WO2010095685A1 - 骨細胞への分化誘導培地及び方法 - Google Patents
骨細胞への分化誘導培地及び方法 Download PDFInfo
- Publication number
- WO2010095685A1 WO2010095685A1 PCT/JP2010/052455 JP2010052455W WO2010095685A1 WO 2010095685 A1 WO2010095685 A1 WO 2010095685A1 JP 2010052455 W JP2010052455 W JP 2010052455W WO 2010095685 A1 WO2010095685 A1 WO 2010095685A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- medium
- differentiation
- cells
- serum
- stem cells
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0654—Osteocytes, Osteoblasts, Odontocytes; Bones, Teeth
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/05—Inorganic components
- C12N2500/10—Metals; Metal chelators
- C12N2500/20—Transition metals
- C12N2500/24—Iron; Fe chelators; Transferrin
- C12N2500/25—Insulin-transferrin; Insulin-transferrin-selenium
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/30—Organic components
- C12N2500/36—Lipids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/30—Organic components
- C12N2500/38—Vitamins
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/30—Organic components
- C12N2500/42—Organic phosphate, e.g. beta glycerophosphate
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/30—Hormones
- C12N2501/38—Hormones with nuclear receptors
- C12N2501/39—Steroid hormones
Definitions
- the present invention relates to a medium composition and method for inducing differentiation of mammalian somatic stem cells or osteoblasts into bone cells under serum-free or low-serum medium conditions.
- Animal serum eg, fetal bovine serum, human serum, etc.
- Human somatic stem cells used for treatment and induction into differentiated cells (eg, bone cells, adipocytes, cardiomyocytes, etc.). It has been.
- animal sera are known to have a risk of infection with unknown viruses and prion diseases in addition to the fact that the component composition is not completely known.
- the patient's own serum that requires cell transplantation is used instead of animal serum, somatic stem cell proliferation culture and target tissue Differentiation into cells has been performed.
- somatic stem cell proliferation culture and target tissue Differentiation into cells has been performed.
- a relatively large amount of blood must be collected from the patient.
- Patent Document 1 is an invention in which human bone marrow-derived mesenchymal stem cells are cultured in a medium containing human serum, and the cultured human bone marrow-derived mesenchymal stem cells are boned in a differentiation-inducing medium containing human serum. An example of guiding to an organization is given.
- Non-patent Document 2 Conventional methods for inducing differentiation from mesenchymal stem cells to bone cells include ⁇ -glycerophosphate, dexamethasone, vitamin C and 10% animal serum (Non-patent Document 2).
- Patent Document 2 discloses a human embryonic stem (ES) containing a lysophospholipid receptor, that is, a ligand of the endothelial cell differentiation gene (Edg) family, such as lysophosphatidic acid (LPA), sphingosine monophosphate (S1P), and the like.
- a serum-free medium for culturing cells is disclosed.
- An object of the present invention is to provide a differentiation-inducing medium, an additive and a method for inducing differentiation from mammalian somatic stem cells to cells having the characteristics of bone cells under serum-free or low-serum medium conditions.
- lysophosphatidine which is a ligand for endothelial cell differentiation gene (Edg) family receptors, in addition to an inducer that has been added to induce differentiation of mammalian somatic stem cells into bone cells. It has been found that a serum-free medium supplemented with acid and selenium, a trace element, can effectively differentiate mammalian somatic stem cells into bone cells. Furthermore, the present inventors have found that it is possible to add melatonin, vitamin D, vitamin A, vitamin K and zinc involved in differentiation from somatic stem cells to bone cells to this serum-free medium.
- the present invention comprises a basic medium for mammalian cell culture, an agent for inducing differentiation of mammalian somatic stem cells into bone cells, a ligand for an endothelial cell differentiation gene (Edg) family receptor, and selenium.
- a medium for inducing differentiation of mammalian somatic stem cells into bone cells which is serum or low serum, is provided.
- the present invention also provides a medium for inducing differentiation of mammalian somatic stem cells into bone cells, comprising a ligand for an endothelial cell differentiation gene (Edg) family receptor and selenium.
- the present invention provides a method for inducing differentiation from somatic stem cells to bone cells, comprising culturing somatic stem cells that can differentiate into bone cells in the medium of the present invention.
- the medium and the medium additive of the present invention can be used under conditions that do not use animal serum that has been added to a conventional differentiation-inducing medium when inducing differentiation from mammalian somatic stem cells to bone cells, that is, under serum-free conditions. It is possible to efficiently induce differentiation from mammalian somatic stem cells into bone cells. Furthermore, problems (eg, the origin of serum, influence on differentiation induction by lots, contamination of unknown / known infectious agents) can be solved by using serum. As a result, stable quality bone cells can be provided.
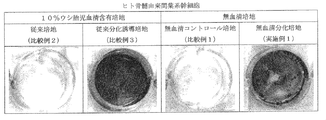
- FIG. 3 is a view of cells that were induced to differentiate from human bone marrow-derived mesenchymal stem cells into bone cells for 21 days in each medium obtained in Example 1 and Comparative Examples 1 to 3, and stained with alizarin red S.
- 6 is a graph showing changes in ALP activity of cells induced to differentiate from human bone marrow-derived mesenchymal stem cells to bone cells in each medium obtained in Example 1 and Comparative Examples 1 to 3 for 7 to 21 days.
- 6 is a graph showing changes in ALP mRNA expression in cells induced to differentiate from human bone marrow-derived mesenchymal stem cells to bone cells in each medium obtained in Example 1 and Comparative Examples 1 to 3 for 7 to 21 days.
- FIG. 1 is a view of cells that were induced to differentiate from human bone marrow-derived mesenchymal stem cells into bone cells for 21 days in each medium obtained in Example 1 and Comparative Examples 1 to 3, and stained with alizarin red S.
- 6 is a graph showing changes in ALP activity of cells induced
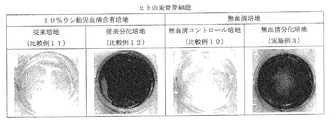
- FIG. 3 is a view of cells that were induced to differentiate from human bone marrow-derived mesenchymal stem cells into bone cells for 14 days in each medium obtained in Example 2 and Comparative Examples 4 to 6, and stained with alizarin red S.
- FIG. 4 is a view of cells that have been induced to differentiate from human osteoblasts into bone cells for 14 days in each medium obtained in Example 3 and Comparative Examples 7 to 9, stained with alizarin red S.
- FIG. 3 is a view of cells that were induced to differentiate from human bone marrow-derived mesenchymal stem cells into bone cells for 14 days in each medium obtained in Example 2 and Comparative Examples 4 to 6, and stained with alizarin red S.
- FIG. 4 is a view of cells that have been induced to differentiate from human osteoblasts into bone cells for 14 days in each medium obtained in Example 3 and Comparative Examples 7 to 9, stained with alizarin red S.
- “somatic stem cells” form each organ in vivo such as osteoblasts, adipocytes, chondrocytes, skin cells, nerve cells, muscle cells, blood cells, fibroblasts, liver cells. It is a cell that can be transdifferentiated into one or more types of tissue cells.
- “Somatic stem cells” are cells excluding embryonic stem cells among stem cells and progenitor cells that have the ability to differentiate into cells having several different functions, and are induced multifunctional stem cells, hematopoietic stem cells, It includes leaf stem cells, neural stem cells, skin stem cells, liver stem cells, pancreatic stem cells and the like.
- the cells that can be induced to differentiate in the medium of the present invention are not limited as long as they are somatic stem cells that can differentiate into mammalian bone cells.
- Preferred examples include bone marrow-derived mesenchymal stem cells, adipose tissue-derived stem cells, and the like. However, it is not limited to these somatic stem cells.
- the medium of the present invention contains a ligand for the endothelial cell differentiation gene (Edg) family receptor as an essential component.
- the Edg family receptors are a group of G protein-coupled receptors with high homology in their gene sequences.
- Edg-1 to Edg-8 have been identified in mammals such as humans, mice, and sheep. ing.
- Edg-2, Edg-4 and Edg-7 function as LPA receptors
- Edg-1, Edg-3, Edg-5, Edg-6 and Edg-8 are known to function as S1P receptors. It has been.
- a “ligand for a receptor” is a substance that specifically binds to the receptor, and includes not only natural ligands existing in the body, but also other natural or synthesized compounds known as agonists and antagonists. Include.
- the ligand for the Edg family receptor includes one or more compounds selected from the group consisting of agonists such as lysophosphatidic acid (LPA) and salts thereof. preferable.
- An agonist for an Edg family receptor is a substance that binds to the Edg family and acts like LPA.
- LPA sphingosine monophosphate
- S1P sphingosine monophosphate
- PAF platelet activating factor
- Sphingosylphosphorylcholine a substance that binds to the Edg family and acts like LPA.
- S1P sphingosine monophosphate
- PAF platelet activating factor
- sphingosylphosphorylcholine Alkyl LPA analogs
- FTY720 Alkyl LPA analogs
- LPA is the following general formula (I): R—O—CH 2 CH (OH) CH 2 PO 4 H 2 (I) (Wherein R is an alkyl group having 10 to 30 carbon atoms, an alkenyl group having 10 to 30 carbon atoms, or an acyl group having 10 to 30 carbon atoms) It is a compound represented by these. In addition, carbon number of the acyl group about R group of said formula (I) does not include carbon number of a carbonyl group.
- LPA As the salt of LPA, a conventionally known salt can be used, and examples thereof include alkali metal salts such as sodium salt and potassium salt, ammonium salt and the like.
- alkali metal salts such as sodium salt and potassium salt, ammonium salt and the like.
- LPA or a salt of LPA include 1-oleoyl lysophosphatidic acid sodium salt and LPA potassium salt.
- Edg ligands can be used alone or in combination of two or more.
- the medium of the present invention contains selenium, which is a trace element.
- Selenium is known to play an important role in the activity of glutathione peroxidase, which decomposes hydrogen peroxide generated in cells into water and oxygen.
- Selenium is usually contained in the medium in the form of a compound such as selenate or sodium selenite.
- Such selenium-containing compounds can be used alone or in combination of two or more.
- the concentration of Edg ligand in the medium of the present invention is preferably 0.01 ⁇ M to 50 ⁇ M, more preferably 0.1 ⁇ M to 10 ⁇ M.
- the selenium concentration is preferably 1 nM to 1 ⁇ M, more preferably 1 nM to 500 nM.
- vitamin A is known to control differentiation induction from embryonic stem cells to nervous system cells.
- Vitamin K is known to have a blood coagulation action and a calcium fixing action on bones.
- Melatonin is known to have an effect of suppressing differentiation from somatic stem cells to adipocytes and a circadian rhythm as a pineal hormone and is related to sleep.
- Zinc is a trace essential element and is known to be an element necessary for the activity of many enzymes.
- vitamin A examples include retinoic acid and its derivatives. Vitamin A can be used alone or in combination of two or more. When vitamin A is contained in the medium, the concentration is not particularly limited, but is usually about 0.1 to 100 ⁇ M, preferably about 1 to 10 ⁇ M.
- vitamin D examples include ergocalciferol, cholecalciferol, derivatives thereof (such as 7-dehydrocholesterol), and metabolites thereof (such as calcitriol).
- Vitamin D can be used alone or in combination of two or more.
- the concentration is not particularly limited, but is usually about 0.1 to 500 nM, preferably about 1 to 100 nM.
- vitamin K examples include phylloquinone, menaquinone, menadione, sodium menadiol diphosphate, and the like. Vitamin K can be used alone or in combination of two or more. When vitamin K is contained in the medium, the concentration is not particularly limited, but is usually about 0.1 to 100 ⁇ M, preferably about 1 to 10 ⁇ M.
- the concentration is not particularly limited, but is usually about 0.1 to 100 nM, preferably about 1 to 50 nM.
- Zinc can be added to the medium in the form of a zinc compound.
- a zinc compound examples include compounds such as zinc chloride (ZnCl 2 ), zinc oxide (ZnO), zinc sulfide (ZnS), and zinc sulfate (ZnSO 4). These zinc compounds can be used alone or in combination of two or more.
- the concentration is not particularly limited, but is usually about 0.1 nM to 100 ⁇ M, preferably about 1 nM to 10 ⁇ M.
- the medium of the present invention contains an agent for inducing differentiation of mammalian somatic stem cells into bone cells.
- Differentiation-inducing agents from somatic stem cells to bone cells are known per se, and known differentiation-inducing agents can also be preferably used in the present invention.
- Preferable examples of known differentiation inducers include those containing ⁇ -glycerophosphate, dexamethasone and vitamin C at the same time.
- Vitamin C known as a water-soluble vitamin, is used for biosynthesis of amino acids, as well as secretion of hormones from the adrenal gland, synthesis of L-carnitine, a carrier for transporting fatty acids to mitochondria, and generation of collagen in connective tissues. It is known that it plays an important role in the hydroxylation reaction that proceeds in the body. Vitamin C may be ascorbic acid or ascorbic acid diphosphate or a salt thereof, or a mixture thereof.
- the concentration of the differentiation-inducing agent is appropriately set according to the type of differentiation-inducing agent used, the type of cell, and the like.
- the differentiation-inducing agent contains ⁇ -glycerophosphate, dexamethasone and vitamin C at the same time, ⁇ -glyceroline
- the acid concentration is preferably 1 mM to 100 mM, more preferably 5 mM to 50 mM
- the dexamethasone concentration is preferably 1 nM to 1 ⁇ M, more preferably 10 nM to 500 nM
- the vitamin C concentration is preferably 10 ⁇ M to 10 mM, more preferably 200 ⁇ M. ⁇ 2 mM.
- the medium of the present invention may be the same as a known medium for mammalian cells except that it contains the above-mentioned two kinds of essential components and the above-mentioned differentiation inducer. Therefore, the medium of the present invention can be obtained by adding the above-described two kinds of essential components and a differentiation inducer conventionally used for inducing differentiation into a follicle to a known basic medium.
- Preferred known serum-free basal media that can be used in the culture medium of the present invention include minimum essential medium (MEM) such as Eagle medium, Dulbecco's modified Eagle medium (DMEM), minimum essential medium ⁇ (MEM- ⁇ ), Mesenchymal cell basal medium (MSCBM), Ham's F-12 and F-10 medium, DMEM / F12 medium, Williams medium E, RPMI-1640 medium, MCDB medium, 199 medium, Fisher medium, Iscove modified Dulbecco medium ( IMDM), McCoy modified medium, and the like. These media are all well-known media in this field.
- MEM minimum essential medium
- DMEM Dulbecco's modified Eagle medium
- MSCBM Mesenchymal cell basal medium
- DMEM / F12 medium Williams medium E
- RPMI-1640 medium RPMI-1640 medium
- MCDB medium 199 medium
- Fisher medium Iscove modified Dulbecco medium
- McCoy modified medium McCoy modified medium
- the medium of the present invention may further contain various additives that are well known to be included in mammalian cell culture media.
- additives include amino acids, inorganic salts, vitamins, and other additives such as carbon sources and antibiotics.
- Amino acids include glycine, L-alanine, L-arginine, L-asparagine, L-aspartic acid, L-cysteine, L-cystine, L-glutamic acid, L-glutamine, L-histidine, L-isoleucine, L- Mention may be made of leucine, L-lysine, L-methionine, L-phenylalanine, L-proline, L-serine, L-threonine, L-tryptophan, L-tyrosine and L-valine.
- Inorganic salts include calcium chloride, copper sulfate, iron (III) nitrate, iron sulfate, magnesium chloride, magnesium sulfate, potassium chloride, sodium hydrogen carbonate, sodium chloride, disodium hydrogen phosphate, sodium dihydrogen phosphate and zinc sulfate. Can be mentioned.
- vitamin B1 As vitamins, choline, vitamin A, vitamin B1, vitamin B2, vitamin B3, vitamin B4, vitamin B5, vitamin B6, vitamin B7, vitamin B12, vitamin B13, vitamin B15, vitamin B17, vitamin Bh, vitamin Bt, vitamin Mention may be made of Bx, vitamin D, vitamin E, vitamin F, vitamin K, vitamin M and vitamin P.
- the amount of each additive may be the same as that of a known medium, and can be appropriately set by a routine test.
- the addition amount of amino acids is usually about 5 mg / L to 500 mg / L, preferably about 10 mg / L to 400 mg / L for each amino acid
- the addition amount of inorganic salts is usually 0 mg / L to About 10 g / L, preferably about 0.01 mg / L to 7 g / L.
- the amount of vitamins added is about 0.01 mg / L to 500 mg / L, preferably 0.05 mg / L for each vitamin. About 300 mg / L.
- FGF fibroblast growth factor
- EGF endothelial cell growth factor
- PDGF platelet derived growth factor
- FGF growth factors
- penicillin streptomycin
- gentamicin kanamycin
- Carbon sources such as glucose, galactose, fructose and sucrose
- antioxidants such as 2-mercaptoethanol, catalase, superoxide dismutase and N-acetylcysteine, and adenosine 5'-monophosphate, corticosterone, ethanolamine, insulin, reduced glutathione, Lipoic acid, hypoxanthine, pheno Mention may be made of other additives such as lured, progesterone, putrescine, pyruvate, thymidine, triiodothyronine, transferrin and lactoferrin. The addition amount of these additives may be the same as the conventional one, and can be appropriately set by a routine test according to the purpose of each additive. Usually, it is about 0.001 mg / L to 5 g / L, particularly about 0.1 to 3 g / L.
- the medium of the present invention can contain one or more of the various additives described above, and usually contains a combination of a plurality of additives.
- glutamic acid is preferable because it appears to exert effects on cell survival and differentiation into bone cells when added to a medium.
- the preferred concentration in the medium is about 1 ⁇ M to 1 mM, more preferably about 25 ⁇ M to 250 ⁇ M.
- the medium of the present invention is serum-free or low-serum, preferably serum-free.
- low serum means a medium containing serum but containing 5% by weight or less, preferably 1% by weight.
- Culture of mammalian somatic cells in the medium of the present invention can be carried out in the same manner as in the past, and is usually performed at a temperature of 30 to 37 ° C., in a 5% CO 2 environment, and 5 to 21% O. Performed in two environments.
- the culture time required for differentiation induction is appropriately set depending on the differentiation inducer used, the type of cells, and the like, and can be appropriately selected while observing the state of the cells. Usually, it is about 10 to 30 days. It is.
- the present invention also provides an additive for constituting the above-described culture medium of the present invention. Therefore, the additive of the present invention contains the above-mentioned Edg ligand and selenium. Moreover, the said differentiation-inducing agent may be further included in these. Further, one or more of the various additives described above may be included. Furthermore, the medium of the present invention can be provided simply by including the components of the basic medium and dissolving in water. It is preferable that the additive of the present invention has a composition that gives the above-described medium of the present invention by dissolving in water or a basic medium. In this case, the mixing ratio of various components contained in the additive is the same as the content ratio of each component in the medium. In addition, as a basic medium, the above-mentioned various culture media conventionally used for culture
- the concentration described in each example is the final concentration in the medium.
- the lysophosphatidic acid (LPA) used was 1-oleoyl lysophosphatidic acid sodium, and the selenium compounds were all sodium selenite.
- Example 1 Comparative Examples 1 to 3 Differentiation induction from human bone marrow-derived mesenchymal stem cells to bone cells under serum-free conditions 1
- a serum-free control medium (Comparative Example 1) was prepared by adding 5 ⁇ M lysophosphatidic acid (LPA), 1 mM ascorbic acid 2-phosphate and 60 nM selenium to the basic medium (DMEM).
- LPA lysophosphatidic acid
- DMEM basic medium
- the final concentration of 10 mM ⁇ -glycerophosphate, 100 nM dexamedazone, and 200 ⁇ M ascorbic acid phosphate were added to DMEM to prepare an osteoblast differentiation basic medium (differentiation basic medium).
- 5 ⁇ M LPA, 1 mM ascorbic acid 2-phosphate, 60 nM selenium and 90 ⁇ M glutamic acid were added to this bone cell differentiation induction basal medium to produce the serum-free differentiation medium of the present invention (Example 1).
- Human bone marrow-derived mesenchymal stem cells (strain name: normal human mesenchymal stem cells (Cryo hMSC), source: LONZA) are seeded in wells of a 12-well culture plate so as to have a cell density of 10,000 cells / cm 2 , Differentiation into bone cells was induced by culturing in each of the above media at 37 ° C. and 5% CO 2 for 7 to 21 days.
- ALP alkaline phosphatase
- a serum-free control medium A (Comparative Example 4) was prepared by adding 5 ⁇ M LPA, 1 mM ascorbic acid 2-phosphate and 100 nM selenium to a basic medium (DMEM).
- Example 1 the final concentration of 10 mM ⁇ -glycerophosphate, 100 nM dexamedazone, and 200 ⁇ M ascorbic acid 2-phosphate was added to DMEM to prepare a bone cell differentiation induction basic medium (differentiation basic medium).
- a bone cell differentiation induction basic medium differentiated basic medium
- 5 ⁇ M LPA, 1 mM ascorbic acid 2-phosphate, 100 nM selenium and 90 ⁇ M glutamic acid were added to this differentiation basic medium, and the serum-free differentiation medium A of the present invention (Example 2-1 ) was manufactured.
- 5 ⁇ M LPA, 1 mM ascorbic acid 2-phosphate, 100 nM selenium, 50 nM cholecalciferol and 5 nM melatonin were added to the differentiation basic medium to add serum-free differentiation medium B (Example 2-2), 5 ⁇ M to DMEM LPA, 1 mM ascorbic acid 2-phosphate, 100 nM selenium, 50 nM cholecalciferol and 2.5 ⁇ M vitamin A acetate to add serum-free differentiation medium C (Example 2-3), 5 ⁇ M in DMEM Serum-free differentiation medium D (Example 2-4) was prepared by adding LPA, 1 mM ascorbic acid 2-phosphate, 100 nM selenium, 50 nM cholecalciferol and 1 ⁇ M vitamin K3.
- human bone marrow-derived mesenchymal stem cells were seeded in wells of a 12-well culture plate at a cell density of 3000 cells / cm 2 , and these media were used at 37 ° C. and 5% CO 2 for 21. Differentiation into bone cells was induced by culturing for days. Bone cells were confirmed by alizarin red S staining.
- the conventional differentiation medium containing serum is also used in the serum-free differentiation mediums B to D in which cholecalciferol, melatonin, vitamin A acetate, and vitamin K3 are further added in combination to the serum-free differentiation medium A. It was confirmed to be differentiated into bone cells to the same extent as (Comparative Example 9). It was confirmed that the conventional medium (Comparative Example 8) and serum-free control mediums A to D (Comparative Examples 4 to 7) did not differentiate into bone cells.
- Example 3 and Comparative Examples 10 to 12 Differentiation induction from human osteoblasts to bone cells under serum-free conditions
- 5 ⁇ M LPA, 1 mM ascorbic acid 2 was added to basal medium (DMEM) containing glutamic acid.
- DMEM basal medium
- a serum-free control medium was prepared by adding phosphoric acid, 60 nM selenium and 90 ⁇ M glutamic acid.
- Example 3 As in Example 1, the final concentration of 10 mM ⁇ -glycerophosphate, 100 nM dexamedazone, and 200 ⁇ M ascorbic acid 2-phosphate was added to DMEM to prepare an osteoblast differentiation basic medium (differentiation basic medium).
- a serum-free differentiation medium (Example 3) of the present invention was prepared by adding 5 ⁇ M LPA, 1 mM ascorbic acid 2-phosphate, 60 nM selenium and 90 ⁇ M glutamic acid to the differentiation basic medium.
- Example 11 10% fetal bovine serum (FBS) was added to each of the basic medium and the differentiation basic medium to produce a conventional medium (Comparative Example 11) and a conventional differentiation medium (Comparative Example 12).
- FBS fetal bovine serum
- Human osteoblasts (strain name: normal human osteoblasts (NHOst), source: LONZA) were seeded in wells of a 12-well culture plate at a cell density of 10,000 cells / cm 2 , Differentiation into bone cells was induced by culturing at 37 ° C. and 5% CO 2 for 14 days. Bone cells were confirmed by alizarin red S staining.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- Biotechnology (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Microbiology (AREA)
- Rheumatology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Cell Biology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP10743811.1A EP2399987B1 (en) | 2009-02-23 | 2010-02-18 | Culture medium and method to induce differentiation into bone cells |
| US13/202,713 US9315777B2 (en) | 2009-02-23 | 2010-02-18 | Culture medium and method for inducing differentiation into bone cells |
| CA2753303A CA2753303A1 (en) | 2009-02-23 | 2010-02-18 | Culture medium and method for inducing differentiation into bone cells |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009-038998 | 2009-02-23 | ||
| JP2009038998A JP5663841B2 (ja) | 2009-02-23 | 2009-02-23 | 骨細胞への分化誘導培地及び方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010095685A1 true WO2010095685A1 (ja) | 2010-08-26 |
Family
ID=42633965
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2010/052455 Ceased WO2010095685A1 (ja) | 2009-02-23 | 2010-02-18 | 骨細胞への分化誘導培地及び方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US9315777B2 (enExample) |
| EP (1) | EP2399987B1 (enExample) |
| JP (1) | JP5663841B2 (enExample) |
| CA (1) | CA2753303A1 (enExample) |
| WO (1) | WO2010095685A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015531787A (ja) * | 2012-09-13 | 2015-11-05 | ザ ボード オブ トラスティーズ オブ ザ レランド スタンフォード ジュニア ユニバーシティー | 卵胞の発達及び卵母細胞の成熟の刺激 |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113025566A (zh) * | 2020-12-30 | 2021-06-25 | 无锡市第九人民医院 | 一种内皮细胞成骨诱导分化培养基及制备方法 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000198701A (ja) * | 1998-11-05 | 2000-07-18 | Bausch & Lomb Surgical Inc | 眼科用の規定された無血清医療溶液およびそれを用いた処置方法 |
| JP2001512304A (ja) * | 1996-12-06 | 2001-08-21 | オシリス セラピューティクス,インコーポレイテッド | ヒト間葉幹細胞の改良された軟骨形成分化 |
| JP2006505248A (ja) | 2002-06-07 | 2006-02-16 | イーエス・セル・インターナショナル・プライヴェート・リミテッド | 幹細胞における分化を制御する方法 |
| JP2006055106A (ja) | 2004-08-23 | 2006-03-02 | National Institute Of Advanced Industrial & Technology | ヒト血清培地を用いるヒト骨髄由来間葉系幹細胞培養法 |
| WO2007080919A1 (ja) * | 2006-01-13 | 2007-07-19 | Japan Science And Technology Agency | 動物細胞を無血清培養するための培地用添加剤、キット及びこれらの利用 |
| JP2007528756A (ja) * | 2003-08-01 | 2007-10-18 | 憲正 中村 | スキャフォールドフリー自己組織性三次元人工組織(Scaffold−freeSelf−Organized3Dsynthetictissue) |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| LV13634B (en) | 2007-05-02 | 2007-12-20 | Jp Biotechnology Sia | Production of ethanol and feedstuffs |
| US20110008893A1 (en) * | 2007-12-28 | 2011-01-13 | Fujirebio Inc. | Medium for mammalian somatic cells and additive therefor |
| JP5515319B2 (ja) * | 2009-02-23 | 2014-06-11 | 富士レビオ株式会社 | 脂肪細胞への分化誘導培地及び方法 |
-
2009
- 2009-02-23 JP JP2009038998A patent/JP5663841B2/ja not_active Expired - Fee Related
-
2010
- 2010-02-18 EP EP10743811.1A patent/EP2399987B1/en not_active Not-in-force
- 2010-02-18 CA CA2753303A patent/CA2753303A1/en not_active Abandoned
- 2010-02-18 WO PCT/JP2010/052455 patent/WO2010095685A1/ja not_active Ceased
- 2010-02-18 US US13/202,713 patent/US9315777B2/en not_active Expired - Fee Related
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001512304A (ja) * | 1996-12-06 | 2001-08-21 | オシリス セラピューティクス,インコーポレイテッド | ヒト間葉幹細胞の改良された軟骨形成分化 |
| JP2000198701A (ja) * | 1998-11-05 | 2000-07-18 | Bausch & Lomb Surgical Inc | 眼科用の規定された無血清医療溶液およびそれを用いた処置方法 |
| JP2006505248A (ja) | 2002-06-07 | 2006-02-16 | イーエス・セル・インターナショナル・プライヴェート・リミテッド | 幹細胞における分化を制御する方法 |
| JP2007528756A (ja) * | 2003-08-01 | 2007-10-18 | 憲正 中村 | スキャフォールドフリー自己組織性三次元人工組織(Scaffold−freeSelf−Organized3Dsynthetictissue) |
| JP2006055106A (ja) | 2004-08-23 | 2006-03-02 | National Institute Of Advanced Industrial & Technology | ヒト血清培地を用いるヒト骨髄由来間葉系幹細胞培養法 |
| WO2007080919A1 (ja) * | 2006-01-13 | 2007-07-19 | Japan Science And Technology Agency | 動物細胞を無血清培養するための培地用添加剤、キット及びこれらの利用 |
Non-Patent Citations (9)
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015531787A (ja) * | 2012-09-13 | 2015-11-05 | ザ ボード オブ トラスティーズ オブ ザ レランド スタンフォード ジュニア ユニバーシティー | 卵胞の発達及び卵母細胞の成熟の刺激 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2399987B1 (en) | 2015-11-04 |
| JP2010193723A (ja) | 2010-09-09 |
| CA2753303A1 (en) | 2010-08-26 |
| EP2399987A4 (en) | 2013-12-25 |
| JP5663841B2 (ja) | 2015-02-04 |
| EP2399987A1 (en) | 2011-12-28 |
| US9315777B2 (en) | 2016-04-19 |
| US20110306132A1 (en) | 2011-12-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5515319B2 (ja) | 脂肪細胞への分化誘導培地及び方法 | |
| EP2233565B1 (en) | Medium for mammalian somatic cells and additive therefor | |
| CN103857789B (zh) | 制备间充质干细胞基础培养基和利用间充质干细胞基础培养基制备细胞治疗产品的方法及用该培养基得到的分化产品 | |
| US10184112B2 (en) | Culture medium additive for use in serum-free culturing of animal cell, kit and use thereof | |
| US10287550B2 (en) | Serum-free chemically defined cell culture medium | |
| EP3263697B1 (en) | Culture medium for culturing mesenchymal stem cell, method for culturing mesenchymal stem cell, and mesenchymal stem cell | |
| Sart et al. | Process engineering of stem cell metabolism for large scale expansion and differentiation in bioreactors | |
| CN110494559B (zh) | 不分化地维持用培养基添加剂 | |
| JP5663841B2 (ja) | 骨細胞への分化誘導培地及び方法 | |
| JP7173007B2 (ja) | リボフラビン誘導体含有培地 | |
| JP2010193723A5 (enExample) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10743811 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2753303 Country of ref document: CA Ref document number: 13202713 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010743811 Country of ref document: EP |