WO2010122984A1 - 非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、および非水電解液二次電池 - Google Patents
非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、および非水電解液二次電池 Download PDFInfo
- Publication number
- WO2010122984A1 WO2010122984A1 PCT/JP2010/056940 JP2010056940W WO2010122984A1 WO 2010122984 A1 WO2010122984 A1 WO 2010122984A1 JP 2010056940 W JP2010056940 W JP 2010056940W WO 2010122984 A1 WO2010122984 A1 WO 2010122984A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- active material
- electrode active
- material layer
- metal oxide
- electrode plate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/621—Binders
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/04—Processes of manufacture in general
- H01M4/0402—Methods of deposition of the material
- H01M4/0404—Methods of deposition of the material by coating on electrode collectors
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/04—Processes of manufacture in general
- H01M4/0471—Processes of manufacture in general involving thermal treatment, e.g. firing, sintering, backing particulate active material, thermal decomposition, pyrolysis
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/131—Electrodes based on mixed oxides or hydroxides, or on mixtures of oxides or hydroxides, e.g. LiCoOx
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/139—Processes of manufacture
- H01M4/1391—Processes of manufacture of electrodes based on mixed oxides or hydroxides, or on mixtures of oxides or hydroxides, e.g. LiCoOx
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/624—Electric conductive fillers
- H01M4/625—Carbon or graphite
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/136—Electrodes based on inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/50—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese
- H01M4/505—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese of mixed oxides or hydroxides containing manganese for inserting or intercalating light metals, e.g. LiMn2O4 or LiMn2OxFy
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- the present invention relates to an electrode plate used for a nonaqueous electrolyte secondary battery such as a lithium ion secondary battery, a method for producing the electrode plate, and a nonaqueous electrolyte secondary battery.
- a non-aqueous electrolyte secondary battery represented by a lithium ion secondary battery has a high energy density and a high voltage, and has a memory effect during charging / discharging (when the battery is charged before it is completely discharged, Since there is no phenomenon in which the capacity decreases, it is used in various fields such as portable devices, notebook computers, and portable devices.
- the non-aqueous electrolyte secondary battery is generally composed of a positive electrode plate, a negative electrode plate, a separator, and a non-aqueous electrolyte solution.
- a positive electrode plate one having an electrode active material layer in which positive electrode active material particles are fixed on the surface of a current collector such as a metal foil is generally used.
- a negative electrode plate one having an electrode active material layer in which negative electrode active material particles are fixed to the surface of a current collector such as copper or aluminum is generally used.
- electrode material particles that are positive electrode active material particles or negative electrode active material particles, a resin binder, and a conductive material (however, the negative electrode active material particles have a conductive effect) If the electrode performance is sufficiently obtained even without a conductive material, the conductive material may be omitted), or, if necessary, use other materials in a solvent.
- a slurry-like electrode active material layer forming composition is prepared by kneading and / or dispersing. And the method of manufacturing the electrode plate provided with the electrode active material layer by applying the electrode active material layer forming composition to the current collector surface, then drying to form a coating film on the current collector, and pressing. Common (eg, JP2006-310010A or JP2006-107750A).
- the electrode active material particles contained in the electrode active material layer forming composition are particulate metal compounds dispersed in the composition, and as such, are applied to the surface of the current collector and dried. Even if pressed, it is difficult to adhere to the surface of the current collector, and it will be peeled off immediately from the current collector. Therefore, a resin binder is added to the electrode active material layer forming composition, and the electrode active material particles are fixed on the current collector with the resin binder to form the electrode active material layer. Therefore, the resin binder is a substantially essential component in the electrode active material layer forming composition.
- lithium ion secondary batteries have been developed for fields that require high input / output characteristics such as electric vehicles, hybrid vehicles, and power tools. Even in the case of a secondary battery used in a relatively small device such as a cellular phone, the device tends to be multifunctional, and therefore, the input / output characteristics are expected to be improved. On the other hand, in order to improve the input / output characteristics of the secondary battery, it is necessary to reduce the impedance of the battery. This is because a battery with high impedance has a problem that its capacity cannot be fully utilized during high-speed charge / discharge.
- the lower limit of the thickness of the electrode active material layer is substantially several tens of ⁇ m. there were.
- a means of reducing the particle diameter of the active material particles used is also effective.
- the particle diameter of the active material particles By reducing the particle diameter of the active material particles, the total surface area of the electrode active material particles contained in the electrode active material layer can be increased, and lithium ions inserted and desorbed in the electrode active material particles The movement distance in the particles can be reduced. Thereby, the behavior of lithium ions becomes smoother, and as a result, the input / output characteristics can be improved.
- the viscosity of the electrode active material layer forming composition tends to increase, and this tendency is particularly high when the particle diameter is 11 ⁇ m or less or even smaller. This was noticeable when substance particles were used. Accordingly, the size of the active material particles that can be used is substantially limited, which has been disadvantageous for the above-described thinning of the electrode active material layer.
- the present invention has been accomplished in view of the above circumstances, and an object of the present invention is to provide an electrode plate having high input / output characteristics and excellent cycle characteristics in an electrode plate for a non-aqueous electrolyte secondary battery. Another object of the present invention is to provide a non-aqueous electrolyte secondary battery with high input / output characteristics by using such an electrode plate, and to provide a method for manufacturing such an electrode plate.
- the inventors of the present invention do not use a resin binder that is generally used, and are crystalline on an electrode on a current collector through a metal oxide that does not exhibit alkali metal ion insertion / release reaction. It was found that the active material particles can be fixed. Further, the present inventors have found that a more desirable input / output characteristic is exhibited by incorporating a carbon component in the electrode active material layer. And based on these knowledge, the present inventors completed the electrode plate for nonaqueous electrolyte secondary batteries of this invention, and the nonaqueous electrolyte secondary battery using the same.
- the present inventors have used electrode active material particles on a current collector through a metal oxide that is crystalline and does not show alkali metal ion insertion / release reaction without using a resin binder.
- a composition containing at least an organometallic compound for producing a metal oxide as a binder and electrode active material particles, and applying this onto a current collector to form a coating film The present inventors have found a method for heating the coating film at an appropriate temperature.
- the coating film by heating the coating film at a temperature higher than the thermal decomposition start temperature of the metal element-containing compound or organometallic compound applied on the current collector and lower than the crystallization temperature of the generated metal oxide, A metal oxide as a binding material is generated, and at this time, the electrode active material particles present around the binding material can be fixed on the current collector, and the temperature during the heating is
- the present inventors have found that the carbon component derived from the organic material or the organometallic compound can be left in the electrode active material layer as a carbon component that is distinguished from the conductive material by adjusting. And based on this knowledge, the present inventors completed invention of the manufacturing method of the electrode plate for nonaqueous electrolyte secondary batteries.
- the electrode plate for a non-aqueous electrolyte secondary battery is: A current collector, An electrode active material layer formed on at least a part of the surface of the current collector, The electrode active material layer contains electrode active material particles, a binder, and a carbon component that is distinguished from a conductive material,
- the binder material is a crystalline metal oxide that does not show alkali metal ion insertion / release reaction.
- the electrode active material layer may further include a conductive material.
- the metal oxide may be Na, Mg, Al, Si, K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni. And any one metal element selected from the group consisting of Cu, Zn, Ga, Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In and Sn It may be a metal oxide or a composite metal oxide containing two or more metal elements selected from the above group.
- the particle diameter of the electrode active material particles may be 11 ⁇ m or less.
- Non-aqueous electrolyte secondary battery A positive electrode plate; A negative electrode plate; A separator provided between the positive electrode plate and the negative electrode plate; An electrolyte solution containing a non-aqueous solvent, At least one of the positive electrode plate and the negative electrode plate is the electrode plate for a non-aqueous electrolyte secondary battery according to claim 1.
- the first method for producing an electrode plate for a non-aqueous electrolyte secondary battery comprises: Formation of an electrode active material layer including at least electrode active material particles, a metal element-containing compound for generating a metal oxide as a binding material, and an organic substance capable of imparting a carbon component distinct from a conductive material An application step of applying the composition to at least a part of the current collector to form a coating film; A heating step performed after the coating step, wherein the coating film is heated to evaporate the solvent, and the metal element-containing compound is pyrolyzed to produce a metal oxide, thereby producing a metal oxide on the current collector.
- a heating step of forming an electrode active material layer containing a metal oxide and the above electrode active material particles The metal element-containing compound used in the coating step is selected so that the metal oxide generated in the heating step is a metal oxide that does not exhibit an alkali metal ion insertion / release reaction,
- the temperature is equal to or higher than the thermal decomposition start temperature of the metal element-containing compound and is equal to or higher than the crystallization temperature of the metal oxide generated in the heating step, and the carbon derived from the organic matter is an electrode active material layer.
- the coating film is heated at a temperature that allows it to remain as a carbon component that is distinguished from the conductive material.
- the metal element-containing compound may be a metal salt.
- a method for producing a second electrode plate for a non-aqueous electrolyte secondary battery according to the present invention comprises: An electrode active material layer-forming composition containing at least electrode active material particles and an organometallic compound for generating a metal oxide as a binding material is applied to at least a part of the current collector to form a coating film An application process for forming A heating step carried out after the coating step, wherein the coating film is heated to evaporate the solvent, and the organometallic compound is pyrolyzed to produce a metal oxide on the current collector.
- a heating step of forming an electrode active material layer containing a metal oxide and the electrode active material particles The organometallic compound used in the coating step is selected so that the metal oxide generated in the heating step is a metal oxide that does not exhibit an alkali metal ion insertion / release reaction,
- the temperature is equal to or higher than the thermal decomposition start temperature of the organometallic compound and is equal to or higher than the crystallization temperature of the metal oxide generated in the heating step, and the carbon derived from the organometallic compound is an electrode active material.
- the coating film is heated at a temperature that allows it to remain as a carbon component that is distinguished from the conductive material in the layer.
- the organometallic compound may be a metal salt.
- a third method for producing an electrode plate for a non-aqueous electrolyte secondary battery comprises: Electrode active material layer forming composition comprising at least electrode active material particles, an organic metal compound for generating a metal oxide as a binder, and an organic material capable of imparting a carbon component distinct from a conductive material An application step of applying an object to at least a part of the current collector to form a coating film; A heating step carried out after the coating step, wherein the coating film is heated to evaporate the solvent, and the organometallic compound is pyrolyzed to produce a metal oxide on the current collector.
- a heating step of forming an electrode active material layer containing a metal oxide and the electrode active material particles The organometallic compound used in the coating step is selected so that the metal oxide generated in the heating step is a metal oxide that does not exhibit an alkali metal ion insertion / release reaction,
- the temperature is not less than the thermal decomposition start temperature of the organometallic compound and not less than the crystallization temperature of the metal oxide produced in the heating step, and at least the carbon derived from the organometallic compound or the organic matter. Is heated at a temperature that allows it to remain in the electrode active material layer as a carbon component distinct from the conductive material.
- the organometallic compound may be a metal salt.
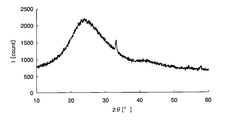
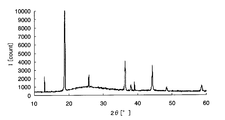
- FIG. 1 is a chart showing X-ray diffraction results of amorphous iron oxide.
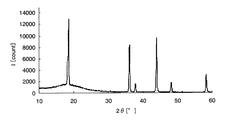
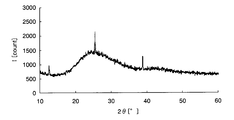
- FIG. 2 is a chart showing X-ray diffraction results of crystalline iron oxide.
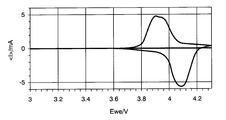
- FIG. 3 is a cyclic voltammogram showing the results of a cyclic voltammetry test using a metal oxide exhibiting a lithium insertion / elimination reaction.
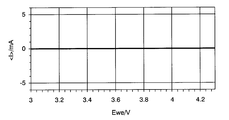
- FIG. 4 is a cyclic voltammogram showing the results of a cyclic voltammetry test using a metal oxide that does not exhibit a lithium insertion / elimination reaction.
- FIG. 5 is an electron micrograph obtained by observing the cross section of Example 1 perpendicular to the current collector surface at a magnification of 3,000 using a scanning electron microscope (SEM).
- SEM scanning electron microscope
- FIG. 6 is a graph showing the X-ray diffraction results of the electrode active material layer of Example 1.
- FIG. 7 is a graph showing the X-ray diffraction results of iron oxide obtained by heating under the same conditions as in Example 1.
- FIG. 8 is a graph showing an X-ray diffraction result of lithium manganate as the positive electrode active material particles.
- FIG. 9 is a graph showing the X-ray diffraction results of the electrode active material layer of Example 1.
- FIG. 10 is a graph showing an X-ray diffraction result of a film formed using the same solution as the raw material solution used in Example 5 except that the positive electrode active material was not added.
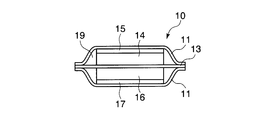
- FIG. 11A is a diagram showing a non-aqueous electrolyte secondary battery.
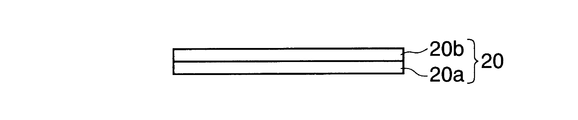
- FIG. 11B is a diagram showing an electrode plate for a non-aqueous electrolyte secondary battery.
- the electrode plate for nonaqueous electrolyte secondary batteries of the present invention the method for producing the electrode plate for nonaqueous electrolyte secondary batteries, and the mode for carrying out the nonaqueous electrolyte secondary battery will be described in order.
- a lithium ion secondary battery will be described as an example of the nonaqueous electrolyte secondary battery of the present invention unless otherwise specified.
- the characteristic of the metal oxide in the present invention “does not show alkali metal ion insertion / release reaction”, unless otherwise specified, lithium ions are used as examples of alkali metal ions, Insertion / desorption will be described.
- the electrode plate of this invention contains both the positive electrode plate and negative electrode plate which are used for a nonaqueous electrolyte secondary battery. Therefore, in the following description, unless otherwise specified, the positive electrode plate and the negative electrode plate will be collectively described as electrode plates, and the positive electrode plate and the negative electrode plate will be described as necessary.
- the electrode plate 20 for a non-aqueous electrolyte secondary battery of the present embodiment includes a current collector 20a and an electrode active material layer 20b formed on at least a part of the surface of the current collector 20a. And.
- the electrode active material layer 20 includes electrode active material particles, a binder, and a carbon component that is distinguished from a conductive material.
- the binding substance is a crystalline metal oxide that does not show alkali metal ion insertion / extraction reaction.
- the electrode active material layer is formed on the current collector by means of a metal oxide in which the electrode active material particles are not a resin binder as in the prior art and are crystalline and do not show an alkali metal ion insertion / release reaction such as lithium ions. And a carbon component that is distinguished from the conductive material.
- the thickness of the electrode active material layer can be appropriately designed in consideration of electric capacity and input / output characteristics required for the electrode plate. Generally, the thickness is designed to be 200 ⁇ m or less, more generally 100 ⁇ m or more and 150 ⁇ m or less. However, particularly in this embodiment, since the electrode active material layer can be formed very thin, an electrode active material having a film thickness of 300 nm or more and 200 ⁇ m or less depends on the particle diameter of the electrode active material particles used. A material layer can be formed. From the viewpoint that a high capacity can be obtained while improving the input / output characteristics, the thickness of the electrode active material layer is particularly preferably 300 nm to 30 ⁇ m, and more preferably 500 nm to 11 ⁇ m.
- the electrode active material particles used have a small particle diameter, and are at least a particle diameter equal to or smaller than the film thickness of the electrode active material layer. This means that this greatly contributes to the improvement of the input / output characteristics.
- the electrode active material layer is thin as described above, the moving distance of the electrons moving between the electrode active material particles and the current collector in the electrode active material layer is shortened. The resistance can be lowered, and as a result, it can contribute to the improvement of the input / output characteristics, which is desirable.
- the lower limit of the thickness of the electrode active material layer mainly depends on the particle diameter of the electrode active material particles used, and as the particle diameter of usable electrode active material particles is reduced, It is possible to make the film thickness thinner than the above range.
- the electrode active material layer preferably has voids to the extent that the electrolytic solution can permeate, and the porosity in the electrode active material layer is generally 15 to 40%, more preferably 20 to 40%. It is. Below, the substance contained in an electrode active material layer is demonstrated concretely.
- Electrode active material particles As the electrode active material particles contained in the electrode active material layer, positive electrode active material particles or negative electrode active materials that can be charged / discharged and exhibit lithium ion insertion / release reactions generally used in electrode plates for non-aqueous electrolyte secondary batteries are used. If it is a substance particle, it will not specifically limit. That is, in this embodiment, on the current collector, a metal oxide is interposed between the particles such as the electrode active material particles or between the electrode active material particles and the current collector, so that the electrode active material layer adheres to each other. The metal oxide is formed and acts as a binder regardless of the type and shape of the electrode active material particles.
- the metal oxide contained in the electrode active material layer of the present embodiment does not show an alkali metal ion insertion / release reaction, it affects the reaction of any electrode active material particles.
- the electrode active material particles can be used without any particular limitation.
- specific examples of the positive electrode active material particles include, for example, LiCoO 2 , LiMn 2 O 4 , LiNiO 2 , LiFeO 2 , Li 4 Ti 5 O 12.
- active material particles such as lithium transition metal composite oxides such as LiFePO 4 .
- the negative electrode active material particles include active material particles made of carbonaceous material such as natural graphite, artificial graphite, amorphous carbon, carbon black, or those obtained by adding different elements to these components, Alternatively, a material that exhibits an insertion / extraction reaction of lithium ions, such as a metal oxide such as Li 4 Ti 5 O 12 , metal lithium and an alloy thereof, tin, silicon, and an alloy thereof can be given.
- the particle diameter of the electrode active material particles used in the present embodiment is not particularly limited, and those having an arbitrary size can be appropriately selected and used. However, the smaller the particle diameter, the larger the total surface area of the electrode active material particles in the electrode active material layer can be increased. It is desirable to do. Thus, the fact that the size of the particle diameter can be selected without any particular limitation is noted as an advantageous effect of the present embodiment. That is, in the production of a conventional electrode plate, it is difficult to use electrode active material particles having a small particle diameter because of a significant increase in the viscosity of the electrode active material layer forming composition.
- the electrode active material particles having an arbitrary particle size can be contained in the electrode active material layer, the surface area of the electrode active material particles in the electrode active material layer is increased.
- the reason why it is possible to use electrode active material particles having a smaller particle diameter is not clear, but a metal oxide is generated instead of the conventional resin binder. This is considered to be due to the addition of a metal element-containing compound or an organometallic compound to the electrode active material layer forming composition.
- the viscosity of the composition becomes high and adjustment thereof is difficult. Yes, the handleability was poor.
- the particle diameter of 11 ⁇ m or less can be easily obtained.
- An electrode plate provided with an electrode active material layer containing electrode active material particles can be obtained. As described above, it is desirable that the particle diameter of the electrode active material particles be 11 ⁇ m or less from the viewpoint of obtaining high input / output characteristics after sufficiently ensuring the handleability of the electrode active material layer forming composition.
- the viscosity of the electrode active material layer forming composition can be obtained even if an electrode plate having a conventional electrode active material layer using a resin binder is used. Became too high to lose fluidity and could not be applied to mass production equipment such as a printing press. Although it is possible to increase the fluidity of the electrode active material layer forming composition by adding a large amount of solvent, it takes a long time to dry and is not substantial, and in particular, production by a winding device is impossible. there were.
- the viscosity of the electrode active material layer forming composition is maintained moderately and the fluidity is good, so that mass production is possible. It can be applied to equipment. Therefore, it is desirable that the particle diameter of the electrode active material particles be 5 ⁇ m or less from the viewpoint of producing an electrode plate exhibiting high input / output characteristics by mass production equipment.
- an electrode active material layer forming composition is used. It was difficult to disperse the electrode active material particles in the material, and this was not feasible.
- the present embodiment even if electrode active material particles having a particle size of 1 ⁇ m or less are used, the dispersibility in the electrode active material layer forming composition is good, and the electrode active material particles of the size are excellent.
- the contained electrode active material layer can be formed on the current collector. Therefore, it is very advantageous and desirable to use electrode active material particles having a particle diameter of 1 ⁇ m or less in the present embodiment. From the above viewpoint, in the present embodiment, the particle diameter of the electrode active material particles is further selected to be 500 nm or less, more preferably 100 nm or less.
- the particle diameter of the electrode active material particles shown in the present invention and the present specification is an average particle diameter (volume median particle diameter: D50) measured by laser diffraction / scattering particle size distribution measurement.
- the particle diameter of the electrode active material contained in the electrode active material layer can be measured using an image analysis type particle size distribution measurement software (manufactured by Mount Tech Co., Ltd., MAC VIEW).
- Binder metal oxide The metal oxide contained as a binder in the electrode active material layer is an oxide of a metal element that is generally understood as a metal, and is a crystalline metal oxide that does not exhibit a lithium ion insertion / release reaction. If it is a thing, it will not specifically limit.
- metal elements examples include Li, Be, Na, Mg, Al, Si, K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In, Sn, Cs, Ba, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg, Tl, Pb, Bi, Fr, Ra, Ce, etc. can be mentioned.
- oxides of metal elements belonging to the third to fifth periods are present as binders in the electrode active material layer.
- the input / output characteristics are improved more favorably, which is preferable. That is, Na, Mg, Al, Si, K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Rb, Sr, Y, Zr, Nb, Mo, Tc, More preferably, a metal oxide containing a metal element selected from the group consisting of Ru, Rh, Pd, Ag, Cd, In, and Sn is present as a binder in the electrode active material layer.
- titanium oxide is inexpensive and easy to handle, and is contained as a binder in the electrode active material layer. In this case, it is possible to show a very excellent effect of improving the input / output characteristics. That is, in the electrode plate for a non-aqueous electrolyte secondary battery according to the present embodiment including an electrode active material layer containing titanium oxide as a binder, a high charge / discharge rate (discharge capacity maintenance) of 80% or more at a discharge rate of 50C. Rate), and can sufficiently cope with a large apparatus such as an automobile.
- the metal oxide in this embodiment includes a metal oxide in which oxygen is bonded to any one of the above metal elements, or two or more metal elements selected from the above metal elements. Any of complex metal oxides may be used.
- metal oxides in which oxygen is bonded to one metal element include sodium oxide, magnesium oxide, aluminum oxide, silicon oxide, potassium oxide, calcium oxide, scandium oxide, titanium oxide, vanadium oxide, chromium oxide, and oxide. Examples thereof include manganese, iron oxide, cobalt oxide, nickel oxide, zinc oxide, gallium oxide, strontium oxide, yttrium oxide, zirconium oxide, molybdenum oxide, ruthenium oxide, tantalum oxide, tungsten oxide, and cerium oxide.
- Examples of composite metal oxides containing two or more metal elements that can be used as the metal oxide in this embodiment include, for example, cerium oxide doped with gadolinium and yttrium. Examples thereof include zirconium oxide, mixed oxide of iron and titanium, oxide mixed with indium and tin, nickel oxide doped with lithium, and the like. The examples of the metal oxide described in this paragraph do not limit the metal oxide in the present invention.
- An object is a crystalline metal oxide that does not show lithium ion insertion / release reaction, and can fix electrode active material particles on a current collector without using a resin binder. Any one may be used.
- the metal oxide mentioned above can be contained in an electrode active material layer by 1 type, or 2 or more types of combination.
- the metal oxide is crystalline, any addition between electrode active material particles or between electrode active material particles and a conductive material is possible. A stronger binding property can be obtained between the components, and as a result, the electrode plate of the present embodiment is presumed to exhibit excellent cycle characteristics.
- Binder compounding ratio In the present embodiment, the mixing ratio of the metal oxide and electrode active material particles in the electrode active material layer is not particularly specified, and the type and size of the electrode active material particles used, the type of metal oxide, the electrode It can be determined as appropriate in consideration of the functions required for. However, in general, the larger the amount of electrode active material particles in the electrode active material layer, the higher the electric capacity of the electrode. From this viewpoint, the electrode active material particles present in the electrode active material layer It can be said that a smaller amount of the metal oxide is more preferable. More specifically, in the electrode active material layer, when the weight ratio of the electrode active material particles is 100 parts by weight, the weight ratio of the metal oxide is 1 part by weight or more and 50 parts by weight or less. be able to.
- the electrode active material particles may not be satisfactorily fixed on the current collector.
- the description of the upper limit of the weight ratio of the metal oxide is not intended to exclude the presence of the metal oxide exceeding the upper limit in the present invention. This shows that the active material particles can be fixed on the current collector with a smaller amount of metal oxide in order to increase the electric capacity of the electrode.
- the metal oxide is specified to be crystalline.
- a crystalline metal oxide means a case where a peak of the metal oxide is detected by analyzing the metal oxide or a sample containing the metal oxide with an X-ray diffractometer. For example, taking iron as an example of the metal element, crystalline iron oxide and amorphous iron oxide will be described using specific analysis results in the respective X-ray diffractometers.
- Samples 1 and 2 were then prepared by applying the above sample solution on a glass substrate. Sample 1 was heated at 300 ° C. for 1 hour, while sample 2 was heated at 500 ° C. for 1 hour. Subsequently, the film-forming surfaces of samples 1 and 2 after heating were scraped to obtain analytical samples 1 and 2, respectively, and composition analysis was performed on these samples.
- peaks can be confirmed around 32 ° and 58 ° on the horizontal axis, and it is understood that crystalline iron oxide is generated on the glass substrate.
- the metal element is an oxide is confirmed by composition analysis, and whether the metal oxide is amorphous from the chart obtained by the X-ray diffractometer, It can be confirmed whether it is crystalline.
- the metal oxide is specified to be crystalline.
- the cycle characteristics of the electrode plate are desirably improved as compared with a conventional electrode plate using a resin binder. Therefore, with this electrode plate, two important problems in the non-aqueous electrolyte secondary battery, which are the intended purposes, namely, improvement of input / output characteristics and improvement of cycle characteristics, are achieved.
- the metal oxide is specified as one that does not show an alkali metal ion insertion / release reaction. The reason is that the metal oxide does not electrochemically react with alkali metal ions such as lithium ions. As a result, no expansion or reactant is generated due to the electrochemical reaction of the metal oxide, and as a result, deterioration due to expansion or deficiency of the metal oxide in the electrode active material layer is suppressed.
- the presence or absence of a lithium ion insertion / elimination reaction of the metal oxide can be confirmed by an electrochemical measurement (cyclic voltammetry: CV) method.
- CV test will be described below.
- the electrode potential is swept from 3.0 V to 4.3 V in the appropriate voltage range of the active material, for example, assuming lithium ions as alkali metal ions and LiMn 2 O 4 as the metal oxide. After that, the work of returning to 3.0 V is repeated about three times.
- the scanning speed is preferably 1 mV / sec. For example, in the case of LiMn 2 O 4 , as shown in FIG.
- an oxidation peak corresponding to the Li elimination reaction of LiMn 2 O 4 appears in the vicinity of about 3.9 V, and the Li insertion reaction occurs in the vicinity of about 4.1 V.
- a corresponding reduction peak appears, whereby the presence or absence of lithium ion insertion / extraction reaction can be confirmed.
- FIG. 4 when no peak appears, it can be determined that there is no lithium ion insertion / release reaction.
- the fact that the metal oxide does not exhibit a lithium ion insertion / release reaction does not mean the electrical property inherent to the metal oxide, but is contained as a binder in the electrode active material layer.
- the metal oxide does not exhibit a lithium ion insertion / release reaction in a voltage range suitable for the electrode active material particles contained in the electrode active material. This is because it is important that the metal oxide does not substantially insert and desorb lithium ions in the electrode plate.
- the presence or absence of lithium ion insertion / desorption reaction of the metal oxide that is expected to be contained in the electrode active material layer can be confirmed as described above. . Therefore, after confirmation in advance, a metal oxide that does not exhibit a lithium ion insertion / release reaction can be present as a binder in the electrode active material layer. On the other hand, whether or not a metal oxide that does not exhibit lithium ion insertion / release reaction is contained in the electrode active material layer in the electrode plate that has already been completed can be confirmed as follows, for example.
- the electrode plate can optionally further contain a conductive material in the electrode active material layer.
- a conductive material in the electrode active material layer, it is possible to ensure better electronic conductivity between each electrode active material and the current collector in the electrode active material layer, and to reduce the volume of the electrode active material layer itself. It is desirable because the resistivity can be lowered efficiently.
- the conductive material those usually used for electrode plates for non-aqueous electrolyte secondary batteries can be used, and conductive carbon materials such as particulate carbon black such as acetylene black and ketjen black are used. Illustrated.
- the average primary particle size of the conductive material is preferably about 20 nm to 50 nm.
- Carbon fiber is known as a different conductive material.
- the carbon fiber can conduct electricity very well in the length direction and can improve the fluidity of electricity.
- the fiber length is about 1 ⁇ m to 20 ⁇ m. Therefore, in addition to the particulate conductive material such as acetylene black described above, the effect of adding the conductive material can be improved by using carbon fiber together.
- the conductivity of the conductive material is generally expressed as an electrical resistivity, and an electrical resistance of about 0.14 to 0.25 ⁇ cm is shown.
- the said average primary particle size is calculated
- the content is not particularly limited, but generally, the proportion of the conductive material is 5 parts by weight or more and 20 parts by weight with respect to 100 parts by weight of the electrode active material particles. It is desirable that the amount is not more than parts by weight.
- the electrode active material layer contains a carbon component (hereinafter also simply referred to as “carbon component”) that is distinguished from the conductive material.
- the carbon component is also distinguished from a conductive material that is optionally added, and particularly in the negative electrode plate, it is present in the electrode active material layer separately from the negative electrode active material particles.
- the presence of the carbon component in the electrode active material layer is generally greater than the amount of carbon derived from the electrode active material or conductive material than the carbon source detected by the composition analysis of the electrode active material layer in the obtained electrode plate. Whether it can be confirmed. However, when the amount of the carbon component is very small, the remaining amount may not be reflected in the composition analysis. The present inventors have confirmed that even such a small amount of carbon component actually contributes to the improvement of the input / output characteristics of the electrode plate by being present in the electrode active material layer. For example, the electrode active material layer forming composition before the addition of the conductive material is applied on the substrate to form a coating film and heated at an appropriate heating temperature, so that a carbon component is present in the formed film. Preliminary experiments will be conducted to confirm this fact.
- the electrode active material layer forming composition to which a conductive material or the like is added is actually applied onto the current collector and heated under the same heating conditions as in the preliminary experiment.
- the electrode plate obtained as described above it is understood that carbon components other than carbon constituting the conductive material remain in the electrode active material layer regardless of the result of the composition analysis of the electrode active material layer.
- TEM transmission electron microscope
- STEM method scanning transmission electron microscope
- Carbon element can be confirmed by element mapping shown by nano-order elemental analysis with a detector.
- the carbon component contained in the electrode active material layer can also be confirmed by evaluating the nano-order state with an EELS spectrometer and obtaining a composition contrast image with a HAADF detector.
- the element mapping and the method using the composition contrast image are particularly useful for the negative electrode plate among the electrode plates. That is, negative electrode active material particles such as graphite or conductive material particles exist in the electrode active material layer as an aggregate of substantially pure carbon atoms.
- the carbon component is not an aggregate of carbon atoms such as a scale of a conductive material, but the carbon component can be confirmed by the presence of carbon elements dispersed in other components. Therefore, in the carbon element mapping, the carbon component can be confirmed by the presence of carbon elements scattered in the electrode active material.
- the carbon component may be contained in the metal oxide in the electrode active material layer. That is, when the metal oxide is generated in the electrode active material layer, a carbon component may be contained to generate these binder materials. Even in such a case, high input / output characteristics and good performance are obtained. Excellent effects such as maintaining proper processing characteristics are exhibited.
- the carbon component is derived from carbon in a substance added to the electrode active material layer forming composition, such as an organic substance or an organometallic compound described later, and formed by adjusting the heating temperature during the manufacturing process. Although it can obtain by making the said carbon remain in the electrode active material layer made, it is not limited to this. More specifically, an electrode active material layer forming composition before adding a carbon material such as a conductive material or negative electrode active material particles composed of graphite is applied onto a substrate to form a coating film, Preliminary experiments are performed to preliminarily confirm that carbon components are present in the formed film by heating at a heating temperature or an appropriate heating atmosphere.
- an electrode active material layer forming composition containing a necessary material is applied onto a current collector, and a heating step is performed under the same conditions as in the preliminary experiment, whereby a negative electrode composed of a conductive material or graphite.
- An electrode plate including an electrode active material layer containing a carbon component other than a carbon material such as active material particles can be produced.
- the input / output characteristics can be further improved by including a carbon component that is distinguished from the conductive material in the electrode active material layer.
- the carbon component by including the carbon component, the flexibility of the electrode active material layer is improved, and the present inventors have shown that excellent processing characteristics can be obtained without using a resin binder. Found by research. Therefore, when processing the electrode plate of the present embodiment, or when manufacturing a non-aqueous electrolyte secondary battery using the manufactured electrode plate of the present embodiment, the electrode plate is placed in a curved state. However, peeling of the electrode active material layer from the current collector and dropping of the active material particles do not occur, and a very excellent electrode plate can be provided.
- the amount of the carbon component distinguished from the conductive material in the electrode active material layer is not particularly limited, and even if it is a minute content that is not reflected in a general composition analysis, This can contribute to the improvement of input / output characteristics.
- the carbon component may be contained in a metal oxide as a binding material. In such a case, the carbon component is contained with respect to 100 mol% of the metal element contained in the metal oxide.
- the content is preferably 10 mol% or more, and the upper limit thereof is not particularly limited, but a content of 50 mol% or less can sufficiently contribute to the improvement of the input / output characteristics and the improvement of the processing characteristics.
- the electrode active material layer contains at least a carbon component that is distinguished from the electrode active material particles, the metal oxide that is the binder, and the conductive material, and the conductive material can be further added. Further optional additives may be contained within the scope not departing from the gist of the invention.
- the current collector is not particularly limited as long as it is generally used as an electrode current collector of an electrode plate for a non-aqueous electrolyte secondary battery.
- aluminum foil or nickel foil can be preferably used as the positive electrode current collector, and copper foil, aluminum foil, nickel foil or the like can be preferably used as the negative electrode current collector.
- the thickness of the current collector is not particularly limited as long as it is a thickness that can generally be used as a current collector for a nonaqueous electrolyte secondary battery electrode plate, but is preferably 10 to 100 ⁇ m, and preferably 15 to 50 ⁇ m. It is more preferable.
- the input / output characteristics of the electrode plate can be evaluated by determining the discharge capacity maintenance rate (%). That is, the discharge capacity retention rate is an evaluation of the discharge rate characteristics, and it is generally understood that the charge rate characteristics are similarly improved in an electrode plate with improved discharge rate characteristics. Therefore, when a desirable discharge capacity maintenance ratio is indicated, it is evaluated that the charge / discharge rate characteristics are improved, and as a result, the input / output characteristics are evaluated as improved. More specifically, the discharge rate 1C is set such that the theoretical value of the discharge capacity (mAh / g) of the active material is completed in 1 hour, and the discharge actually measured at the set discharge rate of 1C.
- the capacity (mAh / g) is set to a discharge capacity maintenance rate of 100%.
- the discharge capacity (mAh / g) when the discharge rate is further increased is measured, and the discharge capacity retention ratio (%) can be obtained from the following equation 1.
- the said discharge capacity is calculated
- the charge / discharge rate characteristics of the electrode plate vary depending on the type and particle diameter of the electrode active material particles used, the amount of the metal oxide that is the binder contained, the thickness of the electrode active material layer, and the like. In general, regarding the charge / discharge rate characteristics of the electrode plate for a non-aqueous electrolyte secondary battery, it is desirable that a discharge capacity maintenance rate of 50% or more is shown at a discharge rate of 50C or more, and more desirably 50% or more. It is desirable that the discharge capacity retention rate be shown at a discharge rate of 100 C or higher, and it can be evaluated that the charge / discharge rate characteristics are high.
- the electrode plate of the present embodiment can exhibit the high charge / discharge rate characteristics described above. However, it is desirable to pay attention to this point because a system capable of withstanding a large current is required when the discharge rate is 2000 C or higher.
- the discharge capacity retention rate is high, and the discharge capacity is 50 C when the discharge rate is 50 C. It is desirable that the maintenance rate be 50% or more, or 80% or more, and even 100%. If it is the electrode plate for nonaqueous electrolyte secondary batteries of this Embodiment, it is possible to show the high discharge maintenance factor shown above.
- the electrode plate for a non-aqueous electrolyte secondary battery according to the present embodiment as described above is crystalline without using a resin binder as in the prior art, and has an alkaline ion insertion / desorption reaction. Due to the presence of a metal oxide not shown, an electrode active material layer is provided in which electrode active material particles are fixed on a current collector. According to such an electrode plate, compared with a conventional electrode plate for a non-aqueous electrolyte secondary battery using a resin binder, even when the same amount of the same electrode active material particles is contained, the output is very high. It is possible to exhibit input characteristics and high cycle characteristics. In addition, the inclusion of a carbon component that is distinct from the conductive material exhibits very desirable input / output characteristics. Moreover, this electrode plate has good film adhesion of the electrode active material layer to the current collector as in the case of a conventional electrode plate using a resin binder, and thus the film formability of the electrode active material layer is good. It is.
- 1st aspect of the manufacturing method of this invention is 1 type, or 2 or more types of metal element content for producing
- An electrode active material layer forming composition containing at least a compound and an organic substance that is capable of imparting a carbon component that is distinguished from a conductive material is prepared, and using this, a coating step described below and heating The steps are performed in order.
- the metal element-containing compound is selected in advance so that the metal oxide generated in the heating step is a metal oxide that does not exhibit an alkali metal ion insertion / release reaction.
- the heating temperature in the heating step is equal to or higher than the thermal decomposition start temperature of the metal element-containing compound and higher than the crystallization temperature of the metal oxide generated in the heating step, and the carbon derived from the organic matter Is set to a temperature at which it can remain in the electrode active material layer as a carbon component distinguished from the conductive material.
- the electrode active material particles and one or more organic metal compounds selected as a material for generating a metal oxide as a binder are at least
- the electrode active material layer forming composition to be contained is prepared, and the coating step and the heating step described later are sequentially performed using the composition.
- the organometallic compound is selected in advance so that the metal oxide generated in the heating step is a metal oxide that does not exhibit an alkali metal ion insertion / release reaction.
- the heating temperature in the heating step is not less than the thermal decomposition start temperature of the organometallic compound, not less than the crystallization temperature of the metal oxide produced in the heating step, and carbon derived from the organometallic compound. Is set to a temperature at which it can remain in the electrode active material layer as a carbon component distinguished from the conductive material.
- a third aspect of the production method of the present invention is a method in which electrode active material particles, one or more organometallic compounds selected as a material for generating a metal oxide as a binder, and a conductive material are used.
- An electrode active material layer-forming composition containing at least an organic substance that is capable of imparting a carbon component that is distinct from the material is prepared, and using this, an application step and a heating step described later are sequentially performed. To do.
- the organometallic compound is selected in advance so that the metal oxide generated in the heating step is a metal oxide that does not exhibit an alkali metal ion insertion / release reaction.
- the heating temperature in the heating step is equal to or higher than the thermal decomposition start temperature of the organometallic compound and is equal to or higher than the crystallization temperature of the metal oxide generated in the heating step, and at least the organometallic compound or the organic matter.
- the temperature is such that the derived carbon can remain in the electrode active material layer as a carbon component distinguished from the conductive material.
- one important point in the preparation of the electrode active material layer forming composition is that a material containing carbon is blended in a material other than a conductive material that is optionally added.
- the carbon component that is distinguished from the conductive material can remain in the produced electrode active material layer. That is, as described above, the electrode active material layer forming composition is blended with at least an organic substance that can impart a carbon component that is different from a conductive material, or an organometallic compound that is a metal oxide generating material. It is necessary to add.
- the production method of the present invention will be described in more detail.
- Electrode active material particles Since the electrode active material particles contained in the electrode active material layer forming composition are the same as the electrode active material particles already described above, the description thereof is omitted here. In addition, in the manufacturing method of an electrode plate, the particle diameter of the electrode active material particle used can select a desired magnitude
- the electrode active material layer forming composition may contain a metal element-containing compound or an organometallic compound as a metal oxide production material to be produced.
- the metal element-containing compound and the organometallic compound may be collectively referred to as a binder generation material.
- the binder material generating material is a metal oxide generating material for fixing the electrode active material particles on the current collector as the binder material.
- the binder generation material When the binder generation material is heated on the substrate at a temperature equal to or higher than the thermal decomposition start temperature, it can be thermally decomposed and oxidized to form a film.
- the present inventors In studying the problems of the present invention, the present inventors have studied the inclusion of electrode active material particles in the metal oxide film when forming the metal oxide film on the substrate, and have earnestly studied. As a result, it has been found that the electrode active material particles can be fixed on the substrate due to the presence of the metal oxide even if the amount of the metal oxide is reduced.
- the present inventors without using a resin binder, under the idea of including electrode active material particles in the binder material to be formed into a film, the binder material generating material, the electrode active material particles, Was prepared, applied on the current collector, and tried to heat.
- the electrode active material particles are collected even if the amount of the binder material generated on the current collector is significantly reduced to the extent that the binder material exists in the electrode active material layer mainly composed of the electrode active material particles. It has been found that it is fixed on the electric body.
- the binder-generating material used in the production method of the present invention contains a metal element that can be thermally decomposed and oxidized to form a film within the scope of the present invention, and Any binding material may be selected as long as the binding material generated on the current collector does not show insertion / release reaction of alkali metal ions such as lithium ions.
- the organometallic compound is a binding material generating material, and the carbon atoms contained in the organometallic compound are contained in the electrode active material layer. It is a compound that can be imparted as a carbon component that is distinguished from the material.
- the binder produced from the binder substance-generating material used does not show alkali metal ion insertion / release reaction.
- the binder material is formed by applying to and heated, and can be confirmed by the cyclic voltammetry method described above.
- the metal element-containing compound includes Li, Be, Na, Mg, Al, Si, K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In, Sn, Cs, Ba, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg, Any compound selected from a general metal element group such as Tl, Pb, Bi, Fr, Ra, and Ce may be used as long as it is a compound containing two or more metal elements.
- the input / output characteristics of the generated electrode plate become higher. It is preferable because of its tendency. That is, Na, Mg, Al, Si, K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Rb, Sr, Y, Zr, Nb, Mo, Tc, A compound containing one or more metal elements selected from Ru, Rh, Pd, Ag, Cd, In, and Sn is preferable as the metal element-containing compound.
- the metal element-containing compound containing the metal element for example, a metal salt is preferably used.
- the metal salt include chloride, nitrate, sulfate, perchlorate, phosphate, bromate and the like.
- chlorides and nitrates are preferable because they are easily available as general-purpose products.
- nitrate is preferably used because it is inexpensive.
- metal salts include magnesium chloride, aluminum nitrate, aluminum chloride, calcium chloride, titanium tetrachloride, vanadium oxosulfate, ammonium chromate, chromium chloride, ammonium dichromate, chromium nitrate, chromium sulfate, manganese nitrate , Manganese sulfate, iron (I) chloride, iron (III) chloride, iron (III) nitrate, iron (II) sulfate, iron (III) sulfate, cobalt chloride, cobalt nitrate, nickel chloride, nickel nitrate, copper chloride, nitric acid Copper, zinc chloride, yttrium nitrate, yttrium chloride, zirconium chloride oxide, zirconium nitrate oxide, zirconium tetrachloride, silver chloride, indium nitrate, tin sulfate,
- the organometallic compound means a compound containing a metal and carbon, and includes both a metal complex containing a carbon element and a metal salt containing a carbon element. More specifically, the organometallic compound may be any one selected from a general metal element group as listed in the above metal element-containing compound, or a compound containing two or more metal elements and carbon. That's fine. In addition, in the organometallic compound, it is preferable that a metal element belonging to 3 to 5 cycles among the metal element group is contained, similarly to the metal element-containing compound.
- the metal salt examples include acetate and oxalate. Among them, acetate is preferably used because it is easily available as a general-purpose product. Specific examples of the metal salt include scandium acetate, chromium acetate, iron (II) acetate, cobalt acetate, nickel acetate, zinc acetate, silver acetate, indium acetate, cerium acetate, Examples thereof include cerium oxalate, lead acetate, lanthanum acetate, strontium acetate, palladium acetate, and barium acetate.
- the metal complex examples include magnesium diethoxide, aluminum acetylacetonate, calcium acetylacetonate dihydrate, calcium di (methoxyethoxide), calcium gluconate monohydrate, calcium citrate tetrahydrate, Calcium salicylate dihydrate, titanium lactate, titanium acetylacetonate, tetraisopropyl titanate, tetranormal butyl titanate, tetra (2-ethylhexyl) titanate, butyl titanate dimer, titanium bis (ethylhexoxy) bis (2-ethyl-3-hydroxy) Hexoxide), diisopropoxytitanium bis (triethanolaminate), dihydroxybis (ammonium lactate) titanium, diisopropoxytitanium bis (ethylacetoacetate) ), Ammonium peroxosodium citrate tetrahydrate, dicyclopentadienyl iron (II), iron (II) lactate trihydrate, iron (III) ace
- the organometallic compound described above is distinguished from the metal element-containing compound described above depending on whether or not it contains carbon. Both the metal element-containing compound and the organometallic compound are binding material-generating materials. However, the difference in their use is that in the organometallic compound, the carbon element contained therein is electrically conductive in the electrode active material layer.
- the carbon component can be left as a distinctive carbon material, and the heating temperature in the heating step needs to be adjusted appropriately in order to leave the carbon element in the electrode active material layer. The heating temperature will be described later.
- the electrode active material layer provided on the electrode plate for a non-aqueous electrolyte secondary battery manufactured by the manufacturing method of the present invention is a binding material capable of fixing the electrode active material particles on the current collector. Any material can be appropriately selected and used as long as it is a material capable of producing a metal oxide.
- an organic substance that is different from the organometallic compound can be used as another compound for providing the carbon component remaining in the electrode active material layer.
- the organic substance include urethane resin, epoxy resin, ethyl cellulose, starch, polyethylene oxide, polyvinyl alcohol, and polyethylene glycol. These organic substances exhibit the effect of adjusting the viscosity when the electrode active material layer forming composition is prepared.
- An organic material that can also act as a viscosity modifier is blended in the electrode active material layer forming composition, and the composition is applied onto a current collector, and then heated at an appropriate temperature to form an electrode active material to be formed. The carbon component can remain in the material layer.
- the electrode active material layer forming composition may be mixed with a conductive material and other additives without departing from the spirit of the present invention.
- the solvent used in the electrode active material layer forming composition can be prepared as an electrode active material layer forming composition to which additives such as electrode active material particles, a binding material generating material, and an organic substance are added, and If it can remove in a heating process after apply
- lower alcohols having a total carbon number of 5 or less such as methanol, ethanol, isopropyl alcohol, propanol, butanol, diketones such as acetylacetone, diacetyl, benzoylacetone, ethyl acetoacetate, ethyl pyruvate, ethyl benzoylacetate, ethyl benzoylformate And ketoesters such as toluene, a single solvent such as toluene, or a mixed solvent composed of a combination of two or more thereof.
- diketones such as acetylacetone, diacetyl, benzoylacetone, ethyl acetoacetate, ethyl pyruvate, ethyl benzoylacetate, ethyl benzoylformate
- ketoesters such as toluene, a single solvent such as toluene, or a mixed solvent composed of
- the electrode active material layer forming composition comprises electrode active material particles, a binder forming material, an organic substance, and other additives added as necessary in an electrode active material layer scheduled to be formed on a current collector. These blending amounts are determined in consideration of the necessary amount.
- the solid content ratio is appropriately adjusted in consideration of the coating property on the current collector in the coating step and the removal of the solvent in the heating step. Generally, the solid content ratio in the electrode active material layer forming composition is adjusted to 30 to 70 wt%.
- the electrode active material layer forming composition prepared as described above is applied onto a current collector to form a coating film.
- the current collector used in the manufacturing method of the present embodiment is the same as the current collector used in the electrode plate for a non-aqueous electrolyte secondary battery, and is omitted here.
- any known coating method can be used as the coating method for the electrode active material layer forming composition.
- a coating film can be formed by applying to any region of the current collector surface by printing, spin coating, dip coating, bar coating, spray coating, or the like.
- the current collector surface is porous, has a large number of irregularities, or has a three-dimensional structure, it can be manually applied in addition to the above method.
- the current collector can further improve the film forming property of the electrode active material layer by performing corona treatment, oxygen plasma treatment, or the like in advance as necessary.
- the amount of the electrode active material layer forming composition applied to the current collector can be arbitrarily determined according to the application of the electrode plate to be produced, etc., but the electrode active material layer in the present embodiment is When it is desired to reduce the film thickness, the electrode active material layer formed by the heating process described later should be thinly applied so that the thickness is about 300 nm to 11 ⁇ m. Can do.
- the electrode active material layer forming composition to the current collector, the electrode active material particles and the binding material generating material (that is, the metal element-containing compound or the organometallic compound), or further A coating film for forming an electrode active material layer containing at least an organic substance (hereinafter sometimes simply referred to as “coating film”) is formed.
- Heating process Next, the heating process for heating the coating film formed in the coating process will be described.
- This heating step heats and thermally decomposes the binder-forming material present in the coating film to produce a crystalline metal oxide containing a metal element contained therein, and in the coating film It is performed for the purpose of removing the contained solvent. Also, at this time, in order to leave carbon in at least one of the organometallic compound in the coating film or the organic substance further added in the electrode active material layer as a carbon component distinct from the conductive material, suitable heating is performed. It is necessary to adjust the temperature or heating atmosphere.
- the heating method is not particularly limited as long as it is a heating method or a heating apparatus that can heat the coating film at a desired heating temperature, and can be appropriately selected and carried out. Specific examples include a method of using a hot plate, an oven, a heating furnace, an infrared heater, a halogen heater, a hot air blower, or the like, or a combination of two or more.
- a hot plate When the current collector to be used is planar, it is preferable to use a hot plate or the like.
- the heating temperature in the heating step is equal to or higher than the thermal decomposition start temperature of the binding substance generating material and equal to or higher than the crystallization temperature of the generated metal oxide, and at least of the organometallic compound and the organic substance to be added.
- the carbon contained in any one of the carbons is determined in a temperature range in which the carbon component that can be distinguished from the conductive material in the electrode active material layer can remain.
- the thermal decomposition starting temperature of the binding substance-generating material varies depending on the type of each compound.
- the metal element-containing compound or organometallic compound contained in the coating film is heated and thermally decomposed, generally, it is rapidly oxidized to form a metal oxide. Therefore, as a preliminary test, a solution containing a metal element-containing compound or an organometallic compound is applied on a substrate and heated, and the laminated film laminated on the substrate is scraped to make a sample. By measuring the oxygen content ratio, it can be determined whether or not a metal oxide is formed, and if a metal oxide has been formed, the metal element-containing compound or organic metal used It is confirmed that the compound was heated on the substrate at a temperature equal to or higher than the thermal decomposition start temperature.
- thermo decomposition start temperature of metal element-containing compound or “thermal decomposition start temperature of organometallic compound” means that the metal element-containing compound or organometallic compound is thermally decomposed by heating and contained in this It can be understood as the temperature at which element oxidation begins.
- the “crystallization temperature” means a temperature at which the metal oxide is crystallized after the metal atom contained in the electrode active material layer forming composition becomes a metal oxide.
- the metal oxide crystallizes at the crystallization temperature, and the crystallinity increases when the temperature is exceeded.
- the term “crystallization” refers to the crystal state in the X-ray diffractometer regardless of the crystallinity. This refers to the case where the peak shown is confirmed.
- the “crystallization temperature” in the present invention does not necessarily coincide with the intrinsic crystallization temperature of the metal oxide, and differs slightly from the intrinsic crystallization temperature depending on the state in the electrode active material layer forming composition. There is a case. Therefore, in consideration of this point, it is desirable to confirm in advance the crystallization temperature of the metal oxide in the electrode active material layer forming coating film.
- the above heating temperature is “below the crystallization temperature” of the metal oxide to be produced when the metal oxide contained in the electrode active material layer formed on the current collector is in an amorphous state. It is a temperature that allows it to exist at.
- the temperature is preliminarily applied by applying a solution containing the binding substance generating material on the substrate, heating at a temperature equal to or higher than the thermal decomposition start temperature of the binding substance generating material, and being made of a metal oxide on the substrate. Form a film, scrape the film into a sample, evaluate the crystallinity using an X-ray diffractometer, and understand that if the crystal peak is not confirmed, it was heated at a temperature below the crystallization temperature. can do.
- specific examples of the temperature at which carbon contained in the organic metal compound or the organic substance can remain in the electrode active material layer as a carbon component that can be distinguished from the conductive material are as follows, for example. That is, when an organometallic compound is used as the compound imparted to the carbon component that is distinguished from the conductive material remaining in the electrode active material layer, the organic group is separated from the organometallic compound, and at least the organic group It is sufficient that a part of the temperature is carbonized without being lost in the heating step and can remain in the electrode active material layer as a carbon component that is distinguished from the conductive material. Similarly, when an organic material is used, it is sufficient that at least a part of the organic group in the organic material is carbonized without disappearing in the heating step and can remain in the electrode active material layer.
- an electrode active material in the electrode plate of the present embodiment using an electrode active material layer-forming composition containing at least electrode active material particles, a metal element-containing compound, and an organic substance.
- the heating temperature in the heating step is not less than the thermal decomposition start temperature of the metal element-containing compound, not less than the crystallization temperature of the metal oxide generated in the heating step, and What is necessary is just to set to the temperature which the carbon derived from the said organic substance can remain
- the heating step is equal to or higher than the thermal decomposition start temperature of the organometallic compound and is equal to or higher than the crystallization temperature of the metal oxide generated in the heating step, and carbon derived from the organometallic compound is an electrode active material layer. What is necessary is just to set to the temperature which can remain
- the electrode active material layer in the electrode plate of the present embodiment is formed using an electrode active material layer forming composition containing at least electrode active material particles, an organometallic compound, and an organic substance.
- the heating temperature in the heating step is equal to or higher than the thermal decomposition start temperature of the organometallic compound and is equal to or higher than the crystallization temperature of the metal oxide generated in the heating step, and the organometallic compound and / or the organic substance. What is necessary is just to set to the temperature which the carbon contained in can remain
- the heating temperature at which a desired component can be present in the electrode active material layer is determined in advance in a preliminary experiment. It is desirable.
- the heating step when determining the heating temperature, it is desirable to sufficiently take into account the heat resistance of the current collector, electrode active material particles, conductive material, and the like used.
- the heat resistance temperature of a copper foil generally used as a current collector for a negative electrode plate is about 200 ° C. because it is oxidized in an air atmosphere, and about 1080 ° C. in an inert gas atmosphere.
- the heat resistance temperature of the aluminum foil is around 660 ° C. For this reason, when the said heating temperature exceeds the said heat-resistant temperature, there exists a possibility that a collector may be damaged.
- the heating atmosphere in the heating step is not particularly limited, and can be appropriately determined in consideration of the material used for manufacturing the electrode plate, the heating temperature, the oxygen potential of the metal element, and the like.
- the heating step can be preferably performed because there is no possibility that the aluminum foil is oxidized even if the heating step is performed in an air atmosphere.
- copper foil is used as the current collector, it is not desirable because it is oxidized when the heating step is performed in an air atmosphere.
- an inert gas atmosphere it is preferable to heat in an inert gas atmosphere, a reducing gas atmosphere, or a mixed gas atmosphere of an inert gas and a reducing gas.
- the heating step is performed in an atmosphere that does not contain sufficient oxygen gas
- the metal oxide is generated in the electrode active material layer
- the oxidation of the metal element in the metal element-containing compound or organometallic compound is performed. Needs to be realized by the combination of oxygen and metal element in the compound contained in the electrode active material layer forming composition, so it is necessary to use the compound containing oxygen element in the compound to be used There is.
- the atmosphere in which the manufacturing method is carried out is not particularly limited to a specific atmosphere, and is an inert gas atmosphere, a reducing gas atmosphere, or a gas atmosphere in which an inert gas and a reducing gas are mixed.
- the manufacturing method can be carried out as appropriate.
- an inert gas atmosphere a manufacturing method can be implemented in the atmosphere of argon gas and nitrogen gas.
- a reducing gas atmosphere a manufacturing method can be implemented in the atmosphere of hydrogen gas and carbon monoxide gas.
- an electrode plate for a non-aqueous electrolyte secondary battery can be manufactured.
- an electrode active material layer-forming composition prepared by containing at least a metal element-containing compound, electrode active material particles, and an organic substance, or an organic metal compound and electrode active material particles that are binding material generating materials.
- the electrode active material layer-forming composition prepared and contained exhibits a viscosity that allows good application to the current collector to be maintained regardless of the particle diameter of the contained electrode active material particles. Therefore, in a conventional electrode active material layer forming composition using a resin binder, it is possible to use electrode active material particles having a small particle diameter, which are difficult to use due to a significant increase in viscosity.
- paintability to the electrical power collector of the said electrode active material formation composition is favorable, it is also possible to apply
- the nonaqueous electrolyte secondary battery 10 is generally provided between the negative electrode 14 and the negative electrode plate 15, the positive electrode 16 and the positive electrode plate 17, and the negative electrode plate 15 and the positive electrode plate 17.
- the separator 13 is provided.
- the negative electrode 14, the negative electrode plate 15, the positive electrode 16, the positive electrode plate 17, and the separator 13 are accommodated in the container 11.
- the separator 13 is made of a polyethylene porous film.
- the container 11 is hermetically sealed with the non-aqueous electrolyte 19 filled in the container 11.
- the electrode plate according to the present embodiment described above is used as the positive electrode plate and / or the negative electrode plate.
- the electrode plate of the present embodiment has very excellent input / output characteristics and cycle characteristics. Therefore, by using such an electrode plate, the performance of the electrode plate is exhibited also in the non-aqueous electrolyte secondary battery of the present embodiment, and the input / output characteristics and cycle characteristics of the battery itself are improved.
- the negative electrode plate is appropriately selected from conventionally known negative electrode plates for non-aqueous electrolyte secondary batteries.
- a copper foil such as an electrolytic copper foil or a rolled copper foil having a thickness of about 5 to 50 ⁇ m is used as a current collector, and an electrode in the negative electrode plate is formed on at least a part of the surface of the current collector.
- coating an active material layer forming composition, drying, and pressing as needed may be used.
- the electrode active material layer forming composition in the negative electrode plate generally includes an active material composed of a carbonaceous material such as natural graphite, artificial graphite, amorphous carbon, carbon black, or a material obtained by adding a different element to these components.
- an active material composed of a carbonaceous material such as natural graphite, artificial graphite, amorphous carbon, carbon black, or a material obtained by adding a different element to these components.
- negative electrode active material particles such as metal lithium and its alloys, tin, silicon, and alloys thereof, materials that can occlude and release lithium ions, and resinous binders, and other additions such as conductive materials as necessary
- the agent is dispersed and mixed, but is not limited thereto.
- the positive electrode plate is appropriately selected from conventionally known positive electrode plates for non-aqueous electrolyte secondary batteries. Can be used.
- a generally known positive electrode plate an aluminum foil having a thickness of about 5 to 50 ⁇ m is used as a current collector, and an electrode active material layer forming composition for forming a positive electrode plate is formed on at least a part of the surface of the current collector.
- a positive electrode plate formed by coating, drying, and pressing as necessary may be used.
- the electrode active material layer forming composition for forming the positive electrode plate generally includes lithium transition metal composite oxides such as LiCoO 2 , LiMn 2 O 4 , LiNiO 2 , LiFeO 2 , Li 4 Ti 5 O 12 , LiFePO 4.
- positive electrode active material particles such as a product, a resin binder, and other additives such as a conductive material as needed are dispersed and mixed, but the present invention is not limited thereto.
- Nonaqueous electrolyte is not particularly limited as long as it is generally used as a nonaqueous electrolyte for a nonaqueous electrolyte secondary battery, but a nonaqueous electrolyte in which a lithium salt is dissolved in an organic solvent is used. Preferably used.
- lithium salt examples include inorganic lithium salts such as LiClO 4 , LiBF 4 , LiPF 6 , LiAsF 6 , LiCl, and LiBr; LiB (C 6 H 5 ) 4 , LiN (SO 2 CF 3 ) 2 , LiC ( Organic compounds such as SO 2 CF 3 ) 3 , LiOSO 2 CF 3 , LiOSO 2 C 2 F 5 , LiOSO 2 C 4 F 9 , LiOSO 2 C 5 F 11 , LiOSO 2 C 6 F 13 , and LiOSO 2 C 7 F 15 Typical examples include lithium salts.
- inorganic lithium salts such as LiClO 4 , LiBF 4 , LiPF 6 , LiAsF 6 , LiCl, and LiBr
- LiB (C 6 H 5 ) 4 LiN (SO 2 CF 3 ) 2
- LiC Organic compounds such as SO 2 CF 3 ) 3 , LiOSO 2 CF 3 , LiOSO 2 C 2 F 5
- Examples of the organic solvent used for dissolving the lithium salt include cyclic esters, chain esters, cyclic ethers, and chain ethers.
- Examples of the cyclic esters include propylene carbonate, butylene carbonate, ⁇ -butyrolactone, vinylene carbonate, 2-methyl- ⁇ -butyrolactone, acetyl- ⁇ -butyrolactone, and ⁇ -valerolactone.
- chain esters examples include dimethyl carbonate, diethyl carbonate, dibutyl carbonate, dipropyl carbonate, methyl ethyl carbonate, methyl butyl carbonate, methyl propyl carbonate, ethyl butyl carbonate, ethyl propyl carbonate, butyl propyl carbonate, propionic acid alkyl ester, Examples include malonic acid dialkyl esters and acetic acid alkyl esters.
- Examples of the cyclic ethers include tetrahydrofuran, alkyltetrahydrofuran, dialkyltetrahydrofuran, alkoxytetrahydrofuran, dialkoxytetrahydrofuran, 1,3-dioxolane, alkyl-1,3-dioxolane, and 1,4-dioxolane.
- Examples of the chain ethers include 1,2-dimethoxyethane, 1,2-diethoxyethane, diethyl ether, ethylene glycol dialkyl ether, diethylene glycol dialkyl ether, triethylene glycol dialkyl ether, and tetraethylene glycol dialkyl ether. It is done.
- a conventionally known structure can be appropriately selected and used.
- the structure which winds a positive electrode plate and a negative electrode plate in the shape of a spiral via the separator like a polyethylene porous film, and accommodates in a battery container is mentioned.
- a structure in which a positive electrode plate and a negative electrode plate cut into a predetermined shape are stacked and fixed via a separator, and this is housed in a battery container may be employed.
- the lead wire attached to the positive electrode plate is connected to the positive electrode terminal provided in the outer container, while the lead wire attached to the negative electrode plate Is connected to a negative electrode terminal provided in the outer container, and the battery container is further filled with a nonaqueous electrification solution and then sealed to produce a nonaqueous electrolyte secondary battery.
- the electrode plate according to the present embodiment in which the input / output characteristics are improved as described above is used as the positive electrode plate and / or the negative electrode plate. Therefore, since the input / output characteristics and cycle characteristics of the electrode plate are improved as described above, the input / output characteristics and cycle characteristics of the non-aqueous electrolyte secondary battery are improved.
- An aluminum plate having a thickness of 15 ⁇ m is prepared as a current collector, and an electrode prepared as described above is formed on one side of the current collector in such an amount that the weight of the electrode active material layer finally obtained is 15 g / m 2.
- the active material layer forming composition was applied with an applicator to form a coating film for forming an electrode active material layer.
- the current collector with the electrode active material layer-forming coating film formed on the surface was placed in a normal temperature electric furnace (muffle furnace, P90, manufactured by Denken) and heated to 260 ° C. over 1 hour. Then, heating was performed for 5 hours while maintaining the temperature at 260 ° C., and then a current collector was installed in the electric furnace heated to 500 ° C., and then heated for 15 minutes while maintaining the temperature at 500 ° C.
- a positive electrode plate for a non-aqueous electrolyte secondary battery according to the above-described embodiment in which an electrode active material layer suitable as a positive electrode active material layer was laminated on the body was obtained.
- the positive electrode plate was taken out of the electric furnace and allowed to stand at room temperature, and then cut into a predetermined size (a disk having a diameter of 15 mm) to obtain Example 1. In addition, it was 22 micrometers when the thickness of the electrode active material layer was measured 10 points
- Example 2 In the preparation of Example 1, the positive electrode plate for a non-aqueous electrolyte secondary battery obtained above was processed into a disk shape of a desired size. In this processing operation, an electrode active material was used. The working electrode could be formed without problems such as peeling of the layers. From this, it was confirmed that the film forming property of the electrode active material layer was good. Also in the examples and comparative examples shown below, good film formation means that, as described above, the positive electrode plate (or negative electrode plate) can be pulled out onto the disc without any problems. .
- the film forming property of the electrode active material layer is evaluated as poor.
- composition analysis test Next, the electrode active material layer in Example 1 was shaved to obtain Sample 1. Then, when composition analysis was performed by X-ray photoelectron spectroscopy (Electron Spectroscopy for Chemical Analysis) using Sample 1, Fe element was 6 Atomic%, Mn element was 8 Atomic%, O element was 53 Atomic%, and C element was 33 Atomic%. was detected. On the other hand, N element was not detected. As a result, it was confirmed that iron nitrate contained in the electrode active material layer forming coating film was thermally decomposed to produce iron oxide.
- Crystallinity evaluation Further, when the crystallinity of the sample 1 was evaluated with an X-ray diffractometer (XRD), the metal oxide contained in the electrode active material layer was found to be crystalline as shown in FIG. It was.
- XRD X-ray diffractometer
- a raw material solution for forming the metal oxide (a solution before adding the positive electrode active material) was applied to a glass plate with a No. 4 bar and heated under the same heating conditions as those for electrode preparation.
- the result of evaluating the crystallinity using an X-ray diffractometer is shown in FIG.
- the crystallinity of M1090 which is a positive electrode active material particle, was evaluated using an X-ray diffractometer, and the results are shown in FIG. FIG.
- FIG. 7 is an X-ray diffraction result of iron oxide obtained by heating the raw material solution, and it was confirmed that the iron oxide was crystalline from the fact that a peak was confirmed.
- FIG. 8 is an X-ray diffraction result of lithium manganate as positive electrode active material particles, and a peak representing crystalline lithium manganate was confirmed. Analysis of FIG. 6 with reference to FIGS. 7 and 8 confirmed that a peak representing the crystallinity of iron oxide was shown in addition to the characteristic peak of crystalline lithium manganate.
- Cyclic voltammetry test (CV test): Further, a CV test was conducted using the positive electrode plate produced in Example 1. Specifically, the operation of first sweeping the electrode potential from 3.0 V to 4.3 V and then returning it to 3.0 V was repeated three times. The scanning speed was 1 mV / sec. The cyclic voltammogram showing the second cycle result corresponds to FIG. 3 described above. As is clear from FIG. 3, an oxidation peak corresponding to the Li elimination reaction of LiMn 2 O 4 was observed near 3.9 V, and a reduction peak corresponding to the Li insertion reaction was observed near 4.1 V. On the other hand, the raw material solution for forming the metal oxide (solution before adding the positive electrode active material) was applied to the aluminum substrate with a Miyabar No.
- LiPF 6 Lithium hexafluorophosphate
- EC ethylene carbonate
- DMC dimethyl carbonate
- Example 1 (15 mm diameter disc, weight of positive electrode active material contained: 2.3 mg / 1.77 cm 2 ) prepared as described above as a positive electrode plate was used as a working electrode, and a metal as a counter electrode and a reference electrode Using the non-aqueous electrolyte prepared above as the lithium plate and electrolyte, a tripolar coin cell was assembled, and this was designated as Example Test Cell 1.
- the example test cell 1 was subjected to the following charge / discharge test.
- Charge / discharge test In the example test cell 1, which is a tripolar coin cell manufactured as described above, first, the working electrode was subjected to a full charge according to the following charging test of the example test cell 1 in order to perform a discharge test of the working electrode.
- Example Test cell 1 was charged at a constant current (240 ⁇ A) under a 25 ° C. environment until the voltage reached 4.3 V. After the voltage reached 4.3 V, the voltage was 4.3 V. The current (discharge rate: 1C) was decreased until the current became 5% or less, and the battery was charged at a constant voltage, fully charged, and then suspended for 10 minutes.
- the “1C” means a current value (current value reaching the discharge end voltage) at which constant current discharge is performed using the tripolar coin cell and discharge is completed in one hour.
- the constant current was set such that a theoretical discharge amount of 90 mAh / g of lithium manganate as an active material was discharged in one hour at the working electrode in Example Test Cell 1.
- Example test cell 1 (Discharge test) Thereafter, the fully charged example test cell 1 was subjected to a constant current (240 ⁇ A) (discharge) in an environment of 25 ° C. until the voltage changed from 4.3 V (full charge voltage) to 3.0 V (discharge end voltage).
- a constant current discharge at a rate of 1 C) the cell voltage (V) on the vertical axis, the discharge time (h) on the horizontal axis, a discharge curve is created, and the working electrode (electrode plate for positive electrode of Example 1)
- the discharge capacity (mAh) was determined and converted to the discharge capacity (mAh / g) per unit weight of the working electrode.
- Discharge capacity maintenance rate at discharge rate 50C 60% or more Discharge capacity maintenance rate at 50C discharge rate 50% or more and less than 60% Discharge capacity maintenance rate at a discharge rate of 50 C 30% or more and less than 50% Discharge capacity maintenance rate at a discharge rate of 50C Less than 30% ...
- Example 2 as the metal element-containing compound Fe (NO 3) 3 ⁇ 9H 2 O [ molecular weight: 404] 10 g of 0.4g was added to methanol 5g, a further polyurethane resin (Arakawa Chemical Industries Co. Ltd. KL-424) A positive electrode plate was produced in the same manner as in Example 1 except that mixing was performed and the electrode active material layer forming composition application amount shown in Table 2 was used, and Example 2 was obtained.
- Example 3 A positive electrode in the same manner as in Example 1 except that a positive electrode active material LiMn 2 O 4 having an average particle size of 0.3 ⁇ m was used and the amount of the electrode active material layer forming composition shown in Table 2 was used.
- a plate was produced as Example 3.
- Example 4 A positive electrode plate was prepared in the same manner as in Example 1 except that the positive electrode active material LiMn 2 O 4 having an average particle size of 10 ⁇ m was used and the amount of the electrode active material layer forming composition shown in Table 2 was set. This was produced as Example 4.
- Example 5 As the metal element-containing compound, Fe (NO 3) 3 ⁇ 9H 2 O [ molecular weight: 404]
- a positive electrode plate was prepared in the same manner as in Example 1 except that the positive electrode active material LiMn 2 O 4 was used and the electrode active material layer forming composition application amount shown in Table 2 was used. .
- Example 6 As an organometallic compound, 4.0 g of Li (CH 3 COO) ⁇ 2H 2 O [molecular weight: 102] was used, and this was added to 16 g of methanol. Further, ethyl cellulose (Nisshinsei Co., Ltd. Etcelle Gr.STD) was used. Example 7 except that 10 g of -7) was mixed, the positive electrode active material LiMn 2 O 4 having an average particle diameter of 1 ⁇ m was used, and the electrode active material layer forming composition application amount shown in Table 2 was used. In the same manner, a positive electrode plate was prepared and used as Example 6.
- Li (CH 3 COO) ⁇ 2H 2 O [molecular weight: 102] was used, and this was added to 16 g of methanol. Further, ethyl cellulose (Nisshinsei Co., Ltd. Etcelle Gr.STD) was used. Example 7 except that 10 g of -7) was mixed, the positive electrode active material LiMn
- Example 7 As an organometallic compound, 12 g of Ni (CH 3 COCHCOCH 3 ) 2 ⁇ 2H 2 O [molecular weight: 293] was used, and this was added to 16 g of ethanol to obtain a positive electrode active material LiMn 2 O 4 having an average particle diameter of 1 ⁇ m. A positive electrode plate was prepared in the same manner as in Example 1 except that the organic material was not used, and the electrode active material layer forming composition application amount shown in Table 2 was used.
- Example 8 As the metal element-containing compound, 4.0 g of Mg (NO 3 ) 2 .6H 2 O [molecular weight: 256] was added to 13 g of water and 3 g of methanol, and further starch (soluble) (Kanto Chemical).
- Example 1 (Example 1), except that 5 g of Co., Ltd.) were mixed, the positive electrode active material LiMn 2 O 4 having an average particle diameter of 1 ⁇ m was used, and the coating amount of the electrode active material layer forming composition shown in Table 2 was used.
- a positive electrode plate was produced and used as Example 8.
- Example 9 6.0 g of Cu (NO 3 ) 2 .3H 2 O [molecular weight: 241] was used as the metal element-containing compound, and this was added to 10 g of methanol and 5 g of acetone, and further cellulose acetate (manufactured by Kanto Chemical Co., Inc.).
- Example 10 As the metal-element-containing compound, Cr (NO 3) 3 ⁇ 9H 2 O [ molecular weight: 400] 5.0 g and titanium diisopropoxy bis acetylacetonate as the organometallic compound [molecular weight: 363.88] ( TC-100) made by Matsumoto Kosho Co., Ltd.
- Example 11 As an organometallic compound, 9.0 g of (CH 3 COCH: C (CH 3 ) O) 2 Co [molecular weight: 257] was used, and this was added to 15 g of methanol and 10 g of toluene, and further phenol resin (Sumitomo).
- Example 12 As a metal element-containing compound, 9.0 g of Mn (NO 3 ) 2 .6H 2 O [molecular weight: 287] was used, and this was added to 10 g of methanol and 10 g of xylene.
- Example 13 As a metal element-containing compound, 7.0 g of Zn (NO 3 ) 2 .6H 2 O [molecular weight: 298] was added to 20 g of methanol, and an epoxy resin (manufactured by DIC: EPICLON 840S) was further added.
- Example 13 A positive electrode plate as in Example 1 except that 5 g was mixed, a positive electrode active material LiMn 2 O 4 having an average particle diameter of 1 ⁇ m was used, and an electrode active material layer forming composition application amount shown in Table 2 was used. was made to be Example 13.
- Example 14 Using 6.0 g of Zr (CH 3 COCHCOCH 3 ) 4 [molecular weight: 488] as an organometallic compound, adding this to 25 g of methanol, and further adding 10 g of an epoxy resin as an organic substance, the average particle diameter
- a positive electrode plate was produced in the same manner as in Example 1 except that it was made as Example 14. Heating conditions are set in a normal temperature electric furnace (muffle furnace, Denken, P90), heated to 260 ° C.
- Example 15 10 g of polyethylene glycol was added as an organic substance, the positive electrode active material LiMn 2 O 4 having an average particle size of 0.3 ⁇ m was used, and the electrode active material layer forming composition coating amount shown in Table 2 was used. Except for this, a positive electrode plate was produced in the same manner as in Example 1, and designated as Example 15.
- Example 16 Except for adding 10 g of polyethylene glycol as an organic substance, using a positive electrode active material LiMn 2 O 4 having an average particle size of 1 ⁇ m, and applying the electrode active material layer forming composition shown in Table 2 Then, a positive electrode plate was produced in the same manner as in Example 1, and designated as Example 16.
- Example 17 Except for adding 10 g of polyethylene glycol as an organic substance, using a positive electrode active material LiMn 2 O 4 having an average particle diameter of 1 ⁇ m, and applying the electrode active material layer forming composition shown in Table 2 Then, a positive electrode plate was produced in the same manner as in Example 1, and designated as Example 17.
- Example 18 Except for adding 10 g of polyethylene glycol as an organic substance, using a positive electrode active material LiMn 2 O 4 having an average particle diameter of 1 ⁇ m, and applying the electrode active material layer forming composition shown in Table 2 A positive electrode plate was produced in the same manner as in Example 1, and designated as Example 18.
- Electrode active material layer thickness About the said Examples 2 thru
- Example 2 Composition analysis test: In the same manner as Sample 1 in Example 1, Samples 2 to 18 were prepared for Examples 2 to 18, and composition analysis was performed using the samples. The results were as follows. In Example 2, 7 atomic% Fe element, 11 atomic% Mn element, 51 atomic% O element, and 31 atomic% C element were detected. On the other hand, N element was not detected. As a result, it was confirmed that iron nitrate contained in the electrode active material layer forming coating film was thermally decomposed to produce iron oxide. In Example 3, Fe atomic element was detected at 6 atomic%, Mn element was detected at 8 atomic%, O element was detected at 55 atomic%, and C element was detected at 31 atomic%. On the other hand, N element was not detected.
- Example 4 As a result, it was confirmed that iron nitrate contained in the electrode active material layer forming coating film was thermally decomposed to produce iron oxide.
- Example 4 7 atomic% of Fe element, 8 atomic% of Mn element, 50 atomic% of O element, and 35 atomic% of C element were detected. On the other hand, N element was not detected. As a result, it was confirmed that iron nitrate contained in the electrode active material layer forming coating film was thermally decomposed to produce iron oxide.
- Example 5 4 atomic% Fe element, 8 atomic% Ti element, 11 atomic% Mn element, 51 atomic% O element, and 26 atomic% C element were detected.
- FIG. 9 is analyzed by combining FIG. 8 showing the crystallinity of M1090, which is the positive electrode active material particle described above, and FIG. 10.
- FIG. 8 shows the crystallinity of M1090, which is the positive electrode active material particle described above
- FIG. 10 shows the characteristic peak of crystalline lithium manganate.
- Example 6 15 atomic% of Mn element, 60 atomic% of O element, and 25 atomic% of C element were detected.
- the analysis result of Li was not obtained.
- the detected amount of carbon component should be further increased.
- Example 7 It was understood that the lithium acetate used as was decomposed in the heating process, thereby forming lithium oxide.
- 11 atomic% of Ni element, 15 atomic% of Mn element, 49 atomic% of O element, and 25 atomic% of C element were detected.
- the carbon component derived from acetylene black and carbon fiber was present in the electrode active material layer.
- carbon components other than the carbon component derived from acetylene black and carbon fiber existed significantly, and the carbon component derived from acetylacetonate existed in the electrode active material layer.
- Example 8 the oxide peak in the crystallinity evaluation of Example 7 was confirmed, thereby confirming that nickel oxide was present in the electrode active material layer.
- Example 8 8 atomic% of the Mg element, 17 atomic% of the Mn element, 52 atomic% of the O element, and 23 atomic% of the C element were detected.
- N element was not detected.
- magnesium nitrate contained in the electrode active material layer-forming coating film was thermally decomposed to produce magnesium oxide.
- 9 atomic% of the Cu element, 14 atomic% of the Mn element, 50 atomic% of the O element, and 27 atomic% of the C element were detected.
- N element was not detected.
- Example 10 As a result, it was confirmed that copper nitrate contained in the electrode active material layer-forming coating film was thermally decomposed to produce copper oxide.
- 5 atomic% Cr element, 6 atomic% Ti element, 9 atomic% Mn element, 47 atomic% O element, and 33 atomic% C element were detected.
- N element was not detected. From the amount of C element detected, it was confirmed that C element was significantly present in addition to acetylene black and carbon fiber-derived C element in the electrode active material layer. Further, from the crystallinity evaluation of Example 10, the peaks of chromium oxide and titanium oxide were confirmed, and it was confirmed that chromium oxide and titanium oxide were present in the electrode active material layer.
- Example 11 Co atomic element 7 atomic%, Mn element 13 atomic%, O element 45 atomic%, and C element 35 atomic% were detected. From the amount of C element detected, it was understood that carbon in cobalt acetate disappeared by heating. As a result, it was confirmed that cobalt acetate contained in the electrode active material layer-forming coating film was thermally decomposed to produce cobalt oxide.
- Example 12 Mn element was detected at 9 atomic%, Mn element was detected at 13 atomic%, O element was detected at 46 atomic%, and C element was detected at 32 atomic%. On the other hand, N element was not detected.
- Example 13 9 atomic% of Zn element, 10 atomic% of Mn element, 45 atomic% of O element, and 36 atomic% of C element were detected. On the other hand, N element was not detected. As a result, it was confirmed that zinc nitrate contained in the electrode active material layer-forming coating film was thermally decomposed to produce zinc oxide. In Example 14, 9 atomic% of Zr element, 15 atomic% of Mn element, 52 atomic% of O element and 24 atomic% of C element were detected.
- Example 15 Fe atomic element was detected at 10 atomic%, Mn element was detected at 12 atomic%, O element was detected at 48 atomic%, and C element was detected at 30 atomic%. On the other hand, N element was not detected. As a result, it was confirmed that iron nitrate contained in the electrode active material layer forming coating film was thermally decomposed to produce iron oxide.
- Example 16 6 atomic% of Fe element, 10 atomic% of Mn element, 51 atomic% of O element, and 33 atomic% of C element were detected. On the other hand, N element was not detected. As a result, it was confirmed that iron nitrate contained in the electrode active material layer forming coating film was thermally decomposed to produce iron oxide.
- Example 17 the Fe element was detected at 7 atomic%, the Mn element at 10 atomic%, the O element at 49 atomic%, and the C element at 34 atomic%. On the other hand, N element was not detected. As a result, it was confirmed that iron nitrate contained in the electrode active material layer forming coating film was thermally decomposed to produce iron oxide.
- Example 18 Fe atomic element was detected at 6 atomic%, Mn element element was detected at 12 atomic%, O element element was detected at 50 atomic%, and C element element was detected at 32 atomic%. On the other hand, N element was not detected. As a result, it was confirmed that iron nitrate contained in the electrode active material layer forming coating film was thermally decomposed to produce iron oxide.
- any electrode active material layer of Examples 2 to 18 includes a carbon component derived from acetylene black and carbon fiber, and a carbon component distinguished from these. was confirmed to exist.
- Crystallinity evaluation of Examples 2 to 18 Further, the crystallinity of the samples 2 to 18 in Examples 2 to 18 was evaluated in the same manner as the sample 1 in Example 1. As a result, it was confirmed that the metal oxides contained in the electrode active material layers exhibited crystallinity in Samples 2 to 18 as well as Sample 1 (illustration of the X-ray diffraction results was omitted).
- Example Test Cells 2 to 18 were produced in the same manner as Example Test Cell 1 in Example 1.
- the disk-shaped size in each Example is the same as that of Example 1, and the weight of the positive electrode active material contained therein is shown in Table 3 or Table 4.
- a charge / discharge test was conducted in the same manner as in Example 1 except that the test cells 2 to 18 were used and the constant current values shown in Table 3 or Table 4 were used.
- the constant current at the time of charging (discharge rate: 1C) is the same as the constant current at the time of discharging (discharge rate: 1C). Description is omitted.
- a slurry-like electrode active material layer forming composition was prepared by stirring with an Excel auto homogenizer (Nippon Seiki Seisakusho Co., Ltd.) at a rotational speed of 7000 rpm for 15 minutes so that the concentration became 55% by weight.
- the electrode active material layer forming composition was applied onto a 15 ⁇ m thick aluminum foil used as the positive electrode current collector so that the coating amount of the electrode active material layer forming composition after drying was 30 g / m 2.
- the viscosity of the electrode active material layer forming composition it was difficult to adjust the viscosity of the electrode active material layer forming composition, the fluidity was deteriorated, application as designed was not possible, and the positive electrode active material layer could not be formed. Therefore, a positive electrode plate for a nonaqueous electrolyte secondary battery could not be produced.
- Example 2 A positive electrode plate for a non-aqueous electrolyte secondary battery was produced in the same manner as in Example 1 except that the metal element-containing compound was not used. And the process which pulls out the disk of a predetermined shape was performed like Example 1, but at this time, an electrode active material layer peels off and the electrode on the disk which can be used for a tripolar coin cell is produced. I could't. That is, the film forming property of the electrode active material layer in the positive electrode plate for a non-aqueous electrolyte secondary battery was poor.
- Comparative Example 3 A slurry-like electrode active material layer forming composition was prepared in the same manner as in Comparative Example 1 except that the positive electrode active material LiMn 2 O 4 having an average particle size of 10 ⁇ m was used. Then, the electrode active material layer forming composition was applied onto a 15 ⁇ m thick aluminum foil used as a positive electrode current collector so that the coating amount of the electrode active material layer forming composition after drying was 30 g / m 2. Then, it was dried in an air atmosphere at 120 ° C. for 20 minutes using an oven to form an electrode active material layer for the positive electrode on the current collector surface.
- Comparative Example 3 For Comparative Example 3, a tripolar coin cell was assembled following Example 1, and a charge / discharge test was performed in the same manner as in Example 1 except for the constant current value, and the discharge capacity and the discharge rate were measured. Table 4 shows the constant current values of Comparative Example 3 and the measurement results.
- Comparative Example 4 An electrode active material layer forming composition was prepared in the same manner as in Comparative Example 1 except that the positive electrode active material LiMn 2 O 4 having an average particle diameter of 1 ⁇ m was used, and the dried electrode on the same aluminum foil as in Comparative Example 1 Application was performed so that the coating amount of the active material layer forming composition was 30 g / m 2 , but the viscosity of the electrode active material layer forming composition was difficult to adjust, the fluidity was poor, and the coating as designed was possible. In other words, the positive electrode active material layer could not be formed. Therefore, a positive electrode plate for a nonaqueous electrolyte secondary battery could not be produced.
- Titanium diisopropoxybis (acetylacetonate), which is a metal element-containing compound (manufactured by Matsumoto Kosho Co., Ltd., TC-100) is used as a binder material generating material in a solution obtained by dissolving 1 g of polyethylene oxide, which is an organic substance, in 9 g of methanol. ) 5.0 g was mixed with to obtain a raw material solution that produced a metal oxide that did not exhibit lithium ion insertion / release reaction.
- a copper plate having a thickness of 10 ⁇ m is prepared as a current collector, and the electrode active material layer prepared as described above is formed on one side of the current collector in an amount such that the weight of the electrode active material layer finally obtained is 15 g / m 2.
- the material layer forming composition was applied with an applicator to form a coating film for forming an electrode active material layer.
- the current collector with the electrode active material layer-forming coating film formed on the surface was heated in an inert gas atmosphere (nitrogen 99.99%) electric furnace (high temperature atmosphere box furnace, manufactured by Koyo Thermo Systems Co., Ltd., KB8610N -VP), heated to 700 ° C.
- a negative electrode plate for a non-aqueous electrolyte secondary battery according to the above-described embodiment in which an appropriate electrode active material layer was laminated as a material layer was obtained.
- the negative electrode plate was allowed to stand until it reached room temperature, then opened to the atmosphere, taken out, and cut into a predetermined size (a disk having a diameter of 15 mm).
- Example 20 A negative electrode plate was produced in the same manner as in Example 19 except that the electrode active material layer forming composition application amount shown in Table 6 was changed, and this was designated as Example 20.
- Example 21 A negative electrode plate was produced in the same manner as in Example 19 except that the particle diameter of the negative electrode active material particles to be used was changed to 10 ⁇ m.
- Example 22 A negative electrode body was produced in the same manner as in Example 19 except that the particle diameter of the negative electrode active material particles used was changed to 1 ⁇ m and the electrode active material layer forming composition application amount shown in Table 6 was changed. This was designated as Example 22.
- Electrode active material layer thickness for Examples 19-22 For Examples 19 to 22, the thickness of the electrode active material layer was measured in the same manner as in Example 1, and the average value was calculated. The results are shown in Table 6.
- Example 19 Composition analysis tests for Examples 19-22: Similarly to Sample 1 in Example 1, Samples 19 to 22 were prepared for Example 19 to Example 22, and composition analysis was performed using the samples. The results were as follows. In Example 19, the Ti element was detected at 13 atomic%, the C element was detected at 62 atomic%, and the O element was detected at 25 atomic%. In Example 20, 15 atomic percent of Ti element, 50 atomic percent of C element, and 35 atomic percent of O element were detected. In Example 21, 12 atomic percent of Ti element, 55 atomic percent of C element, and 33 atomic percent of O element were detected. In Example 22, the Ti element was detected at 14 atomic%, the C element was detected at 53 atomic%, and the O element was detected at 33 atomic%.
- Crystallinity evaluation for Examples 19-22 Further, the crystallinity of the samples 19 to 22 in Examples 19 to 22 was evaluated in the same manner as the sample 1 in Example 1. As a result, it was confirmed that the metal oxide (titanium oxide) contained in the electrode active material layer was crystalline in Samples 19 to 22 as well as Sample 1 (the X-ray diffraction results are shown in the figure). (Omitted).
- Cyclic voltammetry test for Examples 19-22 In order to confirm beforehand whether the metal oxide (namely, titanium oxide) which comprises the electrode active material layer in Example 19 thru
- the raw material solution for producing the metal oxide was applied to an aluminum substrate with a Miyabar No. 4 and heated under the same heating conditions as those for electrode preparation.
- the CV test was conducted in the same manner as described above. As a result, no peak (electrochemical reaction) was confirmed in the cyclic voltammogram showing any cycle result from the first to third cycles. From this, it was confirmed that the titanium oxide as the binding material of Examples 19 to 22 did not show lithium ion insertion / release reaction.
- the CV test was performed using VMP3 manufactured by Bio Logic.
- Example 19-22 Charge / Discharge Test for Examples 19-22: First, similarly to the charge / discharge test in Example 1, a non-aqueous electrolyte was prepared, and instead of using Example 1 as a positive electrode plate, Examples 19 to 22 were used as working electrodes as negative electrode plates. And the Example test cell 19 thru
- the test cell 19 was charged at a constant current (707 ⁇ A) under a 25 ° C. environment until the voltage reached 0.03 V. After the voltage reached 0.03 V, the voltage was 0.03 V.
- the current discharge rate: 1C was reduced until it became 5% or less so as not to fall below, and the battery was charged at a constant voltage, fully charged, and then suspended for 10 minutes.
- the “1C” means a current value (current value reaching the discharge end voltage) at which constant current discharge is performed using the tripolar coin cell and discharge is completed in one hour.
- the constant current was set such that a theoretical discharge amount of 372 mAhr / g of graphite as an active material was discharged in one hour at the working electrode as the example test cell 19. (Discharge test) Thereafter, the fully charged example test cell 19 was subjected to a constant current (707 ⁇ A) (discharge) in an environment of 25 ° C. until the voltage was changed from 0.03 V (full charge voltage) to 2.0 V (discharge end voltage).
- Example 7 (Calculation of discharge capacity maintenance rate (%)) In the same manner as in Example 1, the discharge capacity retention ratio (%) was obtained for Examples 19 to 22. The results are shown in Table 7. Moreover, in the column of “output performance evaluation” in Table 7, the discharge rate characteristics of the electrodes were evaluated as follows. Discharge capacity maintenance rate at discharge rate 50C 80% or more and 100% or less Discharge capacity maintenance rate at a discharge rate of 50C 50% or more and less than 80% Discharge capacity maintenance rate at discharge rate 50C Less than 50% ...
- Example 19 the carbon component distinguished from the electrically conductive material in an electrode active material layer was confirmed as follows by the STEM method mentioned above. First, Example 19 was cut substantially perpendicularly to the current collector surface, and the cross section in the thickness direction of the electrode active material layer was observed by the STEM method to observe the colored region of the carbon component by carbon element mapping. It was confirmed that particles having a diameter of about 15 nm were dispersed. The colored region showing such a carbon component is remarkably smaller than the colored region showing the known conductive material particles, whereby a carbon component that is distinguished from the conductive material is present in the electrode active material layer of Example 19. It was confirmed.
- Cycle characteristics evaluation test for Examples 19 to 22 Using each of Examples 19 to 22, following the above charge test and discharge test, a constant current charge / discharge test at a constant current (7 mA) (discharge rate: 10 C) was performed, and the cycle characteristic evaluation test was repeated 100 times. did. The maintenance rate of the 100th discharge capacity with respect to the first discharge capacity was taken as the 100 cycle capacity maintenance rate. Further, as a cycle characteristic evaluation, the cycle capacity maintenance rate was evaluated as follows. Table 7 shows the results of 100 cycle capacity retention rate and cycle characteristic evaluation. 100 cycle capacity maintenance rate 60% or more 100 cycle capacity maintenance rate less than 60%
- Processing characteristics evaluation (bending evaluation) for Examples 19 to 22 The processing characteristics of Example 19 were evaluated by a cylindrical mandrel method bending test based on JIS K 5600-5-1. Example 19 was sandwiched between test plates in such a direction that the electrode active material layer surface was bent outward, both ends of the test plate were fixed, and bent uniformly at an angle of 180 degrees at a uniform speed over 2 seconds, and the cylindrical mandrel method After performing the bending test, the electrode active material layer surface of Example 1 taken out from the test plate was visually observed and evaluated as follows. In addition, this processing characteristic evaluation was implemented using the mandrel method bending test machine (made by model number REF802 SEPRO). In addition, the processing characteristics of Examples 20 to 22 were evaluated in the same manner as in Example 19.
- Coatability evaluation for Examples 19 to 22 With respect to the applicability of the electrode active material layer forming composition to the current collector, the coating film surface formed on the current collector was visually observed after the application of the negative electrode plate, The evaluation was as follows. The results are shown in Table 6. The surface of the coating was uniform ... Some unevenness was confirmed on a part of the coating surface. A streak or uneven coating was found on the coating surface ... Clear streaks or coating unevenness that cannot be used as a negative electrode plate was confirmed on the coating surface.
- the electrode active material layer forming composition was applied onto a 10 ⁇ m thick copper foil used as the negative electrode current collector so that the coating amount of the electrode active material layer forming composition after drying was 65 g / m 2. This was dried in an air atmosphere at 70 ° C. using an oven to form an electrode active material layer for the negative electrode plate on the current collector. Furthermore, after pressing using a roll press machine so that the thickness of the formed electrode active material layer is about 85 ⁇ m, it is cut into a predetermined size (a disk with a diameter of 15 mm) and 300 ° C. at 70 ° C. A negative electrode plate was produced by vacuum-drying for 5 minutes to obtain Comparative Example 5.
- Examples 1 to 18 and Comparative Examples 1 to 4 of the positive electrode plate described above had a discharge capacity maintenance rate of about 100 when the discharge rate was 1C. However, when the discharge rate was increased, all of the examples maintained a high discharge capacity, whereas Comparative Example 3 showed a significant decrease in the discharge capacity maintenance rate.
- Comparative Example 2 was formed in the same manner as in Example 1 except that no metal oxide was formed in the electrode active material layer, but the film forming property was poor and the electrode active material layer was peeled off. could not be formed. From this, it was confirmed that the metal oxide in the electrode active material layer surely acts as a binder.
- Comparative Example 1 and Comparative Example 4 used a conventional resin binder, but the particle diameter of the positive electrode active material particles used was as small as 5 ⁇ m or less, making it difficult to adjust the viscosity of the electrode active material layer forming composition. As a result, a positive electrode plate could not be produced.
- the particle diameter of the positive electrode active material particles to be used is 5 ⁇ m or less, the positive electrode plate can be formed satisfactorily and the discharge capacity of the obtained positive electrode plate can be maintained. The result was very high.
- Examples 19 to 22 and Comparative Examples 5 to 9 of the negative electrode plate described above showed that Examples 19 to 22 are very excellent in output / input characteristics and cycle characteristics.
- Examples 19 to 22 although the binder material was changed from a resin material to a metal oxide, it was confirmed that the film quality and processing characteristics were excellent as usual.
- the coating suitability the Examples showed excellent properties regardless of the particle size of the active material used, whereas Comparative Example 5 using a negative electrode active material having a particle size of 12 ⁇ m and In No. 6, moderate coating suitability was shown, but when the particle diameter of the active material used was 10 ⁇ m or less, it was shown that the coating suitability was not good.
- the negative electrode plates according to Examples 19 to 22 exhibit very excellent output / input characteristics and cycle characteristics as compared with Comparative Examples 5 to 9, the electrode plates described in the above-described embodiments are used.
- the negative electrode plate is used for a non-aqueous electrolyte secondary battery, it is understood that the input / output characteristics and cycle characteristics of the battery are desirably improved.
- the electrode plate for a non-aqueous electrolyte secondary battery according to the above-described embodiment exhibits a very high discharge capacity retention rate in both the positive electrode plate and the negative electrode plate, and thus has a very high discharge rate characteristic. It was confirmed that it was equipped. As a result, it was inferred that the charge rate characteristics were also high. That is, from the charge / discharge test, it was confirmed that the electrode plate according to the above-described embodiment has excellent input / output characteristics. Further, the processing characteristics and the cycle characteristics evaluation were evaluated using the example of the negative electrode plate as an evaluation of the electrode plate according to the above-described embodiment.
- the excellent properties shown in the evaluation results are not limited to the negative electrode plate made of the electrode plate described in the above embodiment, but the positive electrode plate made of the electrode plate described in the above embodiment. The same properties are also shown in. Therefore, in the non-aqueous electrolyte secondary battery, the electrode plate described in the above-described embodiment is used as a positive electrode plate and / or a negative electrode plate, so that the excellent non-aqueous battery exhibiting superior discharge rate characteristics than the conventional ones. An electrolyte secondary battery can be provided.
- the electrode plate provided with the electrode active material layer of the conventional thickness can also be manufactured, or the electrode provided with the electrode active material layer of very thin thickness It has been shown that boards can be produced. Furthermore, as described above, it was shown that the electrode plate obtained by the manufacturing method described in the above-described embodiment exhibits very desirable discharge rate characteristics.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Secondary Cells (AREA)
- Battery Electrode And Active Subsutance (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201080017448.4A CN102414876B (zh) | 2009-04-24 | 2010-04-19 | 非水电解液二次电池用电极板、非水电解液二次电池用电极板的制备方法及非水电解液二次电池 |
| US13/265,220 US8394537B2 (en) | 2009-04-24 | 2010-04-19 | Electrode plate for non-aqueous electrolyte secondary battery, method for producing the same, and non-aqueous electrolyte secondary battery |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009107181 | 2009-04-24 | ||
| JP2009-107181 | 2009-04-24 | ||
| JP2010024838A JP5136804B2 (ja) | 2009-04-24 | 2010-02-05 | 非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、および非水電解液二次電池 |
| JP2010-024838 | 2010-02-05 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010122984A1 true WO2010122984A1 (ja) | 2010-10-28 |
Family
ID=43011101
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2010/056940 Ceased WO2010122984A1 (ja) | 2009-04-24 | 2010-04-19 | 非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、および非水電解液二次電池 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US8394537B2 (enExample) |
| JP (1) | JP5136804B2 (enExample) |
| CN (1) | CN102414876B (enExample) |
| WO (1) | WO2010122984A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017099137A1 (ja) * | 2015-12-07 | 2017-06-15 | 国立研究開発法人産業技術総合研究所 | カリウムイオン二次電池用正極活物質 |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103531803A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种新型锂电池改性磷酸铁锂正极材料及其制备方法 |
| CN103531783A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种锰酸锂正极材料及其制备方法 |
| CN103531797A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种锂电池用导电改性磷酸铁锂正极材料及其制备方法 |
| CN103531780A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种锂离子电池用正极锰酸锂材料及其制备方法 |
| CN103545484A (zh) * | 2013-10-30 | 2014-01-29 | 合肥恒能新能源科技有限公司 | 一种偶联剂改性的磷酸铁锂复合正极材料及其制备方法 |
| CN103531795A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种锂电池用掺杂改性磷酸铁锂正极材料及其制备方法 |
| CN103531788A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种锂电池用改性锰酸锂正极材料及其制备方法 |
| CN103531798A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种导电改性磷酸铁锂正极材料及其制备方法 |
| CN103531755A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种锂电池用掺杂磷酸铁锂复合材料及其制备方法 |
| CN103545513A (zh) * | 2013-10-30 | 2014-01-29 | 合肥恒能新能源科技有限公司 | 一种改性磷酸铁锂正极材料及其制备方法 |
| CN103545481A (zh) * | 2013-10-30 | 2014-01-29 | 合肥恒能新能源科技有限公司 | 一种石墨烯改性锰酸锂正极材料及其制备方法 |
| CN103531799A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种锂电池用导电性改进磷酸铁锂正极材料及其制备方法 |
| CN103531781A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种锂电池用复合改性锰酸锂材料及其制备方法 |
| CN103531807A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种掺杂改性的磷酸铁锂正极材料及其制备方法 |
| CN103545514A (zh) * | 2013-10-30 | 2014-01-29 | 合肥恒能新能源科技有限公司 | 一种石墨烯改性的磷酸铁锂材料及其制备方法 |
| CN103545511A (zh) * | 2013-10-30 | 2014-01-29 | 合肥恒能新能源科技有限公司 | 一种磷酸铁锂电池正极材料及其制备方法 |
| CN103545506A (zh) * | 2013-10-30 | 2014-01-29 | 合肥恒能新能源科技有限公司 | 一种表面处理的复合锰酸锂材料及其制备方法 |
| CN103545512A (zh) * | 2013-10-30 | 2014-01-29 | 合肥恒能新能源科技有限公司 | 一种大功率锂电池用磷酸铁锂正极材料及其制备方法 |
| CN103531790A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种磷酸铁锂钴酸锂复合材料及其制备方法 |
| CN103531806A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种改变导电性的磷酸铁锂材料及其制备方法 |
| CN103531785A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种添加改性银粉的锰酸锂正极材料及其制备方法 |
| CN103531787A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种铝尖晶石改进锰酸锂正极材料及其制备方法 |
| CN103531782A (zh) * | 2013-10-30 | 2014-01-22 | 合肥恒能新能源科技有限公司 | 一种表面包覆改进锰酸锂正极材料及其制备方法 |
| CN103915601B (zh) * | 2014-04-18 | 2016-02-24 | 北京理工大学 | 一种电极涂覆的方法 |
| EP4024502A4 (en) * | 2019-08-27 | 2022-10-05 | Panasonic Intellectual Property Management Co., Ltd. | SECONDARY BATTERY |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11144736A (ja) * | 1997-11-12 | 1999-05-28 | Mitsubishi Chemical Corp | リチウムイオン電解質電池および該電池の製造方法 |
| JP2001508916A (ja) * | 1996-11-13 | 2001-07-03 | 三菱化学株式会社 | リチウムイオン電池およびその製造方法 |
| JP2005078985A (ja) * | 2003-09-02 | 2005-03-24 | Toshiba Battery Co Ltd | 非水系二次電池用電極及びこれを用いたリチウム二次電池。 |
| JP2008517435A (ja) * | 2004-10-21 | 2008-05-22 | エボニック デグサ ゲーエムベーハー | リチウムイオンバッテリー用の無機セパレータ電極ユニット、その製造方法及びリチウムバッテリーにおけるその使用 |
Family Cites Families (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4902589A (en) | 1988-06-08 | 1990-02-20 | Moli Energy Limited | Electrochemical cells, electrodes and methods of manufacture |
| JP2960834B2 (ja) | 1993-06-07 | 1999-10-12 | シャープ株式会社 | リチウム二次電池 |
| JPH09503092A (ja) | 1993-09-27 | 1997-03-25 | アーサー・ディー・リトル・インコーポレイテッド | エーロゾル法による粉体電極 |
| JPH10208747A (ja) | 1997-01-29 | 1998-08-07 | Hitachi Ltd | 二次電池および二次電池を利用した組電池と機器システム |
| JP2000277119A (ja) | 1999-03-26 | 2000-10-06 | Kyocera Corp | リチウム電池 |
| EP1142834A4 (en) | 1999-11-15 | 2009-06-17 | Mitsubishi Chem Corp | LITHIUM MANGANESE COMPOSITE OXIDE, POSITIVE ELECTRODE MATERIAL FOR LITHIUM SECONDARY ACCUMULATOR, POSITIVE ELECTRODE AND LITHIUM SECONDARY ACCUMULATOR, AND PROCESS FOR THE PREPARATION OF LITHIUM MANGANESE COMPOSITE OXIDE |
| JP2001155739A (ja) | 1999-11-24 | 2001-06-08 | Nissha Printing Co Ltd | 二次電池用正極および二次電池 |
| KR100378014B1 (ko) | 2000-08-21 | 2003-03-29 | 삼성에스디아이 주식회사 | 리튬 이차 전지용 전극 및 리튬 이차 전지 |
| JP4160271B2 (ja) | 2000-08-21 | 2008-10-01 | 三星エスディアイ株式会社 | リチウム二次電池用の電極及びリチウム二次電池 |
| JP4799776B2 (ja) | 2000-08-22 | 2011-10-26 | 富士フイルム株式会社 | 電解質組成物及びそれを用いた電気化学電池 |
| JP3578272B2 (ja) * | 2001-02-22 | 2004-10-20 | 日立マクセル株式会社 | 電気化学素子用電極および電気化学素子 |
| JP3809770B2 (ja) | 2001-02-28 | 2006-08-16 | 財団法人電力中央研究所 | 電池材料の製造方法 |
| EP1403944A4 (en) | 2001-05-15 | 2008-08-13 | Fdk Corp | WATER-FREE ELECTROLYTIC SECONDARY BATTERY AND METHOD FOR PRODUCING AN ANODE MATERIAL THEREFOR |
| JP2003142101A (ja) | 2001-10-31 | 2003-05-16 | Nec Corp | 二次電池用正極およびそれを用いた二次電池 |
| JP2003317707A (ja) | 2002-04-26 | 2003-11-07 | Matsushita Electric Ind Co Ltd | 非水電解質二次電池用負極とその製造方法 |
| JP4767484B2 (ja) | 2002-08-08 | 2011-09-07 | パナソニック株式会社 | 非水電解質二次電池用正極活物質の製造法および正極活物質 |
| EP1547973A4 (en) | 2002-09-05 | 2008-07-30 | Nat Inst Of Advanced Ind Scien | FINE CARBON POWDER WITH METAL OXIDE, METAL-NITRIDE OR METAL CARBIDE COATING, MANUFACTURING METHOD DAF R AND SUPERCONDENSATOR AND SECONDARY BATTERY USING THE FINE CARBON POWDER |
| JP2004103304A (ja) | 2002-09-05 | 2004-04-02 | National Institute Of Advanced Industrial & Technology | 高性能二次電池 |
| JP4610213B2 (ja) | 2003-06-19 | 2011-01-12 | 三洋電機株式会社 | リチウム二次電池及びその製造方法 |
| JP4407211B2 (ja) | 2003-09-02 | 2010-02-03 | 日産自動車株式会社 | 非水電解質二次電池 |
| US7687102B2 (en) | 2003-10-23 | 2010-03-30 | Medtronic, Inc. | Methods and apparatus for producing carbon cathodes |
| US20050130042A1 (en) | 2003-12-11 | 2005-06-16 | Byd America Corporation | Materials for positive electrodes of lithium ion batteries and their methods of fabrication |
| CN1627550A (zh) * | 2003-12-11 | 2005-06-15 | 比亚迪股份有限公司 | 锂离子电池正极材料及其制备方法 |
| WO2006019245A1 (en) | 2004-08-17 | 2006-02-23 | Lg Chem, Ltd. | Lithium secondary batteries with enhanced safety and performance |
| JP4834975B2 (ja) | 2004-09-30 | 2011-12-14 | 大日本印刷株式会社 | 活物質層用塗工組成物、非水電解液二次電池用電極板、及び非水電解液二次電池 |
| JP4193141B2 (ja) | 2005-03-25 | 2008-12-10 | ソニー株式会社 | リチウム二次電池用負極およびリチウム二次電池、並びにそれらの製造方法 |
| JP2006310010A (ja) | 2005-04-27 | 2006-11-09 | Matsushita Electric Ind Co Ltd | リチウムイオン二次電池 |
| FR2890785A1 (fr) | 2005-09-15 | 2007-03-16 | Batscap Sa | Dispositif de stockage d'energie electrique comprenant une couche barriere de protection du collecteur |
| US20070154807A1 (en) | 2005-12-30 | 2007-07-05 | Yevgen Kalynushkin | Nanostructural Electrode and Method of Forming the Same |
| JP5194709B2 (ja) | 2007-10-19 | 2013-05-08 | 住友電気工業株式会社 | 全固体電池およびその製造方法 |
| JP5262143B2 (ja) | 2008-01-31 | 2013-08-14 | トヨタ自動車株式会社 | 正極体およびその製造方法 |
| JP5293020B2 (ja) | 2008-09-10 | 2013-09-18 | 住友化学株式会社 | 非水電解質二次電池用電極合剤、電極および非水電解質二次電池 |
| JP2010129418A (ja) | 2008-11-28 | 2010-06-10 | Sumitomo Chemical Co Ltd | 無機粒子バインダを用いた電極、及びその製造方法 |
-
2010
- 2010-02-05 JP JP2010024838A patent/JP5136804B2/ja not_active Expired - Fee Related
- 2010-04-19 WO PCT/JP2010/056940 patent/WO2010122984A1/ja not_active Ceased
- 2010-04-19 CN CN201080017448.4A patent/CN102414876B/zh not_active Expired - Fee Related
- 2010-04-19 US US13/265,220 patent/US8394537B2/en not_active Expired - Fee Related
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001508916A (ja) * | 1996-11-13 | 2001-07-03 | 三菱化学株式会社 | リチウムイオン電池およびその製造方法 |
| JPH11144736A (ja) * | 1997-11-12 | 1999-05-28 | Mitsubishi Chemical Corp | リチウムイオン電解質電池および該電池の製造方法 |
| JP2005078985A (ja) * | 2003-09-02 | 2005-03-24 | Toshiba Battery Co Ltd | 非水系二次電池用電極及びこれを用いたリチウム二次電池。 |
| JP2008517435A (ja) * | 2004-10-21 | 2008-05-22 | エボニック デグサ ゲーエムベーハー | リチウムイオンバッテリー用の無機セパレータ電極ユニット、その製造方法及びリチウムバッテリーにおけるその使用 |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017099137A1 (ja) * | 2015-12-07 | 2017-06-15 | 国立研究開発法人産業技術総合研究所 | カリウムイオン二次電池用正極活物質 |
| JPWO2017099137A1 (ja) * | 2015-12-07 | 2018-09-27 | 国立研究開発法人産業技術総合研究所 | カリウムイオン二次電池用正極活物質 |
| US10862120B2 (en) | 2015-12-07 | 2020-12-08 | National Institute Of Advanced Industrial Science And Technology | Positive electrode active material for potassium ion secondary cell |
Also Published As
| Publication number | Publication date |
|---|---|
| US8394537B2 (en) | 2013-03-12 |
| JP2010272510A (ja) | 2010-12-02 |
| CN102414876B (zh) | 2014-06-18 |
| JP5136804B2 (ja) | 2013-02-06 |
| CN102414876A (zh) | 2012-04-11 |
| US20120088152A1 (en) | 2012-04-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5136804B2 (ja) | 非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、および非水電解液二次電池 | |
| JP5196197B2 (ja) | 非水電解液二次電池用電極板の製造方法 | |
| JP5212394B2 (ja) | 非水電解液二次電池用電極板の製造方法 | |
| JP4924852B2 (ja) | 非水電解液二次電池用電極板の製造方法 | |
| JP2012079651A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池、および電池パック | |
| JP2011100747A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、および非水電解液二次電池 | |
| JP2012094361A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池および電池パック | |
| JP2011100748A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、および非水電解液二次電池 | |
| JP2012094338A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池、及び電池パック | |
| JP2012084423A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池、および電池パック | |
| JP2011204488A (ja) | 非水電解液二次電池用正極板、非水電解液二次電池用正極板の製造方法、および非水電解液二次電池 | |
| WO2011030846A1 (ja) | 非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、非水電解液二次電池、および電池パック | |
| JP2011204490A (ja) | 非水電解液二次電池用正極板、非水電解液二次電池用正極板の製造方法、および非水電解液二次電池 | |
| JP2012089274A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、非水電解液二次電池、および電池パック | |
| JP2011204489A (ja) | 非水電解液二次電池用正極板、非水電解液二次電池用正極板の製造方法、および非水電解液二次電池 | |
| JP2012089275A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、非水電解液二次電池、および電池パック | |
| JP2011204459A (ja) | 非水電解液二次電池用正極板、非水電解液二次電池用正極板の製造方法、および非水電解液二次電池 | |
| JP2012094334A (ja) | 非水電解液二次電池用正極板、非水電解液二次電池、電池パック、非水電解液二次電池用正極板の製造方法、および正極用電極活物質層の形成方法 | |
| JP2011204491A (ja) | 非水電解液二次電池用正極板、非水電解液二次電池用正極板の製造方法、および非水電解液二次電池 | |
| JP2012074227A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池および電池パック | |
| JP2012190728A (ja) | 非水電解液二次電池用正極板、非水電解液二次電池、および電池パック | |
| JP2012089272A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、非水電解液二次電池、および電池パック | |
| JP2011204470A (ja) | 非水電解液二次電池用正極板、非水電解液二次電池用正極板の製造方法、および非水電解液二次電池 | |
| JP2012089273A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池用電極板の製造方法、非水電解液二次電池、および電池パック | |
| JP2012079447A (ja) | 非水電解液二次電池用電極板、非水電解液二次電池および電池パック |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201080017448.4 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10767041 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13265220 Country of ref document: US |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 10767041 Country of ref document: EP Kind code of ref document: A1 |