WO2011125548A1 - 生分解性樹脂成型体の分解促進剤及びその使用 - Google Patents
生分解性樹脂成型体の分解促進剤及びその使用 Download PDFInfo
- Publication number
- WO2011125548A1 WO2011125548A1 PCT/JP2011/057381 JP2011057381W WO2011125548A1 WO 2011125548 A1 WO2011125548 A1 WO 2011125548A1 JP 2011057381 W JP2011057381 W JP 2011057381W WO 2011125548 A1 WO2011125548 A1 WO 2011125548A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- biodegradable resin
- resin molded
- molded body
- decomposition
- agent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L101/00—Compositions of unspecified macromolecular compounds
- C08L101/16—Compositions of unspecified macromolecular compounds the macromolecular compounds being biodegradable
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L101/00—Compositions of unspecified macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J11/00—Recovery or working-up of waste materials
- C08J11/04—Recovery or working-up of waste materials of polymers
- C08J11/10—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation
- C08J11/16—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with inorganic material
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J11/00—Recovery or working-up of waste materials
- C08J11/04—Recovery or working-up of waste materials of polymers
- C08J11/10—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation
- C08J11/18—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with organic material
- C08J11/22—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with organic material by treatment with organic oxygen-containing compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J11/00—Recovery or working-up of waste materials
- C08J11/04—Recovery or working-up of waste materials of polymers
- C08J11/10—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation
- C08J11/18—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with organic material
- C08J11/22—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with organic material by treatment with organic oxygen-containing compounds
- C08J11/24—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with organic material by treatment with organic oxygen-containing compounds containing hydroxyl groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J11/00—Recovery or working-up of waste materials
- C08J11/04—Recovery or working-up of waste materials of polymers
- C08J11/10—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation
- C08J11/18—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with organic material
- C08J11/22—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with organic material by treatment with organic oxygen-containing compounds
- C08J11/26—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with organic material by treatment with organic oxygen-containing compounds containing carboxylic acid groups, their anhydrides or esters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/01—Use of inorganic substances as compounding ingredients characterized by their specific function
- C08K3/012—Additives activating the degradation of the macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/10—Metal compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/16—Halogen-containing compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/04—Oxygen-containing compounds
- C08K5/07—Aldehydes; Ketones
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/04—Oxygen-containing compounds
- C08K5/09—Carboxylic acids; Metal salts thereof; Anhydrides thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
Definitions
- the present invention relates to a biodegradable resin molded body decomposition accelerator capable of promoting the decomposition of a biodegradable resin molded body containing an oxidative degradation agent.
- the present invention also relates to a biodegradable product having excellent biodegradability.
- the present invention relates to a method for decomposing a biodegradable resin molding that can efficiently decompose a biodegradable resin molding containing an oxidative degradation agent.
- Resin molded products molded from petroleum-based resins such as polyethylene, polypropylene, and polystyrene are used in various fields such as textile products, packaging films, electrical appliances, and industrial materials, and are indispensable for modern society. It has become. On the other hand, these resin molded products are used in large quantities and are disposed of in large quantities, and their disposal is a big problem. For example, there is a problem that toxic gas is generated when the resin molding is incinerated. In addition, since petroleum-based resins exhibit hydrolysis resistance, there is a problem that when the resin molded body is landfilled or disposed of in the mountains, it is not biodegraded in the soil and adversely affects the environment.
- biodegradable resins such as polylactic acid have attracted attention as an alternative to petroleum resins.
- a biodegradable resin is recognized as a material that is friendly to the global environment by being decomposed into microorganisms without destroying the natural environment when disposed in landfills or in the mountains.
- the biodegradable resin is difficult to be molded as compared with petroleum resins.
- the biodegradable resin is susceptible to hydrolysis and thus has a disadvantage of being inferior in terms of durability, strength, and heat resistance. For this reason, in the prior art, substitution of petroleum-based resins with the biodegradable resins is currently limited to some product fields.
- Patent Document 1 an oxidative decomposition agent that lowers the molecular weight of a resin material by oxidative decomposition has been developed.
- the degradation agent can also be applied to high-strength petroleum-based resins (hydrolysis-resistant materials), so that biodegradability is maintained while maintaining the advantages of petroleum resins such as moldability, durability, strength, and heat resistance. It has attracted a lot of attention because it can be prepared.
- the object of the present invention is to provide a technique for improving the natural biodegradation rate of the biodegradable resin molding by accelerating the degradation of the biodegradable resin molding containing the oxidative degradation agent.
- the present inventor has intensively studied to solve the above-mentioned problems, and at the time of disposal of the biodegradable resin molding containing the oxidative degradation agent, the biodegradable resin molding and the chloride salt coexist. It has been found that the degradation rate of the biodegradable resin molded product is significantly increased. The present invention has been completed by further studies based on this finding.
- Item 1 A decomposition accelerator for a biodegradable resin molded article containing an oxidative decomposition agent, comprising a chloride salt as an active ingredient.
- Item 2. Item 2. The decomposition accelerator according to Item 1, wherein the chloride salt is potassium chloride and / or sodium chloride.
- Item 3. Item 3. The decomposition accelerator according to Item 1 or 2, wherein the resin forming the biodegradable resin molded body is polyolefin.
- Item 4. Item 4.
- Agent. Item 5. A decomposition agent for a resin molding, which is used by being blended in a resin molding, comprising an oxidative decomposition agent and a chloride salt as active ingredients.
- Item 6. A biodegradable product comprising a biodegradable resin molded article containing an oxidative degradation agent and the degradation accelerator according to any one of Items 1 to 4.
- Item 6. A biodegradation accelerating resin molded article comprising the degradation agent according to Item 5.
- a biodegradable resin molded body containing an oxidative degradation agent and the biodegradable resin molded body are decomposed by allowing the biodegradable resin molded body to coexist with the degradation accelerator according to any one of Items 1 to 4. Of disassembling the resin molding.
- Item 9. Use of a chloride salt for the production of a decomposition accelerator for a biodegradable resin molding containing an oxidative decomposition agent.
- Item 10. Use of an oxidative degradation agent and a chloride salt for the production of a degradation agent for resin moldings.
- a method for promoting the degradation of a biodegradable resin molded article containing an oxidative degradation agent comprising a step of treating the biodegradable resin molded article in the presence of a chloride salt.
- Item 12 A method for decomposing a biodegradable resin molding containing an oxidative degradation agent, comprising a step of treating the biodegradable resin molding in the presence of a chloride salt.
- Item 13 A method for decomposing a biodegradation-promoting resin molding comprising an oxidative degradation agent and a chloride salt, comprising the step of leaving the biodegradation-promoting resin molding in an outdoor environment.
- the degradation of the biodegradable resin molded product containing the oxidative degradation agent can be specifically accelerated.
- biodegradation can be accelerated without causing environmental pollution.
- the decomposition agent of the present invention when blended with a resin molded body, the resin molded body is accelerated to be decomposed when discarded, and the resin molded body is rapidly reduced in molecular weight to the extent that it can be used by microorganisms. Therefore, rapid natural decomposition is possible without adversely affecting the environment.
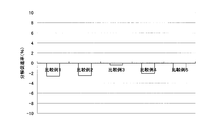
- Test example 1 the result of having measured the decomposition
- Test Example 2 the results of measuring the degradation acceleration rate of the biodegradable resin molded bodies (inner bags) of the biodegradable products (non-pyrogenic composition-containing products) of Examples 3 and 4 are shown.
- Comparative Test Example 1 the results of measuring the degradation acceleration rate of the biodegradable resin molded body (inner bag) of the biodegradable product (non-pyrogenic composition-containing product) of Comparative Example 1-5 are shown.
- Biodegradable resin molded body decomposition accelerator The decomposition accelerator of the present invention is used to promote the decomposition of a biodegradable resin molded body containing an oxidative decomposition agent, and contains a chloride salt as an active ingredient. It is characterized by.
- chloride salt used in the present invention include chloride salts of alkali metals such as potassium chloride, sodium chloride and lithium chloride; chloride salts of alkaline earth metals such as magnesium chloride and calcium chloride;
- the metal chloride include aluminum chloride, copper chloride, iron chloride, zinc chloride, and thallium chloride.
- an alkali metal chloride salt more preferably potassium chloride, sodium chloride, particularly preferably.
- a potassium chloride is mentioned.
- These chloride salts may be used individually by 1 type, and may be used in combination of 2 or more type.
- the degradation accelerator of the present invention is used for promoting the degradation of a biodegradable resin molded article containing an oxidative degradation agent.
- the resin that forms the biodegradable resin molding to which the degradation accelerator of the present invention is applied is not particularly limited as long as it contains an oxidative degradation agent, and preferably includes a thermoplastic resin.
- the thermoplastic resin include polyolefin (polyethylene, polypropylene, etc.), polystyrene, polyvinyl chloride, polyvinylidene chloride, polyamide, polyester, polyvinyl alcohol, polyurethane, ethylene-vinyl acetate copolymer, polycarbonate, and the like. Illustrated. These thermoplastic synthetic resins may be used individually by 1 type, and may be used in combination of 2 or more type.
- thermoplastic resins preferably a resin having hydrolysis resistance such as polyolefin (polyethylene, polypropylene, etc.), polystyrene, polyvinyl chloride, polyvinylidene chloride, polyamide, etc .; more preferably polyolefin; more preferably polyethylene, polypropylene.
- polyolefin polyethylene, polypropylene, etc.
- polystyrene polyvinyl chloride
- polyvinylidene chloride polyamide, etc .
- polyamide polyamide
- polyolefin more preferably polyethylene, polypropylene.
- the shape of the biodegradable resin molding to which the degradation accelerator of the present invention is applied is not particularly limited, and may be any of a sheet shape, a film shape, a pellet shape, a block shape, a fiber shape, and the like.
- the oxidative degradation agent is a substance that oxidizes and decomposes a polymer that forms a resin to reduce the molecular weight of the polymer to such an extent that microbial degradation is possible.
- Oxidative degradation agents are known in the art (e.g., U.S. Pat.Nos.
- the oxidative decomposition agent contained in the resin molding to which the decomposition accelerator of the present invention is applied is not particularly limited, and examples thereof include carboxylic acid metal salts, hydroxycarboxylic acids; transition metal compounds (US Pat. No. 5,308,906), rare earths Examples thereof include compounds and aromatic ketones.
- oxidative decomposition agent a combination of a carboxylic acid metal salt and a hydroxycarboxylic acid (US Pat. No. 5,854,304), a combination of a carboxylic acid metal salt and a filler (US Pat. No. 5,565,503), or the like may be used.
- these oxidative degradation agents may be used alone or in combination of two or more.
- the carboxylic acid metal salt used as the oxidative decomposition agent include a metal salt of an aliphatic carboxylic acid having 10 to 20 carbon atoms, preferably a stearic acid metal salt.
- the metal atom constituting the carboxylic acid metal salt include cobalt, cerium, iron, aluminum, antimony, barium, bismuth, cadmium, chromium, copper, gallium, lanthanum, lead, lithium, magnesium, mercury, molybdenum, nickel, Examples include calcium, rare earth, silver, sodium, strontium, tin, tungsten, vanadium, yttrium, zinc, zirconium and the like.
- stearates of metals such as cobalt, cerium, and iron are particularly preferable.
- hydroxycarboxylic acid used as the oxidative decomposition agent examples include monohydroxytricarboxylic acids such as citric acid; polyhydroxydicarboxylic acids such as trihydroxyglutaric acid and saccharic acid; dihydroxydicarboxylic acids such as tartaric acid; tartronic acid and malic acid.
- monohydroxy dicarboxylic acids such as erythric® acid, arabic acid, mannitic® acid and the like; dihydroxy monocarboxylic acids such as glyoxylic acid and glyceric acid, and the like. These hydroxycarboxylic acids can be used alone or in combination of two or more.
- filler used as one component of the oxidative decomposition agent examples include inorganic carbonate, synthetic carbonate, nepheline syenite, talc, magnesium hydroxide, aluminum hydroxide, diatomaceous earth, natural or synthetic silica, and calcined clay. Illustrated. These fillers desirably have a particle size of less than 150 mesh. These fillers can be used alone or in combination of two or more.
- transition metal compound used as the oxidative decomposition agent include cobalt or magnesium salts, preferably cobalt or magnesium aliphatic carboxylic acid (carbon number 12 to 20) salt, more preferably cobalt stearate, Examples include cobalt oleate, magnesium stearate, and magnesium oleate. These transition metal compounds can be used alone or in combination of two or more.
- rare earth compound used as the oxidative decomposition agent examples include rare earths belonging to Group 3A of the periodic table, or oxides thereof, and more specifically, cerium (Ce), yttrium (Y), neodymium (Nd ), Rare earth oxides, hydroxides, rare earth sulfates, rare earth nitrates, rare earth acetates, rare earth chlorides, rare earth carboxylates, etc., specifically, cerium oxide, sulfuric acid Examples thereof include dicerium, ceric ammonium sulfate, ceric ammonium nitrate, cerium acetate, lanthanum nitrate, cerium chloride, cerium nitrate, cerium hydroxide, cerium octylate, lanthanum oxide, yttrium oxide, and scandium oxide. These rare earth compounds can be used individually by 1 type or in combination of 2 or more types.
- aromatic ketone used as the oxidative decomposition agent examples include benzophenone, anthraquinone, anthrone, acetylbenzophenone, 4-octylbenzophenone and the like. These aromatic ketones can be used singly or in combination of two or more.
- the biodegradable resin molding to be applied is a combination of a carboxylate and a rare earth compound as an oxidative degradation agent. It is suitable to include.
- an oxidative decomposition agent trade name “P-life” (manufactured by P-Life Japan Inc.) can be mentioned.
- the ratio thereof is, for example, 5 to 70 parts by weight, preferably 7 to 60 parts by weight of the rare earth compound per 100 parts by weight of the carboxylate. More preferably, 10 to 50 parts by mass are exemplified.
- an oxidative degradation agent oxidizes and decomposes a polymer that forms a resin molded body under the action of light, heat, air, etc., and lowers the polymer to such an extent that microbial degradation is possible.
- an oxidative degradation agent that exhibits the oxidative degradation action of the polymer by exposure to light (ultraviolet rays) (hereinafter, photorequired oxidative degradation agent) is preferably used.
- a biodegradable resin molded product containing a light-requiring oxidative degradation agent is not decomposed under light-shielding conditions.
- a light-shielding atmosphere (light-shielding space, light-shielding container, light-shielding bag, etc.) at the pre-use stage such as during production, distribution, or storage ), It is possible to maintain a desired function without causing deterioration in durability until use.
- a photorequiring oxidative decomposition agent include an oxidative decomposition agent containing a rare earth compound, and more specifically, trade name “P-life” (manufactured by PLife Japan Inc.) is exemplified. Is done.
- the blending ratio of the oxidative degradation agent in the biodegradable resin molding to which the present invention is applied is appropriately set according to the type of oxidative degradation agent used, the type of resin forming the biodegradable resin molding, and the like. For example, 0.05 to 5% by mass, preferably 0.1 to 3.5% by mass, and more preferably 0.1 to 2.4% by mass with respect to the total mass of the biodegradable resin molded body. Is exemplified.
- the blending ratio of the oxidative degradation agent is 0 with respect to the total mass of the biodegradable resin molding. 0.08 to 0.8% by mass, preferably 0.12 to 0.6% by mass, and more preferably 0.16 to 0.4% by mass.
- the blending ratio of the oxidative degradation agent is 0.4 to 3% by mass, preferably with respect to the total mass of the biodegradable resin molded body. Is 0.6 to 2.5% by mass, more preferably 0.8 to 2% by mass.
- the biodegradable resin molded product to which the present invention is applied can be produced by a known method.
- the following order of manufacturing method is exemplified: (1) A predetermined amount of an oxidative decomposition agent is added to a melt of a resin that forms a biodegradable resin molding, and the solution is molded into a pellet. (2) Next, if necessary, the pellet-shaped molded body is melted and molded into a desired shape.
- the application method of the degradation accelerator of the present invention is not particularly limited as long as it is applied so as to come into contact with the biodegradable resin molded body during the disposal of the biodegradable resin molded body.
- the active ingredient of the decomposition accelerator of the present invention is added to the biodegradable resin molding as it is or after dilution with water or the like as necessary.
- embodiment 1 At the time of production of the product containing the biodegradable resin molding, by adding the active ingredient of the decomposition accelerator of the present invention to the product in advance, Examples thereof include a method in which the biodegradable resin molded product is brought into contact with the active ingredient (hereinafter also referred to as embodiment 2).
- the application method of Embodiment 1 described above is biodegradable into a product in which the active ingredient of the degradation accelerator of the present invention cannot be blended in advance. This is suitable in the case where an adhesive resin molding is used.
- the application method of the said Embodiment 2 is a product which can mix
- the biodegradable resin molding is used for the product for which long-term durability is not requested
- a product to which the embodiment 2 can be applied in this way for example, a product in which the functional composition is surrounded by a sheet-like or film-like biodegradable resin molded body or laminated on the biodegradable resin molded body.
- the functional composition is a composition having specific actions such as cold-retaining action, cooling action, pharmacological action, and exothermic action. A person skilled in the art can appropriately set the composition of the functional composition according to the required action.
- the active ingredient of the degradation accelerator of the present invention is added to the functional composition, and this is surrounded or biodegraded by a sheet-like or film-like biodegradable resin molding.
- the product may be provided by laminating on the conductive resin molding.
- a cryogen a product in which the cryogen composition is surrounded by a sheet or film-shaped biodegradable resin molding
- a body coolant a coolant composition
- the application amount of the degradation accelerator of the present invention varies depending on the shape and type of the biodegradable resin molded body, but for example, the present invention per 100 parts by mass of the biodegradable resin molded body to be applied.
- a range in which the effective component of the decomposition accelerator is 1 to 100 parts by mass, preferably 2.5 to 50 parts by mass, more preferably 5 to 30 parts by mass is exemplified.
- the biodegradable resin molded body is oxidized by leaving it in an outdoor environment in the state of coexisting with the degradation accelerator of the present invention (for example, landfill or disposal in the mountains). Microbial degradation occurs through degradation.
- the period until the biodegradable resin molded body is finally decomposed in the presence of the decomposition accelerator of the present invention is the shape of the biodegradable resin molded body, the type of constituent resin, the discarded environment, etc.
- a biodegradable resin molded body of about 1 g in film or sheet is decomposed to such a degree that the resin molded body can no longer be seen in about 0.5 to 3 years.
- the decomposing agent of the present invention is used by blending with a resin molded body, and comprises an oxidative decomposition agent and a chloride salt as active ingredients. Decomposing agent. In this way, by combining the oxidative decomposition agent and the chloride salt and blending them in the resin molded body, the decomposition of the resin molded body is promoted, and the resin molded body can be rapidly used for microorganisms. It is possible to reduce the molecular weight.
- the oxidative decomposition agent and chloride salt used in the decomposition agent of the present invention are as described in “1. Decomposition accelerator” above.
- the ratio of the oxidative decomposition agent and the chloride salt is not particularly limited.
- the total amount of chloride salt is 4 to 4 per 100 parts by mass of the oxidative decomposition agent.
- the decomposing agent of the present invention is used by being blended with a resin molding.
- the constituent resin, shape, and the like of the resin molded body are the same as those of the biodegradable resin molded body described in “1. Degradation accelerator” above.
- the method of blending the decomposition agent of the present invention into a resin molding can also be performed according to a known method.
- the following production method is exemplified: (1) A predetermined amount of the decomposition agent of the present invention is added to a resin melt forming a resin molding, and the solution is molded into a pellet. (2) Next, if necessary, the pellet-shaped molded body is melted and molded into a desired shape.
- the total mass of the decomposing agent of the present invention is 0.1 to 7 parts by mass, preferably 0.15 to 5 parts by mass, and more preferably 0.2 to 3 parts by mass.
- the resin molded body in which the decomposing agent of the present invention is blended is left in the outdoor environment and treated (for example, landfilled or discarded in the mountains), so that the resin molded body is rapidly reduced in molecular weight by oxidative decomposition, It is then microbially degraded.
- the standard for the period until the resin molded body in which the decomposition agent of the present invention is blended is finally decomposed is the period until the biodegradable resin molded body described in “1. Decomposition accelerator” is decomposed. It is the same.
- Biodegradable product of the present invention is characterized by containing the above-described degradation accelerator and a biodegradable resin molded article containing an oxidative degradation agent. That is, the biodegradable product of the present invention contains the above-described degradation accelerator in combination with a biodegradable resin molded article containing an oxidative degradation agent, and is disposed of in landfills or in the wild after use. The tree biodegradable fat molded product is rapidly decomposed without causing environmental pollution.
- the degradation accelerator contained in the biodegradable product of the present invention is as described in “1. Degradation accelerator” above.
- the type of resin forming the biodegradable resin molded body contained in the biodegradable product of the present invention the shape of the resin molded body, the type and blending ratio of the oxidative degradation agent contained in the biodegradable resin molded body And the like are the same as those of the biodegradable resin molded product to which the above “1. degradation accelerator” is applied.
- the ratio of the degradation accelerator and the biodegradable resin molded body contained in the biodegradable product of the present invention is appropriately set according to the shape and type of the biodegradable resin molded body.
- the active ingredient (chloride salt) of the above-mentioned decomposition accelerator are 5 to 55 parts by weight, preferably 10 to 50 parts by weight, and more preferably 15 to 45 parts by weight per 100 parts by weight of the biodegradable resin molded body. .
- the product of the present invention contains the decomposition accelerator and the biodegradable resin molded body, and the decomposition accelerator and the biodegradable resin molded body are in contact with each other during disposal, the product The form is not particularly limited.
- a product that is surrounded or laminated on a biodegradable resin molded body in the form of a sheet or film is exemplified.
- the biodegradable product of the present invention include a cryogen (a product in which a cryogen composition containing the above-described degradation accelerator is surrounded by a sheet-like or film-like biodegradable resin molding), a body coolant ( Products obtained by enclosing or laminating a cooling agent composition containing the above-described decomposition accelerator in a sheet-like or film-like biodegradable resin molded article, or a medical compress (for compresses containing the above-described decomposition accelerator) Products obtained by laminating a gel-like composition on a sheet-like or film-like biodegradable resin molded article), a body heat-retaining agent (an exothermic composition containing the above-mentioned degradation accelerator is a sheet-like or film-like biodegradable) A product surrounded by a resin molded body) is exemplified.
- a cryogen a product in which a cryogen composition containing the above-described degradation accelerator is surrounded by a sheet-like or film
- the biodegradable resin molded product of the present invention When the biodegradable product of the present invention is left in an outdoor environment and treated (for example, landfilled or disposed of in the mountain), the biodegradable resin molded product is rapidly reduced in molecular weight by oxidative degradation, and then microorganisms Disassembled.
- the standard for the period until the biodegradable resin molded product in the biodegradable product of the present invention is finally decomposed is determined by decomposing the biodegradable resin molded product described in “1. Degradation accelerator” above. It is the same as the period until.
- Biodegradation accelerating resin molding The biodegradation accelerating resin molding of the present invention is characterized in that the resin molding contains a chloride salt and an oxidative degradation agent. That is, the biodegradation promoting resin molded body of the present invention is a resin molded body in which the decomposing agent described in “2. Decomposing agent” is blended.
- the decomposition method of the present invention is characterized in that the biodegradable resin molded body containing an oxidative degradation agent and the above-mentioned decomposition accelerator coexist to decompose the biodegradable resin molded body.
- the decomposition method of the present invention is a method for decomposing a biodegradable resin molded article containing an oxidative decomposition agent using the above decomposition accelerator.
- Decomposition accelerator used in the decomposition method of the present invention biodegradable resin molding to be decomposed, oxidative degradation agent contained in the biodegradable resin molding, the decomposition accelerator and the biodegradable resin molding
- the ratio, the method of coexisting them, and the like are as described in “1. Decomposition accelerator” above.
- the decomposition treatment of the biodegradable resin molded body is performed by leaving it indoors or outdoors in a state where the decomposition accelerator and the biodegradable resin molded body coexist, Preferably, it is carried out by leaving it in an outdoor environment where microorganisms exist (for example, reclamation or disposal in the mountains).
- the period during which the resin molded body is decomposed by the decomposition method of the present invention is the same as the period until the biodegradable resin molded body described in “1.
- Decomposition accelerator” is decomposed.
- the oxidative degradation agent-containing material (containing 80 to 90% oxidative degradation agent) used in the following Examples and Comparative Examples is a trade name “P-life” (manufactured by P-Life Japan Inc .; aliphatic Monocarboxylic acid 50 to 70% by weight, rare earth compound 10 to 20% by weight, and lubricant 10 to 20%).
- Example 1 Biodegradable product (disposable body warmer) 1.
- functional composition 1% by mass of potassium chloride, 55% by mass of iron powder having an average particle size of 50 ⁇ m, 13% by mass of activated carbon having an average particle size of 200 ⁇ m, 26% by mass of water, and leechite having a particle size of 100 ⁇ m
- An exothermic composition was prepared by mixing 3% by mass and 2% by mass of a crosslinked sodium salt of an acrylic acid polymer having a particle size of 380 ⁇ m.
- resin molded body inner bag material of disposable body warmer
- a resin sheet containing 98% by mass of polyethylene and 2% by mass of oxidative degradation agent-containing material was arranged with rotating blades provided with blades on the circumference of a disk-shaped tool net.
- JIS P through the roll Breathable biodegradable resin film (thickness) with pores with a value measured according to 8117-1998 "Paper and board-Air permeability test method-Gurley test machine method" of 13.5 to 14.5 seconds / 100cc 40 ⁇ m) was prepared.
- a biodegradable nonwoven fabric (weight per unit area: 25 g / m 2 ) was prepared by a spunbond method using synthetic fibers containing 99.75% by mass of polypropylene and 0.25% by mass of an oxidative degradation agent-containing material.
- the exothermic composition was accommodated in an inner bag to prepare a biodegradable product (disposable body warmer).
- a biodegradable product (disposable body warmer)
- the prepared biodegradable product was quickly put in an outer bag (air-impermeable and light-shielding) made of a polyvinylidene chloride coated film, and the outer bag was sealed.
- the disposable body warmers were taken out from the outer bag and used at the start of the test.
- Example 2 Biodegradable product (disposable body warmer) A disposable body warmer was prepared under the same conditions as in Example 1 except that 1% by mass of sodium chloride was used instead of 1% by mass of potassium chloride.
- Test Example 1 Evaluation of degradability of biodegradable product (disposable body warmer)
- the disposable body warmers of Examples 1 and 2 were taken out from the outer bag and allowed to stand at room temperature for 24 hours until heat generation was completed. Thereafter, the disposable body warmer that had generated heat was placed in a thermostatic bath at 50 ° C. and stored for 12 days.
- the disposable body warmer is disassembled, the exothermic composition is taken out, the inner bag part that is not perforated is cut into 2 ⁇ 7 cm, and is pulled.
- the tensile strength was measured by pulling in the MD direction with a testing machine (AGS-H, manufactured by Shimadzu Corporation). As a control, the tensile strength was measured after storing under the same conditions as above using only the inner bag containing no exothermic composition. Subsequently, according to the following calculation formula, the decomposition acceleration rate of the inner bag used in Examples 1 and 2 was measured.
- tensile strength falls, so that the decomposition
- FIG. 1 shows the results of measuring the decomposition acceleration rate for the inner bags of the disposable body warmers of Examples 1 and 2.
- the decomposition rate of the inner bag is much higher than that of the inner bag evaluated as a control. It was early.
- the disposable body warmer (Example 1) containing potassium chloride in the exothermic composition (functional composition) has a significantly higher rate of decomposition of the inner bag than the case of containing sodium chloride (Example 2). It was confirmed that it was high and had excellent biodegradability.
- the inner bag of the disposable body warmers of Examples 1 and 2 is provided with a body warmer without any reduction in strength causing problems in use such as brittleness and leakage of the exothermic composition even after the end of heat generation.
- Example 3-4 Biodegradable product (product containing non-pyrogenic composition) 2.2% by mass of potassium chloride, 28.9% by mass of activated carbon having an average particle size of 200 ⁇ m, 57.8% by mass of water, 6.7% by mass of leechite having a particle size of 100 ⁇ m, and partial sodium of an acrylic acid polymer having a particle size of 380 ⁇ m
- a non-pyrogenic composition (functional composition) was prepared by mixing 4.4% by mass of the salt cross-linked product.
- a non-exothermic composition-containing product Example 3 was prepared.
- Example 4 a non-pyrogenic composition-containing product was used under the same conditions as in Example 3 except that 2.2% by mass of sodium chloride was used instead of 2.2% by mass of potassium chloride. Prepared.
- Test Example 2 Evaluation of degradability of biodegradable product (non-pyrogenic composition-containing product)
- Non-pyrogenic composition-containing products of Examples 3 and 4 were stored in a thermostat at 50 ° C. for 3 days, and Storage 1 After 3 days and after storage, the decomposition acceleration rate of the inner bag was measured by the same method as in Test Example 1 above.
- Comparative Test Example 1 Evaluation of degradability of non-pyrogenic composition-containing product-2 Instead of 2.2% by mass of potassium chloride, 2.2% by mass of potassium dihydrogen phosphate (Comparative Example 1), 2.2% by mass of magnesium sulfate (Comparative Example 2), 2.2% by mass of manganese sulfate (Comparative Example) 3) Non-pyrogenic composition under the same conditions as in Example 3 except that 2.2% by mass of potassium sulfate (Comparative Example 4) or 2.2% by mass of sodium sulfate (Comparative Example 5) is used. A containing product was prepared.
- chloride salts have the effect of promoting the degradation of biodegradable resin moldings containing oxidative degradation agents, and are used as degradation accelerators for biodegradable resin moldings containing oxidative degradation agents. It became clear that we could do it. Furthermore, it was confirmed that the combination of a chloride salt and an oxidative decomposition agent is also effective as a decomposition agent for imparting decomposition characteristics to the resin molding.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Sustainable Development (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Separation, Recovery Or Treatment Of Waste Materials Containing Plastics (AREA)
- Biological Depolymerization Polymers (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201180016796.4A CN102822283B (zh) | 2010-03-31 | 2011-03-25 | 生物可降解树脂成型体的降解促进剂及其用途 |
| KR1020127028663A KR20130087370A (ko) | 2010-03-31 | 2011-03-25 | 생분해성 수지 성형체의 분해 촉진제 및 그 사용 |
| GB1216692.2A GB2491527B (en) | 2010-03-31 | 2011-03-25 | Degradation promoter of biodegradable resin molded body, and use thereof |
| US13/634,424 US9056968B2 (en) | 2010-03-31 | 2011-03-25 | Degradation promoter of biodegradable resin molded body, and use thereof |
| HK13102876.0A HK1175798B (en) | 2010-03-31 | 2011-03-25 | Degradation promoter of biodegradable resin molded body, and use thereof |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010082451A JP5733904B2 (ja) | 2010-03-31 | 2010-03-31 | 生分解性樹脂成型体の分解促進剤及びその使用 |
| JP2010-082451 | 2010-03-31 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011125548A1 true WO2011125548A1 (ja) | 2011-10-13 |
Family
ID=44762502
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/057381 Ceased WO2011125548A1 (ja) | 2010-03-31 | 2011-03-25 | 生分解性樹脂成型体の分解促進剤及びその使用 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US9056968B2 (enExample) |
| JP (1) | JP5733904B2 (enExample) |
| KR (1) | KR20130087370A (enExample) |
| CN (1) | CN102822283B (enExample) |
| GB (1) | GB2491527B (enExample) |
| WO (1) | WO2011125548A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPWO2020175276A1 (enExample) * | 2019-02-25 | 2020-09-03 |
Families Citing this family (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103304958A (zh) * | 2013-05-14 | 2013-09-18 | 泉州特米斯高环保科技有限公司 | 一种可降解树脂的生产方法 |
| US20160185920A1 (en) * | 2013-08-08 | 2016-06-30 | Aspen Research Corporation | Methods and systems for promoting and controlling degradation of polymers |
| KR101365615B1 (ko) * | 2013-09-06 | 2014-02-26 | 유재균 | 초기 기계적 강도가 우수한 산화생분해성 필름용 수지 조성물 및 이의 제조방법과 이로부터 제조되는 산화생분해성 필름 |
| CN106032422A (zh) * | 2015-03-13 | 2016-10-19 | 香港纺织及成衣研发中心有限公司 | 一种可降解合成纤维组合物及其制备方法和制品 |
| US11149144B2 (en) | 2015-06-30 | 2021-10-19 | BiologiQ, Inc. | Marine biodegradable plastics comprising a blend of polyester and a carbohydrate-based polymeric material |
| US11359088B2 (en) | 2015-06-30 | 2022-06-14 | BiologiQ, Inc. | Polymeric articles comprising blends of PBAT, PLA and a carbohydrate-based polymeric material |
| US20170002185A1 (en) * | 2015-06-30 | 2017-01-05 | BiologiQ, Inc. | Articles Formed with Biodegradable Materials |
| US11926929B2 (en) | 2015-06-30 | 2024-03-12 | Biologiq, Inc | Melt blown nonwoven materials and fibers including starch-based polymeric materials |
| US10752759B2 (en) | 2015-06-30 | 2020-08-25 | BiologiQ, Inc. | Methods for forming blended films including renewable carbohydrate-based polymeric materials with high blow up ratios and/or narrow die gaps for increased strength |
| US11674018B2 (en) | 2015-06-30 | 2023-06-13 | BiologiQ, Inc. | Polymer and carbohydrate-based polymeric material blends with particular particle size characteristics |
| US11111355B2 (en) | 2015-06-30 | 2021-09-07 | BiologiQ, Inc. | Addition of biodegradability lending additives to plastic materials |
| US11674014B2 (en) | 2015-06-30 | 2023-06-13 | BiologiQ, Inc. | Blending of small particle starch powder with synthetic polymers for increased strength and other properties |
| US11111363B2 (en) | 2015-06-30 | 2021-09-07 | BiologiQ, Inc. | Articles formed with renewable and/or sustainable green plastic material and carbohydrate-based polymeric materials lending increased strength and/or biodegradability |
| US10995201B2 (en) | 2015-06-30 | 2021-05-04 | BiologiQ, Inc. | Articles formed with biodegradable materials and strength characteristics of the same |
| US10920044B2 (en) | 2015-06-30 | 2021-02-16 | BiologiQ, Inc. | Carbohydrate-based plastic materials with reduced odor |
| US11046840B2 (en) | 2015-06-30 | 2021-06-29 | BiologiQ, Inc. | Methods for lending biodegradability to non-biodegradable plastic materials |
| US11879058B2 (en) | 2015-06-30 | 2024-01-23 | Biologiq, Inc | Yarn materials and fibers including starch-based polymeric materials |
| US10919203B2 (en) | 2015-06-30 | 2021-02-16 | BiologiQ, Inc. | Articles formed with biodegradable materials and biodegradability characteristics thereof |
| US11926940B2 (en) | 2015-06-30 | 2024-03-12 | BiologiQ, Inc. | Spunbond nonwoven materials and fibers including starch-based polymeric materials |
| JP7661027B2 (ja) * | 2020-11-10 | 2025-04-14 | 株式会社 伊藤園 | 樹脂成形体及びその製造方法並びに飲食料品 |
| US12201172B2 (en) * | 2021-06-03 | 2025-01-21 | Shen Wei (Usa) Inc. | Eco-friendly wearable dipped article and method of manufacturing |
| JP7779327B2 (ja) * | 2021-11-30 | 2025-12-03 | 日清紡ホールディングス株式会社 | 2種以上の1価有機アニオンを有する海洋生分解促進剤及び海洋生分解性組成物 |
| JPWO2023100257A1 (enExample) * | 2021-11-30 | 2023-06-08 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS49119972A (enExample) * | 1973-03-19 | 1974-11-15 | ||
| JPH0565420A (ja) * | 1991-09-06 | 1993-03-19 | Hagiwara Kogyo Kk | 生物分解性樹脂組成物およびその成形体 |
| JPH07502221A (ja) * | 1991-12-12 | 1995-03-09 | ミネソタ マイニング アンド マニュファクチャリング カンパニー | 分解性の多層構造体 |
| JP2009542871A (ja) * | 2006-07-11 | 2009-12-03 | ディーエスエム アイピー アセッツ ビー.ブイ. | ポリマーおよび酸化触媒を有する組成物 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6482872B2 (en) | 1999-04-01 | 2002-11-19 | Programmable Materials, Inc. | Process for manufacturing a biodegradable polymeric composition |
| US6946506B2 (en) * | 2001-05-10 | 2005-09-20 | The Procter & Gamble Company | Fibers comprising starch and biodegradable polymers |
| EP1878762A1 (en) * | 2006-07-11 | 2008-01-16 | DSMIP Assets B.V. | Oxidisable polymer |
| JP5484161B2 (ja) * | 2010-03-31 | 2014-05-07 | 小林製薬株式会社 | 生分解性使い捨てカイロ |
-
2010
- 2010-03-31 JP JP2010082451A patent/JP5733904B2/ja not_active Expired - Fee Related
-
2011
- 2011-03-25 US US13/634,424 patent/US9056968B2/en active Active
- 2011-03-25 GB GB1216692.2A patent/GB2491527B/en not_active Expired - Fee Related
- 2011-03-25 KR KR1020127028663A patent/KR20130087370A/ko not_active Withdrawn
- 2011-03-25 CN CN201180016796.4A patent/CN102822283B/zh not_active Expired - Fee Related
- 2011-03-25 WO PCT/JP2011/057381 patent/WO2011125548A1/ja not_active Ceased
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS49119972A (enExample) * | 1973-03-19 | 1974-11-15 | ||
| JPH0565420A (ja) * | 1991-09-06 | 1993-03-19 | Hagiwara Kogyo Kk | 生物分解性樹脂組成物およびその成形体 |
| JPH07502221A (ja) * | 1991-12-12 | 1995-03-09 | ミネソタ マイニング アンド マニュファクチャリング カンパニー | 分解性の多層構造体 |
| JP2009542871A (ja) * | 2006-07-11 | 2009-12-03 | ディーエスエム アイピー アセッツ ビー.ブイ. | ポリマーおよび酸化触媒を有する組成物 |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPWO2020175276A1 (enExample) * | 2019-02-25 | 2020-09-03 | ||
| WO2020175276A1 (ja) * | 2019-02-25 | 2020-09-03 | モアディバイス株式会社 | 生分解性鮮度保持フィルム及び生分解性鮮度保持容器 |
| CN113474261A (zh) * | 2019-02-25 | 2021-10-01 | 摩尔装置有限公司 | 生物分解性保鲜膜及生物分解性保鲜容器 |
Also Published As
| Publication number | Publication date |
|---|---|
| GB2491527A (en) | 2012-12-05 |
| GB2491527B (en) | 2016-06-15 |
| CN102822283A (zh) | 2012-12-12 |
| CN102822283B (zh) | 2016-02-24 |
| GB201216692D0 (en) | 2012-10-31 |
| JP5733904B2 (ja) | 2015-06-10 |
| US9056968B2 (en) | 2015-06-16 |
| KR20130087370A (ko) | 2013-08-06 |
| JP2011213836A (ja) | 2011-10-27 |
| US20130018125A1 (en) | 2013-01-17 |
| HK1175798A1 (zh) | 2013-07-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5733904B2 (ja) | 生分解性樹脂成型体の分解促進剤及びその使用 | |
| JP5484161B2 (ja) | 生分解性使い捨てカイロ | |
| CN101027344B (zh) | 多孔可生物降解的薄膜以及从该薄膜获得的卫生用品 | |
| CA2111712C (en) | Biodegradable, liquid impervious films | |
| WO1999045067A1 (en) | Polylactic acid composition and film thereof | |
| SK147793A3 (en) | Disposable absorptive objects with biodegradable backlayers | |
| US20010003797A1 (en) | Degradable disposable diaper | |
| WO1998029506A1 (en) | Water-responsive polymer compositions and method of making the same | |
| KR20030036777A (ko) | 지방족 폴리에스테르수지조성물 및 그것을 함유해서이루어진 필름 | |
| CN103642109B (zh) | 光热协同可控降解高分子复合母料及其制备方法 | |
| CN111605257A (zh) | 含金属氧化物及/或金属氢氧化物的片材 | |
| JP2019166703A (ja) | ポリ(3−ヒドロキシブチレート)系樹脂シート | |
| JP3290496B2 (ja) | 熱可塑性ポリマー組成物 | |
| KR20150134470A (ko) | 산화생분해성을 갖는 열접착형 복합섬유 및 그 제조방법 | |
| JP3474218B2 (ja) | 組成物および積層体 | |
| JP2002035037A (ja) | 生分解性衛生用品 | |
| JP2005036088A (ja) | 食品包装用樹脂組成物及び食品用包装体 | |
| CN103819746A (zh) | 一种可降解环保购物袋 | |
| JPH09291165A (ja) | 生分解性多孔質フィルム | |
| KR20160017761A (ko) | 산화생분해성을 갖는 열접착형 복합섬유 및 그 제조방법 | |
| JP2002051939A (ja) | 携帯用汚物処理袋 | |
| JP3780968B2 (ja) | 生分解性樹脂フィルム | |
| JP3420699B2 (ja) | 機能性エアフィルタ | |
| JP2007254738A (ja) | 環境保護に好適な樹脂ペレット原料の製造方法 | |
| JP2024039153A (ja) | 生分解性ポリオレフィンシート |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180016796.4 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11765449 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13634424 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 1216692 Country of ref document: GB Kind code of ref document: A Free format text: PCT FILING DATE = 20110325 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1216692.2 Country of ref document: GB |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20127028663 Country of ref document: KR Kind code of ref document: A |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11765449 Country of ref document: EP Kind code of ref document: A1 |