WO2014125934A1 - 内燃機関の排ガス浄化装置、排ガス浄化方法及び排ガス浄化触媒 - Google Patents
内燃機関の排ガス浄化装置、排ガス浄化方法及び排ガス浄化触媒 Download PDFInfo
- Publication number
- WO2014125934A1 WO2014125934A1 PCT/JP2014/052138 JP2014052138W WO2014125934A1 WO 2014125934 A1 WO2014125934 A1 WO 2014125934A1 JP 2014052138 W JP2014052138 W JP 2014052138W WO 2014125934 A1 WO2014125934 A1 WO 2014125934A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- exhaust gas
- catalyst
- internal combustion
- nox
- combustion engine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/92—Chemical or biological purification of waste gases of engine exhaust gases
- B01D53/94—Chemical or biological purification of waste gases of engine exhaust gases by catalytic processes
- B01D53/9459—Removing one or more of nitrogen oxides, carbon monoxide, or hydrocarbons by multiple successive catalytic functions; systems with more than one different function, e.g. zone coated catalysts
- B01D53/9477—Removing one or more of nitrogen oxides, carbon monoxide, or hydrocarbons by multiple successive catalytic functions; systems with more than one different function, e.g. zone coated catalysts with catalysts positioned on separate bricks, e.g. exhaust systems
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/002—Mixed oxides other than spinels, e.g. perovskite
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/38—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals
- B01J23/40—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals of the platinum group metals
- B01J23/46—Ruthenium, rhodium, osmium or iridium
- B01J23/468—Iridium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/38—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals
- B01J23/54—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals combined with metals, oxides or hydroxides provided for in groups B01J23/02 - B01J23/36
- B01J23/56—Platinum group metals
- B01J23/63—Platinum group metals with rare earths or actinides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/38—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals
- B01J23/54—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals combined with metals, oxides or hydroxides provided for in groups B01J23/02 - B01J23/36
- B01J23/56—Platinum group metals
- B01J23/64—Platinum group metals with arsenic, antimony, bismuth, vanadium, niobium, tantalum, polonium, chromium, molybdenum, tungsten, manganese, technetium or rhenium
- B01J23/648—Vanadium, niobium or tantalum or polonium
- B01J23/6484—Niobium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/061—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof containing metallic elements added to the zeolite
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/064—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof containing iron group metals, noble metals or copper
- B01J29/072—Iron group metals or copper
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/076—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof containing arsenic, antimony, bismuth, vanadium, niobium, tantalum, polonium, chromium, molybdenum, tungsten, manganese, technetium or rhenium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/08—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the faujasite type, e.g. type X or Y
- B01J29/10—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the faujasite type, e.g. type X or Y containing iron group metals, noble metals or copper
- B01J29/14—Iron group metals or copper
- B01J29/146—Y-type faujasite
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/19—Catalysts containing parts with different compositions
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/024—Multiple impregnation or coating
- B01J37/0246—Coatings comprising a zeolite
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/024—Multiple impregnation or coating
- B01J37/0248—Coatings comprising impregnated particles
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F01—MACHINES OR ENGINES IN GENERAL; ENGINE PLANTS IN GENERAL; STEAM ENGINES
- F01N—GAS-FLOW SILENCERS OR EXHAUST APPARATUS FOR MACHINES OR ENGINES IN GENERAL; GAS-FLOW SILENCERS OR EXHAUST APPARATUS FOR INTERNAL-COMBUSTION ENGINES
- F01N13/00—Exhaust or silencing apparatus characterised by constructional features
- F01N13/009—Exhaust or silencing apparatus characterised by constructional features having two or more separate purifying devices arranged in series
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F01—MACHINES OR ENGINES IN GENERAL; ENGINE PLANTS IN GENERAL; STEAM ENGINES
- F01N—GAS-FLOW SILENCERS OR EXHAUST APPARATUS FOR MACHINES OR ENGINES IN GENERAL; GAS-FLOW SILENCERS OR EXHAUST APPARATUS FOR INTERNAL-COMBUSTION ENGINES
- F01N3/00—Exhaust or silencing apparatus having means for purifying, rendering innocuous, or otherwise treating exhaust
- F01N3/08—Exhaust or silencing apparatus having means for purifying, rendering innocuous, or otherwise treating exhaust for rendering innocuous
- F01N3/10—Exhaust or silencing apparatus having means for purifying, rendering innocuous, or otherwise treating exhaust for rendering innocuous by thermal or catalytic conversion of noxious components of exhaust
- F01N3/103—Oxidation catalysts for HC and CO only
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F01—MACHINES OR ENGINES IN GENERAL; ENGINE PLANTS IN GENERAL; STEAM ENGINES
- F01N—GAS-FLOW SILENCERS OR EXHAUST APPARATUS FOR MACHINES OR ENGINES IN GENERAL; GAS-FLOW SILENCERS OR EXHAUST APPARATUS FOR INTERNAL-COMBUSTION ENGINES
- F01N3/00—Exhaust or silencing apparatus having means for purifying, rendering innocuous, or otherwise treating exhaust
- F01N3/08—Exhaust or silencing apparatus having means for purifying, rendering innocuous, or otherwise treating exhaust for rendering innocuous
- F01N3/10—Exhaust or silencing apparatus having means for purifying, rendering innocuous, or otherwise treating exhaust for rendering innocuous by thermal or catalytic conversion of noxious components of exhaust
- F01N3/18—Exhaust or silencing apparatus having means for purifying, rendering innocuous, or otherwise treating exhaust for rendering innocuous by thermal or catalytic conversion of noxious components of exhaust characterised by methods of operation; Control
- F01N3/20—Exhaust or silencing apparatus having means for purifying, rendering innocuous, or otherwise treating exhaust for rendering innocuous by thermal or catalytic conversion of noxious components of exhaust characterised by methods of operation; Control specially adapted for catalytic conversion
- F01N3/206—Adding periodically or continuously substances to exhaust gases for promoting purification, e.g. catalytic material in liquid form, NOx reducing agents
- F01N3/2066—Selective catalytic reduction [SCR]
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2251/00—Reactants
- B01D2251/20—Reductants
- B01D2251/206—Ammonium compounds
- B01D2251/2062—Ammonia
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/10—Noble metals or compounds thereof
- B01D2255/102—Platinum group metals
- B01D2255/1028—Iridium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/207—Transition metals
- B01D2255/20715—Zirconium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/207—Transition metals
- B01D2255/20723—Vanadium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/207—Transition metals
- B01D2255/2073—Manganese
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/207—Transition metals
- B01D2255/20738—Iron
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/207—Transition metals
- B01D2255/20746—Cobalt
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/20—Metals or compounds thereof
- B01D2255/207—Transition metals
- B01D2255/20753—Nickel
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/50—Zeolites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/40—Nitrogen compounds
- B01D2257/404—Nitrogen oxides other than dinitrogen oxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/40—Nitrogen compounds
- B01D2257/406—Ammonia
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/50—Carbon oxides
- B01D2257/504—Carbon dioxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/70—Organic compounds not provided for in groups B01D2257/00 - B01D2257/602
- B01D2257/702—Hydrocarbons
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2258/00—Sources of waste gases
- B01D2258/01—Engine exhaust gases
- B01D2258/012—Diesel engines and lean burn gasoline engines
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/92—Chemical or biological purification of waste gases of engine exhaust gases
- B01D53/94—Chemical or biological purification of waste gases of engine exhaust gases by catalytic processes
- B01D53/9404—Removing only nitrogen compounds
- B01D53/9409—Nitrogen oxides
- B01D53/9413—Processes characterised by a specific catalyst
- B01D53/9418—Processes characterised by a specific catalyst for removing nitrogen oxides by selective catalytic reduction [SCR] using a reducing agent in a lean exhaust gas
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/92—Chemical or biological purification of waste gases of engine exhaust gases
- B01D53/94—Chemical or biological purification of waste gases of engine exhaust gases by catalytic processes
- B01D53/944—Simultaneously removing carbon monoxide, hydrocarbons or carbon making use of oxidation catalysts
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2229/00—Aspects of molecular sieve catalysts not covered by B01J29/00
- B01J2229/10—After treatment, characterised by the effect to be obtained
- B01J2229/18—After treatment, characterised by the effect to be obtained to introduce other elements into or onto the molecular sieve itself
- B01J2229/186—After treatment, characterised by the effect to be obtained to introduce other elements into or onto the molecular sieve itself not in framework positions
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/38—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals
- B01J23/54—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals combined with metals, oxides or hydroxides provided for in groups B01J23/02 - B01J23/36
- B01J23/66—Silver or gold
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2523/00—Constitutive chemical elements of heterogeneous catalysts
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/50—Catalysts, in general, characterised by their form or physical properties characterised by their shape or configuration
- B01J35/56—Foraminous structures having flow-through passages or channels, e.g. grids or three-dimensional monoliths
- B01J35/57—Honeycombs
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J38/00—Regeneration or reactivation of catalysts, in general
- B01J38/02—Heat treatment
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F01—MACHINES OR ENGINES IN GENERAL; ENGINE PLANTS IN GENERAL; STEAM ENGINES
- F01N—GAS-FLOW SILENCERS OR EXHAUST APPARATUS FOR MACHINES OR ENGINES IN GENERAL; GAS-FLOW SILENCERS OR EXHAUST APPARATUS FOR INTERNAL-COMBUSTION ENGINES
- F01N2510/00—Surface coverings
- F01N2510/06—Surface coverings for exhaust purification, e.g. catalytic reaction
- F01N2510/063—Surface coverings for exhaust purification, e.g. catalytic reaction zeolites
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F01—MACHINES OR ENGINES IN GENERAL; ENGINE PLANTS IN GENERAL; STEAM ENGINES
- F01N—GAS-FLOW SILENCERS OR EXHAUST APPARATUS FOR MACHINES OR ENGINES IN GENERAL; GAS-FLOW SILENCERS OR EXHAUST APPARATUS FOR INTERNAL-COMBUSTION ENGINES
- F01N2610/00—Adding substances to exhaust gases
- F01N2610/02—Adding substances to exhaust gases the substance being ammonia or urea
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02T—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO TRANSPORTATION
- Y02T10/00—Road transport of goods or passengers
- Y02T10/10—Internal combustion engine [ICE] based vehicles
- Y02T10/12—Improving ICE efficiencies
Definitions
- the present invention relates to an exhaust gas purification apparatus, an exhaust gas purification method, and an exhaust gas purification catalyst applicable to an internal combustion engine operated in a lean combustion area such as a diesel engine or a lean burn engine.
- An exhaust gas containing a large amount of carbon monoxide (CO) and nitrogen oxides (NOx) is emitted from an internal combustion engine that burns fuel in a state of excessive oxygen, such as a diesel engine or a lean burn engine.
- exhaust gases of these internal combustion engines include hydrocarbons (HC) which are unburned components of fuel.
- HC hydrocarbons
- ammonia generated by thermal decomposition and hydrolysis of an aqueous urea solution added to the exhaust gas for NOx purification or added to the exhaust gas for NOx purification NH 3 is also included. Therefore, an exhaust gas purification apparatus, an exhaust gas purification method and an exhaust gas purification catalyst applicable to these internal combustion engines are required to be capable of highly oxidizing and purifying CO, NOx, HC and NH 3 in an atmosphere of excessive oxygen.
- the purification technology of NOx conventionally blown with an aqueous urea solution or NH 3 in the exhaust gas flow path, a technique for purifying NOx by reacting NOx with NH 3 are known in the NOx purification catalyst.
- the NOx purification catalyst a catalyst obtained by honeycomb-forming a base metal oxide such as titania (TiO 2 ) or vanadia (V 2 O 5 ) or a ceramic honeycomb monolith such as cordierite is supported by a vanadium-based catalyst or a zeolite-based catalyst. And those with improved heat resistance are used.
- the NOx purification catalyst installed in the exhaust gas passage is exposed to HC.
- the inventors of the present application studied the influence of the inflow of HC on the activity of the NOx purification catalyst.
- the NOx purification catalyst contains a zeolite component
- the NOx purification performance of the NOx purification catalyst decreases and HC Found that CO is partially oxidized to generate CO.
- the exhaust gas purification apparatus described in Non-Patent Documents 1 and 2 nothing is considered about the influence when HC flows in, and as it is, CO, NOx, HC and NH 3 in exhaust gas are highly oxidized and purified. I can not
- the present invention has been made based on the above-described findings, and an object thereof is an exhaust gas purification apparatus for an internal combustion engine capable of highly purifying CO, NOx, HC and NH 3 contained in an exhaust gas with excess oxygen.
- An exhaust gas purification method and an exhaust gas purification catalyst are provided.
- the present invention relates to an exhaust gas flow control system for an internal combustion engine, which discharges an exhaust gas containing oxygen in excess of a stoichiometric amount in oxidation reactions of CO and HC.
- the present invention is configured to install an NOx purification catalyst containing a zeolite component, and to install an iridium (Ir) -containing catalyst downstream of the NOx purification catalyst with respect to the flow direction of the exhaust gas.
- an exhaust gas containing oxygen in excess of the stoichiometric amount in oxidation reactions of CO and HC is brought into contact with a NOx purification catalyst containing a zeolite component, It was made to make it contact.
- the exhaust gas purification catalyst for an internal combustion engine has a porous carrier made of an inorganic compound and a catalytically active component supported on the porous carrier, and the porous single body is made of aluminum (Al), cerium ( Ce), silicon (Si), titanium (Ti), at least one selected from zirconium (Zr), the catalytically active component comprises Ir, niobium (Nb), platinum (Pt), palladium (Pd) It is configured to contain at least one selected from rhodium (Rh) and gold (Au).
- CO, HC, NOx, NH 3 contained in the exhaust gas emitted from the internal combustion engine operated by injecting a lean fuel such as a diesel engine into the combustion chamber can be efficiently purified.
- the exhaust gas emitted from an internal combustion engine represented by a diesel engine mounted on a passenger car, a construction machine or the like often has an oxygen excess atmosphere containing oxygen in excess of the stoichiometric amount.
- the exhaust gases discharged from these internal combustion engines contain CO, HC, NOx, and in some cases, NH 3 components.
- the stoichiometric amount means the amount of O 2 , CO, HC when O 2 and CO, HC contained in the exhaust gas react with each other without excess.
- the meaning of the stoichiometric amount will be described in more detail.
- the present invention relates to an exhaust gas purification apparatus, an exhaust gas purification method, and an exhaust gas purification catalyst which are particularly suitable for purification of exhaust gas such as a diesel engine that exhausts exhaust gas containing oxygen in excess of the stoichiometric amount.
- the inventor of the present invention has installed a NOx purification catalyst containing a zeolite component in the exhaust gas flow path, and an Ir-containing catalyst (Ir-containing catalyst ) by installing the revealed CO in the exhaust gas, HC, NOx, can effectively purify NH 3.
- NOx purification catalyst containing the zeolite component is installed in the exhaust gas flow path and the NH 3 component is added to the exhaust gas flowing into the NOx purification catalyst, NO reacts with NH 3 according to the following reaction formula (3), and NOx is It is purified.
- the NOx purification catalyst is required to have heat resistance performance.
- the heat resistance performance of the NOx purification catalyst can be improved, and further, the temperature range where NOx can be purified can be enhanced, which is preferable.
- the zeolite component is contained in the NOx purification catalyst, it becomes clear that when HC flows into the NOx purification catalyst, the NOx purification performance is lowered and that the HC is partially oxidized to generate CO. did.
- the NH 3 component added to the exhaust gas flow path is also less likely to be consumed on the NOx purification catalyst. That is, there is a possibility that NOx, CO, is NH 3 is discharged from the NOx purification catalyst subsequent stage.
- the inventors of the present application have found that the above problems can be resolved by installing an Ir-containing catalyst downstream of the NOx purification catalyst with respect to the flow direction of the exhaust gas.
- NOx and CO react with each other by the chemical reaction represented by the following reaction formula (4) even in an atmosphere containing oxygen in excess of the stoichiometric amount, and NOx and CO are highly purified. be able to.
- Ir-containing catalysts have the ability to oxidize NH 3 . Therefore, by installing the Ir-containing catalyst downstream of the NOx purification catalyst, it is possible to purify NOx, CO, and NH 3 which flowed out from the NOx purification catalyst upstream. According to the combination of the zeolite-containing NOx purification catalyst and the Ir-containing catalyst, all nitrogen oxides such as NO, NO 2 , N 2 O, N 2 O 3 and the like can be targeted for reduction.
- all hydrocarbons such as CH 4 , C 3 H 6 , C 2 H 4 , C 2 H 2 , C 3 H 8 and the like are to be reduced. It can be done.
- Urea, cyanuric acid, melamine, biuret, and the like can be used as the NH 3 component for NOx reduction added to the exhaust gas flow path, in addition to the NH 3 gas.
- a CO, HC oxidation catalyst may be installed upstream of the NOx purification catalyst with respect to the flow direction of the exhaust gas. If the amount of HC flowing into the NOx purification catalyst is reduced by oxidizing and purifying HC, purification of NOx and NH 3 on the NOx purification catalyst containing zeolite proceeds, so NO on the Ir-containing catalyst in the latter stage -CO reaction, further advances oxidation reaction of NH 3, HC to the outside of the system, CO, NOx, the emission of NH 3 can be highly inhibited.
- diesel engine exhaust gas contains a catalyst poisoning component such as SOx soot, and it is conceivable that HC oxidation performance may be lowered by poisoning the HC oxidation catalyst, but the Ir-containing catalyst contains zeolite. If the catalyst is disposed downstream of the catalyst, the emission of HC, CO, NOx and NH 3 out of the system can be highly suppressed even in that case.
- a catalyst poisoning component such as SOx soot
- the zeolite-containing NOx purification catalyst applied to the present invention is not particularly limited as to the catalytically active component, as long as it is a zeolite-containing catalyst that can purify NOx.
- a NOx purification catalyst at least one selected from vanadium (V), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), Ce, and Zr on a zeolite carrier as a NOx purification catalyst.
- the zeolite-containing NOx purification catalyst has high heat resistance, and can effectively purify NOx even in a temperature range of 350 ° C. or higher.
- Two or more elements of V, Mn, Fe, Co, Ni, Cu, Ce, and Zr used as catalytically active components may be combined. Interaction is caused by combining two or more catalytically active components, which is considered to have high performance.
- the zeolite used as the carrier component has a high specific surface area, and therefore has the effect of improving the degree of dispersion of the catalytically active component to enhance the NOx purification performance. In addition, it is considered that the activity can be improved by being able to support the active component in the ionic state.
- the zeolite is not particularly limited, but the use of a high silica zeolite having a molar ratio of SiO 2 to Al 2 O 3 of 5 or more enhances the heat resistance. Examples of the zeolite used include ⁇ -zeolite, Y-type zeolite, ZSM-5, mordenite, ferrierite and the like.
- the total supported amount of catalytically active components V, Mn, Fe, Co, Ni, Cu, Ce, and Zr is preferably 0.1 wt% or more and 30 wt% or less in terms of element based on the zeolite carrier, more preferably It is 1.0 wt% or more and 10 wt% or less.
- the total amount of V, Mn, Fe, Co, Ni, Cu, Ce, and Zr is 0.1 wt% or less, the supporting effect becomes insufficient, and when it exceeds 30 wt%, the specific surface area of the active component itself decreases and the catalyst This is because the cost is high.
- the Ir-containing catalyst may be any catalyst containing Ir, and no particular limitation is imposed on the other constitution.
- a porous support made of an inorganic compound containing at least one selected from Al, Ce, Si, Ti, and Zr, Ir, Nb, Pt, Pd, Rh, and Au as a catalytically active component is used on a porous support. And at least one selected from the group consisting of
- Ir has the ability to react NOx and CO even in an oxygen-rich atmosphere containing oxygen in excess of the stoichiometric amount, and simultaneously purify NOx and CO. Further, Ir has an oxidation ability of NH 3, NH 3 can also be purified. Furthermore, Ir promotes the reaction between NOx and CO when SOx is contained in the reaction gas. It is considered that when the S component is present in the reaction gas, the electronic state of Ir changes.
- the combination changes the activity and activation temperature of the catalyst. Therefore, an optimum combination of components can be selected according to the properties of the exhaust gas to be applied.
- the activity and activation temperature of the catalyst can be made different.
- the activity of the Ir-containing catalyst can be improved.
- the total supported amount of Ir as the catalytically active component and Nb, Pt, Pd, Rh, and Au is preferably 0.00005 to 10 mol parts in terms of element per 2 mol parts of the porous support, and more preferably 0.0003 mol part to 0.3 mol part. If the total supported amount of Ir and Nb, Pt, Pd, Rh, and Au is less than 0.00005 mol part, the supporting effect becomes insufficient, and if it exceeds 10 mol part, the specific surface area of the active component itself decreases and the catalyst cost Because it becomes higher.
- mol part means the content ratio in conversion of the number of each component. For example, if the supported amount of B component is 1 mol part with respect to 2 mol parts of A component, regardless of the absolute amount of A component, the component A is supported at a ratio of 2 to B component at 1 mol It means being done.
- the Ir-containing catalyst can change the temperature range in which the catalyst is activated by changing the other active component or the carrier used in combination with Ir. It is considered that Ir and other elements are alloyed or the electronic state of Ir changes. Therefore, by installing these two or more types of catalysts along the exhaust gas flow path, the temperature range where NOx and CO can be purified can be expanded, and NOx and CO can be highly purified even if the exhaust gas temperature fluctuates. Can.
- a catalyst for CO and HC purification which is disposed at the front stage of the zeolite-containing NOx purification catalyst, it is possible to use as a catalytically active component on a porous carrier consisting of an inorganic compound containing at least one selected from Al, Ce, Si, Ti and Zr It is possible to use one supporting at least one selected from Pt, Pd, Rh, Au, Ir, ruthenium (Ru), and osmium (Os).
- the CO and HC purification performance can be enhanced by installing the CO and HC purification catalyst at the front stage of the zeolite-containing NOx purification catalyst. That is, by installing the CO, HC purification catalyst, the amount of HC flowing into the subsequent NOx purification catalyst can be reduced, so that high NOx purification performance can be exhibited as a whole.
- HC purification catalyst is using an oxide having a high specific surface area, It is for highly dispersing Pt, Pd, Rh, Au, Ir, Ru, and Os to improve CO and HC purification performance.
- the specific surface area of the porous carrier is preferably in the range of 30 to 800 m 2 / g, and more preferably in the range of 50 to 400 m 2 / g. When the specific surface area of the porous carrier is less than 30 m 2 / g, the required HC purification performance can not be obtained, and when it exceeds 800 m 2 / g, the catalyst cost becomes high.
- the total supported amount of catalytically active components Pt, Pd, Rh, Au, Ir, Ru, Os is preferably 0.00005 to 10 mol parts in terms of element per 2 mol parts of the porous support, and more preferably Is from 0.0003 mol part to 0.3 mol part. If the total supported amount of Pt, Pd, Rh, Au, and Ir is less than 0.00005 mol parts, the supporting effect becomes insufficient, and if it exceeds 10 mol parts, the specific surface area of the active ingredient itself decreases and the catalyst cost It is because it becomes high.
- the reaction between NOx and CO by the Ir-containing catalyst is optimally performed in a temperature range of about 350 ° C. or less.
- the temperature of the exhaust gas having passed through the zeolite-containing NOx purification catalyst may be 350 ° C. or higher.
- the NOx-CO reaction activity on the Ir-containing catalyst can be enhanced by providing a means for reducing the temperature of the exhaust gas having passed through the zeolite-containing NOx purification catalyst.
- a measure such as providing a cooler for adding water to the exhaust gas flow path can be considered.
- the HC When HC continues to flow into the zeolite-containing NOx purification catalyst, the HC may adhere to the zeolite-containing NOx purification catalyst, which may lower the NOx purification performance. In that case, the attached HC can be desorbed by flowing a gas substantially containing no hydrocarbon into the NOx purification catalyst, and the NOx purification performance of the zeolite-containing NOx purification catalyst can be recovered. In addition, when a gas containing substantially no hydrocarbon is made to flow into the NOx purification catalyst to desorb HC, partial oxidation of HC may occur to generate CO. However, even in such a case, since CO can be purified by the Ir-containing catalyst installed in the latter stage, the emission of CO and NOx to the outside of the system can be suppressed.
- does not substantially contain means that the amount of HC contained in the reaction gas is an amount small enough to desorb HC from the NOx purification catalyst, Specifically, the content is 100 ppm or less in terms of carbon element. Furthermore, by raising the temperature of the gas substantially free of HC, recovery of NOx purification performance is promoted.
- the gas temperature is preferably 350 ° C. or more and 550 ° C. or less, and more preferably 400 ° C. or more and 500 ° C. or less. At 350 ° C. or less, the desorption reaction of HC does not proceed, and at 550 ° C. or more, the performance of the catalyst is reduced due to heat load.
- the controller operation of the engine is controlled by a controller to promote combustion in the engine and reduce the amount of HC in the exhaust gas. (See FIG. 11).
- the amount of NOx in the exhaust gas may increase, but since the NOx in the exhaust gas can be highly purified by the zeolite-containing NOx purification catalyst and the Ir-containing catalyst, the amount of NOx released to the atmosphere increases. There is nothing to do.
- the NOx purification performance of the zeolite-containing NOx purification catalyst is lowered by the accumulation of S component in the exhaust gas in the zeolite-containing NOx purification catalyst.
- the temperature of the exhaust gas flowing into the zeolite-containing NOx purification catalyst is set to 350 ° C. or more and 550 ° C. or less, and the gas containing substantially no HC is made to flow into the zeolite-containing NOx purification catalyst. It is possible to desorb the S component from the catalyst, and as a result, it is possible to recover the NOx purification performance.
- the S component is desorbed from the CO, HC purification catalyst, if more CO than HC is present in the exhaust gas, the S component desorption may be promoted.
- the zeolite-containing NOx purification catalyst, the Ir-containing catalyst, the porous carrier used for the CO, HC purification catalyst, or the catalytically active component may be supported on a substrate.
- a heat resistant metal substrate such as cordierite, a ceramic made of Si-Al-O or a stainless steel, which has been conventionally used, is suitable.
- the amount of the porous carrier to be supported is preferably 10 g or more and 300 g or less with respect to 1 liter of the substrate. If it is 10 g or less, the dispersion of the noble metal is reduced, and the catalytic activity is reduced. On the other hand, when it is 300 g or more, in the case where the base material has a honeycomb shape, problems such as clogging of the gas flow path easily occur.
- the zeolite-containing NOx purification catalyst, Ir-containing catalyst, CO, HC purification catalyst can be prepared by, for example, physical preparation methods such as impregnation method, kneading method, coprecipitation method, sol-gel method, ion exchange method, vapor deposition method, A preparation method using a chemical reaction or the like can be used. In particular, by using a preparation method utilizing a chemical reaction, the contact between the raw material of the catalytically active component and the porous carrier becomes strong, and sintering (crystal growth) of the catalytically active component can be prevented.
- zeolite-containing NOx purification catalysts As starting materials for zeolite-containing NOx purification catalysts, Ir-containing catalysts, CO, HC purification catalysts, various compounds such as nitrate compounds, chlorides, acetate compounds, complex compounds, hydroxides, carbonate compounds, organic compounds, metals, Metal oxides can be used.
- the catalyst component is uniformly supported by preparing it by a co-impregnation method using an impregnating solution in which the active component is present in the same solution. be able to.
- the shapes of the zeolite-containing NOx purification catalyst, the Ir-containing catalyst, and the CO and HC purification catalyst can be appropriately adjusted according to the application.
- a honeycomb shape obtained by coating the purification catalyst of the present invention on a honeycomb structure made of various base materials such as cordierite, Si-Al-O, SiC, stainless steel, etc., pellet-like, plate-like, granular, Powder form etc. are mentioned.
- honeycomb shape it is preferable to use a structure made of co-gelite or Si-Al-O, but if there is a possibility that the catalyst temperature may increase, a group which is difficult to react with the catalytically active component It is preferable to use a material (for example, a base material such as a metal honeycomb containing Fe as a main component).
- the honeycomb may be formed of only the porous carrier and the catalytically active component.
- soot and the like in the exhaust gas can be purified.
- the exhaust gas purification apparatus, the exhaust gas purification method, and the exhaust gas purification catalyst according to the present invention are particularly effective for purification of exhaust gas containing oxygen in excess of the stoichiometric amount in oxidation reactions of CO and HC. It is particularly preferred if the oxygen is in excess of the stoichiometric amount.
- a slurry prepared by adding the obtained Fe / zeolite powder and alumina sol to water was coated on a cordierite honeycomb (300 cells / iNC 2 ) and dried by circulating hot air at 150 ° C. for 15 minutes. Furthermore, the obtained sample was fired at 600 ° C. for 1 hour in the atmosphere using an electric furnace to obtain a honeycomb catalyst coated with 250 g of Fe / zeolite per 1 L of apparent volume. This catalyst is referred to as example catalyst 1.
- the performance of the example catalyst 1 was evaluated under the following conditions.
- a honeycomb catalyst having a volume of 6 cm 3 was fixed in a quartz glass reaction tube, and this reaction tube was placed in an electric furnace.
- the reaction gas introduced into the reaction tube has a composition simulating the exhaust gas in an oxygen excess atmosphere, and has 150 ppm of NOx, 180 ppm of NH 3 , 6% of CO 2 , 10% of O 2 , 6% of H 2 O, N 2 : As a residual.
- This gas is used as a reference gas.
- the NOx purification performance of the example catalyst 1 was estimated by the NOx purification rate shown in the following equation.
- the volume space velocity was 45,000 / H.
- the gas temperature was controlled to be heated from 200 ° C. to 500 ° C. while flowing the reaction gas, and the NOx purification performance was measured.
- NOx purification rate (%) ((NOx concentration flowing into the catalyst)-(NOx concentration flowing out from the catalyst)) / (NO concentration flowing into the catalyst) x 100 ⁇ Performance Evaluation of Zeolite-Containing NOx Purification Catalyst Including the Influence of C 3 H 6 >
- the NOx purification performance of the zeolite-containing NOx purification catalyst was measured in the same manner as in the case of using the reference gas except that 300 ppm or 3333 ppm of C 3 H 6 was added to the reaction gas. Furthermore, the rate of conversion of C 3 H 6 to CO and CO 2 over the catalyst was estimated by the HC conversion rate shown in the following equation.
- the NOx purification rate in the temperature range of 250 ° C. or less and 400 ° C. or more was reduced as compared with the case of using the reference gas.
- the NOx purification rate further decreased significantly. Focusing on the activity at 400 ° C., the NOx purification rate dropped significantly from 93% to 25% when compared to the reference gas. From this result, it is clear that the NOx purification rate decreases as the HC concentration in the exhaust gas increases.
- ⁇ HC conversion of zeolite containing NOx purification catalyst> 2 the reference gas, the reference gas to a gas obtained by adding C 3 H 6 of 300 ppm, and in the case of using a gas added C 3 H 6 of 3333ppm based gas, the HC conversion rate of example catalyst 1 Show.
- CO is generated regardless of the C 3 H 6 concentration in the gas.
- the HC ⁇ CO conversion at 400 ° C. is 13%, and it can be seen that about 1300 ppm of CO is generated.
- This Ir-containing catalyst is referred to as example catalyst 3. Furthermore, after impregnating each of the example catalysts 2 and 3 with Nb 2 O 5 sol aqueous solution, they were dried at 120 ° C. and subsequently calcined at 600 ° C. for 1 hour. The amount of Nb added was the same as that of the amount of Ir added.
- the Ir-containing catalysts are referred to as example catalysts 4 and 5, respectively. Table 1 below shows the composition list of the Ir-containing catalyst.
- the reaction gas introduced into the reaction tube is a model gas that simulates the exhaust gas in an oxygen excess atmosphere, and its composition is NOx: 150 ppm, CO: 1500 ppm, O 2 : 3%, SO 2 : 3 ppm, N 2 : residual And Volume space velocity (F / V) was set to 200,000 / H.
- the purification performances of the example catalysts 2 to 5 were determined by determining the NOx and CO purification rates according to the following formula.
- FIG. 3 shows the NOx purification rates of the example catalysts 2 to 5. As is clear from this figure, it was found that the NOx purification rate of about 40% was obtained for each catalyst, and that NO x and CO reacted even in the presence of oxygen to purify NOx.
- the temperature range showing the highest activity is 300 ° C. for Example catalysts 2 and 4, 280 ° C. to 300 ° C. for Example catalyst 3 and 260 ° C. to 270 ° C. for Example catalyst 5, and the presence or absence of heat treatment to SiO 2 It can be seen that the difference differs depending on the presence or absence of Nb addition.
- FIG. 4 shows the CO purification rate of the example catalysts 2 to 5. As is clear from this figure, it is clear that for each catalyst, the CO purification rate exceeds 60% in the temperature range where the NOx purification rate reaches about 40%, and that not only NOx but also CO is also purified. .
- the Ir-containing catalyst can simultaneously purify NOx and CO in an oxygen-rich atmosphere. It is also clear that the use of a catalyst containing Ir and Nb can further enhance the purification rate of NOx and CO.
- the oxidation rate of NH 3 was evaluated for the Ir-containing catalyst.
- reaction gas the thing which remove
- the NH 3 oxidation rate of the Ir-containing catalyst was estimated by the following equation.
- FIG. 5 shows the NH 3 purification rates of the example catalysts 2 and 3 . It can be seen that the NH 3 purification rate exceeds 60% at both of the catalysts above 300 ° C., showing high performance.
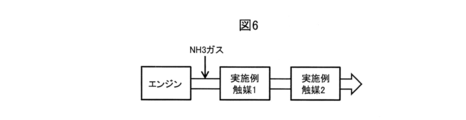
- Example catalyst 1 and Example catalyst 2 are installed in this order from the upstream side of the flow direction of the exhaust gas in the exhaust gas flow path of the engine. It is characterized in that NH 3 gas is added to the upstream side of 1.
- NH 3 gas is added to the upstream side of 1.
- the example catalyst 2 can purify not only NOx and CO but also NH 3 and therefore exhibits high purification performance to NOx, CO, and NH 3 as a whole. it can.
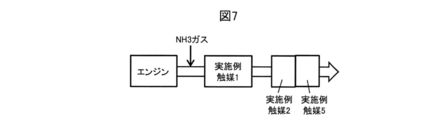
- Example catalyst 1 Example catalyst 2 and Example catalyst 5 are installed in this order from the upstream side of the flow direction of the exhaust gas in the exhaust gas flow path of the engine.
- NH 3 gas is added to the upstream side of the example catalyst 1.
- the example catalysts 2 and 5 exhibit a NOx purification rate of around 40% at around 300 ° C. and around 260 ° C., respectively. Therefore, by arranging the example catalyst 2 and the example catalyst 5 at the latter stage of the example catalyst 1, the temperature range showing a high NOx purification rate can be expanded, and even if the exhaust gas temperature changes, the NOx and CO are effectively purified. can do.
- Example catalyst 1 and Example catalyst 2 are installed in this order from the upstream side of the flow direction of the exhaust gas in the exhaust gas flow path of the engine.
- a cooling device for spraying water into the exhaust gas flow path is provided between the catalyst 1 of the embodiment and the catalyst 2 of the embodiment while adding NH 3 gas on the upstream side of the above.
- it is about 350 ° C. or more that CO is generated from HC in the example catalyst 1.
- the purification of NOx and CO in the example catalyst 2 proceeds at 300 ° C. or less.
- the cooling water supply device by spraying the cooling water from the cooling water supply device to lower the exhaust gas temperature at the rear stage of the example catalyst 1, the NOx and CO purification reaction on the example catalyst 2 can be promoted.
- the installation interval between the example catalyst 1 and the example catalyst 2 is sufficiently large, the exhaust gas temperature falls during this time, and the temperature range is optimal for both catalysts, so the cooling water supply device may be omitted. it can.
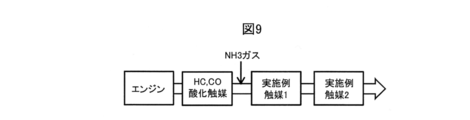
- the fourth example of the exhaust gas purification apparatus includes an HC, CO oxidation catalyst, an example catalyst 1, an example catalyst 2 and an example catalyst 2 in the exhaust gas flow path of the engine from the upstream side in the exhaust gas flow direction. It is characterized in that NH 3 gas is added between the HC, CO oxidation catalyst and the example catalyst 1 while being installed in order.
- NH 3 gas is added between the HC, CO oxidation catalyst and the example catalyst 1 while being installed in order.
- HC and CO oxidation catalysts are disposed at the front stage of the example catalyst 1, HC and CO flowing into the example catalyst 1 can be reduced, and NOx on the example catalyst 1 can be reduced.
- the purification reaction can be promoted, and NOx released to the outside of the system can be reduced.
- the HC, CO oxidation catalyst is poisoned by SOx or the like in the exhaust gas and the HC, CO oxidation performance is lowered, NOx and CO can be purified by the catalyst of Example 2.
- ⁇ Preparation of HC, CO oxidation catalyst> A slurry prepared by adding alumina sol and water to an Al 2 O 3 powder obtained by firing boehmite powder at 600 ° C. for 5 hours in an electric furnace under the atmosphere is a cordierite honeycomb (300 cells / After coating to inc 2 ), it was dried by circulating 150 ° C. hot air for 15 minutes. Furthermore, 200 g of Al 2 O 3 powder was coated per 1 liter of apparent volume of the honeycomb by firing the obtained sample in an electric furnace at 600 ° C. for 1 hour.
- the resulting alumina-coated honeycomb is impregnated with a mixed solution of dinitrodiammine Pt nitric acid solution and Pd nitrate solution, passed through hot air at 150 ° C. for 15 minutes, dried, and fired at 600 ° C. for 1 hour in an electric furnace. went.
- PtPd / Al 2 O 3 honeycomb catalyst powder was obtained in which Pt and Pd each contained 1.5 g and 0.5 g in terms of element per liter of honeycomb.
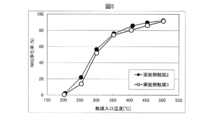
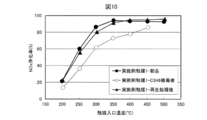
- ⁇ Regeneration treatment of zeolite-containing NOx purification catalyst Part 1> After evaluating NOx purification activity using the reference gas with respect to the example catalyst 1, while maintaining the catalyst inlet temperature at 400 ° C., a gas added with 3333 ppm of C 3 H 6 is allowed to flow for 30 minutes to carry out the catalyst Poisoned to C 3 H 6 Thereafter, the catalyst inlet temperature was lowered to 200 ° C., and the NOx purification activity was evaluated again using the reference gas. Further, the catalyst inlet temperature was maintained at 450 ° C. for 60 minutes while the reference gas was flowing to carry out the catalyst regeneration treatment. Thereafter, the catalyst inlet temperature was lowered to 200 ° C., and the NOx purification activity was evaluated again using the reference gas. The test results are shown in FIG.
- example catalyst 1 after C 3 H 6 poisoning although about 20 points activity compared to new the entire temperature range is reduced, by performing a reproduction process, a new par Recover activity. Therefore, it is understood that the activity of the zeolite-containing NOx purification catalyst can be recovered by circulating the gas whose HC concentration is reduced even if HC flows into the example catalyst 1 and the NOx purification activity decreases.
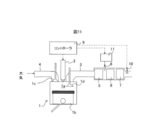
- the exhaust gas purification apparatus of the present embodiment purifies the exhaust gas of the diesel engine 1 and includes a controller 9 for controlling the drive of the engine 1.
- the diesel engine 1 obtains power by compressing the air in the combustion chamber (cylinder) 1a with a piston 1b to a high temperature, supplying fuel from the fuel injection device 2 to the compressed air and causing natural ignition.
- the diesel engine 1 also includes an intake valve 1 c between the intake pipe 4 and the combustion chamber 1 a, and an exhaust valve 1 d between the combustion chamber 1 a and the exhaust pipe 3. Although one intake valve 1c and one intake valve 1d are shown in FIG.
- the number of valves is not limited to this, and a plurality of intake valves 1c and exhaust valves 1d may be provided as necessary.
- Purification catalysts 5, 6, 7 are installed on the outlet side of the exhaust pipe 3, and the exhaust gas discharged from the diesel engine 1 is supplied to the purification catalysts 5, 6, 7 and purified by the purification catalysts 5, 6, 7. After being released to the outside.
- a CO, HC oxidation catalyst, as the purification catalyst 6, a zeolite-containing NOx purification catalyst, and as the purification catalyst 7, an Ir-containing catalyst are installed.
- reference numeral 11 is an NH 3 tank
- reference numeral 8 is a NH 3 injection nozzle for injecting NH 3 between CO, HC oxidation catalyst 5 and zeolite-containing NOx purification catalyst 6, and reference numeral 10 is HC, CO, NOx The sensor is shown.
- the controller 9 determines the degree of deterioration of the HC, CO, and NOx purification performance based on the output signal of the sensor 10 and determines that the HC, CO, and NOx purification performance is lower than a predetermined value,
- the combustion in the engine 1 is promoted to raise the temperature of the exhaust gas flowing into the purification catalysts 5 and 6, and the concentration of HC in the exhaust gas flowing into the purification catalysts 5 and 6 is reduced.
- the controller 9 controls the combustion state of the engine 1 By doing this, the HC and CO purification performance of the CO and HC oxidation catalyst 5 and the zeolite-containing NOx purification catalyst 6 can be recovered.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Crystallography & Structural Chemistry (AREA)
- Combustion & Propulsion (AREA)
- Health & Medical Sciences (AREA)
- General Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Toxicology (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- General Chemical & Material Sciences (AREA)
- Analytical Chemistry (AREA)
- Environmental & Geological Engineering (AREA)
- Biomedical Technology (AREA)
- Catalysts (AREA)
- Exhaust Gas After Treatment (AREA)
- Exhaust Gas Treatment By Means Of Catalyst (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013026934A JP6126858B2 (ja) | 2013-02-14 | 2013-02-14 | 内燃機関の排ガス浄化装置 |
| JP2013-026934 | 2013-02-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014125934A1 true WO2014125934A1 (ja) | 2014-08-21 |
Family
ID=51353943
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/052138 Ceased WO2014125934A1 (ja) | 2013-02-14 | 2014-01-30 | 内燃機関の排ガス浄化装置、排ガス浄化方法及び排ガス浄化触媒 |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP6126858B2 (enExample) |
| WO (1) | WO2014125934A1 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MY191861A (en) * | 2016-07-29 | 2022-07-18 | Basf Corp | Process for the preparation of a zeolitic material having a fau-type framework structure and use thereof in the selective catalytic reduction of nox |
| KR102051857B1 (ko) * | 2017-09-29 | 2019-12-04 | 한국화학연구원 | 고성능 질소산화물 저감 촉매 및 그 제조방법 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001073745A (ja) * | 1999-07-02 | 2001-03-21 | Nissan Motor Co Ltd | 排気ガス浄化システム |
| JP2002285831A (ja) * | 2001-01-27 | 2002-10-03 | Omg Ag & Co Kg | ディーゼルエンジンの排ガスライン中の触媒の触媒活性の再生法 |
| JP2011525950A (ja) * | 2008-06-27 | 2011-09-29 | ユミコア・アクチエンゲゼルシャフト・ウント・コムパニー・コマンディットゲゼルシャフト | ディーゼル排ガスを浄化する方法及び装置 |
| JP2012052546A (ja) * | 2003-08-05 | 2012-03-15 | Basf Catalysts Llc | Scr濾過器を用いた排気処理システムおよび方法 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4250856B2 (ja) * | 2000-05-24 | 2009-04-08 | 三菱自動車工業株式会社 | 筒内噴射型内燃機関 |
-
2013
- 2013-02-14 JP JP2013026934A patent/JP6126858B2/ja not_active Expired - Fee Related
-
2014
- 2014-01-30 WO PCT/JP2014/052138 patent/WO2014125934A1/ja not_active Ceased
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001073745A (ja) * | 1999-07-02 | 2001-03-21 | Nissan Motor Co Ltd | 排気ガス浄化システム |
| JP2002285831A (ja) * | 2001-01-27 | 2002-10-03 | Omg Ag & Co Kg | ディーゼルエンジンの排ガスライン中の触媒の触媒活性の再生法 |
| JP2012052546A (ja) * | 2003-08-05 | 2012-03-15 | Basf Catalysts Llc | Scr濾過器を用いた排気処理システムおよび方法 |
| JP2011525950A (ja) * | 2008-06-27 | 2011-09-29 | ユミコア・アクチエンゲゼルシャフト・ウント・コムパニー・コマンディットゲゼルシャフト | ディーゼル排ガスを浄化する方法及び装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2014155888A (ja) | 2014-08-28 |
| JP6126858B2 (ja) | 2017-05-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7206045B2 (ja) | 排気システム用の亜酸化窒素除去触媒 | |
| KR101974704B1 (ko) | 선택적 암모니아 산화를 위한 이원기능 촉매 | |
| EP3027309B1 (en) | Ammonia slip catalyst | |
| JP6755306B2 (ja) | すす触媒とscr触媒を有する触媒フィルタ | |
| CN103442805B (zh) | 氨氧化催化剂以及使用了其的废气净化装置和废气净化方法 | |
| US8062601B2 (en) | Emission SCR NOX aftertreatment system having reduced SO3 generation and improved durability | |
| WO2012002052A1 (ja) | 選択還元型触媒を用いた排気ガス浄化装置及び排気ガス浄化方法 | |
| EP3548177B1 (en) | Nox adsorber catalyst | |
| EP3024574A1 (en) | Tungsten/titania oxidation catalyst | |
| US10408102B2 (en) | Oxidation catalyst device for exhaust gas purification | |
| CN106457220A (zh) | 废气处理系统 | |
| JP6126858B2 (ja) | 内燃機関の排ガス浄化装置 | |
| JP4704964B2 (ja) | NOx浄化システム及びNOx浄化方法 | |
| JP2015183587A (ja) | 熱機関の排ガス浄化装置、排ガス浄化方法及び排ガス浄化触媒 | |
| US12403425B2 (en) | Exhaust gas treatment system | |
| JP6325042B2 (ja) | 熱機関の排ガス浄化装置 | |
| JP2007239616A (ja) | 排ガスの浄化装置及び排ガスの浄化方法,浄化触媒 | |
| CN121100220A (zh) | 用于处理废气的系统 | |
| JP2013244483A (ja) | 熱機関の排ガス浄化装置及び排ガス浄化方法 | |
| JP6210849B2 (ja) | 熱機関の排ガス浄化装置および排ガス浄化方法 | |
| JP2016217357A (ja) | 熱機関の排ガス浄化装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14751396 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14751396 Country of ref document: EP Kind code of ref document: A1 |