WO2013168588A1 - 複合アミン吸収液、co2又はh2s又はその双方の除去装置及び方法 - Google Patents
複合アミン吸収液、co2又はh2s又はその双方の除去装置及び方法 Download PDFInfo
- Publication number
- WO2013168588A1 WO2013168588A1 PCT/JP2013/062259 JP2013062259W WO2013168588A1 WO 2013168588 A1 WO2013168588 A1 WO 2013168588A1 JP 2013062259 W JP2013062259 W JP 2013062259W WO 2013168588 A1 WO2013168588 A1 WO 2013168588A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- absorption
- amine
- amino
- solution
- tower
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/46—Removing components of defined structure
- B01D53/48—Sulfur compounds
- B01D53/52—Hydrogen sulfide
- B01D53/526—Mixtures of hydrogen sulfide and carbon dioxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/14—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by absorption
- B01D53/1425—Regeneration of liquid absorbents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/14—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by absorption
- B01D53/1456—Removing acid components
- B01D53/1462—Removing mixtures of hydrogen sulfide and carbon dioxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/14—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by absorption
- B01D53/1493—Selection of liquid materials for use as absorbents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/74—General processes for purification of waste gases; Apparatus or devices specially adapted therefor
- B01D53/77—Liquid phase processes
- B01D53/78—Liquid phase processes with gas-liquid contact
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/22—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising organic material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2252/00—Absorbents, i.e. solvents and liquid materials for gas absorption
- B01D2252/20—Organic absorbents
- B01D2252/204—Amines
- B01D2252/20405—Monoamines
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2252/00—Absorbents, i.e. solvents and liquid materials for gas absorption
- B01D2252/20—Organic absorbents
- B01D2252/204—Amines
- B01D2252/2041—Diamines
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2252/00—Absorbents, i.e. solvents and liquid materials for gas absorption
- B01D2252/20—Organic absorbents
- B01D2252/204—Amines
- B01D2252/20421—Primary amines
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2252/00—Absorbents, i.e. solvents and liquid materials for gas absorption
- B01D2252/20—Organic absorbents
- B01D2252/204—Amines
- B01D2252/20426—Secondary amines
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2252/00—Absorbents, i.e. solvents and liquid materials for gas absorption
- B01D2252/20—Organic absorbents
- B01D2252/204—Amines
- B01D2252/20436—Cyclic amines
- B01D2252/20447—Cyclic amines containing a piperazine-ring
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2252/00—Absorbents, i.e. solvents and liquid materials for gas absorption
- B01D2252/20—Organic absorbents
- B01D2252/204—Amines
- B01D2252/20478—Alkanolamines
- B01D2252/20484—Alkanolamines with one hydroxyl group
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2252/00—Absorbents, i.e. solvents and liquid materials for gas absorption
- B01D2252/50—Combinations of absorbents
- B01D2252/504—Mixtures of two or more absorbents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/30—Sulfur compounds
- B01D2257/304—Hydrogen sulfide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/50—Carbon oxides
- B01D2257/504—Carbon dioxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2258/00—Sources of waste gases
- B01D2258/02—Other waste gases
- B01D2258/0283—Flue gases
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02C—CAPTURE, STORAGE, SEQUESTRATION OR DISPOSAL OF GREENHOUSE GASES [GHG]
- Y02C20/00—Capture or disposal of greenhouse gases
- Y02C20/40—Capture or disposal of greenhouse gases of CO2
Definitions
- the present invention relates to an apparatus and method for removing a complex amine absorbent, CO 2 and / or H 2 S, or both.
- an absorption tower for removing CO 2 or H 2 S or both by bringing a gas containing CO 2 or H 2 S or both into contact with an absorbing solution, and CO 2 or H 2 S or It has a regenerator for reproducing a solution which has absorbed the both, the solution was regenerated by removing CO 2 or H 2 S or both in the regeneration tower is reused in the absorption tower, CO 2 or H 2 S or Both of the removing apparatuses are the removing apparatus for CO 2 and / or H 2 S, or both, characterized by using any one of the first to fifth complex amine absorbing liquids.
- a seventh aspect of the present invention is, CO 2 or H 2 S or by contacting the gas with absorbing liquid containing both of CO 2 or H 2 and S or removing the both, CO 2 or H 2 S or both,
- a method for removing CO 2 and / or H 2 S or both wherein the absorbed solution is regenerated, CO 2 and / or H 2 S or both are removed by a regeneration tower, and the regenerated solution is reused by an absorption tower,
- the present invention provides a method for removing CO 2 and / or H 2 S or both, wherein CO 2 and / or H 2 S or both are removed using any one of the first to fifth complex amine absorbing liquids.
- absorption is achieved by dissolving 1) monoethanolamine (MEA) and 2) a primary amine having high steric hindrance represented by the following formula (1) in water to obtain an absorbing solution.
- MEA monoethanolamine
- a primary amine having high steric hindrance represented by the following formula (1) in water to obtain an absorbing solution.
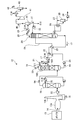
- FIG. 1 is a schematic diagram illustrating the configuration of the CO 2 recovery apparatus according to the first embodiment.
- FIG. 2 is a graph showing the relationship between the primary sterically hindered amine / MEA weight ratio when the total amine concentration in Test Example 1 is 45% by weight and the saturated CO 2 absorption capacity relative ratio.
- FIG. 3 is a relationship diagram between the primary sterically hindered amine / MEA weight ratio and the relative ratio of saturated CO 2 absorption capacity when the total amine concentration in Test Example 1 is 35% by weight.
- FIG. 4 is a graph showing the relationship between the AMP / MEA weight ratio when the total amine concentration in Test Example 2 is 35% by weight and the relative difference ratio of saturated CO 2 concentration.
- FIG. 1 is a schematic diagram illustrating the configuration of the CO 2 recovery apparatus according to the first embodiment.
- FIG. 2 is a graph showing the relationship between the primary sterically hindered amine / MEA weight ratio when the total amine concentration in Test Example 1 is 45% by weight and the saturated
- FIG. 5 is a graph showing the relationship between the AMP / MEA weight ratio when the total amine concentration in Test Example 2 is 40% by weight and the saturated CO 2 concentration difference relative ratio.
- FIG. 6 is a graph showing the relationship between the AMP / MEA weight ratio when the total amine concentration in Test Example 2 is 45% by weight and the relative difference ratio of saturated CO 2 concentration.
- FIG. 7 is a graph showing the relationship between the polyamine / primary amine weight ratio of Test Example 3 and the reaction rate index.
- 1) monoethanolamine (MEA) and 2) a primary amine having a high steric hindrance represented by the above formula (1) are dissolved in water to form an absorbing solution.
- MEA monoethanolamine
- a primary amine having a high steric hindrance represented by the above formula (1) are dissolved in water to form an absorbing solution.
- the total amine concentration in the composite amine absorbing solution is preferably 30 to 70% by weight. This is because if it is outside this range, it will not function well as an absorbent.
- the weight ratio of 2) primary amine having high steric hindrance and 1) monoethanolamine (MEA) is in the range of 0.3 to 2.5, preferably 0.3 to 1.2.
- the range is more preferable, and the range of 0.3 to 0.7 is more preferable. This is because, as shown in a test example to be described later, the absorption performance is lower than the case where 30% by weight of the MEA concentration generally used in the past is used as a standard for the absorption performance. This ratio is changed according to the total amine concentration. When the total amine concentration is 30% by weight, the value approaches 0.3.
- the primary or secondary linear polyamine group includes ethylenediamine (EDA), N, N′-dimethylethylenediamine (DMEDA), N, N′-diethylethylenediamine (DEEDA), propanediamine (PDA), N, N '-Dimethylpropanediamine (DMPDA), and the cyclic polyamine group includes piperazine (PZ), 1-methylpiperazine (1MPZ), 2-methylpiperazine (2MPZ), 2,5-dimethylpiperazine (DMPZ), 1- (2-Aminoethyl) piperazine (AEPRZ) and 1- (2-hydroxyethyl) piperazine (HEP) are preferred.
- the absorption temperature of the absorption tower at the time of contact with the exhaust gas containing CO 2 or the like is usually preferably in the range of 30 to 80 ° C.
- a corrosion inhibitor, a deterioration inhibitor, etc. are added to the absorption liquid used by this invention as needed.
- the absorbent regenerator 20 in reproducing the CO 2 absorbing liquid to remove CO 2 (hereinafter, also referred to as "lean solvent”.) 17 as a CO 2 absorbing solution in the CO 2 absorber 18 Reuse.
- reference numeral 13a denotes a flue
- 13b denotes a chimney
- 34 denotes steam condensed water.
- the CO 2 recovery device may be provided later in order to recover CO 2 from an existing exhaust gas source, or may be provided at the same time as a new exhaust gas source.
- a damper that can be opened and closed is installed in the exhaust gas 14 line, and is opened when the CO 2 recovery device 12 is in operation. Although the exhaust gas source is operating, it is set to close when the operation of the CO 2 recovery device 12 is stopped.
- the exhaust gas 14 from the industrial combustion facility 13 such as a boiler or gas turbine containing CO 2 is pressurized by the exhaust gas blower 22, and then the exhaust gas cooling device. 16, where it is cooled by cooling water 15 and sent to a CO 2 absorption tower 18.
- the reflux water 31 separated and refluxed from the CO 2 entrained gas 28 accompanied by water vapor by the separation drum 30 is supplied to the upper part of the absorption liquid regeneration tower 20 and the circulating wash water 21 side by the reflux water circulation pump 35.
- the regenerated CO 2 absorbing solution (lean solution) 17 is cooled by the rich solution 19 in the rich / lean solution heat exchanger 25, subsequently pressurized by the lean solution pump 32, and further by the lean solution cooler 33. After cooling, it is supplied into the CO 2 absorption tower 18.
- the outline is only described, and a part of the attached devices is omitted.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Analytical Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Environmental & Geological Engineering (AREA)
- Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Organic Chemistry (AREA)
- Gas Separation By Absorption (AREA)
- Treating Waste Gases (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/399,107 US10137407B2 (en) | 2012-05-11 | 2013-04-25 | Complex amine absorbent, and device and method for removing one or both of CO2 and H2S |
| AU2013258901A AU2013258901B2 (en) | 2012-05-11 | 2013-04-25 | Composite amine absorbing solution, and device and method for removing CO2, H2S, or both |
| EP13788078.7A EP2848298B1 (en) | 2012-05-11 | 2013-04-25 | Composite amine absorbing solution and method for removing co2, h2s, or both |
| CA2872593A CA2872593C (en) | 2012-05-11 | 2013-04-25 | Complex amine absorbent, and device and method for removing one or both of co2 and h2s |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012-109948 | 2012-05-11 | ||
| JP2012109948A JP5986796B2 (ja) | 2012-05-11 | 2012-05-11 | 複合アミン吸収液、co2又はh2s又はその双方の除去装置及び方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013168588A1 true WO2013168588A1 (ja) | 2013-11-14 |
Family
ID=49550634
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/062259 Ceased WO2013168588A1 (ja) | 2012-05-11 | 2013-04-25 | 複合アミン吸収液、co2又はh2s又はその双方の除去装置及び方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US10137407B2 (enExample) |
| EP (1) | EP2848298B1 (enExample) |
| JP (1) | JP5986796B2 (enExample) |
| AU (1) | AU2013258901B2 (enExample) |
| CA (1) | CA2872593C (enExample) |
| WO (1) | WO2013168588A1 (enExample) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6841676B2 (ja) | 2017-01-31 | 2021-03-10 | 三菱重工エンジニアリング株式会社 | 複合アミン吸収液、co2又はh2s又はその双方の除去装置及び方法 |
| GB201813839D0 (en) * | 2018-06-26 | 2018-10-10 | Carbon Clean Solutions Ltd | System and process of capturing carbon dioxide from flue gases |
| CN113457382A (zh) * | 2021-07-22 | 2021-10-01 | 浙江天地环保科技股份有限公司 | 一种低粘度的co2捕集混合吸收剂配方 |
| AU2022339829A1 (en) * | 2021-08-31 | 2024-04-11 | Yale University | Systems and methods for carbon sequestration using enhanced weathering |
| CN116371146A (zh) * | 2023-02-24 | 2023-07-04 | 国家能源集团新能源技术研究院有限公司 | 一种二氧化碳吸收剂及其制备方法与应用 |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6119286B2 (enExample) * | 1975-11-18 | 1986-05-16 | Basf Ag | |
| JPH04349921A (ja) * | 1991-05-27 | 1992-12-04 | Mitsubishi Heavy Ind Ltd | 排煙脱炭吸収剤 |
| JPH06343858A (ja) * | 1993-06-08 | 1994-12-20 | Mitsubishi Heavy Ind Ltd | 二酸化炭素吸収剤 |
| JPH0751537A (ja) | 1993-06-30 | 1995-02-28 | Mitsubishi Heavy Ind Ltd | Co2 含有ガス中のco2 を除去する方法 |
| JP2007325996A (ja) * | 2006-06-06 | 2007-12-20 | Mitsubishi Heavy Ind Ltd | 吸収液、co2又はh2s又はその双方の除去装置及び方法 |
| JP2008013400A (ja) | 2006-07-05 | 2008-01-24 | Research Institute Of Innovative Technology For The Earth | 排ガス中の二酸化炭素を吸収及び脱離して回収する方法 |
| JP2008307519A (ja) | 2007-06-18 | 2008-12-25 | Mitsubishi Heavy Ind Ltd | 吸収液、吸収液を用いたco2又はh2s除去装置及び方法 |
| WO2010136425A1 (en) * | 2009-05-26 | 2010-12-02 | Basf Se | Process for recovery of carbon dioxide from a fluid stream, in particular from syngas |
| JP4690659B2 (ja) | 2004-03-15 | 2011-06-01 | 三菱重工業株式会社 | Co2回収装置 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU506199B2 (en) | 1975-06-26 | 1979-12-20 | Exxon Research And Engineering Company | Absorbtion of co2 from gaseous feeds |
| CA1091429A (en) * | 1977-01-06 | 1980-12-16 | Guido Sartori | Process for removing carbon dioxide containing acidic gases from gaseous mixtures |
| US4100257A (en) * | 1977-02-14 | 1978-07-11 | Exxon Research & Engineering Co. | Process and amine-solvent absorbent for removing acidic gases from gaseous mixtures |
| US4100099A (en) * | 1977-03-28 | 1978-07-11 | The Dow Chemical Company | Quaternary salt-polyamine inhibitor for sour gas conditioning solutions |
| JPH0644972B2 (ja) * | 1988-03-11 | 1994-06-15 | ユニオン・カーバイド・コーポレーション | エチレンアミン促進剤を含有する第三級アルカノールアミン吸収剤及びその使用法 |
| JP2809368B2 (ja) * | 1993-04-05 | 1998-10-08 | 関西電力株式会社 | 燃焼排ガス中の二酸化炭素の回収方法 |
| DE19828977A1 (de) * | 1998-06-29 | 1999-12-30 | Basf Ag | Verfahren zur Entfernung saurer Gasbestandteile aus Gasen |
| US6165433A (en) * | 1999-06-10 | 2000-12-26 | Praxair Technology, Inc. | Carbon dioxide recovery with composite amine blends |
| GB2427405A (en) * | 2004-04-07 | 2006-12-27 | Pharmacyclics Inc | Novel hydroxamates as therapeutic agents |
| WO2007093615A1 (de) * | 2006-02-14 | 2007-08-23 | Basf Se | Umrüstung von anlagen zur sauergasentfernung |
| WO2007104800A1 (de) * | 2006-03-16 | 2007-09-20 | Basf Se | Verfahren zum inkontaktbringen zweier phasen, deren kontakt von wärmeentwicklung begleitet ist |
| US7901488B2 (en) * | 2006-10-04 | 2011-03-08 | Board Of Regents, The University Of Texas System | Regeneration of an aqueous solution from an acid gas absorption process by matrix stripping |
| US8318117B2 (en) * | 2008-06-23 | 2012-11-27 | Basf Se | Absorption medium and method for removing sour gases from fluid streams, in particular from flue gases |
| KR101035148B1 (ko) * | 2008-10-28 | 2011-05-17 | 한국전력공사 | 산성가스 분리용 흡수제 |
| DE102010004073A1 (de) * | 2010-01-05 | 2011-07-07 | Uhde GmbH, 44141 | CO2-Entfernung aus Gasen mit niedrigen CO2-Partialdrücken mittels 1,2 Diaminopropan |
| WO2011121635A1 (ja) * | 2010-03-29 | 2011-10-06 | 株式会社 東芝 | 酸性ガス吸収剤、酸性ガス除去装置および酸性ガス除去方法 |
| US9266102B2 (en) * | 2013-03-29 | 2016-02-23 | The University Of Kentucky Research Foundation | Catalysts and methods of increasing mass transfer rate of acid gas scrubbing solvents |
-
2012
- 2012-05-11 JP JP2012109948A patent/JP5986796B2/ja active Active
-
2013
- 2013-04-25 CA CA2872593A patent/CA2872593C/en active Active
- 2013-04-25 AU AU2013258901A patent/AU2013258901B2/en active Active
- 2013-04-25 WO PCT/JP2013/062259 patent/WO2013168588A1/ja not_active Ceased
- 2013-04-25 US US14/399,107 patent/US10137407B2/en active Active
- 2013-04-25 EP EP13788078.7A patent/EP2848298B1/en active Active
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6119286B2 (enExample) * | 1975-11-18 | 1986-05-16 | Basf Ag | |
| JPH04349921A (ja) * | 1991-05-27 | 1992-12-04 | Mitsubishi Heavy Ind Ltd | 排煙脱炭吸収剤 |
| JPH06343858A (ja) * | 1993-06-08 | 1994-12-20 | Mitsubishi Heavy Ind Ltd | 二酸化炭素吸収剤 |
| JPH0751537A (ja) | 1993-06-30 | 1995-02-28 | Mitsubishi Heavy Ind Ltd | Co2 含有ガス中のco2 を除去する方法 |
| JP4690659B2 (ja) | 2004-03-15 | 2011-06-01 | 三菱重工業株式会社 | Co2回収装置 |
| JP2007325996A (ja) * | 2006-06-06 | 2007-12-20 | Mitsubishi Heavy Ind Ltd | 吸収液、co2又はh2s又はその双方の除去装置及び方法 |
| JP2008013400A (ja) | 2006-07-05 | 2008-01-24 | Research Institute Of Innovative Technology For The Earth | 排ガス中の二酸化炭素を吸収及び脱離して回収する方法 |
| JP2008307519A (ja) | 2007-06-18 | 2008-12-25 | Mitsubishi Heavy Ind Ltd | 吸収液、吸収液を用いたco2又はh2s除去装置及び方法 |
| WO2010136425A1 (en) * | 2009-05-26 | 2010-12-02 | Basf Se | Process for recovery of carbon dioxide from a fluid stream, in particular from syngas |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2848298A4 |
Also Published As
| Publication number | Publication date |
|---|---|
| US10137407B2 (en) | 2018-11-27 |
| EP2848298A4 (en) | 2016-02-17 |
| JP2013236987A (ja) | 2013-11-28 |
| CA2872593A1 (en) | 2013-11-14 |
| EP2848298A1 (en) | 2015-03-18 |
| AU2013258901B2 (en) | 2016-07-14 |
| CA2872593C (en) | 2017-12-12 |
| JP5986796B2 (ja) | 2016-09-06 |
| US20150132207A1 (en) | 2015-05-14 |
| AU2013258901A1 (en) | 2014-11-27 |
| EP2848298B1 (en) | 2018-12-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6172884B2 (ja) | 3成分吸収液、co2又はh2s又はその双方の除去装置及び方法 | |
| JP5230080B2 (ja) | 吸収液、co2の除去装置及び方法 | |
| JP2013086079A5 (enExample) | ||
| JP5986796B2 (ja) | 複合アミン吸収液、co2又はh2s又はその双方の除去装置及び方法 | |
| AU2019423879B2 (en) | Composite amine absorption solution, and device and method both for removing CO2 or H2S or both of them | |
| JP5984776B2 (ja) | 複合アミン吸収液、co2又はh2s又はその双方の除去装置及び方法 | |
| JP2013236987A5 (enExample) | ||
| JP6841676B2 (ja) | 複合アミン吸収液、co2又はh2s又はその双方の除去装置及び方法 | |
| KR102844542B1 (ko) | 복합 아민 흡수액, 제거 장치 및 제거 방법 | |
| JP7336245B2 (ja) | Co2、h2s又はそれら双方の吸収液並びにco2又はh2s又はその双方の除去装置及び方法 | |
| AU2012327104B9 (en) | 3-component absorbent solution, and CO2 and/or H2S removal device and method | |
| WO2025032794A1 (ja) | 複合アミン吸収液 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13788078 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2872593 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14399107 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2013788078 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2013258901 Country of ref document: AU Date of ref document: 20130425 Kind code of ref document: A |