WO2020130012A1 - Lds用熱硬化性樹脂組成物および半導体装置の製造方法 - Google Patents
Lds用熱硬化性樹脂組成物および半導体装置の製造方法 Download PDFInfo
- Publication number
- WO2020130012A1 WO2020130012A1 PCT/JP2019/049527 JP2019049527W WO2020130012A1 WO 2020130012 A1 WO2020130012 A1 WO 2020130012A1 JP 2019049527 W JP2019049527 W JP 2019049527W WO 2020130012 A1 WO2020130012 A1 WO 2020130012A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- resin composition
- thermosetting resin
- lds
- component
- mass
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/01—Use of inorganic substances as compounding ingredients characterized by their specific function
- C08K3/013—Fillers, pigments or reinforcing additives
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/20—Oxides; Hydroxides
- C08K3/22—Oxides; Hydroxides of metals
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G59/00—Polycondensates containing more than one epoxy group per molecule; Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups
- C08G59/18—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing
- C08G59/40—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing characterised by the curing agents used
- C08G59/62—Alcohols or phenols
- C08G59/621—Phenols
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G59/00—Polycondensates containing more than one epoxy group per molecule; Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups
- C08G59/18—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing
- C08G59/68—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing characterised by the catalysts used
- C08G59/688—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing characterised by the catalysts used containing phosphorus
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/34—Silicon-containing compounds
- C08K3/36—Silica
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/54—Silicon-containing compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L39/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a single or double bond to nitrogen or by a heterocyclic ring containing nitrogen; Compositions of derivatives of such polymers
- C08L39/04—Homopolymers or copolymers of monomers containing heterocyclic rings having nitrogen as ring member
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L63/00—Compositions of epoxy resins; Compositions of derivatives of epoxy resins
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L63/00—Compositions of epoxy resins; Compositions of derivatives of epoxy resins
- C08L63/04—Epoxynovolacs
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/50—Assembly of semiconductor devices using processes or apparatus not provided for in a single one of the groups H01L21/18 - H01L21/326 or H10D48/04 - H10D48/07 e.g. sealing of a cap to a base of a container
- H01L21/56—Encapsulations, e.g. encapsulation layers, coatings
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/28—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection
- H01L23/29—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection characterised by the material, e.g. carbon
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/28—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection
- H01L23/29—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection characterised by the material, e.g. carbon
- H01L23/293—Organic, e.g. plastic
- H01L23/295—Organic, e.g. plastic containing a filler
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/28—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection
- H01L23/31—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection characterised by the arrangement or shape
- H01L23/3107—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection characterised by the arrangement or shape the device being completely enclosed
- H01L23/3121—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection characterised by the arrangement or shape the device being completely enclosed a substrate forming part of the encapsulation
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/20—Oxides; Hydroxides
- C08K3/22—Oxides; Hydroxides of metals
- C08K2003/2248—Oxides; Hydroxides of metals of copper
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/20—Oxides; Hydroxides
- C08K3/22—Oxides; Hydroxides of metals
- C08K2003/2251—Oxides; Hydroxides of metals of chromium
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L2224/00—Indexing scheme for arrangements for connecting or disconnecting semiconductor or solid-state bodies and methods related thereto as covered by H01L24/00
- H01L2224/01—Means for bonding being attached to, or being formed on, the surface to be connected, e.g. chip-to-package, die-attach, "first-level" interconnects; Manufacturing methods related thereto
- H01L2224/10—Bump connectors; Manufacturing methods related thereto
- H01L2224/15—Structure, shape, material or disposition of the bump connectors after the connecting process
- H01L2224/16—Structure, shape, material or disposition of the bump connectors after the connecting process of an individual bump connector
- H01L2224/161—Disposition
- H01L2224/16151—Disposition the bump connector connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive
- H01L2224/16221—Disposition the bump connector connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive the body and the item being stacked
- H01L2224/16225—Disposition the bump connector connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive the body and the item being stacked the item being non-metallic, e.g. insulating substrate with or without metallisation
- H01L2224/16227—Disposition the bump connector connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive the body and the item being stacked the item being non-metallic, e.g. insulating substrate with or without metallisation the bump connector connecting to a bond pad of the item
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L2224/00—Indexing scheme for arrangements for connecting or disconnecting semiconductor or solid-state bodies and methods related thereto as covered by H01L24/00
- H01L2224/01—Means for bonding being attached to, or being formed on, the surface to be connected, e.g. chip-to-package, die-attach, "first-level" interconnects; Manufacturing methods related thereto
- H01L2224/10—Bump connectors; Manufacturing methods related thereto
- H01L2224/15—Structure, shape, material or disposition of the bump connectors after the connecting process
- H01L2224/16—Structure, shape, material or disposition of the bump connectors after the connecting process of an individual bump connector
- H01L2224/161—Disposition
- H01L2224/16151—Disposition the bump connector connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive
- H01L2224/16221—Disposition the bump connector connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive the body and the item being stacked
- H01L2224/16245—Disposition the bump connector connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive the body and the item being stacked the item being metallic
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L2224/00—Indexing scheme for arrangements for connecting or disconnecting semiconductor or solid-state bodies and methods related thereto as covered by H01L24/00
- H01L2224/01—Means for bonding being attached to, or being formed on, the surface to be connected, e.g. chip-to-package, die-attach, "first-level" interconnects; Manufacturing methods related thereto
- H01L2224/42—Wire connectors; Manufacturing methods related thereto
- H01L2224/47—Structure, shape, material or disposition of the wire connectors after the connecting process
- H01L2224/48—Structure, shape, material or disposition of the wire connectors after the connecting process of an individual wire connector
- H01L2224/481—Disposition
- H01L2224/48151—Connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive
- H01L2224/48221—Connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive the body and the item being stacked
- H01L2224/48225—Connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive the body and the item being stacked the item being non-metallic, e.g. insulating substrate with or without metallisation
- H01L2224/48227—Connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive the body and the item being stacked the item being non-metallic, e.g. insulating substrate with or without metallisation connecting the wire to a bond pad of the item
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L2224/00—Indexing scheme for arrangements for connecting or disconnecting semiconductor or solid-state bodies and methods related thereto as covered by H01L24/00
- H01L2224/01—Means for bonding being attached to, or being formed on, the surface to be connected, e.g. chip-to-package, die-attach, "first-level" interconnects; Manufacturing methods related thereto
- H01L2224/42—Wire connectors; Manufacturing methods related thereto
- H01L2224/47—Structure, shape, material or disposition of the wire connectors after the connecting process
- H01L2224/48—Structure, shape, material or disposition of the wire connectors after the connecting process of an individual wire connector
- H01L2224/481—Disposition
- H01L2224/48151—Connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive
- H01L2224/48221—Connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive the body and the item being stacked
- H01L2224/48245—Connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive the body and the item being stacked the item being metallic
- H01L2224/48247—Connecting between a semiconductor or solid-state body and an item not being a semiconductor or solid-state body, e.g. chip-to-substrate, chip-to-passive the body and the item being stacked the item being metallic connecting the wire to a bond pad of the item
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L2924/00—Indexing scheme for arrangements or methods for connecting or disconnecting semiconductor or solid-state bodies as covered by H01L24/00
- H01L2924/15—Details of package parts other than the semiconductor or other solid state devices to be connected
- H01L2924/181—Encapsulation
- H01L2924/1815—Shape
Definitions
- the present invention relates to a thermosetting resin composition for LDS and a method for manufacturing a semiconductor device.
- Patent Document 1 JP-A-2015-134903 describes a fiber-reinforced resin material obtained by impregnating continuous fibers with a thermoplastic resin composition containing an LDS additive, and a polyamide resin is used as the thermoplastic resin. It is used.
- Patent Document 2 JP-A-2005-163682 discloses a hydrogenated product of a crystalline cyclic olefin ring-opening polymer having repeating units derived from a polycyclic norbornene-based monomer having three or more rings. It describes a polymer composition containing a glass filler and a metal oxide in a specific amount, respectively, whereby it is possible to achieve good electrical characteristics (low dielectric loss tangent), plating adhesion, and reflow heat resistance. Has been done.
- Patent Document 3 Japanese Patent Publication No. 2017-513794 discloses color, flame retardance, process modification, smoke emission, etc. regarding the laser direct structuring process and many additional functions proved in the art.
- Patent Document 3 Japanese Patent Publication No. 2017-513794 discloses color, flame retardance, process modification, smoke emission, etc. regarding the laser direct structuring process and many additional functions proved in the art.
- the present invention provides a technique that is excellent in plating characteristics during fine processing by LDS and that can reduce the wiring width and the wiring interval when forming a circuit.
- thermosetting resin composition for LDS used in LASER DIRECT STRUCTURING (A) a thermosetting resin, (B) an inorganic filler, (C) a non-conductive metal compound that forms a metal nucleus upon irradiation with an active energy ray, (D) a coupling agent,
- the component (A) contains at least one selected from the group consisting of epoxy resins and bismaleimide resins,
- a thermosetting resin composition for LDS which satisfies at least one of the following Condition 1 or Condition 2.
- thermosetting resin composition for granules is granular, and the collapse angle of the thermosetting resin composition for LDS is 35° or less
- thermosetting resin composition for LDS LASER DIRECT STRUCTURING
- a sealing material by sealing the semiconductor element with a cured product of a thermosetting resin composition for LDS (LASER DIRECT STRUCTURING) so as to cover the surface of the semiconductor element; Irradiating a specific portion of the surface of the encapsulant with an active energy ray, A step of hydrophilizing the surface of the sealing material, A step of selectively forming a metal layer in a region of the surface of the encapsulant irradiated with the active energy rays; Including, There is provided a method for manufacturing a semiconductor device, wherein the thermosetting resin composition for LDS is the thermosetting resin composition for LDS in the present invention.
- thermosetting resin composition for LDS (LASER DIRECT STRUCTURING) so as to cover the surface of the semiconductor element; Irradiating a specific portion of the surface of the encapsulant with an active energy ray, A step of hydrophilizing the surface of the sealing material, A step of selectively forming a metal layer in a region of the surface of the encapsulant irradiated with the active energy rays; Including, The thermosetting resin composition for LDS, (A) a thermosetting resin, (B) an inorganic filler, (C) a non-conductive metal compound that forms a metal nucleus upon irradiation with an active energy ray, (D) a coupling agent, There is provided a method for manufacturing a semiconductor device, wherein the component (A) contains at least one selected from the group consisting of epoxy resins and bismaleimide resins.
- thermosetting resin composition for LDS of the present invention there is provided a resin molded article including the cured product of the thermosetting resin composition for LDS of the present invention.
- a resin molded article according to the present invention having a three-dimensional structure, And a three-dimensional circuit formed on the surface of the resin molded product.
- the present invention it is possible to provide a technique that is excellent in plating characteristics during fine processing by LDS and that can reduce the wiring width and the wiring interval when forming a circuit.
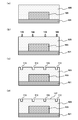
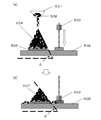
- FIG. 6 is a cross-sectional view showing the manufacturing process of the semiconductor device according to the embodiment. It is a sectional view showing composition of a semiconductor device in an embodiment. It is a schematic diagram showing a measuring method of a collapse angle ( ⁇ ) and a repose angle ( ⁇ ).
- This embodiment provides a resin composition which is excellent in moldability and plating characteristics during fine processing by LDS, and can reduce the wiring width and the wiring interval when forming a circuit.
- thermosetting resin composition for LDS used in LASER DIRECT STRUCTURING (A) a thermosetting resin, (B) an inorganic filler, (C) a non-conductive metal compound that forms a metal nucleus upon irradiation with an active energy ray, (D) a coupling agent,
- the component (A) contains at least one selected from the group consisting of epoxy resins and bismaleimide resins,
- a thermosetting resin composition for LDS wherein the ratio of particles having a particle size of 1 ⁇ m or more and 20 ⁇ m or less in the component (B) is 40% by volume or more and 95% by volume or less with respect to the entire component (B).
- a resin molded product including the cured product of the thermosetting resin composition for LDS in the above embodiment.
- a resin molded article according to the embodiment having a three-dimensional structure, And a three-dimensional circuit formed on the surface of the resin molded product.
- the thermosetting resin composition is a thermosetting resin composition for LDS used for laser direct structuring (LDS).
- LDS is one of the manufacturing methods of a three-dimensional molded circuit component (Molded Interconnect Device: MID).
- MID Molded Interconnect Device
- the surface of a resin molded article containing an LDS additive is irradiated with active energy rays to generate metal nuclei, and the metal nuclei are used as seeds by a plating treatment such as electroless plating.
- a plating pattern is formed in the energy ray irradiation area.
- a conductive member such as a wiring or a circuit can be formed based on this plating pattern.
- thermosetting resin composition for LDS contains the following components (A) to (D).
- Thermosetting resin B) Inorganic filler
- C Non-conductive metal compound
- component (A) is epoxy resin and bis.
- the content of particles having a particle size of 1 ⁇ m or more and 20 ⁇ m or less in the component (B) is 40% by volume or more and 95% by volume, including at least one selected from the group consisting of maleimide resins. % Or less.
- thermosetting resin composition in the thermosetting resin composition, by appropriately selecting the type of the component (A) and the size of the component (B), the thermosetting resin composition is excellent in moldability and thermosetting.
- the cured product of the resin composition is finely processed by LDS, excellent plating characteristics can be obtained, and the wiring width and the wiring interval can be reduced when forming a circuit.
- each component of the thermosetting resin composition of this embodiment will be described.
- the component (A) is a thermosetting resin.
- the component (A) includes at least one selected from the group consisting of an epoxy resin and a bismaleimide resin.
- the component (A) preferably contains an epoxy resin, more preferably an epoxy resin.
- the component (A) preferably contains a bismaleimide resin, more preferably a bismaleimide resin.
- epoxy resin general monomers, oligomers and polymers having two or more epoxy groups in one molecule can be used, and the molecular weight and the molecular structure thereof are not limited.
- the epoxy resin include biphenyl type epoxy resin; bisphenol A type epoxy resin, bisphenol F type epoxy resin, bisphenol type epoxy resin such as tetramethylbisphenol F type epoxy resin, stilbene type epoxy resin, phenol novolac type epoxy resin, cresol novolac type.
- Novolak type epoxy resin such as epoxy resin; polyfunctional epoxy resin such as triphenol methane type epoxy resin, trisphenol type epoxy resin such as alkyl-modified triphenol methane type epoxy resin; phenol aralkyl type epoxy resin having phenylene skeleton A phenol aralkyl type epoxy resin such as a naphthol aralkyl type epoxy resin having a phenylene skeleton, a phenol aralkyl type epoxy resin having a biphenylene skeleton, a naphthol aralkyl type epoxy resin having a biphenylene skeleton; a dihydroxynaphthalene type epoxy resin, a dimer of dihydroxynaphthalene Naphthol type epoxy resin such as epoxy resin obtained by glycidyl etherification; triazine nucleus-containing epoxy resin such as triglycidyl isocyanurate, monoallyl diglycidyl isocyanurate; bridged cyclic hydrocarbon such as dicyclopen
- novolac type epoxy resin polyfunctional Epoxy resin and phenol aralkyl type epoxy resin
- the epoxy resin preferably contains at least one selected from the group consisting of an orthocresol novolac type epoxy resin, a phenol aralkyl type epoxy resin having a biphenylene skeleton, and a triphenylmethane type epoxy resin, and Preferably, at least one selected from the group consisting of orthocresol novolac type epoxy resin and phenol aralkyl type epoxy resin having a biphenylene skeleton is included.
- the bismaleimide resin is a (co)polymer of a compound having two or more maleimide groups.
- the compound having two or more maleimide groups includes, for example, at least one of the compound represented by the following general formula (1) and the compound represented by the following general formula (2).
- R 1 is a divalent organic group having 1 to 30 carbon atoms and may contain at least one kind of oxygen atom and nitrogen atom. From the viewpoint of improving the heat resistance of the cured product, R 1 is more preferably an organic group containing an aromatic ring.
- examples of R 1 include structures represented by the following general formula (1a) or (1b).
- R 31 is a divalent organic group having 1 to 18 carbon atoms, which may contain at least one kind of oxygen atom and nitrogen atom. Further, a plurality of R 32 's each independently represent a hydrogen atom or a substituted or unsubstituted hydrocarbon group having 1 to 4 carbon atoms.
- a plurality of Rs independently exist, and R represents a hydrogen atom, an alkyl group having 1 to 5 carbon atoms or a phenyl group, and preferably a hydrogen atom.
- m is an average value and is a number of 1 or more and 5 or less, preferably a number of more than 1 and 5 or less, more preferably a number of more than 1 and 3 or less, and further preferably a number of more than 1 and 2 or less. is there.
- Examples of the compound represented by the general formula (1) that can be applied in the present embodiment include compounds represented by the following formulas (1-1) to (1-3).
- the plurality of R 2 's each independently represent a hydrogen atom or a substituted or unsubstituted hydrocarbon group having 1 to 4 carbon atoms.
- n is an average value and is a number of 0 or more and 10 or less, preferably a number of 0 or more and 5 or less.
- the component (A) may further contain other thermosetting resin.
- thermosetting resin include benzoxazine resin, phenol resin, urea (urea) resin, melamine resin, unsaturated polyester resin, polyurethane resin, diallyl phthalate resin, silicone resin, cyanate resin, polyimide resin, One or more selected from the group consisting of a polyamide-imide resin and a benzocyclobutene resin can be mentioned.
- the component (A) can contain a resin curing agent such as a phenol resin curing agent described later.
- the content of the component (A) in the thermosetting resin composition is, with respect to the thermosetting resin composition as a whole, from the viewpoint of improving fluidity at the time of molding to improve filling property and molding stability. It is preferably 1% by mass or more, more preferably 2% by mass or more, and further preferably 2.5% by mass or more. On the other hand, the content of the component (A) is preferably 15 mass with respect to the entire thermosetting resin composition from the viewpoint of improving the moisture resistance reliability and reflow resistance, and from the viewpoint of suppressing the warpage of the molded body. % Or less, more preferably 14% by mass or less, and further preferably 13% by mass or less.
- the content with respect to the entire thermosetting resin composition when the thermosetting resin composition contains a solvent, the entire solid content excluding the solvent of the thermosetting resin composition.
- the solid content of the thermosetting resin composition refers to the non-volatile content in the thermosetting resin composition, and refers to the balance excluding volatile components such as water and solvent.
- Component (B) is an inorganic filler.
- Component (B) is, for example, fused silica such as fused crushed silica or fused spherical silica; silica such as crystalline silica or amorphous silica; silicon dioxide; alumina; aluminum hydroxide; silicon nitride; and aluminum nitride. It contains one or more materials selected. From the viewpoint of making mechanical properties or thermal properties of the cured product of the thermosetting resin composition preferable, the component (B) preferably contains fused crushed silica, fused spherical silica, silica such as crystalline silica, and more preferably It is silica.
- the proportion of particles having a particle size of 1 ⁇ m or more and 20 ⁇ m or less improves the plating property during fine processing by LDS, and the wiring width and wiring spacing (line and line) when forming a circuit. From the viewpoint of reducing the (space), it is 40% by volume or more, and more preferably 70% by volume or more, based on the whole of the component (B). Further, the proportion of particles having a particle size of 1 ⁇ m or more and 20 ⁇ m or less in the component (B) is 95% by volume or less from the viewpoint of improving moldability.
- the d 10 particle size of the component (B) is, for example, 0.03 ⁇ m or more, preferably 0.05 ⁇ m or more, more preferably 0.1 ⁇ m or more, and further preferably 0.3 ⁇ m or more. is there.
- the d 10 particle diameter of the component (B) is preferably 3 ⁇ m or less, more preferably 2.0 ⁇ m or less, further preferably 1.0 ⁇ m or less, More preferably, it is 0.8 ⁇ m or less.
- the d 50 particle size of the component (B) is preferably 1.0 ⁇ m or more, and more preferably 2.0 ⁇ m or more, from the viewpoint of improving moldability.
- the d 50 particle diameter of the component (B) is preferably 10 ⁇ m or less, more preferably 7.0 ⁇ m or less, and further preferably 5.0 ⁇ m or less, from the viewpoint of reducing the plating wiring width after laser processing. ..
- the maximum diameter d max of the component (B) is preferably 5.0 ⁇ m or more, more preferably 6.5 ⁇ m or more, still more preferably 8.0 ⁇ m or more, from the viewpoint of improving moldability.
- the maximum diameter d max of the component (B) is preferably 80 ⁇ m or less, more preferably 60 ⁇ m or less, and even more preferably from the viewpoint of reducing the wiring width and the wiring interval (line and space) when forming a circuit. Is 40 ⁇ m or less.
- the maximum diameter d max of the component (B) may be preferably 15 ⁇ m or less, more preferably 12 ⁇ m or less, still more preferably 10 ⁇ m or less.
- the particle size distribution of the particles can be measured on a volume basis using a commercially available laser diffraction particle size distribution measuring device (for example, SALD-7000 manufactured by Shimadzu Corporation).
- the content of the component (B) in the thermosetting resin composition is preferably 65% by mass or more based on the whole thermosetting resin composition from the viewpoint of improving the heat resistance and moisture resistance of the cured product. It is more preferably 70% by mass or more, and further preferably 75% by mass or more. On the other hand, the content of the component (B) is preferably 95 relative to the total amount of the thermosetting resin composition from the viewpoint of more effectively improving the fluidity and filling property during molding of the thermosetting resin composition. It is not more than 90% by mass, more preferably not more than 90% by mass, further preferably not more than 85% by mass.
- the component (C) is a non-conductive metal compound that forms a metal nucleus upon irradiation with active energy rays. Such compounds act as LDS additives.
- the component (C) is not limited as long as it can form a metal nucleus by irradiation with an active energy ray. Although the detailed mechanism is not clear, such a non-conductive metal compound activates (for example, is reduced) when the metal nucleus is activated when irradiated with an active energy ray such as a YAG laser having a wavelength range that can be absorbed. ), it is considered that a metal nucleus capable of metal plating is generated.
- a seed region having a metal nucleus capable of metal plating is formed on the irradiated surface. To be done. By using the obtained seed region, it becomes possible to form a plating pattern such as a circuit on the surface of the cured product of the thermosetting resin composition.
- the component (C) is, for example, (i) a spinel-type metal oxide, (ii) two or more transition metal elements selected from the groups 3 to 12 of the periodic table, and adjacent to the group. And (iii) one or more selected from the group consisting of tin-containing oxides.
- the spinel type structure is one of typical crystal structure types found in AB 2 O 4 type compounds (A and B are metallic elements) which are complex oxides.
- the spinel structure may be either a normal spinel structure or a reverse spinel structure (B(AB)O 4 ) (A and B are partially replaced), but the normal spinel structure is more preferably used.
- a of the forward spinel structure may be copper.
- the metal atom constituting the spinel type metal oxide for example, copper or chromium can be used. That is, the component (C) can contain a spinel-type metal oxide containing copper or chromium.
- copper can be used as the metal atom from the viewpoint of improving the adhesion to the copper plating pattern.
- metal atoms in addition to copper and chromium, trace amounts of metal atoms such as antimony, tin, lead, indium, iron, cobalt, nickel, zinc, cadmium, silver, bismuth, arsenic, manganese, magnesium and calcium are contained. May be These trace metal atoms may be present as oxides. Moreover, the content of each of the trace metal atoms can be 0.001% by mass or less based on the total amount of the metal atoms in the metal oxide.
- the spinel-type metal oxide has high thermal stability and can have durability in an acidic or alkaline aqueous metallization bath.
- the spinel type metal oxide is, for example, by appropriately controlling the dispersibility of the thermosetting resin composition, in a high oxide state, in the unirradiated region on the surface of the cured product of the thermosetting resin composition.
- the metal oxide having a transition metal element of (ii) above is selected from the groups 3 to 12 of the periodic table, and the metal oxide having two or more transition metal elements adjacent to the group. Is.
- the metal belonging to the above transition metal element can be expressed as containing a metal of group n and a metal of group n+1 of the periodic table.
- oxides of these metals may be used alone or in combination of two or more kinds.
- Examples of metals belonging to group n of the periodic table include group 3 (scandium, yttrium), group 4 (titanium, zirconium, etc.), group 5 (vanadium, niobium, etc.), group 6 (chromium, molybdenum, etc.), group 7 (Manganese etc.), group 8 (iron, ruthenium etc.), group 9 (cobalt, rhodium, iridium etc.), group 10 (nickel, palladium, platinum) group 11 (copper, silver, gold etc.), group 12 (zinc) , Cadmium), and Group 13 (aluminum, gallium, indium, etc.).
- Examples of metals of the n+1 group of the periodic table include group 4 (titanium, zirconium, etc.), group 5 (vanadium, niobium, etc.), group 6 (chromium, molybdenum, etc.), group 7 (manganese, etc.), group 8 (iron). , Ruthenium, etc., Group 9 (cobalt, rhodium, iridium, etc.) Group 10 (nickel, palladium, platinum), group 11 (copper, silver, gold, etc.), group 12 (zinc, cadmium, etc.), group 13 (aluminum) , Gallium, indium, etc.).
- the metal oxide having the above-mentioned transition metal element for example, those described in Japanese Patent Publication No. 2004-534408 can be cited.
- the tin-containing oxide of (iii) above is a metal oxide containing at least tin.
- the metal atom constituting the tin-containing oxide may contain antimony in addition to tin.
- Such a tin-containing oxide can contain tin oxide and antimony oxide. More specifically, in the metal component contained in the tin-containing oxide, 90% by mass or more may be tin and 5% by mass or more may be antimony.
- the tin-containing oxide may further contain lead and/or copper as a metal component. Specifically, in the metal component contained in the tin-containing oxide, for example, 90% by mass or more is tin, 5 to 9% by mass is antimony, and 0.01 to 0.1% by mass.
- Such a tin-containing oxide can contain, for example, tin oxide and antimony oxide, and one or more kinds of lead oxide and copper oxide.
- the tin-containing oxide may contain a trace amount of metal atom, which is exemplified as the spinel-type metal oxide.
- the tin-containing oxide may be used in combination with the spinel-type metal oxide (i) or the metal oxide having a transition metal element (ii).
- the content of the component (C) in the thermosetting resin composition is from the viewpoint of improving the plating property in the cured product of the thermosetting resin composition with respect to the entire thermosetting resin composition. For example, it is 2% by mass or more, preferably 4% by mass or more, and more preferably 8% by mass or more.
- the flow of the thermosetting resin composition can be prevented from the viewpoints of suppressing lowering of insulating property and increase of dielectric loss tangent, and when the shape of the component (C) is non-spherical.
- the content of the component (C) is, for example, 20% by mass or less, preferably 18% by mass or less, and more preferably 15% by mass, based on the entire thermosetting resin composition. % Or less.
- the total content of the component (B) and the component (C) in the thermosetting resin composition is preferably the total amount of the thermosetting resin composition from the viewpoint of improving resin mechanical properties and resin strength. It is 70% by mass or more, and more preferably 75% by mass or more. On the other hand, from the viewpoint of improving moldability, the total content of the component (B) and the component (C) is preferably 98% by mass or less, and more preferably 95% by mass, based on the entire thermosetting resin composition. % Or less.
- Component (D) is a coupling agent.
- Component (D) is preferably one or more selected from the group consisting of mercaptosilane, aminosilane and epoxysilane, from the viewpoint of improving moldability by optimizing the viscosity of the thermosetting resin composition. including. From the viewpoint of continuous moldability, mercaptosilane is preferable, from the viewpoint of fluidity, secondary aminosilane is preferable, and from the viewpoint of adhesion, epoxysilane is preferable.
- epoxy silanes include, for example, ⁇ -glycidoxypropyltriethoxysilane, ⁇ -glycidoxypropyltrimethoxysilane, ⁇ -glycidoxypropylmethyldimethoxysilane, ⁇ -(3,4-epoxycyclohexyl)ethyl.
- examples thereof include trimethoxysilane, and preferably ⁇ -glycidoxypropylmethyldimethoxysilane.
- aminosilanes include phenylaminopropyltrimethoxysilane, ⁇ -aminopropyltriethoxysilane, ⁇ -aminopropyltrimethoxysilane, N- ⁇ (aminoethyl) ⁇ -aminopropyltrimethoxysilane, and N- ⁇ (aminoethyl) ⁇ -aminopropylmethyldimethoxysilane, N-phenyl- ⁇ -aminopropyltriethoxysilane, N-phenyl- ⁇ -aminopropyltrimethoxysilane, N- ⁇ (aminoethyl) ⁇ -aminopropyltriethoxysilane, N-( 6-aminohexyl)3-aminopropyltrimethoxysilane, N-(3-(trimethoxysilylpropyl)-1,3-benzenedimethanane, and the like, and preferably N
- Examples of mercaptosilanes include ⁇ -mercaptopropyltrimethoxysilane, 3-mercaptopropylmethyldimethoxysilane, bis(3-triethoxysilylpropyl)tetrasulfide, and bis(3-triethoxysilylpropyl)disulfide.
- Examples thereof include silane coupling agents that exhibit the same function as the mercaptosilane coupling agent by thermal decomposition, and ⁇ -mercaptopropyltrimethoxysilane is preferable.
- silane coupling agents may be preliminarily hydrolyzed and mixed. These silane coupling agents may be used alone or in combination of two or more.
- the content of the component (D) in the thermosetting resin composition is based on the whole thermosetting resin composition from the viewpoint of improving the injection moldability by increasing the flow length of the thermosetting resin composition. It is preferably 0.01% by mass or more, more preferably 0.05% by mass or more, still more preferably 0.1% by mass or more. On the other hand, from the viewpoint of suppressing an increase in water absorption of the cured product of the thermosetting resin composition and obtaining good rust prevention, the content of the component (D) is based on the entire thermosetting resin composition. It is preferably 1% by mass or less, more preferably 0.8% by mass or less, still more preferably 0.6% by mass or less.
- thermosetting resin composition may include components other than the components (A) to (D) described above.
- thermosetting resin composition may further include (E) a curing accelerator.
- Component (E) is a curing accelerator. Any curing accelerator may be used as long as it accelerates the crosslinking reaction between the thermosetting resin and the curing agent, and those used in general thermosetting resin compositions can be used.
- Component (E) is a phosphorus atom-containing compound such as an organic phosphine, a tetra-substituted phosphonium compound, a phosphobetaine compound, an adduct of a phosphine compound and a quinone compound, an adduct of a phosphonium compound and a silane compound; 1,8-diazabicyclo [5.4.0] Undecene-7, benzyldimethylamine, one kind selected from nitrogen atom-containing compounds such as tertiary amines, quaternary salts of the above-mentioned amidines and amines such as 2-methylimidazole, etc. Alternatively, two or more kinds can be included.
- a phosphorus atom-containing compound such as an organic phosphine, a tetra-substituted phosphonium compound, a phosphobetaine compound, an adduct of a phosphine compound and a quinone
- component (E) preferably comprises one or more selected from tetraphenylphosphonium bis(naphthalene-2,3-dioxy)phenyl silicate and traphenylphosphonium-4,4'-sulphonyl diphenolate. ..
- organic phosphine examples include a first phosphine such as ethylphosphine and phenylphosphine; a second phosphine such as dimethylphosphine and diphenylphosphine; and a third phosphine such as trimethylphosphine, triethylphosphine, tributylphosphine and triphenylphosphine.
- first phosphine such as ethylphosphine and phenylphosphine
- second phosphine such as dimethylphosphine and diphenylphosphine
- a third phosphine such as trimethylphosphine, triethylphosphine, tributylphosphine and triphenylphosphine.
- Examples of the tetra-substituted phosphonium compound include compounds represented by the following general formula (6).
- P represents a phosphorus atom.
- R 4 , R 5 , R 6 and R 7 represent an aromatic group or an alkyl group.
- A is selected from a hydroxyl group, a carboxyl group and a thiol group.
- Represents an organic acid, x and y are numbers 1 to 3, z is a number 0 to 3, and x y.
- the compound represented by the general formula (6) can be obtained, for example, as follows, but is not limited thereto. First, a tetra-substituted phosphonium halide, an aromatic organic acid, and a base are mixed in an organic solvent and uniformly mixed to generate an aromatic organic acid anion in the solution system. Then, by adding water, the compound represented by the general formula (6) can be precipitated.
- R 4 , R 5 , R 6 and R 7 bonded to the phosphorus atom are phenyl groups
- AH is a compound having a hydroxyl group in the aromatic ring, that is, phenols.
- A is preferably the anion of the phenols.
- phenols examples include monocyclic phenols such as phenol, cresol, resorcin, and catechol, condensed polycyclic phenols such as naphthol, dihydroxynaphthalene, and anthraquinol, bisphenols such as bisphenol A, bisphenol F, and bisphenol S, Examples include polycyclic phenols such as phenylphenol and biphenol.
- Examples of the phosphobetaine compound include compounds represented by the following general formula (7).
- P represents a phosphorus atom
- R 8 represents an alkyl group having 1 to 3 carbon atoms
- R 9 represents a hydroxyl group
- f is a number of 0 to 5
- g is 0 to It is a number of 3.
- the compound represented by the general formula (7) is obtained, for example, as follows. First, it is obtained through a step of bringing a triaromatic-substituted phosphine, which is a third phosphine, into contact with a diazonium salt and substituting the triaromatic-substituted phosphine with the diazonium group contained in the diazonium salt. However, it is not limited to this.
- Examples of the adduct of the phosphine compound and the quinone compound include compounds represented by the following general formula (8).
- P represents a phosphorus atom.
- R 10 , R 11 and R 12 represent an alkyl group having 1 to 12 carbon atoms or an aryl group having 6 to 12 carbon atoms and are the same as each other;
- R 13 , R 14 and R 15 represent a hydrogen atom or a hydrocarbon group having 1 to 12 carbon atoms and may be the same or different, and R 14 and R 15 are bonded to each other. And may have a ring structure.
- Examples of the phosphine compound used for the adduct of the phosphine compound and the quinone compound include triphenylphosphine, tris(alkylphenyl)phosphine, tris(alkoxyphenyl)phosphine, trinaphthylphosphine, tris(benzyl)phosphine, etc.
- Those having a substituent or a substituent such as an alkyl group and an alkoxyl group are preferable, and examples of the substituent such as an alkyl group and an alkoxyl group include those having a carbon number of 1 to 6.
- Triphenylphosphine is preferable from the viewpoint of easy availability.
- examples of the quinone compound used in the adduct of the phosphine compound and the quinone compound include benzoquinone and anthraquinones, and among them, p-benzoquinone is preferable from the viewpoint of storage stability.
- the adduct can be obtained by contacting and mixing in a solvent in which both the organic tertiary phosphine and the benzoquinone can be dissolved.
- a solvent in which both the organic tertiary phosphine and the benzoquinone can be dissolved.
- ketones such as acetone and methyl ethyl ketone, which have low solubility in adducts, are preferable. However, it is not limited to this.
- R 10 , R 11 and R 12 bonded to the phosphorus atom are phenyl groups, and R 13 , R 14 and R 15 are hydrogen atoms, that is, 1,
- a compound to which 4-benzoquinone and triphenylphosphine are added is preferable from the viewpoint of lowering the elastic modulus of the cured product of the thermosetting resin composition when heated.
- thermosetting resin composition of the present embodiment examples include compounds represented by the following general formula (9), and preferably tetraphenylphosphonium bis. (Naphthalene-2,3-dioxy)phenyl silicate may be mentioned.
- P represents a phosphorus atom and Si represents a silicon atom.
- R 16 , R 17 , R 18 and R 19 are each an organic group having an aromatic ring or a heterocycle, or a fatty group.
- R 20 represents a group group and may be the same or different from each other, wherein R 20 is an organic group bonded to the groups Y 2 and Y 3, and R 21 is a group Y 4 and Y 5 .
- Y 2 and Y 3 represent groups formed by releasing a proton from a proton donating group, and the groups Y 2 and Y 3 in the same molecule bond with a silicon atom to form a chelate structure.
- Y 4 and Y 5 represent groups formed by releasing a proton from a proton donating group, and the groups Y 4 and Y 5 in the same molecule bond with a silicon atom to form a chelate structure.
- R 20 and R 21 may be the same or different from each other, and Y 2 , Y 3 , Y 4 and Y 5 may be the same or different from each other, and Z 1 is an aromatic ring. Or an organic group having a heterocycle, or an aliphatic group.
- R 16 , R 17 , R 18 and R 19 are, for example, phenyl group, methylphenyl group, methoxyphenyl group, hydroxyphenyl group, naphthyl group, hydroxynaphthyl group, benzyl group, methyl group. , Ethyl group, n-butyl group, n-octyl group, cyclohexyl group and the like.
- An aromatic group having a substituent such as a hydroxyl group or an unsubstituted aromatic group is more preferable.
- R 20 is an organic group bonded to Y 2 and Y 3 .
- R 21 is an organic group that binds to groups Y 4 and Y 5 .
- Y 2 and Y 3 are groups in which a proton donating group releases a proton, and the groups Y 2 and Y 3 in the same molecule bond with a silicon atom to form a chelate structure.
- Y 4 and Y 5 are groups formed by releasing a proton from a proton donating group, and the groups Y 4 and Y 5 in the same molecule bond with a silicon atom to form a chelate structure.
- the radicals R 20 and R 21 can be identical or different from one another and the radicals Y 2 , Y 3 , Y 4 and Y 5 can be identical or different from one another.
- the group represented by —Y 2 —R 20 —Y 3 — and Y 4 —R 21 —Y 5 — in the general formula (9) is such that a proton donor releases two protons.
- the proton donor an organic acid having at least two carboxyl groups or hydroxyl groups in the molecule is preferable, and further, a carboxyl group or hydroxyl group at adjacent carbon atoms constituting the aromatic ring is preferable.
- aromatic compound having at least two is preferable, and an aromatic compound having at least two hydroxyl groups at adjacent carbons constituting an aromatic ring is more preferable.
- aromatic compound having at least two hydroxyl groups at adjacent carbons constituting an aromatic ring is more preferable.
- catechol pyrogallol, 1,2-dihydroxynaphthalene, 2,3-dihydroxy.
- examples thereof include alcohol, 1,2-cyclohexanediol, 1,2-propanediol and glycerin, and of these, catechol, 1,2-dihydroxynaphthalene and 2,3-dihydroxynaphthalene are more preferable.
- Z 1 in the general formula (9) represents an organic group or an aliphatic group having an aromatic ring or a heterocycle, and specific examples thereof include a methyl group, an ethyl group, a propyl group, a butyl group, Aliphatic hydrocarbon groups such as hexyl group and octyl group, aromatic hydrocarbon groups such as phenyl group, benzyl group, naphthyl group and biphenyl group, glycidyloxy groups such as glycidyloxypropyl group, mercaptopropyl group and aminopropyl group , A mercapto group, an alkyl group having an amino group, a reactive substituent such as a vinyl group, and the like.
- a methyl group, an ethyl group, a phenyl group, a naphthyl group, and a biphenyl group are preferable from the viewpoint of thermal stability. , And more preferable.
- a silane compound such as phenyltrimethoxysilane and a proton donor such as 2,3-dihydroxynaphthalene are added and dissolved in a flask containing methanol, and then the mixture is allowed to stand at room temperature.
- a sodium methoxide-methanol solution is added dropwise with stirring.
- a solution prepared by dissolving a tetra-substituted phosphonium halide such as tetraphenylphosphonium bromide in methanol prepared in advance therein is added dropwise under stirring at room temperature to precipitate crystals.
- the precipitated crystals are filtered, washed with water, and vacuum dried to obtain an adduct of a phosphonium compound and a silane compound.
- the content of the component (E) is preferably based on the whole thermosetting resin composition from the viewpoint of effectively improving the curability during molding. Is 0.1% by mass or more, more preferably 0.15% by mass or more, and further preferably 0.25% by mass or more.
- the content of the curing accelerator is preferably 1% by mass or less, and more preferably 0.8% by mass, based on the entire thermosetting resin composition. It is as follows.
- thermosetting resin composition may contain at least one kind of organic heat-stable metal chelate complex salt in addition to the non-conductive metal compound as the component (C).
- thermosetting resin composition may further contain a curing agent.
- the curing agent can be roughly classified into three types, for example, a polyaddition type curing agent, a catalyst type curing agent, and a condensation type curing agent. These may be used alone or in combination of two or more.
- polyaddition type curing agent examples include aliphatic polyamines such as diethylenetriamine (DETA), triethylenetetramine (TETA) and metaxylylenediamine (MXDA), diaminodiphenylmethane (DDM), m-phenylenediamine (MPDA), In addition to aromatic polyamines such as diaminodiphenyl sulfone (DDS), polyamine compounds containing dicyandiamide (DICY), organic acid dihydrazide and the like; alicyclic acids such as hexahydrophthalic anhydride (HHPA) and methyltetrahydrophthalic anhydride (MTHPA) Acid anhydrides such as anhydrides, trimellitic anhydride (TMA), pyromellitic anhydride (PMDA), aromatic acid anhydrides such as benzophenone tetracarboxylic acid (BTDA); novolac type phenol resin, polyvinylphenol, aralkyl type Phenolic resin
- catalyst type curing agent examples include tertiary amine compounds such as benzyldimethylamine (BDMA) and 2,4,6-trisdimethylaminomethylphenol (DMP-30); 2-methylimidazole, 2-ethyl-4- Imidazole compounds such as methylimidazole (EMI24); and one or more selected from the group consisting of Lewis acids such as BF 3 complexes.
- BDMA benzyldimethylamine
- DMP-30 2,4,6-trisdimethylaminomethylphenol
- 2-methylimidazole, 2-ethyl-4- Imidazole compounds such as methylimidazole (EMI24)
- Lewis acids such as BF 3 complexes.
- the condensation-type curing agent includes, for example, one or more selected from the group consisting of a resol-type phenol resin; a urea resin such as a methylol group-containing urea resin; and a melamine resin such as a methylol group-containing melamine resin.
- a phenol resin-based curing agent from the viewpoint of improving the balance of flame resistance, moisture resistance, electrical characteristics, curability, storage stability and the like.
- the phenol resin-based curing agent for example, all monomers, oligomers and polymers having two or more phenolic hydroxyl groups in one molecule can be used, and the molecular weight and the molecular structure thereof are not limited.
- Phenolic resin-based curing agents include, for example, novolac type phenolic resins such as phenol novolac resin, cresol novolac resin and bisphenol novolac; polyfunctional phenolic resins such as polyvinyl phenol and triphenol methane type phenolic resin; terpene modified phenolic resin, dicyclohexyl Modified phenol resin such as pentadiene modified phenol resin; phenol aralkyl resin having phenylene skeleton and/or biphenylene skeleton, phenol aralkyl type phenol resin such as naphthol aralkyl resin having phenylene and/or biphenylene skeleton; bisphenol such as bisphenol A and bisphenol F It includes one or more selected from the group consisting of compounds.
- novolac type phenolic resins such as phenol novolac resin, cresol novolac resin and bisphenol novolac
- polyfunctional phenolic resins such as polyvinyl phenol and triphenol methane type
- a novolac type phenol resin from the viewpoint of suppressing warpage of the molded body, it is more preferable to include a novolac type phenol resin, a polyfunctional type phenol resin and a phenol aralkyl type phenol resin. Further, a phenol novolac resin, a phenol aralkyl resin having a biphenylene skeleton, and a formaldehyde-modified triphenylmethane type phenol resin can also be preferably used.
- the content of the curing agent in the thermosetting resin composition achieves excellent fluidity, from the viewpoint of improving the filling property and moldability, with respect to the entire thermosetting resin composition, It is preferably 0.5% by mass or more, more preferably 1% by mass or more, still more preferably 1.5% by mass or more.
- the content of the curing agent is preferably based on the thermosetting resin composition as a whole. Is 9% by mass or less, more preferably 8% by mass or less, and further preferably 7% by mass or less.
- thermosetting resin composition of the present embodiment contains additives such as a release agent, a flame retardant, an ion scavenger, a coloring agent, a low stress agent and an antioxidant, if necessary. be able to. These may be used alone or in combination of two or more.
- the amount of each of these components in the thermosetting resin composition can be about 0.01 to 2% by mass based on the entire thermosetting resin composition.
- the release agent is selected from, for example, natural waxes such as carnauba wax; montanic acid ester waxes such as glycerintrimontanic acid ester; synthetic waxes such as oxidized polyethylene wax; higher fatty acids such as zinc stearate and metal salts thereof; and paraffin.
- the flame retardant can include, for example, one kind or two or more kinds selected from aluminum hydroxide, magnesium hydroxide, zinc borate, zinc molybdate, and phosphazene.
- the ion scavenger may contain one or more selected from hydrotalcites or hydrous oxides of elements selected from magnesium, aluminum, bismuth, titanium and zirconium.
- the colorant may include one kind or two or more kinds selected from carbon black, red iron oxide, and titanium oxide.
- the low-stress agent may include one or more selected from polybutadiene compounds; acrylonitrile-butadiene copolymer compounds; silicone compounds such as silicone oil and silicone rubber.
- thermosetting resin composition of the present embodiment preferably does not contain carbon such as carbon black used as the colorant from the viewpoint of improving the plating property.
- thermosetting resin composition of the present embodiment for example, a mixture is obtained by mixing the respective components of the thermosetting resin composition by a known means. Further, the mixture is melt-kneaded to obtain a kneaded product.
- a kneading method for example, an extrusion kneader such as a single-screw kneading extruder or a twin-screw kneading extruder, or a roll-type kneader such as a mixing roll can be used, but a twin-screw kneading extruder is used. It is preferable.
- the kneaded product can be formed into a predetermined shape.
- thermosetting resin composition obtained in this embodiment contains the components (A) to (D), and the type of the component (A) and the size of the component (B) are appropriately selected. It is possible to improve the plating property when an object is finely processed by LDS, and to reduce the wiring width and the wiring interval when forming a circuit.
- the thermosetting resin composition may have a predetermined shape such as powder, granules, tablets, or sheets. This makes it possible to obtain a thermosetting resin composition suitable for known molding methods such as transfer molding, injection molding, and compression molding.
- the granular thermosetting resin composition is a pulverized product obtained by pulverizing the obtained kneaded product
- the granular thermosetting resin composition is a powder of the thermosetting resin composition (powder Granular kneaded product) is an aggregate obtained by hardening each other or a granulated product obtained by a known granulation method
- the tablet-shaped thermosetting resin composition is a thermosetting resin composition compressed at high pressure.
- thermosetting resin composition for example, from a thermosetting resin composition having a sheet shape or a roll shape that can be wound. It means that it is a resin film.
- the powdery, granular, tablet-shaped or sheet-shaped thermosetting resin composition may be in a semi-cured state (B stage state).
- the molding method of the thermosetting resin composition in the present embodiment includes, for example, mold formation such as injection molding and transfer molding. By using such a molding method, a resin molded product including a cured product of the thermosetting resin composition can be manufactured.
- the resin molded product is a cured product of a thermosetting resin composition containing an LDS additive, specifically, a resin molded product having a three-dimensional structure.
- the shape of the resin molded product is not limited as long as it has a three-dimensional structure, and may have, for example, a curved surface.
- the MID can be obtained by subjecting the resin molded product to LDS.
- the MID has three elements of a three-dimensional shape, the above resin molded product, and a three-dimensional circuit, and is, for example, a component in which a circuit is formed by a metal film on the surface of a resin molded product having a three-dimensional structure.
- the MID can include a resin molded product having a three-dimensional structure and a three-dimensional circuit formed on the surface of the resin molded product.
- the space can be effectively used, and the number of parts can be reduced and the size can be reduced.
- the manufacturing process of the MID is performed by producing a thermosetting resin composition used for LDS, molding the thermosetting resin composition, irradiating the obtained resin molded product with active energy rays, and plating treatment.
- a step of forming a circuit can be included. Further, a surface cleaning step may be added before the plating process.
- the active energy ray with which the resin molded product is irradiated for example, a laser can be used.
- the laser can be appropriately selected from known lasers such as YAG laser, excimer laser, and electromagnetic radiation, and YGA laser is preferable.
- the wavelength of the laser is not limited, but is, for example, 200 nm to 12000 nm. Among these, 248 nm, 308 nm, 355 nm, 532 nm, 1064 nm or 10600 nm may be preferably used.
- the thermosetting resin composition contains the components (A) to (D), and the type of the component (A) and the size of the component (B) are appropriately selected. It is possible to reduce the wiring width and the wiring interval when a circuit is formed on the formed resin molded product by laser irradiation.
- thermosetting resin composition contains the components (A) to (D), and the type of the component (A) and the size of the component (B) are appropriately selected. Excellent in moldability of the resin composition and excellent in plating property of the resin molded product composed of the cured product.
- the resin molded product composed of the cured product of the thermosetting resin composition is not limited to the final product, and may include composite materials and various parts.
- the resin molded product can be used as a component for mobile electronic devices, vehicles and medical devices, other electronic components including electric circuits, a semiconductor encapsulant, and a composite material for forming these.
- the MID can also be applied to mobile phones, smartphones, built-in antennas, sensors, and semiconductor devices.
- thermosetting resin composition for LDS used in LASER DIRECT STRUCTURING (LDS), A thermosetting resin, An inorganic filler, A non-conductive metal compound that forms a metal nucleus by irradiation with an active energy ray; Including a coupling agent,
- the thermosetting resin contains at least one selected from the group consisting of epoxy resins and bismaleimide resins, Provided is a thermosetting resin composition for LDS in which the collapse angle of the thermosetting resin composition for LDS is 35° or less.
- any combination of these configurations, or the expression of the present embodiment converted between the method and the device is also effective as an aspect of the present embodiment.
- a resin molded product according to the present embodiment having a three-dimensional structure It is also possible to obtain a three-dimensional molded circuit component including a three-dimensional circuit formed on the surface of the resin molded product.
- a resin which is excellent in the production stability of a cured product of a resin composition and the plating property during fine processing by LDS, and which can reduce the wiring width and the wiring interval when forming a circuit.
- a composition can be provided.
- thermosetting resin composition is a granular thermosetting resin composition for LDS used for LDS.
- thermosetting resin composition for LDS is granular and contains the following components (A) to (D).
- thermosetting resin composition is granular and contains the following components (A) to (D).
- Thermosetting resin (B) Inorganic filler (C) Non-conductive metal compound (D) Coupling agent that forms a metal nucleus upon irradiation with active energy rays
- Component (A) is an epoxy resin or a bismaleimide resin At least one selected from the group consisting of: Further, the collapse angle of the thermosetting resin composition is 35° or less.
- thermosetting resin composition is used as the resin composition for LDS, the components thereof are appropriately selected, and the thermosetting resin composition is in the form of granules.
- the collapse angle of the composition By appropriately selecting the collapse angle of the composition, the production stability of the thermosetting resin composition at the time of molding is excellent, and the plated property when the cured product of the thermosetting resin composition is finely processed by LDS.
- the wiring width and the wiring interval can be reduced when forming a circuit.
- the disintegration angle of the thermosetting resin composition is an index representing fluidity and is 35° or less. Accordingly, when the granular thermosetting resin composition is conveyed by using a conveying means such as a vibration feeder, it is possible to stably convey the thermosetting resin composition in a granular form without causing sticking or clogging. Thereby, the stability of molding can be improved.
- the collapse angle of the thermosetting resin composition is preferably 35° or less, more preferably 30° or less, and further preferably 25° or less.
- the lower limit of the collapse angle is not limited, the lower the collapse angle is, the more difficult it is to cause sticking or clogging, and is, for example, 1° or more, preferably 10° or more. ..
- the collapse angle is measured by the following method. That is, first, a granular thermosetting resin composition is dropped and deposited from a hole of a funnel onto a horizontal plate having a certain area until it has a certain shape to form a conical granular body. Then, by dropping a weight of a certain weight on the same pedestal as the horizontal plate, a constant impact is given to the granules, and the partially granular thermosetting resin composition spontaneously flows from the horizontal plate. After falling off, the collapse angle of the remaining conical granules can be obtained as the elevation angle from the point on the outer periphery of the bottom surface to the apex of the cone.
- the elevation angle in the granular body before the impact is called the angle of repose
- the difference angle is the difference angle.
- a powder tester manufactured by Hosokawa Micron Co., Ltd.
- the difference angle of the thermosetting resin composition is an index showing the ease of collapse of the granular thermosetting resin composition due to vibration from a conveying device such as a vibration feeder, and improves transport stability. From the viewpoint of improving the molding stability, it may be, for example, 5° or more, preferably 10° or more, more preferably 12° or more, still more preferably 15° or more. Further, the difference angle of the thermosetting resin composition is specifically less than or equal to the angle of repose.
- the d 50 particle size of the thermosetting resin composition is preferably 1.0 mm or less, more preferably 0.5 mm or less, and further preferably 0.3 mm or less from the viewpoint of reducing the plating wiring width after laser processing. Is. Further, from the viewpoint of making the handleability of the thermosetting resin composition more preferable, the d 50 particle size of the thermosetting resin composition is, for example, 100 ⁇ m or more, and preferably 200 ⁇ m or more.
- thermosetting resin composition is measured by using a commercially available laser diffraction particle size distribution measuring device (for example, SALD-7000 manufactured by Shimadzu Corp.) on a volume basis. You can Next, the components contained in the thermosetting resin composition will be described.
- a commercially available laser diffraction particle size distribution measuring device for example, SALD-7000 manufactured by Shimadzu Corp.
- the component (A) is a thermosetting resin.
- Component (A) is selected from the group consisting of an epoxy resin and a bismaleimide resin from the viewpoint of improving the plating property during microfabrication by LDS and reducing the wiring width and the wiring interval when forming a circuit. Including one or more types. From the viewpoint of improving curability, storage stability, heat resistance, moisture resistance, and chemical resistance, the component (A) preferably contains an epoxy resin, more preferably an epoxy resin. On the other hand, from the viewpoint of obtaining more excellent heat resistance, the component (A) preferably contains a bismaleimide resin, more preferably a bismaleimide resin.
- the epoxy resin general monomers, oligomers and polymers having two or more epoxy groups in one molecule can be used, and the molecular weight and the molecular structure thereof are not limited.
- the epoxy resin includes, for example, one type or two or more types selected from the group consisting of the various epoxy resins described in the first embodiment. From the viewpoint of suppressing the warpage of the molded product obtained by curing the thermosetting resin composition and improving the balance of various properties such as filling property, heat resistance, and moisture resistance, among these, novolac type epoxy resin, polyfunctional Epoxy resin and phenol aralkyl type epoxy resin can be preferably used.
- the epoxy resin preferably contains at least one selected from the group consisting of an orthocresol novolac type epoxy resin, a phenol aralkyl type epoxy resin having a biphenylene skeleton, and a triphenylmethane type epoxy resin, and Preferably, at least one selected from the group consisting of orthocresol novolac type epoxy resin and phenol aralkyl type epoxy resin having a biphenylene skeleton is included.
- the bismaleimide resin is a (co)polymer of a compound having two or more maleimide groups.
- the compound having two or more maleimide groups includes, for example, at least one of the compound represented by the general formula (1) and the compound represented by the general formula (2) described in the first embodiment.
- the component (A) may further contain other thermosetting resin.
- a thermosetting resin for example, one kind or two or more kinds selected from the group consisting of the various resins described in the first embodiment can be mentioned.
- the component (A) can contain a resin curing agent such as a phenol resin curing agent described later.
- the content of the component (A) in the thermosetting resin composition is, with respect to the thermosetting resin composition as a whole, from the viewpoint of improving fluidity at the time of molding to improve filling property and molding stability. It is preferably 1% by mass or more, more preferably 2% by mass or more, and further preferably 2.5% by mass or more. On the other hand, the content of the component (A) is preferably 15 mass with respect to the entire thermosetting resin composition from the viewpoint of improving the moisture resistance reliability and reflow resistance, and from the viewpoint of suppressing the warpage of the molded body. % Or less, more preferably 14% by mass or less, and further preferably 13% by mass or less.
- the content with respect to the entire thermosetting resin composition when the thermosetting resin composition contains a solvent, the entire solid content excluding the solvent of the thermosetting resin composition.
- the solid content of the thermosetting resin composition refers to the non-volatile content in the thermosetting resin composition, and refers to the balance excluding volatile components such as water and solvent.
- the thermosetting resin composition may include a curing agent.
- the curing agent can be roughly classified into three types, for example, a polyaddition type curing agent, a catalyst type curing agent, and a condensation type curing agent. These may be used alone or in combination of two or more.
- polyaddition type curing agent examples include various acid anhydrides described above in the first embodiment; various phenol resin-based curing agents described above in the first embodiment; various types described above in the first embodiment.
- the catalyst-type curing agent includes, for example, one or more selected from the group consisting of various types described in the first embodiment.
- the condensation-type curing agent includes, for example, one or more selected from the group consisting of the various resins described above in the first embodiment.
- the phenol resin-based curing agent from the viewpoint of improving the balance of flame resistance, moisture resistance, electrical characteristics, curability, storage stability and the like.
- the phenol resin-based curing agent include those described above in the first embodiment.
- the phenol resin-based curing agent more preferably contains a novolac type phenol resin, a polyfunctional type phenol resin and a phenol aralkyl type phenol resin.
- a phenol novolac resin, a phenol aralkyl resin having a biphenylene skeleton, and a formaldehyde-modified triphenylmethane type phenol resin can also be preferably used.
- the content of the curing agent in the thermosetting resin composition achieves excellent fluidity, from the viewpoint of improving the filling property and moldability, with respect to the entire thermosetting resin composition, It is preferably 0.5% by mass or more, more preferably 1% by mass or more, still more preferably 1.5% by mass or more.
- the content of the curing agent is preferably based on the thermosetting resin composition as a whole. Is 9% by mass or less, more preferably 8% by mass or less, and further preferably 7% by mass or less.
- the component (B) is an inorganic filler.
- the component (B) includes, for example, one kind or two or more kinds of materials selected from the group consisting of various kinds described above in the first embodiment. From the viewpoint of making mechanical properties or thermal properties of the cured product of the thermosetting resin composition preferable, the component (B) preferably contains fused crushed silica, fused spherical silica, silica such as crystalline silica, and more preferably It is silica.
- the d 50 particle diameter of the component (B) may be, for example, 10 ⁇ m or less, preferably 5 ⁇ m or less, more preferably 3 ⁇ m or less, and further preferably 2 ⁇ m or less from the viewpoint of reducing the plating wiring width after laser processing. Is.
- the d 50 particle diameter of the component (B) is, for example, 0.1 ⁇ m or more, and more preferably 0.5 ⁇ m or more.
- the particle size distribution of the particles can be measured on a volume basis using a commercially available laser diffraction particle size distribution measuring device (for example, SALD-7000 manufactured by Shimadzu Corporation).
- the content of the component (B) in the thermosetting resin composition is preferably 65% by mass or more based on the whole thermosetting resin composition from the viewpoint of improving the heat resistance and moisture resistance of the cured product. It is more preferably 70% by mass or more, and further preferably 75% by mass or more. On the other hand, the content of the component (B) is preferably 95 relative to the total amount of the thermosetting resin composition from the viewpoint of more effectively improving the fluidity and filling property during molding of the thermosetting resin composition. It is not more than 90% by mass, more preferably not more than 90% by mass, further preferably not more than 85% by mass.
- the component (C) is a non-conductive metal compound that forms a metal nucleus upon irradiation with active energy rays. Such compounds act as LDS additives.
- the configuration described in the first embodiment can be applied.
- the content of the component (C) in the thermosetting resin composition is from the viewpoint of improving the plating property in the cured product of the thermosetting resin composition with respect to the entire thermosetting resin composition. For example, it is 2% by mass or more, preferably 4% by mass or more, and more preferably 8% by mass or more.
- the flow of the thermosetting resin composition can be prevented from the viewpoints of suppressing lowering of insulating property and increase of dielectric loss tangent, and when the shape of the component (C) is non-spherical.
- the content of the component (C) is, for example, 20% by mass or less, preferably 18% by mass or less, and more preferably 15% by mass, based on the entire thermosetting resin composition. % Or less.
- the total content of the component (B) and the component (C) in the thermosetting resin composition is preferably the total amount of the thermosetting resin composition from the viewpoint of improving resin mechanical properties and resin strength. It is 70% by mass or more, more preferably 75% by mass or more, further preferably 80% by mass or more, and even more preferably 85% by mass or more.
- the total content of the component (B) and the component (C) is preferably 98% by mass or less, and more preferably 95% by mass, based on the entire thermosetting resin composition. % Or less.
- Component (D) is a coupling agent.
- component (D) specifically, the configuration described in the first embodiment can be applied.
- the content of the component (D) in the thermosetting resin composition is based on the whole thermosetting resin composition from the viewpoint of improving moldability by increasing the flow flow length of the thermosetting resin composition. , Preferably 0.01 mass% or more, more preferably 0.05 mass% or more, still more preferably 0.1 mass% or more.
- the content of the component (D) is based on the entire thermosetting resin composition. It is preferably 1% by mass or less, more preferably 0.8% by mass or less, still more preferably 0.6% by mass or less.
- thermosetting resin composition may include components other than the above components.
- thermosetting resin composition may further include (E) a curing accelerator.
- Component (E) is a curing accelerator.
- the curing accelerator specifically, the configuration described in the first embodiment can be applied.
- the content of the component (E) is preferably based on the whole thermosetting resin composition from the viewpoint of effectively improving the curability during molding. Is 0.1% by mass or more, more preferably 0.15% by mass or more, and further preferably 0.25% by mass or more.
- the content of the curing accelerator is preferably 1% by mass or less, and more preferably 0.8% by mass, based on the entire thermosetting resin composition. It is as follows.
- thermosetting resin composition may contain at least one kind of organic heat-stable metal chelate complex salt in addition to the non-conductive metal compound as the component (C).
- thermosetting resin composition of the present embodiment contains additives such as a release agent, a flame retardant, an ion scavenger, a colorant, a stress reducing agent and an antioxidant, if necessary. be able to. These may be used alone or in combination of two or more.
- additives specifically, the configuration described in the first embodiment can be applied.
- the amount of each of these components in the thermosetting resin composition can be about 0.01 to 2% by mass based on the entire thermosetting resin composition.
- thermosetting resin composition of the present embodiment preferably does not contain carbon such as carbon black used as the colorant from the viewpoint of improving the plating property.
- thermosetting resin composition of the present embodiment for example, a mixture is obtained by mixing the respective components of the thermosetting resin composition by a known means. Further, the mixture is melt-kneaded to obtain a kneaded product.
- a kneading method for example, an extrusion kneader such as a single-screw kneading extruder or a twin-screw kneading extruder, or a roll-type kneader such as a mixing roll can be used, but a twin-screw kneading extruder is used. It is preferable.
- the kneaded product can be granulated.
- the granular thermosetting resin composition is specifically an agglomerate or granulated product obtained by solidifying powders (powder-like kneaded material) of the thermosetting resin composition.
- the granular thermosetting resin composition may be in a semi-cured state (B stage state). Then, in the present embodiment, when granulating the kneaded product, the obtained powder, by sieving the granulated product, the collapse angle is a granular thermosetting resin composition in a predetermined range. Obtainable.
- the molding step for producing the thermosetting resin composition in the form of granules include a step of pulverizing the cooled mixture after melt-kneading.
- thermosetting resin composition in the form of granules may be sieved to adjust the size of the granules. Further, for example, the thermosetting resin composition in the form of granules may be treated by a method such as a centrifugal milling method or a hot cut method to adjust the dispersity or fluidity.
- a molding step for producing a pulverized thermosetting resin composition for example, the mixture is pulverized to form a granular thermosetting resin composition, and then the granular thermosetting resin composition is added. Further, a step of pulverizing can be mentioned.
- thermosetting resin composition obtained in the present embodiment is in the form of granules containing a predetermined component and having a predetermined disintegration angle, and therefore has excellent production stability of a cured product of the resin composition.
- the composition can have excellent supply stability and moldability during molding.
- thermosetting resin composition obtained in this embodiment has excellent plating characteristics when the cured product is subjected to fine processing by LDS, and can reduce the wiring width and the wiring interval when forming a circuit.
- thermosetting resin composition in the present embodiment examples include mold forming such as transfer molding, injection molding, compression molding and the like. By using such a molding method, a resin molded product including a cured product of the thermosetting resin composition can be manufactured.
- the resin molded product is a cured product of a thermosetting resin composition containing an LDS additive, specifically, a resin molded product having a three-dimensional structure.
- the shape of the resin molded product is not limited as long as it has a three-dimensional structure, and may have, for example, a curved surface.
- the resin molded product composed of the cured product of the thermosetting resin composition is not limited to the final product and may include composite materials and various parts.
- the resin molded product can be used as a component for mobile electronic devices, vehicles and medical devices, other electronic components including electric circuits, a semiconductor encapsulant, and a composite material for forming these.
- the MID can be obtained by subjecting the resin molded product to LDS.
- the MID has three elements of a three-dimensional shape, the above-mentioned resin molded product, and a three-dimensional circuit, and is, for example, a component in which a circuit is formed by a metal film on the surface of a resin molded product having a three-dimensional structure.
- the MID can include a resin molded product having a three-dimensional structure and a three-dimensional circuit formed on the surface of the resin molded product.
- the manufacturing process of the MID can include, for example, each process described above in the first embodiment. Further, a surface cleaning step may be added before the plating process.
- the active energy ray with which the resin molded product is irradiated for example, a laser can be used.
- the laser for example, the laser described above in the first embodiment can be used.
- the resin composition for LDS is a thermosetting resin composition containing the components (A) to (D), and the shape thereof is a granular shape exhibiting a predetermined collapse angle. It is possible to reduce the wiring width and the wiring interval when a circuit is formed on the resin molded product constituted by the above by laser irradiation.
- the plating treatment either electric field plating or electroless plating may be used.
- a circuit (plating layer) can be formed by performing a plating process on the region irradiated with the laser.
- the plating solution is not limited to any particular one, and widely known plating solutions can be adopted, and a plating solution in which copper, nickel, gold, silver and palladium are mixed as metal components may be used.
- the resin composition for LDS is a thermosetting resin composition containing the components (A) to (D), and the shape thereof is a granular shape having a predetermined collapse angle.
- the resin composition such as excellent supply stability and moldability, is excellent in production stability of a cured product of the resin composition, and is also excellent in plating characteristics of a resin molded product composed of the cured product.
- the present inventors examined the use of microfabrication by LDS in the manufacture of semiconductor devices such as MID.
- This embodiment provides a method for manufacturing a semiconductor device, which is excellent in moldability of an encapsulant and excellent in plating characteristics during microfabrication by LDS, and can reduce a wiring width and a wiring interval when forming a circuit. To do.
- thermosetting resin composition for LDS (LASER DIRECT STRUCTURING) so as to cover the surface of the semiconductor element; Irradiating a specific portion of the surface of the encapsulant with an active energy ray, A step of hydrophilizing the surface of the sealing material, A step of selectively forming a metal layer in a region of the surface of the encapsulant irradiated with the active energy ray; Including, The thermosetting resin composition for LDS, (A) a thermosetting resin, (B) an inorganic filler, (C) a non-conductive metal compound that forms a metal nucleus upon irradiation with an active energy ray, (D) a coupling agent, There is provided a method for manufacturing a semiconductor device, wherein the component (A) contains at least one selected from the group consisting of epoxy resins and bismaleimide resins.
- any combination of these configurations, or the expression of the present embodiment converted between the method and the device is also effective as an aspect of the present embodiment.
- a method for manufacturing a semiconductor device which is excellent in moldability of the encapsulant and excellent in plating characteristics during microfabrication by LDS, and can reduce the wiring width and the wiring interval when forming a circuit. Can be provided.
- FIG. 1A to 1D and FIG. 2 are cross-sectional views showing the manufacturing process of the semiconductor device according to the present embodiment.
- FIG. 2 also shows the configuration of the semiconductor device 100 obtained by such a manufacturing method.
- the semiconductor device 100 (FIG. 2) includes a substrate 101, a semiconductor element 103 mounted on the substrate 101, an encapsulating material 105 for encapsulating the semiconductor element 103, and a predetermined area on a surface of the encapsulating material 105. It has a metal layer 111 provided.
- the sealing material 105 is a cured product of a thermosetting resin composition containing an LDS additive.
- Specific examples of the semiconductor device 100 include molded circuit parts and various semiconductor packages.
- Specific examples of molded circuit parts include those used for automobile members.
- semiconductor packages MAP (Mold Array Package), QFP (Quad Flat Package), SOP (Small Outline Package), CSP (Chip Size Package), QFN (Quad Flat Non-leaded Package), SON (Small).
- BGA Bit Grid Array
- LF-BGA Lead Flame BGA
- FCBGA Fluor Chip BGA
- MAPBGA Molded Array Process BGA
- eWLB embedded Wafer-Level BGA
- Fan-In Examples thereof include semiconductor packages such as type eWLB and Fan-Out type eWLB; SIP (System In package) and the like.
- the method for manufacturing the semiconductor device 100 includes the following steps 1 to 4.
- Step 1 A step of forming the encapsulating material 105 by encapsulating the semiconductor element 103 with a cured product of a thermosetting resin composition for LDS (LASER DIRECT STRUCTURING) so as to cover the surface of the semiconductor element 103 (step 2) Step of irradiating a specific portion of the surface of the encapsulant 105 with an active energy ray (laser 109) (step 3) Step of hydrophilizing the surface of the encapsulant 105 (step 4) Laser on the surface of the encapsulant 105 Step of selectively forming metal layer 111 in the region irradiated with 109
- the thermosetting resin composition for LDS (hereinafter, also simply referred to as “thermosetting resin composition”) is (A) heat.
- the component (A) contains at least one selected from the group consisting of epoxy resins and bismaleimide resins.
- each step will be specifically described first, and the configuration of the thermosetting resin composition for LDS will be described later.
- a sealing material 105 that seals the semiconductor element 103 is formed (FIG. 1A).
- the semiconductor element 103 can be mounted on the substrate 101.
- the substrate 101 is, for example, a wiring substrate such as an interposer or a lead frame.
- the semiconductor element 103 is electrically connected to the substrate 101 by wire bonding, flip chip connection, or the like.
- the molding method of the encapsulant 105 can be selected according to the components contained in the thermosetting resin composition, the shape of the thermosetting resin composition, and the like. Specific examples include compression molding and transfer molding. ..
- a specific site on the surface of the encapsulant 105 is selectively irradiated with an active energy ray such as a laser 109 (FIG. 1B).
- an active energy ray such as a laser 109 (FIG. 1B).
- the laser 109 can be appropriately selected from known lasers such as YAG laser, excimer laser, and electromagnetic radiation, and YGA laser is preferable.
- the wavelength of the laser 109 is also not limited, but is, for example, 200 nm or more and 2000 nm or less, and preferably 240 nm or more and 1100 nm or less from the viewpoint of thinning. Of these, lasers 109 having a wavelength of 248 nm, 308 nm, 355 nm, 515 nm, 532 nm, 1064 nm or 1060 nm are preferable.
- step 3 at least a part of the surface of the encapsulant 105 is hydrophilized to form a hydrophilized surface 107 (FIG. 1D).
- the hydrophilic surface 107 may be a part of the surface of the sealing material 105 or the entire surface thereof.
- the hydrophilic surface 107 may be a flat surface or a curved surface, and may include both of them.
- the hydrophilic surface 107 is preferably formed by the laser 109 in step 2 from the viewpoint of improving the plating property at the time of fine processing by LDS and reducing the wiring width and the wiring interval when forming a circuit. Includes the entire illuminated area.
- Step 3 can be specifically performed before or after step 2.
- the step 3 is preferably a laser irradiation site on the surface of the encapsulant 105, specifically, after the step 2. Specifically, it is a step of making the inner surface of the recess 113 hydrophilic.
- step 3 preferably includes a step of applying a treatment liquid to the surface of the encapsulating material 105.
- a treatment liquid may be applied to the surface of the sealing material 105, and ultrasonic treatment may be performed while heating to about 40 to 60° C.
- the treatment liquid include glycol organic solvents, alcohol organic solvents, and lactone organic solvents.
- glycol organic solvent examples include glycols such as ethylene glycol, diethylene glycol, triethylene glycol, propylene glycol; diethylene glycol monomethyl ether, triethylene glycol monomethyl ether, propylene glycol monomethyl ether, propylene glycol monoethyl ether, dipropylene glycol monomethyl ether.
- glycols such as ethylene glycol, diethylene glycol, triethylene glycol, propylene glycol; diethylene glycol monomethyl ether, triethylene glycol monomethyl ether, propylene glycol monomethyl ether, propylene glycol monoethyl ether, dipropylene glycol monomethyl ether.
- Glycol ethers such as ethers
- glycol ether acetates such as ethylene glycol monomethyl ether acetate, propylene glycol monomethyl ether acetate, diethylene glycol monobutyl ether acetate, and diethylene glycol monoethyl ether acetate.
- the alcohol organic solvent include alcohol ethers having 3 to 8 carbon atoms such as 3-methoxy-3-methyl-1-butanol.
- the lactone-based organic solvent include ⁇ -butyrolactone, ⁇ -methyl- ⁇ -butyrolactone, ⁇ -valerolactone, ⁇ -caprolactone, ⁇ -laurolactone, ⁇ -valerolactone, and hexanolactone.
- the treatment liquid preferably contains a glycol organic solvent, more preferably a glycol hydrophilic organic solvent.
- the metal layer 111 is selectively formed on a predetermined area of the hydrophilic surface 107 by LDS.
- LDS specifically, the surface of a resin molded article containing an LDS additive is irradiated with active energy rays to generate metal nuclei, and the metal nuclei are used as seeds by a plating treatment such as electroless plating, A plating pattern is formed in the energy ray irradiation area.
- a conductive member such as a wiring or a circuit can be formed based on this plating pattern.
- step 4 the metal layer 111 is selectively formed in the laser irradiation site on the surface of the encapsulant 105, specifically, in the recess 113 (FIG. 2).
- the metal layer 111 is specifically a plating film.
- step 4 specifically includes a step of applying a metal to the recess 113 and a step of growing a metal plating layer in the recess 113.
- a circuit (plating layer) can be formed by performing a plating process on the region irradiated with the laser 109 in step 2.
- the plating solution is not limited to any particular one, and widely known plating solutions can be adopted, and a plating solution in which copper, nickel, gold, silver and palladium are mixed as metal components may be used.
- the semiconductor device 100 is obtained through the above steps.
- the encapsulant 105 which is a cured product of the thermosetting resin composition containing the components (A) to (D), is irradiated with a laser 109 to expose the surface of the encapsulant 105 including the laser irradiation region.
- the hydrophilic treatment by forming the metal layer 111 on the hydrophilic surface 107, it is possible to directly form a circuit in a desired region of the semiconductor device 100 such as a semiconductor package. Can be made smaller. Further, it is possible to obtain the semiconductor device 100 which is excellent in the moldability of the encapsulant 105 and the plating property during fine processing by DS.
- the configuration in which the recess 113 is formed at the laser irradiation site and the metal layer 111 is grown in the recess 113 to form a wiring has been described as an example.
- the shape of the recess 113 and the corresponding metal layer 111 The function is not limited to this, and a via may be formed as the recess 113, for example.
- the irradiated portion of the laser 109 is made hydrophilic, the residue after laser irradiation can be effectively removed even when forming a via. As a result, vias can be stably formed with a desired size and pitch.
- step 3 of forming the hydrophilic surface 107 is performed after the step 2 of irradiating the laser 109 has been described as an example, but the steps before and after step 2 and step 3 are not limited to this.
- the step 3 may be performed before the step 2 to form the hydrophilic surface 107, and then the laser 109 may be selectively irradiated to a specific region of the hydrophilic surface 107 in the step 2.
- thermosetting resin composition for LDS contains the components (A) to (D) described above.
- the moldability of the thermosetting resin composition and the heat treatment are improved by hydrophilically treating the encapsulating material 105 and appropriately selecting the types of components contained in the thermosetting resin composition.
- the thermosetting resin composition preferably has the configuration described in the first embodiment.
- the thermosetting resin composition preferably has the configuration described in the second embodiment.
- the component (A) is a thermosetting resin.
- Component (A) is selected from the group consisting of an epoxy resin and a bismaleimide resin from the viewpoint of improving the plating property during microfabrication by LDS and reducing the wiring width and the wiring interval when forming a circuit. Including one or more types. From the viewpoint of improving curability, storage stability, heat resistance, moisture resistance, and chemical resistance, the component (A) preferably contains an epoxy resin, more preferably an epoxy resin. On the other hand, from the viewpoint of obtaining more excellent heat resistance, the component (A) preferably contains a bismaleimide resin, more preferably a bismaleimide resin.
- the epoxy resin general monomers, oligomers and polymers having two or more epoxy groups in one molecule can be used, and the molecular weight and the molecular structure thereof are not limited.
- the epoxy resin includes, for example, one type or two or more types selected from the group consisting of the various epoxy resins described in the first embodiment. From the viewpoint of suppressing the warpage of the molded product obtained by curing the thermosetting resin composition and improving the balance of various properties such as filling property, heat resistance, and moisture resistance, among these, novolac type epoxy resin, polyfunctional Epoxy resin and phenol aralkyl type epoxy resin can be preferably used.
- the epoxy resin preferably contains at least one selected from the group consisting of an orthocresol novolac type epoxy resin, a phenol aralkyl type epoxy resin having a biphenylene skeleton, and a triphenylmethane type epoxy resin, and Preferably, at least one selected from the group consisting of orthocresol novolac type epoxy resin and phenol aralkyl type epoxy resin having a biphenylene skeleton is included.
- the bismaleimide resin is a (co)polymer of a compound having two or more maleimide groups.
- the compound having two or more maleimide groups includes, for example, at least one of the compound represented by the general formula (1) and the compound represented by the general formula (2) described in the first embodiment.
- the component (A) may further contain other thermosetting resin.
- a thermosetting resin for example, one kind or two or more kinds selected from the group consisting of the various resins described in the first embodiment can be mentioned.
- the component (A) can contain a resin curing agent such as a phenol resin curing agent described later.
- the content of the component (A) in the thermosetting resin composition is, with respect to the thermosetting resin composition as a whole, from the viewpoint of improving fluidity at the time of molding to improve filling property and molding stability. It is preferably 1% by mass or more, more preferably 2% by mass or more, and further preferably 2.5% by mass or more. On the other hand, the content of the component (A) is preferably 15 mass with respect to the entire thermosetting resin composition from the viewpoint of improving the moisture resistance reliability and reflow resistance, and from the viewpoint of suppressing the warpage of the molded body. % Or less, more preferably 14% by mass or less, and further preferably 13% by mass or less.
- the content with respect to the entire thermosetting resin composition when the thermosetting resin composition contains a solvent, the entire solid content excluding the solvent of the thermosetting resin composition.
- the solid content of the thermosetting resin composition refers to the non-volatile content in the thermosetting resin composition, and refers to the balance excluding volatile components such as water and solvent.
- the thermosetting resin composition may include a curing agent.
- the curing agent can be roughly classified into three types, for example, a polyaddition type curing agent, a catalyst type curing agent, and a condensation type curing agent. These may be used alone or in combination of two or more.
- polyaddition type curing agent examples include various acid anhydrides described above in the first embodiment; various phenol resin-based curing agents described above in the first embodiment; various types described above in the first embodiment.
- the catalyst-type curing agent includes, for example, one or more selected from the group consisting of various types described in the first embodiment.
- the condensation-type curing agent includes, for example, one or more selected from the group consisting of the various resins described above in the first embodiment.
- the phenol resin-based curing agent from the viewpoint of improving the balance of flame resistance, moisture resistance, electrical characteristics, curability, storage stability and the like.
- the phenol resin-based curing agent include those described above in the first embodiment.
- the phenol resin-based curing agent more preferably contains a novolac type phenol resin, a polyfunctional type phenol resin and a phenol aralkyl type phenol resin.
- a phenol novolac resin, a phenol aralkyl resin having a biphenylene skeleton, and a formaldehyde-modified triphenylmethane type phenol resin can also be preferably used.
- the content of the curing agent in the thermosetting resin composition achieves excellent fluidity, from the viewpoint of improving the filling property and moldability, with respect to the entire thermosetting resin composition, It is preferably 0.5% by mass or more, more preferably 1% by mass or more, still more preferably 1.5% by mass or more.
- the content of the curing agent is preferably based on the thermosetting resin composition as a whole. Is 9% by mass or less, more preferably 8% by mass or less, and further preferably 7% by mass or less.
- the component (B) is an inorganic filler.
- the component (B) includes, for example, one kind or two or more kinds of materials selected from the group consisting of various kinds described above in the first embodiment. From the viewpoint of making mechanical properties or thermal properties of the cured product of the thermosetting resin composition preferable, the component (B) preferably contains fused crushed silica, fused spherical silica, silica such as crystalline silica, and more preferably It is silica.
- the d 50 particle size of the component (B) may be, for example, 10 ⁇ m or less, preferably 5 ⁇ m or less, more preferably 3 ⁇ m or less, and further preferably 2 ⁇ m or less from the viewpoint of reducing the plating wiring width after laser processing. Is.
- the d 50 particle diameter of the component (B) is, for example, 0.1 ⁇ m or more, and more preferably 0.5 ⁇ m or more.
- the particle size distribution of the particles can be measured on a volume basis using a commercially available laser diffraction particle size distribution measuring device (for example, SALD-7000 manufactured by Shimadzu Corporation).
- the content of the component (B) in the thermosetting resin composition is preferably 65% by mass or more based on the whole thermosetting resin composition from the viewpoint of improving the heat resistance and moisture resistance of the cured product. It is more preferably 70% by mass or more, and further preferably 75% by mass or more. On the other hand, the content of the component (B) is preferably 95 relative to the total amount of the thermosetting resin composition from the viewpoint of more effectively improving the fluidity and filling property during molding of the thermosetting resin composition. It is not more than 90% by mass, more preferably not more than 90% by mass, further preferably not more than 85% by mass.
- the component (C) is a non-conductive metal compound that forms a metal nucleus upon irradiation with active energy rays. Such compounds act as LDS additives.
- the configuration described in the first embodiment can be applied.
- the content of the component (C) in the thermosetting resin composition is from the viewpoint of improving the plating property in the cured product of the thermosetting resin composition with respect to the entire thermosetting resin composition. For example, it is 2% by mass or more, preferably 4% by mass or more, and more preferably 8% by mass or more.
- the flow of the thermosetting resin composition can be prevented from the viewpoints of suppressing lowering of insulating property and increase of dielectric loss tangent, and when the shape of the component (C) is non-spherical.
- the content of the component (C) is, for example, 20% by mass or less, preferably 18% by mass or less, and more preferably 15% by mass, based on the entire thermosetting resin composition. % Or less.
- the total content of the component (B) and the component (C) in the thermosetting resin composition is preferably the total amount of the thermosetting resin composition from the viewpoint of improving resin mechanical properties and resin strength. It is 70% by mass or more, more preferably 75% by mass or more, further preferably 80% by mass or more, and even more preferably 85% by mass or more.
- the total content of the component (B) and the component (C) is preferably 98% by mass or less, and more preferably 95% by mass, based on the entire thermosetting resin composition. % Or less.
- Component (D) is a coupling agent.
- component (D) specifically, the configuration described in the first embodiment can be applied.
- the content of the component (D) in the thermosetting resin composition is based on the whole thermosetting resin composition from the viewpoint of improving moldability by increasing the flow flow length of the thermosetting resin composition. , Preferably 0.01 mass% or more, more preferably 0.05 mass% or more, still more preferably 0.1 mass% or more.
- the content of the component (D) is based on the entire thermosetting resin composition. It is preferably 1% by mass or less, more preferably 0.8% by mass or less, still more preferably 0.6% by mass or less.
- thermosetting resin composition may contain components other than the above components.
- thermosetting resin composition may further include (E) a curing accelerator.
- Component (E) is a curing accelerator.
- the curing accelerator specifically, the configuration described in the first embodiment can be applied.
- the content of the component (E) is preferably based on the whole thermosetting resin composition from the viewpoint of effectively improving the curability during molding. Is 0.1% by mass or more, more preferably 0.15% by mass or more, and further preferably 0.25% by mass or more.
- the content of the curing accelerator is preferably 1% by mass or less, and more preferably 0.8% by mass, based on the entire thermosetting resin composition. It is as follows.
- thermosetting resin composition may contain at least one kind of organic heat-stable metal chelate complex salt in addition to the non-conductive metal compound as the component (C).
- thermosetting resin composition can contain additives such as a release agent, a flame retardant, an ion scavenger, a colorant, a low stress agent and an antioxidant, if necessary. These may be used alone or in combination of two or more. For these additives, specifically, the configuration described in the first embodiment can be applied.
- the amount of each of these components in the thermosetting resin composition can be about 0.01 to 2% by mass based on the entire thermosetting resin composition.
- thermosetting resin composition preferably does not contain carbon such as carbon black used as the coloring agent from the viewpoint of improving the plating property.
- thermosetting resin composition As a method for producing a thermosetting resin composition, for example, the components of the thermosetting resin composition are mixed by a known means to obtain a mixture. Further, the mixture is melt-kneaded to obtain a kneaded product.
- a kneading method for example, an extrusion kneader such as a single-screw kneading extruder or a twin-screw kneading extruder, or a roll-type kneader such as a mixing roll can be used, but a twin-screw kneading extruder is used. It is preferable.
- the kneaded product can be formed into a predetermined shape.
- the thermosetting resin composition may have a predetermined shape such as powder, granules, tablets, or sheets. This makes it possible to obtain a thermosetting resin composition suitable for known molding methods such as transfer molding, injection molding, and compression molding.
- the granular thermosetting resin composition is a pulverized product obtained by pulverizing the obtained kneaded product
- the granular thermosetting resin composition is a powder of the thermosetting resin composition (powder Granular kneaded product) is an aggregate obtained by hardening each other or a granulated product obtained by a known granulation method
- the tablet-shaped thermosetting resin composition is a thermosetting resin composition compressed at high pressure.
- thermosetting resin composition for example, from a thermosetting resin composition having a sheet shape or a roll shape that can be wound. It means that it is a resin film.
- the powdery, granular, tablet-shaped or sheet-shaped thermosetting resin composition may be in a semi-cured state (B stage state).
- thermosetting resin composition for LDS of each example was prepared with the formulation shown in Table 1 and evaluated.
- the components used in each example are as follows.
- Inorganic filler A1 amorphous silica/crystalline silica, MUF-4V, manufactured by Tatsumori Co., average particle diameter 3.8 ⁇ m, specific surface area 4.0 m 2 /g
- Inorganic filler A2 Silicon dioxide, SC-2500-SQ, manufactured by Admatechs, average particle diameter 0.6 ⁇ m, specific surface area 6.4 m 2 /g
- Inorganic filler A3 Silicon dioxide, SC-5500-SQ, manufactured by Admatechs, average particle diameter 1.6 ⁇ m, specific surface area 4.4 m 2 /g
- Inorganic filler A4 Silicon dioxide, TS-6026, manufactured by Micron Company, Inc.
- Colorant A1 carbon black, carbon #5, manufactured by Mitsubishi Chemical (coupling agent)
- Coupling agent A1 3-mercaptopropyltrimethoxysilane, S810, coupling agent made by Chisso Corporation A2: 3-glycidoxypropyltrimethoxysilane, S510, coupling agent A3 made by Chisso Corporation: N-phenyl- ⁇ -amino Propyltrimethoxysilane, CF-4083, manufactured by Toray Dow Corning Co.
- Epoxy resin A1 Orthocresol novolac type epoxy resin, EOCN-1020, manufactured by Nippon Kayaku Co., Ltd.
- Epoxy resin A2 Biphenylene skeleton-containing phenol aralkyl type epoxy resin, NC3000, manufactured by Nippon Kayaku Co., Ltd. (hardening agent) Hardener A1: Novolac-type phenol resin, PR-HF-3, Sumitomo Bakelite hardener A2: Biphenylene skeleton-containing phenol aralkyl-type resin, MEH-7851SS, Meiwa Kasei Co.
- Curing accelerator A1 Tetraphenylphosphonium bis(naphthalene-2,3-dioxy)phenyl silicate Curing accelerator A2: Tetraphenylphosphonium-4,4'-sulphonyl diphenolate (release agent) Release Agent A1: Glycerin Trimontanate, Ricolb-WE-4, manufactured by Clariant Japan (additive: non-conductive metal compound) Additive A1: Inorganic composite oxide, 30C965, manufactured by Shepherd Color Co.
- Silicone A1 Polyoxyalkylene epoxy modified dimethyl polysiloxane, FZ-3730, manufactured by Toray Dow Corning (low stress agent)
- Low stress agent A1 Epoxidized polybutadiene, JP-200, manufactured by Nippon Soda Co., Ltd.
- thermosetting resin composition (Preparation of thermosetting resin composition) Raw materials having the blending amounts shown in Table 1 were mixed at room temperature using a mixer, and then roll-kneaded at 70 to 100°C. Next, the obtained kneaded product was cooled and then pulverized to obtain a powdery thermosetting resin composition. Next, for some examples, coarse particles and fine particles were removed using a sieve to obtain a powdery thermosetting resin composition.
- the particle size distribution, d 10 , d 50 and d max of the whole inorganic filler used in each example were measured by a laser diffraction type particle size distribution measuring device (Shimadzu SALD-7000).
- the resin composition obtained in each example was molded and cured under the condition of 175° C. to obtain a cured product.
- the surface of the obtained cured product was irradiated with a YAG laser, and the plating property in the laser irradiation region was evaluated according to the following criteria. ⁇ : There is no unevenness on the plating surface ⁇ : There is some unevenness on the plating surface but there is no unplated part ⁇ : There is unevenness on the plating surface but there is no unplated part ⁇ : Uneven plating part is visible on the plated surface Yes
- thermosetting resin composition for LDS of each example was prepared with the formulation shown in Table 2 and evaluated.
- the components used in each example are as follows.
- Inorganic filler B1 amorphous silica/crystalline silica, MUF-4V, manufactured by Tatsumori Co., Ltd., average particle diameter 3.8 ⁇ m
- Inorganic filler B2 Silicon dioxide, SC-2500-SQ, manufactured by Admatechs, average particle size 0.6 ⁇ m
- Inorganic filler B3 Silicon dioxide, SC-5500-SQ, manufactured by Admatechs, average particle size 1.6 ⁇ m
- Inorganic filler B4 Silicon dioxide, TS-6026, manufactured by Micron Company, average particle size 9.0 ⁇ m
- Epoxy resin B1 Biphenylene skeleton-containing phenol a
- Curing agent B1 Biphenylene skeleton-containing phenol aralkyl type resin, MEH-7851SS, manufactured by Meiwa Kasei Co., Ltd.
- Curing accelerator B1 Tetraphenylphosphonium bis(naphthalene-2,3-dioxy)phenyl silicate
- Curing accelerator B2 Tetraphenylphosphonium-4,4'-sulfonyldiphenolate (release agent) Release Agent B1: Glycerin Trimontanate, Ricolb-WE-4, manufactured by Clariant Japan (additive: non-conductive metal compound)
- Additive B1 Inorganic complex oxide, 30C965, manufactured by Shepherd Color Co.
- Silicone B1 Polyoxyalkylene epoxy modified dimethyl polysiloxane, FZ-3730, manufactured by Toray Dow Corning
- thermosetting resin composition (Preparation of thermosetting resin composition)
- the respective raw materials having the blending amounts shown in Table 2 were mixed at room temperature using a mixer, and then roll-kneaded at 70 to 100°C. Next, the obtained kneaded product was cooled and then pulverized to obtain a powdery thermosetting resin composition. Next, for some examples, coarse particles and fine particles were removed using a sieve to obtain a powdery thermosetting resin composition.
- a 109 g weight 503 which is on the same pedestal 506 as the horizontal plate 505, is dropped three times from a height of 160 mm, and part of the granular thermosetting property is generated by impact.
- the elevation angle ( ⁇ ) of the granules 507 was obtained and the collapse angle was measured using a protractor as shown in FIG. 3( b ). The difference between the angle of repose and the angle of collapse was defined as the difference angle.
- thermosetting resin composition Measurement of average particle diameter d 50 of thermosetting resin composition
- the d 50 of the thermosetting resin composition obtained in each example was measured by a laser diffraction type particle size distribution analyzer (manufactured by Shimadzu Corporation, SALD-7000).
- the particle size distribution and d 50 of the entire inorganic filler used in each example were measured by a laser diffraction particle size distribution analyzer (SALD-7000 manufactured by Shimadzu Corporation).
- the resin composition obtained in each example was molded and cured under the condition of 175° C. to obtain a cured product.
- the surface of the obtained cured product was irradiated with a YAG laser, and the plating property in the laser irradiation region was evaluated according to the following criteria. ⁇ : There is no unevenness on the plating surface ⁇ : There is some unevenness on the plating surface but there is no unplated part ⁇ : There is unevenness on the plating surface but there is no unplated part ⁇ : Uneven plating part is visible on the plated surface Yes
- thermosetting resin composition of each example was prepared with the formulation shown in Table 3, and the encapsulating material was prepared and evaluated using the obtained thermosetting resin composition.
- the raw material components used in each example are as follows.
- Inorganic filler C1 amorphous silica/crystalline silica, MUF-4V, manufactured by Tatsumori Co., average particle diameter 3.8 ⁇ m
- Inorganic filler C2 Silicon dioxide, SC-2500-SQ, manufactured by Admatechs, average particle size 0.6 ⁇ m
- Inorganic filler C3 Silicon dioxide, SC-5500-SQ, manufactured by Admatechs, average particle size 1.6 ⁇ m
- Inorganic filler C4 Silicon dioxide, TS-6026, manufactured by Micron Company, average particle size 9.0 ⁇ m
- Inorganic filler C5 Silicon dioxide, TS-6021, manufactured by Micron Company, Inc.
- Colorant C1 carbon black, carbon #5, manufactured by Mitsubishi Chemical Corporation (coupling agent)
- Coupling agent C1 3-mercaptopropyltrimethoxysilane
- S810 coupling agent manufactured by Chisso Co.
- C2 3-glycidoxypropyltrimethoxysilane
- S510 coupling agent C3 manufactured by Chisso Co.: N-phenyl- ⁇ -amino Propyltrimethoxysilane, CF-4083, manufactured by Toray Dow Corning (thermosetting resin)
- Epoxy resin C1 Orthocresol novolac type epoxy resin, EOCN-1020, manufactured by Nippon Kayaku Co., Ltd.
- Epoxy resin C2 Biphenylene skeleton-containing phenol aralkyl type epoxy resin, NC3000, manufactured by Nippon Kayaku Co., Ltd. (hardening agent) Curing agent C1: Novolac type phenol resin, PR-HF-3, Sumitomo Bakelite Co., Ltd. curing agent C2: Biphenylene skeleton-containing phenol aralkyl type resin, MEH-7851SS, Meiwa Kasei Co., Ltd.
- Curing accelerator C1 Tetraphenylphosphonium bis(naphthalene-2,3-dioxy)phenyl silicate Curing accelerator C2: Tetraphenylphosphonium-4,4'-sulfonyldiphenolate (release agent) Release Agent C1: Glycerin Trimontanate, Ricolb-WE-4, manufactured by Clariant Japan (additive: non-conductive metal compound) Additive C1: Inorganic composite oxide, 30C965, manufactured by Shepherd Color Co.
- Silicone C1 polyoxyalkylene epoxy modified dimethyl polysiloxane, FZ-3730, manufactured by Toray Dow Corning (low stress agent)
- Low stress agent C1 epoxidized polybutadiene, JP-200, manufactured by Nippon Soda Co., Ltd.
- the particle size distribution of the entire inorganic filler used in each example was measured by a laser diffraction particle size distribution analyzer (manufactured by Shimadzu Corporation, SALD-7000).
- thermosetting resin composition (Preparation of thermosetting resin composition) Raw materials having the blending amounts shown in Table 3 were mixed at room temperature using a mixer, and then roll-kneaded at 70 to 100°C. Next, the obtained kneaded product was cooled and then pulverized to obtain a powdery thermosetting resin composition. Next, for some examples, coarse particles and fine particles were removed using a sieve to obtain a powdery thermosetting resin composition.
- the resin composition obtained in each example was molded and cured under the condition of 175° C. to obtain a cured product.
- the obtained cured product was subjected to hydrophilic treatment
- ultrasonic cleaning was performed while heating at 50° C. using dipropylene glycol monomethyl ether as a treatment liquid (frequency 40 kHz, Output 110 W) was performed for 5 minutes to rinse the entire surface of the cured product.
- the surface of the cured product was irradiated with a YAG laser (wavelength 1064 nm), and the plating property in the laser irradiation region was evaluated according to the following criteria.
- thermosetting resin composition for LDS used in LASER DIRECT STRUCTURING (A) a thermosetting resin, (B) an inorganic filler, (C) a non-conductive metal compound that forms a metal nucleus upon irradiation with an active energy ray, (D) a coupling agent,
- the component (A) contains at least one selected from the group consisting of epoxy resins and bismaleimide resins,
- the thermosetting resin composition for LDS wherein the proportion of particles having a particle size of 1 ⁇ m or more and 20 ⁇ m or less in the component (B) is 40% by volume or more and 95% by volume or less with respect to the entire component (B).
- the component (B) has a d 10 particle size of 0.05 ⁇ m or more and 3 ⁇ m or less, A1.
- the thermosetting resin composition for LDS according to item 1. A3.
- (E) A1., which further contains a curing accelerator. Or A2.
- the thermosetting resin composition for LDS according to item 1. A4.
- the thermosetting resin composition for LDS according to claim 1. A5.
- the total content of the component (B) and the component (C) in the thermosetting resin composition for LDS is 70% by mass or more and 98% by mass or less with respect to the entire thermosetting resin composition for LDS. , A1. To A4.
- thermosetting resin composition for LDS used in LASER DIRECT STRUCTURING (LDS), A thermosetting resin, An inorganic filler, A non-conductive metal compound that forms a metal nucleus by irradiation with an active energy ray; Including a coupling agent,
- the thermosetting resin contains at least one selected from the group consisting of epoxy resins and bismaleimide resins, A thermosetting resin composition for LDS, wherein the collapse angle of the thermosetting resin composition for LDS is 35° or less.
- the difference angle of the thermosetting resin composition for LDS is 5° or more, B1.
- the thermosetting resin composition for LDS according to item 1.
- the average particle diameter d 50 of the thermosetting resin composition for LDS is 1.0 mm or less, B1.
- thermosetting resin composition for LDS according to item 1. B4.
- the average particle diameter d 50 of the inorganic filler is 10 ⁇ m or less, B1. Through B3.
- the thermosetting resin composition for LDS according to claim 1. B5.
- the total content of the inorganic filler and the non-conductive metal compound in the thermosetting resin composition for LDS is 80% by mass or more and 98% by mass or less with respect to the entire thermosetting resin composition for LDS. , B1. Through B4.
- thermosetting resin composition for LDS (LASER DIRECT STRUCTURING) so as to cover the surface of the semiconductor element; Irradiating a specific portion of the surface of the encapsulant with an active energy ray, A step of hydrophilizing the surface of the sealing material, A step of selectively forming a metal layer in a region of the surface of the encapsulant irradiated with the active energy ray; Including, The thermosetting resin composition for LDS, (A) a thermosetting resin, (B) an inorganic filler, (C) a non-conductive metal compound that forms a metal nucleus upon irradiation with an active energy ray, (D) a coupling agent, A method for manufacturing a semiconductor device, wherein the component (A) contains at least one selected from the group consisting of epoxy resins and bismaleimide resins.
- the step of hydrophilizing the surface of the encapsulant is a step of hydrophilizing the specific portion of the surface of the encapsulant after the step of irradiating an active energy ray, C1.
- a method of manufacturing a semiconductor device according to item 1. C3.
- the step of hydrophilizing treatment, C1. including a step of applying a treatment liquid containing a glycol-based organic solvent to the surface of the encapsulant. Or C2.
- a method of manufacturing a semiconductor device according to item 1. C4.
- the step of selectively forming the metal layer Applying a metal to the region, Growing a plating layer of the metal in the region, Including C1.
- the method for manufacturing a semiconductor device according to claim 1. C5.
- the wavelength of the active energy ray is 240 nm or more and 1100 nm or less, C1.
Landscapes
- Chemical & Material Sciences (AREA)
- Polymers & Plastics (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Power Engineering (AREA)
- Physics & Mathematics (AREA)
- Computer Hardware Design (AREA)
- General Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Manufacturing & Machinery (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Structures Or Materials For Encapsulating Or Coating Semiconductor Devices Or Solid State Devices (AREA)
- Encapsulation Of And Coatings For Semiconductor Or Solid State Devices (AREA)
- Chemically Coating (AREA)
- Electrodes Of Semiconductors (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020561481A JP7078138B2 (ja) | 2018-12-18 | 2019-12-18 | 半導体装置の製造方法 |
| KR1020217022090A KR102490214B1 (ko) | 2018-12-18 | 2019-12-18 | Lds용 열경화성 수지 조성물 및 반도체 장치의 제조 방법 |
| CN201980084285.2A CN113195631A (zh) | 2018-12-18 | 2019-12-18 | Lds用热固性树脂组合物和半导体装置的制造方法 |
| EP19898553.3A EP3901214A4 (en) | 2018-12-18 | 2019-12-18 | THERMOSETTING RESIN COMPOSITION FOR LDS AND SEMICONDUCTOR DEVICE PRODUCTION METHOD |
| US17/415,432 US20220064402A1 (en) | 2018-12-18 | 2019-12-18 | Thermosetting resin composition for lds and method for manufacturing semiconductor device |
| JP2022080339A JP2022116077A (ja) | 2018-12-18 | 2022-05-16 | Lds用熱硬化性樹脂組成物および半導体装置の製造方法 |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018236604 | 2018-12-18 | ||
| JP2018-236604 | 2018-12-18 | ||
| JP2019025900 | 2019-02-15 | ||
| JP2019-025899 | 2019-02-15 | ||
| JP2019-025900 | 2019-02-15 | ||
| JP2019025899 | 2019-02-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020130012A1 true WO2020130012A1 (ja) | 2020-06-25 |
Family
ID=71100871
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/049527 Ceased WO2020130012A1 (ja) | 2018-12-18 | 2019-12-18 | Lds用熱硬化性樹脂組成物および半導体装置の製造方法 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20220064402A1 (enExample) |
| EP (1) | EP3901214A4 (enExample) |
| JP (2) | JP7078138B2 (enExample) |
| KR (1) | KR102490214B1 (enExample) |
| CN (1) | CN113195631A (enExample) |
| TW (1) | TWI797404B (enExample) |
| WO (1) | WO2020130012A1 (enExample) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112812304A (zh) * | 2021-01-07 | 2021-05-18 | 天津德高化成光电科技有限责任公司 | 一种预聚体、含有该预聚体的封装树脂及封装树脂的应用 |
| JP2022087929A (ja) * | 2020-12-02 | 2022-06-14 | 住友ベークライト株式会社 | 電子装置および電子装置の製造方法 |
| JP2022087923A (ja) * | 2020-12-02 | 2022-06-14 | 住友ベークライト株式会社 | 電子装置および電子装置の製造方法 |
| JP2022117398A (ja) * | 2021-01-29 | 2022-08-10 | 住友ベークライト株式会社 | 封止用樹脂組成物、半導体装置の製造方法、および中空無機フィラーの検出方法 |
| JP2023057246A (ja) * | 2021-10-11 | 2023-04-21 | マクセル株式会社 | 回路部品及び回路部品の製造方法 |
| CN116157915A (zh) * | 2020-08-05 | 2023-05-23 | 信越化学工业株式会社 | 热固性树脂组合物及半导体装置 |
| WO2025205911A1 (ja) * | 2024-03-29 | 2025-10-02 | ナミックス株式会社 | 硬化性樹脂組成物、接着剤組成物、硬化物、半導体装置及び電子部品 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7188309B2 (ja) * | 2019-07-26 | 2022-12-13 | 信越化学工業株式会社 | 熱硬化性マレイミド樹脂組成物及び半導体装置 |
| CN114868465B (zh) * | 2019-12-24 | 2024-12-10 | 住友电木株式会社 | 电磁波屏蔽性框体、逆变器部件、空调部件和汽车部件 |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006233337A (ja) * | 2002-05-22 | 2006-09-07 | Hitachi Maxell Ltd | 成形品 |
| JP2015134903A (ja) | 2013-12-17 | 2015-07-27 | 三菱エンジニアリングプラスチックス株式会社 | 繊維強化樹脂材料、樹脂成形品、メッキ層付樹脂成形品、メッキ層付樹脂成形品の製造方法、および繊維強化樹脂材料の製造方法 |
| JP2015163682A (ja) | 2014-01-30 | 2015-09-10 | 日本ゼオン株式会社 | 重合体組成物及び成形体 |
| JP2016098415A (ja) * | 2014-11-21 | 2016-05-30 | 日立マクセル株式会社 | メッキ膜を有する成形体の製造方法 |
| JP2017513794A (ja) * | 2014-04-09 | 2017-06-01 | ザ シェファード カラー カンパニー | コア‐シェル複合無機金属酸化物、ならびにポリマーおよび樹脂組成物における熱酸化分解を防ぐための調製方法 |
| JP2017179184A (ja) * | 2016-03-31 | 2017-10-05 | 太陽インキ製造株式会社 | 硬化性樹脂組成物、ドライフィルムおよびその硬化物 |
| WO2017199639A1 (ja) * | 2016-05-18 | 2017-11-23 | 住友ベークライト株式会社 | Lds用熱硬化性樹脂組成物、樹脂成形品および三次元成形回路部品 |
| JP2018024812A (ja) * | 2015-12-24 | 2018-02-15 | 三菱エンジニアリングプラスチックス株式会社 | レーザーダイレクトストラクチャリング層形成用組成物、キット、およびメッキ層付樹脂成形品の製造方法 |
| JP2019025899A (ja) | 2017-07-28 | 2019-02-21 | 株式会社ダイセル | 積層体、及び前記積層体を備えたフレキシブルデバイス |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1767663A1 (en) * | 2005-09-23 | 2007-03-28 | Nederlandse Organisatie voor toegepast-natuurwetenschappelijk Onderzoek TNO | Method for partially metallizing a product |
| JP5101026B2 (ja) * | 2005-11-04 | 2012-12-19 | 富士フイルム株式会社 | 導電膜形成方法、導電性パターン形成方法、及び多層配線板の製造方法 |
| JP5218298B2 (ja) * | 2008-07-02 | 2013-06-26 | 信越化学工業株式会社 | 熱硬化性シリコーン樹脂−エポキシ樹脂組成物及び当該樹脂で成形したプレモールドパッケージ |
| CN103597037B (zh) * | 2012-03-23 | 2015-08-19 | 三菱工程塑料株式会社 | 热塑性树脂组合物、树脂成型品和带镀层的树脂成型品的制造方法 |
| CN104221140B (zh) * | 2012-03-29 | 2017-08-25 | 住友电木株式会社 | 树脂组合物和半导体装置 |
| US9783890B2 (en) | 2012-10-26 | 2017-10-10 | Rohm And Haas Electronic Materials Llc | Process for electroless plating and a solution used for the same |
| GB201304770D0 (en) * | 2013-03-15 | 2013-05-01 | Provost Fellows Foundation Scholars And The Other Members Of Board Of | A scalable process for producing exfoliated defect-free, non-oxidised 2-dimens ional materials in large quantities |
| JP2016199779A (ja) * | 2015-04-08 | 2016-12-01 | パナソニックIpマネジメント株式会社 | 修飾金属ナノ粒子,その製造方法,修飾金属ナノインク及び配線層形成方法 |
| WO2017110458A1 (ja) * | 2015-12-24 | 2017-06-29 | 三菱エンジニアリングプラスチックス株式会社 | レーザーダイレクトストラクチャリング層形成用組成物、キット、およびメッキ層付樹脂成形品の製造方法 |
| JP6922158B2 (ja) * | 2016-04-20 | 2021-08-18 | 住友ベークライト株式会社 | 熱硬化性樹脂組成物、樹脂封止基板、および電子装置 |
| JP7124317B2 (ja) * | 2016-10-24 | 2022-08-24 | 東レ株式会社 | 半導体センサおよび複合センサ |
-
2019
- 2019-12-18 WO PCT/JP2019/049527 patent/WO2020130012A1/ja not_active Ceased
- 2019-12-18 US US17/415,432 patent/US20220064402A1/en not_active Abandoned
- 2019-12-18 KR KR1020217022090A patent/KR102490214B1/ko active Active
- 2019-12-18 EP EP19898553.3A patent/EP3901214A4/en not_active Withdrawn
- 2019-12-18 JP JP2020561481A patent/JP7078138B2/ja active Active
- 2019-12-18 TW TW108146486A patent/TWI797404B/zh active
- 2019-12-18 CN CN201980084285.2A patent/CN113195631A/zh active Pending
-
2022
- 2022-05-16 JP JP2022080339A patent/JP2022116077A/ja active Pending
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006233337A (ja) * | 2002-05-22 | 2006-09-07 | Hitachi Maxell Ltd | 成形品 |
| JP2015134903A (ja) | 2013-12-17 | 2015-07-27 | 三菱エンジニアリングプラスチックス株式会社 | 繊維強化樹脂材料、樹脂成形品、メッキ層付樹脂成形品、メッキ層付樹脂成形品の製造方法、および繊維強化樹脂材料の製造方法 |
| JP2015163682A (ja) | 2014-01-30 | 2015-09-10 | 日本ゼオン株式会社 | 重合体組成物及び成形体 |
| JP2017513794A (ja) * | 2014-04-09 | 2017-06-01 | ザ シェファード カラー カンパニー | コア‐シェル複合無機金属酸化物、ならびにポリマーおよび樹脂組成物における熱酸化分解を防ぐための調製方法 |
| JP2016098415A (ja) * | 2014-11-21 | 2016-05-30 | 日立マクセル株式会社 | メッキ膜を有する成形体の製造方法 |
| JP2018024812A (ja) * | 2015-12-24 | 2018-02-15 | 三菱エンジニアリングプラスチックス株式会社 | レーザーダイレクトストラクチャリング層形成用組成物、キット、およびメッキ層付樹脂成形品の製造方法 |
| JP2017179184A (ja) * | 2016-03-31 | 2017-10-05 | 太陽インキ製造株式会社 | 硬化性樹脂組成物、ドライフィルムおよびその硬化物 |
| WO2017199639A1 (ja) * | 2016-05-18 | 2017-11-23 | 住友ベークライト株式会社 | Lds用熱硬化性樹脂組成物、樹脂成形品および三次元成形回路部品 |
| JP2019025899A (ja) | 2017-07-28 | 2019-02-21 | 株式会社ダイセル | 積層体、及び前記積層体を備えたフレキシブルデバイス |
| JP2019025900A (ja) | 2017-07-28 | 2019-02-21 | 株式会社ダイセル | 積層体、及び前記積層体を備えたフレキシブルデバイス |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116157915A (zh) * | 2020-08-05 | 2023-05-23 | 信越化学工业株式会社 | 热固性树脂组合物及半导体装置 |
| EP4195255A4 (en) * | 2020-08-05 | 2024-07-24 | Shin-Etsu Chemical Co., Ltd. | Heat-curable resin composition and semiconductor device |
| JP2022087929A (ja) * | 2020-12-02 | 2022-06-14 | 住友ベークライト株式会社 | 電子装置および電子装置の製造方法 |
| JP2022087923A (ja) * | 2020-12-02 | 2022-06-14 | 住友ベークライト株式会社 | 電子装置および電子装置の製造方法 |
| CN112812304A (zh) * | 2021-01-07 | 2021-05-18 | 天津德高化成光电科技有限责任公司 | 一种预聚体、含有该预聚体的封装树脂及封装树脂的应用 |
| JP2022117398A (ja) * | 2021-01-29 | 2022-08-10 | 住友ベークライト株式会社 | 封止用樹脂組成物、半導体装置の製造方法、および中空無機フィラーの検出方法 |
| JP2023057246A (ja) * | 2021-10-11 | 2023-04-21 | マクセル株式会社 | 回路部品及び回路部品の製造方法 |
| JP7729765B2 (ja) | 2021-10-11 | 2025-08-26 | マクセル株式会社 | 回路部品及び回路部品の製造方法 |
| WO2025205911A1 (ja) * | 2024-03-29 | 2025-10-02 | ナミックス株式会社 | 硬化性樹脂組成物、接着剤組成物、硬化物、半導体装置及び電子部品 |
Also Published As
| Publication number | Publication date |
|---|---|
| KR102490214B1 (ko) | 2023-01-19 |
| CN113195631A (zh) | 2021-07-30 |
| JP2022116077A (ja) | 2022-08-09 |
| KR20210104798A (ko) | 2021-08-25 |
| TW202031787A (zh) | 2020-09-01 |
| EP3901214A4 (en) | 2022-08-31 |
| EP3901214A1 (en) | 2021-10-27 |
| JP7078138B2 (ja) | 2022-05-31 |
| US20220064402A1 (en) | 2022-03-03 |
| TWI797404B (zh) | 2023-04-01 |
| JPWO2020130012A1 (ja) | 2021-09-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7078138B2 (ja) | 半導体装置の製造方法 | |
| JP6265308B1 (ja) | Lds用熱硬化性樹脂組成物、樹脂成形品および三次元成形回路部品 | |
| JP6583278B2 (ja) | 半導体封止用樹脂組成物、半導体装置および構造体 | |
| JP2017125150A (ja) | 樹脂組成物 | |
| JP2024166236A (ja) | 熱硬化性樹脂組成物、及び電子装置 | |
| JP6436107B2 (ja) | 封止用樹脂組成物、電子部品の製造方法、および電子部品 | |
| CN113272949B (zh) | 中空封装体及其制造方法 | |
| CN114364736A (zh) | 半导体密封用树脂组合物和半导体装置 | |
| WO2015146764A1 (ja) | 封止用樹脂組成物、および半導体装置 | |
| JP2017088828A (ja) | 半導体封止用樹脂組成物、半導体装置および構造体 | |
| JP2019006972A (ja) | 封止用樹脂組成物の製造方法及び電子装置の製造方法 | |
| JP7501131B2 (ja) | Lds用熱硬化性樹脂組成物および構造体 | |
| JP2016023279A (ja) | 封止用樹脂組成物、半導体装置および構造体 | |
| CN107531982A (zh) | 密封用树脂组合物和电子部件装置 | |
| JP7476589B2 (ja) | 半導体装置の製造方法 | |
| JP2021102728A (ja) | Lds用熱硬化性樹脂組成物 | |
| JP2021150343A (ja) | 半導体装置およびその製造方法 | |
| JP2017125149A (ja) | 封止用樹脂組成物および電子装置 | |
| JP2022096238A (ja) | Lds用熱硬化性樹脂組成物および電子デバイス |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19898553 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2020561481 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20217022090 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2019898553 Country of ref document: EP Effective date: 20210719 |