WO2011043371A1 - Composé d'oxazole - Google Patents
Composé d'oxazole Download PDFInfo
- Publication number
- WO2011043371A1 WO2011043371A1 PCT/JP2010/067541 JP2010067541W WO2011043371A1 WO 2011043371 A1 WO2011043371 A1 WO 2011043371A1 JP 2010067541 W JP2010067541 W JP 2010067541W WO 2011043371 A1 WO2011043371 A1 WO 2011043371A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- oxazole
- methyl
- pyrazol
- carboxamide
- mixture
- Prior art date
Links
- 0 *c1n[n](*)cc1NC(c1c[o]c(*)n1)=O Chemical compound *c1n[n](*)cc1NC(c1c[o]c(*)n1)=O 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/02—Stomatological preparations, e.g. drugs for caries, aphtae, periodontitis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/06—Antigout agents, e.g. antihyperuricemic or uricosuric agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
Definitions
- the present invention relates to an oxazole compound useful as an active ingredient of a pharmaceutical composition, in particular, a pharmaceutical composition for prevention and / or treatment of a disease involving interleukin 1 receptor-related kinase 4 (IRAK-4).
- IRAK-4 interleukin 1 receptor-related kinase 4
- Interleukin 1 receptor-related kinase 4 is a member of the IRAK family of protein kinases (Molecular Cell 2003, 11 (2), 293-302).
- IRAK-4 is a kinase downstream of all Toll-like receptors (TLRs), interleukin 1 receptor (IL-1R) and interleukin 18 receptor (IL-18R) except TLR-3. It plays an important role in signal transduction systems (Toll / IL-1 receptor signal transduction system, TIR signal transduction system) via various receptors.
- the IRAK family includes IRAK-1, IRAK-2, and IRAK-3 / M members in addition to IRAK-4.
- IRAK-1 and IRAK-4 have kinase activity, while IRAK-2 and IRAK-3 / M are inactive.
- IRAK-4 is believed to phosphorylate IRAK-1.
- Triggered by IRAK-1 phosphorylation, downstream molecules including NF- ⁇ B and MAPK are activated, causing inflammatory cytokines (IL-1, IL-6, IL-23, TNF- ⁇ , etc.) and chemokines Production of mediators that contribute to the inflammatory response, such as IL-8, RANTES, and arachidonic acid metabolites, is promoted.
- the TIR signaling system involving IRAK-4 is thought to contribute to physiological responses such as defense against pathogens, control of inflammation, innate and acquired immunity, cell survival, and proliferation by controlling the production of inflammatory mediators. Yes.
- it has been suggested to be involved in pathological conditions closely related to inflammation such as acute and chronic inflammatory diseases and autoimmune diseases. This includes many diseases, such as rheumatoid arthritis and glomerulonephritis, and other chronic kidney diseases.
- IRAK-4 knockout mice have reduced albuminuria in a streptozotocin-induced diabetes model (Journalourof the American Society of Nephrology 2008, SA-FC476). Therefore, IRAK-4 inhibitors may be highly effective in treating acute and chronic inflammation and autoimmune diseases.
- Non-patent Document 1 a compound of the following formula (A) has an IRAK-4 inhibitory action.
- IRAK-4 inhibitors include benzimidazole derivatives (Patent Document 1), bicyclic heterocyclic derivatives such as thienopyridine (Patent Document 2), imidazopyridazine derivatives (Patent Document 3), benzimidazole derivatives having an indazole side chain ( Patent Document 4), imidazopyridine derivatives (Non-Patent Document 2) and the like have been reported.
- Patent Document 1 benzimidazole derivatives
- Patent Document 2 bicyclic heterocyclic derivatives such as thienopyridine
- Patent Document 3 imidazopyridazine derivatives
- Patent Document 4 benzimidazole derivatives having an indazole side chain
- Non-Patent Document 2 imidazopyridine derivatives
- Patent Document 5 It has been reported that the compounds of the following formula (B) (Patent Document 5) and the following formula (C) (Patent Document 6) have trkA receptor inhibitory activity and show utility for lower urinary tract diseases and pain, respectively. Has been. However, this document does not disclose the activity relating to IRAK-4, nor does it disclose the compounds described in the present application. (See the official gazette for symbols in the formula.)
- Patent Documents 7 to 9 show that the compound of formula (D) has an immunomodulatory action. However, this document does not disclose the activity relating to IRAK-4, nor does it disclose the compounds described in the present application. (See the official gazette for symbols in the formula.)
- An object of the present invention is to provide a compound useful as an active ingredient of a pharmaceutical composition, for example, a pharmaceutical composition for treating a disease involving IRAK-4.
- the present invention relates to a compound of formula (I) or a salt thereof, and a pharmaceutical composition containing the compound of formula (I) or a salt thereof, and an excipient.
- Ring A is a monocyclic heteroaryl
- R 1 is a monocyclic or bicyclic heteroaryl optionally substituted with 1 to 3 R 10
- R 2 is -C (O) NH 2 , -C (O) NH-R 0 , -C (O) NH-R 00 -OH, -C (O) NH-R 00 -OR 0 , -C ( O) N (R 0 ) 2 , -C (O) NH-cycloalkyl, -C (O) NH-heterocycloalkyl, -C (O) NH- (pyrazolyl optionally substituted with R 0 ), -C (O) -R 0 , -C (O) -cycloalkyl, -S (O) 2 NH 2 , -S (O) 2 NH-R 0 , -S (O) 2 NH-cycloalkyl,- R 00 -OH,
- the present invention also includes a pharmaceutical composition for preventing and / or treating a disease involving IRAK-4, which comprises a compound of formula (I) or a salt thereof, that is, a compound of formula (I) or a salt thereof.
- the present invention relates to a preventive and / or therapeutic agent for diseases involving IRAK-4.
- the present invention also relates to the use of a compound of formula (I) or a salt thereof for the manufacture of a pharmaceutical composition for prevention and / or treatment of a disease involving IRAK-4, and a disease involving IRAK-4.
- a disease involving IRAK-4 comprising administering to a subject an effective amount of a compound of formula (I) or a salt thereof and a compound of formula (I) or a salt thereof for use in the prevention and / or treatment of
- the present invention relates to a method for preventing and / or treating
- the compound of formula (I) has an IRAK-4 inhibitory action and can be used as a prophylactic and / or therapeutic agent for diseases involving IRAK-4.
- inflammatory diseases or autoimmune diseases include inflammatory diseases or autoimmune diseases, particularly musculoskeletal and connective tissue diseases (rheumatoid arthritis, osteoarthritis, systemic lupus erythematosus, Sjogren's syndrome, scleroderma, gout, etc.), Rejection in organ transplantation, auto-inflammatory diseases (Muckle-Wells syndrome, etc.), genitourinary diseases (diabetic nephropathy, glomerulonephritis such as IgA nephropathy, chronic renal failure), liver disease (nonalcoholic steatohepatitis, etc.
- autoimmune diseases particularly musculoskeletal and connective tissue diseases (rheumatoid arthritis, osteoarthritis, systemic lupus erythematosus, Sjogren's syndrome, scleroderma, gout, etc.), Rejection in organ transplantation, auto-inflammatory diseases (Muckle-Wells syndrome, etc.), genitourinary
- Infectious diseases (sepsis, etc.), digestive system diseases (ulcerative colitis, Crohn's disease, etc.), skin inflammatory diseases (atopic dermatitis, psoriasis, etc.), endocrine diseases (diabetes, etc.), cardiovascular diseases (arteries) Sclerosis etc.), central diseases (multiple sclerosis etc.), respiratory diseases (asthma, chronic obstructive pulmonary disease etc.) and cancer.
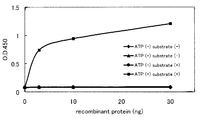
- FIG. 1 shows the phosphorylation activity of an IRAK-1 peptide substrate in a rat IRAK-4 recombinant protein assay system.
- the vertical axis shows the absorbance at 450 nm.
- the horizontal axis indicates the amount of rat IRAK-4 recombinant protein.
- alkyl includes linear alkyl and branched alkyl. Accordingly, “lower alkyl” is straight-chain or branched alkyl having 1 to 6 carbon atoms (hereinafter referred to as C 1-6 ), for example, methyl, ethyl, n-propyl, isopropyl, n- Butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl, n-hexyl group and the like.
- C 1-6 straight-chain or branched alkyl having 1 to 6 carbon atoms
- Another embodiment is C 1-4 alkyl, and yet another embodiment is methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, and still another embodiment. , Methyl.
- Alkylene is a divalent group formed by removing any one hydrogen atom of the above “alkyl”. Accordingly, “lower alkylene” means a linear or branched C 1-6 alkylene such as methylene, ethylene, trimethylene, tetramethylene, pentamethylene, hexamethylene, propylene, methylmethylene, methylethylene, And ethylethylene, 1,2-dimethylethylene, 1,1,2,2-tetramethylethylene group and the like. Another embodiment is C 1-5 alkylene, and yet another embodiment is methylene, ethylene, trimethylene, tetramethylene, pentamethylene.
- Halogen means F, Cl, Br, I.
- Halogeno lower alkyl is lower alkyl substituted with one or more halogens. Another embodiment is lower alkyl substituted with 1 to 5 halogens, and yet another embodiment is difluoromethyl, trifluoromethyl, difluoroethyl, trifluoroethyl.
- Cycloalkyl is a C 3-10 saturated hydrocarbon ring group, which may have a bridge.
- Another embodiment is C 3-6 cycloalkyl, yet another embodiment is C 7-10 cycloalkyl, and yet another embodiment is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, adamantyl. In still another embodiment, they are cyclopropyl, cyclobutyl, cyclopentyl, and cyclohexyl.
- the “aryl” is a C 6-14 monocyclic to tricyclic aromatic hydrocarbon ring group, and includes a cyclic group condensed with a C 5-8 cycloalkene at a double bond site thereof.
- Another embodiment is phenyl or naphthyl, and yet another embodiment is phenyl.
- Heterocycle refers to i) a 3-8 membered, alternatively 5-7 membered monocyclic heterocycle containing from 1 to 4 heteroatoms selected from oxygen, sulfur and nitrogen, and Ii) the monocyclic heterocycle is condensed with one or two rings selected from the group consisting of a monocyclic heterocycle, a benzene ring, a C 5-8 cycloalkane, and a C 5-8 cycloalkene, or It means a cyclic group selected from bi to tricyclic heterocycles containing 1 to 5 heteroatoms selected from oxygen, sulfur and nitrogen, formed by spiro bonds. Ring atoms such as sulfur or nitrogen may be oxidized to form oxides or dioxides.
- heterocycle examples include the following embodiments.
- monocyclic saturated heterocyclic groups (a) those containing 1 to 4 nitrogen atoms, such as aziridinyl, azetidinyl, pyrrolidinyl, pyrazolidinyl, imidazolidinyl, piperidyl, piperazinyl, azepanyl, diazepanyl, azocanyl; (B) those containing 1 to 3 nitrogen atoms and 1 to 2 oxygen atoms and / or 1 to 2 sulfur atoms, for example oxazolidinyl, isoxazolidinyl, thiazolidinyl, isothiazolidinyl, morpholinyl , Thiomorpholinyl, homomorpholinyl, thiazepanyl; (C) those containing 1 to 2 oxygen atoms, such as oxiranyl, oxetanyl, tetrahydrofuranyl, tetrahydropyr
- (1) monocyclic unsaturated heterocyclic groups (a) those containing 1 to 4 nitrogen atoms, such as pyrrolyl, pyrrolinyl, pyrazolyl, pyrazolinyl, imidazolyl, imidazolinyl, triazolyl, tetrazolyl, pyridyl, dihydropyridinyl, Tetrahydropyridinyl, pyridazinyl, pyrimidinyl, dihydropyrimidinyl, pyrazinyl, triazinyl, dihydrotriazinyl, azepinyl; (B) those containing 1 to 3 nitrogen atoms and 1 to 2 oxygen atoms and / or 1 to 2 sulfur atoms, such as oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl, thiadiazolyl, dihydrothiazinyl, oxazinyl
- a condensed polycyclic saturated heterocyclic group (a) one containing 1 to 5 nitrogen atoms, such as quinuclidinyl, 7-azabicyclo [2.2.1] heptyl; (B) those containing 1 to 4 nitrogen atoms, and 1 to 3 oxygen atoms and / or 1 to 3 sulfur atoms, such as 2-oxa-5-azabicyclo [2.2.1] heptyl, trithia Asia Zaindenyl, dioxoloimidazolidinyl; (C) those containing 1 to 3 oxygen atoms and / or 1 to 3 sulfur atoms, such as oxabicyclo [2.2.1] heptyl, 2,6-dioxabicyclo [3.2.2] octyl;
- nitrogen-containing heterocycle means (1)-(a), (1)-(b), (2)-(a), (2)-(b) among the above “heterocycle”.
- (3)-(a), (3)-(b), (4)-(a), and (4)-(b) and the like contain at least one nitrogen atom.
- Heteroaryl is an aromatic ring group in (2) of the above “heterocycle” or a hetero ring group having at least one aromatic ring in (4) as a constituent element, As an aspect, it means a group having a bond on a carbon atom constituting the ring.
- Monocyclic heteroaryls such as pyrrolyl, pyrazolyl, imidazolyl, triazolyl, tetrazolyl, pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl, triazinyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl, thiadiazolyl, furyl, thienyl; Indolyl, indolinyl, isoindolyl, isoindolinyl, indolizinyl, indazolyl, benzimidazolyl, pyrrolopyridinyl, pyrazolopyridinyl, imidazolazolidinyl, benzotriazolyl, purinyl, benzodioxolyl, quinolyl, tetrahydroquinolinyl, isoquinolinyl, te
- Heterocycloalkyl is a heterocyclic group in which the bond between ring atoms consists of only a single bond, as in (1) and (3) of the above “heterocycle”.
- the group which has a bond in the carbon atom which comprises is meant.
- Optionally substituted amino means NH 2 , or an amino group substituted with one or two arbitrary groups, “heteroaryl” having a bond on the nitrogen atom constituting the ring, and Includes “heterocycloalkyl”.
- heteroaryl and “heterocycloalkyl” may be substituted.
- Examples of the substituent in the above-mentioned “amino group substituted with one or two arbitrary groups” include R 0 , halogeno lower alkyl, cycloalkyl, heterocycloalkyl, —C (O) OR 0 , — C (O) -R 0, -C (O) - halogeno-lower alkyl, -R 00 - cycloalkyl, -R 00 - heterocycloalkyl, -R 00 - phenyl, -R 00 - heteroaryl, -R 00 -C (O) OH, -R 00 -C (O) OR 0 , -R 00 -cyano, -R 00 -OH, -R 00 -OR 0 and -R 00 -S (O) 2 -R 0
- cycloalkyl is optionally substituted with halogen, oxo, —OH or —
- heteroaryl and “heterocycloalkyl” having a bond on the nitrogen atom constituting the ring include 1-aziridinyl, 1-azetidinyl, 1-pyrrolidinyl, 1-pyrazolidinyl, 1-imidazolidinyl, 1-piperidyl 1-piperazinyl, 1-azepanyl, 1-diazepanyl, 1-azocanyl, 3-oxazolidinyl, 2-isoxazolidinyl, 3-thiazolidinyl, 2-isothiazolidinyl, 4-morpholinyl, 4-thiomorpholinyl, 4- Homomorpholinyl, 4-thiazepanyl, 1-pyrrolyl, 1-pyrazolyl, 1-imidazolyl, 1-triazolyl, 1-tetrazolyl, 1-indolyl, 1-indolinyl, 2-isoindolyl, 2-isoindolinyl, 1-indazolyl, 1-benzimid
- Examples of the substituent in the “heteroaryl” and “heterocycloalkyl” having a bond at the nitrogen atom constituting the ring include R 0 , halogen, halogeno lower alkyl, —OH, —OR 0 , —R 00 -OH and a group selected from the group consisting of -R 00 -OR 0.
- “optionally substituted” means unsubstituted or having 1 to 5 substituents. In one aspect, it is unsubstituted or has 1 to 3 substituents. In another aspect, it is unsubstituted or has one substituent. In another aspect, it is unsubstituted. In still another embodiment, it has one substituent. In addition, when it has a some substituent, those substituents may be the same or may mutually differ.
- One embodiment of the compound of formula (I) of the present invention is a compound of formula (II), (III) or (IV), another embodiment is a compound of formula (II), and another embodiment is , A compound of formula (III), and yet another embodiment is a compound of formula (IV).
- R 2a is -C (O) NH 2 , -C (O) NH-R 0 , -C (O) NH-cycloalkyl, -C (O) -R 0 , -C (O) -cycloalkyl, -S (O) 2 NH 2 , -S (O) 2 NH-R 0 , oxadiazolyl or tetrazolyl optionally substituted by R 0 ,
- oxadiazolyl in R 2a may be substituted with R 0 , -R 00 -OH or -R 00 -OR 0
- R 3a is, H, R 0, cycloalkyl, heterocycloalkyl, phenyl, pyridyl, -R 00 - cycloalkyl, -R 00 - heterocycloalkyl, -R 00 -OH or, -R 00 -OR 0
- R 2b is -C (O) NH 2 , -C (O) NH-R 0 , -C (O) NH-cycloalkyl, -C (O) -R 0 , -C (O) -cycloalkyl, -S (O) 2 NH 2 , -S (O) 2 NH-R 0 , oxadiazolyl or tetrazolyl optionally substituted by R 0 ,
- oxadiazolyl in R 2b may be substituted with R 0 , -R 00 -OH or -R 00 -OR 0
- R 3b is, H, R 0, cycloalkyl, heterocycloalkyl, phenyl, pyridyl, -R 00 - cycloalkyl, -R 00 - heterocycloalkyl, -R 00 -OH or, -R 00 -OR 0
- R 2c is -C (O) NH 2 , -C (O) NH-R 0 , -C (O) NH-cycloalkyl, -C (O) -R 0 , -C (O) -cycloalkyl, -S (O) 2 NH 2 , -S (O) 2 NH-R 0 , oxadiazolyl or tetrazolyl optionally substituted by R 0 ,
- oxadiazolyl in R 2c may be substituted with R 0 , -R 00 -OH or -R 00 -OR 0
- R 3c is, H, R 0, cycloalkyl, heterocycloalkyl, phenyl, pyridyl, -R 00 - cycloalkyl, -R 00 - heterocycloalkyl, -R 00 -OH or, -R 00 -OR 0
- An embodiment of the A ring in formula (I) is pyrazolyl or thiazolyl, another embodiment is pyrazolyl, and yet another embodiment is thiazolyl.
- R 1 As an embodiment of R 1 , each of pyridyl, pyridazinyl, pyrimidinyl, furyl, thienyl, indazolyl, benzimidazolyl, pyrrolopyridinyl, imidazopyridinyl, quinolyl, which may be substituted with 1 to 3 R 10 , or Benzoxazolyl, and in another embodiment, each is pyridyl or quinolyl optionally substituted by 1 to 3 R 10 , and in another embodiment, 1 to 3 R Pyridin-4-yl or quinolin-4-yl which may be substituted with 10 and, in another embodiment, pyridine-4-yl which may be substituted with 1 to 3 R 10 In still another embodiment, pyridin-4
- R 2 , R 2a , R 2b and R 2c include —C (O) NH 2 , —C (O) NH—R 0 , —S (O) 2 NH 2 or 5 -(Methoxymethyl) -1,3,4-oxadiazol-2-yl, and in another embodiment, -C (O) NH 2 or -C (O) NH-R 0 , another aspect is a -C (O) NH 2.
- R 3 R 3a , a certain aspect of the R 3b and R 3c, R 0, heterocycloalkyl, phenyl, pyridyl, -R 00 - heterocycloalkyl, -R 00 -OH or, -R 00- OR 0 , where heterocycloalkyl, phenyl and pyridyl may be substituted with a group selected from the group consisting of R 0 , halogen or —OR 0 , and in another embodiment, R 0 , heterocycloalkyl, pyridyl, —R 00 -heterocycloalkyl or —R 00 —OR 0 , in yet another embodiment, R 0 , and in another embodiment, —R 00 -OR 0 , yet another embodiment is heterocycloalkyl, yet another embodiment is -R 00 -heterocycloalkyl, and yet another embodiment is R 0 , halogen or ,
- phenyl is optionally substituted with a methoxy group. .
- R 10 R 0
- halogen optionally substituted amino, or —R 00 — (optionally substituted amino)
- another embodiment is R 0 .
- it is halogen, in another embodiment it is optionally substituted amino, and in yet another embodiment, it is methyl.
- R 10 As an aspect of “optionally substituted amino” in R 10 , (i) —NH 2 , (ii) 1 or 2 R 0 , halogeno lower alkyl, cycloalkyl, heterocycloalkyl , -C (O) -R 0, -C (O) - halogeno-lower alkyl, -R 00 - cycloalkyl, -R 00 - heterocycloalkyl, -R 00 -OH and from -R 00 -OR 0
- a compound comprising a combination of two or more groups described in (1) to (6) or a compound thereof Examples thereof include salts, and specific examples thereof include the following combinations.
- R 1 is pyridin-4-yl substituted at the 2-position with R 10
- R 2a is —C (O) NH 2
- R 3a is R 0
- heterocycloalkyl pyridyl, — R 00 - heterocycloalkyl or
- compounds of formula (II) is -R 00 -OR 0.
- Examples of specific compounds included in the present invention include the following compounds. 2- (2-aminopyridin-4-yl) -N- (3-carbamoyl-1-methyl-1H-pyrazol-4-yl) -1,3-oxazole-4-carboxamide, N- (3-carbamoyl-1-methyl-1H-pyrazol-4-yl) -2- (2-methylpyridin-4-yl) -1,3-oxazole-4-carboxamide, 2- (2-aminopyridin-4-yl) -N- [3-carbamoyl-1- (2-methoxyethyl) -1H-pyrazol-4-yl] -1,3-oxazole-4-carboxamide, N- [3-carbamoyl-1- (tetrahydro-2H-pyran-4-yl) -1H-pyrazol-4-yl] -2- (2-methylpyridin-4-yl) -1,3-oxazole-4
- tautomers and geometric isomers may exist depending on the type of substituent.
- the compound of the formula (I) may be described in only one form of an isomer, but the present invention includes other isomers, separated isomers, or those isomers. Also includes mixtures.
- the compound of formula (I) may have an asymmetric carbon atom or axial asymmetry, and optical isomers based on this may exist.
- the present invention also includes separated optical isomers of the compound of formula (I) or a mixture thereof.
- the present invention includes a pharmaceutically acceptable prodrug of the compound of formula (I).
- Pharmaceutically acceptable prodrugs are compounds having groups that can be converted to amino groups, hydroxyl groups, carboxyls, etc. by solvolysis or under physiological conditions. Examples of groups that form prodrugs include those described in Prog. Med., 5, 2157-2161 (1985) and “Development of Pharmaceuticals” (Yodogawa Shoten, 1990), Volume 7, Molecular Design 163-198. It is done.
- the salt of the compound of the formula (I) is a pharmaceutically acceptable salt of the compound of the formula (I), and may form an acid addition salt or a salt with a base depending on the type of substituent. is there.
- inorganic acids such as hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid, nitric acid, phosphoric acid, formic acid, acetic acid, trifluoroacetic acid, propionic acid, oxalic acid, malonic acid, succinic acid, fumaric acid
- Organic acids such as acid, maleic acid, lactic acid, malic acid, mandelic acid, tartaric acid, dibenzoyltartaric acid, ditoluoyltartaric acid, citric acid, methanesulfonic acid, ethanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, aspartic acid, glutamic acid Acid addition salts with, inorganic bases such
- the present invention also includes various hydrates and solvates of the compound of formula (I) and salts thereof, and crystalline polymorphic substances.
- the present invention also includes compounds labeled with various radioactive or non-radioactive isotopes.
- the compound of the formula (I) and a salt thereof can be produced by applying various known synthesis methods utilizing characteristics based on the basic structure or the type of substituent.
- it is effective in terms of production technology to replace the functional group with an appropriate protecting group (a group that can be easily converted into the functional group) at the stage from the raw material to the intermediate.
- protecting groups include protecting groups described in “Greene's Protective Groups in Organic Synthesis (4th edition, 2006)” by PGM Wuts and TW Greene, What is necessary is just to select suitably according to these reaction conditions.
- the desired compound after carrying out the reaction by introducing the protective group, the desired compound can be obtained by removing the protective group as necessary.
- the prodrug of the compound of the formula (I) introduces a specific group at the stage from the raw material to the intermediate as in the case of the protective group, or further reacts with the obtained compound of the formula (I).
- the reaction can be carried out by applying a method known to those skilled in the art, such as ordinary esterification, amidation, dehydration and the like.
- typical production methods of the compound of the formula (I) will be described. Each manufacturing method can also be performed with reference to the reference attached to the said description.
- the manufacturing method of this invention is not limited to the example shown below.
- the compound of the formula (I) can be obtained by reacting the carboxylic acid derivative (1) with the amino compound (2).
- the carboxylic acid derivative (1) and the amino compound (2) are used in an equivalent amount or in an excess amount, and the mixture is heated in the presence of a condensing agent in a solvent inert to the reaction from under cooling. Under stirring, preferably at -20 ° C to 60 ° C, usually for 0.1 hour to 5 days.
- the solvent used here are not particularly limited, but are aromatic hydrocarbons such as benzene, toluene or xylene, halogenated hydrocarbons such as dichloromethane, 1,2-dichloroethane or chloroform, diethyl ether, tetrahydrofuran.
- Ethers such as dioxane, dimethoxyethane, N, N-dimethylformamide, N-methyl-2-pyrrolidone, dimethyl sulfoxide, ethyl acetate, acetonitrile or water, and mixtures thereof.
- condensing agents include, but are not limited to, 1- (3-dimethylaminopropyl) -3-ethylcarbodiimide, dicyclohexylcarbodiimide, 1,1′-carbonyldiimidazole, diphenyl phosphate azide, and phosphorus oxychloride. Is not to be done.
- the reaction may be preferable for the reaction to use an additive such as 1-hydroxybenzotriazole.
- an additive such as 1-hydroxybenzotriazole.
- Performing the reaction in the presence of an organic base such as triethylamine, N, N-diisopropylethylamine, or N-methylmorpholine, or an inorganic base such as potassium carbonate, sodium carbonate, or potassium hydroxide may facilitate the reaction. May be advantageous.
- the method of making it react with an amino compound (2) after converting the carboxyl group part of a carboxylic acid derivative (1) into a reactive derivative can also be used.
- Examples of reactive derivatives of carboxylic acids include acid halides obtained by reacting with halogenating agents such as phosphorus oxychloride, thionyl chloride, oxalyl chloride, mixed acid anhydrides obtained by reacting with isobutyl chloroformate, Examples include active esters obtained by condensation with 1-hydroxybenzotriazole and the like.
- the reaction of these reactive derivatives with the amino derivative (2) is carried out in a solvent inert to the reaction of halogenated hydrocarbons, aromatic hydrocarbons, ethers, etc., under cooling to heating, preferably- It can be carried out at 78 to 60 ° C.
- Compound (5) can be obtained by a coupling reaction between compound (3) and compound (4) or an ester thereof.
- the compounds (3) and (4) or the ester thereof are used in an equivalent amount or one of them in excess, and a mixture thereof is used in a solvent inert to the reaction in the presence of a base and a palladium catalyst at room temperature to This is usually carried out by stirring for 0.1 hour to 5 days under heating under reflux.
- This reaction is preferably performed in an inert gas atmosphere.
- solvent used here are not particularly limited, but aromatic hydrocarbons, ethers, halogenated hydrocarbons, alcohols such as methanol, ethanol, 2-propanol, butanol, N, N-dimethyl Examples include formamide, dimethyl sulfoxide, and mixed solvents thereof.
- base inorganic bases such as sodium carbonate, potassium carbonate and sodium hydroxide are preferable.
- palladium catalyst tetrakis (triphenylphosphine) palladium (0), dichlorobis (triphenylphosphine) palladium (II), palladium (II) chloride-1,1′-bis (diphenylphosphino) ferrocene and the like are preferable.
- Compound (1) can be obtained by hydrolysis reaction of compound (5).
- the hydrolysis reaction can be carried out with reference to Greene and Wuts, “Protective Groups in Organic Synthesis”, 3rd edition, John Wiley & Sons Inc, 1999. [Reference] A. d. Meijere and F. Diederich, “Metal-Catalyzed Cross-Coupling Reactions”, 1st edition, VCH Publishers Inc., 1997 The Chemical Society of Japan, “5th edition, Experimental Chemistry Course (Volume 13)” Maruzen, 2005
- Compound (8) can be obtained by an amidation reaction between compound (6) and compound (7).
- Compound (9) can be produced from compound (8), and compound (10) can be produced from compound (9) with reference to, for example, the methods described in Organic Letters 2000, 2 (8), 1165-1168.
- Compound (1a) can be obtained from compound (10) by a hydrolysis reaction.
- the hydrolysis reaction can be performed with reference to Greene and Wuts, “Protective Groups in Organic Synthesis”, 3rd edition, John Wiley & Sons Inc, 1999.
- the compounds of formula (I) are isolated and purified as free compounds, their salts, hydrates, solvates or polymorphic substances.
- the salt of the compound of formula (I) can also be produced by subjecting it to a conventional salt formation reaction. Isolation and purification are carried out by applying ordinary chemical operations such as extraction, fractional crystallization, and various fractional chromatography. Various isomers can be produced by selecting an appropriate raw material compound, or can be separated by utilizing a difference in physicochemical properties between isomers.
- optical isomers can be obtained by general optical resolution of racemates (for example, fractional crystallization leading to diastereomeric salts with optically active bases or acids, chromatography using chiral columns, etc.) It can also be produced from a suitable optically active raw material compound.
- Test Example 1 Rat IRAK-4 Inhibition Test 1-1: Preparation of Rat IRAK-4 Recombinant Protein a) Rat IRAK-4 Cloning rat IRAK-4 FLAGF primer shown in SEQ ID NO: 5 and rat IRAK shown in SEQ ID NO: 6 Using the -4R primer, the rat IRAK-4 gene was cloned from the rat spleen cDNA library by PCR. Pyrobest (registered trademark) DNA Polymerase (TAKARA BIO, # R005A) was used for PCR.
- amino acid sequence represented by SEQ ID NO: 2 The amino acid sequence represented by SEQ ID NO: 2, the human IRAK-4 amino acid sequence (NM_001114182) and the mouse IRAK-4 amino acid sequence (NM_029926) registered in the GenBank Reference Sequence (NCBI, RefSeq) database were aligned by the Clustal W method. . As a result, the amino acid sequence represented by SEQ ID NO: 2 showed 84.1% and 92.6% homology with human and mouse IRAK-4 amino acid sequences, respectively.
- Rat IRAK-4 recombinant protein was prepared from Sf9 cells infected with rat IRAK-4 expression baculovirus using Anti-FLAG M2 affinity gel (Sigma).
- the base sequence of the rat IRAK-4 fusion gene expressed by the rat IRAK-4 expression baculovirus is shown in SEQ ID NO: 3, and the deduced amino acid sequence of the fusion protein is shown in SEQ ID NO: 4.
- a His tag derived from the pFastBac HT vector and a FLAG tag used for purification of the recombinant protein are fused downstream of the start codon Met.
- a vector-derived sequence is fused before the stop codon.
- IRAK-4 activity can be confirmed by phosphorylation activity of IRAK-1 peptide substrate (Proceedings of the National Academy of Sciences 2002, 99 (8), 5567-5572) .
- the recombinant protein obtained above was reacted in the presence / absence of IRAK-1 peptide substrate and in the presence / absence of ATP to examine the IRAK-4 activity of the recombinant protein.
- Neutravidin 500 ng (Sigma) was added to the plate and allowed to stand overnight.
- Tween-20 Tris-Buffered Saline Tween-20 (TBST) (50 mM Tris-Cl (pH 7.5), 150 mM NaCl, 0.05% Tween20), and 0 or 12.5 pmol biotin-IRAK-1 peptide substrate (biotin -spacer-FGL ARF SRF AGS SPS QSS MVA RTQ TVR GTL A-COOH (Proceedings of the National Academy of Sciences 2002, 99 (8), 5567-5572) was added and allowed to stand for 1 hour.
- TST Tris-Buffered Saline Tween-20
- rat IRAK-4 inhibitory activity Avidin 150 ng (Sigma) was added to the plate and allowed to stand overnight. The next day, the plate was washed twice with TBST, 17.5 pmol of biotin-IRAK-1 peptide substrate was added, and allowed to stand for 1 hour. Thereafter, the plate was washed 3 times with TBST, added with a blocking solution, and allowed to stand for 1 hour. After washing 3 times with TBST, rat IRAK-4 enzyme, test compound, ATP (final concentration is 80 ⁇ M) in reaction buffer of 25 mM Tris-Cl (pH 7.5), 10 mM MgCl 2 , 1 mM DTT, 0.1% Bovine serum albumin And added to the plate.
- IRAK-4 inhibitory activity of the test compound was defined as the concentration of the test compound that suppresses IRAK-4 activity by 50% (IC 50 value). The inhibition rate when no enzyme was added was 100%, the inhibition rate when no test compound was added was 0%, and the IC 50 value of the compound was calculated by the logistic method.
- Test Example 2 IL-1 ⁇ -stimulated IL-6 production inhibition test using human lung epithelial cell line A549 A549 cells were seeded on a 96-well plate at 4 ⁇ 104 cells / 160 ⁇ L / well (1st day) and cultured overnight. . On the next day, a test compound (20 ⁇ L / well) was added and incubated for 1 hour, and then IL-1 ⁇ (R & D Systems, Inc.) was added to a final concentration of 1 ng / mL (20 ⁇ L / well). At this time, each plate was provided with wells to which IL-1 ⁇ was not added as an unstimulated group.
- the culture supernatant was collected and the amount of IL-6 in the supernatant was measured.
- the collected culture supernatant was frozen and stored at -20 ° C or lower, and then dissolved and measured.
- the amount of IL-6 in the supernatant was quantified using Human IL-6 DuoSet Economy Pack (R & D Systems, Inc.) according to the method attached to the kit and measured as absorbance at 450 nm.
- the IL-6 production inhibitory activity of the test compound was defined as the concentration of the test compound that suppresses IL-6 production by 50% (IC 50 value).
- the inhibition rate of the unstimulated group was 100%, the inhibition rate when no test compound was added was 0%, and the IC 50 value of the compound was calculated by the logistic method.
- Test Example 3 Rat Collagen-Induced Arthritis Model 3-1: Induction of Rat Collagen Arthritis, Drug Administration Dissolve bovine-derived type II collagen (collagen technical workshop) in 0.1M acetic acid aqueous solution to make 2 mg / mL solution, etc.
- a collagen emulsion with a final concentration of 1 mg / mL was made by emulsifying with an amount of Incomplete Freund Adjuvant (Difco Laboratories). 7 weeks old female Lewis rats were divided into groups using body weight as an index, and rats other than the untreated group were anesthetized, and 10 or more ridges were placed on the back from which 0.5 mL of the above collagen emulsion was removed.
- Intradermal administration was divided into two sites on the left and right. This first immunization day was defined as day 0. Further, on the seventh day, 0.1 mL of the above-mentioned collagen emulsion was intradermally administered at two locations on the ridge and boosted. No treatment group was immunized. The test compound was continuously orally administered from the first immunization day (or the day after the first immunization day) to the 21st day, or from the 7th day (or 8th day) to the 21st day of the first immunization day.
- Table 2 shows the results of suppression of hindlimb swelling by rat collagen-induced arthritis of some compounds. In addition, 3 mg / kg was administered twice a day.
- Test Example 4 Anti-glomerular basement membrane antibody-induced glomerulonephritis model A rat anti-glomerular basement membrane (GBM) antibody was prepared by immunizing a rat (Japanese white variety healthy) with rat GBM. WKY rats (Nippon Charles River) were male, 7 weeks old, and weighed about 180 g. Nephritis was induced by injecting anti-GBM serum (0.1 mL) into the WKY rat through the tail vein. The rats were divided into groups according to body weight, and the test compound was orally administered twice a day from immediately after the onset of nephritis until the last day of the experiment. Urine collection was performed on days 3, 7, 14, and 21 after the initiation of nephritis.
- GBM rat anti-glomerular basement membrane
- Protein concentration Micro TP Test Wako (Pyrogallol Red Method, Wako Pure Chemical Industries)
- NAG activity NAG Test Shionogi (Yoshio Shionogi)
- Creatinine CRE-EN kainos (enzymatic method, kainos)
- BUN BUN Kainos (Kainos)
- Cholesterol Detamina TC555 (Kyowa Medics)
- Example 7 showed the effect of suppressing glomerulonephritis at the minimum effective dose of 0.3 mg / kg twice a day.
- Test Example 5 db / db mouse albuminuria model
- the animals are db / db mice (male, body weight of about 30 g, CLEA Japan) which are spontaneous type II diabetes mice and control mice (male, body weight of about 25 g). , Japan Marie).
- the db / db mice were divided into groups so that the urinary albumin concentration and blood glucose level at 7 weeks of age were almost uniform, and test compounds were orally administered twice daily from 8 weeks of age.
- Urine collection was performed every week after the start of the test until the 11th week of age. At the end of the experiment, blood was collected from the abdominal vena cava and kidneys were collected. Urine volume, albumin concentration, creatinine concentration and MCP-1 concentration were measured from urine samples.
- Creatinine and glucose concentrations were measured from plasma samples. Each parameter was measured using the following method or kit.
- Albumin concentration ELISA method
- Creatinine CRE-EN kainos (enzyme method, kainos)
- MCP-1 DuoSet mouse MCP-1 (R & D Systems)
- Glucose Glucose CII-Test Wako (Wako Pure Chemical Industries)
- the compound of Example 7 showed an inhibitory effect on diabetic nephropathy at the minimum effective dose of 0.3 mg / kg twice a day.
- the compound of the formula (I) has an IRAK-4 inhibitory activity and an activity to inhibit IL-1-stimulated IL-6 production in the cell line. Moreover, it was confirmed that the compound of Formula (I) has the inhibitory effect with respect to the inflammatory reaction of arthritis, glomerulonephritis, and diabetic nephropathy.
- the compounds of formula (I) are used as pharmaceuticals, in particular diseases related to IRAK-4, such as inflammatory diseases or autoimmune diseases, in particular musculoskeletal and connective tissue diseases (rheumatoid arthritis, osteoarthritis, Systemic lupus erythematosus, Sjogren's syndrome, scleroderma, gout, etc.) rejection in organ transplantation, autoinflammatory diseases (Muckle-Wells syndrome, etc.), genitourinary diseases (diabetic nephropathy, IgA nephropathy, etc.) , Chronic renal failure), liver disease (non-alcoholic steatohepatitis, etc.), infection (sepsis, etc.), digestive system diseases (ulcerative colitis, Crohn's disease, etc.), skin inflammatory diseases (atopic dermatitis, psoriasis) Etc.), endocrine diseases (diabetes, etc.), cardiovascular diseases (arteriosclerosis, etc.), inflammatory
- Test Example 6 Cytochrome P450 (CYP) 3A4 enzyme inhibition test 6-1: Inhibition test I (calculation of residual rate I) Using a 96-well plate, substrate 2 ⁇ M (midazolam), test compound 5 ⁇ M, human liver microsome (0.1 mg protein / mL) 0.1 mM EDTA, 1 mM NADPH-containing 100 mM phosphate buffer (pH 7.4), total volume 150 ⁇ L Incubated for 20 minutes at 37 ° C. Thereafter, 130 ⁇ L of an aqueous solution containing 80% acetonitrile was added to stop the reaction. Thereafter, the sample was analyzed by LC / MS / MS, and the residual ratio I was calculated using Equation 1 below.
- Residual rate I (%) A i , I / A 0 , I x 100
- a i , I amount of metabolite produced after reaction in the presence of test compound in inhibition test I
- a 0 , I amount of metabolite produced after reaction in the absence of test compound in inhibition test I
- inhibition test II calculation of residual rate II
- Residual rate II (%) A i , II / A 0 , II / (A i , I / A 0 , I ) x 100
- a i , II amount of metabolite produced after reaction in the presence of test compound in inhibition test II
- a 0 , II amount of metabolite produced after reaction in the absence of test compound in inhibition test II
- the compound of Example 7 showed a CYP3A4 residual rate of 99% during the reversible inhibition test and a CYP3A4 residual rate of 107% during the time-dependent test.
- a pharmaceutical composition containing one or more compounds of the formula (I) or a salt thereof as an active ingredient is an excipient normally used in the art, that is, a pharmaceutical excipient, a pharmaceutical carrier, etc.
- Administration is oral by tablet, pill, capsule, granule, powder, liquid, etc., or injection such as intraarticular, intravenous, intramuscular, suppository, eye drops, ophthalmic ointment, transdermal solution, ointment Any form of parenteral administration using an agent, a transdermal patch, a transmucosal solution, a transmucosal patch, an inhalant, etc. may be used.
- a solid composition for oral administration tablets, powders, granules and the like are used.
- one or more active ingredients are mixed with at least one inert excipient.
- the composition may contain an inert additive such as a lubricant, a disintegrant, a stabilizer and a solubilizing agent according to a conventional method. If necessary, tablets or pills may be coated with a sugar coating or a film of a gastric or enteric substance.
- Liquid compositions for oral administration include pharmaceutically acceptable emulsions, solutions, suspensions, syrups or elixirs and the like, and commonly used inert diluents such as purified water. Or it contains ethanol.
- the liquid composition may contain solubilizers, wetting agents, auxiliaries such as suspending agents, sweeteners, flavors, fragrances, and preservatives.
- the injection for parenteral administration contains a sterile aqueous or non-aqueous solvent, suspension, or emulsion.
- aqueous solvent include distilled water for injection or physiological saline.
- Non-aqueous solvents include alcohols such as ethanol.
- Such compositions may further contain isotonic agents, preservatives, wetting agents, emulsifiers, dispersants, stabilizers, or solubilizing agents. These are sterilized by, for example, filtration through a bacteria-retaining filter, blending with a bactericide, or irradiation. These can also be used by producing a sterile solid composition and dissolving or suspending it in sterile water or a sterile solvent for injection before use.
- External preparations include ointments, plasters, creams, jellies, poultices, sprays, lotions, eye drops, eye ointments and the like.
- ointment bases commonly used ointment bases, lotion bases, aqueous or non-aqueous solutions, suspensions, emulsions, and the like.
- a transmucosal agent such as an inhalant or a nasal agent is used in a solid, liquid or semi-solid form, and can be produced according to a conventionally known method.
- known excipients, and further pH adjusters, preservatives, surfactants, lubricants, stabilizers, thickeners and the like may be appropriately added.
- an appropriate device for inhalation or insufflation can be used.
- a known device such as a metered dose inhalation device or a nebulizer
- the compound is administered alone or as a powder in a formulated mixture or as a solution or suspension in combination with a pharmaceutically acceptable carrier. be able to.
- the dry powder inhaler or the like may be for single or multiple administration, and a dry powder or a powder-containing capsule can be used. Alternatively, it may be in the form of a pressurized aerosol spray using a suitable propellant such as chlorofluoroalkane, hydrofluoroalkane, or a suitable gas such as carbon dioxide.
- a suitable propellant such as chlorofluoroalkane, hydrofluoroalkane, or a suitable gas such as carbon dioxide.
- the appropriate daily dose is about 0.001 to 100 mg / kg, preferably 0.1 to 30 mg / kg, more preferably 0.1 to 10 mg / kg per body weight. Or in 2 to 4 divided doses.
- the daily dose is suitably about 0.0001 to 10 mg / kg per body weight, and is administered once to several times a day.
- a transmucosal agent about 0.001 to 100 mg / kg per body weight is administered once to several times a day. The dosage is appropriately determined according to the individual case in consideration of symptoms, age, sex and the like.
- the compound of the formula (I) can be used in combination with various therapeutic or preventive agents for diseases for which the compound of the formula (I) is considered to be effective.
- the combination may be administered simultaneously, separately separately, or at desired time intervals.
- a pharmaceutical preparation containing various therapeutic or prophylactic agents for the diseases for which the compound of formula (I) is considered to be effective, and a compound of formula (I), even if the coadministration preparation is formulated separately It may be a composition.
- the production method of the compound of formula (I) and its raw material compound will be described in more detail based on Examples.
- this invention is not limited to the compound as described in the following Example.
- the manufacturing method of a raw material compound is shown to a manufacture example.
- the production method of the compound of the formula (I) is not limited to the production methods of the specific examples shown below, and the compound of the formula (I) may be a combination of these production methods or a person skilled in the art. It can also be produced by methods that are self-evident.
- HCl in the structural formula indicates a hydrochloride, and the number before HCl indicates a molar ratio.
- 2HCl means dihydrochloride.
- Production Example 7 A mixture of methyl 2- (2-chloropyridin-4-yl) -1,3-oxazole-4-carboxylate (960 mg), m-chloroperbenzoic acid (903 mg) and methylene chloride (20 mL) The mixture was stirred at 40 ° C. for 10 hours. After allowing to cool, a saturated aqueous sodium thiosulfate solution and a saturated aqueous sodium hydrogen carbonate solution were added, and the mixture was extracted with chloroform. The organic layer was washed successively with saturated aqueous sodium hydrogen carbonate solution and saturated brine, and then dried over anhydrous magnesium sulfate.
- the filtrate was concentrated under reduced pressure, and a mixture of THF and 1M hydrochloric acid (1: 1, 10 mL) was added to the residue, followed by stirring at room temperature for 2 hours.
- the reaction mixture was washed with diethyl ether, and then the pH was adjusted to about 10 by adding 2M aqueous potassium carbonate solution to the obtained aqueous layer. After extracting with chloroform, the organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate.
- Production Example 30 6-chloropyridin-3-yl) -4-nitro-1H-pyrazole-3-carboxamide (222 mg), 10% palladium on carbon (50% water content, 177 mg), ammonium formate (262 mg), and A mixture of methanol (6.66 mL) was stirred at 70 ° C. for 1 hour. After adding ammonium formate (209 mg), the mixture was further stirred at 70 ° C. for 1 hour.
- Tables 3 to 25 show the structure, production method, and physicochemical data of each of the production example compounds.
- Example 1 4-Amino-1-methyl-1H-pyrazole-3-carboxamide (250 mg), 2- ⁇ 2-[(tert-butoxycarbonyl) (2-methoxyethyl) amino] pyridin-4-yl ⁇ -1,3
- a mixture of -oxazole-4-carboxylic acid (713 mg), WSC ⁇ HCl (564 mg), HOBt (397 mg) and DMF (10 mL) was stirred at room temperature for 2 hours.
- the reaction mixture was concentrated, water was added, and the mixture was extracted with ethyl acetate.
- the organic layer was washed successively with saturated aqueous sodium hydrogen carbonate solution and saturated brine, and dried over anhydrous magnesium sulfate.
- Example 6 4-Amino-1- (tetrahydro-2H-pyran-4-yl) -1H-pyrazole-3-carboxamide (753 mg), 2- (2-methylpyridin-4-yl) -1,3-oxazole-4 -To a mixture of carboxylic acid (731 mg), HOBt (726 mg) and DMF (18.3 mL) was added WSC ⁇ HCl (1.03 g) under ice cooling, and the mixture was stirred at room temperature for 4 hours.
- Example 7 N- [3-carbamoyl-1- (tetrahydro-2H-pyran-4-yl) -1H-pyrazol-4-yl] -2- (2-methylpyridin-4-yl) -1,3-oxazole-4 -To a mixture of carboxamide (45 mg) in methanol (1.8 mL) was added 4M hydrogen chloride / 1,4-dioxane solution (286 ⁇ L), and the mixture was stirred at room temperature for 1 hour.
- Example 8 tert-butyl ⁇ 4- [4-( ⁇ 3- [5- (methoxymethyl) -1,3,4-oxadiazol-2-yl] -1-methyl-1H-pyrazol-4-yl ⁇ carbamoyl)
- a mixture of -1,3-oxazol-2-yl] pyridin-2-yl ⁇ carbamate (84 mg), TFA (1 mL) and methylene chloride (2.2 mL) was stirred at room temperature for 6 hours.
- the reaction mixture was concentrated under reduced pressure, ethyl acetate was added to the residue, and the resulting solid was collected by filtration.
- Example 9 tert-butyl ⁇ 4- [4-( ⁇ 3-[(2,5-dimethyl-1H-pyrrol-1-yl) sulfonyl] -1-methyl-1H-pyrazol-4-yl ⁇ carbamoyl) -1,3 -Oxazol-2-yl] pyridin-2-yl ⁇ (2,2,2-trifluoroethyl) carbamate (155 mg) and 1,4-dioxane (2 mL) were mixed with 6M hydrochloric acid (7 mL). After adding at room temperature, the temperature was raised to 80 ° C. and stirred for 3 hours.
- Example 10 2-Oxalyl chloride (41 ⁇ L) was added to a mixture of 2- ⁇ 2-[(tert-butoxycarbonyl) amino] pyridin-4-yl ⁇ -1,3-oxazole-4-carboxylic acid (120 mg) and methylene chloride at room temperature. After the addition, 1 drop of DMF was added and stirred at room temperature for 30 minutes. To the reaction mixture, a mixture of 5-amino-2-methyl-1,3-thiazole-4-carboxamide (62 mg), triethylamine (110 ⁇ L) and methylene chloride was added under ice cooling, and the mixture was stirred for 1 hour.
- reaction mixture was diluted with chloroform, washed successively with water and saturated brine, and dried over anhydrous magnesium sulfate.
- the solvent was distilled off under reduced pressure, and the resulting solid was washed with methanol and then dried under reduced pressure to obtain tert-butyl (4- ⁇ 4-[(4-carbamoyl-2-methyl-1,3-thiazole-5- Yl) carbamoyl] -1,3-oxazol-2-yl ⁇ pyridin-2-yl) carbamate (46 mg) was obtained as a pale yellow solid.
- Example 11 tert-butyl (4- ⁇ 4-[(4-carbamoyl-2-methyl-1,3-thiazol-5-yl) carbamoyl] -1,3-oxazol-2-yl ⁇ pyridin-2-yl) carbamate ( 46 mg) was suspended in methanol (3 mL), 4M hydrogen chloride / 1,4-dioxane solution (600 ⁇ L) was added, and the mixture was stirred at room temperature overnight. Furthermore, 4M hydrogen chloride / 1,4-dioxane solution (1 mL) was added, and the mixture was stirred at 60 ° C.
- Example 52 tert-butyl rel-2- ⁇ 2-[(1R, 2R, 4S) -bicyclo [2.2.1] hept-2-ylamino] pyridin-4-yl ⁇ -1,3-oxazole-4-carboxylate (115 mg) was dissolved in methylene chloride (2.3 mL), TFA (198 ⁇ L) was added, and the mixture was stirred at room temperature for 3 hours.
- Example 53 N-ethyl in a mixture of rel- (1S, 2R, 4R) -7-oxabicyclo [2.2.1] heptan-2-amine hydrochloride (673 mg), benzotrifluoride (11.25 mL), chloroform (22.5 mL) -N-isopropylpropan-2-amine (1.16 mL) was added, and the mixture was stirred at room temperature for 15 min. Methyl 2- (1-oxidepyridin-4-yl) -1,3-oxazole-4-carboxylate (495 mg) was added, and the mixture was cooled to ⁇ 10 ° C. and p-toluenesulfonic anhydride (1.469 g ) Was gradually added.
- Tables 46 to 67 show structures of other compounds of the present invention. These can be easily synthesized by using the above-described production methods, the methods described in the Examples and methods obvious to those skilled in the art, or variations thereof.
- the pharmaceutical composition of the present invention is suitable for inflammatory diseases, autoimmune diseases and the like. It can be used as a prophylactic or therapeutic agent for diseases related to IRAK-4.
- the description of “Artificial Sequence” is described in the numerical heading ⁇ 223> of the sequence listing.
- the nucleotide sequence represented by the sequence number 3 in the sequence listing is derived from the expression vector from 1-78 and 1492-1578, from the synthetic primer, from 79-111, from 112- No. 1491 is a fusion gene consisting of rat IRAK-4 ORF.
- the amino acid sequence represented by the sequence of SEQ ID NO: 4 is a deduced amino acid sequence encoded by the fusion gene represented by the sequence of SEQ ID NO: 3.
- the base sequences represented by the sequences of SEQ ID NOs: 5 and 6 are artificially synthesized primer sequences.
Landscapes
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Pulmonology (AREA)
- Diabetes (AREA)
- Rheumatology (AREA)
- Urology & Nephrology (AREA)
- Pain & Pain Management (AREA)
- Dermatology (AREA)
- Physical Education & Sports Medicine (AREA)
- Emergency Medicine (AREA)
- Communicable Diseases (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Endocrinology (AREA)
- Obesity (AREA)
- Vascular Medicine (AREA)
- Oncology (AREA)
- Hematology (AREA)
- Transplantation (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Gastroenterology & Hepatology (AREA)
- Plural Heterocyclic Compounds (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
L'invention porte sur un nouveau et excellent procédé prophylactique et/ou thérapeutique pour des maladies associées à la kinase-4 associée au récepteur de l'interleukine-1 (IRAK-4), qui est basé sur un effet inhibiteur de la kinase-4 associée au récepteur de l'interleukine-1 (IRAK-4). De façon spécifique, l'invention porte sur un dérivé amide d'acide oxazolecarboxylique qui est caractérisé en ce qu'un oxazole qui est substitué par un groupe hétéroaryle en position 2 est lié à un noyau hétéroaryle par l'intermédiaire d'un carboxamide. Le dérivé amide d'acide oxazolecarboxylique a un puissant effet inhibiteur sur l'IRAK-4 et peut servir d'agent prophylactique et/ou thérapeutique pour des maladies associées à l'IRAK-4 telles que des maladies inflammatoires et des maladies auto-immunes.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009233048A JP2012254939A (ja) | 2009-10-07 | 2009-10-07 | オキサゾール化合物 |
| JP2009-233048 | 2009-10-07 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011043371A1 true WO2011043371A1 (fr) | 2011-04-14 |
Family
ID=43856824
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2010/067541 WO2011043371A1 (fr) | 2009-10-07 | 2010-10-06 | Composé d'oxazole |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP2012254939A (fr) |
| WO (1) | WO2011043371A1 (fr) |
Cited By (81)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013533318A (ja) * | 2010-08-11 | 2013-08-22 | ミレニアム ファーマシューティカルズ, インコーポレイテッド | ヘテロアリールおよびその使用 |
| JP2013544879A (ja) * | 2010-12-07 | 2013-12-19 | アクテリオン ファーマシューティカルズ リミテッド | Alx受容体アゴニストとしてのヒドロキシル化アミノトリアゾール誘導体 |
| JP2013544878A (ja) * | 2010-12-07 | 2013-12-19 | アクテリオン ファーマシューティカルズ リミテッド | Alx受容体アゴニストとしてのオキサゾリル−メチルエーテル誘導体 |
| US8765746B2 (en) | 2010-10-13 | 2014-07-01 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8796268B2 (en) | 2010-08-11 | 2014-08-05 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8796314B2 (en) | 2009-01-30 | 2014-08-05 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8815271B2 (en) | 2010-11-03 | 2014-08-26 | Dow Agrosciences, Llc. | Pesticidal compositions and processes related thereto |
| US8901153B2 (en) | 2012-04-27 | 2014-12-02 | Dow Agrosciences, Llc. | Pesticidal compositions and processes related thereto |
| WO2015006181A1 (fr) | 2013-07-11 | 2015-01-15 | Merck Sharp & Dohme Corp. | Inhibiteurs amidopyrazole substitués de kinases associées aux récepteurs de l'interleukine (irak -4) |
| JP2015503622A (ja) * | 2012-01-13 | 2015-02-02 | ブリストル−マイヤーズ スクイブ カンパニーBristol−Myers Squibb Company | キナーゼ阻害剤として有用なトリアゾリルまたはトリアジアゾリル置換されたピリジル化合物 |
| JP2015503618A (ja) * | 2012-01-10 | 2015-02-02 | ニンバス アイリス, インコーポレイテッド | Irak阻害剤およびその使用 |
| JP2015503620A (ja) * | 2012-01-13 | 2015-02-02 | ブリストル−マイヤーズ スクイブ カンパニーBristol−Myers Squibb Company | キナーゼ阻害剤として有用な複素環置換されたピリジル化合物 |
| US9024031B1 (en) | 2014-08-19 | 2015-05-05 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9029555B1 (en) | 2014-07-31 | 2015-05-12 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9029554B1 (en) | 2013-10-17 | 2015-05-12 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9029556B1 (en) | 2014-07-31 | 2015-05-12 | Dow Argosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9029411B2 (en) | 2008-01-25 | 2015-05-12 | Millennium Pharmaceuticals, Inc. | Thiophenes and uses thereof |
| WO2015068856A1 (fr) | 2013-11-08 | 2015-05-14 | Takeda Pharmaceutical Company Limited | Pyrazole pour le traitement de troubles auto-immuns |
| US9044017B2 (en) | 2013-10-17 | 2015-06-02 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9062038B2 (en) | 2010-08-11 | 2015-06-23 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| WO2015104662A1 (fr) | 2014-01-10 | 2015-07-16 | Aurigene Discovery Technologies Limited | Composés d'imidazole utilisables en tant qu'inhibiteurs de l'irak4 |
| US9085564B2 (en) | 2013-10-17 | 2015-07-21 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9085552B1 (en) | 2014-09-12 | 2015-07-21 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9090601B2 (en) | 2009-01-30 | 2015-07-28 | Millennium Pharmaceuticals, Inc. | Thiazole derivatives |
| US9102654B2 (en) | 2013-10-17 | 2015-08-11 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9102655B2 (en) | 2013-10-17 | 2015-08-11 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9108946B2 (en) | 2013-10-17 | 2015-08-18 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9139589B2 (en) | 2009-01-30 | 2015-09-22 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US9137998B2 (en) | 2013-10-22 | 2015-09-22 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9144241B2 (en) | 2013-10-22 | 2015-09-29 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| JP2015529235A (ja) * | 2012-09-26 | 2015-10-05 | エフ・ホフマン−ラ・ロシュ・アクチェンゲゼルシャフト | 環状エーテルピラゾール−4−イル−ヘテロシクリル−カルボキサミド化合物と使用方法 |
| US9149040B2 (en) | 2013-10-22 | 2015-10-06 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9155304B2 (en) | 2013-10-22 | 2015-10-13 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9174962B2 (en) | 2013-10-17 | 2015-11-03 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9199964B1 (en) | 2014-07-31 | 2015-12-01 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9282740B2 (en) | 2013-10-22 | 2016-03-15 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9282739B2 (en) | 2012-04-27 | 2016-03-15 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US9295260B2 (en) | 2013-10-22 | 2016-03-29 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9295258B2 (en) | 2013-10-22 | 2016-03-29 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| EP2903613A4 (fr) * | 2012-10-08 | 2016-04-20 | Merck Sharp & Dohme | Inhibiteurs d'activité d'irak4 |
| US9445597B2 (en) | 2013-10-22 | 2016-09-20 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9474276B2 (en) | 2013-10-22 | 2016-10-25 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| WO2016172560A1 (fr) * | 2015-04-22 | 2016-10-27 | Rigel Pharmaceuticals, Inc. | Composés de pyrazole et procédé de fabrication et d'utilisation de ces composés |
| US9491944B2 (en) | 2013-10-22 | 2016-11-15 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9497966B2 (en) | 2013-10-22 | 2016-11-22 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9549560B2 (en) | 2013-10-22 | 2017-01-24 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9598440B2 (en) | 2012-10-08 | 2017-03-21 | Merck Sharp & Dohme Corp. | Inhibitors of IRAK4 activity |
| US9655365B2 (en) | 2011-10-26 | 2017-05-23 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US9708288B2 (en) | 2012-04-27 | 2017-07-18 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US9788546B2 (en) | 2013-10-22 | 2017-10-17 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9788545B2 (en) | 2013-10-22 | 2017-10-17 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9801376B2 (en) | 2013-10-22 | 2017-10-31 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9801383B2 (en) | 2013-10-22 | 2017-10-31 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9808008B2 (en) | 2013-10-22 | 2017-11-07 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| WO2018052058A1 (fr) * | 2016-09-15 | 2018-03-22 | 武田薬品工業株式会社 | Composé hétérocyclique |
| US9951086B2 (en) | 2013-12-19 | 2018-04-24 | Bayer Pharma Aktiengesellschaft | Indazolecarboxamides, processes for their preparation, pharmaceutical preparations comprising them and their use for producing medicaments |
| WO2018089199A1 (fr) * | 2016-10-26 | 2018-05-17 | Rigel Pharmaceuticals, Inc. | Dérivés d'oxazole destinés à être utilisés en tant qu'inhibiteurs d'irak et leur procédé de préparation |
| US9988376B2 (en) | 2013-07-03 | 2018-06-05 | Glaxosmithkline Intellectual Property Development Limited | Benzothiophene derivatives as estrogen receptor inhibitors |
| US9993514B2 (en) | 2013-07-03 | 2018-06-12 | Glaxosmithkline Intellectual Property Development Limited | Compounds |
| US10100033B2 (en) | 2016-12-29 | 2018-10-16 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US10233155B2 (en) | 2016-12-29 | 2019-03-19 | Dow Agrosciences Llc | Processes for the preparation of pesticide compounds |
| EP3466955A1 (fr) | 2014-01-13 | 2019-04-10 | Aurigene Discovery Technologies Limited | Procédé pour la préparation de dérivés de oxazolo[4,5-b]pyridine et thiazolo[4,5-b]pyridine en tant qu'inhibiteurs de irak4 pour le traitement du cancer |
| WO2019111218A1 (fr) | 2017-12-08 | 2019-06-13 | Cadila Healthcare Limited | Nouveaux composés hétérocycliques utilisés en tant qu'inhibiteurs d'irak4 |
| US10336762B2 (en) | 2017-02-16 | 2019-07-02 | Gilead Sciences, Inc. | Pyrrolo[1,2-b]pyridazine derivatives |
| WO2019133531A1 (fr) | 2017-12-26 | 2019-07-04 | Kymera Therapeutics, Inc. | Agents de dégradation de kinases irak et leurs utilisations |
| JP2019533687A (ja) * | 2016-10-26 | 2019-11-21 | ライジェル ファーマシューティカルズ, インコーポレイテッド | Irak阻害剤としてのピラゾールアミド化合物 |
| US10774076B2 (en) | 2015-08-04 | 2020-09-15 | Rigel Pharmaceuticals, Inc. | Benzazole compounds and methods for making and using the compounds |
| US10875866B2 (en) | 2018-07-13 | 2020-12-29 | Gilead Sciences, Inc. | Pyrrolo[1,2-B]pyridazine derivatives |
| US11065231B2 (en) | 2017-11-17 | 2021-07-20 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of interleukin-1 receptor- associated kinase 4 polypeptides |
| US11117889B1 (en) | 2018-11-30 | 2021-09-14 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11292792B2 (en) | 2018-07-06 | 2022-04-05 | Kymera Therapeutics, Inc. | Tricyclic CRBN ligands and uses thereof |
| US11358948B2 (en) | 2017-09-22 | 2022-06-14 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| US11370787B2 (en) | 2019-08-30 | 2022-06-28 | Rigel Pharmaceuticals, Inc. | Pyrazole compounds, formulations thereof, and a method for using the compounds and/or formulations |
| US20220289710A1 (en) * | 2018-11-02 | 2022-09-15 | Merck Sharp & Dohme Llc | 2-amino-n-heteroaryl-nicotinamides as nav1.8 inhibitors |
| US11485743B2 (en) | 2018-01-12 | 2022-11-01 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| US11512080B2 (en) | 2018-01-12 | 2022-11-29 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| US11591332B2 (en) | 2019-12-17 | 2023-02-28 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11623932B2 (en) | 2017-09-22 | 2023-04-11 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| US11685750B2 (en) | 2020-06-03 | 2023-06-27 | Kymera Therapeutics, Inc. | Crystalline forms of IRAK degraders |
| US11707457B2 (en) | 2019-12-17 | 2023-07-25 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11993594B2 (en) | 2022-09-27 | 2024-05-28 | Rigel Pharmaceuticals, Inc. | IRAK inhibitors and method for making and using |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008006793A1 (fr) * | 2006-07-14 | 2008-01-17 | Glaxo Group Limited | Indoles |
| WO2008009954A1 (fr) * | 2006-07-21 | 2008-01-24 | Astex Therapeutics Limited | Utilisation médicale d'inhibiteurs de kinases dépendants de la cycline |
| WO2009054468A1 (fr) * | 2007-10-24 | 2009-04-30 | Astellas Pharma Inc. | Composé à base d'azolecarboxamide ou son sel |
| US20090149517A1 (en) * | 2007-12-10 | 2009-06-11 | Ulrich Bothe | Novel 2-substituted tiazole-4-carboxamide derivatives, their preparation and use as pharmaceuticals |
-
2009
- 2009-10-07 JP JP2009233048A patent/JP2012254939A/ja not_active Withdrawn
-
2010
- 2010-10-06 WO PCT/JP2010/067541 patent/WO2011043371A1/fr active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008006793A1 (fr) * | 2006-07-14 | 2008-01-17 | Glaxo Group Limited | Indoles |
| WO2008009954A1 (fr) * | 2006-07-21 | 2008-01-24 | Astex Therapeutics Limited | Utilisation médicale d'inhibiteurs de kinases dépendants de la cycline |
| WO2009054468A1 (fr) * | 2007-10-24 | 2009-04-30 | Astellas Pharma Inc. | Composé à base d'azolecarboxamide ou son sel |
| US20090149517A1 (en) * | 2007-12-10 | 2009-06-11 | Ulrich Bothe | Novel 2-substituted tiazole-4-carboxamide derivatives, their preparation and use as pharmaceuticals |
Non-Patent Citations (1)
| Title |
|---|
| BUCKLEY, G.M. ET AL.: "IRAK-4 inhibitors. Part 1: A series of amides", BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, vol. 18, no. 11, 2008, pages 3211 - 3214, XP022711199, DOI: doi:10.1016/j.bmcl.2008.04.058 * |
Cited By (170)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9029411B2 (en) | 2008-01-25 | 2015-05-12 | Millennium Pharmaceuticals, Inc. | Thiophenes and uses thereof |
| US9090601B2 (en) | 2009-01-30 | 2015-07-28 | Millennium Pharmaceuticals, Inc. | Thiazole derivatives |
| US8796314B2 (en) | 2009-01-30 | 2014-08-05 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US9139589B2 (en) | 2009-01-30 | 2015-09-22 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US9062038B2 (en) | 2010-08-11 | 2015-06-23 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8796268B2 (en) | 2010-08-11 | 2014-08-05 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8796271B2 (en) | 2010-08-11 | 2014-08-05 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8859768B2 (en) | 2010-08-11 | 2014-10-14 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| JP2013533318A (ja) * | 2010-08-11 | 2013-08-22 | ミレニアム ファーマシューティカルズ, インコーポレイテッド | ヘテロアリールおよびその使用 |
| US8765746B2 (en) | 2010-10-13 | 2014-07-01 | Millennium Pharmaceuticals, Inc. | Heteroaryls and uses thereof |
| US8815271B2 (en) | 2010-11-03 | 2014-08-26 | Dow Agrosciences, Llc. | Pesticidal compositions and processes related thereto |
| US9422278B2 (en) | 2010-11-03 | 2016-08-23 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| JP2013544879A (ja) * | 2010-12-07 | 2013-12-19 | アクテリオン ファーマシューティカルズ リミテッド | Alx受容体アゴニストとしてのヒドロキシル化アミノトリアゾール誘導体 |
| JP2013544878A (ja) * | 2010-12-07 | 2013-12-19 | アクテリオン ファーマシューティカルズ リミテッド | Alx受容体アゴニストとしてのオキサゾリル−メチルエーテル誘導体 |
| US9655365B2 (en) | 2011-10-26 | 2017-05-23 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| JP2015503618A (ja) * | 2012-01-10 | 2015-02-02 | ニンバス アイリス, インコーポレイテッド | Irak阻害剤およびその使用 |
| JP2015503620A (ja) * | 2012-01-13 | 2015-02-02 | ブリストル−マイヤーズ スクイブ カンパニーBristol−Myers Squibb Company | キナーゼ阻害剤として有用な複素環置換されたピリジル化合物 |
| US10227340B2 (en) | 2012-01-13 | 2019-03-12 | Bristol-Myers Squibb Company | Thiazolyl- or thiadiazolyl-substituted pyridyl compounds useful as kinase inhibitors |
| JP2015503622A (ja) * | 2012-01-13 | 2015-02-02 | ブリストル−マイヤーズ スクイブ カンパニーBristol−Myers Squibb Company | キナーゼ阻害剤として有用なトリアゾリルまたはトリアジアゾリル置換されたピリジル化合物 |
| US8901153B2 (en) | 2012-04-27 | 2014-12-02 | Dow Agrosciences, Llc. | Pesticidal compositions and processes related thereto |
| US9708288B2 (en) | 2012-04-27 | 2017-07-18 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US9282739B2 (en) | 2012-04-27 | 2016-03-15 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US9591857B2 (en) | 2012-04-27 | 2017-03-14 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| US9931323B2 (en) | 2012-09-26 | 2018-04-03 | Genentech, Inc. | Cyclic ether pyrazol-4-yl-heterocyclyl-carboxamide compounds and methods of use |
| JP2015529235A (ja) * | 2012-09-26 | 2015-10-05 | エフ・ホフマン−ラ・ロシュ・アクチェンゲゼルシャフト | 環状エーテルピラゾール−4−イル−ヘテロシクリル−カルボキサミド化合物と使用方法 |
| US9586948B2 (en) | 2012-10-08 | 2017-03-07 | Merck Sharp & Dohme Corp. | Inhibitors of IRAK4 activity |
| US9598440B2 (en) | 2012-10-08 | 2017-03-21 | Merck Sharp & Dohme Corp. | Inhibitors of IRAK4 activity |
| EP2903613A4 (fr) * | 2012-10-08 | 2016-04-20 | Merck Sharp & Dohme | Inhibiteurs d'activité d'irak4 |
| US9988376B2 (en) | 2013-07-03 | 2018-06-05 | Glaxosmithkline Intellectual Property Development Limited | Benzothiophene derivatives as estrogen receptor inhibitors |
| US9993514B2 (en) | 2013-07-03 | 2018-06-12 | Glaxosmithkline Intellectual Property Development Limited | Compounds |
| WO2015006181A1 (fr) | 2013-07-11 | 2015-01-15 | Merck Sharp & Dohme Corp. | Inhibiteurs amidopyrazole substitués de kinases associées aux récepteurs de l'interleukine (irak -4) |
| US9670164B2 (en) | 2013-10-17 | 2017-06-06 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9540342B2 (en) | 2013-10-17 | 2017-01-10 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9796682B2 (en) | 2013-10-17 | 2017-10-24 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9126974B2 (en) | 2013-10-17 | 2015-09-08 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9723839B2 (en) | 2013-10-17 | 2017-08-08 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9044017B2 (en) | 2013-10-17 | 2015-06-02 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9670178B2 (en) | 2013-10-17 | 2017-06-06 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9174962B2 (en) | 2013-10-17 | 2015-11-03 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9199942B2 (en) | 2013-10-17 | 2015-12-01 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9661849B2 (en) | 2013-10-17 | 2017-05-30 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9862702B2 (en) | 2013-10-17 | 2018-01-09 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9255082B2 (en) | 2013-10-17 | 2016-02-09 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9255083B2 (en) | 2013-10-17 | 2016-02-09 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US10315999B2 (en) | 2013-10-17 | 2019-06-11 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9260396B2 (en) | 2013-10-17 | 2016-02-16 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9085564B2 (en) | 2013-10-17 | 2015-07-21 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9102654B2 (en) | 2013-10-17 | 2015-08-11 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9580405B2 (en) | 2013-10-17 | 2017-02-28 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9901095B2 (en) | 2013-10-17 | 2018-02-27 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9108946B2 (en) | 2013-10-17 | 2015-08-18 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9908864B2 (en) | 2013-10-17 | 2018-03-06 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9988356B2 (en) | 2013-10-17 | 2018-06-05 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9414594B2 (en) | 2013-10-17 | 2016-08-16 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9102655B2 (en) | 2013-10-17 | 2015-08-11 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9029554B1 (en) | 2013-10-17 | 2015-05-12 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9550751B2 (en) | 2013-10-17 | 2017-01-24 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9433215B2 (en) | 2013-10-17 | 2016-09-06 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9434712B2 (en) | 2013-10-17 | 2016-09-06 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9447048B2 (en) | 2013-10-17 | 2016-09-20 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US9282740B2 (en) | 2013-10-22 | 2016-03-15 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9295258B2 (en) | 2013-10-22 | 2016-03-29 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9788546B2 (en) | 2013-10-22 | 2017-10-17 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9491944B2 (en) | 2013-10-22 | 2016-11-15 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9149040B2 (en) | 2013-10-22 | 2015-10-06 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9497966B2 (en) | 2013-10-22 | 2016-11-22 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9497967B2 (en) | 2013-10-22 | 2016-11-22 | Doe AgroSciences LLC | Synergistic pesticidal compositions and related methods |
| US9523100B2 (en) | 2013-10-22 | 2016-12-20 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9137998B2 (en) | 2013-10-22 | 2015-09-22 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9445597B2 (en) | 2013-10-22 | 2016-09-20 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9549560B2 (en) | 2013-10-22 | 2017-01-24 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9155304B2 (en) | 2013-10-22 | 2015-10-13 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9144241B2 (en) | 2013-10-22 | 2015-09-29 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9788545B2 (en) | 2013-10-22 | 2017-10-17 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9295260B2 (en) | 2013-10-22 | 2016-03-29 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9474276B2 (en) | 2013-10-22 | 2016-10-25 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9808008B2 (en) | 2013-10-22 | 2017-11-07 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9801383B2 (en) | 2013-10-22 | 2017-10-31 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| USRE48057E1 (en) | 2013-10-22 | 2020-06-23 | Dow Agrosciences Llc | Pesticidal compositions and related methods |
| US9801376B2 (en) | 2013-10-22 | 2017-10-31 | Dow Agrosciences Llc | Synergistic pesticidal compositions and related methods |
| US9890145B2 (en) | 2013-11-08 | 2018-02-13 | Takeda Pharmaceutical Company Limited | Heterocyclic compound |
| US9321757B2 (en) | 2013-11-08 | 2016-04-26 | Takeda Pharmaceutical Company Limited | Heterocyclic compound |
| CN105899505A (zh) * | 2013-11-08 | 2016-08-24 | 武田药品工业株式会社 | 用于治疗自身免疫病症的吡唑 |
| WO2015068856A1 (fr) | 2013-11-08 | 2015-05-14 | Takeda Pharmaceutical Company Limited | Pyrazole pour le traitement de troubles auto-immuns |
| JP2016535772A (ja) * | 2013-11-08 | 2016-11-17 | 武田薬品工業株式会社 | 自己免疫疾患治療のためのピラゾール |
| US9951086B2 (en) | 2013-12-19 | 2018-04-24 | Bayer Pharma Aktiengesellschaft | Indazolecarboxamides, processes for their preparation, pharmaceutical preparations comprising them and their use for producing medicaments |
| WO2015104662A1 (fr) | 2014-01-10 | 2015-07-16 | Aurigene Discovery Technologies Limited | Composés d'imidazole utilisables en tant qu'inhibiteurs de l'irak4 |
| EP3466955A1 (fr) | 2014-01-13 | 2019-04-10 | Aurigene Discovery Technologies Limited | Procédé pour la préparation de dérivés de oxazolo[4,5-b]pyridine et thiazolo[4,5-b]pyridine en tant qu'inhibiteurs de irak4 pour le traitement du cancer |
| EP4353324A2 (fr) | 2014-01-13 | 2024-04-17 | Aurigene Oncology Limited | Dérivés hétérocyclyles bicycliques comme inhibiteurs de irak4 |
| EP3805233A1 (fr) | 2014-01-13 | 2021-04-14 | Aurigene Discovery Technologies Limited | Enantiomers (r) et (s) de n-(5-(3-hydroxypyrrolidin-1-yl)-2-morpholinooxazolo[4,5-b]pyridin-6-yl)-2-(2-methylpyridin-4-yl)oxazole-carboxamide en tant qu'inhibiteurs d'irak4 pour le traitement du cancer |
| US9029556B1 (en) | 2014-07-31 | 2015-05-12 | Dow Argosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9371310B2 (en) | 2014-07-31 | 2016-06-21 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9249122B1 (en) | 2014-07-31 | 2016-02-02 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9199964B1 (en) | 2014-07-31 | 2015-12-01 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9840490B2 (en) | 2014-07-31 | 2017-12-12 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9611247B2 (en) | 2014-07-31 | 2017-04-04 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9255081B1 (en) | 2014-07-31 | 2016-02-09 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US10035786B2 (en) | 2014-07-31 | 2018-07-31 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1h-pyrazol-1-yl)pyridine |
| US9580403B2 (en) | 2014-07-31 | 2017-02-28 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9573931B2 (en) | 2014-07-31 | 2017-02-21 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9029555B1 (en) | 2014-07-31 | 2015-05-12 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US10005758B2 (en) | 2014-08-19 | 2018-06-26 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9024031B1 (en) | 2014-08-19 | 2015-05-05 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9809570B2 (en) | 2014-08-19 | 2017-11-07 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9522900B2 (en) | 2014-08-19 | 2016-12-20 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9115115B1 (en) | 2014-08-19 | 2015-08-25 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9663489B2 (en) | 2014-09-12 | 2017-05-30 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9156813B1 (en) | 2014-09-12 | 2015-10-13 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9422265B2 (en) | 2014-09-12 | 2016-08-23 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9085552B1 (en) | 2014-09-12 | 2015-07-21 | Dow Agrosciences Llc | Process for the preparation of 3-(3-chloro-1H-pyrazol-1-yl)pyridine |
| US9896430B2 (en) | 2014-09-12 | 2018-02-20 | Dow Agrosciences Llc | Process for the preparation of 3-(3-CHLORO-1H-pyrazol-1-yl)pyridine |
| EA039043B1 (ru) * | 2015-04-22 | 2021-11-25 | Райджел Фармасьютикалз, Инк. | Пиразольные соединения и способ получения и применения данных соединений |
| WO2016172560A1 (fr) * | 2015-04-22 | 2016-10-27 | Rigel Pharmaceuticals, Inc. | Composés de pyrazole et procédé de fabrication et d'utilisation de ces composés |
| US10208074B2 (en) * | 2015-04-22 | 2019-02-19 | Rigel Pharmaceuticals, Inc. | Pyrazole compounds and method for making and using the compounds |
| US11370808B2 (en) | 2015-04-22 | 2022-06-28 | Rigel Pharmaceuticals, Inc. | Pyrazole compounds and methods for making and using the compounds |
| JP7050879B2 (ja) | 2015-04-22 | 2022-04-08 | ライジェル ファーマシューティカルズ, インコーポレイテッド | ピラゾール化合物ならびにこの化合物を作製および使用するための方法 |
| AU2016252869B2 (en) * | 2015-04-22 | 2020-10-22 | Rigel Pharmaceuticals, Inc. | Pyrazole compounds and method for making and using the compounds |
| US20190153006A1 (en) * | 2015-04-22 | 2019-05-23 | Rigel Pharmaceuticals, Inc. | Pyrazole compounds and methods for making and using the compounds |
| JP2018513177A (ja) * | 2015-04-22 | 2018-05-24 | ライジェル ファーマシューティカルズ, インコーポレイテッド | ピラゾール化合物ならびにこの化合物を作製および使用するための方法 |
| CN113750094A (zh) * | 2015-04-22 | 2021-12-07 | 里格尔药品股份有限公司 | 吡唑化合物以及制备和使用该化合物的方法 |
| US10208075B2 (en) * | 2015-04-22 | 2019-02-19 | Rigel Pharmaceuticals, Inc. | Pyrazole compounds and method for making and using the compounds |
| US9982000B2 (en) | 2015-04-22 | 2018-05-29 | Rigel Pharmaceuticals, Inc. | Pyrazole compounds and method for making and using the compounds |
| CN107438603B (zh) * | 2015-04-22 | 2021-08-10 | 里格尔药品股份有限公司 | 吡唑化合物以及制备和使用该化合物的方法 |
| CN107438603A (zh) * | 2015-04-22 | 2017-12-05 | 里格尔药品股份有限公司 | 吡唑化合物以及制备和使用该化合物的方法 |
| JP2021020924A (ja) * | 2015-04-22 | 2021-02-18 | ライジェル ファーマシューティカルズ, インコーポレイテッド | ピラゾール化合物ならびにこの化合物を作製および使用するための方法 |
| US11299486B2 (en) | 2015-08-04 | 2022-04-12 | Rigel Pharmaceuticals, Inc. | Benzazole compounds and methods for making and using the compounds |
| US10774076B2 (en) | 2015-08-04 | 2020-09-15 | Rigel Pharmaceuticals, Inc. | Benzazole compounds and methods for making and using the compounds |
| WO2018052058A1 (fr) * | 2016-09-15 | 2018-03-22 | 武田薬品工業株式会社 | Composé hétérocyclique |
| US10941140B2 (en) | 2016-10-26 | 2021-03-09 | Rigel Pharmaceuticals, Inc. | IRAK inhibitors and method for making and using |
| CN110099899A (zh) * | 2016-10-26 | 2019-08-06 | 里格尔药品股份有限公司 | 用作irak抑制剂的噁唑衍生物及其制备方法 |
| US11939317B2 (en) | 2016-10-26 | 2024-03-26 | Rigel Pharmaceuticals, Inc. | Amide compounds and method for making and using |
| JP2019533687A (ja) * | 2016-10-26 | 2019-11-21 | ライジェル ファーマシューティカルズ, インコーポレイテッド | Irak阻害剤としてのピラゾールアミド化合物 |
| US11492349B2 (en) | 2016-10-26 | 2022-11-08 | Rigel Pharmaceuticals, Inc. | IRAK inhibitors and method for making and using |
| US10370367B2 (en) | 2016-10-26 | 2019-08-06 | Rigel Pharmaceuticals, Inc. | IRAK inhibitors and method for making and using |
| JP7170634B2 (ja) | 2016-10-26 | 2022-11-14 | ライジェル ファーマシューティカルズ, インコーポレイテッド | Irak阻害剤としての使用のためのオキサゾール誘導体およびその調製のための方法 |
| JP7379641B2 (ja) | 2016-10-26 | 2023-11-14 | ライジェル ファーマシューティカルズ, インコーポレイテッド | Irak阻害剤としてのピラゾールアミド化合物 |
| CN110099899B (zh) * | 2016-10-26 | 2024-01-02 | 里格尔药品股份有限公司 | 用作irak抑制剂的噁唑衍生物及其制备方法 |
| JP2019537577A (ja) * | 2016-10-26 | 2019-12-26 | ライジェル ファーマシューティカルズ, インコーポレイテッド | Irak阻害剤としての使用のためのオキサゾール誘導体およびその調製のための方法 |
| JP7170635B2 (ja) | 2016-10-26 | 2022-11-14 | ライジェル ファーマシューティカルズ, インコーポレイテッド | Irak阻害剤としてのピラゾールアミド化合物 |
| JP7379640B2 (ja) | 2016-10-26 | 2023-11-14 | ライジェル ファーマシューティカルズ, インコーポレイテッド | Irak阻害剤としての使用のためのオキサゾール誘導体およびその調製のための方法 |
| US11530194B2 (en) | 2016-10-26 | 2022-12-20 | Rigel Pharmaceuticals, Inc. | Amide compounds and method for making and using |
| WO2018089199A1 (fr) * | 2016-10-26 | 2018-05-17 | Rigel Pharmaceuticals, Inc. | Dérivés d'oxazole destinés à être utilisés en tant qu'inhibiteurs d'irak et leur procédé de préparation |
| US10100033B2 (en) | 2016-12-29 | 2018-10-16 | Dow Agrosciences Llc | Processes for the preparation of pesticidal compounds |
| US10233155B2 (en) | 2016-12-29 | 2019-03-19 | Dow Agrosciences Llc | Processes for the preparation of pesticide compounds |
| US10336762B2 (en) | 2017-02-16 | 2019-07-02 | Gilead Sciences, Inc. | Pyrrolo[1,2-b]pyridazine derivatives |
| US11358948B2 (en) | 2017-09-22 | 2022-06-14 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| US11623932B2 (en) | 2017-09-22 | 2023-04-11 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| US11065231B2 (en) | 2017-11-17 | 2021-07-20 | Arvinas Operations, Inc. | Compounds and methods for the targeted degradation of interleukin-1 receptor- associated kinase 4 polypeptides |
| WO2019111218A1 (fr) | 2017-12-08 | 2019-06-13 | Cadila Healthcare Limited | Nouveaux composés hétérocycliques utilisés en tant qu'inhibiteurs d'irak4 |
| US10874743B2 (en) | 2017-12-26 | 2020-12-29 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11318205B1 (en) | 2017-12-26 | 2022-05-03 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| WO2019133531A1 (fr) | 2017-12-26 | 2019-07-04 | Kymera Therapeutics, Inc. | Agents de dégradation de kinases irak et leurs utilisations |
| US11723980B2 (en) | 2017-12-26 | 2023-08-15 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11512080B2 (en) | 2018-01-12 | 2022-11-29 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| US11485743B2 (en) | 2018-01-12 | 2022-11-01 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| US11932635B2 (en) | 2018-01-12 | 2024-03-19 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| US11292792B2 (en) | 2018-07-06 | 2022-04-05 | Kymera Therapeutics, Inc. | Tricyclic CRBN ligands and uses thereof |
| US11897882B2 (en) | 2018-07-06 | 2024-02-13 | Kymera Therapeutics, Inc. | Tricyclic crbn ligands and uses thereof |
| US11535622B2 (en) | 2018-07-13 | 2022-12-27 | Gilead Sciences, Inc. | Pyrrolo[1,2-b] pyridazine derivatives |
| US10875866B2 (en) | 2018-07-13 | 2020-12-29 | Gilead Sciences, Inc. | Pyrrolo[1,2-B]pyridazine derivatives |
| US20220289710A1 (en) * | 2018-11-02 | 2022-09-15 | Merck Sharp & Dohme Llc | 2-amino-n-heteroaryl-nicotinamides as nav1.8 inhibitors |
| US11352350B2 (en) | 2018-11-30 | 2022-06-07 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11807636B2 (en) | 2018-11-30 | 2023-11-07 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11117889B1 (en) | 2018-11-30 | 2021-09-14 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11370787B2 (en) | 2019-08-30 | 2022-06-28 | Rigel Pharmaceuticals, Inc. | Pyrazole compounds, formulations thereof, and a method for using the compounds and/or formulations |
| US11591332B2 (en) | 2019-12-17 | 2023-02-28 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11779578B2 (en) | 2019-12-17 | 2023-10-10 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11707457B2 (en) | 2019-12-17 | 2023-07-25 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11685750B2 (en) | 2020-06-03 | 2023-06-27 | Kymera Therapeutics, Inc. | Crystalline forms of IRAK degraders |
| US11993594B2 (en) | 2022-09-27 | 2024-05-28 | Rigel Pharmaceuticals, Inc. | IRAK inhibitors and method for making and using |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2012254939A (ja) | 2012-12-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2011043371A1 (fr) | Composé d'oxazole | |
| CA2971640C (fr) | Modulateurs de cot et procedes d'utilisation associes | |
| CN108368127B (zh) | 化合物及其作为ep4受体拮抗剂的用途 | |
| CN108383836B (zh) | 作为mek抑制剂的杂环基化合物 | |
| US11918592B2 (en) | Inhibitors of cyclin dependent kinase 7 (CDK7) | |
| CA2957046C (fr) | Derives de pyrimidine substitues par un heterocyclyle eventuellement condenses utiles pour le traitement des maladies inflammatoires, metaboliques, oncologiques et auto-immunes | |
| US9580418B2 (en) | Bicyclic aromatic carboxamide compounds useful as Pim kinase inhibitors | |
| JP5925688B2 (ja) | アミド誘導体およびその用途 | |
| US20130150364A1 (en) | Heterocyclic compound | |
| US8148411B2 (en) | 2-hetarylthiazole-4-carboxamide derivatives, their preparation and use as pharmaceuticals | |
| KR20050115252A (ko) | 피라졸로[1,5-a]피리미딘 유도체 | |
| JP2016528298A (ja) | Pimキナーゼ阻害剤として有用なフロピリジン及びチエノピリジンカルボキシアミド化合物 | |
| EP3280707A1 (fr) | Dérivés de quinazolinone bicyclique | |
| CA2728683A1 (fr) | Nouvelles phenylpyrazinones en tant qu'inhibiteurs de kinases | |
| WO2010058846A1 (fr) | 4,6-diaminonicotinamide | |
| TW200417546A (en) | New compounds | |
| US8163739B2 (en) | 2-arylthiazole-4-carboxamide derivatives, their preparation and use as pharmaceuticals | |
| JP2008013527A (ja) | チエノ[3,2−d]ピリミジン−2,4−ジアミン誘導体 | |
| WO2012043505A1 (fr) | Nouveau dérivé de pipéridine et produit pharmaceutique l'incluant | |
| CN111406054A (zh) | 作为组蛋白脱乙酰基酶6抑制剂的1,2,4-噁二唑衍生物 | |
| MX2013004814A (es) | Derivados de triazol como ligandos para receptores de acido gamma-aminobutirico (gaba). | |
| US7897626B2 (en) | 2-substituted thiazole-4-carboxamide derivatives, their preparation and use as pharmaceuticals | |
| AU2011305667A1 (en) | Oxadiazole inhibitors of leukotriene production | |
| JP7233130B2 (ja) | Irak4阻害剤としての新規な三環式化合物 | |
| WO2012099200A1 (fr) | Dérivé de pyrazole |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10822041 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 10822041 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: JP |