WO2017221883A1 - 抗体-薬物コンジュゲート - Google Patents
抗体-薬物コンジュゲート Download PDFInfo
- Publication number
- WO2017221883A1 WO2017221883A1 PCT/JP2017/022509 JP2017022509W WO2017221883A1 WO 2017221883 A1 WO2017221883 A1 WO 2017221883A1 JP 2017022509 W JP2017022509 W JP 2017022509W WO 2017221883 A1 WO2017221883 A1 WO 2017221883A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- antibody
- gene
- drug
- fab
- conjugate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6807—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug or compound being a sugar, nucleoside, nucleotide, nucleic acid, e.g. RNA antisense
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7105—Natural ribonucleic acids, i.e. containing only riboses attached to adenine, guanine, cytosine or uracil and having 3'-5' phosphodiester links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6889—Conjugates wherein the antibody being the modifying agent and wherein the linker, binder or spacer confers particular properties to the conjugates, e.g. peptidic enzyme-labile linkers or acid-labile linkers, providing for an acid-labile immuno conjugate wherein the drug may be released from its antibody conjugated part in an acidic, e.g. tumoural or environment
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/0091—Purification or manufacturing processes for gene therapy compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2881—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against CD71
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/60—Medicinal preparations containing antigens or antibodies characteristics by the carrier linked to the antigen
- A61K2039/6031—Proteins
- A61K2039/6056—Antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/62—Medicinal preparations containing antigens or antibodies characterised by the link between antigen and carrier
- A61K2039/627—Medicinal preparations containing antigens or antibodies characterised by the link between antigen and carrier characterised by the linker
Definitions
- the present invention relates to an antibody-drug conjugate (ADC), particularly a conjugate of an anti-CD71 antibody antigen-binding fragment (eg, Fab ') and a drug.
- ADC antibody-drug conjugate
- the present invention provides a composition comprising the above conjugate for delivering a drug to muscle.
- CD71 is a transferrin receptor, which binds to iron-bound holotransferrin, transfers it into cells by receptor-mediated endocytosis, and functions to take up iron into the cells. CD71 is thought to be recycled onto the cell membrane after being taken into the cell by endocytosis from the cell membrane.
- Non-Patent Documents 1 to 4 Since transferrin is taken into cells by CD71, studies have been conducted to incorporate a drug into CD71-expressing cells by conjugating transferrin and the drug (Non-Patent Documents 1 to 4). It has been reported that a conjugate of transferrin and DNA cannot be taken into a cell by itself, but DNA is taken into a cell when polyethyleneimine is complexed with a conjugate (Non-Patent Document). 5).
- Muscle is said to be a difficult tissue for drug delivery.
- the reason for this is considered that the endothelium of the blood vessel in the muscle has the blood vessel spread on the paving stone and does not have a hole for the substance to pass through. Since the structure of the vascular endothelium serves as a barrier, it is difficult to efficiently deliver the drug to the muscle (Non-patent Documents 6 and 7).
- Non-Patent Document 8 discloses conjugates of antibody monomers and siRNA against Her2, TENB2, and the like.
- Non-Patent Document 9 discloses Biseptic Digixigenin-binding Antibodies in which antibodies against Her2, IGF1-R, CD22 and the like and siRNA are bound to the antibody via digoxigenin.
- Non-Patent Document 10 discloses that siRNA is delivered to muscles by Fab ′ fragment of anti-CD71 antibody, but the inventors have published a part of the contents of the basic application after the priority date of the present application.
- Patent Document 1 discloses that the presence or absence of an antitumor effect is determined by the amino acid sequence of an anti-CD71 antibody, and the amino acid sequence of an antibody having an antitumor effect.
- An object of the present invention is to provide a method for effectively delivering a drug such as a nucleic acid to a tissue or a cell such as a muscle.
- the present inventors have found that a conjugate of an anti-CD71 antibody or an antigen-binding fragment thereof and a drug is successfully delivered to muscle.
- a drug delivered to muscle can exert a desired activity in the muscle (for example, when a conjugate of an anti-CD71 antibody or an antigen-binding fragment thereof and siRNA against myostatin is delivered to muscle. It has been found to have an effect of increasing the amount).
- the present invention has been made based on such findings.
- a conjugate of an anti-CD71 antibody or an antigen-binding fragment thereof and a drug (sometimes referred to herein as a conjugate of the present invention).
- a conjugate of the present invention [2] The conjugate according to [1] above, wherein the antigen-binding fragment is Fab ′.
- the anti-CD71 antibody or antigen-binding fragment thereof is Fab ′ of an anti-CD71 antibody.
- a composition for use in delivering a drug to at least one selected from cardiac muscle and skeletal muscle comprising the conjugate according to any one of [1] to [9] above.
- a medicament comprising the conjugate according to any one of [1] to [9] above.
- a method for preventing and / or treating a muscular disease comprising administering an effective amount of the conjugate according to any one of [1] to [9] to a mammal.
- a method for producing a conjugate of Fab ′ of an anti-CD71 antibody and a drug Reacting a thiol group of the Fab ′ with a drug having a functional group reactive with the thiol group, or binding to a linker having a functional group reactive with the thiol group of the Fab ′ and the thiol group Reacting with a drug.
- a drug can be effectively delivered to cells or tissues such as muscles such as skeletal muscle and cardiac muscle.
- a disease of a cell or tissue such as muscle can be prevented or treated by effectively delivering the drug to a cell or tissue such as muscle such as skeletal muscle and myocardium.
- FIG. 1 shows an example of preparing a conjugate in which the antibody is Fab ′ and the drug is siRNA as an example of the antibody-drug conjugate.
- FIG. 1A shows an example of introducing a thiol-reactive group into siRNA.
- FIG. 1B shows a scheme in which an antibody is digested and reduced to Fab ′, and the conjugate is obtained by reacting the thiol group of Fab ′ with the thiol-reactive group of siRNA.
- FIG. 1C shows a chromatogram from size exclusion chromatography-HPLC.
- FIG. 1D is a chromatogram with conjugate peaks, indicating that the conjugate is obtained with high purity.
- FIG. 1E shows the absorption spectrum of Fab ′, FIG.
- FIG. 1F shows the absorption spectrum of siRNA
- FIG. 1G shows the absorption spectrum of the conjugate.
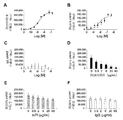
- FIG. 2 shows, as an example of an antibody-drug conjugate, an anti-CD71 antibody (clone RI7 217.1.3) whose antibody was purchased from BioXcell (West Riverside, NH) (referred to herein as “clone RI7”). The evaluation results of the binding ability of the conjugate in which the drug is siRNA are shown.
- FIG. 2A shows the binding activity of clone RI7 to CD71 immobilized on the plate surface.
- FIG. 2B shows the binding activity to CD71 of a conjugate of clone RI7 Fab ′ and siRNA (specifically, siRNA against HPRT).
- FIG. 2C shows that the conjugate of siRNA and the isotype control IgG 2a Fab ′ does not react to CD71.
- FIG. 2D shows that the conjugate of clone RI7 Fab ′ and siRNA (specifically siRNA against HPRT) competes with clone RI7 for binding to CD71.
- FIG. 2E shows that the conjugate of clone RI7 Fab ′ and siRNA (specifically siRNA against HPRT) competes weakly with transferrin (Tf).
- Figure 2F is a conjugate of a Fab of clone Ri7 'and siRNA (siRNA specifically for HPRT) is the isotype control IgG 2a indicates that no conflict.
- FIG. 1 shows that the conjugate of siRNA and the isotype control IgG 2a indicates that no conflict.
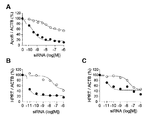
- FIG. 3 shows gene silencing (knockdown) by a conjugate of clone RI7 Fab ′ and siRNA on cultured cells.
- FIG. 3A shows silencing of gene expression of ApoB in cells when conjugates of clone RI7 Fab ′ and siRNA against siApoB (siApoB) are added to the medium for primary hepatocytes that weakly express CD71.
- FIG. 3B shows B cells stimulated with anti-IgM as an example of mitogen
- FIG. 3C shows siRNA against Fab ′ of clone RI7 and HPRT against T cells stimulated with L-PHA as an example of mitogen (siHPRT).
- FIG. 3A black circles indicate GalNAc-siApoB, and white circles indicate a conjugate of clone RI7 Fab ′ and siApoB.
- FIG. 3B and FIG. 3C a black circle indicates a conjugate of clone RI7 Fab ′ and siHPRT, and a white circle indicates a conjugate of isotype control IgG 2a and siHPRT.
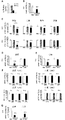
- FIG. 4 shows the in vivo kinetics and function of a conjugate in which the antibody is Clone RI7 Fab 'and the drug is siRNA.
- FIG. 4A shows the amount of conjugate in plasma 6 hours and 24 hours after administration.
- FIG. 4B shows the expression level of ApoB in the liver 24 hours after administration of a conjugate of siRNA to Fab 'of Clone RI7 and ApoB (10 mg / kg body weight, intravenously).
- FIG. 4D and 4E show silencing for HPRT in gastrocnemius muscle (FIG. 4D) and myocardium (FIG. 4E) 72 hours after administration of conjugate of siRNA to FabRI of clone RI7 and HPRT (10 mg / kg body weight, intravenous).
- FIG. 4F shows that HPRT silencing does not occur with the negative control siRNA.
- siNC means a negative control siRNA.
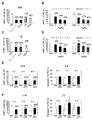
- FIG. 6 is a diagram showing the influence on silencing by the difference in linker.
- FIG. 6A shows HPRT silencing 48 hours after administration of conjugate (10 mg / kg body weight, intravenously) of clone RI7 Fab ′ and siRNA to HPRT according to the type of linker.
- the linker was obtained by reacting a functional group having the same name in FIG. 1A with a thiol group of Fab ′ according to the scheme of FIG. 1B.
- FIG. 1A shows HPRT silencing 48 hours after administration of conjugate (10 mg / kg body weight, intravenously) of clone RI7 Fab ′ and siRNA to HPRT according to the type of linker.
- the linker was obtained by reacting a functional group having the same name in FIG. 1A with a thiol group of Fab ′ according to the scheme of FIG. 1B.
- FIG. 6B shows silencing in gastrocnemius muscle 7 days after administration of a conjugate of siRNA to Fab 'of clone RI7 and HPRT (10 mg / kg body weight, intravenous), maleimide linker (non-cleavable) and Val-Cit linker ( The results of comparison with “Cleavageable” are shown.
- FIG. 6C shows a comparison of silencing by various linkers in the myocardium 48 hours after administration of a conjugate (10 mg / kg body weight, i.v.) of siRNA to Fab 'of clone RI7 and HPRT.
- FIG. 6D shows silencing in the myocardium 7 days after administration of a conjugate of clone RI7 Fab ′ and siRNA to HPRT (10 mg / kg body weight, intravenous), maleimide linker (non-cleavable) and Val-Cit linker ( The results of comparison with “Cleavageable” are shown.
- n 4
- statistical analysis was performed by Dunnet's test (*: p ⁇ 0.05, ***: p ⁇ 0.001).
- FIG. 6E shows HPRT or myostatin (negative control) in gastrocnemius muscle at 2 weeks, 3 weeks or 4 weeks after administration of conjugate of siRNA to clone RT7 Fab ′ and HPRT (10 mg / kg body weight, intravenous) Indicates silencing.
- FIG. 6F shows silencing for HPRT or myostatin (negative control) in the myocardium after administration of FIG. 6E.
- n 4 and statistical analysis was performed by student's t-test (**: p ⁇ 0.05, **: p ⁇ 0.01, ***: p ⁇ 0. 001).
- FIG. 7 is a diagram showing the effect of different administration methods on silencing.
- FIG. 7 is a diagram showing the effect of different administration methods on silencing.
- FIG. 7B shows silencing for HPRT in the myocardium 7 days after administration.
- FIG. 7C shows silencing for HPRT 7 days after administration in gastrocnemius muscle treated with 1 ⁇ g of various conjugates or negative control by intramuscular administration.
- FIG. 7D shows silencing in the contralateral gastrocnemius muscle that is untreated.
- n 4 and statistical analysis was performed by Dunner's test (**: p ⁇ 0.05; *** p ⁇ 0.001).
- FIG. 7F shows silencing for HPRT or myostatin after 3, 7, or 14 days in the untreated contralateral gastrocnemius muscle.
- n 4 and statistical analysis is by student's test (*: p ⁇ 0.05; ***: p ⁇ 0.001).
- FIG. 8 shows the effect of conjugation of siRNA to Clone RI7 Fab ′ and myostatin in peripheral arterial disease (PAD) mice.
- FIG. 8A shows silencing for myostatin or HPRT by a conjugate administered intramuscularly into the gastrocnemius muscle of one leg of a normal mouse.
- FIG. 8B shows silencing for myostatin or HPRT in the gastrocnemius muscle of the contralateral paw that is untreated.
- FIG. 8C shows silencing of the conjugate of clone RI7 Fab ′ with siRNA (siMSTN) against myostatin in PAD model mice by femoral artery ligation (FAL).
- FIG. 8C shows silencing for myostatin following intramuscular administration of a clone RI7 Fab ′ and siMSTN conjugate (20 ⁇ g / FAL completed leg) at weekly intervals for 4 weeks.
- FIG. 8C shows gastrocnemius muscle weight in mice treated as in FIG. 8C.
- n 2-5, and statistical analysis was performed by student's t test (##: p ⁇ 0.001 compared to FAL control).
- FIG. 8E shows a hematoxylin-eosin stained image of a thin layer section of the gastrocnemius muscle of a mouse treated as in FIG. 8C. An arrowhead indicates a muscle fiber, and the regenerated muscle fiber is characterized by a nucleus located in the center.
- FIG. 8F shows the average value of the cross-sectional area of muscle fibers in the gastrocnemius muscle shown in FIG. 8E.
- FIG. 8F statistical analysis was performed by the student's t test ($$: p ⁇ 0.01 with respect to the sham control (sham); ##: p ⁇ 0.001 with respect to the FAL control).
- FIG. 8G the result of the running performance test by the treadmill test of the mouse
- n 12 and statistical analysis was performed by the student's t test (#: p ⁇ 0.05 vs. FAL control).
- FIG. 8G 12
- FIG. 9 shows gene silencing (knockdown) by conjugate of Fab ′ of siRNA and OKT9 antibody to cultured human cells.
- Fab ′ of siRNA and OKT9 antibody For human chronic myeloid leukemia cells expressing CD71 (K562), conjugate of Fab 'of OKT9 antibody and siRNA against HPRT (represented by “CD71-siRNA (HPRT)” in the figure) is added to the medium.
- Fig. 2 is a diagram showing silencing of HPRT gene expression in cells in the case of a conjugate using Fab 'of isotype control IgG 2a instead of OKT9 antibody (in the figure, represented by "IgG-siRNA (HPRT)”). Was used as a negative control.
- subject means an animal (eg, a mammal (eg, a primate (eg, human))).
- antibody means an immunoglobulin and includes polyclonal antibodies and monoclonal antibodies.
- a monoclonal antibody is preferable.
- the origin of the antibody is not particularly limited, and examples thereof include non-human animal antibodies (for example, non-human mammal antibodies) and human antibodies.
- the antibody may be a chimeric antibody or a humanized antibody.
- the antibody may also be a bispecific antibody.
- the origin of the antibody is preferably a humanized antibody or a human antibody.
- the antibody is preferably a chimeric monoclonal antibody, a humanized monoclonal antibody or a human monoclonal antibody.
- Chimeric monoclonal antibodies and humanized monoclonal antibodies can be prepared from antibodies of non-human animals by a method known per se.
- the antibody has a structure in which two heavy chains and two light chains are associated.
- the heavy chain consists of a heavy chain variable region (VH) and a heavy chain constant region (CH1, CH2 and CH3) and a hinge region located between the heavy chain variable region and the heavy chain constant region.
- the light chain consists of a light chain variable region (VL) and a light chain constant region (CL).
- Each heavy and light chain variable region has three complementarity determining regions (CDRs), which characterize the antigen specificity of the antibody.
- the CDRs are called heavy chain CDR1, heavy chain CDR2, and heavy chain CDR3, and light chain CDR1, light chain CDR2, and light chain CDR3 from the N-terminal side of the heavy chain and light chain, respectively.
- antigen-binding fragment means a part of an antibody that maintains the binding to an antigen.
- the antigen-binding fragment can comprise the heavy chain variable region or the light chain variable region of the antibody of the present invention, or both.
- the antigen-binding fragment may be chimerized or humanized.
- Antigen-binding fragments include, for example, Fab, Fab ′, F (ab ′) 2 , Fv, scFv (single chain Fv), diabody, sc (Fv) 2 (single chain (Fv) 2 ), and half-molecule type Ig. In the present invention, antigen-binding fragments of these antibodies can be used.
- Such antibody fragments are not particularly limited, and can be obtained, for example, by treating an antibody with an enzyme.
- Fab can be obtained by digesting an antibody with papain.
- F (ab ′) 2 can be obtained by digesting the antibody with pepsin, and Fab ′ can be obtained by further reducing this.
- treating means therapy or prevention.
- the “drug” means, for example, a physiologically active substance (eg, protein (eg, enzyme), nucleic acid (eg, DNA or modified nucleic acid), lipid, peptide, sugar, low molecular weight compound useful in the living body, Metabolites and secondary metabolites), contrast agents, fluorescent dyes and the like (sometimes referred to herein as physiologically active substances).
- a physiologically active substance eg, protein (eg, enzyme), nucleic acid (eg, DNA or modified nucleic acid), lipid, peptide, sugar, low molecular weight compound useful in the living body, Metabolites and secondary metabolites), contrast agents, fluorescent dyes and the like (sometimes referred to herein as physiologically active substances).
- drug includes, in addition to a physiologically active substance, a drug in a form capable of releasing the physiologically active substance in a cell (for example, a prodrug such as a physiologically active substance and a physiologically active substance). Vesicle).
- physiologically active substance means a substance that acts on a specific physiological regulation function of a living body.
- a contrast agent or a fluorescent dye as a “drug”
- an image diagnosis of muscles can be performed.
- a physiologically active substance is preferable, and as the physiologically active substance, “nucleic acid” is preferable.
- nucleic acid includes natural nucleic acids such as natural DNA and natural RNA, modified nucleic acids such as antisense oligonucleotides (ASO), modified DNA and modified RNA, artificial nucleic acids and combinations thereof. It is done.
- modified nucleic acid include a fluorescent dye-modified nucleic acid, a biotinylated nucleic acid, and a nucleic acid into which a cholesteryl group has been introduced.
- RNA may be 2′-O-methyl modified, 2′-fluoro modified, or 2′-methoxyethyl (MOE) modified to the base to enhance stability, thereby phosphodiester bonds in the nucleic acid backbone.
- the artificial nucleic acid examples include a nucleic acid in which a 2 ′ oxygen atom and a 4 ′ carbon atom are cross-linked.
- an artificial nucleic acid for example, a locked nucleic acid (LNA) which is a crosslinked DNA in which a 2′-position oxygen atom and a 4′-position carbon atom are crosslinked via methylene, a 2′-position oxygen atom and ENA in which 4′-position carbon atom is bridged through ethylene, BNA COC in which 2′-position oxygen atom and 4′-position carbon atom are bridged through —CH 2 OCH 2 —, 2′-position oxygen A bridged nucleic acid (BNA) such as BNA NC in which the atom and the 4′-position carbon atom are bridged via —NR—CH 2 — ⁇ where R is a methyl or hydrogen atom ⁇ ; CMOE in which the oxygen atom and the 4′-position carbon
- RNA examples include artificial RNA for gene silencing such as siRNA and shRNA, non-coding RNA such as microRNA (miRNA) and aptamer, and natural RNA such as mRNA. These RNAs can be modified to stabilize in vivo.
- siRNA and shRNA non-coding RNA such as microRNA (miRNA) and aptamer
- mRNA microRNA
- RNAs can be modified to stabilize in vivo.
- conjugate means a conjugate in which two substances are linked by a covalent bond.
- the two substances may be directly connected or may be connected via a linker.
- one of the two substances is an antibody or an antigen-binding fragment thereof, and the other is a drug (for example, a physiologically active substance).
- the linker may be a cleavable linker or a non-cleavable linker.
- antibody-drug conjugate means a conjugate (ADC) of an antibody and a drug.
- ADC conjugate
- affinity for an antigen is imparted to the drug, which can increase the efficiency of delivering the drug to a target in vivo site.
- an antibody-drug conjugate is used in a sense that also includes a conjugate of an antigen-binding fragment of an antibody and a drug.
- CD71 is known as a transferrin receptor having a function of taking up iron into cells. CD71 is expressed in hepatocytes. CD71 is not expressed in normal quiescent lymphocytes, but is expressed in lymphocytes when activated by mitogen or the like. Furthermore, CD71 is highly expressed in muscle (for example, myocardium and gastrocnemius). Apotransferrin (ie, non-iron conjugate) binds to CD71 when bound to two Fe 3+ ions to form holotransferrin (ie, iron conjugate). The complex of CD71 and holotransferrin is translocated into the cell by receptor-mediated endocytosis.
- CD71 and transferrin dissociate in an endosomal environment, and transferrin moves into the cell while CD71 is recycled to the cell membrane. Thus, transferrin is thought to translocate into cells by proper binding to CD71 and proper dissociation.
- Examples of CD71 include human CD71 (for example, having the sequence described in GenBank accession number XM 015113112) and mouse CD71 (for example, having the sequence described in GenBank accession number NM 011638).
- the cell can be a cell that expresses CD71 (eg, a eukaryotic cell, eg, a mammalian cell, eg, a primate cell, eg, a human cell) or a tissue or organ containing them.
- CD71 expressed in the cells may be endogenous CD71 or exogenous CD71.
- CD71 expressed in the cells may be induced in expression.
- anti-CD71 antibody means an antibody that recognizes CD71 or an antibody that binds to CD71.
- the anti-CD71 antibody is preferably an antibody that recognizes human CD71.
- the anti-CD71 antibody is preferably a monoclonal antibody, and more preferably an anti-CD71 chimeric monoclonal antibody, an anti-CD71 humanized monoclonal antibody or an anti-CD71 human monoclonal antibody.
- human monoclonal antibody means any antibody in which the sequences of the variable domain and constant domain are human sequences.
- the term has a sequence derived from a human gene, but has been altered, for example, to reduce possible immunogenicity, increase affinity, remove cysteines that can cause undesirable folding, etc.
- the term also encompasses such antibodies produced recombinantly in non-human cells that can be glycosylated that are not characteristic of human cells. These antibodies can be prepared in various forms, as described below.
- chimeric monoclonal antibody means a monoclonal antibody in which regions of antibodies from two or more different species are linked.
- Examples of chimeric monoclonal antibodies include monoclonal antibodies in which the variable region of a mouse antibody is linked to the constant region of a human antibody.
- the term “chimeric human monoclonal antibody” includes the VH and VL of antibodies of non-human mammalian species, and the CH and CL domains of human antibodies.
- One or more CDRs of a chimeric antibody can be derived from a human antibody.
- the CDRs from a human antibody can be combined with the CDRs of an antibody from a non-human mammal such as a mouse or rat.
- all CDRs can be derived from human antibodies.
- CDRs from multiple human antibodies can be combined in a chimeric antibody.
- the chimeric antibody may comprise CDR1 of the light chain of the first human antibody, CDR2 of the light chain of the second human antibody and CDR3 of the light chain of the third human antibody, It may be derived from one or more other antibodies.
- humanized monoclonal antibody refers to a monoclonal antibody derived from a non-human mammal, wherein an amino acid residue characteristic of the antibody sequence of the non-human mammal species is located at the corresponding position of the human antibody. Substituted with the found residue. This “humanization” process is thought to reduce the immunogenicity of the resulting antibody in humans.
- Antibodies derived from non-human mammals can be humanized using techniques well known in the art (eg, techniques described in Winter et al., Immunol. Today, 14: 43-46 (1993)).
- Humanized monoclonal antibodies can be produced, for example, by recombinant DNA technology that replaces the CH1, CH2, CH3, hinge domain, and / or framework domain of antibodies from non-human mammals with the corresponding human sequences (eg, WO 92/02190, And US Pat. Nos. 5,530,101, 5,585,089, 5,693,761, 5,693,792, 5,714,350, and 5,777,085. Can be engineered by using the technique described in No. 1).
- the term “humanized monoclonal antibody” includes within its meaning chimeric human monoclonal antibodies and CDR-grafted antibodies.
- the CDR-grafted antibody of the present invention can be obtained by replacing the VH and VL CDRs of human antibodies with the VH and VL CDRs of non-human animal antibodies, respectively.
- muscle means striated muscle and smooth muscle.
- the striated muscle means a muscle having a striated structure, and examples thereof include skeletal muscle and myocardium.
- Smooth muscle means a muscle without a striated structure, and an example thereof is a visceral muscle.
- vascular endothelial cell means a cell covering the inside of a blood vessel, and is usually a flat and thin cell.
- Vascular endothelial cells control the passage of substances inside and outside the blood vessels and the passage of white blood cells. Therefore, in order for a drug administered into a blood vessel to move to the tissue parenchyma, it is required to pass through a layer of vascular endothelial cells.
- a conjugate of an anti-CD71 antibody and a drug can be provided.
- a conjugate of an anti-CD71 antibody antigen-binding fragment (eg, Fab ') and a drug can also be provided.
- a conjugate of an anti-CD71 antibody Fab 'and a nucleic acid can be provided.
- These conjugates can be effectively delivered to, for example, muscle (eg, myocardium and gastrocnemius).
- These conjugates can also be administered, for example, by intravenous, intramuscular, subcutaneous, or intraperitoneal administration.
- conjugates can also be administered intravenously or intramuscularly, thereby effectively delivering to muscle (eg, myocardium and gastrocnemius) and even into cells.
- This conjugate expresses CD71 (hereinafter sometimes referred to as “CD71 positive”) cells (for example, muscle, cancer cells, immune cells (for example, lymphocytes such as B cells, T cells) and hepatocytes. Etc.) can be suitably used to deliver drugs.
- CD71 positive for example, muscle, cancer cells, immune cells (for example, lymphocytes such as B cells, T cells) and hepatocytes. Etc.
- CD71 positive for example, muscle, cancer cells, immune cells (for example, lymphocytes such as B cells, T cells) and hepatocytes. Etc.) can be suitably used to deliver drugs.
- CD71 positive cells for example, muscle, cancer cells, immune cells (for example, lymphocytes such as B cells, T cells) and hepatocytes. Etc.) can be suitably used to deliver drugs.
- vesicles having a particle size of about 300 nm or less than 300 nm can be taken into cells by endocytosis. Moreover, even a macromolecule can be taken into a cell together with CD71. Therefore, in one aspect of the present invention, vesicles having a particle size of about 300 nm or less than 300 nm and having an anti-CD71 antibody or an antigen-binding fragment thereof on the surface are also provided.
- the vesicle may contain a drug inside.
- vesicles containing physiologically active substances are also referred to as “drugs”.
- the type of vesicle is not particularly limited, and examples thereof include liposomes using lipid molecules, Lipid Nanoparticle (LNP), and the like.
- the particle size of the vesicle is not particularly limited, and the upper limit is usually 300 nm, preferably 250 nm, and more preferably 200 nm.
- the lower limit of the particle size of the vesicle is not particularly limited, and may be 30 nm, 40 nm, 60 nm, or 80 nm.
- the anti-CD71 antibody or antigen-binding fragment thereof and the drug can be linked via a linker.
- the linker that the conjugate of the present invention may have represents a moiety that links an anti-CD71 antibody or an antigen-binding fragment thereof and a drug.
- the linker may be a cleavable linker or a non-cleavable linker, as shown in Examples described later.
- cleavable means capable of cleaving in an intracellular environment (for example, in a low pH environment in an endosome and in an intracellular reducing environment), and “non-cleavable” means a cell It means not cleaved or substantially cleaved in the internal environment.
- the linker may not be a linker that is actively decomposed outside the cell so that the drug cannot reach the cell, and the linker is not particularly limited, and various linkers can be used.
- a cleavable linker and a non-cleavable linker can be used as the linker.
- the cleavable linker include a linker having a —SS— bond (eg, SS linker, DMSS linker) that cleaves in a reducing environment in a cell, and a hydrazone that is cleaved by low pH in endosomes.
- a linker having a bond in its constituent, a linker having an orthoester bond in its constituent, and a linker having a peptide bond in its constituent that is cleaved by cathepsin B for example, a linker having a valine-citrulline bond in its molecule (Val -Cit linker)
- cathepsin B for example, a linker having a valine-citrulline bond in its molecule (Val -Cit linker)
- the cleavable linker includes a linker having a sugar chain that is cleaved by a sugar chain degrading enzyme such as glucuronidase, and can be preferably used in the present invention.
- non-cleavable linker examples include a thiol-reactive group that is a maleimide group, and can be cleaved in an intracellular environment (for example, in a low pH environment in an endosome and in a reducing environment in a cell) during its construction.
- linkers that do not have a binding site sometimes referred to herein as maleimide linkers
- the maleimide linker is distinguished from a linker having a maleimide group and cleavable (for example, DMSS linker, Val-Cit linker) and other cleavable linker (for example, SS linker).
- the linker is preferably a maleimide linker, a Val-Cit linker, an SS linker, or a DMSS linker.
- the anti-CD71 antibody or antigen-binding fragment thereof used in the present invention is preferably one that can bind to CD71 stronger than blood transferrin or can bind to CD71 at a site different from transferrin.
- an anti-CD71 antibody or an antigen-binding fragment thereof when used, it can bind to CD71 in vivo regardless of the presence of transferrin in the blood.
- the anti-CD71 antibody or antigen-binding fragment thereof binds to CD71, it can be taken together into the cell by the action of spontaneously taking up CD71 into the cell.
- the evaluation of whether an anti-CD71 antibody or an antigen-binding fragment thereof can bind to CD71 more strongly than blood transferrin is carried out, for example, by examining the binding activity of anti-CD71 antibody or an antigen-binding fragment thereof and blood transferrin to CD71. This can be done by comparison.
- the comparison of the binding activity of the antibody or antigen-binding fragment thereof and transferrin to CD71 can be performed, for example, by competitive binding activity evaluation.
- the well plate incubated with CD71 is washed, and then blocked using D-PBS ( ⁇ ) containing 1% bovine serum albumin (BSA) or the like. After further washing, the anti-CD71 antibody or antigen-binding fragment thereof and transferrin are incubated in the well. After washing, it is labeled with an appropriate label (for example, a fluorescent label), and competitive binding activity can be evaluated from the detection intensity.
- the anti-CD71 antibody or the antigen-binding fragment thereof binds to a site different from transferrin with respect to CD71. For example, in the evaluation of the competitive binding activity, the anti-CD71 antibody or the antigen-binding fragment thereof to be competed is used.
- transferrin can be labeled with different distinguishable labels and evaluated by their respective detection intensity or overall detection intensity.
- anti-CD71 antibody or antigen-binding fragment thereof labeled with a distinguishable fluorescent label and transferrin are used, if these mixed fluorescence is detected, the antibody or It can be evaluated that both the antigen-binding fragment and transferrin bind to CD71, and these bind to different sites with respect to CD71.
- the anti-CD71 antibody is a chimeric monoclonal antibody of clone RI7 217.1.3, a humanized monoclonal antibody of clone RI7 217.1.3, or a human monoclonal antibody of clone RI7 217.1.3, an OKT9 antibody (BioXcell A chimeric monoclonal antibody of BE0023), a humanized monoclonal antibody of OKT9 antibody (BioXcell: BE0023) or a human monoclonal antibody of OKT9 antibody (BioXcell: BE0023), a chimeric monoclonal antibody of anti-human CD71 antibody (5E9C11) A humanized monoclonal antibody of anti-human CD71 antibody (5E9C11) or a human monoclonal antibody
- an antibody comprising a band, antibody comprising a heavy chain CDRl ⁇ 3 and the light chain CDRl ⁇ 3 having the same amino acid sequence with any of these antibodies are preferred.
- the amino acid sequence of CDRs can be determined according to Kabat numbering.
- those Fab ′ are preferable.
- the anti-CD71 antibody or antigen-binding fragment used in the present invention is not particularly limited.
- the CDR amino acid sequence is the above clone RI7 217.1.3 (BioXcell), OKT9 antibody (BioXcell: BE0023).
- an antigen-binding fragment thereof an antibody whose epitope is completely or partially identical for binding to human CD71 (or an antibody with overlapping epitopes), or an antigen-binding fragment thereof, or An antibody having an amino acid sequence having one to several amino acid additions, insertions, deletions, or substitutions relative to the amino acid sequences of these antibodies, or an antigen-binding fragment thereof is preferable.
- heavy chain CDR1 having the amino acid sequence represented by SEQ ID NO: 26, SEQ ID NO: A heavy chain variable region comprising a heavy chain CDR2 having the amino acid sequence represented by SEQ ID NO: 27 and a heavy chain CDR3 having the amino acid sequence represented by SEQ ID NO: 28, and a light chain CDR1 having the amino acid sequence represented by SEQ ID NO: 29, the sequence
- An antibody comprising a light chain variable region comprising a light chain CDR2 having the amino acid sequence represented by number 30 and a light chain CDR3 having the amino acid sequence represented by SEQ ID NO: 31, or an antigen-binding fragment thereof, or a sequence thereof
- a light chain variable region having the amino acid sequence shown by SEQ ID No. 25 Is preferably an antibody or antigen binding fragment thereof comprising.
- these CDRs are referred to as Kabat et al. al, Ann. NY Acad Sci 190: 382-93 (1971), or a database of antibody amino acid sequences created by Kabat et al. ([Sequence ofteProteins of Immunological Interest US Dept.Health and198HumanSer 198S). And can be found by examining homology (for example, refer to GenBank accession number: AAA51064.1 for the heavy chain variable region of OKT9 and GenBank accession number: AAA51130.1 for the light chain variable region of OKT9) can do).
- “Substantial identity” means that two amino acid sequences have at least 70%, 75% or 80% sequence identity, preferably at least 90% or 95% sequence identity, more preferably at least 97%, Means having 98% or 99% sequence identity. In certain embodiments, amino acids at positions that are not identical in sequence may be substituted with conservative amino acids.
- % sequence identity refers to the percentage of residues in two matching sequences when the two amino acid sequences are aligned so that the amino acid (or conservative amino acid) matches are maximized.
- Amino acid sequence identity can be determined by any method known to those of skill in the art. For example, the algorithm of Karlin and Altshul (Proc. Natl. Acad. Sci. USA 87: 2264-2268, 1990 and Proc. Natl. Acad. Sci. USA 90: 5873-5877, 1993) can be determined. It can be determined by the BLAST program (J. Mol. Biol. 215: 403-410, 1990) using such an algorithm. A program for determining amino acid sequence identity is available, for example, on the Internet website of National Center for Biotechnology Information.
- an anti-CD71 antibody or an antigen-binding fragment thereof is a chimeric monoclonal antibody of clone RI7 217.1.3, a humanized monoclonal antibody of clone RI7 217.1.3, or a human monoclonal antibody of clone RI7 217.1.3 Or an antigen-binding fragment thereof (preferably Fab ′);
- the drug is a bioactive substance (eg, a nucleic acid (preferably mRNA, siRNA or ASO (more preferably siRNA or ASO)); and
- a linker preferably, a maleimide linker, a Val-Cit linker, an SS linker, and a DMSS linker
- an anti-CD71 antibody or an antigen-binding fragment thereof (i) an anti-CD71 antibody or an antigen-binding fragment thereof, (Ia) a heavy chain comprising a heavy chain CDR1 having the amino acid sequence represented by SEQ ID NO: 26, a heavy chain CDR2 having the amino acid sequence represented by SEQ ID NO: 27, and a heavy chain CDR3 having the amino acid sequence represented by SEQ ID NO: 28 A light region comprising a variable region, a light chain CDR1 having the amino acid sequence represented by SEQ ID NO: 29, a light chain CDR2 having the amino acid sequence represented by SEQ ID NO: 30, and a light chain CDR3 having the amino acid sequence represented by SEQ ID NO: 31.
- an antibody comprising a chain variable region, or an antigen-binding fragment thereof
- An antibody preferably a human antibody or a antigen-binding fragment thereof comprising a heavy chain variable region having the amino acid sequence represented by SEQ ID NO: 24 and a light chain variable region having the amino acid sequence represented by SEQ ID NO: 25
- an antibody preferably a human antibody
- an antibody preferably a human antibody
- an antibody preferably a human antibody
- an antibody preferably a human antibody
- compositions for use in delivering a drug to muscle comprising anti-CD71 antibody or antigen-binding property thereof
- Compositions comprising conjugates of fragments and drugs
- the composition can be administered by intravenous administration, subcutaneous administration, intraperitoneal administration, intramuscular administration, or the like.
- Fab ′ of an anti-CD71 antibody is preferable.
- the conjugate of Fab ' which is an antigen-binding fragment of anti-CD71 antibody, and a drug was taken up into cells expressing CD71. Therefore, a conjugate of an anti-CD71 antibody antigen-binding fragment and a drug can bind to CD71 in vivo and be taken into CD71-expressing cells.
- a conjugate of an anti-CD71 antibody Fab ′ and a drug can be provided.
- Fab ′ obtained by reducing F (ab ′) 2 has a thiol group. Therefore, Fab ′ and a drug can be bonded via an S atom (sulfur atom) contained in the thiol group of Fab ′.
- Fab ′ can be obtained from an antibody by a method known per se.
- Fab ′ can be obtained by subjecting the antibody to pepsin degradation to obtain F (ab ′) 2 and further reducing this using a reducing agent such as cysteamine.
- the drug in the conjugate of Fab ′ and a drug, may be linked to Fab ′ via a linker or not.
- conjugates of Fab ′ and drugs methods known to those skilled in the art can be used to introduce linkers and prepare conjugates.
- a linker introduced into the drug with respect to the thiol group of Fab ′ from the viewpoint of simplicity of reaction and the biotoxicity of the resulting conjugate. It can be linked by reacting with a thiol reactive group therein. (See, for example, FIG. 1B).
- Fab ' is excellent in the balance between the binding affinity with the membrane protein to be recycled and the dissociation property in the endosome. Therefore, conjugates of Fab ', which is an antigen-binding fragment, and a drug can be preferably used for drug delivery into cells.
- a conjugate of Fab ′ of an anti-CD71 antibody and siRNA is provided.
- siRNA is about 15 kDa, and is rapidly removed from the blood by clearance by the kidney.
- siRNA can exist more stably in blood.
- a conjugate of an anti-CD71 antibody or an antigen-binding fragment thereof for example, Fab '
- siRNA or ASO against myostatin Conjugation of an anti-CD71 antibody or antigen-binding fragment thereof (eg, Fab ′) and siRNA or ASO to myostatin can reduce myostatin expression in muscle or myocardium, thereby increasing muscle mass .
- a medicament for use in increasing muscle mass in a subject in need thereof, comprising an anti-CD71 antibody or an antigen-binding fragment thereof (eg, Fab ′) and siRNA or ASO for myostatin A medicament comprising the conjugate.
- the medicament may be administered by, for example, intravenous administration, subcutaneous administration, intraperitoneal administration, or intramuscular administration.
- siRNA for myostatin examples include siRNA having a sense strand having the base sequence of SEQ ID NO: 15 (or SEQ ID NO: 5) and an antisense strand having the base sequence of SEQ ID NO: 16 (or SEQ ID NO: 6).
- siRNA for myostatin the RNA described in 3) of (2) in Example 1 can be used.
- myostatin include human myostatin (for example, having the sequence described in GenBank accession number NM_005259.2).
- an antisense or an antisense strand is a DNA fragment or RNA fragment having a sequence complementary to a certain sequence of DNA or RNA. For example, it can be complementarily bound to mRNA. Includes what can be done. Therefore, the antisense strand in siRNA means an RNA strand that binds complementarily to the target mRNA.
- the sense strand means the strand that is not the antisense strand in the nucleic acid constituting the duplex.
- siRNA having a modification for stabilization can be used as siRNA.
- modification for stabilization include 2'-O-methyl modification and 2'-fluoro modification.
- RNA may be modified alternately with 2'-O-methyl and 2'-fluoro modifications to consecutive bases.
- the drug can be siRNA.
- a thiol reactive group can be introduced into the sense strand or antisense strand of siRNA.
- a thiol reactive group can be introduced into the sense strand of the siRNA (eg, the 3 'end of the sense strand).
- the drug may be an antisense oligonucleic acid (ASO).
- ASO antisense oligonucleic acid
- a thiol reactive group can be introduced at the aminated 5 'end or 3' end of an antisense oligonucleic acid.
- a thiol reactive group can be introduced at the 5 'end of ASO.
- ASO and a thiol reactive group may be linked via a spacer, for example, a spacer composed of a nucleic acid having 1 to several bases.
- the drug can be a substance having anticancer activity.
- Substances with anti-cancer activity include tumor size reduction (delay or cessation), tumor metastasis inhibition, tumor growth inhibition (delay or cessation), and alleviation of one or more symptoms associated with cancer.
- the substance which produces at least one of these is mentioned.
- Specific examples include, but are not limited to, toxins, anticancer agents, and radioisotopes.
- the toxin include Pseudomonas aeruginosa exotoxin (PE) or a cytotoxic fragment thereof (eg, PE38), diphtheria toxin, ricin A and the like.
- Anticancer agents include, for example, adriamycin, daunomycin, mitomycin, cisplatin, vincristine, epirubicin, methotrexate, 5-fluorouracil, aclacinomycin, nitrogen mustard, cyclophosphamide, bleomycin, daunorubicin, doxorubicin, vincristine, vinblastine Low molecular weight compounds such as vindesine, tamoxifen, dexamethasone, and cytokines that activate immunocompetent cells (eg, human interleukin 2, human granulocyte macrophage colony stimulating factor, human macrophage colony stimulating factor, human interleukin 12), etc. Examples include proteins. Examples of radioisotopes include 32 P, 14 C, 125 I, 3 H, 131 I, 211 At, 90 Y, and the like.
- substances having an anti-cancer action include substances that change gene expression in immune cells and / or tumor cells. By altering gene expression in immune cells and / or tumor cells, it is possible to maintain or enhance the action of immune cells on tumor cells and tumor tissues.
- examples of the substance that changes gene expression in immune cells include siRNA capable of silencing such as PD1 gene, endogenous T cell receptor gene, and nucleic acids such as ASO.
- examples of the substance that changes gene expression in tumor cells include siRNA, ASO and other nucleic acids capable of silencing immune checkpoints such as PD-L1 gene and PD-L2 gene.
- An antibody can be obtained by a person skilled in the art by immunizing an animal with an antigen according to a conventional method.
- a monoclonal antibody is obtained by fusing spleen cells of an animal immunized with an antigen and myeloma to form a hybridoma, cloning a hybridoma that produces an antibody that binds to the antigen, and It can be obtained as a produced antibody.
- Anti-CD71 antibodies can be obtained using CD71 as an antigen.
- the anti-CD71 antibody may be obtained by immunizing an animal with virus-like particles (VLP) that express CD71.
- VLP virus-like particles
- Anti-CD71 antibodies may be obtained by further selecting those that do not bind to VLPs that do not display CD71 for the purpose of excluding antibodies against VLPs.
- the anti-CD71 antibody may be obtained by immunizing an animal with cells expressing CD71.
- An anti-CD71 antibody that does not bind to cells that do not present CD71 may be further selected and used for the purpose of excluding antibodies against cells.
- Antigen-binding fragments of antibodies can be obtained by treating the obtained antibodies by methods well known to those skilled in the art.
- An antibody that competes with an antibody (desired antibody) for binding to CD71 can be obtained by a competition assay.
- a competition assay for example, blocking at least 20%, preferably at least 20-50%, more preferably at least 50%, 60%, 70%, 80%, or 90% binding of the desired antibody to CD71. If possible, the antibody can compete with the desired antibody for binding to CD71. Competing antibodies can be confirmed by cross-blocking assays, preferably by competitive ELISA assays.
- CD71 is coated, for example, on a microtiter plate, and the labeled desired antibody is added and incubated to form a bond between the antigen and the desired antibody. Thereafter, a candidate competing antibody is added to the well, incubated, washed, and the amount of the desired antibody bound (label remaining amount) is quantified to determine whether the antibody has competed. .
- Antibodies that bind completely or partially to the same epitope as an antibody for CD71 can be obtained by a competition assay as described above. Antibodies that bind completely or partially to the same epitope as certain antibodies for CD71 may also be obtained by hydrogen-deuterium exchange mass spectrometry (HDX MS). Hydrogen-deuterium exchange mass spectrometry can identify the protein binding surface (substitution is unlikely to occur on the binding surface) by detecting the change in mass when deuterium in the solution replaces the amide hydrogen on the protein surface. it can.
- Hydrogen-deuterium exchange mass spectrometry can identify the protein binding surface (substitution is unlikely to occur on the binding surface) by detecting the change in mass when deuterium in the solution replaces the amide hydrogen on the protein surface. it can.
- An antibody / drug conjugate can be prepared by those skilled in the art using a method well known as a method for preparing an antibody-drug conjugate (ADC). The same applies to the conjugate of an antigen-binding fragment of an antibody and a drug.
- ADC antibody-drug conjugate
- a method for producing a conjugate of Fab ′, which is an antigen-binding fragment, and a drug which introduces a thiol-reactive group into a drug or a carbon chain having a substituent bonded to the drug.
- a method for producing a conjugate of Fab ′, which is an antigen-binding fragment, and a drug the group introducing a thiol-reactive group into a drug or a carbon chain having a substituent bonded to the drug.
- a reaction comprising reacting a thiol group of Fab ′, which is an antigen-binding fragment, with a drug or a thiol-reactive group introduced into the drug.
- a thiol-reactive group, a group that introduces a thiol-reactive group, or a carbon chain having a substituent is a spacer introduced into ASO (for example, a spacer composed of a nucleic acid of 1 to several bases). It can introduce
- those having a linker can be produced by a method known per se, for example, a carbon chain having a substituent on a drug (preferably a carbon chain having an amino group at the end of the carbon chain).
- a carbon chain having a substituent on a drug preferably a carbon chain having an amino group at the end of the carbon chain.
- the number of carbon atoms in the carbon chain having the above substituent may be 1 to 10, preferably 2 to 8, more preferably 4 to 6, and even more preferably 6.
- Examples of the substituent of the carbon chain having the above-described substituent include an amino group and a thiol group, and preferably an amino group.
- an alkyl chain having 6 carbon atoms having an amino group at the terminal (sometimes referred to herein as a C6 amino chain) can be particularly preferably used.
- the bond between the drug and the carbon chain having a substituent is a commercially available carrier for 3 ′ or 5′-amino modification or amidite reagent (for example, but not limited to, 3′-PT- It can be carried out by a nucleic acid synthesis reaction by a phosphoramidite method using Amino-Modifier C6 CPG or 5'-DMS (O) MT-Amino-Modifier C6 (Glen Research).
- the thiol-reactive group means a functional group having reactivity with a specific site (thiol group) of the anti-CD71 antibody or binding fragment thereof.
- the thiol reactive group include a maleimide group, a bromoacetamide group, a pyridyldithio group, a vinyl sulfone group, and an iodoacetamide group.
- the group that introduces a thiol-reactive group means a reagent having a thiol-reactive group.
- a reagent include maleimidocaproyl (mc); maleimidocaproyl-p-aminobenzylcarbamate; Royl-peptide-aminobenzylcarbamate (eg, maleimidocaproyl-L-phenylalanine-L-lysine-p-aminobenzylcarbamate and maleimidocaproyl-L-valine-L-citrulline-p-aminobenzylcarbamate (vc)); N-maleimidocaproyl-valyl-citrulyl-p-aminobenzylcarbamate p-nitrophenyl ester (mc-Val-Cit-PABC-PNP); N-succinimidyl 3- (2-pyridyldithio) proprionate (N-succinimid N-succin
- Bonding between an amino group and a group that introduces a thiol-reactive group can be performed by, for example, a condensation reaction or an addition reaction. More specifically, for example, an excess amount of BMPS is added to a drug having a carbon chain having an amino group bound thereto, and the mixture is kept at room temperature for about 2 to 4 hours. After the amino group has completely reacted, unreacted The BMPS can be removed by ultrafiltration or the like.
- a group capable of introducing a thiol-reactive group can be introduced into a vesicle as a drug.
- an amino group and a group for introducing a thiol-reactive group can be bound by a condensation reaction, an addition reaction, or the like. More specifically, for example, an excessive amount of BMPS is added to the drug, and the mixture is kept at room temperature for about 2 to 4 hours. After the amino group has completely reacted, unreacted BMPS is removed by ultrafiltration or the like. Can be implemented.
- the thiol-reactive group and the —SH group can be bound by, for example, an oxidation-reduction reaction, a condensation reaction, an addition reaction, or the like. More specifically, for example, it can be carried out by mixing approximately equal amounts of an antibody or antigen-binding fragment thereof and a drug into which a thiol-reactive group has been introduced, and incubating at room temperature for 4 to 16 hours. Excess reactants can be removed by chromatography.
- the method for producing a conjugate of Fab ′ that is an antigen-binding fragment and a drug wherein Fab ′ that is an antigen-binding fragment and the drug
- a method comprising reacting a thiol-reactive group of
- the conjugate of Fab ′ and drug can be separated from unreacted substances such as Fab ′ and drug using separation techniques such as size exclusion chromatography and HPLC.
- the conjugate of the present invention can be used as a medicament as it is or mixed with a pharmacologically acceptable carrier (for example, excipient).
- a pharmacologically acceptable carrier for example, excipient
- the conjugate of the present invention can be used as a medicament such as a therapeutic agent for muscle diseases.
- a muscular disease For example, a muscular disease, a muscular atrophy disease, an arteriosclerotic disease, and the disease relevant to the heart are mentioned.
- muscular dystrophy for example, Duchenne muscular dystrophy, Becker muscular dystrophy, Emery-Dreifuss muscular dystrophy, facioscapulohumeral muscular dystrophy, distal muscular dystrophy, distal muscular dystrophy, limb-girdle muscular dystrophy, Fukuyama congenital Muscular dystrophy, myotonic dystrophy), periodic limb paralysis, diaphragm paralysis, diaphragmatic relaxation, distal myopathy, myotonia syndrome, mitochondrial disease, muscle wasting disease.
- muscular dystrophy for example, Duchenne muscular dystrophy, Becker muscular dystrophy, Emery-Dreifuss muscular dystrophy, facioscapulohumeral muscular dystrophy, distal muscular dystrophy, distal muscular dystrophy, limb-girdle muscular dystrophy, Fukuyama congenital Muscular dystrophy, myotonic dystrophy
- periodic limb paralysis diaphragm paralysis, diaphragmatic relaxation, distal
- amyotrophic diseases include sarcopenia (age-related muscular atrophy), disuse muscular atrophy, cachexia, amyotrophic lateral sclerosis, and spinal muscular atrophy.
- arteriosclerotic diseases include peripheral arterial occlusion.
- Heart-related diseases include, for example, angina pectoris (including exertional angina pectoris, variant angina), acute coronary syndromes (unstable angina pectoris, acute myocardial infarction, transition to heart failure after myocardial infarction) ), Heart failure (including HFrEF, HFpEF, acute heart failure, chronic heart failure, decompensated heart failure), cardiomyopathy (including dilated cardiomyopathy, hypertrophic cardiomyopathy, restrictive cardiomyopathy), pulmonary heart, asymptomatic Myocardial ischemia, arrhythmia (conduction disorder, sinus node dysfunction, ectopic supraventricular rhythm, atrioventricular block, atrial fibrillation, atrial flutter, reentrant supraventricular tachycardia (SVT, PSVT), Wolf -Parkinson-White (WPW) syndrome, leg and bundle block, ventricular extrasystole, ventricular tachycardia, ventricular fibrillation, sudden cardiac death) valvular abnormalities (aor
- the conjugate of the anti-CD71 antibody of the present invention or an antigen-binding fragment thereof and a substance having anticancer activity can be used as a medicament for treating cancer, such as a therapeutic agent for cancer.
- cancer such as a therapeutic agent for cancer.
- CD71 positive cancer for example, blood cancer (leukemia, erythroleukemia), lymphoma, colon cancer, breast cancer, mesothelioma, liver cancer, kidney cancer, small cell lung cancer, malignant melanoma, medulloblastoma Neuroblastoma, cervical cancer, ovarian cancer, glioma, glioblastoma and the like.
- liver diseases include hemochromatosis, Wilson's disease, glycogenosis, amino acid metabolism disorder, urea cycle metabolism disorder, porphyria, constitutional jaundice, fibrotic polycystic liver disease, nonalcoholic steatohepatitis, B Examples include hepatitis C, liver fibrosis, cirrhosis, hereditary ATTR amyloidosis (familial amyloid polyneuropathy (FAP)), ⁇ -1 antitrypsin deficiency (AATD), and the like.
- FAP hereditary ATTR amyloidosis
- AATD ⁇ -1 antitrypsin deficiency
- drugs that target various genes, mRNA, miRNA, proteins, etc. can be used.
- a drug can act on a target gene or the like to suppress production of a protein or the like that should be originally expressed, such as various genes, mRNA, miRNA, and protein.
- examples include nucleic acids such as siRNA, shRNA, ASO, aptamer, miRNA, etc., and modified nucleic acids for the target.
- the target protein may be produced in cells or tissues, and examples thereof include nucleic acids such as mRNA encoding the target protein and modified nucleic acids thereof.
- the drug target of the conjugate in the present invention is not particularly limited, and examples thereof include the following targets.
- ⁇ Targets related to cancer and immunity> Clusterin gene, Nucleolin gene, AKT1 protein kinase gene, BIRC5 gene, MAGEC1 gene, MAGEC2 gene, CTAG1 gene, TPBG gene, Hsp27 gene, ⁇ -catenin (catenin) gene, CXCL12 (SDF-1) Gene, STAT-3 gene, PKN3 gene, PLK1 gene, mutant KRAS (G12D) gene, Grb-2 gene, Androgen receptor gene, TGF ⁇ gene (TGF ⁇ -1 gene, TGF ⁇ -2 gene, TGF ⁇ -3 gene ) STAT-3 gene, VEGF gene, KSP (Eg5) gene, CEBPA gene, Nek2 gene, p53 gene, MUC1 gene, TPBG gene, HIF -1 ⁇ gene, RPN2 gene, EphA2 gene, RRM1 gene, CDC45 gene, six-1 gene, I
- ⁇ Targets for liver disease > HSP47 gene, ⁇ 1-antitrypsin (antitrypsin) gene, ALAS-1 gene, DGAT2 gene, Hydroxyacid oxidase (hydroxy acid oxidase) gene, TGF ⁇ -2 gene, Transthyretin (transthyretin) gene, PCSK9 gene, or these genes Molecules such as encoded proteins or mRNA encoding the proteins encoded by these genes, miR-103, miR-107.
- ⁇ Targets related to skeletal muscle> Myostatin gene, dystrophin gene, SMN2 gene, DMPK gene, SOD1 gene, PABPN1 gene, cPLA2 gene, AMPA type Glu receptor 1 gene, AMPA type Glu receptor 3 gene, FOXO-1 gene, C9orf72 gene, ActRIIB gene MiR-155, which encodes a molecule such as the DUX4 gene, the NLRP3 gene, or a protein encoded by these genes, or a protein encoded by these genes.
- ⁇ Target for myocardium Myostatin (myostatin) gene, ApoA gene, STIM1 gene, EGF1 gene, VEGF-A gene, Phospholamban (phospholamban) gene, Serca2a gene, Sarcolipin gene, or proteins encoded by these genes, or these molecules MRNA encoding a protein encoded by the above gene, ⁇ 1-adrenergic receptor, TL receptor 4, or gene or mRNA encoding these receptors, miR-15, miR-195, miR-208, miR-92a MiR-34a, miR-25, miR-486.
- the medicament containing the conjugate of the present invention may contain a pharmacologically acceptable carrier (for example, an excipient).
- excipients that can be contained in the medicament include salts, solvents (eg, water and ethanol), buffers, sugars and sugar alcohols, surfactants, isotonic agents, preservatives, antioxidants, chelating agents. Excipients.
- the medicament containing the conjugate of the present invention can be administered, for example, intravenously, subcutaneously, intraperitoneally or intramuscularly. Therefore, the medicament containing the conjugate of the present invention may be formulated according to a conventional method as a medicament for intravenous administration, subcutaneous, intraperitoneal or intramuscular administration.
- the conjugate may be prepared as a medicament using a medium suitable for administration (eg, sterile pyrogen-free water) prior to use.
- the composition may contain an excipient in the same manner as the medicine.
- a method of delivering a drug to muscle comprising administering to the subject a conjugate of the drug and an anti-CD71 antibody or antigen-binding fragment thereof.
- a method is provided.
- Fab ′ can be used as the anti-CD71 antibody or antigen-binding fragment.
- a method for delivering a drug to immune cells such as hepatocytes, B cells or T cells, or cancer cells in a subject, comprising the drug and an anti-CD71 antibody or an antigen-binding fragment thereof.
- a method is provided that includes administering a conjugate to a subject.
- the daily dose of the conjugate of the present invention varies depending on the patient's condition, body weight, type of drug, route of administration, etc.
- the daily dose may be about 1 to about 1000 mg, preferably about 3 to about 300 mg, more preferably about 10 to about 200 mg as the drug in the conjugate of the present invention.
- the daily dose can be administered in multiple doses (eg, once, twice or three times).
- a method for increasing muscle mass in a muscle in a subject, wherein said subject comprises a conjugate of an anti-CD71 antibody or antigen-binding fragment thereof and siRNA or shRNA against myostatin.
- a method is provided that includes administering a gate.
- a method for treating peripheral arterial disease in a subject in the subject wherein the subject comprises a conjugate of an anti-CD71 antibody or antigen-binding fragment thereof and siRNA or shRNA against myostatin.
- a method for treating cancer in a subject comprising administering to said subject a conjugate of an anti-CD71 antibody or antigen-binding fragment thereof and a substance having anticancer activity.
- a method is provided.
- a method for treating liver disease in a subject comprising: a conjugate of an anti-CD71 antibody or an antigen-binding fragment thereof and a substance having a therapeutic and / or preventive effect on liver disease.
- a method comprising administering is provided.
- a method for treating an immune disease in a subject comprising a conjugate of an anti-CD71 antibody or an antigen-binding fragment thereof and a substance having an effect of treating and / or preventing an immune disease.
- a method comprising administering is provided.
- CD71 may be induced in immune cells prior to administration of a conjugate with a substance having therapeutic and / or prophylactic effects for immune diseases.
- the present invention there is provided the use of a conjugate of an antibody that binds to a cell surface antigen or an antigen-binding fragment thereof and a drug for delivering the drug into a cell.
- the cell may be a cell that takes up membrane proteins into the cell by endocytosis.
- the cell surface antigen is a membrane protein (for example, a receptor), and the membrane protein can be taken into the cell by endocytosis.
- Fab ′ can be used as an antibody or antigen-binding fragment thereof that binds to a cell surface antigen.
- an anti-CD71 antibody or an antigen-binding fragment thereof for delivering a drug to muscles (for example, myocardium and gastrocnemius muscle).
- the present invention also provides the use of an anti-CD71 antibody or antigen-binding fragment thereof for increasing muscle mass.
- an anti-CD71 antibody or antigen-binding fragment thereof in the manufacture of a composition for delivering a drug to muscle (eg, myocardium and gastrocnemius).
- the present invention also provides the use of an anti-CD71 antibody or antigen-binding fragment thereof in the manufacture of a medicament for use in increasing muscle mass.
- the present invention also provides the use of an anti-CD71 antibody or antigen-binding fragment thereof and siRNA or shRNA against myostatin for treating peripheral arterial disease in a subject in need thereof.
- the present invention further provides the use of an anti-CD71 antibody or antigen-binding fragment thereof in the preparation of a medicament for treating peripheral arterial disease in a subject in need thereof.
- the present invention still further provides the use of an anti-CD71 antibody or antigen-binding fragment thereof and siRNA or shRNA against myostatin in the preparation of a medicament for treating peripheral arterial disease in a subject in need thereof.
- Fab 'can be used as the antigen-binding fragment.
- an anti-CD71 antibody or antigen-binding fragment thereof for delivering a drug to immune cells such as hepatocytes, B cells or T cells, or cells such as cancer cells (particularly CD71 positive cells).
- an anti-CD71 antibody in the manufacture of a composition for delivering a drug to immune cells such as hepatocytes, B cells or T cells, or cells such as cancer cells (especially CD71 positive cells) or Use of the antigen-binding fragment is provided.
- the present invention also provides an anti-CD71 antibody in the manufacture of a composition for delivering a drug to immune cells such as hepatocytes, B cells or T cells, or cells such as cancer cells (particularly CD71 positive cells).
- the use of an antigen-binding fragment thereof and a drug, or the use of a conjugate of an anti-CD71 antibody or antigen-binding fragment thereof and a drug is provided.
- the use of an anti-CD71 antibody or an antigen-binding fragment thereof and a drug, or an anti-CD71 antibody or an antigen-binding fragment thereof, in the manufacture of a medicament for treating cancer in a subject in need thereof Use of a conjugate of a drug with a drug is provided.
- the drug can be a substance having anti-cancer activity.
- Example 1 Preparation of anti-CD71 antibody-drug conjugate
- an anti-CD71 antibody-siRNA conjugate was prepared as an example of an anti-CD71 antibody-drug conjugate.
- Conjugates produced as non-limiting examples in this example are as follows. Each of the following conjugates is a conjugate of RNA and an antibody fragment. Specifically, RNA and Fab ′ are linked via a linker.
- the conjugates represented by the following formulas (I) to (IV) represent conjugates in which RNA and Fab ′ are linked by a maleimide linker, a Val-Cit linker, an SS linker, and a DMSS linker, respectively.
- Anti-CD71 antibody As the anti-CD71 antibody in this example, an anti-CD71 antibody (clone RI7 217.1.3) purchased from BioXcell (West Riverside, NH) was used.
- the control IgG in this example is isotype control IgG 2a (BP0089) purchased from BioXcell (in this specification, sometimes referred to as “IgG 2a ”. In Table 1 below, it is described as “IgG”. ) was used.
- siRNA The sequences of siRNA for ApoB, hypoxanthine-phosphoribosyl-transferase (HPRT), and myostatin (MSTN), and siRNA (siNC) used as a negative control in this example were as follows.
- siApoB Sense strand 5′- G g A a U c U u A u A u U u G a U c C a A— (CH 2 ) 6 NH 2 -3 ′ (SEQ ID NO: 1); Antisense strand: 5′-pu U g G a U c A a A u A u A a G aU U C cs C su-3 ′ (SEQ ID NO: 2); 2) siHPRT Sense strand: 5'- U c C u A u G a C u G u A g A u U u U a U - (CH 2) 6 NH 2 -3 '( SEQ ID NO: 3); Antisense strand: 5′-pa U a A a A u C u A c A g U c A u A g G as A su-3 ′ (SEQ ID NO: 4); 3) siMSTN Sense strand: 5'- G a G a
- N-succinimidyl 3-maleimidopropionate (BMPS) (Acme Bioscience, Palo Alto, Calif.), N-succinimidyl 3- [2-pyridyldithio] propionate (SPDP) (Thermo Fisher Scientific Inc, Waltham, Mass.) N-maleimidocaproyl-valyl-citrulyl-p-aminobenzylcarbamate p-nitrophenyl ester (mc-Val-Cit-PABC-PNP) or N-succinimidyl 4-methyl-4- (2-pyridyldithio) pentanate ( Synchem, Elk Grove Village, IL).
- SPDP N-succinimidyl 3-maleimidopropionate
- SPDP N-succinimidyl 3- [2-pyridyldithio] propionate
- SPDP N-maleimidocaproyl-valyl-citrulyl-p-a
- DMSS dimethyl SS
- HPLC-MS high performance liquid chromatography-mass spectrometry
- MWCO 3K Amicon Ultra ultrafiltration devices
- the conjugate was prepared according to the scheme shown in FIG. 1B. Specifically, clone RI7 was subjected to pepsin degradation to obtain its F (ab ′) 2 and further reduced with cysteamine to obtain its Fab ′ fragment. The resulting Fab ′ fragment has two thiol groups used to make the conjugate.
- the Fab ′ fragment was purified using a Sephadex G-25 column equilibrated with a buffer (30 mM HEPES (pH 7.0), 150 mM NaCl), and the maleimide group or (2-pyridyldithio) pentanate of the siRNA obtained above was used.
- the reaction was allowed to conjugate with the group in elution buffer. These reactions were monitored using size exclusion chromatography-HPLC using TSKgel G2000SWxL (7.8 mm ⁇ 300 mm, TOSOH) using 20 mM phosphate-300 mM NaCl buffer (pH 7.0). After overnight incubation at room temperature, the Fab′-siRNA conjugate was separated from unreacted Fab ′ and siRNA using SEC-HPLC (see FIG. 1C). Fab′-siRNA conjugates could be separated from unreacted Fab ′ and siRNA (see FIG. 1D).
- the Fab′-siRNA conjugate is concentrated using an Amicon Ultra ultrafiltration device (MWCO 10 kDa), and the siRNA is quantified using a Quant-it RiboGreen RNA assay kit (Life technologies, Carlsbad, Calif.). Quantified using ATTO-TAG FQ Derivatization Reagent (FQ; 3- (2-furoyl) quinoline-2-carboxaldehyde) (Invitrogen, Carlsbad, CA). Hereinafter, the concentration and weight of the conjugate are expressed as the concentration and weight of siRNA. Plasma siRNA was extracted using Trizol and then reverse transcribed using TaqMan MicroRNA Reverse Transcription Kit (Life technologies, Carlsbad, CA). These samples were quantified by real-time PCR. According to FIG.
- the absorption spectrum of Fab ′ of the anti-CD71 antibody was measured and found to have a peak at 280 nm.
- the absorption spectrum of siHRPT had a peak at 260 nm.
- the isolated clone RI7 Fab′-siRNA conjugate was found to show a spectrum that is the sum of both absorbance spectra.
- Various other linkers were similarly prepared, and it was verified that the clone RI7 Fab′-siRNA conjugate could be isolated.
- Table 1 shows the molecular number ratio between siRNA and protein. * In the table, the linker of formula (I) is indicated as maleimide linker, the linker of formula (II) is indicated as Val-Cit linker, the linker of formula (III) is indicated as SS linker, and the linker of formula (IV) The linker is denoted as DMSS linker.
- Example 2 CD71 binding assay
- the ability of the clone RI7 Fab'-siRNA conjugate prepared above to bind to CD71 was verified.
- conjugate of this example clone RI7 Fab′-siHPRT linked with a maleimide linker was used.
- the sample was washed twice with D-PBS (-) containing 0.05% Tween 20, and a sample of the conjugate (100 ng in terms of siRNA) was incubated for 2 hours at room temperature in the presence or absence of a competitor reagent. Washed 4 times with D-PBS (-) containing 0.05% Tween 20, added RiboGreen solution for siRNA detection, and measured fluorescence (excitation light 485nm, fluorescence 535nm) with Envision 2104 Multilabel Reader (PerkinElmer) .
- the EC 50 for binding of clone RI7 was 1.5 nM. This suggests that CD71 covering the plate maintains its natural conformation.
- Ribogreen solution diluted 200-fold with PBS is added to each well, and the fluorescence derived from Ribogreen generated by binding to the RNA of the conjugate bound to the CD71 protein is produced.
- the EC 50 was 29 nM. In contrast, no binding was observed with IgG 2a -siHPRT.
- Example 3 In vitro validation of silencing GalNAc-siApoB was synthesized as described in JK Nair et al., J. Am. Chem. Soc. 136 (2014) 16958-16961. GalNAc-siApoB is known to bind to the asialoglycoprotein receptor on the surface of hepatocytes and be taken up into the cell, causing silencing in the cell. In this example, by contrasting with this molecule, silencing of clone RI7 Fab′-siApoB in hepatocytes and silencing of clone RI7 Fab′-siHPRT in B cells and T cells were verified. In both conjugates, maleimide was used as the linker.
- ACTB As an internal reference, ACTB, GAPDH or ribosomal protein largeP0 (Rplp0) was quantified using ACTB, GAPDH, or Rplp0 control reagent (Applied Biosystems, Forster City, CA), respectively, and the quantification values of ApoB and HPRT are These were normalized by the amount of mRNA.
- hepatocytes were used as cells for verification of silencing of clone RI7 Fab′-siApoB in this Example.
- the gene expression level was standardized by ⁇ -actin expression level.
- the clone RI7 Fab′-siApoB has an EC 50 for suppression of ApoB gene expression of 11 nM, showing 46% silencing at 900 nM (open circles in FIG. 3A).
- GalNAc-siApoB had an EC 50 of 0.29 nM and showed 90% silencing at 900 nM (black circle in FIG. 3A).
- B cells and T cells were isolated from the spleen of mice (BALB / cA Jcl, 8 weeks old, male).
- Cells were isolated using BMAC isolation kit mouse (Milteny Biotec, Bergisch Gladbach, Germany) and Pan T cell isolation kit II mouse (Milteny Biotec, Bergisch Gladbach, Germany) using AutoMACS.
- BMAC isolation kit mouse Milteny Biotec, Bergisch Gladbach, Germany
- Pan T cell isolation kit II mouse Melteny Biotec, Bergisch Gladbach, Germany
- Cells were seeded in a 96-well plate at a concentration of 10,000 cells / well, and B cells were reacted with various concentrations of clone RI7 Fab′-siHPRT in the presence of 10 ⁇ g / mL anti-mouse IgM (SouthernBiotech, Birmingham, AL). I let you.
- T cells were reacted with various concentrations of clone RI7 Fab′-siHPRT in the presence of 5 ⁇ g / mL phytohemagglutinin-L 4 (L-PHA) (Wako Pure Chemical Industries, Ltd., Japan). After 72 hours, mRNA expression was quantified by real-time PCR. The results were as shown in FIGS. 3B and C. As a negative control, IgG 2a Fab′-siHPRT was used.
- FIG. 3B it was revealed that HPRT was silenced by clone RI7 Fab′-siHPRT in B cells activated with anti-IgM.
- FIG. 3C it was revealed that HPRT was silenced by Clone RI7 Fab′-siHPRT in T cells activated with L-PHA.
- the black circles are the results with the anti-CD71 antibody Fab′-siHPRT
- the white circles are the results with the Fab′-siHPRT of IgG 2a .
- Example 4 Silencing in vivo Endogenous gene silencing by the anti-CD71 antibody Fab'-siRNA in vivo was verified in mice.
- conjugate of this example clone RI7 Fab′-siApoB linked with a maleimide linker was used.
- Clone RI7 Fab'-siApoB was intravenously administered to mice at a dose of 10 mg / kg body weight. As shown in FIG. 4A, clone RI7 Fab'-siApoB was detected in blood even 24 hours after administration.
- mice (Balb / c, 7 weeks old, female) administered with clone RI7 Fab'-siApoB was taken out, and total RNA was extracted using the Trizol extraction method. After extraction, mRNA was confirmed by quantitative PCR as described in Example 3.
- CD71 is highly expressed in the myocardium and gastrocnemius muscle (see FIG. 5).
- Example 5 Effect of linker on antibody-drug conjugate (ADC) In this example, the effect of linker on ADC was examined.
- linkers we compared non-cleavable maleimide linkers, Val-Cit linkers that are sensitive to cathepsin B and cleave in endosomes, SS linkers that cleave in reducing environments, and DMSS linkers that cleave more sensitively in reducing environments ( The effect of the linker on the ADC was confirmed.
- Clone RI7 Fab'-siHPRT linked with a Val-Cit linker had 66% silencing of HPRT in the gastrocnemius muscle when administered intravenously at a dose of 10 mg / kg body weight, but was linked with a maleimide linker.
- Clone RI7 Fab'-siHPRT had 65% HPRT silencing (FIGS. 6A and B). Thus, it was shown that whether or not the linker is cleaved by itself does not affect gene silencing by ADC (see FIG. 1A).
- silencing was 27% in the clone RI7 Fab'-siHPRT linked by the SS linker, and silencing was 48% in the clone RI7 Fab'-siHPRT linked by the DMSS linker (FIG. 6A). .
- This result was similar in the myocardium when intravenously administered at a dose of 10 mg / kg body weight (FIG. 6C). Also in the myocardium, it was shown that whether or not the linker is cleaved per se does not affect gene silencing by ADC (FIGS. 6C and D).
- muscular vascular endothelial cells are known to have low substance permeability because they are spread on the blood vessel wall and do not have pores.
- Fab'-drug conjugates were able to deliver drugs into muscle, suggesting that they were superior in both efficiency through the vascular endothelium and uptake into muscle cells.
- HPRT silencing effect in the gastrocnemius muscle was confirmed over time for mice that were administered intravenously at a dose of 10 mg / kg body weight for the clone RI7 Fab'-siHPRT linked with a maleimide linker.
- HPRT silencing in the gastrocnemius muscle continued even 4 weeks after administration.
- myostatin which is another gene, was observed (FIG. 6E right), and it was confirmed that silencing that lasted for up to 4 weeks was sequence-specific silencing.
- mice administered once intravenously at a dose of 10 mg / kg body weight in the myocardium, HPRT silencing in the gastrocnemius muscle persisted but gradually weakened even after 4 weeks of administration (FIG. 6F).
- the change in the expression level of myostatin in the myocardium was not significant (FIG. 6F).
- Example 6 Relationship between administration route and silencing of anti-CD71 antibody Fab′-siRNA
- silencing of anti-CD71 antibody Fab′-siRNA by intravenous administration, intraperitoneal administration and subcutaneous administration was compared. The difference in silencing depending on the route of administration was examined.
- clone RI7 Fab′-siHPRT linked with a maleimide linker was used as a conjugate in this example.
- Clone RI7 Fab'-siHPRT was administered intravenously (i.v.), intraperitoneally (i.p.) or subcutaneously (s.c.) at a dose of 10 mg / kg body weight, and silencing was verified 7 days later. The results were as shown in FIGS. 7A and B.
- the siHPRT bound to the C6 amino chain showed the same effect when 1 ⁇ g of the anti-CD71 antibody Fab′-siHPRT was administered at a dose of 630 ⁇ g. This result means that the effect of silencing was enhanced on the order of 10 3 by conjugation with anti-CD71 antibody.
- 87% HPRT silencing was also confirmed 14 days after administration (FIG. 7E left panel). According to FIG. 7E right panel, it can be seen that the expression level of myostatin was not significantly changed in this mouse, and sequence-specific silencing was observed in the left panel of FIG. 7E. Even at this dose, as shown in the left panel of FIG. 7F, silencing was about 20% in the gastrocnemius on the opposite side.
- Example 7 Effect of anti-CD71 antibody Fab'-siMSTN in peripheral arterial disease model
- the effect of anti-CD71 antibody Fab'-siMSTN in a peripheral arterial disease model was examined.
- clone RI7 Fab′-siMSTN linked with a maleimide linker was used as a conjugate in this example.
- a peripheral arterial disease (PAD) model was obtained by anesthetizing a mouse (C57BL / 6J, 10 weeks old, male) purchased from CLEA Japan, Inc. with isoflurane, and the proximal and distal parts of the femoral artery and the femur. An incision was made proximal to the deep artery, ligated, and then sutured closed. Hind leg ischemia caused by femoral artery ligation (FAL) damages muscles, and this model is used as a PAD model to verify the effects of various pharmaceuticals.
- FAL femoral artery ligation
- mice were intramuscularly administered 20 ⁇ g / foot of clone RI7 Fab'-siMSTN (or PBS as a control) once a week for 4 weeks.
- a peripheral arterial disease (PAD) model in which both femoral arteries of a mouse were ligated was prepared, and clone RI7 Fab'-siMSTN was intramuscularly administered according to the above administration schedule.
- PID peripheral arterial disease
- clone RI7 Fab′-siMSTN For peripheral arterial disease (PAD) model, 20 ⁇ g / foot of clone RI7 Fab′-siMSTN was administered four times at a frequency of once a week to verify the therapeutic effect.
- Femoral artery ligation (FAL) decreased myostatin mRNA expression in PBS-treated gastrocnemius muscle (see FIG. 8C) but did not change muscle weight (see FIG. 8D).
- the muscle mass was increased by 17% compared to the control group administered with PBS (see FIG. 8D).
- the average cross-sectional area of muscle fibers in the gastrocnemius muscle and the number of regenerated muscle fibers with nuclei in the center were measured using HALO TM image analysis systems (Indica Labs, Inc. Corrales, NM). The results were as shown in FIGS. 8E and F. As shown in FIGS. 8E and F, the PAD model treated with clone RI7 Fab′-siMSTN had an increased average cross-sectional area of muscle fibers in the gastrocnemius muscle.
- Example 8 Evaluation of gene silencing by anti-human CD71 antibody Fab'-siRNA in chronic myeloid leukemia cell line K562
- siRNA against human HPRT gene was used as siRNA.
- an OKT9 Fab′-siRNA conjugate was prepared in the same manner as described in Example 1.
- a maleimide linker of formula (I) was used as siRNA for human HPRT.
- siRNA for human HPRT siRNA having the sequences shown in SEQ ID NOs: 3 and 4 was used.
- Quantitative PCR was performed using ViiA7 Real-Time PCR System (Applied Biosystems, Foster City, CA), and silencing efficiency was calculated by the comparative Ct method. At this time, 36B4 gene was used as an internal standard. For quantitative PCR, the following were used as primers and probes.
- h36B4 amplification forward primer (SEQ ID NO: 20): 5'-CGTCTTGCTCGAGATGTGATG-3 ' Reverse primer for h36B4 amplification (SEQ ID NO: 21): 5'-CCAGCAGGTCAGCAAAGAATT-3 ' h36B4 detection probe (SEQ ID NO: 22): 5 '-(FAM) -CCATCACATTGTAGCCCTCTGTGTGCTC- (TAMRA) -3'
- Example 9 Evaluation of gene silencing by antisense oligonucleic acid (ASO)
- ASO antisense oligonucleic acid
- MALAT1-ASO an antisense oligonucleic acid against the MALAT1 gene
- Antisense oligonucleic acid 5′- a CTAgttcactgaaTGC-3 ′ against MALAT1 gene (SEQ ID NO: 23)
- upper case letters indicate that the nucleic acid is BNA NC
- lower case letters indicate DNA
- all nucleic acids have undergone phosphorothioate modification.
- the underlined adenine was used as a spacer and linked to the linker and C with a phosphodiester bond.
- ASO with maleimide group introduced Aqueous solution of MALAT1-ASO (1680 nmol, consigned to Gene Design Co., Ltd.) in which an amino group was introduced by binding a C6 amino chain via a (adenine) as a spacer DMSO, 10 ⁇ PBS aqueous solution and distilled water were added. Subsequently, BMPS (1680 nmol) dissolved in DMSO was added and reacted at room temperature for 30 minutes. Ultrafiltration dialysis was performed to remove reagent residues and the like, and MALAT1-ASO (1630 nmol) into which the target maleimide group was introduced was obtained.
- MALAT1-ASO (1680 nmol, consigned to Gene Design Co., Ltd.
- (ASO) represents an antisense oligo (ASO), and ASO and a linker are linked at the 5 'end of ASO.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Organic Chemistry (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biotechnology (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Neurology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Manufacturing & Machinery (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicinal Preparation (AREA)
- Peptides Or Proteins (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16/311,464 US20190240346A1 (en) | 2016-06-20 | 2017-06-19 | Antibody-drug conjugate |
| EP17815351.6A EP3473270B1 (en) | 2016-06-20 | 2017-06-19 | Antibody-drug conjugate |
| CN201780038198.4A CN109310765A (zh) | 2016-06-20 | 2017-06-19 | 抗体-药物偶联物 |
| DK17815351.6T DK3473270T5 (da) | 2016-06-20 | 2017-06-19 | Konjugat af antistof-lægemiddel |
| JP2018524078A JP6823269B2 (ja) | 2016-06-20 | 2017-06-19 | 抗体−薬物コンジュゲート |
| EP22210104.0A EP4212176A1 (en) | 2016-06-20 | 2017-06-19 | Antibody-drug conjugate |
| US18/637,050 US20250082772A1 (en) | 2016-06-20 | 2024-04-16 | Antibody-drug conjugate |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016122187 | 2016-06-20 | ||
| JP2016-122187 | 2016-06-20 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/311,464 A-371-Of-International US20190240346A1 (en) | 2016-06-20 | 2017-06-19 | Antibody-drug conjugate |
| US18/637,050 Division US20250082772A1 (en) | 2016-06-20 | 2024-04-16 | Antibody-drug conjugate |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017221883A1 true WO2017221883A1 (ja) | 2017-12-28 |
Family
ID=60784523
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/022509 Ceased WO2017221883A1 (ja) | 2016-06-20 | 2017-06-19 | 抗体-薬物コンジュゲート |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US20190240346A1 (enExample) |
| EP (2) | EP3473270B1 (enExample) |
| JP (4) | JP6823269B2 (enExample) |
| CN (1) | CN109310765A (enExample) |
| DK (1) | DK3473270T5 (enExample) |
| WO (1) | WO2017221883A1 (enExample) |
Cited By (50)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10160969B2 (en) | 2014-01-16 | 2018-12-25 | Wave Life Sciences Ltd. | Chiral design |
| US10167309B2 (en) | 2012-07-13 | 2019-01-01 | Wave Life Sciences Ltd. | Asymmetric auxiliary group |
| US10280192B2 (en) | 2011-07-19 | 2019-05-07 | Wave Life Sciences Ltd. | Methods for the synthesis of functionalized nucleic acids |
| JP2019513371A (ja) * | 2016-04-01 | 2019-05-30 | アビディティー バイオサイエンシーズ エルエルシー | 核酸ポリペプチド組成物とその使用 |
| US10307434B2 (en) | 2009-07-06 | 2019-06-04 | Wave Life Sciences Ltd. | Nucleic acid prodrugs and methods of use thereof |
| US10329318B2 (en) | 2008-12-02 | 2019-06-25 | Wave Life Sciences Ltd. | Method for the synthesis of phosphorus atom modified nucleic acids |
| US10428019B2 (en) | 2010-09-24 | 2019-10-01 | Wave Life Sciences Ltd. | Chiral auxiliaries |
| WO2020028864A1 (en) * | 2018-08-02 | 2020-02-06 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating facioscapulohumeral muscular dystrophy |
| WO2020028836A1 (en) * | 2018-08-02 | 2020-02-06 | Dyne Therapeutics, Inc. | Muscle-targeting complexes and uses thereof in treating muscle atrophy |
| WO2020028861A1 (en) * | 2018-08-02 | 2020-02-06 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| WO2020028832A1 (en) * | 2018-08-02 | 2020-02-06 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| WO2020256721A1 (en) * | 2019-06-19 | 2020-12-24 | Synthis, Llc | Antib0dy-alk5 inhibitor conjugates and their uses |
| CN112153987A (zh) * | 2018-04-10 | 2020-12-29 | 伊纳泰里斯公司 | 抗体-药物缀合物及其用于治疗癌症的用途 |
| US10881743B2 (en) | 2017-12-06 | 2021-01-05 | Avidity Biosciences, Inc. | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| US10994020B2 (en) | 2017-01-06 | 2021-05-04 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and methods of inducing exon skipping |
| CN112912399A (zh) * | 2018-08-02 | 2021-06-04 | 达因疗法公司 | 肌肉靶向复合物及其用于治疗蓬佩病的用途 |
| CN112930197A (zh) * | 2018-08-02 | 2021-06-08 | 达因疗法公司 | 肌肉靶向复合物及其用途 |
| CN113166240A (zh) * | 2018-08-02 | 2021-07-23 | 达因疗法公司 | 肌肉靶向复合物及其用于治疗弗里德赖希共济失调的用途 |
| US11110180B2 (en) | 2017-10-04 | 2021-09-07 | Avidity Biosciences Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| US11142767B2 (en) | 2017-07-21 | 2021-10-12 | The Governors Of The University Of Alberta | Antisense oligonucleotides that bind to exon 51 of human dystrophin pre-mRNA |
| US20210324101A1 (en) * | 2018-08-02 | 2021-10-21 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating hypertrophic cardiomyopathy |
| US11168141B2 (en) | 2018-08-02 | 2021-11-09 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| EP3830130A4 (en) * | 2018-08-02 | 2022-05-18 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating centronuclear myopathy |
| EP3829645A4 (en) * | 2018-08-02 | 2022-05-18 | Dyne Therapeutics, Inc. | MUSCLE TARGETING COMPLEXES AND THEIR USES FOR THE TREATMENT OF PROGRESSIVE FIBRODYSPLASIA OSSIFIANS |
| US11525137B2 (en) | 2020-03-19 | 2022-12-13 | Avidity Biosciences, Inc. | Compositions and methods of treating Facioscapulohumeral muscular dystrophy |
| US11578090B2 (en) | 2019-06-06 | 2023-02-14 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| US11583593B2 (en) | 2016-01-14 | 2023-02-21 | Synthis Therapeutics, Inc. | Antibody-ALK5 inhibitor conjugates and their uses |
| WO2023027125A1 (ja) | 2021-08-24 | 2023-03-02 | ペプチドリーム株式会社 | ヒトトランスフェリンレセプター結合抗体-ペプチドコンジュゲート |
| WO2023026994A1 (ja) | 2021-08-21 | 2023-03-02 | 武田薬品工業株式会社 | ヒトトランスフェリンレセプター結合ペプチド-薬物コンジュゲート |
| JP2023512442A (ja) * | 2020-01-10 | 2023-03-27 | ダイン セラピューティクス,インコーポレーテッド | 顔面肩甲上腕型筋ジストロフィーを処置するための筋標的化複合体およびそれらの使用 |
| US11633498B2 (en) | 2021-07-09 | 2023-04-25 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| US11638761B2 (en) | 2021-07-09 | 2023-05-02 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating Facioscapulohumeral muscular dystrophy |
| US11648318B2 (en) | 2021-07-09 | 2023-05-16 | Dyne Therapeutics, Inc. | Anti-transferrin receptor (TFR) antibody and uses thereof |
| JP2023535444A (ja) * | 2020-07-23 | 2023-08-17 | ダイン セラピューティクス,インコーポレーテッド | 顔面肩甲上腕型筋ジストロフィーを処置するための筋標的化複合体およびそれらの使用 |
| US11771776B2 (en) | 2021-07-09 | 2023-10-03 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US11827702B2 (en) | 2021-09-01 | 2023-11-28 | Biogen Ma Inc. | Anti-transferrin receptor antibodies and uses thereof |
| US11833221B2 (en) | 2021-09-01 | 2023-12-05 | Ionis Pharmaceuticals, Inc. | Oligomeric compounds for reducing DMPK expression |
| JP2024501872A (ja) * | 2020-12-31 | 2024-01-16 | ダイン セラピューティクス,インコーポレーテッド | 顔面肩甲上腕筋ジストロフィーを処置するための筋標的化複合体およびそれらの使用 |
| US11911484B2 (en) | 2018-08-02 | 2024-02-27 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| US11912779B2 (en) | 2021-09-16 | 2024-02-27 | Avidity Biosciences, Inc. | Compositions and methods of treating facioscapulohumeral muscular dystrophy |
| US11931421B2 (en) | 2022-04-15 | 2024-03-19 | Dyne Therapeutics, Inc. | Muscle targeting complexes and formulations for treating myotonic dystrophy |
| US11969475B2 (en) | 2021-07-09 | 2024-04-30 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating facioscapulohumeral muscular dystrophy |
| US12006499B2 (en) | 2019-06-06 | 2024-06-11 | Avidity Biosciences, Inc. | Una amidites and uses thereof |
| US12018087B2 (en) | 2018-08-02 | 2024-06-25 | Dyne Therapeutics, Inc. | Muscle-targeting complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide and methods of delivering oligonucleotide to a subject |
| US12071621B2 (en) | 2022-04-05 | 2024-08-27 | Avidity Biosciences, Inc. | Anti-transferrin receptor antibody-PMO conjugates for inducing DMD exon 44 skipping |
| US12097263B2 (en) | 2018-08-02 | 2024-09-24 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| US12128109B2 (en) | 2021-07-09 | 2024-10-29 | Dyne Therapeutics, Inc. | Muscle targeting complexes and formulations for treating dystrophinopathies |
| JP2024545136A (ja) * | 2021-12-06 | 2024-12-05 | ノースイースト ファーマスーティカル グループ カンパニー リミテッド | がん治療のための抗TfR1抗体MAb11-22.1複合体 |
| US12370264B1 (en) | 2018-08-02 | 2025-07-29 | Dyne Therapeutics, Inc. | Complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide and method of delivering oligonucleotide to a subject |
| US12497615B2 (en) | 2019-04-25 | 2025-12-16 | Avidity Biosciences, Inc. | Nucleic acid compositions and methods of multi-exon skipping |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017221883A1 (ja) * | 2016-06-20 | 2017-12-28 | 武田薬品工業株式会社 | 抗体-薬物コンジュゲート |
| WO2020247738A1 (en) * | 2019-06-07 | 2020-12-10 | Dyne Therapeutics, Inc. | Methods of preparing protein-oligonucleotide complexes |
| CA3163283A1 (en) * | 2020-01-10 | 2021-07-15 | Romesh R. Subramanian | Muscle targeting complexes and uses thereof for modulation of genes associated with muscle health |
| JP2023509793A (ja) * | 2020-01-10 | 2023-03-09 | ダイン セラピューティクス,インコーポレーテッド | 筋緊張性ジストロフィーを処置するための筋標的化複合体およびその使用 |
| BR112022014771A2 (pt) * | 2020-01-31 | 2022-10-11 | Dyne Therapeutics Inc | Anticorpo anti-receptor de transferrina (tfr) e usos do mesmo |
| WO2021154477A1 (en) * | 2020-01-31 | 2021-08-05 | Dyne Therapeutics, Inc. | Anti-transferrin receptor (tfr) antibody and uses thereof |
| MX2022011880A (es) | 2020-03-27 | 2022-10-20 | Avidity Biosciences Inc | Composiciones y metodos para tratar distrofia muscular. |
| KR20230162925A (ko) | 2021-03-26 | 2023-11-29 | 가부시키가이샤 후지미인코퍼레이티드 | 연마 방법 및 반도체 기판의 제조 방법, 그리고 연마용조성물 세트 |
| US12239710B2 (en) * | 2021-04-14 | 2025-03-04 | Aro Biotherapeutics Company | FN3 domain-siRNA conjugates and uses thereof |
| CA3214552A1 (en) | 2021-04-14 | 2022-10-20 | Russell C. Addis | Cd71 binding fibronectin type iii domains |
| CN120882749A (zh) | 2023-03-08 | 2025-10-31 | 渤健马萨诸塞州股份有限公司 | 抗转铁蛋白受体抗体及其用途 |
| WO2025007063A1 (en) * | 2023-06-30 | 2025-01-02 | Avidity Biosciences, Inc. | Compositions and methods of using pln-targeting antibody-oligonucleotide conjugates |
| WO2025024334A1 (en) | 2023-07-21 | 2025-01-30 | Marrow Therapeutics, Inc. | Hematopoietic cell targeting conjugates and related methods |
| WO2025090898A1 (en) | 2023-10-26 | 2025-05-01 | Biogen Ma Inc. | Anti-transferrin receptor antibodies and uses thereof cross reference to related applications |
| WO2025090121A1 (en) * | 2023-10-27 | 2025-05-01 | Yale University | Conjugate and methods of treatment |
| WO2025168041A1 (zh) * | 2024-02-08 | 2025-08-14 | 成都新泽利医药科技有限公司 | 治疗2型炎症性疾病的组合物和方法 |
| WO2025207959A1 (en) | 2024-03-27 | 2025-10-02 | Biogen Ma Inc. | Anti-transferrin receptor antibodies and uses thereof |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1992002190A1 (en) | 1990-08-09 | 1992-02-20 | Silverman Harvey N | Method and system for whitening teeth |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5693792A (en) | 1992-02-18 | 1997-12-02 | Otsuga Kagaku Kabushiki Kaisha | β-lactam and cephem compounds and processes for their production |
| US5714350A (en) | 1992-03-09 | 1998-02-03 | Protein Design Labs, Inc. | Increasing antibody affinity by altering glycosylation in the immunoglobulin variable region |
| US5777085A (en) | 1991-12-20 | 1998-07-07 | Protein Design Labs, Inc. | Humanized antibodies reactive with GPIIB/IIIA |
| JP2003531821A (ja) * | 1999-12-29 | 2003-10-28 | イムノージェン インコーポレーテッド | 改変型ドキソルビシンおよびダウノルビシンを含む細胞傷害性薬剤ならびにその治療上の使用 |
| WO2007000671A2 (en) | 2005-06-15 | 2007-01-04 | Monoclonal Antibodies Therapeutics | Anti-cd71 monoclonal antibodies and uses thereof for treating malignant tumour cells |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5616418A (en) * | 1979-07-20 | 1981-02-17 | Teijin Ltd | Antitumor protein complex and its preparation |
| GB8903021D0 (en) * | 1989-02-10 | 1989-03-30 | Celltech Ltd | Chemical compounds |
| GB8903022D0 (en) * | 1989-02-10 | 1989-03-30 | Celltech Ltd | Chemical compounds |
| JP4824025B2 (ja) * | 2004-06-07 | 2011-11-24 | マクロジェニックス ウエスト,インコーポレイテッド | トランスフェリンレセプター抗体 |
| US8354102B2 (en) * | 2007-05-15 | 2013-01-15 | Hoffmann-La Roche Inc. | Antibodies directed to mGluR7 |
| US10550195B2 (en) * | 2014-07-11 | 2020-02-04 | The Regents Of The University Of California | Tumor selective macropinocytosis-dependent rapidly internalizing antibodies |
| MA45328A (fr) * | 2016-04-01 | 2019-02-06 | Avidity Biosciences Llc | Compositions acide nucléique-polypeptide et utilisations de celles-ci |
| WO2017221883A1 (ja) * | 2016-06-20 | 2017-12-28 | 武田薬品工業株式会社 | 抗体-薬物コンジュゲート |
-
2017
- 2017-06-19 WO PCT/JP2017/022509 patent/WO2017221883A1/ja not_active Ceased
- 2017-06-19 CN CN201780038198.4A patent/CN109310765A/zh active Pending
- 2017-06-19 EP EP17815351.6A patent/EP3473270B1/en active Active
- 2017-06-19 US US16/311,464 patent/US20190240346A1/en active Pending
- 2017-06-19 JP JP2018524078A patent/JP6823269B2/ja active Active
- 2017-06-19 EP EP22210104.0A patent/EP4212176A1/en active Pending
- 2017-06-19 DK DK17815351.6T patent/DK3473270T5/da active
-
2020
- 2020-12-21 JP JP2020211507A patent/JP7138965B2/ja active Active
-
2022
- 2022-08-31 JP JP2022137571A patent/JP7536328B2/ja active Active
-
2024
- 2024-04-16 US US18/637,050 patent/US20250082772A1/en active Pending
- 2024-07-31 JP JP2024124515A patent/JP2024153831A/ja active Pending
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5585089A (en) | 1988-12-28 | 1996-12-17 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5693761A (en) | 1988-12-28 | 1997-12-02 | Protein Design Labs, Inc. | Polynucleotides encoding improved humanized immunoglobulins |
| WO1992002190A1 (en) | 1990-08-09 | 1992-02-20 | Silverman Harvey N | Method and system for whitening teeth |
| US5777085A (en) | 1991-12-20 | 1998-07-07 | Protein Design Labs, Inc. | Humanized antibodies reactive with GPIIB/IIIA |
| US5693792A (en) | 1992-02-18 | 1997-12-02 | Otsuga Kagaku Kabushiki Kaisha | β-lactam and cephem compounds and processes for their production |
| US5714350A (en) | 1992-03-09 | 1998-02-03 | Protein Design Labs, Inc. | Increasing antibody affinity by altering glycosylation in the immunoglobulin variable region |
| JP2003531821A (ja) * | 1999-12-29 | 2003-10-28 | イムノージェン インコーポレーテッド | 改変型ドキソルビシンおよびダウノルビシンを含む細胞傷害性薬剤ならびにその治療上の使用 |
| WO2007000671A2 (en) | 2005-06-15 | 2007-01-04 | Monoclonal Antibodies Therapeutics | Anti-cd71 monoclonal antibodies and uses thereof for treating malignant tumour cells |
Non-Patent Citations (33)
| Title |
|---|
| "GenBank", Database accession no. AAA51064.1 |
| "GenBank", Database accession no. AAA51130.1 |
| "GenBank", Database accession no. NM 005259.2 |
| "GenBank", Database accession no. NM 011638 |
| "GenBank", Database accession no. XM 011513112 |
| B. RIPPE ET AL., PHYSIOL. REV., vol. 74, 1994, pages 163 - 219 |
| B. SCHNEIDER ET AL., MOLECULAR THERAPY-NUCLEIC ACIDS, vol. 1, 2012, pages e46 |
| BEDUNEAU A ET AL.: "Design of targeted lipid nanocapsules by conjugation of whole antibodies and antibody Fab' fragments", BIOMATERIALS, vol. 28, no. 33, 2007, pages 4978 - 4990, XP022233266, ISSN: 0142-9612 * |
| DEBINSKI W ET AL.: "Monovalent immunotoxin containing truncated form of Pseudomonas exotoxin as potent antitumor agent", CANCER RESEARCH, vol. 52, no. 19, 1 October 1992 (1992-10-01), pages 5379 - 5385, XP055563011, ISSN: 0008-5472 * |
| DOMINGO DL ET AL.: "Transferrin receptor as a target for antibody-drug conjugates", METHODS IN ENZYMOLOGY, vol. 112, 1985, pages 238 - 247, XP009514215, ISSN: 0076-6879 * |
| E. CHANG ET AL., HUM GENE THER., vol. 8, 1997, pages 467 - 475 |
| E. WAGNER ET AL., PNAS, vol. 87, 1990, pages 3410 - 3414 |
| HUDSON AJ ET AL.: "Cellular delivery of hammerhead ribozymes conjugated to a transferrin receptor antibody", INTERNATIONAL JOURNAL OF PHARMACEUTICS, vol. 182, no. 1, 1999, pages 49 - 58, XP000997331, ISSN: 0378-5173 * |
| ISHIKAWA E ET AL.: "Preparation of monomeric Fab'-horseradish peroxidase conjugate using thiol groups in the hinge and its evaluation in enzyme immunoassay and immunohistochemical staining", ANNALS OF THE NEW YORK ACADEMY OF SCIENCES, vol. 420, 1983, pages 74 - 89, XP009089104, ISSN: 0077-8923 * |
| J. MOL. BIOL., vol. 215, 1990, pages 403 - 410 |
| J.A. WOLFF ET AL., ADV. GENET., vol. 54, 2005, pages 1 - 20 |
| J.K. NAIR ET AL., J. AM. CHEM. SOC., vol. 136, 2014, pages 16958 - 16961 |
| K.W. LIANG ET AL., MOLECULAR THERAPY, vol. 1, no. 3, 2000, pages 236 - 243 |
| KABAT ET AL.: "Sequence of Proteins of Immunological Interest", 1983, US DEPT. HEALTH AND HUMAN SERVICES |
| KABAT, ANN. NY ACAD SCI, vol. 190, 1971, pages 382 - 93 |
| KANJIRO MIYATA ET AL.: "Polymer nanotechnology for nucleic acid delivery", DRUG DELIVERY SYSTEM, vol. 31, no. 1, January 2016 (2016-01-01), pages 44 - 53, XP055563024 * |
| KARLIN; ALTSHUL, PROC. NATL. ACAD. SCI. USA, vol. 87, 1990, pages 2264 - 2268 |
| M. DAVIS ET AL., BIOCONJUG. CHEM., vol. 14, 2003, pages 1122 - 1132 |
| M. TEMPONI ET AL., CANCER RES., vol. 52, 1992, pages 2497 - 2503 |
| NORMAND-SDIQUI N ET AL.: "Oligonucleotide delivery: Uptake of rat transferrin receptor antibody (OX-26) conjugates into an in vitro immortalised cell line model of the blood-brain barrier", INTERNATIONAL JOURNAL OF PHARMACEUTICS, vol. 163, no. 1-2, 1998, pages 63 - 71, XP008074504, ISSN: 0378-5173 * |
| PROC. NATL. ACAD. SCI. USA, vol. 90, 1993, pages 5873 - 5877 |
| SCHNYDER A ET AL.: "Targeting of skeletal muscle in vitro using biotinylated immunoliposomes", THE BIOCHEMICAL JOURNAL, vol. 377, no. 1, 2004, pages 61 - 67, XP055563015, ISSN: 0264-6021 * |
| SEKYERE EO ET AL.: "Examination of the distribution of the transferrin homologue, melanotransferrin (tumour antigen p97), in mouse and human", BIOCHIMICA ET BIOPHYSICA ACTA, vol. 1722, no. 2, 2005, pages 131 - 142, XP055563028, ISSN: 0006-3002 * |
| SUGO T ET AL.: "Development of antibody-siRNA conjugate targeted to cardiac and skeletal muscles", JOURNAL OF CONTROLLED RELEASE, vol. 237, 29 June 2016 (2016-06-29), pages 1 - 13, XP029679981, ISSN: 0168-3659 * |
| T. SUGO ET AL., JOURNAL OF CONTROLLED RELEASE, vol. 237, 2016, pages 1 - 13 |
| T.L. CUELLAR ET AL., NUCLEIC ACID RESEARCH, vol. 43, no. 2, 2015, pages 1189 - 1203 |
| WINTER ET AL., IMMUNOL. TODAY, vol. 14, 1993, pages 43 - 46 |
| Y. SATO ET AL., FASEB J., vol. 14, 2000, pages 2018 - 2118 |
Cited By (148)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10329318B2 (en) | 2008-12-02 | 2019-06-25 | Wave Life Sciences Ltd. | Method for the synthesis of phosphorus atom modified nucleic acids |
| US10307434B2 (en) | 2009-07-06 | 2019-06-04 | Wave Life Sciences Ltd. | Nucleic acid prodrugs and methods of use thereof |
| US10428019B2 (en) | 2010-09-24 | 2019-10-01 | Wave Life Sciences Ltd. | Chiral auxiliaries |
| US10280192B2 (en) | 2011-07-19 | 2019-05-07 | Wave Life Sciences Ltd. | Methods for the synthesis of functionalized nucleic acids |
| US10167309B2 (en) | 2012-07-13 | 2019-01-01 | Wave Life Sciences Ltd. | Asymmetric auxiliary group |
| US10160969B2 (en) | 2014-01-16 | 2018-12-25 | Wave Life Sciences Ltd. | Chiral design |
| US11987558B1 (en) | 2016-01-14 | 2024-05-21 | Synthis Therapeutics, Inc. | ALK5 inhibitors and their uses |
| US11583593B2 (en) | 2016-01-14 | 2023-02-21 | Synthis Therapeutics, Inc. | Antibody-ALK5 inhibitor conjugates and their uses |
| US12473377B2 (en) | 2016-01-14 | 2025-11-18 | Synthis Therapeutics, Inc. | Antibody-ALK5 inhibitor conjugates and their uses |
| US12234290B2 (en) | 2016-04-01 | 2025-02-25 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| JP2019513371A (ja) * | 2016-04-01 | 2019-05-30 | アビディティー バイオサイエンシーズ エルエルシー | 核酸ポリペプチド組成物とその使用 |
| US10487149B2 (en) | 2016-04-01 | 2019-11-26 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| US10550188B2 (en) | 2016-04-01 | 2020-02-04 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| US10787519B2 (en) | 2016-04-01 | 2020-09-29 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| US10800848B2 (en) | 2016-04-01 | 2020-10-13 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| US12351637B2 (en) | 2016-04-01 | 2025-07-08 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| US10994020B2 (en) | 2017-01-06 | 2021-05-04 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and methods of inducing exon skipping |
| US11400163B2 (en) | 2017-01-06 | 2022-08-02 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and methods of inducing exon skipping |
| US11179472B2 (en) | 2017-01-06 | 2021-11-23 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and methods of inducing exon skipping |
| US11311627B1 (en) | 2017-01-06 | 2022-04-26 | Avidity Biosciences Llc | Nucleic acid-polypeptide compositions and methods of inducing exon skipping |
| US12064483B2 (en) | 2017-01-06 | 2024-08-20 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and methods of inducing exon skipping |
| US11142767B2 (en) | 2017-07-21 | 2021-10-12 | The Governors Of The University Of Alberta | Antisense oligonucleotides that bind to exon 51 of human dystrophin pre-mRNA |
| US11891603B2 (en) | 2017-07-21 | 2024-02-06 | The Governors Of The University Of Alberta | Antisense oligonucleotides that bind to exon 51 of human dystrophin pre-mRNA |
| US11364302B1 (en) | 2017-10-04 | 2022-06-21 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| US12364769B2 (en) | 2017-10-04 | 2025-07-22 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| US11110180B2 (en) | 2017-10-04 | 2021-09-07 | Avidity Biosciences Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| US10881743B2 (en) | 2017-12-06 | 2021-01-05 | Avidity Biosciences, Inc. | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| US11712478B2 (en) | 2017-12-06 | 2023-08-01 | Avidity Biosciences, Inc. | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| US11583591B2 (en) | 2017-12-06 | 2023-02-21 | Avidity Biosciences Llc | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| US11872287B2 (en) | 2017-12-06 | 2024-01-16 | Avidity Biosciences, Inc. | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| US11576980B2 (en) | 2017-12-06 | 2023-02-14 | Avidity Biosciences, Inc. | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| US11554176B2 (en) | 2017-12-06 | 2023-01-17 | Avidity Biosciences, Inc. | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| US11497814B2 (en) | 2017-12-06 | 2022-11-15 | Avidity Biosciences, Inc. | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| US12263224B2 (en) | 2017-12-06 | 2025-04-01 | Avidity Biosciences, Inc. | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| US11253607B2 (en) | 2017-12-06 | 2022-02-22 | Avidity Biosciences, Inc. | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| US11246941B2 (en) | 2017-12-06 | 2022-02-15 | Avidity Biosciences, Inc. | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| CN112153987B (zh) * | 2018-04-10 | 2024-01-09 | 伊纳泰里斯公司 | 抗体-药物缀合物及其用于治疗癌症的用途 |
| CN112153987A (zh) * | 2018-04-10 | 2020-12-29 | 伊纳泰里斯公司 | 抗体-药物缀合物及其用于治疗癌症的用途 |
| US12357703B2 (en) | 2018-08-02 | 2025-07-15 | Dyne Therapeutics, Inc. | Muscle-targeting complexes comprising an anti-transferin receptor antibody linked to an oligonucleotide and method of use thereof to induce exon skipping |
| US12319743B2 (en) | 2018-08-02 | 2025-06-03 | Dyne Therapeutics, Inc. | Complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide and method of delivering oligonucleotide to a subject |
| US11248056B1 (en) | 2018-08-02 | 2022-02-15 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| EP3830259A4 (en) * | 2018-08-02 | 2022-05-04 | Dyne Therapeutics, Inc. | MUSCLE TARGETED COMPLEXES AND THEIR USES IN THE TREATMENT OF FACIOSCAPULOHUMERAL MUSCULAR DYSTROPHY |
| EP3829649A4 (en) * | 2018-08-02 | 2022-05-04 | Dyne Therapeutics, Inc. | ANTI-MUSCLE COMPLEXES AND THEIR USES |
| EP3829596A4 (en) * | 2018-08-02 | 2022-05-11 | Dyne Therapeutics, Inc. | MUSCLE TARGETED COMPLEXES AND THEIR USES IN THE TREATMENT OF MYOTONIC DYSTROPHY |
| EP3829594A4 (en) * | 2018-08-02 | 2022-05-11 | Dyne Therapeutics, Inc. | MUSCLE TARGETING COMPLEXES AND THEIR USES FOR THE TREATMENT OF HYPERTROPHIC CARDIOMYOPATHY |
| EP3829635A4 (en) * | 2018-08-02 | 2022-05-11 | Dyne Therapeutics, Inc. | MUSCLE TARGETING COMPLEXES AND THEIR USES FOR THE TREATMENT OF MUSCLE ATROPHY |
| EP3830130A4 (en) * | 2018-08-02 | 2022-05-18 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating centronuclear myopathy |
| EP3829645A4 (en) * | 2018-08-02 | 2022-05-18 | Dyne Therapeutics, Inc. | MUSCLE TARGETING COMPLEXES AND THEIR USES FOR THE TREATMENT OF PROGRESSIVE FIBRODYSPLASIA OSSIFIANS |
| JP2021533197A (ja) * | 2018-08-02 | 2021-12-02 | ダイン セラピューティクス, インコーポレーテッドDyne Therapeutics, Inc. | ポンペ病を処置するための筋標的化複合体およびそれらの使用 |
| US11369689B2 (en) | 2018-08-02 | 2022-06-28 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| EP3830127A4 (en) * | 2018-08-02 | 2022-06-29 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating pompe disease |
| US11390682B2 (en) | 2018-08-02 | 2022-07-19 | Dyne Therapeutics, Inc. | Methods of intravenouisly delivering anti-transferrin antibody/oligonucleotide complexes to subjects having muscular dystrophy |
| JP2021532832A (ja) * | 2018-08-02 | 2021-12-02 | ダイン セラピューティクス, インコーポレーテッドDyne Therapeutics, Inc. | フリートライヒ運動失調症を処置するための筋標的化複合体およびそれらの使用 |
| JP2021533198A (ja) * | 2018-08-02 | 2021-12-02 | ダイン セラピューティクス, インコーポレーテッドDyne Therapeutics, Inc. | 筋標的化複合体およびそれらの使用 |
| US11497815B2 (en) | 2018-08-02 | 2022-11-15 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US11518816B2 (en) | 2018-08-02 | 2022-12-06 | Dyne Therapeutics, Inc. | Methods of delivering an oligonucleotide to a subject having facioscapulohumeral muscular dystrophy |
| US12173079B2 (en) | 2018-08-02 | 2024-12-24 | Dyne Therapeutics, Inc. | Muscle-targeting complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide |
| JP7611825B2 (ja) | 2018-08-02 | 2025-01-10 | ダイン セラピューティクス,インコーポレーテッド | ジストロフィン異常症を処置するための筋標的化複合体およびそれらの使用 |
| JP2021532831A (ja) * | 2018-08-02 | 2021-12-02 | ダイン セラピューティクス, インコーポレーテッドDyne Therapeutics, Inc. | ジストロフィン異常症を処置するための筋標的化複合体およびそれらの使用 |
| WO2020028864A1 (en) * | 2018-08-02 | 2020-02-06 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating facioscapulohumeral muscular dystrophy |
| JP2021533200A (ja) * | 2018-08-02 | 2021-12-02 | ダイン セラピューティクス, インコーポレーテッドDyne Therapeutics, Inc. | 顔面・肩甲・上腕筋ジストロフィーを処置するための筋標的化複合体およびそれらの使用 |
| JP2021533199A (ja) * | 2018-08-02 | 2021-12-02 | ダイン セラピューティクス, インコーポレーテッドDyne Therapeutics, Inc. | 筋強直症ジストロフィーを処置するための筋標的化複合体およびそれらの使用 |
| US11168141B2 (en) | 2018-08-02 | 2021-11-09 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US12496352B2 (en) | 2018-08-02 | 2025-12-16 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| JP7576542B2 (ja) | 2018-08-02 | 2024-10-31 | ダイン セラピューティクス,インコーポレーテッド | 筋標的化複合体およびそれらの使用 |
| JP2025026842A (ja) * | 2018-08-02 | 2025-02-26 | ダイン セラピューティクス,インコーポレーテッド | 筋標的化複合体およびそれらの使用 |
| EP3830131A4 (en) * | 2018-08-02 | 2023-04-19 | Dyne Therapeutics, Inc. | MUSCLE TARGETED COMPLEXES AND THEIR USES IN THE TREATMENT OF FREDERICH'S ATAXIA |
| US11633496B2 (en) | 2018-08-02 | 2023-04-25 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| IL280425B2 (en) * | 2018-08-02 | 2025-12-01 | Dyne Therapeutics Inc | Muscle-targeted conjugates and their uses in the treatment of myotonia dystrophica |
| US12478687B2 (en) | 2018-08-02 | 2025-11-25 | Dyne Therapeutics, Inc. | United states |
| US20210324101A1 (en) * | 2018-08-02 | 2021-10-21 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating hypertrophic cardiomyopathy |
| US12460011B2 (en) | 2018-08-02 | 2025-11-04 | Dyne Therapeutics, Inc. | Complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide and method of delivering an oligonucleotide to a subject |
| US12428487B2 (en) | 2018-08-02 | 2025-09-30 | Dyne Therapeutics, Inc. | Complexes comprising an anti-transferrin receptor antibody linked to an oligonicleotide and method of delivering oligonucleotide to a subject |
| US11111309B2 (en) | 2018-08-02 | 2021-09-07 | Dyne Therapeutics, Inc. | Method of reducing expression of DUX4 in a muscle cell by administering an anti-transferrin receptor antibody linked to an oligonucleotide targeting DUX4 |
| CN113166240A (zh) * | 2018-08-02 | 2021-07-23 | 达因疗法公司 | 肌肉靶向复合物及其用于治疗弗里德赖希共济失调的用途 |
| IL280425B1 (en) * | 2018-08-02 | 2025-08-01 | Dyne Therapeutics Inc | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| US12370264B1 (en) | 2018-08-02 | 2025-07-29 | Dyne Therapeutics, Inc. | Complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide and method of delivering oligonucleotide to a subject |
| US11787869B2 (en) | 2018-08-02 | 2023-10-17 | Dyne Therapeutics, Inc. | Methods of using muscle targeting complexes to deliver an oligonucleotide to a subject having facioscapulohumeral muscular dystrophy or a disease associated with muscle weakness |
| US11795234B2 (en) | 2018-08-02 | 2023-10-24 | Dyne Therapeutics, Inc. | Methods of producing muscle-targeting complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide |
| US11795233B2 (en) | 2018-08-02 | 2023-10-24 | Dyne Therapeutics, Inc. | Muscle-targeting complex comprising an anti-transferrin receptor antibody linked to an oligonucleotide |
| CN112955154A (zh) * | 2018-08-02 | 2021-06-11 | 达因疗法公司 | 肌肉靶向复合物及其用于治疗强直性肌营养不良的用途 |
| US11833217B2 (en) | 2018-08-02 | 2023-12-05 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| WO2020028836A1 (en) * | 2018-08-02 | 2020-02-06 | Dyne Therapeutics, Inc. | Muscle-targeting complexes and uses thereof in treating muscle atrophy |
| US12173078B2 (en) | 2018-08-02 | 2024-12-24 | Dyne Therapeutics, Inc. | Complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide |
| JP7708957B2 (ja) | 2018-08-02 | 2025-07-15 | ダイン セラピューティクス,インコーポレーテッド | 筋標的化複合体およびそれらの使用 |
| CN112930197A (zh) * | 2018-08-02 | 2021-06-08 | 达因疗法公司 | 肌肉靶向复合物及其用途 |
| JP7705793B2 (ja) | 2018-08-02 | 2025-07-10 | ダイン セラピューティクス,インコーポレーテッド | 筋強直症ジストロフィーを処置するための筋標的化複合体およびそれらの使用 |
| CN112912399A (zh) * | 2018-08-02 | 2021-06-04 | 达因疗法公司 | 肌肉靶向复合物及其用于治疗蓬佩病的用途 |
| CN112912499A (zh) * | 2018-08-02 | 2021-06-04 | 达因疗法公司 | 肌肉靶向复合物及其用于治疗面肩肱型肌营养不良的用途 |
| US11911484B2 (en) | 2018-08-02 | 2024-02-27 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| US12097263B2 (en) | 2018-08-02 | 2024-09-24 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| US12329825B1 (en) | 2018-08-02 | 2025-06-17 | Dyne Therapeutics, Inc. | Muscle targeting complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide and method of use thereof to induce exon skipping of exon 44 of dystrophin in a subject |
| US12325753B2 (en) | 2018-08-02 | 2025-06-10 | Dyne Therapeutics, Inc. | Method of using an anti-transferrin receptor antibody to deliver an oligonucleotide to a subject having facioscapulohumeral muscular dystrophy |
| US11286305B2 (en) | 2018-08-02 | 2022-03-29 | Dyne Therapeutics, Inc. | Complex comprising anti-transferrin receptor antibody covalently linked to an oligonucleotide that targets DUX4 RNA |
| WO2020028832A1 (en) * | 2018-08-02 | 2020-02-06 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US12263225B2 (en) | 2018-08-02 | 2025-04-01 | Dyne Therapeutics, Inc. | Muscle-targeting complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide and methods of use thereof to target dystrophin and to treat Duchenne muscular dystrophy |
| US12005124B2 (en) | 2018-08-02 | 2024-06-11 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US12280122B2 (en) | 2018-08-02 | 2025-04-22 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| US12012460B2 (en) | 2018-08-02 | 2024-06-18 | Dyne Therapeutics, Inc. | Muscle-targeting complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide |
| US12018087B2 (en) | 2018-08-02 | 2024-06-25 | Dyne Therapeutics, Inc. | Muscle-targeting complexes comprising an anti-transferrin receptor antibody linked to an oligonucleotide and methods of delivering oligonucleotide to a subject |
| WO2020028861A1 (en) * | 2018-08-02 | 2020-02-06 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| US12497615B2 (en) | 2019-04-25 | 2025-12-16 | Avidity Biosciences, Inc. | Nucleic acid compositions and methods of multi-exon skipping |
| US12006499B2 (en) | 2019-06-06 | 2024-06-11 | Avidity Biosciences, Inc. | Una amidites and uses thereof |
| US12365704B2 (en) | 2019-06-06 | 2025-07-22 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| US11578090B2 (en) | 2019-06-06 | 2023-02-14 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and uses thereof |
| WO2020256721A1 (en) * | 2019-06-19 | 2020-12-24 | Synthis, Llc | Antib0dy-alk5 inhibitor conjugates and their uses |
| JP2023512442A (ja) * | 2020-01-10 | 2023-03-27 | ダイン セラピューティクス,インコーポレーテッド | 顔面肩甲上腕型筋ジストロフィーを処置するための筋標的化複合体およびそれらの使用 |
| US12049629B2 (en) | 2020-03-19 | 2024-07-30 | Avidity Biosciences, Inc. | Compositions and methods of treating Facioscapulohumeral muscular dystrophy |
| US11999955B2 (en) | 2020-03-19 | 2024-06-04 | Avidity Biosciences, Inc. | Compositions and methods of treating facioscapulohumeral muscular dystrophy |
| US12104156B2 (en) | 2020-03-19 | 2024-10-01 | Avidity Biosciences, Inc. | Compositions and methods of treating facioscapulohumeral muscular dystrophy |
| US11555190B2 (en) | 2020-03-19 | 2023-01-17 | Avidity Biosciences, Inc. | Compositions and methods of treating Facioscapulohumeral muscular dystrophy |
| US11525137B2 (en) | 2020-03-19 | 2022-12-13 | Avidity Biosciences, Inc. | Compositions and methods of treating Facioscapulohumeral muscular dystrophy |
| JP2023535444A (ja) * | 2020-07-23 | 2023-08-17 | ダイン セラピューティクス,インコーポレーテッド | 顔面肩甲上腕型筋ジストロフィーを処置するための筋標的化複合体およびそれらの使用 |
| JP2024501872A (ja) * | 2020-12-31 | 2024-01-16 | ダイン セラピューティクス,インコーポレーテッド | 顔面肩甲上腕筋ジストロフィーを処置するための筋標的化複合体およびそれらの使用 |
| US12403203B2 (en) | 2021-07-09 | 2025-09-02 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US12440575B2 (en) | 2021-07-09 | 2025-10-14 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US12144868B2 (en) | 2021-07-09 | 2024-11-19 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US12128109B2 (en) | 2021-07-09 | 2024-10-29 | Dyne Therapeutics, Inc. | Muscle targeting complexes and formulations for treating dystrophinopathies |
| US12239717B2 (en) | 2021-07-09 | 2025-03-04 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US12239716B2 (en) | 2021-07-09 | 2025-03-04 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US12102687B2 (en) | 2021-07-09 | 2024-10-01 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| US11633498B2 (en) | 2021-07-09 | 2023-04-25 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating myotonic dystrophy |
| US11638761B2 (en) | 2021-07-09 | 2023-05-02 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating Facioscapulohumeral muscular dystrophy |
| US11986537B2 (en) | 2021-07-09 | 2024-05-21 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US11648318B2 (en) | 2021-07-09 | 2023-05-16 | Dyne Therapeutics, Inc. | Anti-transferrin receptor (TFR) antibody and uses thereof |
| US12329824B1 (en) | 2021-07-09 | 2025-06-17 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US11672872B2 (en) | 2021-07-09 | 2023-06-13 | Dyne Therapeutics, Inc. | Anti-transferrin receptor antibody and uses thereof |
| US11839660B2 (en) | 2021-07-09 | 2023-12-12 | Dyne Therapeutics, Inc. | Anti-transferrin receptor antibody and uses thereof |
| US11679161B2 (en) | 2021-07-09 | 2023-06-20 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating facioscapulohumeral muscular dystrophy |
| US11844843B2 (en) | 2021-07-09 | 2023-12-19 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating facioscapulohumeral muscular dystrophy |
| US12144867B2 (en) | 2021-07-09 | 2024-11-19 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US12397062B2 (en) | 2021-07-09 | 2025-08-26 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US11969475B2 (en) | 2021-07-09 | 2024-04-30 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating facioscapulohumeral muscular dystrophy |
| US11759525B1 (en) | 2021-07-09 | 2023-09-19 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating facioscapulohumeral muscular dystrophy |
| US11771776B2 (en) | 2021-07-09 | 2023-10-03 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| WO2023026994A1 (ja) | 2021-08-21 | 2023-03-02 | 武田薬品工業株式会社 | ヒトトランスフェリンレセプター結合ペプチド-薬物コンジュゲート |
| WO2023027125A1 (ja) | 2021-08-24 | 2023-03-02 | ペプチドリーム株式会社 | ヒトトランスフェリンレセプター結合抗体-ペプチドコンジュゲート |
| US11833221B2 (en) | 2021-09-01 | 2023-12-05 | Ionis Pharmaceuticals, Inc. | Oligomeric compounds for reducing DMPK expression |
| US11827702B2 (en) | 2021-09-01 | 2023-11-28 | Biogen Ma Inc. | Anti-transferrin receptor antibodies and uses thereof |
| US12157774B2 (en) | 2021-09-16 | 2024-12-03 | Avidity Biosciences, Inc. | Compositions and methods of treating facioscapulohumeral muscular dystrophy |
| US11912779B2 (en) | 2021-09-16 | 2024-02-27 | Avidity Biosciences, Inc. | Compositions and methods of treating facioscapulohumeral muscular dystrophy |
| US12071485B2 (en) | 2021-09-16 | 2024-08-27 | Avidity Biosciences, Inc. | Compositions and methods of treating facioscapulohumeral muscular dystrophy |
| US12486328B2 (en) | 2021-09-16 | 2025-12-02 | Avidity Biosciences, Inc. | Compositions and methods of treating facioscapulohumeral muscular dystrophy |
| JP2024545136A (ja) * | 2021-12-06 | 2024-12-05 | ノースイースト ファーマスーティカル グループ カンパニー リミテッド | がん治療のための抗TfR1抗体MAb11-22.1複合体 |
| US12071621B2 (en) | 2022-04-05 | 2024-08-27 | Avidity Biosciences, Inc. | Anti-transferrin receptor antibody-PMO conjugates for inducing DMD exon 44 skipping |
| US12359202B2 (en) | 2022-04-05 | 2025-07-15 | Avidity Biosciences, Inc. | Anti-transferrin receptor antibody-PMO conjugates for inducing DMD exon 44 skipping |
| US12440574B2 (en) | 2022-04-15 | 2025-10-14 | Dyne Therapeutics, Inc. | Muscle targeting complexes and formulations for treating myotonic dystrophy |
| US11931421B2 (en) | 2022-04-15 | 2024-03-19 | Dyne Therapeutics, Inc. | Muscle targeting complexes and formulations for treating myotonic dystrophy |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3473270A4 (en) | 2020-02-26 |
| CN109310765A (zh) | 2019-02-05 |
| JP2022174146A (ja) | 2022-11-22 |
| DK3473270T5 (da) | 2024-09-09 |
| EP4212176A1 (en) | 2023-07-19 |
| US20190240346A1 (en) | 2019-08-08 |
| EP3473270A1 (en) | 2019-04-24 |
| JP7138965B2 (ja) | 2022-09-20 |
| JP2021052791A (ja) | 2021-04-08 |
| JP7536328B2 (ja) | 2024-08-20 |
| EP3473270B1 (en) | 2022-11-30 |
| JP2024153831A (ja) | 2024-10-29 |
| DK3473270T3 (da) | 2023-01-09 |
| JPWO2017221883A1 (ja) | 2019-02-28 |
| JP6823269B2 (ja) | 2021-02-03 |
| US20250082772A1 (en) | 2025-03-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7138965B2 (ja) | 抗体-薬物コンジュゲート | |
| JP7654757B2 (ja) | 抗トランスフェリン受容体抗体およびその使用 | |
| US20240325558A1 (en) | Muscle targeting complexes and uses thereof for treating myotonic dystrophy | |
| JP5758888B2 (ja) | 二重特異性ジゴキシゲニン結合抗体 | |
| US20250025570A1 (en) | Muscle targeting complexes for treating facioscapulohumeral muscular dystrophy | |
| RU2766586C2 (ru) | Фармацевтическая композиция для лечения и/или предупреждения злокачественной опухоли | |
| US20240110184A1 (en) | Muscle targeting complexes and uses thereof for treating facioscapulohumeral muscular dystrophy | |
| JP2024534488A (ja) | ジストロフィノパチーを処置するための筋標的化複合体の投与 | |
| JP2023527638A (ja) | 顔面肩甲上腕型筋ジストロフィーを処置するための組成物および方法 | |
| EP3808773A2 (en) | Axl-targeting antibody, antibody-drug conjugate, preparation method therefor, and use thereof | |
| US10947316B2 (en) | Antibody-drug conjugates containing anti-Globo H antibodies and uses thereof | |
| US20210032316A1 (en) | Inverse Agonistic Anti-US28 Antibodies | |
| JP2024526259A (ja) | 抗protac抗体および複合体 | |
| WO2025036480A1 (zh) | 化合物以及含有该化合物的抗体偶联药物 | |
| EP4655007A1 (en) | Antibody oligonucleotide conjugates | |
| WO2024197302A1 (en) | Compositions and methods for delivering antibody oligonucleotide conjugates for exon skipping | |
| WO2024088283A1 (zh) | 人源化的l1cam抗体药物偶联物 | |
| TWI817107B (zh) | 寡核苷酸共軛物及其製備與應用 | |
| EP3875118A1 (en) | Use of antibody-drug conjugates and antibodies for drug delivery | |
| CN118440948A (zh) | 一种rna及含有rna的药物 | |
| KR20240099484A (ko) | 표적 세포로의 활성 제약 성분의 적혈구-매개 전달을 위한 치료 화합물 | |
| WO2025081059A1 (en) | Carriers for drug conjugates |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| ENP | Entry into the national phase |
Ref document number: 2018524078 Country of ref document: JP Kind code of ref document: A |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17815351 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2017815351 Country of ref document: EP Effective date: 20190121 |