WO2017175733A1 - バイオマス固体燃料 - Google Patents
バイオマス固体燃料 Download PDFInfo
- Publication number
- WO2017175733A1 WO2017175733A1 PCT/JP2017/013990 JP2017013990W WO2017175733A1 WO 2017175733 A1 WO2017175733 A1 WO 2017175733A1 JP 2017013990 W JP2017013990 W JP 2017013990W WO 2017175733 A1 WO2017175733 A1 WO 2017175733A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- solid fuel
- biomass
- less
- water
- immersion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L5/00—Solid fuels
- C10L5/40—Solid fuels essentially based on materials of non-mineral origin
- C10L5/44—Solid fuels essentially based on materials of non-mineral origin on vegetable substances
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L5/00—Solid fuels
- C10L5/40—Solid fuels essentially based on materials of non-mineral origin
- C10L5/44—Solid fuels essentially based on materials of non-mineral origin on vegetable substances
- C10L5/442—Wood or forestry waste
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L5/00—Solid fuels
- C10L5/02—Solid fuels such as briquettes consisting mainly of carbonaceous materials of mineral or non-mineral origin
- C10L5/06—Methods of shaping, e.g. pelletizing or briquetting
- C10L5/08—Methods of shaping, e.g. pelletizing or briquetting without the aid of extraneous binders
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L5/00—Solid fuels
- C10L5/02—Solid fuels such as briquettes consisting mainly of carbonaceous materials of mineral or non-mineral origin
- C10L5/26—After-treatment of the shaped fuels, e.g. briquettes
- C10L5/28—Heating the shaped fuels, e.g. briquettes; Coking the binders
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L5/00—Solid fuels
- C10L5/02—Solid fuels such as briquettes consisting mainly of carbonaceous materials of mineral or non-mineral origin
- C10L5/34—Other details of the shaped fuels, e.g. briquettes
- C10L5/36—Shape
- C10L5/363—Pellets or granulates
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L5/00—Solid fuels
- C10L5/40—Solid fuels essentially based on materials of non-mineral origin
- C10L5/44—Solid fuels essentially based on materials of non-mineral origin on vegetable substances
- C10L5/445—Agricultural waste, e.g. corn crops, grass clippings, nut shells or oil pressing residues
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L9/00—Treating solid fuels to improve their combustion
- C10L9/08—Treating solid fuels to improve their combustion by heat treatments, e.g. calcining
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2200/00—Components of fuel compositions
- C10L2200/04—Organic compounds
- C10L2200/0461—Fractions defined by their origin
- C10L2200/0469—Renewables or materials of biological origin
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2290/00—Fuel preparation or upgrading, processes or apparatus therefore, comprising specific process steps or apparatus units
- C10L2290/06—Heat exchange, direct or indirect
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2290/00—Fuel preparation or upgrading, processes or apparatus therefore, comprising specific process steps or apparatus units
- C10L2290/08—Drying or removing water
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2290/00—Fuel preparation or upgrading, processes or apparatus therefore, comprising specific process steps or apparatus units
- C10L2290/28—Cutting, disintegrating, shredding or grinding
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2290/00—Fuel preparation or upgrading, processes or apparatus therefore, comprising specific process steps or apparatus units
- C10L2290/32—Molding or moulds
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E50/00—Technologies for the production of fuel of non-fossil origin
- Y02E50/10—Biofuels, e.g. bio-diesel
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E50/00—Technologies for the production of fuel of non-fossil origin
- Y02E50/30—Fuel from waste, e.g. synthetic alcohol or diesel
Definitions
- the present invention relates to a biomass solid fuel.
- the present invention has been made to solve this problem, and an object of the present invention is to provide a biomass solid fuel that suppresses collapse due to rainwater and reduces COD of wastewater while suppressing cost increase. It is in.
- the biomass solid fuel of the present invention is a biomass solid fuel obtained by molding biomass powder as a raw material, and the connection or adhesion between the biomass powders is maintained after immersion in water.
- the fuel ratio (fixed carbon / volatile content) is preferably 0.15 to 1.50, more preferably 0.17 to 1.50, and still more preferably 0.20 to 1.50
- anhydrous base high calorific value is preferably 4500-7000 (kcal / kg), more preferably 4700-7000 (kcal / kg)
- the molar ratio O / C of oxygen O to carbon C is preferably 0.00.
- the molar ratio H / C of hydrogen H to carbon C is preferably 0.70 to 1.40.
- the solid fuel of the present invention is obtained through a molding process of crushing biomass after crushing, compressing and molding biomass powder that has become scrap or powder into a lump, and a heating process for heating the lump after the molding process
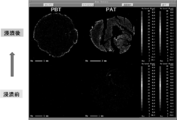
- the molded solid material thus produced is used as fuel (corresponding to PBT described later).
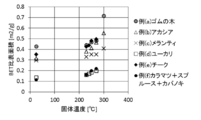
- the biomass solid fuel of the present invention has preferable properties (for example, water resistance) by adjusting, for example, the species of biomass used as a raw material, its part, the heating temperature in the heating step (sometimes described as solid temperature in the figure), and the like. Property, grindability) can be obtained.
- the industrial analysis values, elemental analysis values, and higher calorific values in this specification are based on JIS M 8812, 8813, and 8814.
- biomass as a raw material is also simply referred to as “raw material” or “biomass”, and is obtained by a molding process, and the mass before the heating process is also referred to as “unheated mass”.
- biomass solid fuel is also simply referred to as “solid fuel”.
- the total content of rubber tree, acacia, meranti, eucalyptus, teak, larch, spruce, and birch is preferably 50% by weight or more in the total weight of the raw material biomass. More preferably, it is 80 weight% or more, and may be 100 weight%.
- the biomass solid fuel of the present invention is manufactured by a method including a molding step and a heating step subsequent thereto.
- a block is formed using a known molding technique.
- the lump is preferably a pellet or briquette and can be of any size.
- the heating step the molded lump is heated.
- the biomass solid fuel obtained after the heating step preferably has an immersion water COD (chemical oxygen demand) of 3000 ppm or less when immersed in water.
- the biomass solid fuel preferably has a COD ratio represented by (COD of biomass solid fuel after heating step / COD of unheated biomass solid fuel) of 1.00 or less, and 0.98 or less. More preferably.
- COD (chemical oxygen demand) of immersion water when biomass solid fuel is immersed in water also simply referred to as “COD”) refers to the preparation of an immersion water sample for COD measurement in 1973. This is the COD value analyzed according to JIS K0102 (2010) -17, in accordance with the Agency Notification No. 13 (b) Method for testing metals contained in industrial waste.
- the biomass solid fuel obtained after the heating step preferably has a grindability index (HGI) based on JIS M 8801 of 15 or more and 70 or less, more preferably 20 or more and 60 or less. It is preferable that a BET specific surface area of 0.11m 2 /g ⁇ 0.80m 2 / g, more preferably 0.15m 2 /g ⁇ 0.80m 2 / g, 0.3 ⁇ 0 more preferably .8m is 2 / g, more preferably more that is 0.3 ⁇ 0.7m 2 / g. Further, the equilibrium moisture after immersion in water is preferably 10 to 65 wt%, more preferably 15 to 65 wt%, further preferably 15 to 50 wt%, and further preferably 15 to 45 wt%. preferable.
- HGI grindability index
- the method for producing a biomass solid fuel according to the present invention includes a molding step of forming biomass powder of crushed and pulverized biomass to obtain an unheated lump, and heating to obtain a heated solid by heating the unheated lump
- the heating temperature in the heating step is preferably 150 ° C. to 400 ° C. By setting the temperature of the heating step within this range, a biomass solid fuel having the above characteristics can be obtained.
- the heating temperature is appropriately determined depending on the shape and size of the biomass as a raw material and the lump, but is preferably 150 to 400 ° C, more preferably 200 to 350 ° C, further preferably 230 to 300 ° C, and more preferably 230 to 290 ° C. Is particularly preferred.
- the heating time in the heating step is not particularly limited, but is preferably 0.2 to 3 hours.
- the particle size of the biomass powder is not particularly limited, but is preferably about 100 to 3000 ⁇ m on average, more preferably 400 to 1000 ⁇ m on average.
- the measuring method of the particle size of biomass powder may use a well-known measuring method.
- the connection or adhesion between the biomass powders is maintained by solid crosslinking, so the particle size of the biomass powders is not particularly limited as long as it can be molded.
- a known range may be used as long as the particle size is in a range where both cost and moldability are compatible.
- B / A 0.6 to 1 where A is the bulk density of the unheated block before the heating step and B is the bulk density of the heated solid after the heating step.
- the value of the bulk density A is not particularly limited as long as it is a known range in which biomass powder is molded to obtain an unheated lump. Moreover, since the bulk density changes depending on the type of raw material biomass, it may be set as appropriate. The bulk density can be measured by the method described in Examples described later. Further, H2 / H1 is preferably 1.1 to 4.0, where HGI of unheated lump (hard glove grindability index of JIS M8801) is H1, and HGI of the heated solid is H2. 1.1 to 2.5 is more preferable.
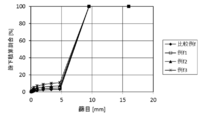
- the solid fuel of the present invention preferably has an expansion coefficient of 20% or less before and after immersion in water, and preferably has a volume expansion coefficient of about 173% or less, and 160% or less. Is more preferable. More preferably, the expansion coefficient of diameter and length is 15% or less, and the volume expansion coefficient is approximately 152% or less. More preferably, the expansion coefficient of the diameter and the length is 13% or less, and the volume expansion coefficient is about 140% or less. More preferably, the expansion coefficient of diameter and length is 11% or less, and the volume expansion coefficient is approximately 137% or less. It is even more preferable that the length expansion coefficient is 10% or less. Thus, when the expansion coefficient after immersion in water is within a certain range, the biomass solid fuel of the present invention does not collapse even by immersion and has water resistance.
- the length expansion coefficient, the diameter expansion coefficient, and the volume expansion coefficient can be measured by the methods described in the examples.
- the characteristics of the biomass solid fuel may be determined within a suitable range depending on the species of biomass used as a raw material.
- preferred types of biomass raw materials used in the present invention, properties of the obtained solid fuel (corresponding to PBT described later), and production methods thereof will be described respectively, but these are only examples, and the present invention. Is not limited to these.
- COD is preferably 2500 ppm or less, more preferably 1100 ppm or less, further preferably 600 ppm or less, and the COD ratio is preferably 1.00 or less, more preferably 0.60 or less, and even more preferably 0.55 or less.
- the equilibrium moisture after immersion in water (corresponding to the solid moisture in the examples) is preferably 15 wt% to 45 wt%, more preferably 15 wt% to 40 wt%, and 15 wt% to 32 wt%. Further preferred.
- the BET specific surface area is 0.43m 2 /g ⁇ 0.80m 2 / g, more preferably 0.44m 2 /g ⁇ 0.80m 2 / g, 0.47m 2 / g ⁇ More preferably, it is 0.80 m 2 / g.
- the fuel ratio is preferably 0.20 to 1.50, more preferably 0.25 to 1.50.
- the anhydrous base high calorific value is preferably 4700 to 7000 kcal / kg, more preferably 5000 to 7000 kcal / kg, and further preferably 5100 to 7000 kcal / kg.
- the molar ratio O / C of oxygen O to carbon C is preferably 0.10 to 0.65, and more preferably 0.15 to 0.60.
- the molar ratio H / C between hydrogen H and carbon C is preferably 0.70 to 1.38, more preferably 0.70 to 1.35.
- the volume expansion rate is preferably 158% or less, more preferably 142% or less, and even more preferably 115% or less.
- the heating temperature in the heating step is preferably 230 to 350 ° C, more preferably 250 to 300 ° C, and further preferably 255 to 290 ° C.
- Solid fuel b As an aspect of the present invention, the properties of a biomass solid fuel (hereinafter sometimes referred to as solid fuel b) when the raw material is an acacia xylem are as follows.
- COD is preferably 400 ppm or less, more preferably 350 ppm or less, further preferably 250 ppm or less, and the COD ratio is preferably 1.00 or less, more preferably 0.98 or less, and even more preferably 0.81 or less.
- HGI is preferably 20 to 70, more preferably 35 to 65, and still more preferably 40 to 65.
- the fuel ratio is preferably 0.21 to 0.90, more preferably 0.21 to 0.88, and still more preferably 0.21 to 0.85.
- the anhydrous base high calorific value is preferably 4790 to 7000 kcal / kg, more preferably 4800 to 7000 kcal / kg, and further preferably 4900 to 6500 kcal / kg.
- the molar ratio O / C of oxygen O to carbon C is preferably 0.25 to 0.62, more preferably 0.28 to 0.61, and still more preferably 0.30 to 0.61.
- the diameter expansion coefficient is preferably 15% or less, more preferably 9% or less, and further preferably 7% or less.
- the volume expansion rate is preferably 143% or less, more preferably 127% or less, and even more preferably 119% or less.
- the heating temperature in the heating step is preferably 200 to 350 ° C., more preferably 220 to 300 ° C., and further preferably 230 to 290 ° C.
- Solid fuel c As one aspect of the present invention, the properties of a biomass solid fuel (hereinafter sometimes referred to as solid fuel c) when the raw material is meranti are as follows.
- COD is preferably 550 ppm or less, more preferably 500 ppm or less, further preferably 300 ppm or less, and the COD ratio is preferably 0.98 or less, more preferably 0.89 or less, and further preferably 0.54 or less.
- the equilibrium moisture after immersion in water is preferably 15 wt% to 30 wt%, more preferably 15 wt% to 27 wt%, and even more preferably 17 wt% to 26 wt%.
- the HGI is preferably 25 to 70, more preferably 30 to 70, and further preferably 30 to 60.
- the BET specific surface area is preferably 0.30 to 0.45 m 2 / g, more preferably 0.30 to 0.41 m 2 / g, and 0.33 to 0.40 m 2 / g. More preferably.
- the fuel ratio is preferably 0.19 to 0.80, more preferably 0.20 to 0.80, still more preferably 0.20 to 0.50, and particularly preferably 0.21 to 0.5.
- the anhydrous base high calorific value is preferably 4800 to 7000 kcal / kg, more preferably 4800 to 6500 kcal / kg, and further preferably 4900 to 6000 kcal / kg.
- the molar ratio O / C of oxygen O to carbon C is preferably 0.30 to 0.62, more preferably 0.30 to 0.61, and still more preferably 0.35 to 0.61.
- the molar ratio H / C between hydrogen H and carbon C is preferably 0.90 to 1.30, more preferably 0.95 to 1.30, and even more preferably 1.00 to 1.30.
- the length expansion coefficient is preferably 10% or less, more preferably 8% or less, and further preferably 6% or less.
- the volume expansion rate is preferably 145% or less, more preferably 135% or less, and even more preferably 128% or less.
- the heating temperature in the heating step is preferably 200 to 350 ° C., more preferably 220 to 300 ° C., and further preferably 230 to 290 ° C.
- Solid fuel d solid fuel d
- properties of a biomass solid fuel (hereinafter sometimes referred to as solid fuel d) when the raw material is eucalyptus are as follows.
- COD is preferably 900 ppm or less, more preferably 800 ppm or less, further preferably 650 ppm or less, and the COD ratio is preferably 0.95 or less, more preferably 0.84 or less, and even more preferably 0.68 or less.
- the equilibrium moisture after immersion in water (corresponding to the solid moisture in the examples) is preferably 13 wt% to 25 wt%, more preferably 15 wt% to 24 wt%, and 15 wt% to 23 wt%. Further preferred.
- the BET specific surface area is 0.135m 2 /g ⁇ 0.210m 2 / g, more preferably 0.140m 2 /g ⁇ 0.210m 2 / g, 0.150m 2 / g ⁇ More preferably, it is 0.195 m 2 / g.
- HGI is preferably 25 to 50, more preferably 27 to 45, and further preferably 30 to 40.
- the HGI ratio (described later) is preferably 1.1 to 4.0, more preferably 1.4 to 2.3, and still more preferably 1.5 to 2.0.
- the molar ratio O / C of oxygen O to carbon C is preferably 0.35 to 0.65, more preferably 0.40 to 0.63, and still more preferably 0.45 to 0.59.
- the molar ratio H / C between hydrogen H and carbon C is preferably 1.00 to 1.24, more preferably 1.05 to 1.24, and still more preferably 1.05 to 1.169.
- the length expansion coefficient is preferably 6.0% or less, more preferably 5.5% or less, and even more preferably 4.5% or less.
- the heating temperature in the heating step is preferably 200 to 350 ° C., more preferably 220 to 300 ° C., and further preferably 231 to 265 ° C.
- the equilibrium moisture after immersion in water (corresponding to the solid moisture in the example) is preferably 15 wt% to 30 wt%, more preferably 17 wt% to 29 wt%, and preferably 17 wt% to 28 wt%. Further preferred.
- HGI is preferably 21 to 45, more preferably 22 to 40, and even more preferably 25 to 38.
- the HGI ratio (described later) is preferably 1.1 to 4.0, more preferably 1.1 to 2.0, and still more preferably 1.3 to 1.9.
- the fuel ratio is preferably 0.23 to 0.60, more preferably 0.27 to 0.55, and further preferably 0.27 to 0.49.
- the anhydrous base high calorific value is preferably 4600 to 6000 kcal / kg, more preferably 4780 to 5500 kcal / kg, and further preferably 4800 to 5350 kcal / kg.
- the length expansion coefficient is preferably 6.0% or less, more preferably 4.5% or less, and even more preferably 4.0% or less.
- the volume expansion rate is preferably 140% or less, more preferably 131% or less, and even more preferably 128% or less.
- the heating temperature in the heating step is preferably 200 to 350 ° C., more preferably 230 to 300 ° C., and further preferably 235 to 269 ° C.
- solid fuel f a biomass solid fuel (hereinafter sometimes referred to as solid fuel f) when the raw material is a mixture of larch, spruce, and birch.
- the mixing ratio of larch, spruce and birch is not particularly limited.
- larch: spruce: birch 30 to 70:25 to 65: 0 to 25 may be mixed.
- COD is preferably 1200 ppm or less, more preferably 1000 ppm or less, further preferably 850 ppm or less, and the COD ratio is preferably 0.33 or less, more preferably 0.28 or less, and further preferably 0.24 or less.
- the equilibrium moisture after immersion in water is preferably 15 wt% to 30 wt%, more preferably 15 wt% to 28 wt%, and even more preferably 15 wt% to 27 wt%.
- the BET specific surface area is 0.150m 2 /g ⁇ 0.250m 2 / g, more preferably 0.160m 2 /g ⁇ 0.250m 2 / g, 0.170m 2 / g ⁇ More preferably, it is 0.250 m 2 / g.
- the HGI is preferably 18 to 45, more preferably 21 to 40, and still more preferably 22 to 35.
- the HGI ratio (described later) is preferably 1.1 to 4.0, more preferably 1.2 to 2.4, and still more preferably 1.2 to 2.2.
- the fuel ratio is preferably 0.165 to 0.35, more preferably 0.17 to 0.35, and still more preferably 0.18 to 0.30.
- the anhydrous base high calorific value is preferably 4800 to 6000 kcal / kg, more preferably 4900 to 5700 kcal / kg, and further preferably 5000 to 5500 kcal / kg.
- the molar ratio O / C of oxygen O to carbon C is preferably 0.45 to 0.64, more preferably 0.47 to 0.62, and further preferably 0.50 to 0.61.
- the molar ratio H / C between hydrogen H and carbon C is preferably 1.0 to 1.3, more preferably 1.1 to 1.3, and even more preferably 1.10 to 1.29.
- the diameter expansion coefficient is preferably 15.0% or less, more preferably 13.0% or less, and even more preferably 10.0% or less.
- the length expansion coefficient is preferably 7.0% or less, more preferably 6.0% or less, and even more preferably 4.5% or less.
- the present inventors in the order of the process of performing the heating process of heating the unheated lump after the molding process, the components derived from biomass that is the raw material without using a binder. It is speculated that a biomass solid fuel with high water resistance can be produced that is used to maintain the connection or adhesion between biomass powders and does not collapse even when immersed in water. As a result of the analysis by the present inventors, the following knowledge about the mechanism by which the biomass solid fuel acquires water resistance was obtained.
- the present inventor has three types of biomass solid fuels having different production methods, specifically, unheated solid fuel formed by pulverized biomass (sometimes referred to as “White Pellet:“ WP ”), and pulverization.
- the solid fuel obtained by molding and heating the obtained biomass (Pelletizing Before Torrefaction: may be described as “PBT”) is subjected to FT-IR analysis, GC-MS analysis, SEM observation, etc.
- the water resistance mechanism of solid fuel was analyzed. Note that no binder is used in either WP or PBT.
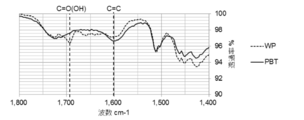
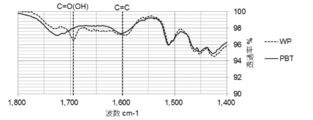
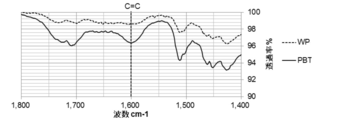
- FIGS. 5 to 8 show an example of the result of FT-IR analysis of the biomass solid fuel
- FIG. 9 shows the result of the GC-MS analysis of the acetone extract of the biomass solid fuel (refer to the examples for details).
- FIG. 4 is a diagram showing a mechanism (estimation) of solid bridge development in PBT.
- the liquid resulting from the melting of abietic acid becomes a gap between the pulverized biomass (also referred to as “biomass powder”) (consolidated by molding after pulverization and adjacent) Elution to the gap between the biomass powders, and further, abietic acid evaporates and undergoes thermal decomposition, and the hydrophobic substance adheres to the gaps between the biomass powders to develop crosslinking (solid crosslinking).
- attachment of biomass powder is maintained by the abietic acid derived from the biomass etc. which are raw materials, without adding a binder. Therefore, it is considered that the biomass powders are connected or adhered to each other to suppress water entry and improve water resistance.
- Abietic acid or a derivative thereof has a melting point of about 139 to 142 ° C and a boiling point of about 250 ° C. Therefore, it is inferred that heating causes abietic acid or the like to melt near the melting point to cause liquid crosslinking, and abietic acid or the like thermally decomposes near the boiling point to develop solid crosslinking.
- Terpenes such as abietic acid are generally contained in biomass (Hokkaido Prefectural Forest Products Experiment Station Monthly Report No. 171, April 1966, Japan Wood Conservation Society “Wood Preservation” Vol. 34-2 (2008), etc.) . Although there is a slight difference in the content depending on the type of biomass ("Use of essential oils” Ohira Goro, Report of the 6th Research Subcommittee of the Japan Wood Society, Table 1, Table 1 of the Japan Wood Society 1999), etc. It is considered that water resistance is imparted by heating at least 230 ° C. to 250 ° C. for biomass in general because water resistance (not disintegrated even after immersion in water, see Table 2) is manifested by heating at a temperature of °C or higher. .
- PBT also improves the strength of solid fuel due to the development of solid cross-linking, and has good grindability (HGI, ball mill grindability) without adding a binder by heating at least 230 ° C to 250 ° C as well as water resistance.
- HGI ball mill grindability
- good handling properties mechanical durability, pulverization test
- COD is reduced in PBT. This is because the tar content of the biomass raw material is volatilized by heating, and at the same time, the solid fuel surface of PBT is covered with solidified abietic acid, and the solid fuel surface is hydrophobic. This is considered to be because the elution of tar remaining in the biomass raw material is suppressed.
- biomass solid fuels were produced by the following production methods. Tables 1 to 4 show properties of these solid fuels.
- Example a Rubber tree>
- a biomass solid fuel was produced as follows using a rubber tree as a raw material biomass.
- Comparative example a is an unheated biomass solid fuel (WP) that has been molded after crushing and pulverization and has not undergone a heating step. Also in Comparative Example a, no binder is used. The properties of the solid fuel of Comparative Example a are also shown in Tables 1 and 2. In the unheated biomass solid fuel (WP) of Comparative Example a, the pellet collapsed after being immersed in water for 168 hours, and each property could not be measured.
- the solid yield is the weight ratio before and after heating (100 ⁇ dry weight after heating / dry weight before heating (%)), and the heat yield is the calorific value ratio before and after heating (higher heat generation after heating).
- Tables 1 and 3 show the results of the high calorific value (anhydrous basis), the fuel ratio calculated based on the industrial analysis value (the air dry basis), and the elemental analysis value (anhydrous basis), and the results obtained based on this.
- the molar ratio of oxygen O, carbon C, and hydrogen H is shown.
- HGI is based on JIS M 8801 as described above, and the higher the value, the better the grindability.
- the HGI ratio is calculated by 100 ⁇ HGI after heating / HGI before heating (%).
- “HHV” is higher calorific value (anhydrous basis)
- FC is fixed carbon (air-dry base)
- VM volatile matter (air-dry base)
- fuel ratio is “FC / VM” Represents the calculated value.
- pellet length and diameter were measured.
- the pellet length 10 pellets before immersion were randomly selected for each solid fuel, and electronic calipers (Mitutoyo: CD-15CX, repetition accuracy was 0.01 mm, rounded off to two decimal places. ).
- the length was measured up to the tip.
- the pellet diameter was also measured using the same electronic caliper. The measured values of the pellet length and diameter are average values of 10 pieces.
- m1 is a container weight
- m2 is a container weight + sample weight
- V is a container volume.
- the “pore” here is a pore having a diameter of 2 nm to 100 nm.
- FIG. 2 shows the average pore diameter of each solid fuel surface
- FIG. 3 shows the total pore volume of each solid fuel.
- Tables 2 and 4 show CODs of the immersion water when immersed in water. Tables 2 and 4 also show the diameter, length, pH, solid moisture, and mechanical durability after immersing the biomass solid fuel in water for 168 hours.
- the measuring method of each property is as follows.
- pellet length and diameter were measured in the same manner as before immersion in water.
- the pellet length was measured by electronic calipers (manufactured by Mitutoyo: CD-15CX, repetition accuracy of 0.01 mm, rounded off 2 decimal places) for 10 randomly selected before immersion. When the pellet end was slanted, the length was measured up to the tip. The pellet diameter was also measured using the same electronic caliper. The measured values of the pellet length and diameter are average values of 10 pieces.
- Example b Acacia>
- the temperature was raised to the target temperature (heating temperature described in Table 1) and heated in the same manner as in Examples a1 to a4 except that acacia was used as the raw material biomass.
- Properties of the biomass solid fuel b (Examples b1 to b4) obtained after the heating step were measured by the same method as in Example a. The results are shown in Tables 1 and 2.
- Comparative Example b (WP) the properties were measured using the same raw materials as in Examples b1 to b4 except that the heating step was not performed.
- the binders are not used in any of Examples b1 to b4 and Comparative Example b. Since the moisture after immersion in water is after immersion for 168 hours, it is considered that the moisture in the solid fuel b has substantially reached equilibrium.
- Comparative Example b collapsed immediately after being immersed in water.
- Examples b1 to b4 even after immersion in water (168 hours), the connection or adhesion between the biomass powders was maintained and did not collapse. Thereby, since the solid shape was maintained even after immersion, moisture measurement was possible, and the expression of water resistance could be confirmed.
- Examples b1 to b4 have advantageous properties as solid fuels that are often stored outdoors. Further, the results of HGI and ball mill grindability showed that grindability was improved in Examples b1 to b4 as compared with Comparative Example b.
- Regarding COD COD was reduced in Examples b2, b3 and b4 as compared with Comparative Example b.
- Examples b1 to b3 were excellent, and the solid fuels of Examples b2 and b3 exhibited particularly excellent physical properties. In addition, in Examples b2 to b4, it was shown that the expansion coefficient was particularly low.
- Example c Meranti>
- the raw material biomass was heated to the target temperature (heating temperature described in Table 1) in the same manner as in Examples a1 to a4, except that meranti was used.
- Tables 1 and 2 show the properties of the biomass solid fuel c obtained after the heating step.
- Comparative Example c (WP) the properties were measured using the same raw materials as in Examples c1 to c4 except that the heating step was not performed.
- the water after immersion in water is considered to be balanced because it is after 168 hours of immersion.
- no binder is used.
- the measuring method of each property of the biomass solid fuel is the same as in Example a.
- Comparative Example c collapsed immediately after being immersed in water.
- Examples c1 to c4 the connection or adhesion between the biomass powders is maintained and the water resistance is improved without being destroyed even after being immersed in water. From the results of HGI and ball mill grindability, it was shown that grindability was improved in Examples c1 to c4 as compared with Comparative Example c.
- Regarding COD it was reduced in Examples c1 to c4 as compared with Comparative Example c1.
- Examples c1 to c3 were excellent from the viewpoint of COD, mechanical durability, and solid yield, and Examples c2 and c3 were particularly excellent.
- Examples c2 to a4 it was shown that the expansion coefficient was particularly low.
- Example d Eucalyptus>
- the target temperature (described in Table 3) was used in the same manner as in Examples a1 to a4, except that eucalyptus was used as the raw material biomass and was molded into a pellet shape having a diameter of 6 mm in the molding process. The temperature was raised to (heating temperature) and heated. Properties of the biomass solid fuel d (Example d1 to Example d4) obtained after the heating step were measured by the same method as in Example a. The results are shown in Tables 3 and 4.
- Comparative Example d WP
- the properties were measured using the same raw materials as in Examples d1 to d4 except that the heating step was not performed.
- the binders are not used in any of Examples d1 to d4 and Comparative Example d. Since the moisture after immersion in water is after immersion for 168 hours, it is considered that the moisture in the solid fuel d has substantially reached equilibrium.
- Comparative Example d collapsed immediately after being immersed in water.
- Examples d1 to d4 even after immersion in water (168 hours), the connection or adhesion between the biomass powders was maintained and did not collapse. Thereby, since the solid shape was maintained even after immersion, moisture measurement was possible, and the expression of water resistance could be confirmed.
- Examples d1 to d4 have advantageous characteristics as a solid fuel that is often stored outdoors. Further, the results of HGI and ball mill grindability showed that grindability was improved in Examples d1 to d4 as compared with Comparative Example d. Regarding COD, COD was reduced in Examples d1 to d4 as compared with Comparative Example d.
- Comparative Example f WP
- the properties were measured using the same raw materials as in Examples f1 to f3 except that the heating step was not performed.
- binders are not used in any of Examples f1 to f3 and Comparative Example f. Since the moisture after immersion in water is after immersion for 168 hours, it is considered that the moisture in the solid fuel f has substantially reached equilibrium.
- Examples f1 to f3 were excellent, and the solid fuels of Examples f2 and f3 showed particularly excellent physical properties. In addition, it was shown that the expansion rate was particularly low in Examples f2 to f3.
- the present inventors investigated the thermal properties of the biomass solid fuel of the present invention and the solid fuel obtained through the process of steam explosion of the biomass as described in Patent Document 1, respectively, and the biomass solid fuel of the present invention It was found that the fuel is excellent in ignitability.
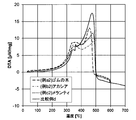
- ⁇ TG and DTA> TG and DTA were measured using a differential thermal and thermogravimetric simultaneous measurement apparatus STA7300 manufactured by Hitachi High-Tech Science. 5 mg of a sample whose particle size was adjusted to 45-90 ⁇ m with a cutter mill was measured at 4 vol. While flowing a% oxygen-nitrogen mixed gas at 200 cc / min, the temperature was raised to 600 ° C. and held for 60 min.
- Comparative Example q has a low weight loss and therefore has less volatile content and lower ignitability than Examples (a2), (b2) and (c2). Also, from the DTA results, it can be said that the heat generation occurred in the comparative example q from the high temperature side as compared with the examples (a2), (b2) and (c2), and the ignitability is low.
- the reason for this is that in Comparative Example q, biomass is obtained in the order of pulverization / drying, steam explosion, molding, and heating in the order of biomass, but the organic components float on the surface of the biomass powder during steam explosion. This is presumed to be caused by volatilization by subsequent heating (carbonization).
- the PBT of the present invention including Example (a2), Example (b2), and Example (c2), it is presumed that there is a large amount of residual volatile matter that does not go through the explosion process.
- the biomass solid fuel of the present invention does not include a steam explosion process, it is considered that the biomass solid fuel is excellent in ignitability in addition to being able to suppress the cost as compared with Comparative Example q.
- the terpenes forming the solid bridge of PBT also have a large residual amount for the same reason, and a stronger solid bridge can be obtained. Therefore, the PBT of the present invention is superior in strength and water resistance as compared with Comparative Example q. It is guessed.
- FIGS. 5 to 9 show FT of biomass solid fuel r (solid fuel (PBT) obtained by heating at 250 ° C. after being pulverized into a pellet shape) obtained from the red pine as a raw material by the same method as in Example a2 above.
- FIG. 6 is a diagram showing the results of -IR analysis. The same raw material is pulverized and unheated after molding (WP) is also shown. On both the outer surface of the pellet (FIG. 5) and the center of the cross section (FIG. 6), the amount of COOH groups is WP> PBT, and the amount of C ⁇ C bonds is PBT> WP.
- the COOH group elution amount in the acetone extract is WP> PBT, which indicates that PBT has few hydrophilic COOH groups. Furthermore, in the solid after acetone extraction (FIG. 8), PBT has more C ⁇ C bonds than WP. Therefore, it turns out that PBT is excellent in water resistance.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Environmental & Geological Engineering (AREA)
- Geology (AREA)
- Geochemistry & Mineralogy (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Combustion & Propulsion (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Forests & Forestry (AREA)
- Wood Science & Technology (AREA)
- Ecology (AREA)
- Biodiversity & Conservation Biology (AREA)
- Agronomy & Crop Science (AREA)
- Solid Fuels And Fuel-Associated Substances (AREA)
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| MYPI2018001687A MY186936A (en) | 2016-04-06 | 2017-04-03 | Biomass solid fuel |
| CA3019888A CA3019888A1 (en) | 2016-04-06 | 2017-04-03 | Biomass solid fuel |
| AU2017247418A AU2017247418B2 (en) | 2016-04-06 | 2017-04-03 | Biomass solid fuel |
| US16/090,473 US11390823B2 (en) | 2016-04-06 | 2017-04-03 | Biomass solid fuel |
| RU2018138545A RU2746855C2 (ru) | 2016-04-06 | 2017-04-03 | Твердое топливо из биомассы |
| JP2018510598A JP6501037B2 (ja) | 2016-04-06 | 2017-04-03 | バイオマス固体燃料 |
| KR1020187031769A KR102431476B1 (ko) | 2016-04-06 | 2017-04-03 | 바이오매스 고체 연료 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016076441 | 2016-04-06 | ||
| JP2016-076441 | 2016-04-06 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017175733A1 true WO2017175733A1 (ja) | 2017-10-12 |
Family
ID=60001528
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/013990 Ceased WO2017175733A1 (ja) | 2016-04-06 | 2017-04-03 | バイオマス固体燃料 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US11390823B2 (enExample) |
| JP (3) | JP6501037B2 (enExample) |
| KR (1) | KR102431476B1 (enExample) |
| AU (1) | AU2017247418B2 (enExample) |
| CA (1) | CA3019888A1 (enExample) |
| MY (1) | MY186936A (enExample) |
| RU (1) | RU2746855C2 (enExample) |
| WO (1) | WO2017175733A1 (enExample) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019069849A1 (ja) * | 2017-10-04 | 2019-04-11 | 宇部興産株式会社 | バイオマス固体燃料 |
| WO2019131983A1 (ja) * | 2017-12-28 | 2019-07-04 | 日本製紙株式会社 | 固体燃料の製造方法 |
| WO2020229824A1 (en) | 2019-05-13 | 2020-11-19 | Hamer, Christopher | Process for producing solid biomass fuel |
| WO2021024001A1 (en) | 2019-08-08 | 2021-02-11 | Hamer, Christopher | Process for producing solid biomass fuel |
| WO2021156628A1 (en) | 2020-02-06 | 2021-08-12 | Hamer, Christopher | Process for producing solid biomass fuel |
| GB202117376D0 (en) | 2021-12-01 | 2022-01-12 | Bai hong mei | Process for producing solid biomass fuel |
| WO2022079427A1 (en) | 2020-10-12 | 2022-04-21 | Hamer, Christopher | Process for producing solid biomass fuel |
| JP2022542058A (ja) * | 2019-07-22 | 2022-09-29 | ホン メイ バイ | 固体バイオマス燃料を生成するための方法 |
| WO2023156796A1 (en) | 2022-02-18 | 2023-08-24 | Hamer, Christopher | Process for producing solid biomass fuel |
| JP2024504972A (ja) * | 2021-01-21 | 2024-02-02 | カーボン テクノロジー ホールディングス, エルエルシー | 反応性が緩和されたバイオカーボンペレット |
| WO2024062247A1 (en) | 2022-09-20 | 2024-03-28 | Hamer, Christopher | Solid biomass fuel anti-coking additive |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102417652B1 (ko) | 2021-11-05 | 2022-07-07 | 주식회사 엠티에스 | 아임계수를 이용한 바이오매스 고형화 연료 제조 장치 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008136477A1 (ja) * | 2007-04-27 | 2008-11-13 | Mitsubishi Heavy Industries Environment Engineering Co., Ltd. | バイオコークス製造装置及び製造方法 |
| JP2014098097A (ja) * | 2012-11-14 | 2014-05-29 | Daio Paper Corp | 固形燃料の製造方法及び固形燃料 |
| WO2014087949A1 (ja) * | 2012-12-05 | 2014-06-12 | 宇部興産株式会社 | バイオマス固体燃料 |
| JP2015067789A (ja) * | 2013-09-30 | 2015-04-13 | 日本製紙株式会社 | 固体燃料の製造方法及び固体燃料 |
| WO2016056608A1 (ja) * | 2014-10-07 | 2016-04-14 | 宇部興産株式会社 | バイオマス固体燃料 |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR19990037945A (ko) * | 1999-02-26 | 1999-05-25 | 장성민 | 생나무를 이용한 연료용 팰렛 제조방법 |
| JP2000319676A (ja) | 1999-05-11 | 2000-11-21 | Nisshin Kogyo Kk | 固形化燃料及びその製造方法 |
| WO2006077652A1 (ja) | 2005-01-24 | 2006-07-27 | Osaka Industrial Promotion Organization | 木質バイオマス固形燃料及びその製法 |
| US7942942B2 (en) | 2006-05-21 | 2011-05-17 | Paoluccio John A | Method and apparatus for biomass torrefaction, manufacturing a storable fuel from biomass and producing offsets for the combustion products of fossil fuels and a combustible article of manufacture |
| NZ606220A (en) | 2006-10-26 | 2014-05-30 | Xyleco Inc | Processing biomass |
| PL2300575T3 (pl) * | 2008-06-26 | 2017-09-29 | Accordant Energy, Llc | Przerobiony surowiec paliwowy do zastępowania węgla kopalnego w elektrowniach węglowych |
| JP2012528008A (ja) | 2009-05-26 | 2012-11-12 | アメリカン ペレット サプライ エルエルシー | 圧縮されたバイオマスによるペレット及びブリケット |
| US20110179701A1 (en) | 2010-01-27 | 2011-07-28 | G-Energy Technologies, Llc | Torrefaction of ligno-cellulosic biomasses and mixtures |
| MY161924A (en) | 2010-08-17 | 2017-05-15 | Ube Industries | Solid fuel, and method and apparatus for producing same |
| EP2457978A1 (en) * | 2010-11-24 | 2012-05-30 | Evonik Degussa GmbH | Process for pyrolysis of lignin-rich biomass, carbon-rich solid obtained and use thereof as soil amendment or adsorbent |
| AU2012258767B2 (en) | 2011-05-23 | 2017-01-05 | Virent, Inc. | Production of chemicals and fuels from biomass |
| BR112015006734B1 (pt) | 2012-09-28 | 2021-12-28 | Nippon Paper Industries Co., Ltd | Combustível sólido e seu método de produção |
| ES2927465T3 (es) * | 2012-12-21 | 2022-11-07 | Jiangsu Hengtron Nanotech Co Ltd | Materiales cátodos LMFP con rendimiento electroquímico mejorado |
| KR101308869B1 (ko) | 2013-04-12 | 2013-09-16 | 안상우 | 초본계 분말과 보풀된 폐지를 주원료로 하는 친환경 고형 연료 및 그 제조방법 |
-
2017
- 2017-04-03 MY MYPI2018001687A patent/MY186936A/en unknown
- 2017-04-03 RU RU2018138545A patent/RU2746855C2/ru active
- 2017-04-03 WO PCT/JP2017/013990 patent/WO2017175733A1/ja not_active Ceased
- 2017-04-03 KR KR1020187031769A patent/KR102431476B1/ko active Active
- 2017-04-03 AU AU2017247418A patent/AU2017247418B2/en active Active
- 2017-04-03 JP JP2018510598A patent/JP6501037B2/ja active Active
- 2017-04-03 US US16/090,473 patent/US11390823B2/en active Active
- 2017-04-03 CA CA3019888A patent/CA3019888A1/en active Pending
-
2019
- 2019-03-13 JP JP2019046217A patent/JP7003950B2/ja active Active
-
2021
- 2021-11-02 JP JP2021179603A patent/JP7267382B2/ja active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008136477A1 (ja) * | 2007-04-27 | 2008-11-13 | Mitsubishi Heavy Industries Environment Engineering Co., Ltd. | バイオコークス製造装置及び製造方法 |
| JP2014098097A (ja) * | 2012-11-14 | 2014-05-29 | Daio Paper Corp | 固形燃料の製造方法及び固形燃料 |
| WO2014087949A1 (ja) * | 2012-12-05 | 2014-06-12 | 宇部興産株式会社 | バイオマス固体燃料 |
| JP2015067789A (ja) * | 2013-09-30 | 2015-04-13 | 日本製紙株式会社 | 固体燃料の製造方法及び固体燃料 |
| WO2016056608A1 (ja) * | 2014-10-07 | 2016-04-14 | 宇部興産株式会社 | バイオマス固体燃料 |
Cited By (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11939549B2 (en) | 2017-10-04 | 2024-03-26 | Mitsubishi Ube Cement Corporation | Biomass solid fuel |
| JPWO2019069849A1 (ja) * | 2017-10-04 | 2020-10-22 | 宇部興産株式会社 | バイオマス固体燃料 |
| WO2019069849A1 (ja) * | 2017-10-04 | 2019-04-11 | 宇部興産株式会社 | バイオマス固体燃料 |
| JP7467577B2 (ja) | 2017-10-04 | 2024-04-15 | Ube三菱セメント株式会社 | バイオマス固体燃料 |
| JP2023025203A (ja) * | 2017-10-04 | 2023-02-21 | Ube三菱セメント株式会社 | バイオマス固体燃料 |
| WO2019131983A1 (ja) * | 2017-12-28 | 2019-07-04 | 日本製紙株式会社 | 固体燃料の製造方法 |
| JPWO2019131983A1 (ja) * | 2017-12-28 | 2020-12-24 | 日本製紙株式会社 | 固体燃料の製造方法 |
| JP7261176B2 (ja) | 2017-12-28 | 2023-04-19 | 日本製紙株式会社 | 固体燃料の製造方法 |
| WO2020229824A1 (en) | 2019-05-13 | 2020-11-19 | Hamer, Christopher | Process for producing solid biomass fuel |
| JP2020186362A (ja) * | 2019-05-13 | 2020-11-19 | バイ ホン メイBai, Hong Mei | 固体バイオマス燃料の製造方法 |
| JP7622970B2 (ja) | 2019-05-13 | 2025-01-28 | ホン メイ バイ | 固体バイオマス燃料の製造方法 |
| US11920100B2 (en) | 2019-05-13 | 2024-03-05 | Hong Mei Bai | Process for producing solid biomass fuel |
| JP2022542058A (ja) * | 2019-07-22 | 2022-09-29 | ホン メイ バイ | 固体バイオマス燃料を生成するための方法 |
| WO2021024001A1 (en) | 2019-08-08 | 2021-02-11 | Hamer, Christopher | Process for producing solid biomass fuel |
| JP2022543297A (ja) * | 2019-08-08 | 2022-10-11 | ホン メイ バイ | 固体バイオマス燃料を生成するための方法 |
| JP7713730B2 (ja) | 2019-08-08 | 2025-07-28 | ホン メイ バイ | 固体バイオマス燃料を生成するための方法 |
| WO2021156628A1 (en) | 2020-02-06 | 2021-08-12 | Hamer, Christopher | Process for producing solid biomass fuel |
| JP2023544871A (ja) * | 2020-10-12 | 2023-10-25 | ホン メイ バイ | 固体バイオマス燃料を生成するための方法 |
| WO2022079427A1 (en) | 2020-10-12 | 2022-04-21 | Hamer, Christopher | Process for producing solid biomass fuel |
| JP2024504972A (ja) * | 2021-01-21 | 2024-02-02 | カーボン テクノロジー ホールディングス, エルエルシー | 反応性が緩和されたバイオカーボンペレット |
| JP2024504969A (ja) * | 2021-01-21 | 2024-02-02 | カーボン テクノロジー ホールディングス, エルエルシー | 調整可能な粉砕性指数を有するバイオカーボンペレット |
| JP2024506244A (ja) * | 2021-01-21 | 2024-02-13 | カーボン テクノロジー ホールディングス, エルエルシー | 調整可能な粉砕性指数を有するバイオカーボンペレットを製造するためのプロセス |
| WO2023099900A1 (en) | 2021-12-01 | 2023-06-08 | Hamer, Christopher | Process for producing solid biomass fuel |
| GB202117376D0 (en) | 2021-12-01 | 2022-01-12 | Bai hong mei | Process for producing solid biomass fuel |
| WO2023156796A1 (en) | 2022-02-18 | 2023-08-24 | Hamer, Christopher | Process for producing solid biomass fuel |
| WO2024062247A1 (en) | 2022-09-20 | 2024-03-28 | Hamer, Christopher | Solid biomass fuel anti-coking additive |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2017247418A1 (en) | 2018-11-01 |
| MY186936A (en) | 2021-08-26 |
| CA3019888A1 (en) | 2017-10-12 |
| JP2019123879A (ja) | 2019-07-25 |
| JP7003950B2 (ja) | 2022-01-21 |
| US11390823B2 (en) | 2022-07-19 |
| US20190119593A1 (en) | 2019-04-25 |
| AU2017247418B2 (en) | 2019-12-19 |
| JPWO2017175733A1 (ja) | 2019-01-10 |
| JP7267382B2 (ja) | 2023-05-01 |
| JP6501037B2 (ja) | 2019-04-17 |
| JP2022017460A (ja) | 2022-01-25 |
| KR102431476B1 (ko) | 2022-08-12 |
| RU2746855C2 (ru) | 2021-04-21 |
| RU2018138545A3 (enExample) | 2020-05-19 |
| KR20180133444A (ko) | 2018-12-14 |
| RU2018138545A (ru) | 2020-05-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7267382B2 (ja) | バイオマス固体燃料 | |
| JP7467577B2 (ja) | バイオマス固体燃料 | |
| JP7289800B2 (ja) | バイオマス固体燃料 | |
| WO2017175737A1 (ja) | バイオマス炭化物の冷却装置 | |
| NZ747132B2 (en) | Cooling apparatus for carbonized biomass |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 2018510598 Country of ref document: JP |
|
| ENP | Entry into the national phase |
Ref document number: 3019888 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2017247418 Country of ref document: AU Date of ref document: 20170403 Kind code of ref document: A Ref document number: 20187031769 Country of ref document: KR Kind code of ref document: A |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17779111 Country of ref document: EP Kind code of ref document: A1 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17779111 Country of ref document: EP Kind code of ref document: A1 |