WO2017150305A1 - ニッケル粉の製造方法 - Google Patents
ニッケル粉の製造方法 Download PDFInfo
- Publication number
- WO2017150305A1 WO2017150305A1 PCT/JP2017/006623 JP2017006623W WO2017150305A1 WO 2017150305 A1 WO2017150305 A1 WO 2017150305A1 JP 2017006623 W JP2017006623 W JP 2017006623W WO 2017150305 A1 WO2017150305 A1 WO 2017150305A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- nickel
- nickel powder
- insoluble solid
- mixed slurry
- added
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/16—Making metallic powder or suspensions thereof using chemical processes
- B22F9/18—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds
- B22F9/24—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds starting from liquid metal compounds, e.g. solutions
- B22F9/26—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds starting from liquid metal compounds, e.g. solutions using gaseous reductors
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/05—Metallic powder characterised by the size or surface area of the particles
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2301/00—Metallic composition of the powder or its coating
- B22F2301/15—Nickel or cobalt
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2304/00—Physical aspects of the powder
- B22F2304/10—Micron size particles, i.e. above 1 micrometer up to 500 micrometer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2999/00—Aspects linked to processes or compositions used in powder metallurgy
Definitions
- the present invention relates to a method for producing a fine nickel powder that can be used as a seed crystal from a solution containing a nickel sulfate ammine complex, and is particularly applicable to a process for controlling the number of generated particles to a necessary amount.
- a method for producing a fine nickel powder an atomizing method in which molten nickel is dispersed in gas or water to obtain a fine powder, or nickel disclosed in Patent Document 1 is volatilized and reduced in the gas phase.
- a dry method such as a CVD method for obtaining powder is known.
- a method for producing nickel powder by a wet process a method using a reducing agent disclosed in Patent Document 2 or a method of spraying a nickel solution in a reducing atmosphere at a high temperature disclosed in Patent Document 3
- a spray pyrolysis method for obtaining nickel powder by a pyrolysis reaction is known.
- these methods are expensive because they require expensive reagents and a large amount of energy.
- Non-Patent Document 1 a method for obtaining nickel powder by supplying hydrogen gas to a nickel sulfate ammine complex solution and reducing nickel ions in the complex solution as shown in Non-Patent Document 1 is industrially inexpensive and useful. .
- the nickel powder particles obtained are easily coarsened, and it has been difficult to produce a fine powder that can be used for seed crystals.
- seed crystals when generating and growing particles from an aqueous solution, a small amount of fine crystals called seed crystals coexist, supplying a reducing agent there, and growing seed crystals to obtain a powder having a predetermined particle size.
- the method is used.
- the seed crystal used in this method is often obtained by pulverizing a product, etc., but it is time consuming and leads to an increase in cost because the yield is reduced. Further, the seed crystal having the optimum particle size and properties is not always obtained by pulverization.
- the present invention provides a fine nickel powder that becomes a seed crystal necessary for the production of nickel powder from a solution containing the nickel sulfate ammine complex, according to the amount necessary for the production of the nickel powder.
- a method for producing nickel powder to be produced is provided.
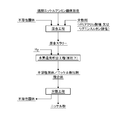
- the first invention of the present invention for solving such a problem is that a nickel complex ion formed by continuously supplying and stirring a solution containing a nickel sulfate ammine complex, an insoluble solid, and a dispersing agent into a reaction vessel. Hydrogen gas is blown into the solution containing nickel, and the nickel complex ions in the solution containing nickel complex ions are reduced to form a complex with nickel particle precipitates on the surface of the insoluble solid, and the complex containing the complex is reduced. After the slurry is generated, the reducing slurry is extracted from the reaction vessel.
- the second invention of the present invention is a mixing step of forming a mixed slurry by adding a seed crystal insoluble solid and a polyacrylate or lignin sulfonate as a dispersant to a solution containing a nickel sulfate ammine complex,
- the mixed slurry is charged into the reaction tank, hydrogen gas is blown into the mixed slurry in the reaction tank, and nickel complex ions in the mixed slurry are reduced to form nickel particle precipitates on the surface of the insoluble solid.
- It is a nickel powder manufacturing method characterized in that the precipitate is made into nickel powder through the reduction precipitation step in order.
- the amount of polyacrylate added is added to the mixed slurry.
- the nickel powder production method is characterized in that the amount is more than 1.0% by weight and not more than 10.0% by weight of the insoluble solid.
- the fifth invention of the present invention is characterized in that the amount of polyacrylate added in the fourth invention is 2.0 to 6.0% by weight with respect to the weight of the seed crystal insoluble solid. It is a manufacturing method of powder.
- the sixth invention of the present invention is a method for producing nickel powder, characterized in that the polyacrylate in the fourth and fifth inventions is sodium polyacrylate (PAA).
- PAA sodium polyacrylate
- the dispersant added in the mixing step of the second and third inventions uses lignin sulfonic acid
- the added amount of lignin sulfonic acid is insoluble added to the mixed slurry.
- the nickel powder production method is characterized in that the amount is 2.0% by weight or more and 20.0% by weight or less of the weight of the solid.
- a fine nickel powder optimal for a seed crystal used for producing nickel powder economically and efficiently is obtained in a necessary amount. Accordingly, it is possible to provide a manufacturing method, and it has a remarkable industrial effect.
- FIG. 5 is a graph showing changes in nickel concentration in solutions after completion of reaction when sodium polyacrylate is used in Examples 1 to 4. It is a figure which shows the change by the reaction time of the nickel density
- the present invention relates to a method for producing nickel powder by adding a dispersant and a seed crystal insoluble solid to a nickel sulfate ammine complex solution and blowing hydrogen gas into the nickel sulfate ammine complex solution.
- a method for producing nickel powder, characterized by producing nickel powder characterized by producing nickel powder.
- the nickel sulfate ammine complex solution used in the present invention is not particularly limited, but one or a mixture selected from nickel and cobalt mixed sulfide, crude nickel sulfate, nickel oxide, nickel hydroxide, nickel carbonate, nickel powder and the like.
- a nickel leaching solution solution containing nickel obtained by dissolving nickel-containing materials such as industrial intermediates with sulfuric acid or ammonia in accordance with the components, and solvent purification, ion exchange, neutralization, etc.
- a solution obtained by adding ammonia to a solution obtained by removing the impurity element in the solution by applying the step to form a nickel sulfate ammine complex solution is suitable.
- a dispersant is first added to the nickel sulfate ammine complex solution.
- the dispersant used in this step is not particularly limited as long as it is a polyacrylate or lignin sulfonate, but as a polyacrylate that can be obtained industrially at low cost, calcium acrylate, sodium polyacrylate, Among potassium polyacrylate and lignin sulfonate, calcium lignin sulfonate, sodium lignin sulfonate, and potassium lignin sulfonate are preferable.
- ammonium sulfate concentration in the solution is preferably in the range of 10 to 500 g / L for both of the production methods shown in FIG. If it is 500 g / L or more, the solubility is exceeded and crystals are deposited. In addition, since ammonium sulfate is newly generated by the reaction, it is difficult to achieve less than 10 g / L.
- insoluble solid that is insoluble in the complex solution and becomes a matrix for precipitation is added to the nickel sulfate ammine complex solution whose dispersant concentration is adjusted as described above.
- the insoluble solid added here is not particularly limited as long as it has a low solubility in a nickel sulfate ammine complex solution, an ammonium sulfate aqueous solution or an alkaline solution.
- nickel powder, iron powder, alumina powder, zirconia powder, silica Powder etc. can be used.
- the present invention is not a method of precipitating powder using a seed crystal that has been generally used in the past, and making the whole seed crystal into a product, but after the necessary precipitation on the surface of the insoluble solid is completed, the precipitate is precipitated and grown as an insoluble solid
- the product is separated from the product, and only the powder portion of the separated precipitate is to be used as the product. According to such a method of the present invention, the influence on the product due to the impurities of the seed crystal itself is avoided.
- the addition amount of the insoluble solid is not particularly limited, and an amount that can be mixed by stirring when added to the nickel sulfate ammine complex solution is selected according to the kind of the solid. As an example, an amount of about 50 to 100 g / L may be added.
- the shape and size are not particularly limited, but the nickel deposits on the surface may be separated by colliding with each other or applying vibration as described later. A surface having a gentle shape is suitable so that objects can be effectively separated.
- the insoluble solid of the present invention Prior to depositing nickel, it is preferable to use the insoluble solid of the present invention after applying impact or impact in advance to remove the deposits on the surface of the insoluble solid. Furthermore, the insoluble solid after separating the nickel deposits can be used again after being subjected to pretreatment such as washing as necessary.
- the insoluble solid is used as a seed crystal and a dispersant is added.
- the added insoluble solid forms a sufficiently dispersed state in the complex solution, and the surface of the insoluble solid is finely formed.
- a suitable amount of addition in the range of 1.0 to 20.0% by weight of the insoluble solid added to the complex solution is desirable, especially polyacrylate Lignin sulfonate is preferred.
- the amount added is added to the mixed slurry. More than 1.0% by weight of the insoluble solid and 10.0% by weight or less, desirably 2.0% by weight or more and 6.0% by weight or less.
- the addition amount When the addition amount is 1.0% by weight or less, nickel powder does not precipitate. When the addition amount is 2.0% by weight or more, the insoluble solid is sufficiently dispersed, and the number of nickel powders generated in proportion to the addition amount can be controlled. It is preferable.
- the upper limit tends to increase even if the upper limit exceeds 6.0% by weight, but it is not preferable that too many seed crystals are formed because the handling and the dispersing agent aggregate together, and considering the effect commensurate with the amount added. It is 10.0 weight% or less, More preferably, it is 6.0 weight% or less.
- the amount added is 2 of the weight of the insoluble solid added to the mixed slurry.
- the amount is not less than 0.0% by weight and not more than 20.0% by weight. If the addition amount is 2.0% by weight or less, nickel powder cannot be obtained, and it is necessary to exceed 2.0% by weight. However, if the addition amount exceeds 5.0% by weight, it is generated in proportion to the addition amount. It is preferable because the number of nickel powders can be controlled.
- the “reduction / precipitation step” in which the nickel complex ions in the mixed slurry are reduced with hydrogen to form a composite in which nickel precipitates are formed on the surface of the insoluble solid is a method of performing batch processing and continuous It is possible to adopt a method of performing processing.
- the “reduction / precipitation process” in which the reduction / precipitation process is performed in batch processing is performed by charging a mixed slurry formed by adding a dispersant and an insoluble solid into a reaction tank of a high pressure resistant high temperature vessel.
- hydrogen gas is blown into the mixed slurry stored in the slurry to reduce the nickel complex ions in the mixed slurry, thereby forming a reduced slurry containing a composite formed with nickel as precipitates on the surface of the insoluble solid contained. .
- the reaction temperature at this time is preferably in the range of 150 to 200 ° C. If the reaction temperature is lower than 150 ° C., the reduction efficiency is lowered. Even if the reaction temperature is 200 ° C. or higher, there is no influence on the reaction. Further, the pressure during the reaction is preferably 1.0 to 4.0 MPa. If the pressure is less than 1.0 MPa, the reaction efficiency decreases, and if it exceeds 4.0 MPa, there is no effect on the reaction and the loss of hydrogen gas increases.
- the mixed slurry formed by adding the dispersant and the insoluble solid is continuously supplied into the reaction vessel of the high pressure resistant high temperature vessel, and hydrogen gas is continuously blown into the mixed slurry flowing in the reaction vessel.
- nickel complex ions in the mixed slurry are reduced to obtain a reduced slurry containing a composite in which nickel precipitates are formed on the surface of the contained insoluble solid.
- the obtained reduction slurry is continuously extracted from the reaction vessel and collected, and is supplied to the next step. That is, by making the reduction reaction process a continuous process, it is possible to reduce the time required for replacing the slurry and setting the conditions for the reduction process, and an improvement in production efficiency can be expected. Further, by controlling the amount of the mixed slurry flowing in, the production amount can be adjusted, the reaction tank can be reduced in capacity, the cost for capital investment and repair can be reduced, and it is economical.
- the reaction temperature in such a reduction / precipitation step is preferably in the range of 150 to 200 ° C. If the reaction temperature is lower than 150 ° C., the reduction efficiency is lowered. Even if the reaction temperature is 200 ° C. or higher, there is no influence on the reaction.
- the pressure in the gas phase part of the reaction tank during the reaction is preferably 1.0 to 4.0 MPa. If the pressure is less than 1.0 MPa, the reaction efficiency decreases, and if it exceeds 4.0 MPa, there is no effect on the reaction and the loss of hydrogen gas increases.
- the insoluble solid forms a sufficiently dispersed state in the mixed slurry, and in such a state, as a finer powdery precipitate on the surface of the insoluble solid.
- Nickel precipitates can be formed, and nickel can be extracted and recovered from the nickel sulfate ammine complex solution, and further the amount of nickel powder produced by precipitation can be adjusted by adjusting the amount of dispersant added.
- This step is performed when an insoluble solid is used, and the nickel precipitate formed in the reduction / precipitation step is attached to the surface of the insoluble solid and cannot be used in that state.
- the nickel precipitate is separated from the insoluble solid and recovered.
- a specific separation method for example, in order to prevent oxidation due to heat generation, put insoluble solids together in water, rotate and collide with insoluble solids to separate surface nickel deposits, and screen to obtain nickel powder , Rotating on a wet sieve and sieving the separated nickel precipitates at the same time to obtain nickel powder, or applying ultrasonic waves in the liquid to give vibration and separating, sieving to obtain nickel powder, etc.
- any material having an opening smaller than the size of the insoluble solid can be used.
- the nickel powder produced as described above can be used, for example, as a nickel paste, which is an internal constituent material of a multilayer ceramic capacitor, and the particles are grown by repeating the hydrogen reduction using the recovered nickel powder as a seed crystal. High-purity nickel metal can be produced.
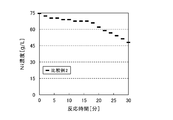
- the reduced slurry of the sample was taken out from the sample port of the autoclave every two minutes after the supply of hydrogen gas was started, and the nickel concentration of the filtrate was analyzed by solid-liquid separation. As the reaction proceeds, nickel precipitates as a powder, and the nickel concentration of the filtrate decreases accordingly. As shown in FIG. 2, it was possible to reduce and recover 80% or more of nickel in 30 minutes calculated from the change in concentration.
- the supply of hydrogen gas was stopped and the inner cylinder can was cooled. After cooling, the slurry in the inner cylinder can was filtered, and 42.7 g of the deposited nickel powder was recovered. When the collected nickel powder was observed, it was confirmed that the nickel powder was fine enough to be used for seed crystals.
- Nickel powder was produced and recovered under the same conditions and method as in Example 1 except that sodium polyacrylate was added in an amount of 4.5 g corresponding to 6% by weight of the seed crystal. The result is shown in FIG. As shown in FIG. 2, as in Example 1, 80% or more of nickel was reduced and recovered in 30 minutes.
- Nickel powder was produced and recovered under the same conditions and method as in Example 1 except that 7.5 g corresponding to 10% by weight of the seed crystal was added. The result is shown in FIG. As shown in FIG. 2, as in Example 1, 80% or more of nickel was reduced and recovered in 30 minutes.
- Nickel powder was produced and recovered under the same conditions and method as in Example 1 except that 0.75 g of sodium polyacrylate was added corresponding to 1% by weight of the seed crystal. The result is shown in FIG. As shown in FIG. 2, about 50% of nickel was reduced and recovered in 30 minutes calculated from the change in concentration.
- Nickel powder was prepared in the same manner as in Example 1 except that the dispersant and the insoluble solid were not added and the other liquid composition and reduction conditions were the same.
- the nickel concentration of the sampled solution decreased from 75 g / L to about 45 g / L.
- the nickel powder could not be recovered from the solution after the completion of the hydrogen gas blowing, and it was confirmed that plate-like nickel scaling was generated on the side wall and the stirrer in the inner cylinder can.
- Nickel powder was produced in the same manner as in Example 1 except that 75 g of nickel powder was added as an insoluble solid without adding a dispersant. The result is shown in FIG. As shown in FIG. 3, only about 20% of nickel could be reduced in 30 minutes calculated from the concentration change.
- nickel powder having an average particle size (D50) of 85 ⁇ m was added as an insoluble solid serving as a precipitation matrix to prepare a desired mixed slurry.
- the sodium polyacrylate added here corresponds to 0.2% by weight, 1.0% by weight, 2.0% by weight, 4.0% by weight, and 6.0% by weight, respectively, of the insoluble solid amount in pure content. Is.
- the prepared mixed slurry is charged into an inner can of the autoclave, and heated and maintained at 185 ° C. while stirring, and hydrogen gas is blown into the autoclave so that the pressure in the autoclave becomes 3.5 MPa. Supplied. After 60 minutes had passed since the supply of hydrogen gas, the supply of hydrogen gas was stopped and the inner cylinder can was cooled.
- the slurry in the inner cylinder can is filtered to recover a complex of insoluble solid and nickel precipitate, and then a wet sieve having an opening of 75 ⁇ m is used to apply vibration to the matrix insoluble solid and the surface. The nickel precipitates were separated and nickel powder was recovered.
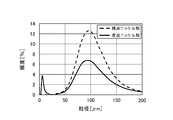
- the recovered nickel powder under the sieve was measured for particle size by a particle size distribution device (trade name: 9320-X100, manufactured by Microtrac Co., Ltd.) to obtain the particle size distribution.
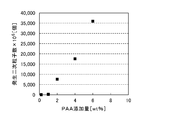
- FIG. 4 shows the relationship between the number of nickel powders calculated using the above equation (1) and the amount of sodium polyacrylate added.
- FIG. 4 shows that there is a correlation between the amount of sodium polyacrylate added and the number of nickel powders, and the amount of nickel powder generated can be adjusted by the amount of sodium polyacrylate added.
- the amount of sodium polyacrylate added is 1.0% by weight or less, nickel powder cannot be obtained, but when it exceeds 1.0% by weight, the number of nickel powders generated in proportion to the amount added can be controlled. Recognize.
- the nickel sulfate ammine complex solution prepared above and the seed crystal slurry are continuously supplied to the autoclave by a pump, and hydrogen gas is blown from a cylinder while the autoclave is kept at 185 ° C. while stirring. Hydrogen gas was supplied and held so that the pressure in the can was 3.5 MPa. At that time, after blowing hydrogen gas, the supply amount and discharge amount of nickel sulfate ammine complex solution and seed crystal slurry are adjusted so that the residence time in the autoclave is 1 hour, and the liquid amount in the autoclave is constant. The reacted slurry was continuously extracted from the autoclave and collected.
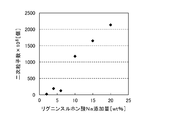
- the number of nickel powders was calculated by a calculation method using the above formula (1). As a result, the number of particles increased as shown in Table 1, and it can be seen from the particle size distribution shown in FIG. 6 that fine nickel powder was generated.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Powder Metallurgy (AREA)
- Manufacture And Refinement Of Metals (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2017227207A AU2017227207B2 (en) | 2016-03-04 | 2017-02-22 | Nickel powder production method |
| CA3016415A CA3016415A1 (en) | 2016-03-04 | 2017-02-22 | Method for producing nickel powder |
| EP17759771.3A EP3424626A4 (en) | 2016-03-04 | 2017-02-22 | PROCESS FOR PRODUCING NICKEL POWDER |
| CN201780015054.7A CN108778577A (zh) | 2016-03-04 | 2017-02-22 | 镍粉的制造方法 |
| US16/081,980 US20190009343A1 (en) | 2016-03-04 | 2017-02-22 | Method for producing nickel powder |
| PH12018501872A PH12018501872A1 (en) | 2016-03-04 | 2018-09-03 | Method for producing nickel powder |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016042668A JP6641632B2 (ja) | 2016-03-04 | 2016-03-04 | ニッケル粉の製造方法 |
| JP2016-042668 | 2016-03-04 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017150305A1 true WO2017150305A1 (ja) | 2017-09-08 |
Family
ID=59743856
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/006623 Ceased WO2017150305A1 (ja) | 2016-03-04 | 2017-02-22 | ニッケル粉の製造方法 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US20190009343A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP3424626A4 (cg-RX-API-DMAC7.html) |
| JP (1) | JP6641632B2 (cg-RX-API-DMAC7.html) |
| CN (1) | CN108778577A (cg-RX-API-DMAC7.html) |
| AU (1) | AU2017227207B2 (cg-RX-API-DMAC7.html) |
| CA (1) | CA3016415A1 (cg-RX-API-DMAC7.html) |
| PH (1) | PH12018501872A1 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2017150305A1 (cg-RX-API-DMAC7.html) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6624464B2 (ja) * | 2017-12-21 | 2019-12-25 | 住友金属鉱山株式会社 | ニッケル粉の製造方法 |
| JP7034439B2 (ja) * | 2018-06-19 | 2022-03-14 | 住友金属鉱山株式会社 | ニッケル粉の回収方法 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015122534A1 (ja) * | 2014-02-17 | 2015-08-20 | 国立大学法人高知大学 | ニッケル粉の製造方法 |
| JP5796696B1 (ja) * | 2015-01-22 | 2015-10-21 | 住友金属鉱山株式会社 | ニッケル粉の製造方法 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2853380A (en) * | 1957-05-20 | 1958-09-23 | Sherritt Gordon Mines Ltd | Method of recovering metal values from solutions |
| US3833351A (en) * | 1973-02-15 | 1974-09-03 | Univ Eng Inc | Continuous preparation of pure metals by hydrogen reduction |
| FI106635B (fi) * | 1999-11-09 | 2001-03-15 | Outokumpu Oy | Menetelmä nikkelin pelkistämiseksi vesiliuoksesta |
| JP2005528981A (ja) * | 2002-06-12 | 2005-09-29 | スルザー メテコ(カナダ)インコーポレイテッド | 担持型触媒の製造のための湿式製錬方法 |
| CN103273074B (zh) * | 2013-03-27 | 2014-12-10 | 深圳市中金岭南科技有限公司 | 一种连续生产超细镍粉工艺方法 |
| CA2939809C (en) * | 2014-02-21 | 2017-08-22 | Kochi University, National University Corporation | Method for producing nickel powder |
| JP6459879B2 (ja) * | 2015-09-28 | 2019-01-30 | 住友金属鉱山株式会社 | ニッケル粉の製造方法、反応設備の運転方法 |
-
2016
- 2016-03-04 JP JP2016042668A patent/JP6641632B2/ja active Active
-
2017
- 2017-02-22 AU AU2017227207A patent/AU2017227207B2/en not_active Ceased
- 2017-02-22 CA CA3016415A patent/CA3016415A1/en not_active Abandoned
- 2017-02-22 WO PCT/JP2017/006623 patent/WO2017150305A1/ja not_active Ceased
- 2017-02-22 US US16/081,980 patent/US20190009343A1/en not_active Abandoned
- 2017-02-22 CN CN201780015054.7A patent/CN108778577A/zh active Pending
- 2017-02-22 EP EP17759771.3A patent/EP3424626A4/en not_active Withdrawn
-
2018
- 2018-09-03 PH PH12018501872A patent/PH12018501872A1/en unknown
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015122534A1 (ja) * | 2014-02-17 | 2015-08-20 | 国立大学法人高知大学 | ニッケル粉の製造方法 |
| JP5796696B1 (ja) * | 2015-01-22 | 2015-10-21 | 住友金属鉱山株式会社 | ニッケル粉の製造方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3424626A4 * |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2017227207B2 (en) | 2019-11-21 |
| CA3016415A1 (en) | 2017-09-08 |
| EP3424626A4 (en) | 2019-10-30 |
| CN108778577A (zh) | 2018-11-09 |
| AU2017227207A1 (en) | 2018-09-27 |
| EP3424626A1 (en) | 2019-01-09 |
| PH12018501872A1 (en) | 2019-05-15 |
| JP2017155319A (ja) | 2017-09-07 |
| JP6641632B2 (ja) | 2020-02-05 |
| US20190009343A1 (en) | 2019-01-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10220446B2 (en) | Method for producing nickel powder | |
| WO2016117138A1 (ja) | ニッケル粉の製造方法 | |
| JP2015166488A5 (cg-RX-API-DMAC7.html) | ||
| JP5796696B1 (ja) | ニッケル粉の製造方法 | |
| JP6816755B2 (ja) | ニッケル粉の製造方法 | |
| WO2017150305A1 (ja) | ニッケル粉の製造方法 | |
| JP6241617B2 (ja) | コバルト粉の製造方法 | |
| JP5881091B2 (ja) | ニッケル粉の製造方法 | |
| JP2017155319A5 (cg-RX-API-DMAC7.html) | ||
| JP7007650B2 (ja) | ニッケル粉の製造方法 | |
| JP2018141203A (ja) | 種晶用ニッケル粉末の製造方法 | |
| JP2018178232A (ja) | ニッケル粉の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 3016415 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2017227207 Country of ref document: AU Date of ref document: 20170222 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2017759771 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2017759771 Country of ref document: EP Effective date: 20181004 |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17759771 Country of ref document: EP Kind code of ref document: A1 |