WO2017188315A1 - 早期胃癌検出キット及びその使用 - Google Patents
早期胃癌検出キット及びその使用 Download PDFInfo
- Publication number
- WO2017188315A1 WO2017188315A1 PCT/JP2017/016543 JP2017016543W WO2017188315A1 WO 2017188315 A1 WO2017188315 A1 WO 2017188315A1 JP 2017016543 W JP2017016543 W JP 2017016543W WO 2017188315 A1 WO2017188315 A1 WO 2017188315A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- gastric cancer
- early
- tissue sample
- detection
- detecting
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
Definitions
- the present invention relates to an early gastric cancer detection kit and use thereof.
- This application claims priority based on Japanese Patent Application No. 2016-088283 filed in Japan on April 26, 2016, the contents of which are incorporated herein by reference.
- Cancer / testis antigen is a general term for proteins that are not expressed except in cancer cells and testis.
- Kitakyushu lug cancer antigen-1 (hereinafter referred to as “KK-LC-1”), was originally identified as an antigen that is specifically expressed in lung cancer cell lines ( For example, see Non-Patent Document 1.)
- gastric cancer is found early, and in cases where lymph node metastasis or distant metastasis is not observed, the prognosis is good by curative resection.
- the results of treatment for the 5-year survival rate for gastric cancer are about 90% for Stage I, about 70% for Stage II, about 50% for Stage III, and about 10% for Stage IV. For this reason, early detection of gastric cancer is important.
- gastric cancers are often asymptomatic at an early stage. Therefore, it is difficult to detect gastric cancer early due to subjective symptoms. Subjective symptoms include stomach discomfort, nausea, black stool, anemia, weight loss, ease of fatigue, fever, and loose stool. After such subjective symptoms appear, gastric cancer is often found in a state of progression and metastasis. Therefore, a technique for detecting gastric cancer at an early stage is required.
- an object of the present invention is to provide a technique for detecting early gastric cancer.
- the present invention includes the following aspects.
- a method for detecting early gastric cancer cells comprising a step of detecting the expression of KK-LC-1 in a gastric tissue sample.
- a technique for detecting early gastric cancer can be provided.
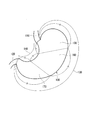
- FIG. 1 It is a schematic diagram which shows a stomach, an esophagus, and a duodenum.
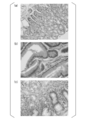
- A is a photomicrograph of a representative gastric tissue sample derived from the fundus gland. The magnification is 400 times.
- B is a photomicrograph of a representative stomach tissue sample derived from the pyloric gland. The magnification is 400 times.
- C is a photomicrograph of a representative gastric tissue sample derived from the border region of the fundus gland and pyloric gland. The magnification is 400 times.
- the present invention specifically hybridizes to a KK-LC-1 specific binding substance, a primer set for amplifying KK-LC-1 gene cDNA, or mRNA of KK-LC-1 gene.
- An early gastric cancer detection kit is provided.
- early gastric cancer means precancerous or stage I gastric cancer.
- the precancerous state means a state that is normal in appearance but is considered to be able to develop cancer if left untreated.
- Stage I gastric cancer refers to gastric cancer classified as IA or IB in the TNM classification proposed by the International Union for Cancer (UICC).
- KK-LC-1 expression is also observed in apparently normal gastric tissue samples derived from gastric cancer patients. Therefore, early gastric cancer can be detected by detecting the expression of KK-LC-1 in a gastric tissue sample derived from a subject.
- the expression of KK-LC-1 may be detected at the protein level or at the mRNA level.
- the RefSeq ID of the human KK-LC-1 gene is NM_001017978.
- kits of this embodiment examples of the specific binding substance include antibodies, antibody fragments, aptamers, and the like.
- An antibody can be produced, for example, by immunizing an animal such as a mouse with KK-LC-1 protein or a fragment thereof as an antigen. Alternatively, for example, it can be prepared by screening a phage library. Examples of antibody fragments include Fv, Fab, scFv and the like.
- the anti-KK-LC-1 antibody may be a monoclonal antibody or a polyclonal antibody. A commercially available antibody may also be used. Further, the anti-KK-LC-1 antibody may be humanized. Humanized antibodies are less prone to problems when administered to humans.
- An aptamer is a substance having a specific binding ability to a target substance.
- aptamers include nucleic acid aptamers and peptide aptamers.
- the nucleic acid aptamer having a specific binding ability to the target peptide can be selected by, for example, the systematic evolution of ligand by exponential enrichment (SELEX) method.
- Peptide aptamers having specific binding ability to the target peptide can be selected by, for example, the two-hybrid method using yeast.
- the above specific binding substance may be labeled with a fluorescent dye.
- labeled with a fluorescent dye means that the above-mentioned specific binding substance is directly labeled with a fluorescent dye, and the secondary antibody that binds to the above-mentioned specific binding substance is a fluorescent dye. Includes cases where it is labeled.
- KK-LC-1 protein is localized on the cell membrane surface.
- the inventors have further clarified that the C-terminal side of the KK-LC-1 protein exists on the surface of the cell membrane in a state exposed to the outside of the cell.
- a specific binding substance labeled with a fluorescent dye is brought into contact with the stomach of a subject, and the state of staining with the fluorescent dye is confirmed using an endoscope or the like. Expression can be confirmed. That is, the specific binding substance is labeled with a fluorescent dye and may be used for detection by an endoscope.
- the endoscope may have a built-in microscope.
- the specific binding substance may be labeled with a positron nuclide. That is, the specific binding substance may be a positron emission tomography (PET) probe labeled with a positron nuclide.
- PET positron emission tomography
- PET is a computed tomography technique that utilizes positron detection.
- the positron nuclides used for PET diagnosis include 11 C (half-life 20.4 minutes), 13 N (half-life 9.97 minutes), 15 O (half-life 2.04 minutes), 18 F (half-life 109. 8 minutes), 64 Cu (half life 12.7 hours), 89 Zr (half life 78.91 hours) and the like.
- KK-LC-1 protein-specific PET probe By labeling the specific binding substance with any of the positron nuclides described above, a KK-LC-1 protein-specific PET probe can be produced. By using the specific binding substance as a probe for PET, early gastric cancer can be detected with high accuracy and minimally invasive.
- a chelating agent such as DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) is used as the antibody or aptamer.
- DOTA 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- the method include binding and then chelating DOTA with a positron nuclide such as 64 Cu.
- the method of binding DOTA to an antibody or aptamer include a method of reacting an antibody or aptamer with DOTA-NHS (N-hydroxysuccinimide) ester.
- the KK-LC-1 protein in the serum or plasma of the subject may be detected by an ELISA method using the above specific binding substance. Thereby, early gastric cancer can be detected with high accuracy with minimal invasiveness.
- KK-LC-1 expression may be detected at the mRNA level.
- Examples of the method for detecting at the mRNA level include reverse transcription of RNA prepared from a stomach tissue sample into cDNA, and gene amplification using this cDNA as a template.
- the early gastric cancer detection kit of this embodiment may be a primer set for amplifying the cDNA of the KK-LC-1 gene.
- the base sequence of the primer set is not particularly limited as long as the cDNA of the KK-LC-1 gene can be specifically amplified.
- the set of the sense primer shown in SEQ ID NO: 1 and the antisense primer shown in SEQ ID NO: 2 also good.
- RNA prepared from a gastric tissue sample is contacted with a probe that specifically hybridizes to mRNA of the KK-LC-1 gene, and KK-LC-
- a probe that specifically hybridizes to mRNA of the KK-LC-1 gene is contacted with a probe that specifically hybridizes to mRNA of the KK-LC-1 gene, and KK-LC-
- detection of the binding of one gene mRNA to the probe More specifically, examples include DNA microarray analysis and Northern blotting.
- the probe is not particularly limited as long as it specifically hybridizes to mRNA of the KK-LC-1 gene.
- the probe may be fixed on a carrier to constitute a DNA microarray or the like.
- the early gastric cancer detection kit of this embodiment may further include a Helicobacter pylori infection detection agent.
- Helicobacter pylori infection detection agent Early gastric cancer can be detected with higher accuracy by detecting whether or not it is infected with Helicobacter pylori together with the expression of KK-LC-1. Detection of Helicobacter pylori infection may be performed using a stomach tissue sample, or may be performed using the serum of a subject from which the stomach tissue sample is derived.
- the Helicobacter pylori infection detection drug is not particularly limited, and examples include a detection drug that detects the presence of Helicobacter pylori, or a detection drug that detects an inflammatory reaction or immune response caused by Helicobacter pylori infection.
- Examples of the detection agent for detecting the presence of Helicobacter pylori include a detection agent for detecting a gene or protein derived from Helicobacter pylori.
- proteins derived from Helicobacter pylori include urease such as urease A and urease B; flagellar proteins such as FlaA, FlaB, FliD, FliK, FlgE, and FlgM; vacuolated toxin (VacA); toxin-associated protein (CagA); Examples include sphere-activating protein (NapA).
- Examples of the gene derived from Helicobacter pylori include the gene encoding the aforementioned protein derived from Helicobacter pylori.
- a detection agent for detecting an inflammatory reaction or immune response caused by Helicobacter pylori infection a detection agent for detecting anti-Helicobacter pylori IgG antibody in serum derived from a subject, a gastric mucosal epithelium by Helicobacter pylori infection
- detection agents for detecting IL-8 released from cells detection agents for detecting TNF- ⁇ , IL-1, IL-6 and the like induced by IL-8 production.
- detection agents for detecting Helicobacter pylori infection include detection agents by the urea test method.
- This detection drug is as follows. First, urea labeled with a stable isotope or a radioisotope is orally administered to a subject. Then, urea labeled with isotopes is hydrolyzed by urease of Helicobacter pylori, and CO 2 labeled with ammonia and isotopes is generated and released from exhaled breath. Therefore, this exhalation is collected, and the ratio of CO 2 labeled with an isotope and CO 2 existing in nature is measured. Helicobacter pylori infection can be detected based on the measured ratio.
- the present invention provides a method for detecting early gastric cancer cells comprising the step of detecting the expression of KK-LC-1 in a gastric tissue sample.
- the expression of KK-LC-1 may be detected in the same manner as described above, and may be detected at the protein level or at the mRNA level.
- a gastric tissue sample a biopsy sample collected from a subject using an endoscope, a gastric lavage fluid, or the like can be used.
- FIG. 1 is a schematic diagram showing the stomach 100, the esophagus 110, and the duodenum 120.
- the stomach 100 is divided into three parts, and the corresponding points are connected to the upper part of the stomach 100. It is described that it is divided into three areas, that is, an area 150, a middle area 160, and a lower area 170, and this area division is also applied in this specification.
- the detection rate of early gastric cancer can be improved when the gastric tissue sample is derived from the lower stomach region.
- gastric glands There are three types of gastric glands: fundic gland, cardia gland and pyloric gland. As will be described later in Examples, when the gastric tissue sample is derived from the pyloric gland, the detection rate of early gastric cancer can be further improved.
- early gastric cancer can be detected with higher accuracy by confirming the expression of KK-LC-1 in a stomach tissue sample derived from the lower stomach region or the pyloric gland. More preferably, the gastric tissue sample is derived from the pyloric gland in the lower stomach region.
- the method for detecting early gastric cancer cells of the present embodiment may further comprise a step of detecting Helicobacter pylori infection in the stomach tissue sample.
- the method for detecting Helicobacter pylori infection is not particularly limited, and the same method as described above can be used.
- Example 1 The expression of KK-LC-1 in gastric tissue samples from gastric cancer patients was examined. As gastric tissue samples, not only samples derived from tumor sites but also samples derived from apparently normal tissues were examined. We also examined the area from which the gastric tissue samples were derived and whether the stomach cancer patients were infected with Helicobacter pylori.
- stomach tissue sample was taken from the resected tissue. We examined patients who could be collected.
- the patient's preoperative serum was collected. Subsequently, the abundance of anti-Helicobacter pylori IgG in the patient's preoperative serum was measured by ELISA to confirm whether the patient was infected with Helicobacter pylori.
- stomach tissue sample derived from the tumor site and 1 to 4 apparently normal stomach tissue samples distal to the tumor site were collected from the fresh stomach tissue after gastrectomy.
- the appearance of normal tissue was recorded from the upper stomach region, the middle stomach region, and the lower stomach region.
- total RNA was prepared from each collected tissue to prepare cDNA.
- tissue adjacent to each collected tissue sample was also collected, and after fixing with formalin, a paraffin-embedded block was prepared.
- a sliced section was prepared from the prepared paraffin-embedded block and stained with hematoxylin and eosin. Subsequently, by microscopic observation of the stained tissue, it was identified whether the stomach tissue sample originated from the fundus gland, the pyloric gland, the fundus gland, or the boundary area of the pyloric gland.
- FIG. 2 (a) is a photomicrograph of a representative gastric tissue sample derived from the fundus gland. The magnification is 400 times.
- FIG. 2 (b) is a photomicrograph of a representative gastric tissue sample derived from the pyloric gland. The magnification is 400 times.
- FIG. 2 (c) is a photomicrograph of a representative gastric tissue sample derived from the border region between the fundus gland and the pyloric gland. The magnification is 400 times.
- KK-LC-1 was confirmed by RT-PCR using the prepared cDNA as a template.
- a primer for KK-LC-1 amplification a sense primer (SEQ ID NO: 1) and an antisense primer (SEQ ID NO: 2) were used.
- SEQ ID NO: 1 a sense primer
- SEQ ID NO: 2 an antisense primer
- Table 1 shows the results of counting the expression of KK-LC-1 for gastric tissue samples derived from tumor sites. As a result, expression of KK-LC-1 was observed in 77.9% of gastric tissue samples derived from the tumor site. This result indicates that KK-LC-1 is useful as a gastric cancer marker.
- Table 2 shows the results of totalizing the region from which the gastric tissue sample is derived and the expression of KK-LC-1 for the apparently normal gastric tissue sample.
- KK-LC-1 expression was observed in 5 cases (21.7%) out of 23 cases in which one apparently normal gastric tissue sample was collected. Further, out of 38 cases obtained from two apparently normal gastric tissue samples, 23 cases (60.5%) expressed KK-LC-1 in any of the gastric tissue samples. Further, out of 15 cases where four apparently normal gastric tissue samples were collected, 11 cases (73.3%) expressed KK-LC-1 in any of the gastric tissue samples. Therefore, it was revealed that the detection rate of early gastric cancer was improved by collecting as many apparently normal gastric tissue samples as possible and examining the expression of KK-LC-1.
- Table 3 shows the results of aggregating the region from which the gastric tissue sample is derived and the expression of KK-LC-1 for gastric tissue samples that are apparently normal only for patients infected with Helicobacter pylori.
- Example 2 The expression of cancer / testis antigens in gastric tissue samples derived from tumor sites of gastric cancer patients was examined and tabulated according to the stage of gastric cancer. As cancer / testis antigens, expression of KK-LC-1, MAGE-A1, MAGE-A3, MAGE-A4, SSX-4, and NY-ESO-1 was examined. In addition, the expression of CEA and CA19-9, which are gastric cancer markers, was also examined by ELISA using preoperative serum of gastric cancer patients as a sample.
- KK-LC-1 The expression of KK-LC-1 was examined by RT-PCR using the sense primer shown in SEQ ID NO: 1 and the antisense primer shown in SEQ ID NO: 2. The presence or absence of KK-LC-1 expression was determined based on the presence or absence of the amplification product in the 40-cycle PCR reaction.
- MAGE-A1, MAGE-A3, MAGE-A4, SSX-4 and NY-ESO-1 was examined by 50 cycles of real-time PCR reaction using a commercially available kit. All of the kits used were manufactured by Thermo Scientifics.
- the MAGE-A1 was detected using the model “Hs00607097_m1” and the MAGE-A3 was detected using the model “Hs00366532_m1”.
- -A4 detection uses kit "Hs00365979_m1”
- SSX-4 detection uses kit "Hs02341532_m1”
- NY-ESO-1 detection uses kit "Hs00265824_m1" did.
- CEA expression was measured using a commercially available kit (model “Lumipulse CEA-N BDX06T Ref: 292662”, Fujirebio Inc.).
- the expression of CA19-9 was measured using a commercially available kit (model “Lumipulse CA19-9-N UDX10T Ref: 292655”, Fujirebio Inc.).
- Table 4 shows the results of counting the expression of each cancer / testis antigen and gastric cancer marker according to the stage of gastric cancer. As a result, it was revealed that the expression frequency of KK-LC-1 was remarkably high in the tumor site of stage I gastric cancer compared to other cancer / testis antigens or gastric cancer markers. This result further supports that KK-LC-1 is useful as a marker capable of detecting early gastric cancer.
- the present invention can provide a technique for detecting early gastric cancer.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Hematology (AREA)
- Biochemistry (AREA)
- Urology & Nephrology (AREA)
- Physics & Mathematics (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Microbiology (AREA)
- Analytical Chemistry (AREA)
- Food Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicinal Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Biophysics (AREA)
- Cell Biology (AREA)
- Plant Pathology (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Investigating Or Analysing Biological Materials (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016-088283 | 2016-04-26 | ||
| JP2016088283A JP6320447B2 (ja) | 2016-04-26 | 2016-04-26 | 前癌状態の胃癌検出キット及び前癌状態の胃癌細胞の検出方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017188315A1 true WO2017188315A1 (ja) | 2017-11-02 |
Family
ID=60159652
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/016543 Ceased WO2017188315A1 (ja) | 2016-04-26 | 2017-04-26 | 早期胃癌検出キット及びその使用 |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP6320447B2 (enExample) |
| WO (1) | WO2017188315A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109628457A (zh) * | 2019-02-03 | 2019-04-16 | 淮海工学院 | 单链dna核酸适配体及其筛选方法与用途 |
| CN112661812A (zh) * | 2021-03-17 | 2021-04-16 | 南京鼓楼医院 | 一种kk-lc-1抗原靶向结合肽及其衍生物、探针与应用 |
| CN117700493A (zh) * | 2023-12-20 | 2024-03-15 | 南京鼓楼医院 | 一种kk-lc-1靶向结合蛋白及其衍生物、试剂盒和应用 |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102627788B1 (ko) * | 2018-06-21 | 2024-01-23 | 고리츠다이가쿠호징 나고야시리츠다이가쿠 | 위암 바이오마커 및 그 용도 |
| JP7150312B2 (ja) * | 2018-07-31 | 2022-10-11 | 学校法人北里研究所 | 胃癌細胞におけるkk-lc-1の発現を予測する方法及びキット |
| JP6797423B2 (ja) * | 2018-12-20 | 2020-12-09 | 学校法人北里研究所 | 胃組織試料が前癌状態であるか否かを判定する方法及びキット |
| WO2024048753A1 (ja) * | 2022-09-02 | 2024-03-07 | 学校法人北里研究所 | ヘリコバクター・ピロリ除菌後の患者が胃癌を発症するリスクを評価する方法、ポリヌクレオチド及びキット |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015128418A (ja) * | 2013-12-05 | 2015-07-16 | 学校法人北里研究所 | 胃癌細胞の検出方法、及び胃癌細胞検出キット |
| JP2016138840A (ja) * | 2015-01-28 | 2016-08-04 | 学校法人北里研究所 | 癌細胞の検出方法及び癌細胞検出キット |
-
2016
- 2016-04-26 JP JP2016088283A patent/JP6320447B2/ja active Active
-
2017
- 2017-04-26 WO PCT/JP2017/016543 patent/WO2017188315A1/ja not_active Ceased
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015128418A (ja) * | 2013-12-05 | 2015-07-16 | 学校法人北里研究所 | 胃癌細胞の検出方法、及び胃癌細胞検出キット |
| JP2016138840A (ja) * | 2015-01-28 | 2016-08-04 | 学校法人北里研究所 | 癌細胞の検出方法及び癌細胞検出キット |
Non-Patent Citations (2)
| Title |
|---|
| SHIDA, A. ET AL.: "Frequent High Expression of Kita-Kyushu Lung Cancer Antigen-1 (KK-LC-1) in Gastric Cancer", ANTICANCER RESEARCH, vol. 35, 2015, pages 3575 - 3580, XP055436396 * |
| TAKASHI FUKUYAMA ET AL.: "Igan ni Okeru Gan/Seiso Kogen Kitakyushu lung cancer antigen- 1(KK-LC-1) no Hatsugen to Helicobacter pylori Kansen tono Kanrensei", ANNUAL MEETING OF THE JAPANESE ASSOCIATION OF CANCER IMMUNOLOGY, 2013, pages 146 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109628457A (zh) * | 2019-02-03 | 2019-04-16 | 淮海工学院 | 单链dna核酸适配体及其筛选方法与用途 |
| CN112661812A (zh) * | 2021-03-17 | 2021-04-16 | 南京鼓楼医院 | 一种kk-lc-1抗原靶向结合肽及其衍生物、探针与应用 |
| CN117700493A (zh) * | 2023-12-20 | 2024-03-15 | 南京鼓楼医院 | 一种kk-lc-1靶向结合蛋白及其衍生物、试剂盒和应用 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2017195803A (ja) | 2017-11-02 |
| JP6320447B2 (ja) | 2018-05-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6320447B2 (ja) | 前癌状態の胃癌検出キット及び前癌状態の胃癌細胞の検出方法 | |
| US10712343B2 (en) | Molecular analysis of tumor samples | |
| Ma et al. | The prognostic values of CA125, CA19. 9, NSE, AND SCC for stage I NSCLC are limited | |
| ES2856979T3 (es) | Procedimientos de identificación de un individuo en función de si se va a tratar con quimioterapia en base a moléculas marcadoras y usos relacionados | |
| Coosemans et al. | Increased immunosuppression is related to increased amounts of ascites and inferior prognosis in ovarian cancer | |
| JP2024059621A (ja) | がんの診断及び治療のための組成物及び方法 | |
| WO2016049045A1 (en) | Pancreatic cancer diagnostic | |
| JP6361943B2 (ja) | 補体因子bタンパク質に特異的に結合する抗体及び糖鎖抗原19−9タンパク質に特異的に結合する抗体を含む膵臓癌診断用キット | |
| JP7150312B2 (ja) | 胃癌細胞におけるkk-lc-1の発現を予測する方法及びキット | |
| Lee et al. | A high-sensitivity cfDNA capture enables to detect the BRAF V600E mutation in papillary thyroid carcinoma | |
| JP6707248B2 (ja) | 胃癌細胞の検出方法、及び胃癌細胞検出キット | |
| RU2010149266A (ru) | Улучшенные способы иммуноанализа | |
| KR102599695B1 (ko) | 마커 사람 부고환 단백질 4 (he4) 기반의 폐 선암 재발의 발견 방법 및 관련 용도 | |
| Potempa-Jeziorowska | Salivary biomarkers for monitoring the course of cancer development–real perspectives | |
| Jafari et al. | Imaging Techniques and Biochemical Biomarkers: New Insights into Diagnosis of Pancreatic Cancer | |
| El-Gezawy et al. | Expression of circulating annexin A2 in hepatic diseases and hepatocellular carcinoma | |
| Voronova et al. | Evaluation and diagnostic potential of plasma biomarkers in bladder cancer | |
| KR20160021531A (ko) | 위암 진단용 마커 | |
| Oni et al. | Detection of incipient pancreatic cancer with novel tumor-specific antibodies in mouse models | |
| US20240159754A1 (en) | Lung cancer diagnosis using biomarkers from exosomes | |
| Hanif et al. | Significance of prostate specific antigen in prostate cancer patients and in non cancerous prostatic disease patients | |
| JP2004511758A (ja) | 大腸癌の診断、モニタリング、ステージング、イメージングおよび処置の方法 | |
| 島田英昭 | Combinational antibody detection approach increases the clinical validity of colorectal cancer screening | |
| Shirin et al. | Clinical Significance of CA125 level with clinicopathological variables and peritoneal dissemination in patients with gastric carcinoma | |
| JPWO2005049864A1 (ja) | 癌診断方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17789597 Country of ref document: EP Kind code of ref document: A1 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17789597 Country of ref document: EP Kind code of ref document: A1 |