitution, deletion, insertion and / or addition of one or more amino acid residues to the cloned naturally occurring fibroin gene sequence. Can be obtained at. Substitutions, deletions, insertions and / or additions of amino acid residues can be carried out by methods well known to those skilled in the art, such as partial mutagenesis. Specifically, Nucleic Acid Res. It can be carried out according to the method described in the literature such as 10, 6487 (1982), Methods in Enzymeology, 100, 448 (1983).
- the modified fibroin is, for example, a fibroin containing a domain sequence represented by the formula 1: [(A) n motif-REP] m or the formula 2: [(A) n motif-REP] m- (A) n motif. There may be.
- the modified fibroin may further have an amino acid sequence (N-terminal sequence and C-terminal sequence) added to either or both of the N-terminal side and the C-terminal side of the domain sequence.
- the N-terminal sequence and the C-terminal sequence are not limited to this, but are typically regions that do not have the repetition of the amino acid motif characteristic of fibroin, and consist of about 100 residues of amino acids.
- domain sequence refers to a fibroin-specific crystalline region (typically corresponding to the (A) n motif of an amino acid sequence) and an amorphous region (typically to REP of an amino acid sequence). It is an amino acid sequence that produces (corresponding to)), and is an amino acid represented by the formula 1: [(A) n motif-REP] m or the formula 2: [(A) n motif-REP] m- (A) n motif. Means an array.
- the (A) n motif indicates an amino acid sequence mainly composed of alanine residues, and the number of amino acid residues is 2 to 27.
- the number of amino acid residues of the n motif may be 2 to 20, 4 to 27, 4 to 20, 8 to 20, 10 to 20, 4 to 16, 8 to 16, or 10 to 16. Further, the ratio of the number of alanine residues to the total number of amino acid residues in the (A) n motif may be 40% or more, 60% or more, 70% or more, 80% or more, 83% or more, 85% or more, It may be 86% or more, 90% or more, 95% or more, or 100% (meaning that it is composed only of alanine residues). At least seven of the (A) n motifs present in the domain sequence may be composed of only alanine residues.
- REP shows an amino acid sequence consisting of 2 to 200 amino acid residues.
- REP may be an amino acid sequence composed of 10 to 200 amino acid residues.
- m indicates an integer of 2 to 300, and may be an integer of 10 to 300.
- the plurality of (A) n motifs may have the same amino acid sequence or different amino acid sequences.
- the plurality of REPs may have the same amino acid sequence or different amino acid sequences.
- modified fibroin examples include modified fibroin (first modified fibroin) derived from the large spitting tube bookmark thread protein produced in the large bottle-shaped gland of spider, and modified fibroin with a reduced content of glycine residues.
- first modified fibroin modified fibroin
- second modified fibroin modified fibroin with reduced n-motif content
- third modified fibroin modified fibroin
- glycine residue content modified fibroin with reduced.
- n-motif content reduced It has a modified fibroin (fourth modified fibroin), a modified fibroin having a domain sequence containing a region having a locally high hydrophobicity index (fifth modified fibroin), and a domain sequence having a reduced content of glutamine residues.
- Modified fibroin (sixth modified fibroin) can be mentioned.
- the modified fibroin (first modified fibroin) derived from the large spider canal bookmark thread protein produced in the large bottle-shaped gland of the spider is a domain sequence represented by the formula 1: [(A) n motif-REP] m.
- Examples include proteins containing.
- n is preferably an integer of 3 to 20, more preferably an integer of 4 to 20, further preferably an integer of 8 to 20, and even more preferably an integer of 10 to 20 in Equation 1.
- An integer of ⁇ 16 is even more preferred, an integer of 8-16 is particularly preferred, and an integer of 10-16 is most preferred.
- the number of amino acid residues constituting REP in Formula 1 is preferably 10 to 200 residues, more preferably 10 to 150 residues, and 20 to 100 residues. Is even more preferable, and 20 to 75 residues are even more preferable.
- the total number of residues of glycine residue, serine residue and alanine residue contained in the amino acid sequence represented by the formula 1: [(A) n motif-REP] m is an amino acid residue. It is preferably 40% or more, more preferably 60% or more, and further preferably 70% or more with respect to the total number.
- the first modified fibroin comprises the unit of the amino acid sequence represented by the formula 1: [(A) n motif-REP] m, and the C-terminal sequence is the amino acid sequence shown in any of SEQ ID NOs: 1 to 3.
- it may be a protein having an amino acid sequence having 90% or more homology with the amino acid sequence shown in any one of SEQ ID NOs: 1 to 3.
- the amino acid sequence shown in SEQ ID NO: 1 is the same as the amino acid sequence consisting of 50 residues at the C-terminal of the amino acid sequence of ADF3 (GI: 1263287, NCBI), and the amino acid sequence shown in SEQ ID NO: 2 is a sequence. It is the same as the amino acid sequence in which 20 residues were removed from the C end of the amino acid sequence shown in SEQ ID NO: 1, and the amino acid sequence shown in SEQ ID NO: 3 was obtained by removing 29 residues from the C end of the amino acid sequence shown in SEQ ID NO: 1. It has the same amino acid sequence.
- first modified fibroin 90% or more sequence identity with the amino acid sequence shown by (1-i) SEQ ID NO: 4 or the amino acid sequence shown by (1-ii) SEQ ID NO: 4
- the sequence identity is preferably 95% or higher.
- the amino acid sequence represented by SEQ ID NO: 4 is the amino acid sequence of ADF3 to which the amino acid sequence (SEQ ID NO: 5) consisting of a starting codon, a His10 tag and an HRV3C protease (Human rhinovirus 3C protease) recognition site is added to the N-terminal.
- the 13th repeat region is increased approximately twice and mutated so that the translation terminates at the 1154th amino acid residue.
- the amino acid sequence at the C-terminal of the amino acid sequence shown in SEQ ID NO: 4 is the same as the amino acid sequence shown in SEQ ID NO: 3.
- the modified fibroin of (1-i) may consist of the amino acid sequence shown in SEQ ID NO: 4.
- the modified fibroin (second modified fibroin) having a reduced content of glycine residue has an amino acid sequence in which the domain sequence has a reduced content of glycine residue as compared with the naturally occurring spider fibroin.
- the second modified fibroin has an amino acid sequence corresponding to at least one or more glycine residues in REP substituted with another amino acid residue as compared to naturally occurring spider fibroin. Can be done.
- the second modified fibroin has a domain sequence of GGX and GPGXX in REP as compared with the naturally occurring spider fibroin (where G is a glycine residue, P is a proline residue, and X is an amino acid other than glycine. Indicating a residue.) At least one motif sequence having an amino acid sequence corresponding to one or more glycine residues in the motif sequence being replaced with another amino acid residue. May be.
- the ratio of the motif sequence in which the above-mentioned glycine residue is replaced with another amino acid residue may be 10% or more with respect to the total motif sequence.
- the second modified fibroin contains the domain sequence represented by the formula 1: [(A) n motif-REP] m, and from the above domain sequence, the above domain sequence from the (A) n motif located closest to the C-terminal side.
- the total number of amino acid residues in the amino acid sequence consisting of XGX (where X indicates amino acid residues other than glycine) contained in all REPs in the sequence excluding the sequence up to the C-terminal of is z, and the above domain sequence. Therefore, when the total number of amino acid residues in the sequence excluding the sequence from the (A) n motif located most on the C-terminal side to the C-terminal of the above domain sequence is w, z / w is 30% or more.
- the number of alanine residues with respect to the total number of amino acid residues in the n motif may be 83% or more, preferably 86% or more, more preferably 90% or more, and 95% or more. It is even more preferably 100% (meaning that it is composed only of alanine residues).
- the second modified fibroin is preferably one in which the content ratio of the amino acid sequence consisting of XGX is increased by substituting one glycine residue of the GGX motif with another amino acid residue.
- the content ratio of the amino acid sequence consisting of GGX in the domain sequence is preferably 30% or less, more preferably 20% or less, further preferably 10% or less, 6 % Or less is even more preferable, 4% or less is even more preferable, and 2% or less is particularly preferable.
- the content ratio of the amino acid sequence consisting of GGX in the domain sequence can be calculated by the same method as the method for calculating the content ratio (z / w) of the amino acid sequence consisting of XGX described below.
- z / w (%) can be calculated by dividing z by w.
- z / w is preferably 50.9% or more, more preferably 56.1% or more, further preferably 58.7% or more, and 70% or more. Is even more preferable, and 80% or more is even more preferable.
- the upper limit of z / w is not particularly limited, but may be, for example, 95% or less.
- the second modified fibroin is modified, for example, from the cloned naturally occurring spider fibroin gene sequence by substituting at least a part of the base sequence encoding the glycine residue to encode another amino acid residue.
- one glycine residue in the GGX motif and the GPGXX motif may be selected as the glycine residue to be modified, or may be substituted so that z / w is 50.9% or more. It can also be obtained, for example, by designing an amino acid sequence satisfying the above embodiment from the amino acid sequence of naturally occurring spider fibroin and chemically synthesizing a nucleic acid encoding the designed amino acid sequence.
- the amino acid sequence corresponding to the deletion, insertion and / or addition may be modified.
- the above-mentioned other amino acid residues are not particularly limited as long as they are amino acid residues other than glutamine residues, but are valine (V) residue, leucine (L) residue, isoleucine (I) residue, and methionine ( Hydrophilic amino acid residues such as M) residue, proline (P) residue, phenylalanine (F) residue and tryptophan (W) residue, glutamine (Q) residue, asparagine (N) residue, serine (S) ) Residues, hydrophilic amino acid residues such as lysine (K) residues and glutamine (E) residues are preferred, valine (V) residues, leucine (L) residues, isoleucine (I) residues and glutamine ( Q) Residues are more preferred, and glutamine (Q) residues are even more preferred.
- the second modified fibroin (2-i) the amino acid sequence represented by SEQ ID NO: 6, SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9, or (2-ii) SEQ ID NO: 6, sequence.
- modified fibroins comprising an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in No. 7, SEQ ID NO: 8 or SEQ ID NO: 9.
- the modified fibroin of (2-i) will be described.
- the amino acid sequence shown in SEQ ID NO: 6 is obtained by substituting GQX for all GGX in the REP of the amino acid sequence shown in SEQ ID NO: 10, which corresponds to naturally occurring spider fibroin.
- every two (A) n motifs are deleted from the N-terminal side to the C-terminal side from the amino acid sequence shown in SEQ ID NO: 6, and the amino acid sequence is further before the C-terminal sequence.
- One [(A) n motif-REP] is inserted into.
- amino acid sequence shown in SEQ ID NO: 8 two alanine residues are inserted on the C-terminal side of each (A) n motif of the amino acid sequence shown in SEQ ID NO: 7, and a part of glutamine (Q) residue is further added. It is substituted with a serine (S) residue and a part of the amino acid on the N-terminal side is deleted so as to have almost the same molecular weight as that of SEQ ID NO: 7.
- the amino acid sequence shown in SEQ ID NO: 9 is a region of 20 domain sequences existing in the amino acid sequence shown in SEQ ID NO: 11 (however, several amino acid residues on the C-terminal side of the region are substituted). This is a sequence in which the His tag is added to the C-terminal of the sequence obtained by repeating the above four times.
- the value of z / w in the amino acid sequence shown by SEQ ID NO: 10 is 46.8%.
- the z / w values in the amino acid sequence shown in SEQ ID NO: 6, the amino acid sequence shown in SEQ ID NO: 7, the amino acid sequence shown in SEQ ID NO: 8, and the amino acid sequence shown in SEQ ID NO: 9 are 58.7%, respectively. It is 70.1%, 66.1% and 70.0%.
- the values of x / y in the jagged ratio (described later) of 1: 1.8 to 11.3 of the amino acid sequences shown in SEQ ID NO: 10, SEQ ID NO: 6, SEQ ID NO: 7, SEQ ID NO: 8 and SEQ ID NO: 9 are They are 15.0%, 15.0%, 93.4%, 92.7% and 89.3%, respectively.
- the modified fibroin of (2-i) may consist of the amino acid sequence represented by SEQ ID NO: 6, SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9.
- the modified fibroin of (2-ii) contains an amino acid sequence having 90% or more sequence identity with the amino acid sequence represented by SEQ ID NO: 6, SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9.
- the modified fibroin of (2-ii) is also a protein containing a domain sequence represented by the formula 1: [(A) n motif-REP] m.
- the sequence identity is preferably 95% or more.
- the modified fibroin of (2-ii) has 90% or more sequence identity with the amino acid sequence represented by SEQ ID NO: 6, SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9, and is contained in REP.
- X indicates an amino acid residue other than glycine.
- the second modified fibroin may contain a tag sequence at either or both of the N-terminus and the C-terminus. This enables isolation, immobilization, detection, visualization and the like of modified fibroin.
- an affinity tag using specific affinity (binding, affinity) with other molecules can be mentioned.
- affinity tag a histidine tag (His tag)
- His tag is a short peptide in which about 4 to 10 histidine residues are lined up, and has the property of specifically binding to metal ions such as nickel. Therefore, isolation of modified fibroin by metal chelation chromatography (chromatography) is performed. Can be used for.
- Specific examples of the tag sequence include the amino acid sequence shown in SEQ ID NO: 12 (amino acid sequence including His tag sequence and hinge sequence).
- tag sequences such as glutathione-S-transferase (GST) that specifically binds to glutathione and maltose-binding protein (MBP) that specifically binds to maltose can also be used.
- GST glutathione-S-transferase
- MBP maltose-binding protein
- an "epitope tag” that utilizes an antigen-antibody reaction can also be used.
- a peptide (epitope) exhibiting antigenicity as a tag sequence
- an antibody against the epitope can be bound.
- the epitope tag include HA (peptide sequence of hemagglutinin of influenza virus) tag, myc tag, FLAG tag and the like.
- a tag sequence that can be separated with a specific protease can also be used.
- the modified fibroin from which the tag sequence has been separated can also be recovered.
- the second modified fibroin comprising a tag sequence, (2-iii) SEQ ID NO: 13, SEQ ID NO: 14, SEQ ID NO: 14, or the amino acid sequence set forth by SEQ ID NO: 15 or (2-iv).
- modified fibroins comprising an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 13, SEQ ID NO: 11, SEQ ID NO: 14 or SEQ ID NO: 15.
- amino acid sequences represented by SEQ ID NO: 16, SEQ ID NO: 17, SEQ ID NO: 13, SEQ ID NO: 11, SEQ ID NO: 14, and SEQ ID NO: 15, are SEQ ID NO: 10, SEQ ID NO: 18, SEQ ID NO: 6, SEQ ID NO: 7, and SEQ ID NO: 8, respectively.
- amino acid sequence shown in SEQ ID NO: 12 is added to the N-terminal of the amino acid sequence shown in SEQ ID NO: 9.
- the modified fibroin of (2-iii) may consist of the amino acid sequence represented by SEQ ID NO: 13, SEQ ID NO: 11, SEQ ID NO: 14 or SEQ ID NO: 15.
- the modified fibroin of (2-iv) contains an amino acid sequence having 90% or more sequence identity with the amino acid sequence represented by SEQ ID NO: 13, SEQ ID NO: 11, SEQ ID NO: 14 or SEQ ID NO: 15.
- the modified fibroin of (2-iv) is also a protein containing a domain sequence represented by the formula 1: [(A) n motif-REP] m.
- the sequence identity is preferably 95% or more.
- the modified fibroin of (2-iv) has 90% or more sequence identity with the amino acid sequence set forth in SEQ ID NO: 13, SEQ ID NO: 11, SEQ ID NO: 14 or SEQ ID NO: 15 and is contained in REP.
- X indicates an amino acid residue other than glycine.
- the second modified fibroin may contain a secretory signal for releasing the protein produced in the recombinant protein production system to the outside of the host.
- the sequence of the secretory signal can be appropriately set according to the type of host.
- the modified fibroin (third modified fibroin) having a reduced content of (A) n motif has a domain sequence of which the content of (A) n motif is reduced as compared with the naturally occurring spider silk fibroin.
- the third modified fibroin may have an amino acid sequence corresponding to a 10-40% deletion of the (A) n motif from naturally occurring spider silk fibroin.
- the third modified fibroin has a domain sequence of 1 to 3 (A) n motifs, one (A), at least from the N-terminal side to the C-terminal side, as compared with the naturally occurring spider silk fibroin. It may have an amino acid sequence corresponding to the deletion of the n-motif.
- the third modified fibroin has a domain sequence of at least two consecutive deletions of the (A) n motif from the N-terminal side toward the C-terminal side and one deletion as compared with the naturally occurring spider silk fibroin.
- (A) It may have an amino acid sequence corresponding to the deletion of the n-motif being repeated in this order.
- the third modified fibroin may have an amino acid sequence corresponding to the deletion of the (A) n motif at least every other two from the N-terminal side to the C-terminal side in the domain sequence. ..
- the third modified fibroin contains a domain sequence represented by the formula 1: [(A) n motif-REP] m, and two adjacent [(A) n motifs from the N-terminal side to the C-terminal side.
- - When the number of amino acid residues in the REP of the unit is sequentially compared and the number of amino acid residues in the REP having a small number of amino acid residues is 1, the ratio of the number of amino acid residues in the other REP is 1.8 to 1.
- x is the maximum value of the sum of the amino acid residues of two adjacent [(A) n-motif-REP] units, which is 11.3, and y is the total number of amino acid residues of the domain sequence.
- the number of alanine residues with respect to the total number of amino acid residues in the n motif may be 83% or more, preferably 86% or more, more preferably 90% or more, and 95% or more. It is even more preferably 100% (meaning that it is composed only of alanine residues).
- FIG. 1 shows a domain sequence obtained by removing the N-terminal sequence and the C-terminal sequence from spider silk fibroin. From the N-terminal side (left side), the domain sequence consists of (A) n motif-first REP (50 amino acid residue)-(A) n motif-second REP (100 amino acid residue)-(A) n. Motif-Third REP (10 amino acid residues)-(A) n motif-Fourth REP (20 amino acid residues)-(A) n motif-Fifth REP (30 amino acid residues)-(A) It has an arrangement called n motifs.
- Two adjacent [(A) n motif-REP] units are sequentially selected from the N-terminal side to the C-terminal side so as not to overlap. At this time, there may be a [(A) n motif-REP] unit that is not selected.
- pattern 1 (comparison between the first REP and the second REP, and comparison between the third REP and the fourth REP)
- pattern 2 (comparison between the first REP and the second REP, and a comparison).
- 4th REP and 5th REP comparison Pattern 3 (2nd REP and 3rd REP comparison, and 4th REP and 5th REP comparison
- Pattern 4 (1st REP and (Comparison of the second REP) is shown. There are other selection methods.
- the number of amino acid residues of each REP in the two selected adjacent [(A) n motif-REP] units is compared.
- the comparison is made by finding the ratio of the number of amino acid residues of the other when the one with the smaller number of amino acid residues is set to 1.
- the 5th REP is assumed to be 1 when the 4th REP having a smaller number of amino acid residues is 1.
- each pattern add up the total number of amino acid residues of the two adjacent [(A) n motif-REP] units shown by the solid line (not only the number of REP but also the number of amino acid residues of (A) n motif. be.). Then, the total values added are compared, and the total value (maximum value of the total value) of the pattern in which the total value is maximum is defined as x. In the example shown in FIG. 1, the total value of pattern 1 is the maximum.
- x / y (%) can be calculated by dividing x by the total number of amino acid residues y in the domain sequence.
- x / y is preferably 50% or more, more preferably 60% or more, further preferably 65% or more, still more preferably 70% or more. It is preferably 75% or more, even more preferably 80% or more, and particularly preferably 80% or more.
- the upper limit of x / y is not particularly limited and may be, for example, 100% or less.
- x / y is preferably 89.6% or more, and when the jagged ratio is 1: 1.8 to 3.4, x.
- / Y is preferably 77.1% or more, and when the jagged ratio is 1: 1.9 to 8.4, x / y is preferably 75.9% or more, and the jagged ratio is 1. In the case of 1.9 to 4.1, x / y is preferably 64.2% or more.
- x / y is 46.4% or more. Is more preferable, 50% or more is more preferable, 55% or more is further preferable, 60% or more is even more preferable, 70% or more is even more preferable, and 80% or more is used. It is particularly preferable to have.
- the upper limit of x / y is not particularly limited and may be 100% or less.
- the third modified fibroin lacks one or more of the sequences encoding the (A) n motif so that x / y is 64.2% or more from the cloned naturally occurring spider silk fibroin gene sequence. It can be obtained by losing it. Further, for example, from the amino acid sequence of naturally occurring spider fibroin, an amino acid sequence corresponding to the deletion of one or more (A) n motifs so that x / y is 64.2% or more is designed. It can also be obtained by chemically synthesizing a nucleic acid encoding the designed amino acid sequence.
- one or more amino acid residues are further substituted, deleted, inserted and /.
- the amino acid sequence corresponding to the addition may be modified.
- the third modified fibroin (3-i) the amino acid sequence represented by SEQ ID NO: 18, SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9, or (3-ii) SEQ ID NO: 18, sequence.
- modified fibroins comprising an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in No. 7, SEQ ID NO: 8 or SEQ ID NO: 9.
- the modified fibroin of (3-i) will be described.
- the amino acid sequence shown by SEQ ID NO: 18 lacks (A) n motifs every other from the N-terminal side to the C-terminal side from the amino acid sequence shown by SEQ ID NO: 10, which corresponds to naturally occurring spider fibroin. It was lost, and one [(A) n motif-REP] was inserted in front of the C-terminal sequence.
- the amino acid sequence shown in SEQ ID NO: 7 is obtained by substituting GQX for all GGX in the REP of the amino acid sequence shown in SEQ ID NO: 18.
- amino acid sequence shown in SEQ ID NO: 8 two alanine residues are inserted on the C-terminal side of each (A) n motif of the amino acid sequence shown in SEQ ID NO: 7, and a part of glutamine (Q) residue is further added. It is substituted with a serine (S) residue and a part of the amino acid on the N-terminal side is deleted so as to have almost the same molecular weight as that of SEQ ID NO: 7.
- the amino acid sequence shown in SEQ ID NO: 9 is a region of 20 domain sequences existing in the amino acid sequence shown in SEQ ID NO: 11 (however, several amino acid residues on the C-terminal side of the region are substituted). This is a sequence in which the His tag is added to the C-terminal of the sequence obtained by repeating the above four times.
- the value of x / y in the amino acid sequence shown in SEQ ID NO: 10 (corresponding to naturally occurring spider silk fibroin) in a jagged ratio of 1: 1.8 to 11.3 is 15.0%.
- the value of x / y in the amino acid sequence shown by SEQ ID NO: 18 and the amino acid sequence shown by SEQ ID NO: 7 is 93.4%.
- the value of x / y in the amino acid sequence shown in SEQ ID NO: 8 is 92.7%.
- the value of x / y in the amino acid sequence shown in SEQ ID NO: 9 is 89.3%.
- the values of z / w in the amino acid sequences represented by SEQ ID NO: 10, SEQ ID NO: 18, SEQ ID NO: 7, SEQ ID NO: 8 and SEQ ID NO: 9 are 46.8%, 56.2%, 70.1% and 66. 1% and 70.0%.
- the modified fibroin of (3-i) may consist of the amino acid sequence represented by SEQ ID NO: 18, SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9.
- the modified fibroin of (3-ii) contains an amino acid sequence having 90% or more sequence identity with the amino acid sequence represented by SEQ ID NO: 18, SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9.
- the modified fibroin of (3-ii) is also a protein containing a domain sequence represented by the formula 1: [(A) n motif-REP] m.
- the sequence identity is preferably 95% or more.
- the modified fibroin of (3-ii) has 90% or more sequence identity with the amino acid sequence represented by SEQ ID NO: 18, SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9, and has N-terminal to C-terminal sides.
- the number of amino acid residues of REP of two adjacent [(A) n motif-REP] units is sequentially compared and the number of amino acid residues of REP having a small number of amino acid residues is set to 1, the other The amino acid residue of two adjacent [(A) n-motif-REP] units having a ratio of the number of amino acid residues of REP of 1.8 to 11.3 (giza ratio of 1: 1.8 to 11.3).
- x is the maximum value of the total value obtained by adding the radix and the total number of amino acid residues in the domain sequence is y, it is preferable that x / y is 64.2% or more.
- the third modified fibroin may contain the above-mentioned tag sequence at either or both of the N-terminal and the C-terminal.
- the third modified fibroin comprising a tag sequence, (3-iii) the amino acid sequence set forth in SEQ ID NO: 17, SEQ ID NO: 11, SEQ ID NO: 14 or SEQ ID NO: 15, or (3-iv) sequence.
- modified fibroins comprising an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in No. 17, SEQ ID NO: 11, SEQ ID NO: 14 or SEQ ID NO: 15.
- amino acid sequences represented by SEQ ID NO: 16, SEQ ID NO: 17, SEQ ID NO: 13, SEQ ID NO: 11, SEQ ID NO: 14, and SEQ ID NO: 15, are SEQ ID NO: 10, SEQ ID NO: 18, SEQ ID NO: 6, SEQ ID NO: 7, and SEQ ID NO: 8, respectively.
- amino acid sequence shown in SEQ ID NO: 12 is added to the N-terminal of the amino acid sequence shown in SEQ ID NO: 9.
- the modified fibroin of (3-iii) may consist of the amino acid sequence represented by SEQ ID NO: 17, SEQ ID NO: 11, SEQ ID NO: 14 or SEQ ID NO: 15.
- the modified fibroin of (3-iv) contains an amino acid sequence having 90% or more sequence identity with the amino acid sequence represented by SEQ ID NO: 17, SEQ ID NO: 11, SEQ ID NO: 14 or SEQ ID NO: 15.
- the modified fibroin of (3-iv) is also a protein containing a domain sequence represented by the formula 1: [(A) n motif-REP] m.
- the sequence identity is preferably 95% or more.
- the modified fibroin of (3-iv) has 90% or more sequence identity with the amino acid sequence represented by SEQ ID NO: 17, SEQ ID NO: 11, SEQ ID NO: 14 or SEQ ID NO: 15, and has N-terminal to C-terminal sides.
- the number of amino acid residues of REP of two adjacent [(A) n motif-REP] units is sequentially compared and the number of amino acid residues of REP having a small number of amino acid residues is set to 1, the other Let x be the maximum value of the total value obtained by adding the number of amino acid residues of two adjacent [(A) n motif-REP] units in which the ratio of the number of amino acid residues of REP is 1.8 to 11.3.
- x / y is preferably 64.2% or more.
- the third modified fibroin may contain a secretory signal for releasing the protein produced in the recombinant protein production system to the outside of the host.
- the sequence of the secretory signal can be appropriately set according to the type of host.
- the modified fibroin (fourth modified fibroin) having a reduced content of glycine residue and (A) n motif has a domain sequence higher than that of naturally occurring spider fibroin (A). In addition to having a reduced content of n motifs, it has an amino acid sequence with a reduced content of glycine residues.
- the domain sequence of the fourth modified fibroin lacks at least one or more (A) n motifs as compared to naturally occurring spider fibroin, plus at least one or more glycine residues in the REP. It can be said that the group has an amino acid sequence corresponding to the substitution of another amino acid residue.
- the fourth modified fibroin includes the above-mentioned modified fibroin having a reduced content of glycine residue (second modified fibroin) and (A) modified fibroin having a reduced content of n motif (third). It is a modified fibroin that also has the characteristics of modified fibroin). Specific embodiments and the like are as described in the second modified fibroin and the third modified fibroin.
- the fourth modified fibroin (4-i) the amino acid sequence represented by SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9, (4-ii) SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: Examples thereof include modified fibroins containing an amino acid sequence having 90% or more sequence identity with the amino acid sequence shown in 9. Specific embodiments of the modified fibroin comprising the amino acid sequence set forth in SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9 are as described above.
- a modified fibroin (fifth modified fibroin) having a domain sequence containing a region with a large local hydrophobicity index has one or more amino acids in the REP whose domain sequence is compared with that of naturally occurring spider fibroin.
- Local hydrophobicity index corresponding to the fact that the residue was replaced with an amino acid residue with a large hydrophobicity index and / or that one or more amino acid residues with a large hydrophobicity index were inserted in REP. It may have an amino acid sequence containing a large region of.
- the region having a large hydrophobicity index locally is composed of consecutive 2 to 4 amino acid residues.
- the amino acid residue having a large hydrophobicity index described above is an amino acid selected from isoleucine (I), valine (V), leucine (L), phenylalanine (F), cysteine (C), methionine (M) and alanine (A). It is more preferably a residue.
- one or more amino acid residues in REP were replaced with amino acid residues having a high hydrophobicity index as compared with naturally occurring spider fibroin, and / or 1 in REP.
- one or more amino acid residues are substituted, deleted, or inserted as compared with the naturally occurring spider fibroin.
- / or there may be a modification of the amino acid sequence corresponding to the addition.
- the fifth modified fibroin is hydrophobic, for example, from the gene sequence of cloned naturally occurring spider silk fibroin to one or more hydrophilic amino acid residues in REP (eg, amino acid residues having a negative hydrophobicity index). It can be obtained by substituting an amino acid residue (eg, an amino acid residue having a positive hydrophobicity index) and / or inserting one or more hydrophobic amino acid residues in the REP. Also, for example, one or more hydrophilic amino acid residues in REP have been replaced with hydrophobic amino acid residues from the amino acid sequence of naturally occurring spider silk fibroin, and / or one or more hydrophobic amino acids in REP.
- an amino acid sequence corresponding to the insertion of a residue can also be obtained by designing an amino acid sequence corresponding to the insertion of a residue and chemically synthesizing a nucleic acid encoding the designed amino acid sequence.
- one or more hydrophilic amino acid residues in the REP were replaced with hydrophobic amino acid residues from the amino acid sequence of naturally occurring spider silk fibroin, and / or one or more hydrophobic in the REP.
- the amino acid sequence corresponding to the substitution, deletion, insertion and / or addition of one or more amino acid residues may be further modified.
- the fifth modified fibroin contains the domain sequence represented by the formula 1: [(A) n motif-REP] m, and extends from the (A) n motif located most on the C-terminal side to the C-terminal of the above-mentioned domain sequence.
- Let p be the total number of amino acid residues contained in the region where the average value of the hydrophobicity index of consecutive 4 amino acid residues is 2.6 or more in all REPs contained in the sequence excluding the sequence from the above domain sequence.
- hydrophobicity index of amino acid residues a known index (Hydrotherapy index: Kyte J, & Dollittle R (1982) "A simple method for dispensing the hydropathic character of lip. 105-132) is used.
- hydrophobicity index of each amino acid hydrophobicity index, hereinafter also referred to as “HI” is as shown in Table 1 below.
- the average value of the hydrophobicity index is obtained for all four consecutive amino acid residues (each amino acid residue is used to calculate the average value 1 to 4 times).
- a region in which the average value of the hydrophobicity index of consecutive four amino acid residues is 2.6 or more is specified. Even if a certain amino acid residue corresponds to a plurality of "consecutive 4 amino acid residues having an average value of 2.6 or more of hydrophobicity index", it should be included as one amino acid residue in the region. become.
- the total number of amino acid residues contained in the region is p. Further, the total number of amino acid residues contained in the sequence A is q.
- the average value of the hydrophobicity index of 4 consecutive amino acid residues is 2.
- the hydrophobicity index of the consecutive four amino acid residues is present.
- p / q is preferably 6.2% or more, more preferably 7% or more, further preferably 10% or more, and more preferably 20% or more. Even more preferably, it is even more preferably 30% or more.
- the upper limit of p / q is not particularly limited, but may be, for example, 45% or less.
- the fifth modified fibroin is, for example, the amino acid sequence of the cloned naturally occurring spider silk fibroin, one or more hydrophilic amino acid residues (eg, hydrophobic) in the REP so as to satisfy the above p / q condition.
- Amino acid residues with a negative sex index are replaced with hydrophobic amino acid residues (eg, amino acid residues with a positive hydrophobicity index) and / or one or more hydrophobic amino acid residues in the REP.
- the amino acid residue having a large hydrophobicity index is not particularly limited, but isoleucine (I), valine (V), leucine (L), phenylalanine (F), cysteine (C), methionine (M) and alanine (A). ), More preferably valine (V), leucine (L) and isoleucine (I).
- the fifth modified fibroin (5-i) the amino acid sequence represented by SEQ ID NO: 19, SEQ ID NO: 20 or SEQ ID NO: 21, or (5-ii) SEQ ID NO: 19, SEQ ID NO: 20 or SEQ ID NO: A modified fibroin containing an amino acid sequence having 90% or more sequence identity with the amino acid sequence shown in 21 can be mentioned.
- the modified fibroin of (5-i) will be described.
- the amino acid sequence shown in SEQ ID NO: 22 is an amino acid sequence in which alanine residues in the (A) n motif of naturally occurring spider fibroin are continuous deleted so that the number of consecutive alanine residues is five. Is.
- the amino acid sequence shown in SEQ ID NO: 19 has two amino acid sequences (VLI) consisting of three amino acid residues inserted every other REP in the amino acid sequence shown in SEQ ID NO: 22 and is shown in SEQ ID NO: 22. A part of the amino acid on the C-terminal side is deleted so that the molecular weight of the amino acid sequence is almost the same as that of the amino acid sequence.
- amino acid sequence shown in SEQ ID NO: 23 two alanine residues are inserted on the C-terminal side of each (A) n motif with respect to the amino acid sequence shown in SEQ ID NO: 22, and a part of glutamine (Q) remains.
- the group was replaced with a serine (S) residue, and some amino acids on the C-terminal side were deleted so as to have substantially the same molecular weight as the amino acid sequence shown in SEQ ID NO: 22.
- the amino acid sequence shown by SEQ ID NO: 20 is obtained by inserting an amino acid sequence (VLI) consisting of three amino acid residues every other REP into the amino acid sequence shown by SEQ ID NO: 23.
- the amino acid sequence shown in SEQ ID NO: 21 is the amino acid sequence shown in SEQ ID NO: 23, in which two amino acid sequences (VLI) consisting of three amino acid residues are inserted every other REP.
- the modified fibroin of (5-i) may consist of the amino acid sequence represented by SEQ ID NO: 19, SEQ ID NO: 20 or SEQ ID NO: 21.
- the modified fibroin of (5-ii) contains an amino acid sequence having 90% or more sequence identity with the amino acid sequence represented by SEQ ID NO: 19, SEQ ID NO: 20 or SEQ ID NO: 21.
- the modified fibroin of (5-ii) is also a protein containing a domain sequence represented by the formula 1: [(A) n motif-REP] m.
- the sequence identity is preferably 95% or more.
- the modified fibroin of (5-ii) has 90% or more sequence identity with the amino acid sequence represented by SEQ ID NO: 19, SEQ ID NO: 20 or SEQ ID NO: 21, and is located most on the C-terminal side (A) n.
- P / q is preferably 6.2% or more.
- the fifth modified fibroin may contain a tag sequence at either or both of the N-terminal and the C-terminal.
- modified fibroins comprising an amino acid sequence having 90% or more sequence identity with the amino acid sequence set forth in No. 25 or SEQ ID NO: 26.
- amino acid sequences represented by SEQ ID NO: 24, SEQ ID NO: 25 and SEQ ID NO: 26 are the amino acid sequences represented by SEQ ID NO: 12 (His tag) at the N-terminal of the amino acid sequences represented by SEQ ID NO: 19, SEQ ID NO: 20 and SEQ ID NO: 21, respectively. (Including array and hinge array) is added.
- the modified fibroin of (5-iii) may consist of the amino acid sequence represented by SEQ ID NO: 24, SEQ ID NO: 25 or SEQ ID NO: 26.
- the modified fibroin of (5-iv) contains an amino acid sequence having 90% or more sequence identity with the amino acid sequence represented by SEQ ID NO: 24, SEQ ID NO: 25 or SEQ ID NO: 26.
- the modified fibroin of (5-iv) is also a protein containing a domain sequence represented by the formula 1: [(A) n motif-REP] m.
- the sequence identity is preferably 95% or more.
- the modified fibroin of (5-iv) has 90% or more sequence identity with the amino acid sequence represented by SEQ ID NO: 24, SEQ ID NO: 25 or SEQ ID NO: 26, and is located most on the C-terminal side (A) n.
- P / q is preferably 6.2% or more.
- the fifth modified fibroin may contain a secretory signal for releasing the protein produced in the recombinant protein production system to the outside of the host.
- the sequence of the secretory signal can be appropriately set according to the type of host.
- Modified fibroin having a domain sequence with a reduced content of glutamine residues has an amino acid sequence with a reduced content of glutamine residues as compared to naturally occurring spider silk fibroin. ..
- the sixth modified fibroin preferably contains at least one motif selected from the GGX motif and the GPGXX motif in the amino acid sequence of REP.
- the content of the GPGXX motif is usually 1% or more, may be 5% or more, and is preferably 10% or more.
- the upper limit of the GPGXX motif content is not particularly limited and may be 50% or less, or 30% or less.
- GPGXX motif content is a value calculated by the following method.
- the total number of GPGXX motifs contained in the region is tripled (3).
- the GPGXX motif content is calculated as s / t, where t is the total number of amino acid residues in all REPs excluding the motif.
- the target of "the sequence obtained by excluding the sequence from the (A) n motif located on the most C-terminal side to the C-terminal of the domain sequence from the domain sequence” is "the most C-terminal side".
- (A) Sequence from n motif to C end of domain sequence ”(sequence corresponding to REP) may include a sequence characteristic of spider silk fibroin and a sequence having low correlation with m. When is small (that is, when the domain sequence is short), it affects the calculation result of the GPGXX motif content, and this effect is to be eliminated.
- the "GPGXX motif” is located at the C-terminal of the REP, even if the "XX" is, for example, "AA”, it is treated as a "GPGXX motif".
- FIG. 3 is a schematic diagram showing the domain sequence of spider silk fibroin.
- the sixth modified fibroin preferably has a glutamine residue content of 9% or less, more preferably 7% or less, further preferably 4% or less, and particularly preferably 0%. ..
- the "glutamine residue content” is a value calculated by the following method.
- the region is included in all REPs included in the sequence (the sequence corresponding to "region A” in FIG. 3) excluding the sequence from the (A) n motif located on the side to the C-terminal of the domain sequence from the domain sequence, the region is included.
- the total number of glutamine residues contained is u, and the sequence from the (A) n motif located most on the C-terminal side to the C-terminal of the domain sequence is removed from the domain sequence, and (A) all REPs excluding the n motif.
- the glutamine residue content is calculated as u / t, where t is the total number of amino acid residues.
- the reason for targeting is the above-mentioned reason. The same is true.
- the sixth modified fibroin had its domain sequence deleted from one or more glutamine residues in REP or replaced with other amino acid residues as compared to naturally occurring spider fibroin. It may have a corresponding amino acid sequence.
- the "other amino acid residue” may be an amino acid residue other than the glutamine residue, but is preferably an amino acid residue having a larger hydrophobicity index than the glutamine residue.
- the hydrophobicity index of amino acid residues is as shown in Table 1.
- amino acid residues having a larger hydrophobicity index than glutamine residues include isoleucine (I), valine (V), leucine (L), phenylalanine (F), cysteine (C), and methionine (M).
- Amino acid residues selected from alanine (A), glycine (G), threonine (T), serine (S), tryptophan (W), tyrosine (Y), proline (P) and histidine (H). can.
- amino acid residues selected from isoleucine (I), valine (V), leucine (L), phenylalanine (F), cysteine (C), methionine (M) and alanine (A) are more preferable.
- Isoleucine (I), valine (V), leucine (L) and phenylalanine (F) are more preferably amino acid residues.
- the sixth modified fibroin preferably has a hydrophobicity of REP of -0.8 or more, more preferably -0.7 or more, further preferably 0 or more, and 0.3 or more. Is even more preferable, and 0.4 or more is particularly preferable.
- the upper limit of the hydrophobicity of REP is not particularly limited and may be 1.0 or less, or 0.7 or less.
- the "hydrophobicity of REP” is a value calculated by the following method. The most C-terminal in spider silk fibroin containing a domain sequence represented by formula 1: [(A) n motif-REP] m or formula 2: [(A) n motif-REP] m- (A) n motif. In all REPs included in the sequence (the sequence corresponding to "region A" in FIG.
- the reason for targeting is the above-mentioned reason. The same is true.
- the sixth modified fibroin had its domain sequence deleted of one or more glutamine residues in REP as compared to naturally occurring spider fibroin and / or one or more glutamines in REP.
- the modification corresponding to the substitution of the residue with another amino acid residue there is a modification of the amino acid sequence corresponding to the substitution, deletion, insertion and / or addition of one or more amino acid residues. May be good.
- the sixth modified fibroin is, for example, deleted one or more glutamine residues in REP from the cloned naturally occurring spider fibroin gene sequence and / or one or more glutamine residues in REP. Can be obtained by substituting with another amino acid residue. Also, for example, one or more glutamine residues in REP have been deleted from the amino acid sequence of naturally occurring spider silk fibroin, and / or one or more glutamine residues in REP have been replaced with other amino acid residues. It can also be obtained by designing an amino acid sequence corresponding to the substitution and chemically synthesizing a nucleic acid encoding the designed amino acid sequence.
- the sixth modified fibroin (6-i) in SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 32, SEQ ID NO: 33 or SEQ ID NO: 43.
- a modified fibroin containing the indicated amino acid sequence, or (6-ii), represented by SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 32, SEQ ID NO: 33 or SEQ ID NO: 43 examples thereof include modified fibroins containing an amino acid sequence having 90% or more sequence identity with the amino acid sequence.
- the amino acid sequence (Met-PRT410) shown in SEQ ID NO: 7 is based on the base sequence and amino acid sequence of Nephila clavipes (GenBank accession number: P468401, GI: 11744415), which is a naturally occurring fibroin, (A) n.
- the amino acid sequence in which the alanine residues are continuous in the motif is modified to improve the productivity, such as increasing the number of consecutive alanine residues to five.
- Met-PRT410 since Met-PRT410 has not been modified for the glutamine residue (Q), the glutamine residue content is about the same as the glutamine residue content of naturally occurring fibroin.
- amino acid sequence (M_PRT888) shown in SEQ ID NO: 27 is obtained by substituting VL for all QQs in Met-PRT410 (SEQ ID NO: 7).
- M_PRT525) In the amino acid sequence (M_PRT525) shown in SEQ ID NO: 34, two alanine residues are inserted into the region (A5) where the alanine residues are continuous with respect to Met-PRT410 (SEQ ID NO: 7), and the molecular weight of Met-PRT410 is adjusted. Two domain sequences on the C-terminal side were deleted so that they were almost the same, and 13 glutamine residues (Q) were replaced with serine residues (S) or proline residues (P).
- amino acid sequence (M_PRT699) shown in SEQ ID NO: 32 is obtained by substituting VL for all QQs in M_PRT525 (SEQ ID NO: 34).
- the amino acid sequence shown by SEQ ID NO: 43 (Met-PRT966) contains all the QQs in the amino acid sequence shown by SEQ ID NO: 9 (the amino acid sequence before the amino acid sequence shown by SEQ ID NO: 42 is added to the C terminal) VF. And the remaining Q is replaced with I.
- amino acid sequences represented by SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 32, SEQ ID NO: 33 and SEQ ID NO: 43 all have a glutamine residue content of 9% or less. There is (Table 2).
- the modified fibroin of (6-i) comprises the amino acid sequence represented by SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 32, SEQ ID NO: 33 or SEQ ID NO: 43. There may be.
- the modified fibroin of (6-ii) is 90% or more of the amino acid sequence represented by SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 32, SEQ ID NO: 33 or SEQ ID NO: 43. It contains an amino acid sequence having the sequence identity of.
- the modified fibroin of (6-ii) is also a domain represented by the formula 1: [(A) n motif-REP] m or the formula 2: [(A) n motif-REP] m- (A) n motif. It is a protein containing a sequence.
- the sequence identity is preferably 95% or more.
- the modified fibroin of (6-ii) preferably has a glutamine residue content of 9% or less. Further, the modified fibroin of (6-ii) preferably has a GPGXX motif content of 10% or more.
- the sixth modified fibroin may contain a tag sequence at either or both of the N-terminal and the C-terminal. This enables isolation, immobilization, detection, visualization and the like of modified fibroin.
- the sixth modified fibroin containing the tagged sequence (6-iii) SEQ ID NO: 35, SEQ ID NO: 36, SEQ ID NO: 37, SEQ ID NO: 38, SEQ ID NO: 39, SEQ ID NO: 40, SEQ ID NO: 41 or A modified fibroin containing the amino acid sequence set forth in SEQ ID NO: 44, or (6-iv) SEQ ID NO: 35, SEQ ID NO: 36, SEQ ID NO: 37, SEQ ID NO: 38, SEQ ID NO: 39, SEQ ID NO: 40, SEQ ID NO: 41 or SEQ ID NO: A modified fibroin containing an amino acid sequence having 90% or more sequence identity with the amino acid sequence shown in 44 can be mentioned.
- amino acid sequences represented by SEQ ID NO: 35, SEQ ID NO: 36, SEQ ID NO: 37, SEQ ID NO: 38, SEQ ID NO: 39, SEQ ID NO: 40, SEQ ID NO: 41 and SEQ ID NO: 44 are SEQ ID NO: 27, SEQ ID NO: 28 and SEQ ID NO: 29, respectively.
- SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 32, SEQ ID NO: 33 and SEQ ID NO: 43 added to the N-terminal of the amino acid sequence represented by SEQ ID NO: 12 (including His tag sequence and hinge sequence). It is a thing.
- amino acid sequences shown in SEQ ID NO: 44 all have a glutamine residue content of 9% or less (Table 3).
- the modified fibroin of (6-iii) comprises the amino acid sequence represented by SEQ ID NO: 35, SEQ ID NO: 36, SEQ ID NO: 37, SEQ ID NO: 38, SEQ ID NO: 39, SEQ ID NO: 40, SEQ ID NO: 41 or SEQ ID NO: 44. There may be.
- the modified fibroin of (6-iv) is 90% or more of the amino acid sequence represented by SEQ ID NO: 35, SEQ ID NO: 36, SEQ ID NO: 37, SEQ ID NO: 38, SEQ ID NO: 39, SEQ ID NO: 40, SEQ ID NO: 41 or SEQ ID NO: 44. It contains an amino acid sequence having the sequence identity of.
- the modified fibroin of (6-iv) is also a domain represented by the formula 1: [(A) n motif-REP] m or the formula 2: [(A) n motif-REP] m- (A) n motif. It is a protein containing a sequence.
- the sequence identity is preferably 95% or more.
- the modified fibroin of (6-iv) preferably has a glutamine residue content of 9% or less. Further, the modified fibroin of (6-iv) preferably has a GPGXX motif content of 10% or more.
- the sixth modified fibroin may contain a secretory signal for releasing the protein produced in the recombinant protein production system to the outside of the host.
- the sequence of the secretory signal can be appropriately set according to the type of host.
- the modified fibroin is at least two or more of the characteristics of the first modified fibroin, the second modified fibroin, the third modified fibroin, the fourth modified fibroin, the fifth modified fibroin, and the sixth modified fibroin. It may be a modified fibroin having the above-mentioned characteristics.
- the modified fibroin may be a hydrophilic modified fibroin or a hydrophobic modified fibroin.
- Hydrophobic modified fibroin is the sum of the hydrophobicity index (HI) of all amino acid residues constituting the modified fibroin, and then the sum is divided by the total number of amino acid residues (mean HI) is 0 or more. Is a modified fibroin.
- the hydrophobicity index is as shown in Table 1.
- the hydrophilic modified fibroin is a modified fibroin having the above-mentioned average HI of less than 0.
- hydrophobic modified fibroin examples include the above-mentioned sixth modified fibroin. More specific examples of the hydrophobically modified fibroin include the amino acid sequences set forth in SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 29, SEQ ID NO: 30, SEQ ID NO: 31, SEQ ID NO: 32, SEQ ID NO: 33 or SEQ ID NO: 43. Examples thereof include modified fibroins containing the amino acid sequences set forth in SEQ ID NO: 35, SEQ ID NO: 37, SEQ ID NO: 38, SEQ ID NO: 39, SEQ ID NO: 40, SEQ ID NO: 41 or SEQ ID NO: 44.
- hydrophilic modified fibroin examples include the above-mentioned first modified fibroin, second modified fibroin, third modified fibroin, fourth modified fibroin, and fifth modified fibroin. More specific examples of the hydrophilic spider silk protein include the amino acid sequence set forth in SEQ ID NO: 4, the amino acid sequence set forth in SEQ ID NO: 6, SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9, the amino acid sequence represented by SEQ ID NO: 13, SEQ ID NO: 13. 11.
- Amino acid sequence represented by SEQ ID NO: 14 or SEQ ID NO: 15 amino acid sequence represented by SEQ ID NO: 18, SEQ ID NO: 7, SEQ ID NO: 8 or SEQ ID NO: 9, SEQ ID NO: 17, SEQ ID NO: 11, SEQ ID NO: 14 or SEQ ID NO: Examples include the amino acid sequence set forth in 15, the modified fibroin comprising the amino acid sequence set forth in SEQ ID NO: 19, SEQ ID NO: 20 or SEQ ID NO: 21.
- modified fibroin can be used alone or in combination of two or more.
- the modified fibroin expresses the nucleic acid, for example, by a host transformed with an expression vector having a nucleic acid sequence encoding the modified fibroin and one or more regulatory sequences operably linked to the nucleic acid sequence. It can be produced by.
- the method for producing the nucleic acid encoding the modified fibroin is not particularly limited.
- the nucleic acid can be produced by a method of amplifying and cloning by a polymerase chain reaction (PCR) or the like using a gene encoding modified fibroin, or a method of chemically synthesizing the nucleic acid.
- the chemical synthesis method of nucleic acid is not particularly limited, and for example, AKTA oligopilot plus 10/100 (GE Healthcare Japan Co., Ltd.) based on the amino acid sequence information of the spider silk protein obtained from the NCBI web database or the like.
- the gene can be chemically synthesized by a method of linking an oligonucleotide automatically synthesized by the above method by PCR or the like.
- a nucleic acid encoding the modified fibroin consisting of an amino acid sequence in which an amino acid sequence consisting of a start codon and a His10 tag is added to the N-terminal may be synthesized.
- the regulatory sequence is a sequence that controls the expression of the recombinant protein in the host (for example, promoter, enhancer, ribosome binding sequence, transcription termination sequence, etc.), and can be appropriately selected depending on the type of host.
- an inducible promoter that functions in the host cell and can induce the expression of the desired modified fibroin may be used.
- An inducible promoter is a promoter that can control transcription by the presence of an inducing substance (expression inducer), the absence of a repressor molecule, or physical factors such as an increase or decrease in temperature, osmotic pressure, or pH value.
- the type of expression vector can be appropriately selected according to the type of host, such as a plasmid vector, a viral vector, a cosmid vector, a phosmid vector, and an artificial chromosome vector.
- a vector that can be autonomously replicated in a host cell or can be integrated into the chromosome of the host and contains a promoter at a position where a nucleic acid encoding modified fibroin can be transcribed is preferably used.
- any of prokaryotes and eukaryotes such as yeast, filamentous fungi, insect cells, animal cells and plant cells can be suitably used.
- the expression vector is a vector containing a promoter, a ribosome-binding sequence, a nucleic acid encoding a modified fibroin, and a transcription termination sequence while being capable of self-sustaining replication in the prokaryote. It is preferable to have. It may contain a gene that controls a promoter.
- Examples of protozoa include microorganisms belonging to the genera Escherichia, Brevibacillus, Serratia, Bacillus, Microbacterium, Brevibacillus, Corinebacterium, Pseudomonas and the like.
- Examples of microorganisms belonging to the genus Escherichia include Escherichia coli and the like.
- Examples of microorganisms belonging to the genus Brevibacillus include Brevibacillus agri and the like.
- Examples of microorganisms belonging to the genus Serratia include Serratia marcescens and the like.
- Examples of microorganisms belonging to the genus Bacillus include Bacillus satirus and the like.
- microorganisms belonging to the genus Microbacterium include Microbacterium, Ammonia Phyllum and the like.
- microorganisms belonging to the genus Brevibacterium include Brevibacterium divaricatum and the like.
- microorganisms belonging to the genus Corynebacterium include Corynebacterium and Ammonia Genes.
- microorganisms belonging to the genus Pseudomonas include Pseudomonas putida and the like.
- the vector into which the nucleic acid encoding the modified fibroin is introduced includes, for example, pBTrp2 (manufactured by Behringer-Mannheim), pGEX (manufactured by Pharmacia), pUC18, pBluescriptII, pSupex, pET22b, pCold, pUB110, pNCO2 (Japanese Unexamined Patent Publication No. 2002-238569) and the like can be mentioned.
- Examples of eukaryotic hosts include yeast and filamentous fungi (mold, etc.).
- yeast include yeasts belonging to the genus Saccharomyces, Pichia, Schizosaccharomyces and the like.
- Examples of the filamentous fungus include filamentous fungi belonging to the genus Aspergillus, the genus Penicillium, the genus Trichoderma, and the like.
- examples of the vector into which the nucleic acid encoding the modified fibroin is introduced include YEp13 (ATCC37115) and YEp24 (ATCC37051).
- any method for introducing an expression vector into the host cell any method can be used as long as it is a method for introducing DNA into the host cell.
- a method using calcium ions [Proc. Natl. Acad. Sci. USA, 69, 2110 (1972)]
- electroporation method electroporation method
- spheroplast method protoplast method
- lithium acetate method competent method and the like.
- nucleic acid by a host transformed with an expression vector in addition to direct expression, secretory production, fusion protein expression, etc. can be performed according to the method described in Molecular Cloning 2nd Edition. ..
- the modified fibroin can be produced, for example, by culturing a transformed host in a culture medium, producing and accumulating the modified fibroin in the culture medium, and collecting the modified fibroin from the culture medium.
- the method of culturing the transformed host in the culture medium can be carried out according to the method usually used for culturing the host.
- the culture medium contains a carbon source, a nitrogen source, inorganic salts and the like that can be assimilated by the host, and the culture of the host is efficiently performed.
- a natural medium or a synthetic medium may be used as long as it can be used.
- the carbon source may be any material that can be assimilated by the host, for example, glucose, fructose, sucrose, carbohydrates containing them such as molasses, starch and starch hydrolysates, and organic acids such as acetic acid and propionic acid. , And alcohols such as ethanol and propanol can be used.
- nitrogen source examples include ammonium salts of inorganic or organic acids such as ammonia, ammonium chloride, ammonium sulfate, ammonium acetate and ammonium phosphate, other nitrogen-containing compounds, and peptone, meat extract, yeast extract and corn steep liquor. Casein hydrolyzate, soybean meal and soybean meal hydrolyzate, various fermented cells and digested products thereof can be used.
- inorganic salts for example, primary potassium phosphate, secondary potassium phosphate, magnesium phosphate, magnesium sulfate, sodium chloride, ferrous sulfate, manganese sulfate, copper sulfate and calcium carbonate can be used.
- Culturing of prokaryotes such as Escherichia coli or eukaryotes such as yeast can be performed under aerobic conditions such as shaking culture or deep aeration stirring culture.
- the culture temperature is, for example, 15-40 ° C.
- the culture time is usually 16 hours to 7 days.
- the pH of the culture medium during culture is preferably maintained at 3.0 to 9.0.
- the pH of the culture medium can be adjusted by using an inorganic acid, an organic acid, an alkaline solution, urea, calcium carbonate, ammonia or the like.
- antibiotics such as ampicillin and tetracycline may be added to the culture medium as needed during the culture.
- an inducer may be added to the medium as needed.
- isopropyl- ⁇ -D-thiogalactopyranoside or the like is used when culturing a microorganism transformed with an expression vector using the lac promoter
- indol acrylic is used when culturing a microorganism transformed with an expression vector using the trp promoter. Acids and the like may be added to the medium.
- Modified fibroin produced by a transformed host can be isolated and purified by methods commonly used for protein isolation and purification. For example, when the modified fibroin is expressed in a lysed state in the cells, after the culture is completed, the host cells are collected by centrifugation, turbid in an aqueous buffer solution, and then an ultrasonic crusher, a French press, or a manton gaulin. Crush the host cells with a homogenizer, dynomil, or the like to obtain a cell-free extract.
- a method usually used for isolating and purifying a protein that is, a solvent extraction method, a salting-out method using ammonium sulfate, a desalting method, or an organic solvent is used.
- Precipitation method anion exchange chromatography method using a resin such as diethylaminoethyl (DEAE) -cepharose, DIAION HPA-75 (manufactured by Mitsubishi Kasei Co., Ltd.), positive using a resin such as S-Sepharose FF (manufactured by Pharmacia).
- Ion exchange chromatography method hydrophobic chromatography method using resin such as butyl Sepharose, phenyl Sepharose, gel filtration method using molecular sieve, affinity chromatography method, chromatofocusing method, electrophoresis method such as isoelectric point electrophoresis Purified preparations can be obtained by using the above methods alone or in combination.
- the insoluble material of the modified fibroin is recovered as a precipitate fraction by similarly collecting the host cell, crushing it, and centrifuging it.
- the recovered insoluble form of modified fibroin can be solubilized with a protein denaturing agent.
- a purified specimen of modified fibroin can be obtained by the same isolation and purification method as described above.
- the modified fibroin When the modified fibroin is secreted extracellularly, the modified fibroin can be recovered from the culture supernatant. That is, a purified sample can be obtained by treating the culture by a method such as centrifugation to obtain a culture supernatant and using the same isolation and purification method as described above from the culture supernatant.
- the shape of the structural protein is not particularly limited, but it is fibrous or powder from the viewpoint that it is excellent in dispersibility in the matrix resin and the effect of promoting the opening of the fiber bundle of the reinforcing fiber is more remarkable. It is preferably in the shape. That is, the fiber-spreading agent of the present embodiment preferably contains structural protein fibers and / or structural protein powder.
- the structural protein fiber may be a spun of the above-mentioned protein.
- the structural protein fiber is preferably a spider silk fibroin fiber, that is, a polypeptide derived from a natural spider silk protein (artificial spider silk protein) spun.
- Structural protein fibers can be produced by known spinning methods. That is, for example, when producing a structural protein fiber, first, the structural protein is dissolved in a solvent such as dimethyl sulfoxide (DMSO), N, N-dimethylformamide (DMF), or hexafluoroisopronol (HFIP) to promote dissolution. It is added together with an inorganic salt as an agent and dissolved to prepare a dope solution. Then, using this doping solution, spinning can be performed by a known spinning method such as wet spinning, dry spinning, or dry-wet spinning to obtain a desired structural protein fiber.
- DMSO dimethyl sulfoxide
- DMF N, N-dimethylformamide
- HFIP hexafluoroisopronol
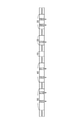
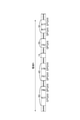
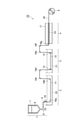
- FIG. 1 is a schematic view showing an example of a spinning device for producing structural protein fibers.
- the spinning device 10 shown in FIG. 1 is an example of a spinning device for dry / wet spinning, and has an extrusion device 1, a coagulation bath 20, a washing bath 21, and a drying device 4 in order from the upstream side. ..
- the extrusion device 1 has a storage tank 7, in which the doping liquid (spinning stock solution) 6 is stored.
- the coagulation liquid 11 (for example, methanol) is stored in the coagulation bath 20.
- the doping liquid 6 is pushed out from a nozzle 9 provided with an air gap 19 between the doping liquid 11 and the coagulating liquid 11 by a gear pump 8 attached to the lower end of the storage tank 7.
- the extruded doping liquid 6 is supplied into the coagulating liquid 11 via the air gap 19.
- the solvent is removed from the dope liquid 6 and the protein is coagulated.
- the coagulated protein is guided to the washing tub 21, washed with the washing liquid 12 in the washing tub 21, and then sent to the drying device 4 by the first nip roller 13 and the second nip roller 14 installed in the washing tub 21. Be done.
- the rotation speed of the second nip roller 14 is set to be faster than the rotation speed of the first nip roller 13, the structural protein fiber 36 stretched at a magnification corresponding to the rotation speed ratio can be obtained.
- the structural protein fibers stretched in the washing liquid 12 are removed from the washing tub 21 and then dried when passing through the drying device 4, and then wound up by a winder. In this way, the structural protein fiber is finally obtained by the spinning device 10 as the wound product 5 wound around the winder.
- 18a to 18g are thread guides.
- the coagulation liquid 11 may be any solution that can be desolvated, and examples thereof include lower alcohols having 1 to 5 carbon atoms such as methanol, ethanol and 2-propanol, and acetone.
- the coagulant 11 may contain water as appropriate.
- the temperature of the coagulating liquid 11 is preferably 0 to 30 ° C.
- the distance through which the coagulated protein passes through the coagulation liquid 11 (substantially, the distance from the thread guide 18a to the thread guide 18b) may be long enough to efficiently remove the solvent, for example, 200 to 500 mm. Is.
- the residence time in the coagulation liquid 11 may be, for example, 0.01 to 3 minutes, preferably 0.05 to 0.15 minutes. Further, stretching (pre-stretching) may be performed in the coagulating liquid 11.
- the stretching performed in the washing bath 21 when obtaining the structural protein fiber may be a so-called moist heat stretching performed in warm water, a solution obtained by adding an organic solvent or the like to warm water, or the like.
- the temperature of this moist heat stretching may be, for example, 50 to 90 ° C, preferably 75 to 85 ° C.
- the undrawn yarn (or the pre-drawn yarn) can be drawn, for example, 1 to 10 times, and preferably 2 to 8 times.
- the structural protein fiber is preferably a short fiber (that is, a fiber having a fiber length of 5 mm or less).
- the fiber length of the structural protein fiber is preferably 5 mm or less, more preferably 1 mm or less.
- Such a structural protein fiber is more excellent in dispersibility in the matrix resin, and the effect of promoting the opening of the fiber bundle of the reinforcing fiber can be obtained more remarkably.
- the lower limit of the fiber length of the structural protein fiber is not particularly limited, and may be, for example, 0.1 mm or more, or 0.25 mm or more.
- the structural protein fiber may be one that has been cut using a fiber cutting machine so as to have the above-mentioned suitable fiber length. That is, the structural protein fiber may be a chopped fiber.
- the structural protein powder may be, for example, a structural protein crushed using a crusher, or may be finely cut with a fiber cutting machine or the like. From the viewpoint that a structural protein powder having excellent dispersibility can be easily obtained, the structural protein powder may be, for example, crushed or cut structural protein fibers.
- the fiber-spreading agent of the present embodiment may be blended at the time of compounding the fiber bundle of the reinforcing fiber and the matrix resin.
- the fiber-reinforced composite material may be produced by mixing a fiber bundle of reinforcing fibers, a matrix resin, and a fiber-spreading agent of the present embodiment.
- the fiber-reinforced composite material may be produced by combining a resin sheet containing a matrix resin and a fiber-spreading agent with a fiber bundle of reinforcing fibers.
- the blending amount of the fiber-spreading agent of the present embodiment is preferably blended so as to satisfy the suitable content range in the fiber-reinforced composite material described later.
- the fiber-spreading agent of the present embodiment also has an effect of suppressing the orientation of fiber bundles of reinforcing fibers in the fiber-reinforced composite material. That is, the fiber-spreading agent of the present embodiment is an orientation inhibitor that suppresses the orientation of the fiber bundle by being blended in a fiber-reinforced composite material containing a fiber bundle in which a plurality of reinforcing fibers are bundled and a matrix resin. You may.
- the fiber-reinforced composite material of the present embodiment includes a fiber bundle of reinforcing fibers, a matrix resin, and the above-mentioned defibrating agent.
- the matrix resin a known resin used for the fiber-reinforced composite material can be used without particular limitation.
- the matrix resin include vinyl ester resin, unsaturated polyester, phenol resin, melamine resin, epoxy resin, polyamide resin, methyl methacrylate, polypropylene, nylon, polyethylene, polystyrene, polyacetal, polycarbonate, ABS, AES, PET, and PBT. , PPS, LCP, PEEK, polyurethane, urea resin, silicon resin and the like.
- the matrix resin may be a thermoplastic resin or a thermosetting resin.
- the content of the matrix resin is not particularly limited, but may be, for example, 35% by mass or more, preferably 45% by mass or more.
- the content of the matrix resin may be, for example, 65% by mass or less, preferably 55% by mass or less.
- the reinforcing fiber a known reinforcing fiber used for the fiber-reinforced composite material can be used without particular limitation.
- the reinforcing fiber for example, carbon fiber, boron fiber, aramid fiber, polyethylene fiber, Zylon (registered trademark) fiber and the like can be preferably used.
- the reinforcing fibers include synthetic fibers such as polyamide fibers and polyimide fibers, metal fibers such as alumina fibers, titanium fibers, steel fibers and copper fibers, and plant-derived natural fibers such as bamboo fibers, kenaf fibers and bagas fibers.
- Inorganic fibers such as glass fibers and basalt fibers can also be used.
- the fiber length of the reinforcing fiber is not particularly limited, and may be a so-called long fiber (for example, a fiber having a fiber length of more than 50 mm), a short fiber (for example, a fiber having a fiber length of 50 mm or less), or a continuous fiber. There may be.
- the reinforcing fiber may be carbon fiber.
- the effect of promoting the opening of the fiber bundle by the opening agent is more remarkable.
- the carbon fiber short fibers having a fiber length of 50 mm or less are more preferable.
- the content of the reinforcing fiber is not particularly limited, but may be, for example, 25% by mass or more, preferably 35% by mass or more.
- the content of the reinforcing fiber may be, for example, 55% by mass or less, preferably 45% by mass or less.
- the content of the fiber-spreading agent is not particularly limited, but may be, for example, 0.5% by mass or more, preferably 1% by mass or more.
- the content of the fiber-spreading agent may be, for example, 10% by mass or less, preferably 5% by mass or less.
- the fiber reinforced composite material may further contain components other than the above.
- other components include fillers, curing agents, low shrinkage agents, internal mold release agents and the like.
- the filler examples include calcium carbonate, aluminum hydride, barium sulfate, clay, mica and the like.
- the content of the filler is not particularly limited, and may be, for example, 0.5% by mass or more, preferably 1% by mass or more, 50% by mass or less, and preferably 20% by mass or less.
- the manufacturing method of the fiber reinforced composite material is not particularly limited.
- Examples of the method of compounding the matrix resin and the reinforcing fiber include a hand lay-up method, a spray-up method, and an SMC press in which a sheet-like material in which the reinforcing fiber and the resin component are mixed in advance is compression-molded with a mold.
- a method, an RTM method in which a resin is injected into a laminated mold in which fibers are spread, such as injection molding, an autoclave molding method, a deautoclave molding method, or the like can be used.
- thermoplastic resin injection thermosetting resin BMC (Bulk Moding Compound) injection
- GMT Glass-Mat reinforced Thermoplastics
- a known compounding method such as a compounding method can be used.
- the timing of adding the defibrating agent is not particularly limited, and for example, it may be added in advance to the resin component in the above-mentioned compounding method, or may be added at the same time as the compounding.
- the first aspect and the second aspect will be described below as specific examples of the method for producing the fiber-reinforced composite material.
- the production method according to the first aspect includes a step of mixing reinforcing fibers with a resin composition containing a resin component and a fiber-spreading agent.
- the method of mixing the resin composition and the reinforcing fiber is not particularly limited, and may be, for example, an extruder, a kneader, or the like.
- the resin component in the first aspect is a component that forms a matrix resin of a fiber-reinforced composite material, and may be, for example, a thermoplastic resin or a thermosetting resin.
- the resin composition may further contain a curing agent, and the above-mentioned production method may further include a step of curing the resin composition.
- the curing agent and curing method are not particularly limited, and known curing agents and curing methods can be applied depending on the type of thermosetting resin and the like.
- the manufacturing method according to the second aspect is a sheet forming step of leveling a resin composition containing a resin component and a fiber spreading agent into a sheet to form a resin sheet, and arranging reinforcing fibers on the resin sheet.
- the compounding step of compounding the resin sheet and the reinforcing fiber is included.
- the resin component in the second aspect is a component that forms the matrix resin of the fiber-reinforced composite material, and may be, for example, a thermoplastic resin or a thermosetting resin.
- the resin composition may further contain a curing agent, and the above-mentioned production method may further include a step of curing the resin composition.
- the curing agent and curing method are not particularly limited, and known curing agents and curing methods can be applied depending on the type of thermosetting resin and the like.
- the molding method of the resin composition is not particularly limited, and examples thereof include a method such as compression molding.
- the method of combining the resin sheet and the reinforcing fiber is not particularly limited, and examples thereof include a method of spraying the reinforcing fiber on the resin sheet.
- the fiber reinforced composite material is not particularly limited.
- the fiber-reinforced composite material can be suitably used for members such as frames, inner panels, and outer panels of structures such as automobiles, railroad vehicles, ships, aircraft, rockets, artificial satellites, and robots.
- fiber-reinforced composite materials include automobile brake discs, wheels, fuel tanks, hoods, roofs, side doors, back doors, luggage inner panels, outer panels, exterior parts, structural parts, undercarriage parts, engine-related parts, and FC. It can be suitably used for parts for cars / EV cars, other parts and the like.
- exterior parts include bumpers, rocker moldings, pillars, roof moldings, and the like.
- structural parts include bumper ring hoses, rear floor pans, crash boxes, dash panels, rear partitions, seat back frames, braces and the like.
- the lower parts include a lower absorber, an undercover, and the like.
- engine-related parts include engine covers, engine undercovers, cylinder head covers, oil pans, radiator supports, timing belts and chain covers, and the like.
- parts for FC vehicles and EV vehicles include stack frames, stack end plates, FC cells, inverter covers, inverter cases, rotor motors, stator motors, reactors and the like.
- other parts include brackets, anchors, pedals, sheet metal parts, and the like.
- the fiber reinforced composite material can be suitably used for the front leg door, main leg door, floor beam, engine cover, aileron, wing body fairing, horizontal stabilizer, vertical stabilizer, rudder, elevator, etc. of an aircraft.
- the fiber-reinforced composite material is used for ship masts, drone frames, suitcases, cargo containers, housings for electronic and electrical equipment (for example, personal computers, displays, projectors, cameras, mobile phones, smartphones, tablets, etc.).
- Fishing gear eg fishing rods, reels
- golf club shafts, golf club heads, rackets eg tennis, badminton, squash, table tennis
- car frames front forks, rims, baseball bats, skis , Hockey stick, ski stock, bamboo sword for kendo, Japanese bow, western bow, table tennis table, cue for billiard, stick for gate ball, material for construction and civil engineering, blade for wind power generation (windmill), fly wheel, pipes, parabolic antenna , Prosthesis, wheelchair, bed, portable slope, pine needle stick, artificial bone and the like.
- the fiber-spreading agent of the above-described embodiment also has an effect of suppressing the orientation of fiber bundles of reinforcing fibers in the fiber-reinforced composite material. Therefore, one aspect of the present invention is an orientation inhibitor that is blended in a fiber-reinforced composite material containing a fiber bundle in which a plurality of reinforcing fibers are bundled and a matrix resin to suppress the orientation of the fiber bundles of the reinforcing fibers. be able to.
- Example 1-1 [Protein production] (1) Preparation of expression vector As a protein contained in the undiluted spinning solution, the sequence number is based on the base sequence and amino acid sequence of fibroin (GenBank accession number: P468401, GI: 11744415) derived from Nephila clavipes. A modified fibroin having 15 (spider silk fibroin, hereinafter also referred to as “PRT799”) was designed.
- the amino acid sequence shown by SEQ ID NO: 15 has an amino acid sequence in which amino acid residues are substituted, inserted or deleted for the purpose of improving productivity with respect to the amino acid sequence of fibroin derived from Nephila clavipes. Further, the amino acid sequence (tag sequence and hinge sequence) shown by SEQ ID NO: 12 is added to the N-terminal.
- nucleic acid encoding the modified fibroin PRT799 having the designed amino acid sequence shown by SEQ ID NO: 15 was synthesized.
- An NdeI site was added to the nucleic acid at the 5'end and an EcoRI site was added downstream of the stop codon.
- the nucleic acid was cloned into a cloning vector (pUC118). Then, the nucleic acid was cut out by restriction enzyme treatment with NdeI and EcoRI, and then recombinant into the protein expression vector pET-22b (+) to obtain an expression vector.
- the seed culture solution was added to a jar fermenter to which 500 mL of the production medium (Table 5) was added so that the OD600 was 0.05.
- the temperature of the culture solution was kept at 37 ° C., and the culture was controlled at a constant pH of 6.9.
- the dissolved oxygen concentration in the culture solution was maintained at 20% of the dissolved oxygen saturation concentration.
- the feed solution (glucose 455 g / 1 L, Yeast Extract 120 g / 1 L) was added at a rate of 1 mL / min.
- the temperature of the culture solution was kept at 37 ° C., and the culture was controlled at a constant pH of 6.9. Further, the dissolved oxygen concentration in the culture solution was maintained at 20% of the dissolved oxygen saturation concentration, and the culture was carried out for 20 hours. Then, 1 M of isopropyl- ⁇ -thiogalactopyranoside (IPTG) was added to the culture medium to a final concentration of 1 mM to induce the expression of modified fibroin.
- IPTG isopropyl- ⁇ -thiogalactopyranoside
- the washed precipitate was suspended in 8M guanidine buffer (8M guanidine hydrochloride, 10 mM sodium dihydrogen phosphate, 20 mM NaCl, 1 mM Tris-HCl, pH 7.0) to a concentration of 100 mg / mL at 60 ° C. Stir with a stirrer for 30 minutes to dissolve. After dissolution, dialysis was performed with water using a dialysis tube (cellulose tube 36/32 manufactured by Sanko Junyaku Co., Ltd.). The white aggregated protein obtained after dialysis was recovered by centrifugation, water was removed by a freeze-dryer, and the freeze-dried powder was recovered to obtain the desired modified fibroin protein (PRT799).
- 8M guanidine buffer 8M guanidine hydrochloride, 10 mM sodium dihydrogen phosphate, 20 mM NaCl, 1 mM Tris-HCl, pH 7.0
- a known dry-wet spinning was performed using the obtained doping liquid and the spinning device 10 shown in FIG. 4 to obtain a monofilament of spider silk fibroin.
- dry-wet spinning was performed under the following conditions.
- Extrude nozzle diameter 0.1 mm
- Extrusion speed 327.6 ml / h
- Coagulant (methanol) temperature 2 ° C
- Winding speed 99.5m / min
- Stretching ratio 4.52 times Drying temperature: 80 ° C
- Air gap length 5 mm
- a vinyl ester resin (Neopol 8025 manufactured by Japan U-Pica Co., Ltd.), calcium carbonate (Whiten SB blue manufactured by Bikita Powder Industry Co., Ltd.), the above-mentioned defibrating agent (A-1), and other additives are kneaded with a mixer.
- the resin composition was obtained.
- carbon fiber manufactured by Taiwan Plastics Co., Ltd., TC36P 12K (fiber bundle diameter 7 ⁇ m) cut into 1 inch (about 25 mm) is sprayed on it, and further.
- the obtained molded product was cut into a test piece of 100 ⁇ 25 mm to obtain a test piece for a bending test, and the bending characteristics of the test piece were measured using a bending tester (manufactured by Shimadzu Corporation, AG-20kNX).
- the test speed was 5 mm / min and the distance between the fulcrums was 80 mm.
- Table 6 The results are shown in Table 6.
- Example 2-1 A flat plate-shaped molded body (fiber reinforced composite material) having a size of 300 mm ⁇ 300 mm ⁇ 2 mm was obtained in the same manner as in Example 1-1.
- the 300 mm ⁇ 300 mm surface of the obtained molded body was divided into 12 ⁇ 10, and images were taken with a microscope (VHX-5000 manufactured by KEYENCE) for three randomly selected sections (1 section: 25 mm ⁇ 30 mm). The orientation of the fiber bundle of the carbon fiber was evaluated from the image.
- Comparative Example 2-1 A flat plate-shaped molded product (fiber reinforced composite material) having a size of 300 mm ⁇ 300 mm ⁇ 2 mm was obtained in the same manner as in Comparative Example 1-1. With respect to the obtained molded product, the orientation of the fiber bundles of carbon fibers was evaluated in the same manner as in Example 2-1. The results are shown in FIG. 5 (b).
- Example 2-2 [Protein production] A modified fibroin having the amino acid sequence represented by SEQ ID NO: 44 (spider silk fibroin, hereinafter also referred to as “PRT966”) was designed, and a nucleic acid encoding the modified fibroin PRT966 having the designed amino acid sequence represented by SEQ ID NO: 44 was prepared. Synthesized. Using the nucleic acid, modified fibroin (PRT966) was obtained in the same manner as in [Protein production] of Example 1-1.
- PRT966 spike silk fibroin
- a short fibrous spreading agent (A-2) having an average length of 0.5 mm was obtained in the same manner as in Example 1-1 except that the above-mentioned PRT966 was used instead of PRT799.
- Example 2-1 and Comparative Example 2-1 are compared, and Example 2-2 and Comparative Example 2-2 are compared, the examples have a more random orientation than the comparative example, and the fibers are opened. It was confirmed that the agent can also be used as an orientation inhibitor.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Gastroenterology & Hepatology (AREA)
- Materials Engineering (AREA)
- Textile Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- Polymers & Plastics (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Peptides Or Proteins (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2022536341A JPWO2022014525A1 (enExample) | 2020-07-15 | 2021-07-12 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020121461 | 2020-07-15 | ||
| JP2020-121461 | 2020-07-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022014525A1 true WO2022014525A1 (ja) | 2022-01-20 |
Family
ID=79554737
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2021/026099 Ceased WO2022014525A1 (ja) | 2020-07-15 | 2021-07-12 | 開繊剤 |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JPWO2022014525A1 (enExample) |
| WO (1) | WO2022014525A1 (enExample) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018116979A1 (ja) * | 2016-12-20 | 2018-06-28 | Spiber株式会社 | 繊維強化樹脂材および積層体 |
| JP2019044117A (ja) * | 2017-09-05 | 2019-03-22 | 国立研究開発法人農業・食品産業技術総合研究機構 | 繊維強化複合材料及びその製造方法 |
| WO2019066028A1 (ja) * | 2017-09-29 | 2019-04-04 | 内浜化成株式会社 | 繊維強化複合材、繊維強化複合材用添加材及び繊維強化複合材の製造方法 |
| WO2020067518A1 (ja) * | 2018-09-28 | 2020-04-02 | 小島プレス工業株式会社 | 射出成形体、射出成形用組成物、及び射出成形体の製造方法 |
-
2021
- 2021-07-12 WO PCT/JP2021/026099 patent/WO2022014525A1/ja not_active Ceased
- 2021-07-12 JP JP2022536341A patent/JPWO2022014525A1/ja active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018116979A1 (ja) * | 2016-12-20 | 2018-06-28 | Spiber株式会社 | 繊維強化樹脂材および積層体 |
| JP2019044117A (ja) * | 2017-09-05 | 2019-03-22 | 国立研究開発法人農業・食品産業技術総合研究機構 | 繊維強化複合材料及びその製造方法 |
| WO2019066028A1 (ja) * | 2017-09-29 | 2019-04-04 | 内浜化成株式会社 | 繊維強化複合材、繊維強化複合材用添加材及び繊維強化複合材の製造方法 |
| WO2020067518A1 (ja) * | 2018-09-28 | 2020-04-02 | 小島プレス工業株式会社 | 射出成形体、射出成形用組成物、及び射出成形体の製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2022014525A1 (enExample) | 2022-01-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7285485B2 (ja) | 繊維強化複合材、繊維強化複合材用添加材及び繊維強化複合材の製造方法 | |
| WO2018116979A1 (ja) | 繊維強化樹脂材および積層体 | |
| JP7088511B2 (ja) | フィブロイン様タンパク質を含むコンポジット成形組成物及びその製造方法 | |
| US11174572B2 (en) | Composite molding composition including fibroin-like protein, and method for producing composite molding composition | |
| JP7372678B2 (ja) | 成形体及びその製造方法 | |
| JP7142854B2 (ja) | 繊維強化樹脂成形体および繊維強化樹脂成形体の製造方法 | |
| WO2019151429A1 (ja) | タンパク質繊維の製造方法 | |
| WO2018164189A1 (ja) | タンパク質成形体及びこれを製造する方法、並びにタンパク質溶液 | |
| WO2022014525A1 (ja) | 開繊剤 | |
| JP7366359B2 (ja) | 射出成形体、射出成形用組成物、及び射出成形体の製造方法 | |
| JP7323894B2 (ja) | 軟質ポリウレタンフォーム、自動車用シートパッド、及び軟質ポリウレタンフォームの製造方法 | |
| JP7724501B2 (ja) | 繊維強化複合材の製造方法 | |
| JP2024052853A (ja) | 難燃性組成物 | |
| WO2020027153A1 (ja) | 改変フィブロイン繊維及びその製造方法 | |
| JPWO2020067554A1 (ja) | 成形体の製造方法および構造タンパク質成形体 | |
| JP7356115B2 (ja) | 樹脂成形品及びその製造方法 | |
| JP2022001669A (ja) | タンパク質繊維の製造方法 | |
| WO2019151437A1 (ja) | タンパク質紡績糸の製造方法 | |
| JPWO2019151436A1 (ja) | タンパク質捲縮ステープルの製造方法 | |
| JPWO2020067553A1 (ja) | タンパク質紡績糸の製造方法 | |
| JP2020121962A (ja) | タンパク質フィルム及びタンパク質フィルムの製造方法 | |
| WO2019151432A1 (ja) | 油剤付着タンパク質捲縮繊維の製造方法 | |
| WO2025187819A1 (ja) | 繊維強化複合材及びその製造方法 | |
| JPWO2019066006A1 (ja) | 撚糸の製造方法、仮撚り糸の製造方法、及び糸の撚り加工方法 | |
| JP2021055197A (ja) | タンパク質繊維の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21841429 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2022536341 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 21841429 Country of ref document: EP Kind code of ref document: A1 |