WO2011125547A1 - 生分解性使い捨てカイロ - Google Patents
生分解性使い捨てカイロ Download PDFInfo
- Publication number
- WO2011125547A1 WO2011125547A1 PCT/JP2011/057380 JP2011057380W WO2011125547A1 WO 2011125547 A1 WO2011125547 A1 WO 2011125547A1 JP 2011057380 W JP2011057380 W JP 2011057380W WO 2011125547 A1 WO2011125547 A1 WO 2011125547A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- disposable body
- inner bag
- body warmer
- mass
- exothermic composition
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F7/00—Heating or cooling appliances for medical or therapeutic treatment of the human body
- A61F7/02—Compresses or poultices for effecting heating or cooling
- A61F7/03—Compresses or poultices for effecting heating or cooling thermophore, i.e. self-heating, e.g. using a chemical reaction
- A61F7/032—Compresses or poultices for effecting heating or cooling thermophore, i.e. self-heating, e.g. using a chemical reaction using oxygen from the air, e.g. pocket-stoves
- A61F7/034—Flameless
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F7/00—Heating or cooling appliances for medical or therapeutic treatment of the human body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F7/00—Heating or cooling appliances for medical or therapeutic treatment of the human body
- A61F7/08—Warming pads, pans or mats; Hot-water bottles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F7/00—Heating or cooling appliances for medical or therapeutic treatment of the human body
- A61F2007/0001—Body part
- A61F2007/0029—Arm or parts thereof
- A61F2007/0036—Hand
Definitions
- the present invention relates to a biodegradable disposable body warmer. More specifically, the present invention relates to a disposable body warmer that is excellent in biodegradability after use while sufficiently maintaining the physical characteristics (strength) of the inner bag during use.
- Conventional general disposable body warmers contain a heat-generating composition that generates heat in the presence of air in a breathable inner bag (storage bag), and is taken out from the non-breathable outer bag when used.
- Disposable body warmers are excellent in convenience as a body warmer and may be supplied at a low price.
- the disposable body warmers are disposed of by landfill or incineration after use.
- the inner bags used in conventional disposable body warmers are made of petroleum plastic materials (hydrolysis-resistant materials) such as polyethylene, polypropylene, and polystyrene. Even when it is treated, it is not biodegraded in the soil and has a problem of adversely affecting the environment.
- Patent Document 1 discloses a technique in which a sheet material in which a nonwoven fabric in which cellulose-based natural fibers are fixed with chitosan and a film sheet mainly composed of starch is laminated is used as an inner bag of a biodegradable disposable body warmer. Has been proposed.
- Patent Document 2 proposes a technique for using a laminate of a polylactic acid film and natural fibers as an inner bag of a biodegradable disposable body warmer. Furthermore, Patent Document 3 proposes a biodegradable disposable body warmer in which an exothermic composition containing iron powder as a main component and containing a non-sodium salt is contained in a biodegradable inner bag.
- the disposable body warmer is subjected to physical loads such as kneading and folding during use, and the heating load due to heat generation is also imposed, and the inner bag is exposed to a harsh environment, so the inner bag can withstand a harsh environment. It is required to have physical characteristics (strength).
- the inner bag using the hydrolyzable biodegradable plastic has a disadvantage that it is weak in strength and gradually becomes brittle when the disposable body warmer is used. For this reason, the conventionally reported biodegradable disposable body warmers have problems that the feeling of use deteriorates or the exothermic composition easily leaks with the lapse of time of use.
- Patent Document 4 an oxidative decomposition agent that lowers the molecular weight of a plastic material by oxidative decomposition has been developed.
- the decomposing agent can be applied to a high-strength petroleum-based plastic material (hydrolysis-resistant material), and thus has attracted attention as a technology that replaces hydrolyzable biodegradable plastics.
- hydrolysis-resistant material hydrolysis-resistant material
- An object of the present invention is to provide a disposable body warmer that is excellent in biodegradability after use while the inner bag has sufficient physical characteristics (strength) during use.
- the present inventor has intensively studied to solve the above-mentioned problems. As a result, a disposable body warmer containing an exothermic composition containing potassium chloride and / or sodium chloride in an inner bag containing an oxidative degradation agent is used. It has been found that the biodegradability after use can be improved while maintaining sufficient physical properties (strength). The present invention has been completed by further studies based on this finding.
- a biodegradable disposable body warmer characterized in that an exothermic composition containing at least one selected from the group consisting of potassium chloride and sodium chloride is contained in an inner bag containing an oxidative degradation agent.
- Item 2. The biodegradable disposable body warmer according to claim 1, wherein the inner bag is a laminate in which a breathable resin film is laminated on a woven fabric or a nonwoven fabric.
- Item 3. Item 3. The biodegradable disposable body warmer according to Item 2, wherein the resin forming the breathable resin film is polyolefin.
- Item 5. The biodegradable disposable body warmer according to any one of Items 1 to 4, wherein the exothermic composition contains potassium chloride, iron powder, a water retention agent, and water.
- Item 6. Item 6. The biodegradation according to any one of Items 1 to 5, wherein the oxidative degradation agent is at least one selected from the group consisting of carboxylic acid metal salts, hydroxycarboxylic acids, transition metal compounds, rare earth compounds, and aromatic ketones. Sex disposable warmer.
- Item 7. Item 7. The biodegradable disposable body warmer according to any one of Items 1 to 6, wherein the oxidative degradation agent is a combination of a carboxylate and a rare earth compound.
- the decomposition of the inner bag by the oxidative decomposition agent can be specifically promoted by potassium chloride and / or sodium chloride contained in the exothermic composition. Even if it is subjected to waste disposal, etc., it decomposes quickly and does not cause environmental pollution.
- biodegradable disposable body warmer of the present invention physical properties (strength) that cause problems in use such as the inner bag becomes brittle and the exothermic composition leaks out from the inner bag when the body heats. Since no deterioration occurs, it has sufficient practical value.
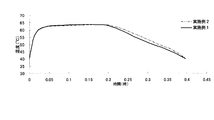
- Test Example 1 the results of measuring the temporal change in the heat generation temperature for the disposable body warmers (Examples 1 and 2) are shown.
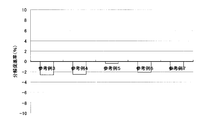
- Test example 1 the result of having measured the decomposition
- the reference test example 1 the result of having measured the decomposition
- Comparative Test Example 1 the results of measuring the decomposition acceleration rate for the inner bag of the non-pyrogenic composition-containing product (Reference Example 3-7) are shown.
- the biodegradable disposable body warmer of the present invention is characterized in that an exothermic composition containing potassium chloride and / or sodium chloride is contained in an inner bag containing an oxidative decomposition agent.
- an exothermic composition containing potassium chloride and / or sodium chloride is contained in an inner bag containing an oxidative decomposition agent.
- an exothermic composition containing potassium chloride and / or sodium chloride is used as a heat source of the disposable body warmer.
- Potassium chloride and sodium chloride contained in the exothermic composition specifically promote the degradation of the inner bag by the oxidative degradation agent described later, and improve the biodegradation characteristics after use of the biodegradable disposable body warmer of the present invention. Demonstrate.
- potassium chloride and sodium chloride activate the surface of the iron powder in the exothermic composition, and also exert the effect of promoting the oxidation reaction of iron.
- potassium chloride has a remarkably high effect of specifically accelerating the degradation of the inner bag by the oxidative degradation agent, and is preferably used in the present invention.
- potassium chloride or sodium chloride may be used alone or in combination.
- the blending ratio of potassium chloride and / or sodium chloride in the exothermic composition is not particularly limited, but for example, 0.5 to 10% by mass based on the total mass of the exothermic composition About 0.5 to 5% by mass, and more preferably about 0.5 to 3% by mass.
- potassium chloride and / or sodium chloride in the exothermic composition is, for example, 4 parts per 100 parts by mass of the oxidative degradation agent contained in the inner bag. It is desirable to satisfy about 2 to 2800 parts by mass, preferably about 10 to 2600 parts by mass, and more preferably about 50 to 24000 parts by mass.
- the exothermic composition is one that generates heat upon contact with air, and includes components required for the heat generation in addition to potassium chloride.
- the component required for the exotherm is not particularly limited, but as a suitable example of the exothermic composition used in the present invention, at least one selected from the group consisting of potassium chloride and sodium chloride, iron powder , A water retention agent, and a composition containing water.
- the total mass of potassium chloride and / or sodium chloride, iron powder, water retention agent and water is preferably about 80 to 100% by mass.
- Iron powder has a function of generating heat by reacting with oxygen in the air.
- examples of the iron powder used in the exothermic composition include reduced iron and cast iron. These can be used individually by 1 type or in combination of 2 or more types.
- the shape of the iron powder is not particularly limited and may be granular or fibrous.
- the particle size of the granular iron powder is not particularly limited, but it is usually desirable to satisfy the range of about 10 to 300 ⁇ m, preferably about 10 to 100 ⁇ m.
- the above-mentioned particle size of the iron powder is an electric vibration in which 100 g of a sample to be measured (iron powder or the like) is provided with 700 ⁇ m, 650 ⁇ m, 500 ⁇ m, 400 ⁇ m, 300 ⁇ m, 250 ⁇ m, 100 ⁇ m, 50 ⁇ m, and 10 ⁇ m sieves in order from the top. It can be calculated by passing through a sieve and vibrating for 15 minutes, and then measuring the amount left and passed through each sieve. For example, when iron powder having a particle size of 10 to 300 ⁇ m is used, the iron powder that passes through all 300 ⁇ m sieves and remains on any or all of the 10 to 250 ⁇ m sieves may be used.
- the mixing ratio of the iron powder in the exothermic composition is not particularly limited. For example, it is about 30 to 80% by mass, preferably about 45 to 65% by mass, based on the total mass of the exothermic composition. Can be mentioned.
- a water retention agent is a substance having a function of retaining water.
- the water retention agent used in the exothermic composition is not particularly limited, and examples thereof include porous substances and water absorbent resins.
- porous material used as the water retention agent include activated carbon, wood powder, perlite, vermiculite, leechite, and the like.
- Activated carbon can take air into the micropores on the surface and promote the supply of oxygen, or keep the heat so that the heat radiation temperature does not vary.
- Activated carbon has a very porous internal structure and therefore provides particularly good water retention.
- activated carbon not only absorbs water well, but also absorbs water vapor evaporated by the generation of heat of the exothermic composition, and helps prevent escape of water vapor.
- activated carbon can also serve as a water retention material.
- activated carbon can also absorb the odor caused by the oxidation of iron powder.
- activated carbon for example, activated carbon prepared from coconut shell, wood, charcoal, coal, bone charcoal and the like can be suitably used. Examples of the shape of the activated carbon include granular and fibrous shapes.
- activated carbons may be used alone or in combination of two or more.
- the particle size is preferably about 10 to 300 ⁇ m, more preferably about 10 to 100 ⁇ m.
- the measuring method of the said particle size it is the same as that of the case of the particle size of the said iron powder.
- activated carbon, leechite and vermiculite are preferable, and activated carbon and leechite are more preferable, and activated carbon is particularly preferable.
- These porous materials may be used alone or in combination of two or more.
- water-absorbing resins used as water retention agents include isobutylene-maleic anhydride copolymer, polyvinyl alcohol-acrylic acid copolymer, starch-acrylate graft copolymer, and polyacrylic acid.
- examples include cross-linked salts, acrylate-acrylate copolymers, acrylate-acrylamide copolymers, and cross-linked polyacrylonitrile salts.
- a cross-linked polyacrylate is preferable.
- These water-absorbing resins can be used singly or in combination of two or more.
- the particle size of the water absorbent resin is usually about 100 to 500 ⁇ m, preferably about 250 to 400 ⁇ m. About the measuring method of the said particle size, it is the same as that of the case of the particle size of the said iron powder.
- the water retention agent may be a porous material or a water-absorbing resin, or a combination thereof.
- the water retention agent used in the exothermic composition is preferably a porous material, a combination of a porous material and a water absorbent resin; more preferably activated carbon, activated carbon and another porous material (a porous material other than activated carbon); A combination of water-absorbing resins; more preferably, a combination of activated carbon, leechite, and a cross-linked polyacrylate.
- the mixing ratio of the water retention agent in the exothermic composition is not particularly limited, but for example, about 2 to 30% by mass, preferably about 5 to 20% by mass, based on the total mass of the exothermic composition. Can be mentioned. More specifically, when a porous material is used alone as the water retention agent, it is about 10 to 30% by mass, preferably about 10 to 20% by mass, based on the total mass of the exothermic composition. . Further, when the water-absorbing resin is used alone as the water retention agent, it is about 2 to 10% by mass, preferably about 2 to 7% by mass, based on the total mass of the exothermic composition.
- the porous material and the water-absorbing resin are used in combination as the water retention agent, the porous material is 5 to 20% by mass and the water-absorbing resin is 1 to 10% by mass, based on the total mass of the exothermic composition.
- Preferred examples include 7 to 20% by mass of a porous substance and 1 to 5% by mass of a water absorbent resin.
- a combination of activated carbon, another porous material, and a water-absorbing resin is used as a water retention agent, 3 to 20% by mass of activated carbon and 1 to other porous material per total mass of the exothermic composition.
- 10% by mass and 1 to 10% by mass of a water-absorbing resin preferably 5 to 15% by mass of activated carbon, 1 to 5% by mass of another porous substance, and 1 to 5% by mass of a water-absorbing resin.
- water blended in the exothermic composition for example, distilled water or tap water can be used.
- the mixing ratio of water in the exothermic composition is not particularly limited. For example, it is about 1 to 40% by mass, preferably about 20 to 30% by mass, based on the total mass of the exothermic composition. It is done.
- the exothermic composition may contain other additives that can be blended in the exothermic composition as necessary.
- additives include metal salts other than potassium chloride or sodium chloride.
- metal salts other than potassium chloride or sodium chloride include sulfates such as ferric sulfate, potassium sulfate, sodium sulfate, manganese sulfate, and magnesium sulfate; cupric chloride, sodium chloride, calcium chloride, manganese chloride, Examples include chlorides such as magnesium chloride and cuprous chloride.
- the exothermic composition is prepared by mixing each component.
- the exothermic composition may be prepared in a vacuum or in an inert gas atmosphere as necessary in order to suppress heat generation during preparation.
- the exothermic composition can be prepared by mixing, for example, according to the method described in US Pat. No. 4,649,895.
- an inner bag containing an oxidative degradation agent is used as an inner bag for containing the exothermic composition.
- the inner bag is not particularly limited as long as it has air permeability and can contain the exothermic composition. However, in order to suppress leakage of the contained exothermic composition and further improve the feeling of use of the disposable body warmer.
- a laminate in which a breathable resin film is laminated on a woven fabric or a nonwoven fabric is preferable.
- the breathable resin film is disposed on the inner side of the warmer (the side in contact with the exothermic composition), and the woven or non-woven fabric is disposed on the outer side of the warmer (the side in contact with the outside world).
- the resin forming the breathable resin film used in the laminate is not particularly limited, but a thermoplastic synthetic resin is preferably used.
- a thermoplastic synthetic resin is preferably used.
- the thermoplastic synthetic resin constituting the breathable resin film include polyolefin (polyethylene, polypropylene, etc.), polystyrene, polyvinyl chloride, polyvinylidene chloride, polyamide, polyester, polyvinyl alcohol, polyurethane, and ethylene-vinyl acetate. Examples thereof include a copolymer and polycarbonate.
- thermoplastic resins polyolefin (polyethylene, polypropylene, etc.), polystyrene, polyvinyl chloride, polyvinylidene chloride, polyamide are preferred from the viewpoint of maintaining strength during use and providing excellent biodegradability after use.
- a resin having hydrolysis resistance such as polyolefin; more preferably polyolefin; more preferably polyethylene, polypropylene; and particularly preferably polyethylene.
- thermoplastic synthetic resins may be used individually by 1 type, and may be used in combination of 2 or more type.
- air permeability is ensured by providing pores for allowing air to pass through. It is desirable that the pores are of a level that does not allow the contained exothermic composition to pass through.
- a method of providing pores in a resin film is also known. For example, a method of forming pores by passing a resin film through a roll in which rotary blades provided with blades are arranged on the circumference of a disk-shaped tool net; calcium carbonate Examples thereof include a method of stretching a resin sheet containing an inorganic filler such as and forming pores in the stretched resin film.
- the fiber material forming the woven or non-woven fabric laminated with the breathable resin film includes synthetic fibers such as nylon, vinylon, polyester, rayon, acetate, acrylic, polyolefin (polyethylene, polypropylene, etc.) and polyvinyl chloride. And natural fibers such as cotton, hemp, and silk.
- synthetic fibers such as nylon, vinylon, polyester, rayon, acetate, acrylic, polyolefin (polyethylene, polypropylene, etc.) and polyvinyl chloride.
- natural fibers such as cotton, hemp, and silk.
- the basis weight of the woven or non-woven fabric is preferably about 20 to 100 g / m 2 .
- the lamination of the breathable resin film and the woven or non-woven fabric can be usually performed by a laminating method. Any conventionally known method can be applied to the laminating method. For example, a method of laminating by thermal bonding, a method of laminating with an adhesive such as a hot melt adhesive, an acrylic adhesive, or a urethane adhesive can be used. Further, in the laminate used for the inner bag, the breathable resin film and the woven or non-woven fabric may be bonded all over, or may be partially bonded to ensure flexibility. .
- the inner bag used in the present invention includes an oxidative decomposition agent (sometimes referred to as a chemical decomposition agent).
- an oxidative decomposition agent sometimes referred to as a chemical decomposition agent.
- the oxidative degradation agent is contained in the inner bag
- its inclusion mode is not particularly limited.
- the inner bag is a laminate of a breathable resin film and a woven or non-woven fabric
- it may be contained in at least one of the breathable resin film and a laminate of the woven or non-woven fabric
- the oxidative degradation agent is a substance that oxidizes and decomposes the polymer that forms the inner bag and lowers the polymer to such an extent that microbial degradation is possible.
- Oxidative degradation agents are known in the art (e.g., U.S. Pat.Nos. 3,840,512, 3,994,855, 4,101,720, 4,156,666, 4,256,851, 4,360,60, 4,461,853, 4,476,255, 4,517,318, 4,931,488, 4,983,645, 5,096,939, 5,308,906, 5,565,503, 5,854,304; WO88 / 09354, WO92 / 11298, WO94 / 13735, WO00 / 59996, etc.).
- the oxidative decomposition agent include carboxylic acid metal salts, hydroxycarboxylic acids; transition metal compounds (US Pat. No.
- oxidative decomposition agent a combination of a carboxylic acid metal salt and a hydroxycarboxylic acid (US Pat. No. 5,854,304), a combination of a carboxylic acid metal salt and a filler (US Pat. No. 5,565,503), or the like may be used.
- these oxidative degradation agents may be used alone or in combination of two or more.
- the carboxylic acid metal salt used as the oxidative decomposition agent include a metal salt of an aliphatic carboxylic acid having 10 to 20 carbon atoms, preferably a stearic acid metal salt.
- the metal atom constituting the carboxylic acid metal salt include cobalt, cerium, iron, aluminum, antimony, barium, bismuth, cadmium, chromium, copper, gallium, lanthanum, lead, lithium, magnesium, mercury, molybdenum, nickel, Examples include calcium, rare earth, silver, sodium, strontium, tin, tungsten, vanadium, yttrium, zinc, zirconium and the like.
- Preferred examples of the carboxylic acid metal salt used as the oxidative decomposition agent include stearates of metals such as cobalt, cerium, and iron.
- hydroxycarboxylic acid used as the oxidative decomposition agent examples include monohydroxytricarboxylic acids such as citric acid; polyhydroxydicarboxylic acids such as trihydroxyglutaric acid and saccharic acid; dihydroxydicarboxylic acids such as tartaric acid; tartronic acid and malic acid.
- monohydroxy dicarboxylic acids such as erythric® acid, arabic acid, mannitic® acid and the like; dihydroxy monocarboxylic acids such as glyoxylic acid and glyceric acid, and the like. These hydroxycarboxylic acids can be used alone or in combination of two or more.
- filler used as one component of the oxidative decomposition agent examples include inorganic carbonate, synthetic carbonate, nepheline syenite, talc, magnesium hydroxide, aluminum hydroxide, diatomaceous earth, natural or synthetic silica, and calcined clay. Illustrated. These fillers desirably have a particle size of less than 150 mesh. These fillers can be used alone or in combination of two or more.
- transition metal compound used as the oxidative decomposition agent include cobalt or magnesium salts, preferably cobalt or magnesium aliphatic carboxylic acid (carbon number 12 to 20) salt, more preferably cobalt stearate, Examples include cobalt oleate, magnesium stearate, and magnesium oleate. These transition metal compounds can be used alone or in combination of two or more.
- rare earth compound used as the oxidative decomposition agent examples include rare earths belonging to Group 3A of the periodic table, or oxides thereof, and more specifically, cerium (Ce), yttrium (Y), neodymium (Nd ), Rare earth oxides, hydroxides, rare earth sulfates, rare earth nitrates, rare earth acetates, rare earth chlorides, rare earth carboxylates, etc., specifically, cerium oxide, sulfuric acid Examples thereof include dicerium, ceric ammonium sulfate, ceric ammonium nitrate, cerium acetate, lanthanum nitrate, cerium chloride, cerium nitrate, cerium hydroxide, cerium octylate, lanthanum oxide, yttrium oxide, and scandium oxide. These rare earth compounds can be used individually by 1 type or in combination of 2 or more types.
- aromatic ketone used as the oxidative decomposition agent examples include benzophenone, anthraquinone, anthrone, acetylbenzophenone, 4-octylbenzophenone and the like. These aromatic ketones can be used singly or in combination of two or more.
- a combination of a carboxylate and a rare earth compound is preferably used as an oxidative degradation agent from the viewpoint of further promoting biodegradability after use.
- brand name "P-life” made by P life Japan Inc.
- an oxidative degradation agent oxidizes and decomposes a polymer that forms a resin molded body under the action of light, heat, air, etc., and lowers the polymer to such an extent that microbial degradation is possible.
- an oxidative degradation agent that exhibits the oxidative degradation action of the polymer by exposure to light (hereinafter, photorequired oxidative degradation agent) is preferably used.
- Inner bags containing light-requiring oxidative degradation agents are not decomposed under light-shielding conditions, so store them in a light-shielded atmosphere (light-shielding space, light-shielding container, light-shielding bag, etc.) at the pre-use stage such as during manufacturing, distribution, and storage. This makes it possible to maintain a desired function as an inner bag without causing deterioration in durability until use.
- a photorequiring oxidative decomposition agent include an oxidative decomposition agent containing a rare earth compound, and more specifically, trade name “P-life” (manufactured by PLife Japan Inc.) is exemplified. Is done.
- the blending ratio of the oxidative degradation agent blended in the inner bag is appropriately set according to the type of oxidative degradation agent used, the type of constituent material of the inner bag, etc. Examples are 0.48 to 3.8% by mass, preferably 0.72 to 3.1% by mass, and more preferably 0.96 to 2.4% by mass, based on the weight.
- the oxidative degradation agent is 0.4 to 0.4 based on the total weight of the air-permeable resin film. 3% by mass, preferably 0.6-2.5% by mass, more preferably 0.8-2% by mass; and the oxidative degradation agent is 0.08-0. Examples are 8% by mass, preferably 0.12 to 0.6% by mass, and more preferably 0.16 to 0.4% by mass.
- the inner bag containing the oxidative decomposition agent can be produced by a known method.
- the following production method is exemplified: (1) A predetermined amount of oxidative decomposition agent is added to the polymer melt forming the inner bag, and the solution is molded into pellets. (2) Next, the pellet-shaped molded body is melted and molded into the shape of the material (fibrous, film, etc.) that forms the inner bag. (3) The material molded into a fiber, film, etc. is shaped into the inner bag To process.
- Biodegradable disposable body warmer The biodegradable disposable body warmer of the present invention is prepared by adhering and sealing the ends of the inner bag in a state where the exothermic composition is contained in the inner bag.
- the disposable hand warmer thus prepared is provided in an airtight state by an outer bag (preferably light-shielding) that does not allow air to permeate and is used as a warmer by removing the inner bag from the outer bag before use. Is done.
- an outer bag preferably light-shielding
- the disposal method of the biodegradable disposable body warmer of the present invention is not limited, but it has a remarkable characteristic that it has excellent biodegradability, so it is desirable to be subjected to landfill treatment.
- the biodegradable disposable body warmer of the invention is disposed of in landfills or in the mountains after use, the inner bag is subjected to microbial decomposition through oxidative degradation.
- the period until the biodegradable disposable body warmer of the invention is finally decomposed varies depending on the discarded environment, it is normally decomposed to the extent that the inner bag is not visible in about 0.5 to 3 years. .
- the oxidative degradation agent-containing material (containing 80 to 90% oxidative degradation agent) used in the following Examples and Comparative Examples is a trade name “P-life” (manufactured by P-Life Japan Inc .; aliphatic Monocarboxylic acid 50 to 70% by weight, rare earth compound 10 to 20% by weight, and lubricant 10 to 20%).
- Example 1 Preparation of disposable body warmers Preparation of exothermic composition 55% by mass of iron powder with an average particle size of 50 ⁇ m, 13% by mass of activated carbon with an average particle size of 200 ⁇ m, 1% by mass of potassium chloride, 26% by mass of water, 3% by mass of leechite with a particle size of 100 ⁇ m, An exothermic composition was prepared by mixing 2% by mass of a crosslinked sodium salt of an acrylic acid polymer having a diameter of 380 ⁇ m.
- a resin sheet containing 98% by mass of polyethylene and 2% by mass of an oxidative degradation agent-containing material is passed through a roll in which rotary blades with blades are arranged on the circumference of a disk-shaped tool net.
- a non-woven fabric (weighing 25 g / m 2 ) was prepared by the spunbond method.
- the prepared disposable body warmer was quickly put in an outer bag (air-impermeable and light-shielding) made of a polyvinylidene chloride coated film, and the outer bag was sealed.
- the disposable body warmers were taken out from the outer bag and used at the start of the test.
- Example 2 Preparation of Disposable Cairo A disposable warmer was prepared under the same conditions as in Example 1 except that 1% by mass of sodium chloride was used instead of 1% by mass of potassium chloride.
- Test Example 1 Evaluation of decomposability and exothermicity of disposable warmers The disposable warmers of Examples 1 and 2 were taken out of the outer bag and left at room temperature for 24 hours until the heat generation was completed. Thereafter, the disposable body warmer that had generated heat was placed in a thermostatic bath at 50 ° C. and stored for 12 days.
- the disposable body warmer is disassembled, the exothermic composition is taken out, the inner bag part that is not perforated is cut into 2 ⁇ 7 cm, and is pulled.
- the tensile strength was measured by pulling in the MD direction with a testing machine (AGS-H, manufactured by Shimadzu Corporation). As a control, the tensile strength was measured after storing under the same conditions as above using only the inner bag containing no exothermic composition. Subsequently, according to the following calculation formula, the decomposition acceleration rate of the inner bag used in Examples 1 and 2 was measured.
- tensile strength falls, so that the decomposition
- the change over time in the exothermic temperature was measured using the temperature measuring method of JIS S-4100 “Disposable warmers”. Specifically, first, using a heating device comprising a heater and a circulating water bath, an underlay material and a covering material were stacked on the heating portion, and the temperature of the heating portion was raised to 30 ° C. and held at ⁇ 1 ° C.
- FIG. 1 shows the results of measuring the temporal change of the heat generation temperature for the disposable warmers of Examples 1 and 2. Moreover, the result of having measured the decomposition
- the decomposition rate was remarkably faster than the inner bag evaluated as a control.
- the disposable body warmer (Example 1) containing potassium chloride in the exothermic composition has a significantly higher decomposition rate of the inner bag than the case of containing sodium chloride (Example 2), and has excellent biodegradation. It was confirmed that it has sex.
- the inner bag of the disposable body warmers of Examples 1 and 2 is provided with a body warmer without any reduction in strength causing problems in use such as brittleness and leakage of the exothermic composition even after the end of heat generation. The physical properties that should have been maintained.
- Reference Test Example 1 Evaluation of degradability of a product containing a non-pyrogenic composition-1 Activated carbon 28.9% by mass with an average particle size of 200 ⁇ m, 2.2% by mass of potassium chloride, 57.8% by mass of water, 6.7% by mass of leechite with a particle size of 100 ⁇ m, and acryl polymer partial sodium of 380 ⁇ m A non-pyrogenic composition was prepared by mixing 4.4% by mass of the salt cross-linked product. By storing 20 g of the obtained non-pyrogenic composition in the inner bag used in Example 1 above, a non-pyrogenic composition-containing product (Reference Example 1) was prepared.
- Reference Example 2 a non-pyrogenic composition-containing product was used under the same conditions as Reference Example 1 except that 2.2% by weight of sodium chloride was used instead of 2.2% by weight of potassium chloride. Prepared.
- composition-containing products were stored in a thermostat at 50 ° C. for 3 days, and the decomposition acceleration rate of the inner bag was measured by the same method as in Test Example 1 after 1 day and after 3 days of storage.
- Reference Test Example 2 Evaluation of degradability of a product containing a non-pyrogenic composition-2 Instead of 2.2% by mass of potassium chloride, 2.2% by mass of potassium dihydrogen phosphate (Reference Example 3), 2.2% by mass of magnesium sulfate (Reference Example 4), 2.2% by mass of manganese sulfate (Reference Example) 5) Non-pyrogenic composition under the same conditions as Reference Example 1 except that 2.2% by mass of potassium sulfate (Reference Example 6) or 2.2% by mass of sodium sulfate (Reference Example 7) was used. A containing product was prepared.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Emergency Medicine (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Thermotherapy And Cooling Therapy Devices (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/634,451 US9668913B2 (en) | 2010-03-31 | 2011-03-25 | Biodegradable disposable hand warmer |
| CN201180016852.4A CN103096848B (zh) | 2010-03-31 | 2011-03-25 | 生物可降解一次性取暖袋 |
| GB1216695.5A GB2491752B (en) | 2010-03-31 | 2011-03-25 | Biodegradable disposable body warmer |
| KR1020127028578A KR20130019410A (ko) | 2010-03-31 | 2011-03-25 | 생분해성 일회용 손난로 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010082456A JP5484161B2 (ja) | 2010-03-31 | 2010-03-31 | 生分解性使い捨てカイロ |
| JP2010-082456 | 2010-03-31 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011125547A1 true WO2011125547A1 (ja) | 2011-10-13 |
Family
ID=44762501
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/057380 Ceased WO2011125547A1 (ja) | 2010-03-31 | 2011-03-25 | 生分解性使い捨てカイロ |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US9668913B2 (enExample) |
| JP (1) | JP5484161B2 (enExample) |
| KR (1) | KR20130019410A (enExample) |
| CN (1) | CN103096848B (enExample) |
| GB (1) | GB2491752B (enExample) |
| WO (1) | WO2011125547A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105012068A (zh) * | 2015-06-16 | 2015-11-04 | 刘静 | 热敷碗及制作方法 |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5733904B2 (ja) * | 2010-03-31 | 2015-06-10 | 小林製薬株式会社 | 生分解性樹脂成型体の分解促進剤及びその使用 |
| US10448646B2 (en) * | 2013-07-30 | 2019-10-22 | Kobayashi Pharmaceutical Co., Ltd. | Attracting tool |
| WO2019151472A1 (ja) * | 2018-02-05 | 2019-08-08 | フェリック株式会社 | 温度制御剤ならびにそれを用いた発熱組成物及び温熱材 |
| US11306946B2 (en) | 2019-10-30 | 2022-04-19 | Ignik Outdoors, Inc. | Air-activated device-warming systems and methods |
| JP7686958B2 (ja) | 2020-11-30 | 2025-06-03 | 富士フイルムビジネスイノベーション株式会社 | 樹脂粒子 |
| KR102640316B1 (ko) * | 2023-03-21 | 2024-02-27 | (주)제일산업화학 | 친환경 쓰레기 종량제 봉투의 제조방법 및 이로부터 제조된 친환경 쓰레기 종량제 봉투 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002301099A (ja) * | 2001-04-09 | 2002-10-15 | Sanpo Kagaku Kk | 使い捨てカイロ |
| JP2003250830A (ja) * | 2002-03-06 | 2003-09-09 | Osaka Kagaku Gokin Kk | 生分解性使い捨てカイロ |
| JP2006307113A (ja) * | 2005-05-02 | 2006-11-09 | Funen:Kk | 樹脂組成物及び樹脂組成物を用いた樹脂成型品 |
Family Cites Families (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1346650A (en) | 1970-09-18 | 1974-02-13 | Sekisui Chemical Co Ltd | Photo-degradable resin compositions |
| US4476255A (en) * | 1971-11-01 | 1984-10-09 | Owens-Illinois, Inc. | Photoreactive plastic composition and articles degradable by ultraviolet radiation and a process for their manufacture |
| US4101720A (en) * | 1972-03-01 | 1978-07-18 | Owens-Illinois, Inc. | Degradable plastic |
| US3994855A (en) * | 1972-04-18 | 1976-11-30 | Ab Akerlund & Rausing | Degradable polymer composition and process for preparing the same |
| US4360606A (en) | 1972-10-26 | 1982-11-23 | Owens-Illinois, Inc. | Photo-degradable polymer compositions |
| US3840512A (en) | 1972-11-09 | 1974-10-08 | Ici Ltd | Degradable plastics composition |
| US4156666A (en) | 1975-10-31 | 1979-05-29 | Shiseido Company, Ltd. | Degradable synthetic resin compositions |
| IL52974A0 (en) * | 1977-09-21 | 1977-11-30 | Plastopil Hazorea | Agricultural process film products for carrying out the process and controllably degradable polymer compositions for making said film products |
| US4256851A (en) * | 1977-12-12 | 1981-03-17 | Owens-Illinois, Inc. | Degradable plastic composition containing unsaturated alcohol or ester thereof |
| JPS55126817U (enExample) * | 1979-03-05 | 1980-09-08 | ||
| JPS6220583A (ja) | 1985-07-18 | 1987-01-29 | Kiribai Kagaku Kogyo Kk | 発熱剤組成物 |
| CH671961A5 (enExample) | 1987-02-27 | 1989-10-13 | Amrotex Ag | |
| GB8712009D0 (en) | 1987-05-21 | 1987-06-24 | Folk Drive Eng Ltd | Degradable plastics |
| US4938645A (en) | 1989-05-30 | 1990-07-03 | Phillips Plastics Corporation | Tee tree fastener |
| US5096939A (en) * | 1989-06-07 | 1992-03-17 | Techmer Pm | Degradable polymeric compositions |
| US4983645A (en) * | 1989-07-11 | 1991-01-08 | Mobil Oil Corporation | Photoinitiators as light sensitive degradants for polyethylene |
| CA2098911A1 (en) | 1990-12-21 | 1992-06-22 | Graham M. Chapman | Photodegradable plastic composition |
| US5308906A (en) * | 1991-12-11 | 1994-05-03 | Kimberly-Clark Corporation | Extrudable elastomeric composition having controlled rate of degradation |
| US5416133A (en) | 1992-08-24 | 1995-05-16 | Gaia Research Limited Partnership | Chemically degradable polyolefin films |
| US5352716A (en) | 1992-12-16 | 1994-10-04 | Ecostar International, L.P. | Degradable synthetic polymeric compounds |
| JPH0675436U (ja) | 1993-04-02 | 1994-10-25 | フジテクノ株式会社 | 使い捨てカイロの包袋用シート材 |
| EP0786240A4 (en) * | 1994-10-14 | 2000-01-19 | Japan Pionics | THERMOGENIC SHEET AND MANUFACTURING METHOD |
| US5854304A (en) * | 1994-12-14 | 1998-12-29 | Epi Environmental Products Inc. | Degradable/compostable concentrates, process for making degradable/compostable packaging materials and the products thereof |
| US6482872B2 (en) | 1999-04-01 | 2002-11-19 | Programmable Materials, Inc. | Process for manufacturing a biodegradable polymeric composition |
| WO2005058213A1 (ja) * | 2003-12-16 | 2005-06-30 | Kao Corporation | 蒸気温熱具 |
| JPWO2006006654A1 (ja) * | 2004-07-14 | 2008-05-01 | マイコール株式会社 | ヒートクロス及びその製造方法 |
| CN1315975C (zh) * | 2004-10-13 | 2007-05-16 | 上海超迪科技有限公司 | 化学发热材料及发热袋 |
| AU314334S (en) * | 2006-09-08 | 2007-05-31 | Wyeth Corp | Therapeutic device |
| CN101338182B (zh) * | 2007-07-06 | 2010-12-29 | 上海超迪科技有限公司 | 化学发热组成物及发热袋 |
| US20090112231A1 (en) * | 2007-10-31 | 2009-04-30 | Luizzi Joseph M | Epilatory product comprising an air-activated heat-generating module |
-
2010
- 2010-03-31 JP JP2010082456A patent/JP5484161B2/ja not_active Expired - Fee Related
-
2011
- 2011-03-25 CN CN201180016852.4A patent/CN103096848B/zh not_active Expired - Fee Related
- 2011-03-25 WO PCT/JP2011/057380 patent/WO2011125547A1/ja not_active Ceased
- 2011-03-25 GB GB1216695.5A patent/GB2491752B/en not_active Expired - Fee Related
- 2011-03-25 KR KR1020127028578A patent/KR20130019410A/ko not_active Withdrawn
- 2011-03-25 US US13/634,451 patent/US9668913B2/en active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002301099A (ja) * | 2001-04-09 | 2002-10-15 | Sanpo Kagaku Kk | 使い捨てカイロ |
| JP2003250830A (ja) * | 2002-03-06 | 2003-09-09 | Osaka Kagaku Gokin Kk | 生分解性使い捨てカイロ |
| JP2006307113A (ja) * | 2005-05-02 | 2006-11-09 | Funen:Kk | 樹脂組成物及び樹脂組成物を用いた樹脂成型品 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105012068A (zh) * | 2015-06-16 | 2015-11-04 | 刘静 | 热敷碗及制作方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5484161B2 (ja) | 2014-05-07 |
| CN103096848A (zh) | 2013-05-08 |
| US9668913B2 (en) | 2017-06-06 |
| US20130008425A1 (en) | 2013-01-10 |
| CN103096848B (zh) | 2017-03-08 |
| JP2011212170A (ja) | 2011-10-27 |
| KR20130019410A (ko) | 2013-02-26 |
| GB2491752B (en) | 2016-10-26 |
| GB2491752A (en) | 2012-12-12 |
| GB201216695D0 (en) | 2012-10-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5733904B2 (ja) | 生分解性樹脂成型体の分解促進剤及びその使用 | |
| JP5484161B2 (ja) | 生分解性使い捨てカイロ | |
| CN101338182B (zh) | 化学发热组成物及发热袋 | |
| KR102241625B1 (ko) | 소취 필터 | |
| US9920954B2 (en) | Heating tool | |
| USRE32026E (en) | Structure of warmer | |
| TWI690310B (zh) | 發熱件 | |
| US20010003797A1 (en) | Degradable disposable diaper | |
| WO2006006648A1 (ja) | 発熱組成物、発熱体及び発熱体の製造方法 | |
| US10448646B2 (en) | Attracting tool | |
| JPWO2014157726A1 (ja) | 発熱組成物およびそれを用いた使い捨てカイロ | |
| JP7295668B2 (ja) | ストーマ装具用繊維製品及びストーマ装具 | |
| JP2829989B2 (ja) | 炊飯米包装体 | |
| JP3420699B2 (ja) | 機能性エアフィルタ | |
| JP2002051939A (ja) | 携帯用汚物処理袋 | |
| JP2003334419A (ja) | モールド印刷成型シート | |
| JP4080340B2 (ja) | ペット用保温マット | |
| JPH07213264A (ja) | 酸素吸収剤 | |
| JP2003236938A (ja) | 機能性成形体の製造方法及びこの方法を用いて製造された機能性成形体 | |
| JP2024039153A (ja) | 生分解性ポリオレフィンシート | |
| JP2002095453A (ja) | 鮮度保持材 | |
| WO2003055336A1 (en) | Deoxidizer | |
| WO2014157725A1 (ja) | 発熱組成物及びそれを用いた使い捨てカイロ | |
| HK1210690B (en) | Heat generator |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180016852.4 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11765448 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13634451 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 1216695 Country of ref document: GB Kind code of ref document: A Free format text: PCT FILING DATE = 20110325 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1216695.5 Country of ref document: GB |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20127028578 Country of ref document: KR Kind code of ref document: A |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11765448 Country of ref document: EP Kind code of ref document: A1 |