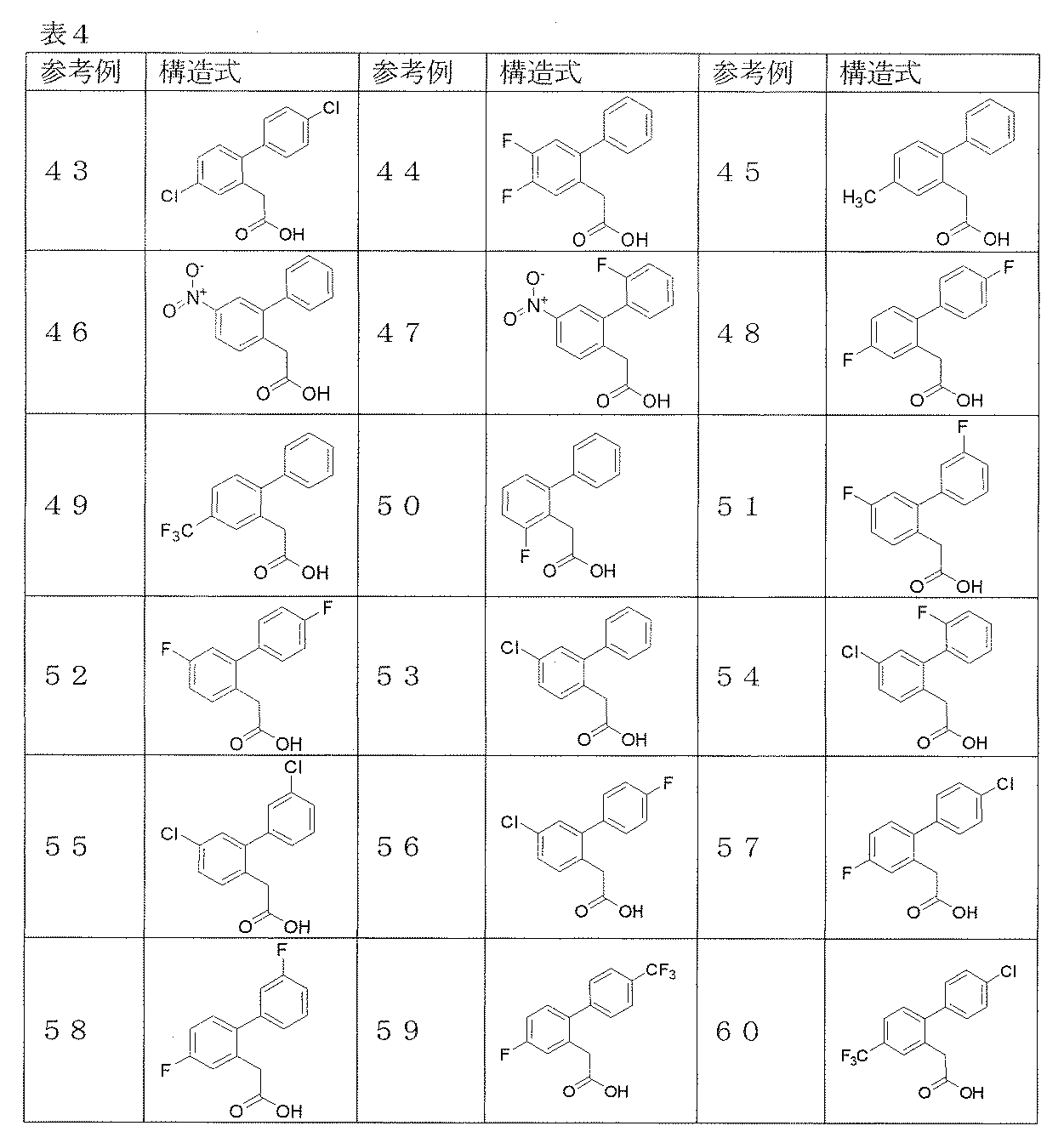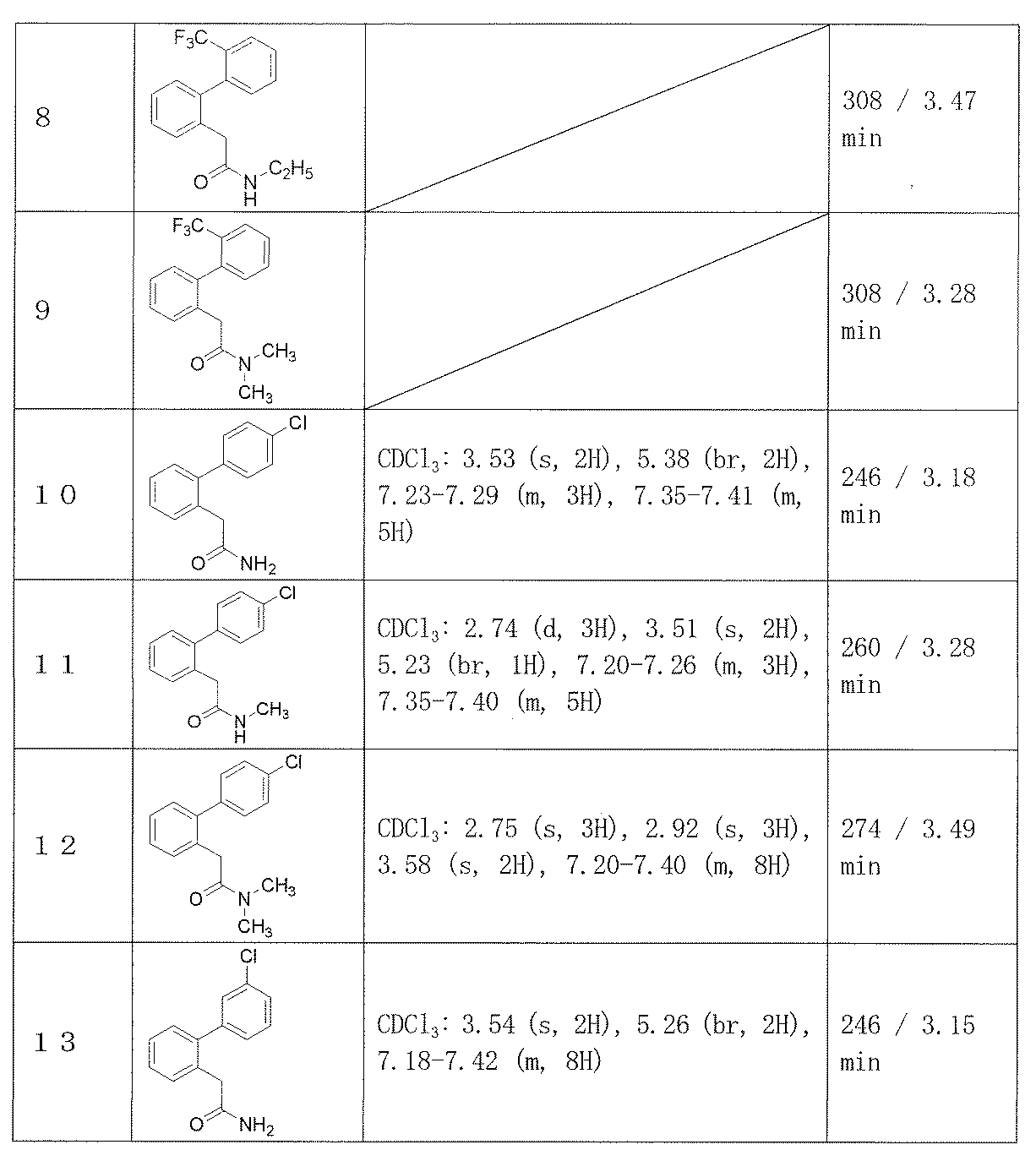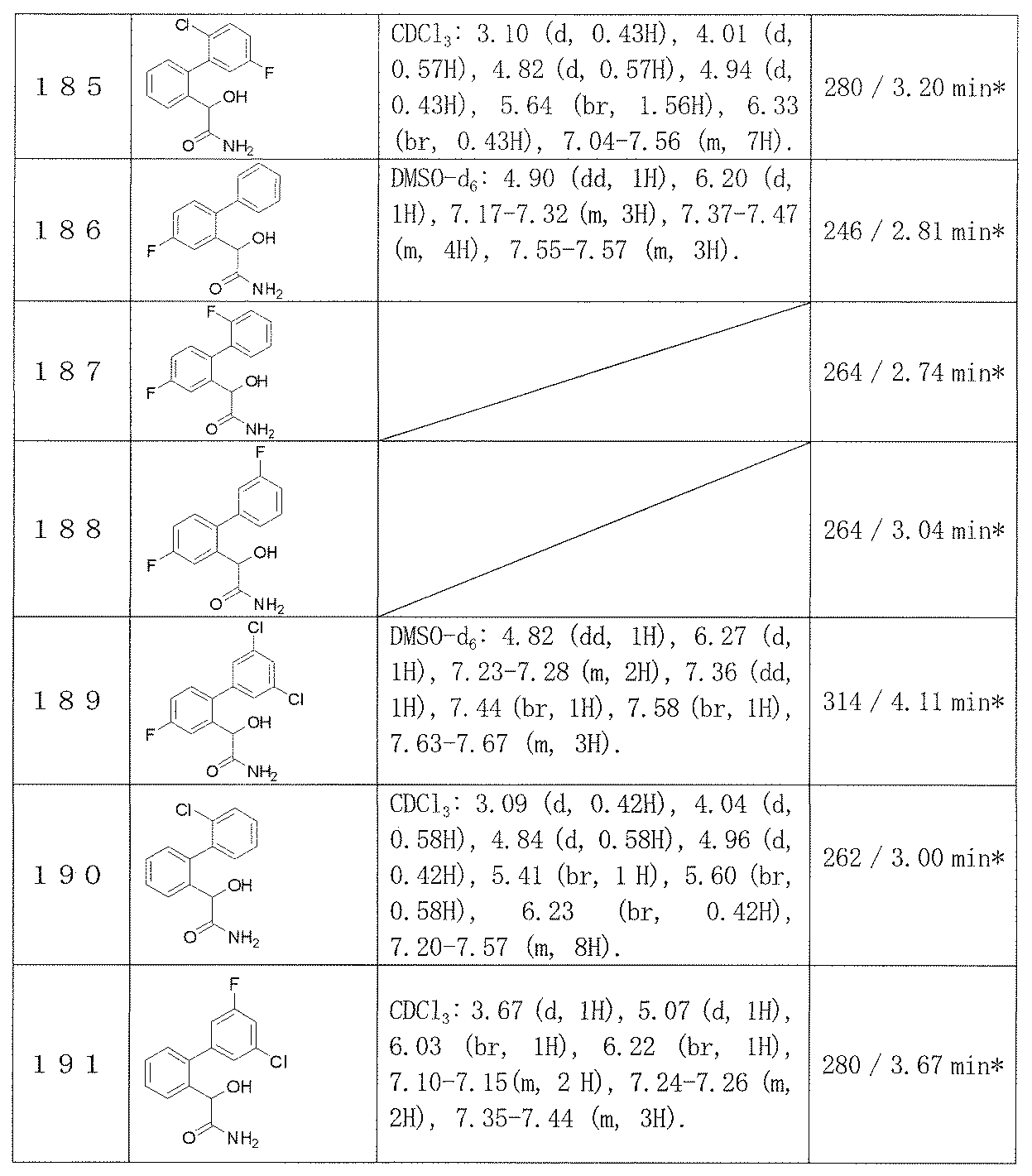WO2010055911A1 - ビフェニルアセトアミド誘導体 - Google Patents
ビフェニルアセトアミド誘導体 Download PDFInfo
- Publication number
- WO2010055911A1 WO2010055911A1 PCT/JP2009/069355 JP2009069355W WO2010055911A1 WO 2010055911 A1 WO2010055911 A1 WO 2010055911A1 JP 2009069355 W JP2009069355 W JP 2009069355W WO 2010055911 A1 WO2010055911 A1 WO 2010055911A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- acetamide
- fluorobiphenyl
- biphenyl
- alkyl
- atom
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 CC(C)CCN1*C1 Chemical compound CC(C)CCN1*C1 0.000 description 3
- YMXMDERILJQYMS-UHFFFAOYSA-N CC(C(O)=O)c1ccccc1-c1ccccc1 Chemical compound CC(C(O)=O)c1ccccc1-c1ccccc1 YMXMDERILJQYMS-UHFFFAOYSA-N 0.000 description 1
- NYGBSRIHGKEKFQ-UHFFFAOYSA-N OC(C(c1ccccc1-c1ccccc1)(F)F)=O Chemical compound OC(C(c1ccccc1-c1ccccc1)(F)F)=O NYGBSRIHGKEKFQ-UHFFFAOYSA-N 0.000 description 1
- FTIZUOJARGPUNB-UHFFFAOYSA-N OC(C(c1ccccc1-c1ccccc1)F)=O Chemical compound OC(C(c1ccccc1-c1ccccc1)F)=O FTIZUOJARGPUNB-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C235/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms
- C07C235/02—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton
- C07C235/32—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton the carbon skeleton containing six-membered aromatic rings
- C07C235/34—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton the carbon skeleton containing six-membered aromatic rings having the nitrogen atoms of the carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/16—Amides, e.g. hydroxamic acids
- A61K31/165—Amides, e.g. hydroxamic acids having aromatic rings, e.g. colchicine, atenolol, progabide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/20—Hypnotics; Sedatives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/01—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C233/02—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having nitrogen atoms of carboxamide groups bound to hydrogen atoms or to carbon atoms of unsubstituted hydrocarbon radicals
- C07C233/11—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having nitrogen atoms of carboxamide groups bound to hydrogen atoms or to carbon atoms of unsubstituted hydrocarbon radicals with carbon atoms of carboxamide groups bound to carbon atoms of an unsaturated carbon skeleton containing six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C255/00—Carboxylic acid nitriles
- C07C255/49—Carboxylic acid nitriles having cyano groups bound to carbon atoms of six-membered aromatic rings of a carbon skeleton
- C07C255/57—Carboxylic acid nitriles having cyano groups bound to carbon atoms of six-membered aromatic rings of a carbon skeleton containing cyano groups and carboxyl groups, other than cyano groups, bound to the carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C317/00—Sulfones; Sulfoxides
- C07C317/44—Sulfones; Sulfoxides having sulfone or sulfoxide groups and carboxyl groups bound to the same carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C323/00—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups
- C07C323/50—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and carboxyl groups bound to the same carbon skeleton
- C07C323/62—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and carboxyl groups bound to the same carbon skeleton having the sulfur atom of at least one of the thio groups bound to a carbon atom of a six-membered aromatic ring of the carbon skeleton
-
- C—CHEMISTRY; METALLURGY
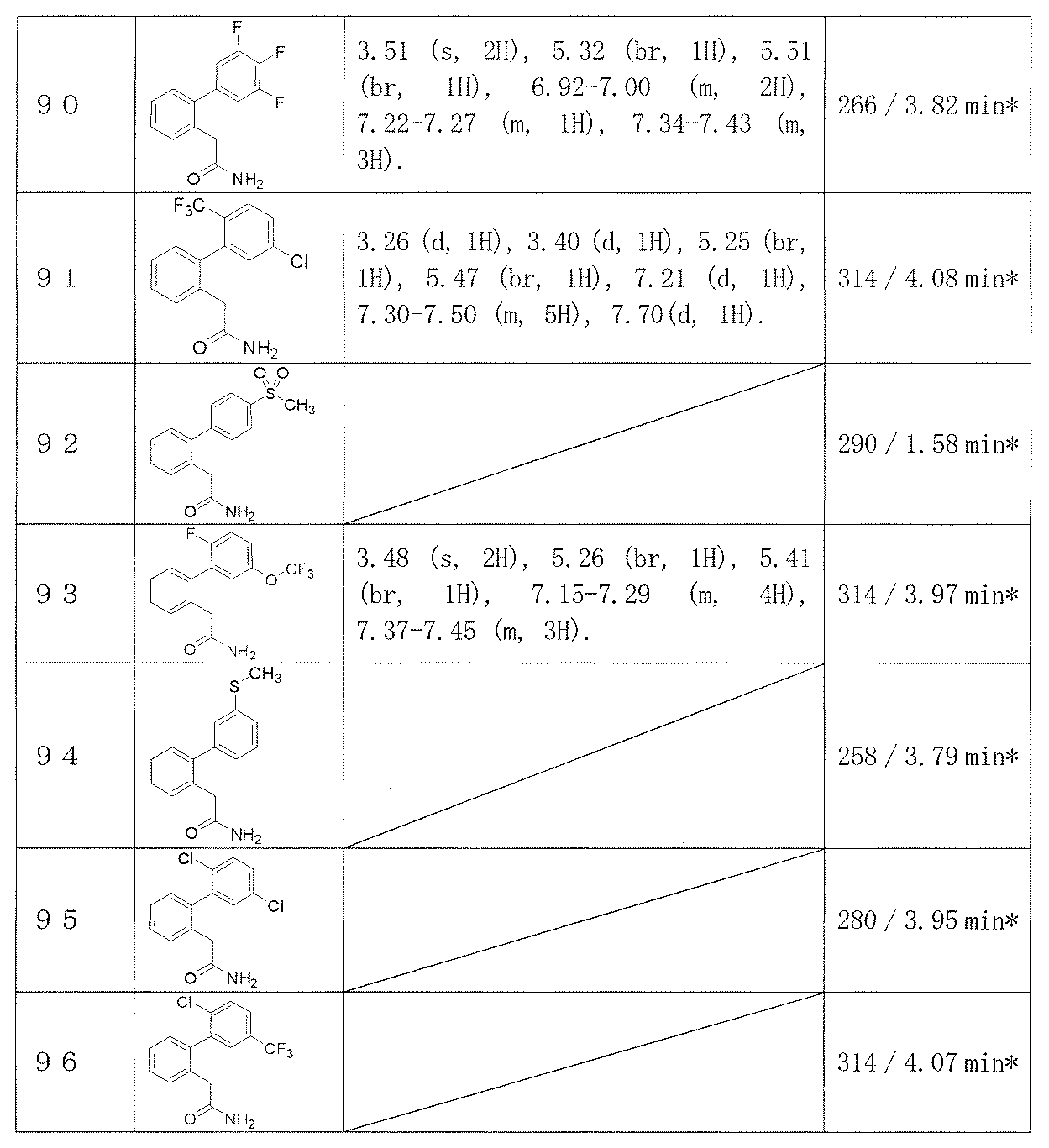
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/02—Systems containing only non-condensed rings with a three-membered ring
Definitions
- the present invention relates to a novel biphenylacetamide derivative useful as a therapeutic agent for epilepsy.
- Epilepsy is a chronic disease in which paroxysmal movement, consciousness, sensory abnormalities, and behavioral abnormalities resulting from excessive excitation of cranial nerve cells are repeated. And one in three cases of this disease is refractory epilepsy that shows resistance to existing drug treatments.
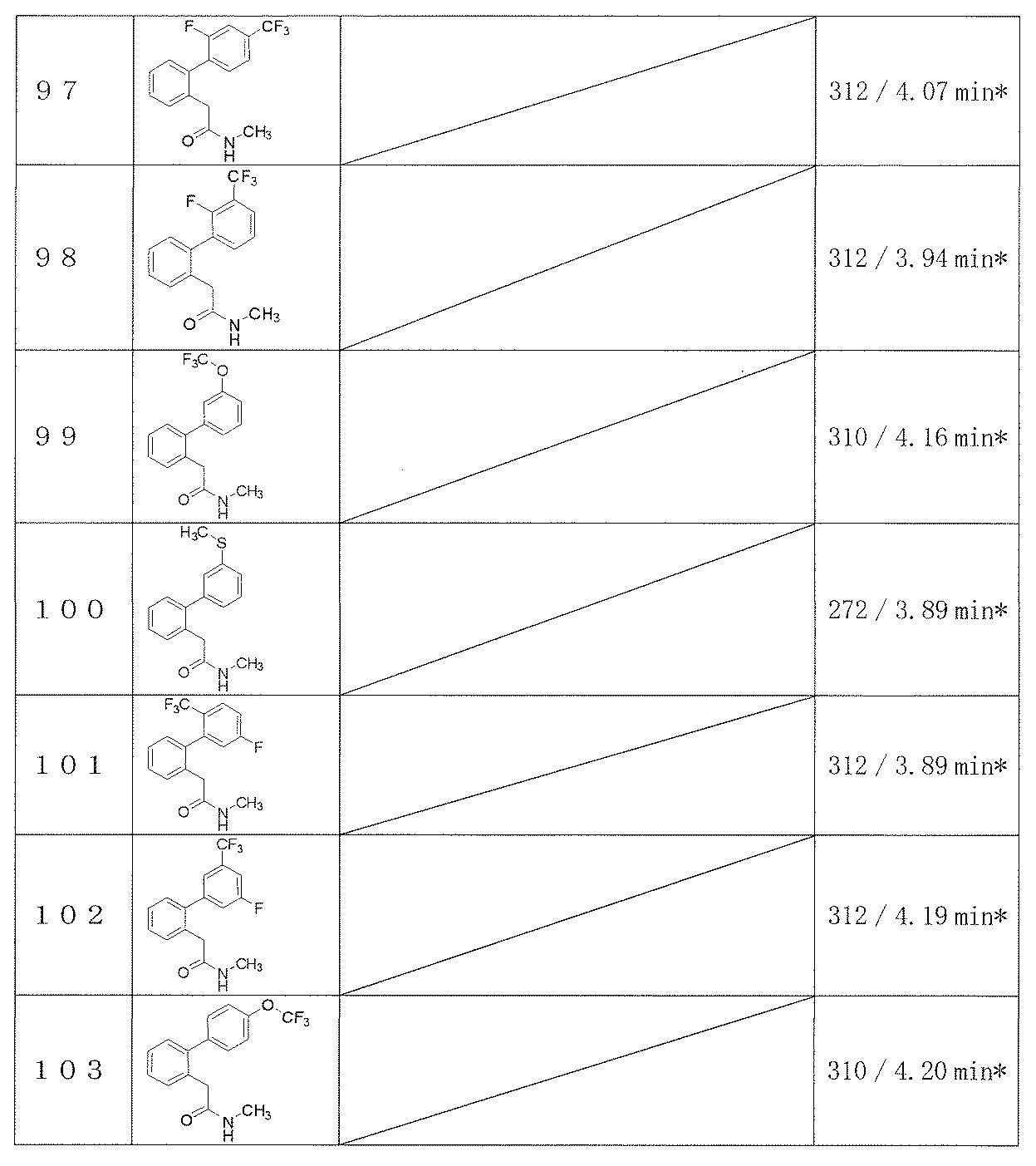
- Epilepsy seizures are broadly classified into partial seizures and generalized seizures. Partial seizures indicate behavioral abnormalities and electroencephalographic abnormalities that start in a limited area of the unilateral cerebral hemisphere. Partial seizures are further classified into simple partial seizures, complex partial seizures, and secondary generalized tonic-clonic seizures.
- generalized seizures are seizures that do not show a localized seizure origin and manifest symptoms in bilateral hemispheric synchronization. The usual clinical symptoms of generalized seizures are characteristic systemic motor symptoms with loss of consciousness.
- the electroencephalogram at the time of seizure is bilaterally synchronized. Absence attack (Absence ⁇ seizure), atypical absence attack (Atypical absence seizure), myoclonic attack (Myoclonic seizure), tonic seizure (Clonic seizure), clonic seizure, tonic-clonic seizure ), Weakness attacks (Atonic ⁇ ⁇ ⁇ seizure), West syndrome (West syndrome) and Lennox-Gastaut syndrome are classified as generalized seizures.
- antiepileptic drugs have no specific mechanism of action, and various mechanisms are considered to act in a complex manner. Therefore, some of the drugs that have been developed as antiepileptic drugs so far have been widely used in multiple neurological and mental disorders (Pawel, DZ et al., Non-epilepsy use of antiepileptic drugs, Pharmacological Reports, 2006, 58, 1-12).
- bipolar disorder manic-depressive disorder
- mood modulation has few specific treatments (lithium only)
- some antiepileptic drugs have a mood-stable effect (mood stabilizer) is recognized and used.
- Valproic acid has been used.
- valproic acid has been used in clinical practice as well as lithium (Angel, I and Horovitz, T., Bipolar disorder and valproic acid) as a first-line drug for bipolar depression.
- lithium Angel, I and Horovitz, T., Bipolar disorder and valproic acid
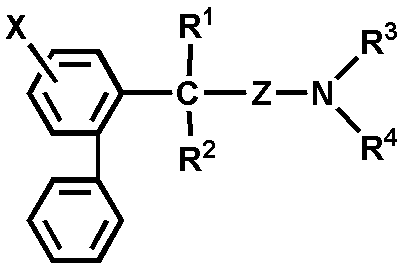
- Patent Document 1 describes biphenylalkylamines represented by the following general formula having an antiarrhythmic action.
- X is hydrogen or halogen;
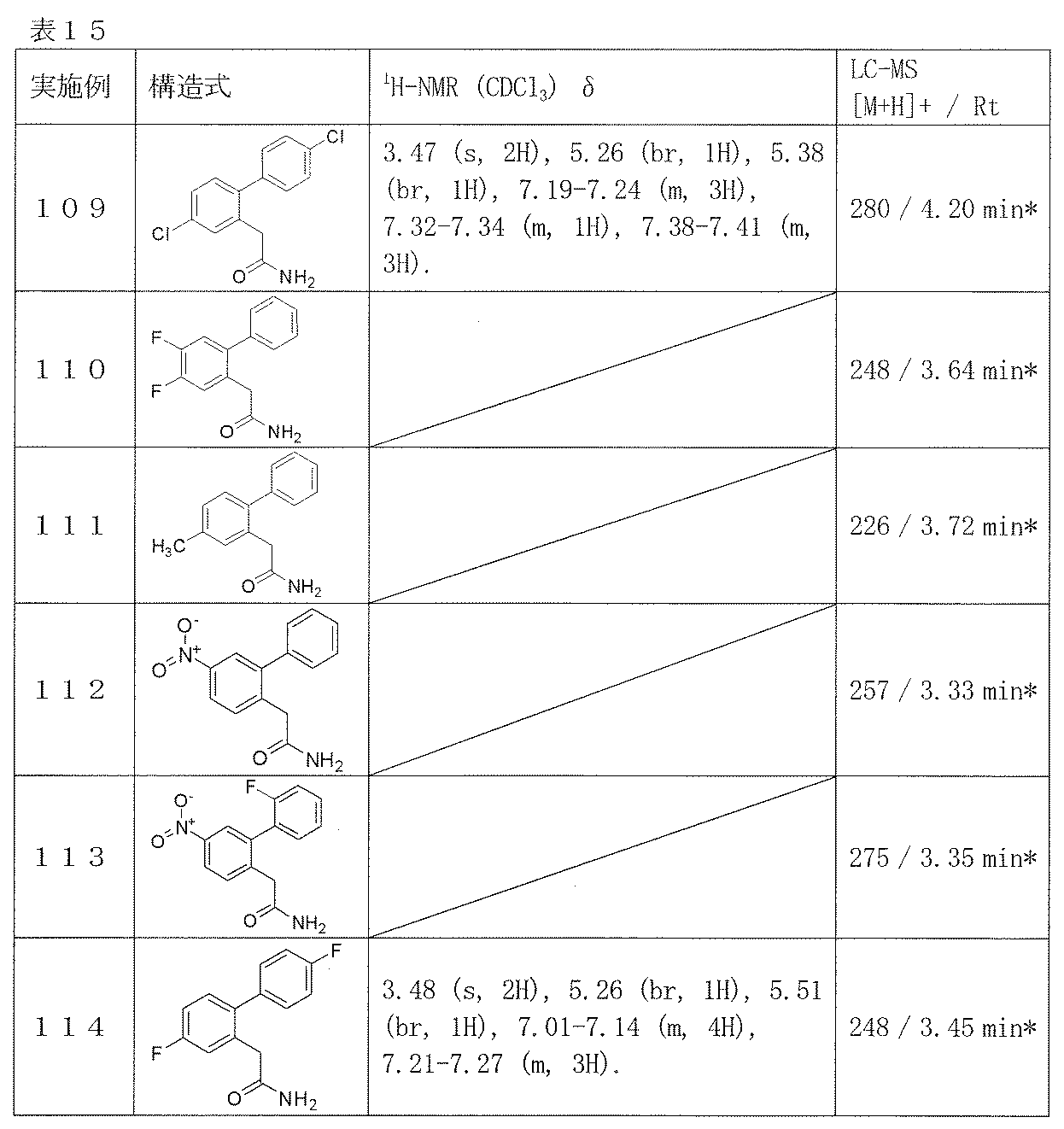
- R 1 is hydrogen, hydroxyl, CN, CONH 2 or COOR 5 where R 5 is hydrogen or lower alkyl;
- R 2 is hydrogen or C 1-3 alkyl;
- Z is — (CH 2 ) n — or Wherein n is 2, 3 or 4;
- R 3 and R 4 are each selected from pyrrolidino, piperidino and morpholino together with hydrogen, lower alkyl, lower alkenyl, phenylalkyl or nitrogen atom A group forming a cyclic structure.
- biphenyl and an amino group are bonded via 3 or more carbon atoms, and the chemical structure is different from the compound represented by the following formula (I).
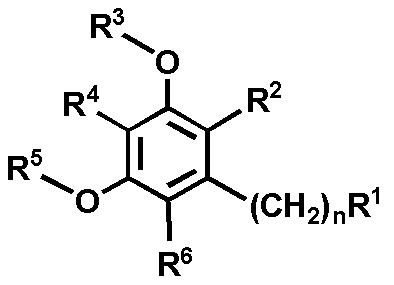
- Patent Document 2 describes a benzene derivative represented by the following general formula useful as an Hsp90 family protein inhibitor.
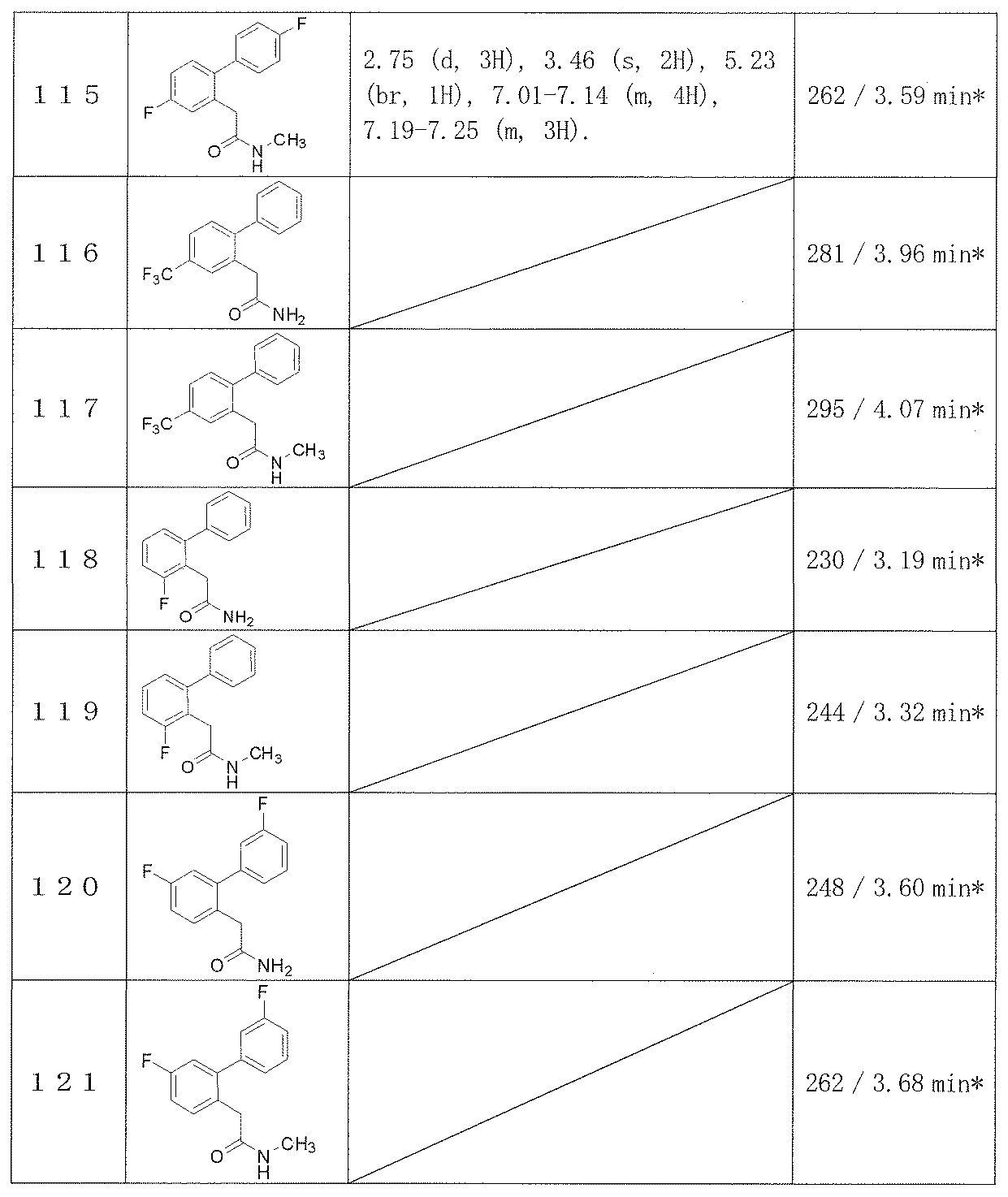
- R 1 is a hydrogen atom, hydroxy, cyano, carboxy, nitro, halogen, substituted or unsubstituted lower alkyl, —CONR 7 R 8 (wherein R 7 and R 8 are the same or different, and a hydrogen atom, substituted or non-substituted R 2 represents substituted or unsubstituted lower alkyl, substituted or unsubstituted lower alkenyl, substituted or unsubstituted lower alkynyl, substituted or unsubstituted aryl, etc .; R 3 and R 5 is the same or different and represents a hydrogen atom, substituted or unsubstituted lower alkyl or the like; R 4 and R 6 are the same or different and represent a hydrogen atom, hydroxy, halogen, cyano, substituted or unsubstit
- An object of the present invention is to provide a novel compound useful as an antiepileptic drug.
- a novel compound represented by the following formula (A) exhibits a strong anticonvulsant action and completed the present invention.
- a biphenylacetamide derivative represented by the following formula (A) (hereinafter sometimes referred to as “the compound of the present invention”) is provided.
- R 1 , R 2 and R 3 are bonded one by one to any substitutable carbon atom on the benzene ring to which they are bonded, and are the same or different and represent a hydrogen atom, a fluorine atom, a chlorine atom, bromine Represents an atom, C 1-6 alkyl, C 1-6 alkoxy substituted with 1 to 3 fluorine atoms, C 1-6 alkyl-S (O) n — or cyano, wherein the alkyl or alkyl-S (O N ⁇ may be substituted with 1 to 5 fluorine atoms, wherein any two groups of R 1 , R 2 and R 3 are 2′-methyl and 3′-methyl.
- R 4 and R 5 are bonded one by one to any substitutable carbon atom on the benzene ring to which they are bonded, and are the same or different and each represents a hydrogen atom, a fluorine atom, a chlorine atom, C 1-6 Alkyl, C 1-6 alkoxy substituted with 1 to 3 fluorine atoms, C 1-6 alkyl-S (O) n —, cyano or nitro, wherein alkyl or alkyl-S (O) n — is Optionally substituted with 1 to 5 fluorine atoms, R 6 and R 7 are the same or different and each represents a hydrogen atom, a fluorine atom, methyl, ethyl, a hydroxyl group or C 1-6 alkoxy (provided that when one is a hydroxyl group, the other is a fluorine atom, a hydroxyl group or C 1 -6 not alkoxy), or together with the
- R 1 , R 2 , R 3 , R 4 and R 5 are all hydrogen atoms and any one of R 6 and R 7 is alkoxy, R 8 and R 9 are the same or different, and Atom, C 1-3 alkyl, C 3-7 cycloalkyl-C 1-3 alkyl or C 3-6 cycloalkyl. ] Or a hydrate or solvate thereof.
- R 8 and R 9 are the same or different and each is a hydrogen atom, C 1-3 alkyl, C 3-7 cycloalkyl-C 1-3 alkyl or C 3-6 cycloalkyl, Cycloalkyl-alkyl and cycloalkyl may be substituted with 1 to 5 fluorine atoms, where R 1 , R 2 , R 3 , R 4 and R 5 are all hydrogen atoms, R Any one of 8 and R 9 is a hydrogen atom, Item 3. The compound according to Item 2, or a hydrate or solvate thereof.
- R 1 , R 2 and R 3 are the same or different and are a hydrogen atom, a fluorine atom, a chlorine atom, a bromine atom, trifluoromethyl, trifluoromethoxy, C 1-3 alkyl-S (O) n -Or cyano, and the alkyl-S (O) n- may be substituted with 1 to 5 fluorine atoms, Item 2.
- R 1 , R 2 and R 3 are the same or different and are a hydrogen atom, a fluorine atom, a chlorine atom, a bromine atom, trifluoromethyl, or trifluoromethoxy.
- Item 2. The compound according to Item 2 or 3, or a hydrate or solvate thereof.
- R 4 and R 5 are the same or different and are a hydrogen atom, a fluorine atom, a chlorine atom, C 1-3 alkyl, trifluoromethyl, or trifluoromethoxy.
- Item 7 The compound according to any one of Items 2 to 6, or a hydrate or solvate thereof.
- R 6 and R 7 are the same or different and are a hydrogen atom, methyl, ethyl, hydroxyl group or C 1-3 alkoxy (provided that one of them is a hydroxyl group, the other is a hydroxyl group or C 1-3 alkoxy) is not), Item 8.
- R 1 , R 2 and R 3 are the same or different and are a hydrogen atom, a fluorine atom, a chlorine atom, a bromine atom, trifluoromethyl or trifluoromethoxy
- R 4 and R 5 are the same or different and are a hydrogen atom, a fluorine atom, a chlorine atom, trifluoromethyl or trifluoromethoxy
- R 6 and R 7 are the same or different and are a hydrogen atom, methyl, hydroxyl group or methoxy (provided that one is a hydroxyl group, the other is not a hydroxyl group or methoxy)
- R 8 is a hydrogen atom, methyl or ethyl
- R 9 is a hydrogen atom, Item 3.
- R 1, R 2, R 3, R 4 and R 5 are not all simultaneously hydrogen atoms, the compound or a hydrate or solvate thereof, as claimed in claim 2 or claim 9.
- a pharmaceutical composition comprising the compound according to any one of items 1 to 12, or a hydrate or solvate thereof, and a pharmaceutically acceptable carrier.
- An antiepileptic drug comprising as an active ingredient the compound according to any one of items 1 to 12, or a hydrate or solvate thereof.
- a mood stabilizer for bipolar disorder comprising the compound according to any one of items 1 to 12 or a hydrate or solvate thereof as an active ingredient.
- a method for treating epilepsy comprising administering to a patient with epilepsy a therapeutically effective amount of the compound according to any one of items 1 to 12, or a hydrate or solvate thereof.
- R 1 to R 9 and n in the compound of formula (I) in Item 2 include the following.
- R 1 to R 3 are the same or different and are preferably a hydrogen atom, a fluorine atom, a chlorine atom, a bromine atom, trifluoromethyl or trifluoromethoxy, more preferably the same or different, a hydrogen atom, a fluorine atom, chlorine An atom, trifluoromethyl or trifluoromethoxy, or the same or different, a hydrogen atom, a fluorine atom or a chlorine atom;
- R 4 and R 5 are the same or different and are preferably a hydrogen atom, a fluorine atom, a chlorine atom, trifluoromethyl or trifluoromethoxy, more preferably the same or different, a hydrogen atom, a fluorine atom or a chlorine atom.
- R 6 and R 7 are the same or different and are preferably a hydrogen atom, a fluorine atom, methyl, a hydroxyl group or methoxy, more preferably the same or different, a hydrogen atom, methyl, a hydroxyl group or methoxy, or the same or different.
- a hydrogen atom or a hydroxyl group, or both are hydrogen atoms.
- R 8 is preferably a hydrogen atom, methyl or ethyl, and R 9 is preferably a hydrogen atom. More preferably, R 8 is a hydrogen atom or methyl, and R 9 is a hydrogen atom.
- n is preferably 2.
- Preferable specific examples of R 1 to R 9 and n described above may limit the compounds of the above-mentioned items by one or any combination thereof, and the compounds of the formula (I) limited by these may also be used. This is one aspect of the present invention.
- antiepileptic drugs exhibit equally strong anticonvulsant activity in any of the model animals used for the evaluation of antiepileptic drugs, antiepileptic drugs exhibiting a broad therapeutic spectrum (eg, simple partial seizures, complex partial seizures) Prophylaxis against generalized seizures such as seizures and secondary seizures, absence seizures, myoclonic seizures, clonic seizures, tonic seizures, tonic clonic seizures, weakness seizures, West syndrome and Lennox-Gastaut syndrome (Or therapeutic agent).
- the group of compounds of the present invention is also expected as a prophylactic and / or therapeutic agent for intractable epileptic seizures where conventional drug treatment is not successful.
- the compound of the present invention exhibits a pathological improvement effect at a dose equivalent to the dose showing anticonvulsant activity in a model animal in a manic state and a depressed state, which is a pathological background of bipolar disorder. It can be expected as a mood stabilizer for disorders.
- the compounds of the present invention may exist in the form of hydrates and / or solvates, these hydrates and / or solvates are also included in the compounds of the present invention.
- the compound of the present invention may have one or more asymmetric carbon atoms, and may cause geometric isomerism and axial chirality, so that it exists as several stereoisomers. There is. In the present invention, these stereoisomers, mixtures thereof and racemates are included in the compounds represented by formula (A) and formula (I) of the present invention.
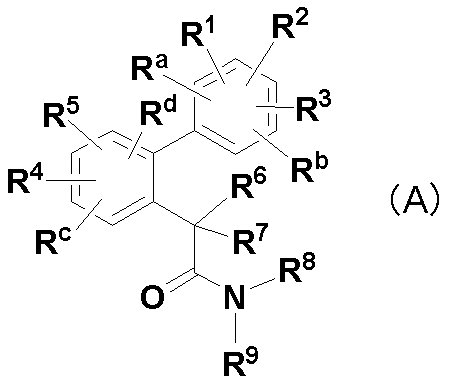
- substitution position of R 1 , R 2 , R 3 , R a and R b in the compound of the formula (A) of the present invention is any carbon atom of 5 substitutable carbon atoms on the benzene ring to which they are bonded. They are bonded one by one, and the substitution positions of R 4 , R 5 , R c, and R d are bonded one by one to any of the four substitutable carbon atoms on the benzene ring to which they are bonded. .
- substitution position of R 1 , R 2 and R 3 in the compound of the formula (1) of the present invention is any carbon atom among five substitutable carbon atoms on the benzene ring to which they are bonded.
- One of the three, and R 4 and R 5 are substituted at any two of the four substitutable carbon atoms on the benzene ring to which they are attached. Join one by one.
- the substitution position numbers of R 1 , R 2 and R 3 in the compound of the formula (1) of the present invention are represented by the following formula (I).
- any two groups of R 1 , R 2, and R 3 are 2′-methyl and 3′-methyl, and two methyl groups are substituted at positions 2 ′ and 3 ′ in the following formula (I).
- Alkyl means a linear or branched saturated hydrocarbon.
- C 1-3 alkyl or “C 1-6 alkyl” has 1 to 3 or 1 carbon atoms. Each group represents 6 to 6. Specific examples thereof include methyl, ethyl, propyl, isopropyl and the like in the case of “C 1-3 alkyl”, and in the case of “C 1-6 alkyl”, in addition to the above, butyl, isobutyl, s— Examples include butyl, t-butyl, pentyl, isopentyl, neopentyl, hexyl and the like.
- C 3-6 cycloalkyl means a monocyclic saturated hydrocarbon having from 3 to 6 carbon atoms. Specific examples thereof include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl and the like.
- C 3-7 cycloalkyl-C 1-3 alkyl means a monocyclic saturated hydrocarbon having 3 to 7 carbon atoms, a straight-chain or branched saturated hydrocarbon having 1 to 3 carbon atoms
- An alkyl group bonded to any carbon atom of Specific examples thereof include cyclopropylmethyl, cyclobutylmethyl, 1-cyclopropylethyl, 2-cyclopropylethyl, 1-cyclopropylpropyl, 2-cyclopropylpropyl, 1-cyclobutylethyl, 2-cyclobutylethyl, Examples include cyclopentylmethyl and cyclohexylmethyl.
- C 1-6 alkoxy means linear or branched alkoxy having 1 to 6 carbon atoms. Specific examples thereof include methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, s-butoxy, t-butoxy, pentyloxy, hexyloxy and the like.
- C 1-6 alkoxy substituted with 1 to 3 fluorine atoms means that 1 to 3 substitutable hydrogen atoms of the above alkoxy are substituted with fluorine atoms. Specific examples include difluoromethoxy, trifluoromethoxy, 2,2,2-trifluoroethoxy, 3,3,3-trifluoropropoxy, 4-fluorobutoxy, 4,4,4-trifluorobutoxy and the like. .
- C 1-6 alkyl-S (O) n — means a —S (O) n — group to which a linear or branched alkyl having 1 to 6 carbon atoms is bonded.
- alkylthio eg, methylthio, ethylthio, etc.
- alkylsulfinyl eg, methylsulfinyl, ethylsulfinyl, etc.
- alkylsulfonyl eg, methane
- Alkyl (including methyl and ethyl) optionally substituted with 1 to 5 fluorine atoms, alkyl-S (O) n —, alkoxy, cycloalkyl and cycloalkyl-alkyl are alkyl, alkoxy, cyclo It means that 1 to 5 substitutable hydrogen atoms on any carbon of alkyl and cycloalkyl-alkyl are replaced by fluorine atoms, and specific examples thereof include trifluoromethyl, fluoroethyl, Examples include trifluoroethyl, trifluoromethanesulfonyl, trifluoromethoxy, 2-fluorocyclopropyl, (2-fluorocyclopropyl) methyl, and the like. When the alkyl has 1 carbon, the number of fluorine atoms that may be substituted is 3 or less.
- R 1 to R 3 , R a , and R b include a hydrogen atom, a fluorine atom, a chlorine atom, a bromine atom, C 1-6 alkyl, C 1-6 alkoxy substituted with 1 to 3 fluorine atoms, C 1-6 alkyl-S (O) n — or cyano may be mentioned (the alkyl or alkyl-S (O) n — may be substituted with 1 to 5 fluorine atoms, and n is 0 And represents a hydrogen atom, a fluorine atom, a chlorine atom, a bromine atom, trifluoromethyl, trifluoromethoxy, methylthio, methanesulfonyl or cyano.
- a hydrogen atom, a fluorine atom, a chlorine atom, trifluoromethyl, trifluoromethoxy or cyano can be mentioned, more preferably a hydrogen atom, a fluorine atom, a chlorine atom or trifluoromethyl can be mentioned, and particularly preferably, Examples thereof include a hydrogen atom, a fluorine atom or trifluoromethyl, or a hydrogen atom, a fluorine atom or a chlorine atom.
- R 4 , R 5 , R c , and R d are each a hydrogen atom, a fluorine atom, a chlorine atom, C 1-6 alkyl, C 1-6 alkoxy substituted with 1 to 3 fluorine atoms, C 1- 6 alkyl-S (O) n —, cyano or nitro (the alkyl or alkyl-S (O) n — may be substituted with 1 to 5 fluorine atoms, and n is 0 to 2), preferably a hydrogen atom, a fluorine atom, a chlorine atom, methyl, ethyl, trifluoromethyl, trifluoromethoxy, methylthio, methanesulfonyl or cyano.
- a hydrogen atom, a fluorine atom, a chlorine atom, methyl, ethyl, trifluoromethyl, trifluoromethoxy or cyano is mentioned, and still more preferably a hydrogen atom, a fluorine atom, a chlorine atom, trifluoromethyl or trifluoromethoxy.
- a hydrogen atom or a fluorine atom is mentioned.
- R 6 and R 7 are each a hydrogen atom, a fluorine atom, methyl, ethyl, hydroxyl group or C 1-6 alkoxy, or C 6-6 cycloalkyl together with R 6 , R 7 and a carbon atom bonded thereto.
- the methyl, ethyl, alkoxy and cycloalkyl may be substituted with 1 to 5 fluorine atoms
- a hydrogen atom, methyl, ethyl, hydroxyl group, methoxy or Ethoxy is mentioned.
- a hydrogen atom, methyl, or a hydroxyl group is mentioned.
- R 8 and R 9 include a hydrogen atom, C 1-6 alkyl, C 3-7 cycloalkyl-C 1-3 alkyl or C 3-6 cycloalkyl (the alkyl, cycloalkylalkyl and cycloalkyl are Optionally substituted with 1 to 5 fluorine atoms).
- R 8 is preferably a hydrogen atom, methyl, ethyl or cyclopropyl. Particularly preferred is a hydrogen atom or methyl.
- R 9 is preferably a hydrogen atom, methyl or ethyl. Particularly preferred is a hydrogen atom or methyl.
- N may be an integer of 0 to 2, and preferably 0 or 2. Particularly preferably, 2 is mentioned.
- Epilepsy in the present invention is intended for chronic diseases in which cranial movements, consciousness, sensory abnormalities and behavioral abnormalities derived from excessive excitement of cranial nerve cells are intended, and simple partial seizures, complex partial seizures and secondary generalization Includes partial seizures such as seizures, absence seizures, myoclonic seizures, clonic seizures, tonic seizures, tonic clonic seizures, weak seizures, general seizures such as West syndrome and Lennox-Gastaut syndrome.
- the antiepileptic drug is intended to be a prophylactic and / or therapeutic drug for the above diseases
- the epilepsy treatment method is intended to be a preventive and / or therapeutic method for the above diseases.
- the bipolar disorder in the present invention means a disease that repeats a manic state and a depressive state to cause mood modulation
- the mood stabilizer for bipolar disorder in the present invention is a preventive and / or therapeutic agent for the above-mentioned diseases.
- a method for treating bipolar disorder intends a method for preventing and / or treating the above-mentioned diseases.
- the compound of the formula (I) of the present invention can be synthesized by the following procedure.
- the compound of formula (A) can also be synthesized and purified by introducing the substituents R a to R d into the starting material and performing the same procedure as described below.
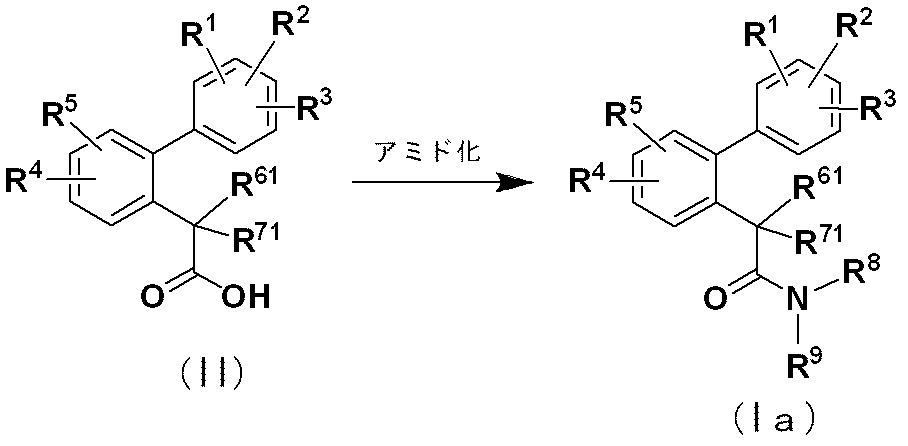
- R 1 to R 5 , R 8 and R 9 are the same as defined in item 1, and R 61 and R 71 are the same or different and represent a hydrogen atom, a fluorine atom, methyl, ethyl or C 1- Represents 6 alkoxy, or together with R 61 , R 71 and the carbon atom to which they are attached, constitutes a C 3-6 cycloalkyl.
- the amidation reaction of compound (II) can be performed according to a conventional method.
- this reaction is achieved by converting compound (II) into a reactive derivative (for example, a lower alkyl ester, an active ester, an acid anhydride, an acid halide, etc.) and reacting with ammonia or various amines.
- a reactive derivative for example, a lower alkyl ester, an active ester, an acid anhydride, an acid halide, etc.
- the active ester include p-nitrophenyl ester, N-hydroxysuccinimide ester, pentafluorophenyl ester and the like.
- Specific examples of the acid anhydride include mixed acid anhydrides with ethyl chlorocarbonate, isobutyl chlorocarbonate, isovaleric acid, pivalic acid and the like.
- Compound (Ia) can also be produced by reacting compound (II) with ammonia or various amines in the presence of a condensing agent.
- a condensing agent include N, N′-dicyclohexylcarbodiimide, 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide monohydrochloride, N, N′-carbonyldiimidazole, benzotriazol-1-yl -Oxytris (pyrrolidino) phosphonium-hexafluorophosphate and the like.
- These condensing agents can be used alone or in combination with these condensing agents and peptide synthesis reagents such as N-hydroxysuccinimide and N-hydroxybenzotriazole.
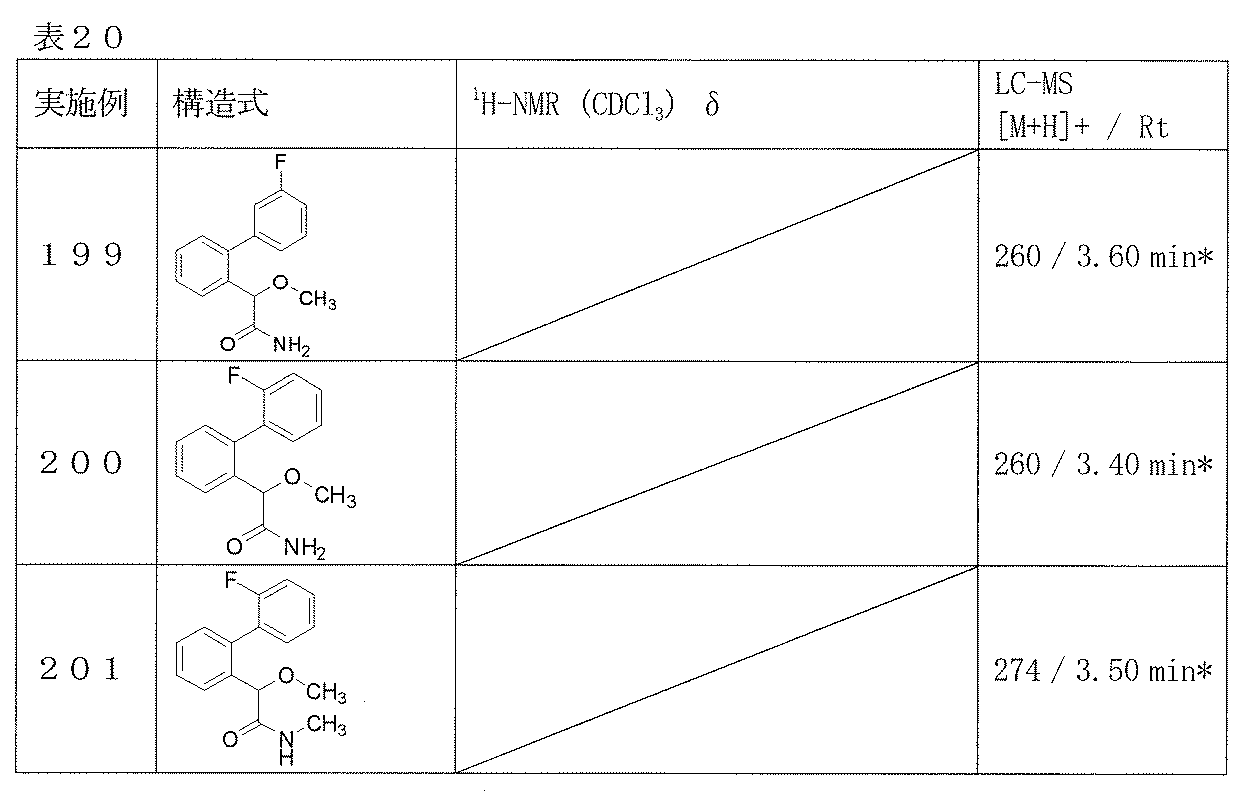
- the reaction of compound (II) with ammonia or various amines is carried out in a solvent or without a solvent.
- the solvent should be selected according to the type of raw material compound, etc., and include, for example, toluene, THF, dioxane, DME, dichloromethane, chloroform, ethyl acetate, acetone, acetonitrile, DMF, DMSO, etc. Alternatively, it can be used as a mixed solvent.
- Ammonia or various amines may be used in the form of an acid addition salt such as an aqueous solution or hydrochloride, and a free base may be generated in the reaction system. This reaction is usually carried out in the presence of a base.
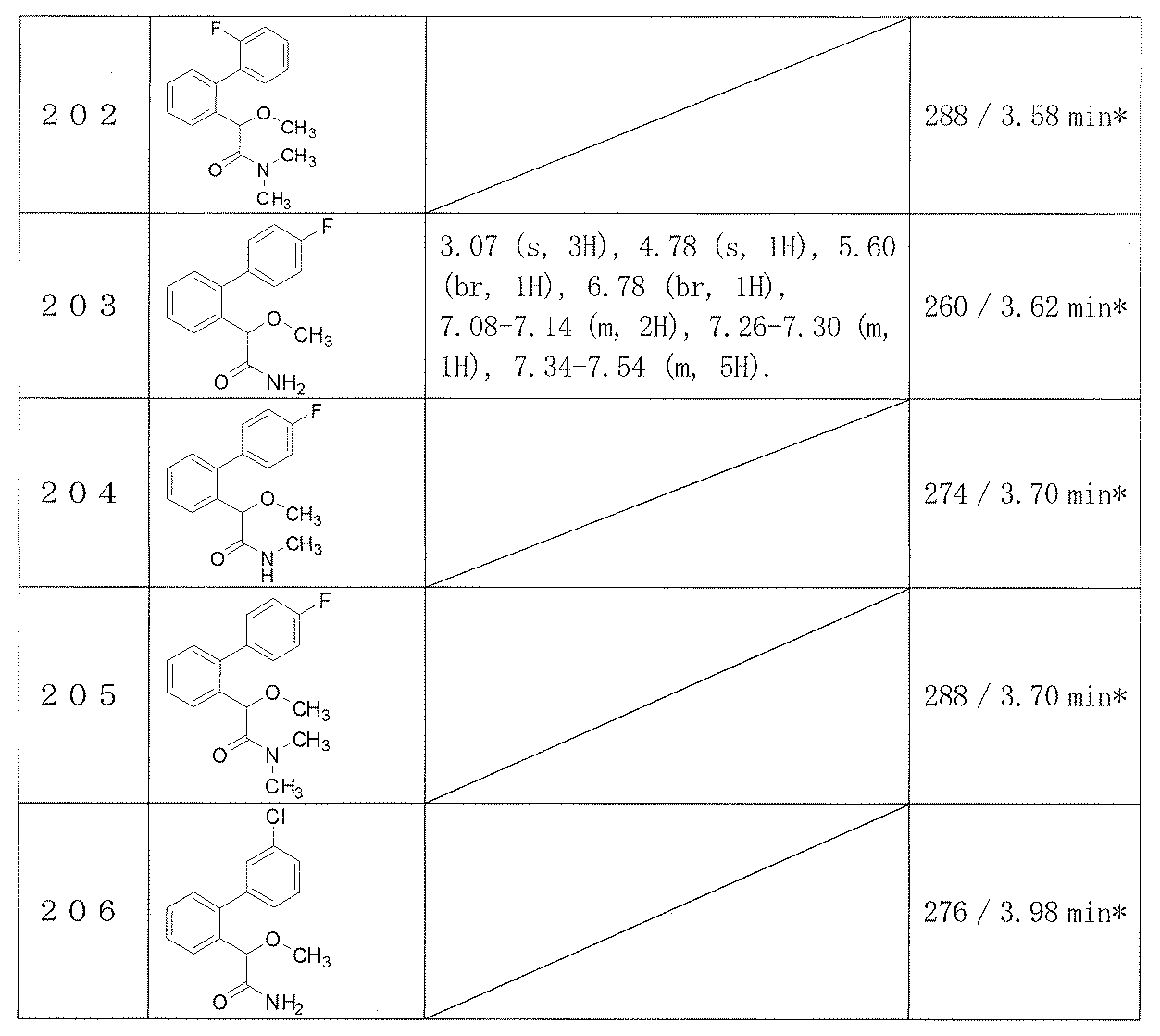
- the base used include inorganic bases such as potassium carbonate and sodium hydrogen carbonate, triethylamine, ethyldiisopropylamine, N-methylmorpholine, pyridine. And organic bases such as 4-dimethylaminopyridine. While the reaction temperature varies depending on the kind of raw material compound used, it is generally about ⁇ 30 ° C. to about 150 ° C., preferably about ⁇ 10 ° C. to about 70 ° C.
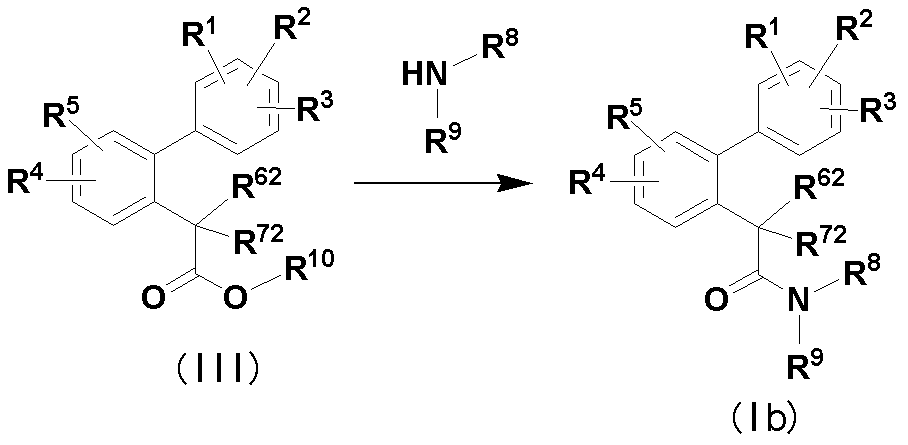
- R 1 to R 5 , R 8 and R 9 are the same as defined in item 1, and one of R 62 and R 72 is a hydroxyl group and the other is a hydrogen atom, methyl or ethyl. And R 10 is C 1-6 alkyl.
- the reaction of compound (III) with ammonia or various amines is carried out in a solvent or without a solvent.
- the solvent should be selected according to the type of the raw material compound, etc., for example, toluene, THF, dioxane, DME, dichloromethane, chloroform, ethyl acetate, acetone, acetonitrile, DMF, DMSO, methanol, ethanol, ethylene A glycol etc. are mentioned, It can use individually or as a mixed solvent.
- the reaction temperature varies depending on the kind of raw material compound used, it is generally about ⁇ 30 ° C. to about 150 ° C., preferably about ⁇ 10 ° C. to about 70 ° C.
- R 1 to R 5 , R 8 and R 9 are the same as defined in item 1, and R 61 and R 71 are the same or different and represent a hydrogen atom, a fluorine atom, methyl, ethyl or C 1- Represents 6 alkoxy, or together with R 61 , R 71 and the carbon atom bonded thereto, C 3-6 cycloalkyl is formed, X is a chlorine atom, a bromine atom, an iodine atom or a triflate (CF 3 SO 3- ).
- compound (VI) is coupled with various substituted phenylboronic acids, compound (Ic) is obtained.
- Compound (Ic) is prepared by mixing compound (VI) with a boron reagent such as various substituted phenylboronic acids [PhB (OH) 2 ], an organometallic reagent such as various substituted phenylzinc chlorides, and the like in a suitable solvent. It can be obtained by carrying out a cross-coupling reaction under a transition metal catalyst such as a palladium catalyst typified by phosphine palladium.
- inorganic bases such as alkali metal carbonates (for example, sodium carbonate, potassium carbonate, cesium carbonate, etc.) and potassium phosphate
- organic bases such as triethylamine, diisopropylethylamine, and chloride It can also be carried out in the presence of an inorganic salt such as lithium or cesium fluoride.
- the various boron reagents or organometallic reagents used in the coupling reaction with compound (VI) are commercially available, or are prepared from commercially available reagents according to known methods.
- Specific examples of the solvent should be selected according to the type of raw material compound and the type of reagent used. For example, toluene, THF, dioxane, DME, ethyl acetate, acetone, acetonitrile, DMF or methanol, ethanol, isopropanol, t -Alcohols such as butanol and water can be mentioned, and these can be used alone or as a mixed solvent. While the reaction temperature varies depending on the raw material compound used and the type of reagent, etc., it is generally 0 to 200 ° C., preferably 60 to 150 ° C.
- the compounds of the formulas (Ia), (Ib) and (Ic) produced by the above production methods 1, 2, and 3 can be isolated and purified by usual methods such as chromatography and recrystallization.
- the raw material compounds used in the above production methods 1, 2, and 3 can be produced by the following method.
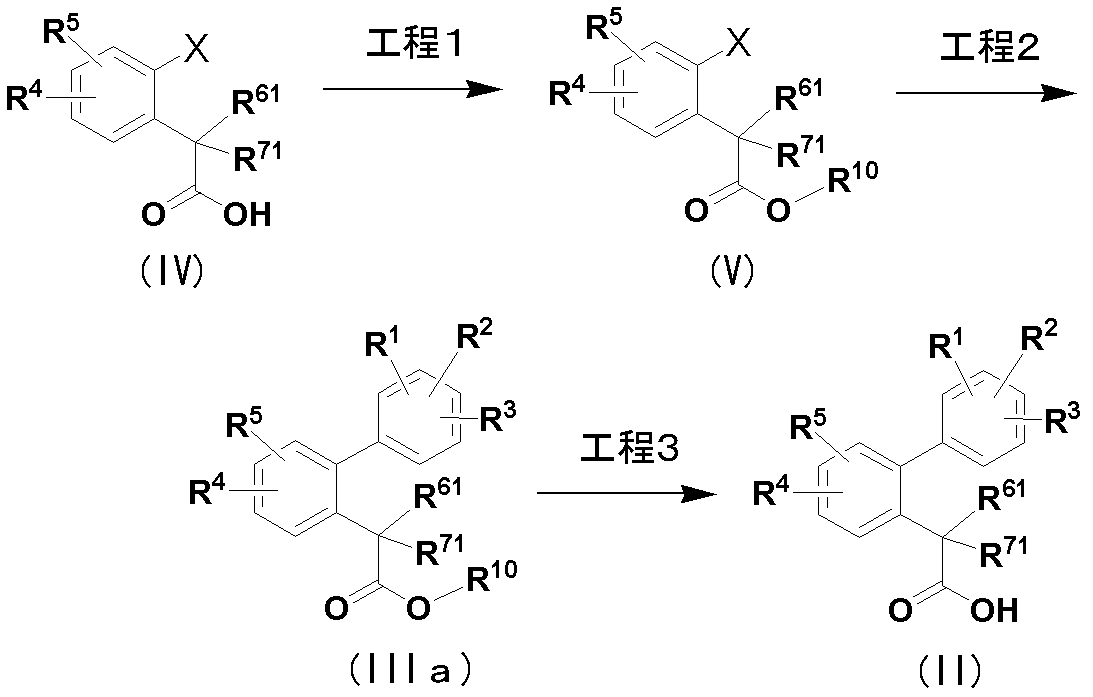
- Compound (II) used in Production Method 1 is produced according to the method shown by the following reaction formula.
- R 1 to R 5 , R 61 and R 71 are the same as defined in Production Method 1, R 10 is C 1-6 alkyl, and X is a chlorine atom, bromine atom, iodine atom or trif Rate (CF 3 SO 3- ).
- Step 1 Compound (V) is obtained by esterifying compound (IV).
- the esterification reaction in step 1 can be performed according to a conventional method. For example, this reaction is carried out by contacting compound (IV) with various alcohols or alkyl halides in an appropriate solvent under acidic or basic conditions.
- the compound (IV) can be synthesized by reacting a diazo compound such as diazomethane or trimethylsilyldiazomethane.
- the solvent should be selected according to the type of raw material compound, etc., and include, for example, alcohols such as THF, dioxane, DME, acetone, acetonitrile, DMF, toluene or methanol, ethanol, isopropanol, etc. Alternatively, it can be used as a mixed solvent.
- the acid used include mineral acids such as hydrochloric acid, sulfuric acid and hydrofluoric acid, and organic acids such as trifluoroacetic acid, methanesulfonic acid, trifluoromethanesulfonic acid and p-toluenesulfonic acid.
- the base used include alkali metal hydroxides such as sodium hydroxide, potassium hydroxide and lithium hydroxide, alkoxy alkali metals such as t-butoxy potassium, sodium carbonate, potassium carbonate and lithium carbonate.
- alkali metal carbonate is mentioned. While the reaction temperature varies depending on the raw material compound used and the type of reagent, etc., it is generally 0 to 150 ° C., preferably 20 to 100 ° C.
- Step 2 Compound (IIIa) is compound (V) and boron reagent such as various substituted phenylboronic acids [PhB (OH) 2 ], organometallic reagents such as various substituted phenyl zinc chlorides, etc. in an appropriate solvent.
- boron reagent such as various substituted phenylboronic acids [PhB (OH) 2 ], organometallic reagents such as various substituted phenyl zinc chlorides, etc.
- boron reagent such as various substituted phenylboronic acids [PhB (OH) 2 ]
- organometallic reagents such as various substituted phenyl zinc chlorides, etc.
- a transition metal catalyst such as a palladium catalyst typified by tetrakistriphenylphosphine palladium.
- inorganic bases such as alkali metal carbonates (for example, sodium carbonate, potassium carbonate, cesium carbonate, etc.) and potassium phosphate, organic bases such as triethylamine, diisopropylethylamine, and chloride
- organic bases such as triethylamine, diisopropylethylamine, and chloride
- an inorganic salt such as lithium or cesium fluoride.
- the compound (IIIa) in which R 61 and R 71 are fluorine can also be synthesized using a known method or a method analogous thereto. (WO2009019633, US2004254166, Tetrahedron Letters, 27, 6103-6106 (1986))
- boron reagents or organometallic reagents used in Step 2 are commercially available, or are prepared from commercially available reagents according to a known method.
- Specific examples of the solvent should be selected according to the type of raw material compound and the type of reagent used. For example, toluene, THF, dioxane, DME, ethyl acetate, acetone, acetonitrile, DMF or methanol, ethanol, isopropanol, t -Alcohols such as butanol and water can be mentioned, and these can be used alone or as a mixed solvent. While the reaction temperature varies depending on the raw material compound used and the type of reagent, etc., it is generally 0 to 200 ° C., preferably 60 to 150 ° C.
- Step 3 Compound (II) is obtained by hydrolyzing compound (IIIa).
- the hydrolysis reaction in step 3 can be performed according to a conventional method. For example, this reaction is carried out by contacting compound (IIIa) with water in an appropriate solvent under acidic or basic conditions.
- the solvent should be selected according to the type of the raw material compound, and include, for example, THF, dioxane, DME, acetone, acetonitrile, DMF, DMSO or alcohols such as methanol, ethanol, isopropanol, and water.
- the acid used include mineral acids such as hydrochloric acid and sulfuric acid.
- the base used include alkali metal hydroxides such as sodium hydroxide, potassium hydroxide and lithium hydroxide, alkoxy alkali metals such as t-butoxy potassium, sodium carbonate, potassium carbonate and lithium carbonate.
- alkali metal carbonate is mentioned.
- the reaction temperature varies depending on the raw material compound used and the type of reagent, etc., it is generally 0 to 150 ° C., preferably 20 to 100 ° C.
- compound (II) can also be produced by subjecting compound (IV) to the same reaction as in step 1 without passing through steps 2 and 3.
- the compound (III) used in the production method 2 is produced in the same manner as the method represented by the above reaction formula.
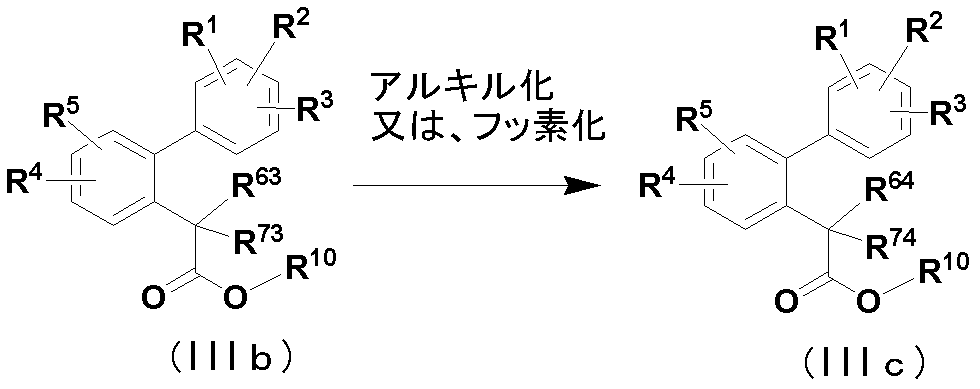
- the compound (IIIa) [compound of the following formula (IIIc)] in which R 61 and / or R 71 used in the above method is a C 1-6 alkyl or fluorine atom is produced according to the method shown in the following reaction formula.
- R 1 to R 5 are the same as defined in item 1, and at least one of R 63 and R 73 is a hydrogen atom or a hydroxyl group, and when only one is a hydrogen atom or a hydroxyl group, the other is Is the same as the definition of R 6 and R 7 in Item 1, wherein at least one of R 64 and R 74 is C 1-6 alkyl or fluorine, and the other is the definition of R 6 and R 7 in Item 1.
- R 10 is C 1-6 alkyl.
- the alkylation or fluorination reaction can be performed according to a conventional method.
- compound (IIIb) and an alkyl halide, dimethyl sulfate, (diethylamino) sulfur trifluoride, bis (2-methoxyethyl) aminosulfur trifluoride and the like are obtained under neutral or basic conditions in an appropriate solvent. This is done by contacting them.
- Specific examples of the solvent should be selected according to the type of raw material compound, etc. For example, alcohols such as toluene, hexane, diethyl ether, THF, dioxane, DME, acetone, acetonitrile, DMF or methanol, ethanol, isopropanol, etc.
- alkali metal hydroxides such as sodium hydroxide, potassium hydroxide and lithium hydroxide
- alkali metal hydrides such as sodium hydride and potassium hydride
- sodium methoxide sodium ethoxide
- Alkoxy alkali metals such as t-butoxy potassium
- alkali carbonates such as sodium carbonate, potassium carbonate and lithium carbonate, lithium diisopropylamide, lithium hexamethyldisilazide, sodium hexamethyldisilazide, potassium hexamethyldisilazide, etc.
- reaction temperature varies depending on the raw material compound used and the type of reagent, etc., it is generally ⁇ 78 to 150 ° C., preferably ⁇ 20 to 70 ° C.
- Compound (IIIb) is produced by a method similar to the production method of compound (IIIa).
- Compound (II) can also be produced from compound (IIIc) by a method similar to the method of deriving compound (IIIa) to compound (II) (step 3).
- Compound (IV) is a commercially available compound, or can be produced from a known compound according to a known method.
- Compound (VI) used in Production Method 3 can be obtained, for example, by subjecting Compound (IV) to an amidation reaction in Production Method 1 or 2.
- the compound of formula (I) produced by each of the above production methods can be isolated and purified by conventional methods such as chromatography, recrystallization and reprecipitation.
- chromatography recrystallization and reprecipitation.
- the compound of formula (I) is a racemate, according to a conventional method such as an optical resolution method by chromatography using an optically active column, a preferential crystallization method, a diastereomer method, etc. And can be separated and purified.
- the novel biphenylacetamide derivative of the present invention is useful as an antiepileptic drug as described later.
- the administration route of the compound of the present invention may be any of oral administration, parenteral administration and rectal administration, and the daily dose varies depending on the type of compound, administration method, patient symptom / age and the like.
- oral administration it is usually 1 to 200 mg, preferably 5 to 100 mg, more preferably 10 to 70 mg per kg body weight of the mammal, and in the case of human, usually 50 to 5000 mg, preferably 100 to 4000 mg, more preferably 500. Up to 3000 mg can be administered in 1 to several divided doses.
- parenteral administration such as intravenous injection
- it is usually 0.1 to 100 mg, preferably 1 to 10 mg, more preferably 4 to 5 mg, more preferably 50 to 1000 mg, preferably 100 to 400 mg per kg body weight of a mammal. More preferably, 200 to 300 mg can be administered.
- the compound of the present invention is usually administered in the form of a preparation prepared by mixing with a pharmaceutical carrier when used for pharmaceutical use as described above.
- a pharmaceutical carrier a non-toxic substance that is commonly used in the pharmaceutical field and does not react with the compound of the present invention is used.
- citric acid glutamic acid, glycine, lactose, inositol, glucose, mannitol, dextran, sorbitol, cyclodextrin, starch, partially pregelatinized starch, sucrose, methyl paraoxybenzoate, propyl paraoxybenzoate, and aluminate metasilicate
- Magnesium sulfate, synthetic aluminum silicate crystalline cellulose, sodium carboxymethylcellulose, hydroxypropyl starch, carboxymethylcellulose calcium, ion exchange resin, methylcellulose, gelatin, gum arabic, pullulan, hydroxypropylcellulose, low-substituted hydroxypropylcellulose, hydroxypropylmethylcellulose , Polyvinylpyrrolidone, polyvinyl alcohol, alginic acid, sodium alginate Light anhydrous silicic acid, magnesium stearate, talc, tragacanth, bentonite, bee gum, carboxyvinyl
- Examples of the dosage form include tablets, capsules, granules, powders, syrups, suspensions, injections, suppositories, eye drops, ointments, coatings, patches, inhalants and the like. These preparations can be prepared according to a conventional method. In the case of a liquid preparation, it may be dissolved or suspended in water or other appropriate medium at the time of use. Tablets and granules may be coated by a known method. In addition, these formulations may contain other therapeutically valuable ingredients.
- s is a single line
- d is a double line
- t is a triple line
- q is a quadruple line
- m is a multiple line
- br is a broad line.
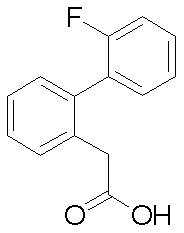
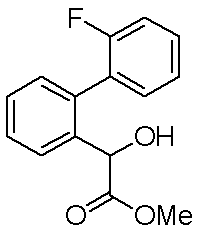
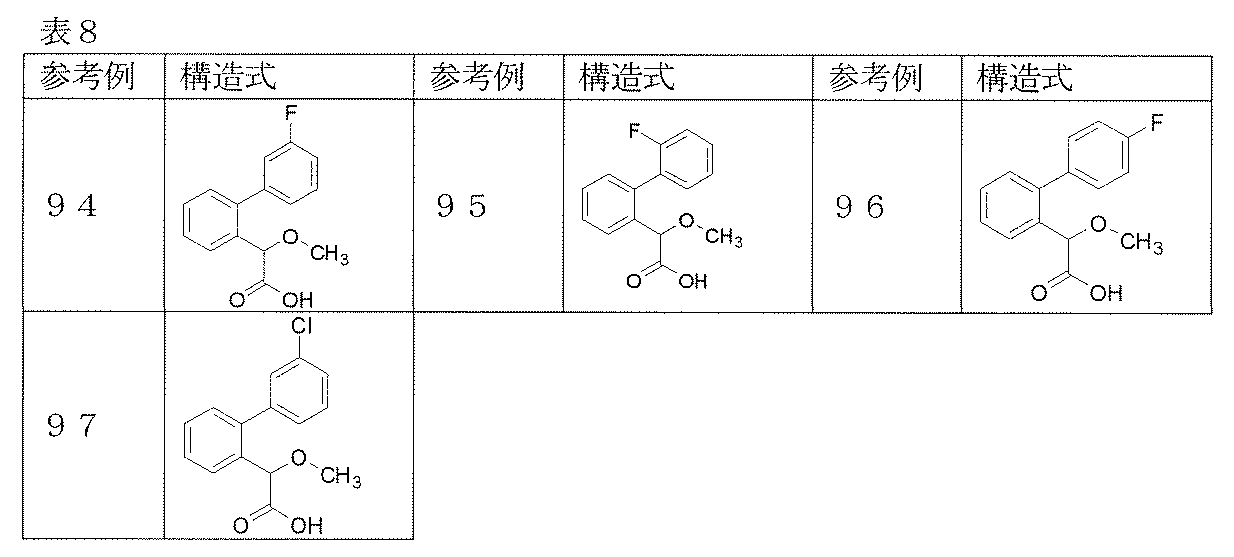
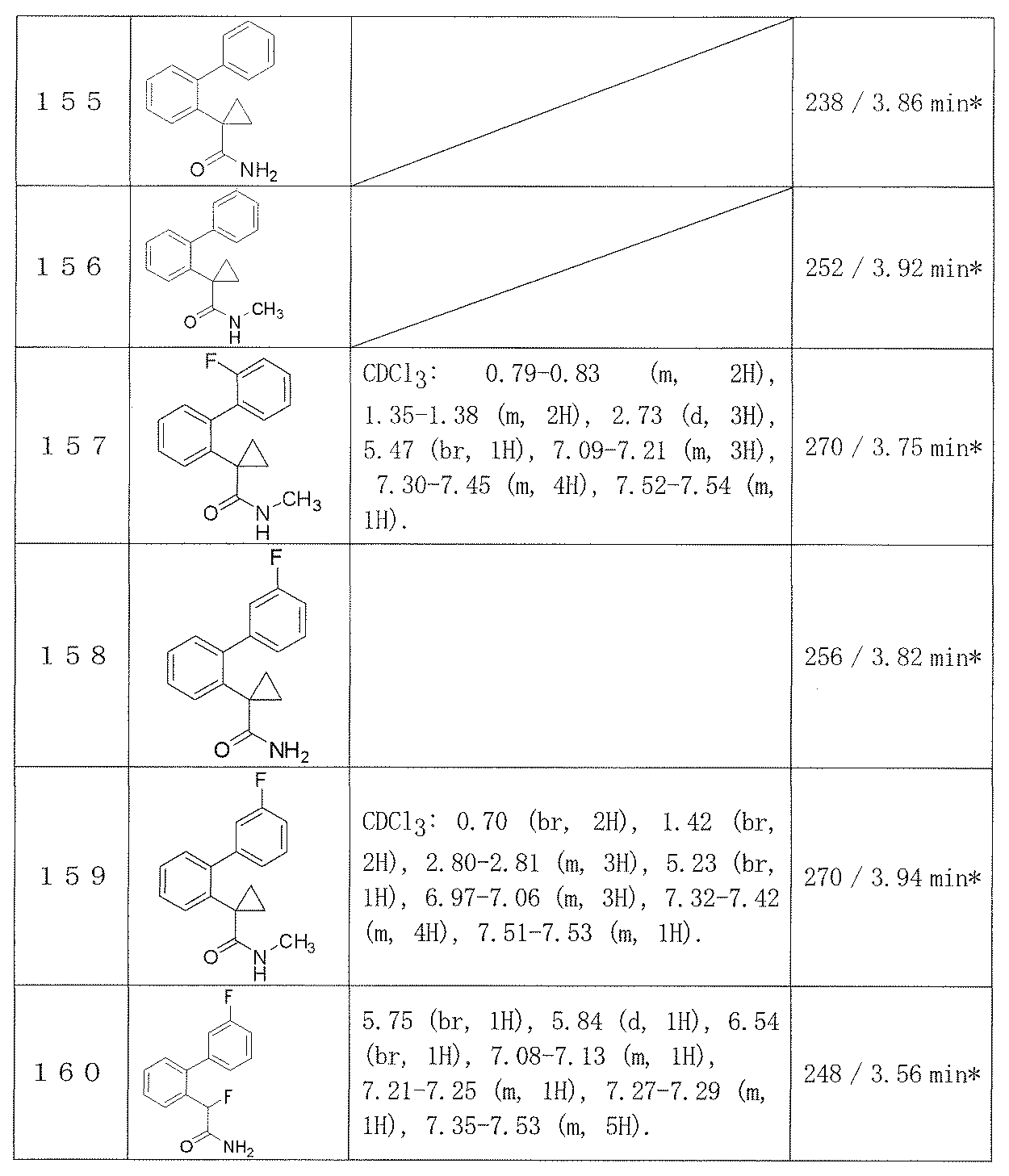
- Reference example 1 (2'-Fluorobibiphenyl-2-yl) acetic acid
- Step 1 To a solution of 2-bromophenylacetic acid (12.8 g) and potassium carbonate (16.4 g) in DMF (50 ml), a solution of ethyl iodide (5.7 ml) in DMF (20 ml) is added dropwise at 0 ° C. The mixture was warmed to room temperature and stirred overnight. After completion of the reaction, water was added to the reaction solution and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate and concentrated. The residue was purified by silica gel column chromatography to give ethyl (2-bromophenyl) acetate (13.3 g) as an oil.
- Step 2 Ethyl (2-bromophenyl) acetate (4.01 g), 2-fluorophenylboronic acid (3.46 g), tetrakis (triphenylphosphine) palladium (0) (0.95 g), potassium carbonate ( 6.82 g) of dioxane-water (10: 1) (44 ml) was heated to reflux at 110 ° C. for 8 hours under a nitrogen stream. After completion of the reaction, the reaction mixture was cooled to room temperature, water was added and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate and concentrated. The residue was purified by silica gel column chromatography to obtain ethyl (2'-fluorobiphenyl-2-yl) acetate (3.87 g) as an oil.
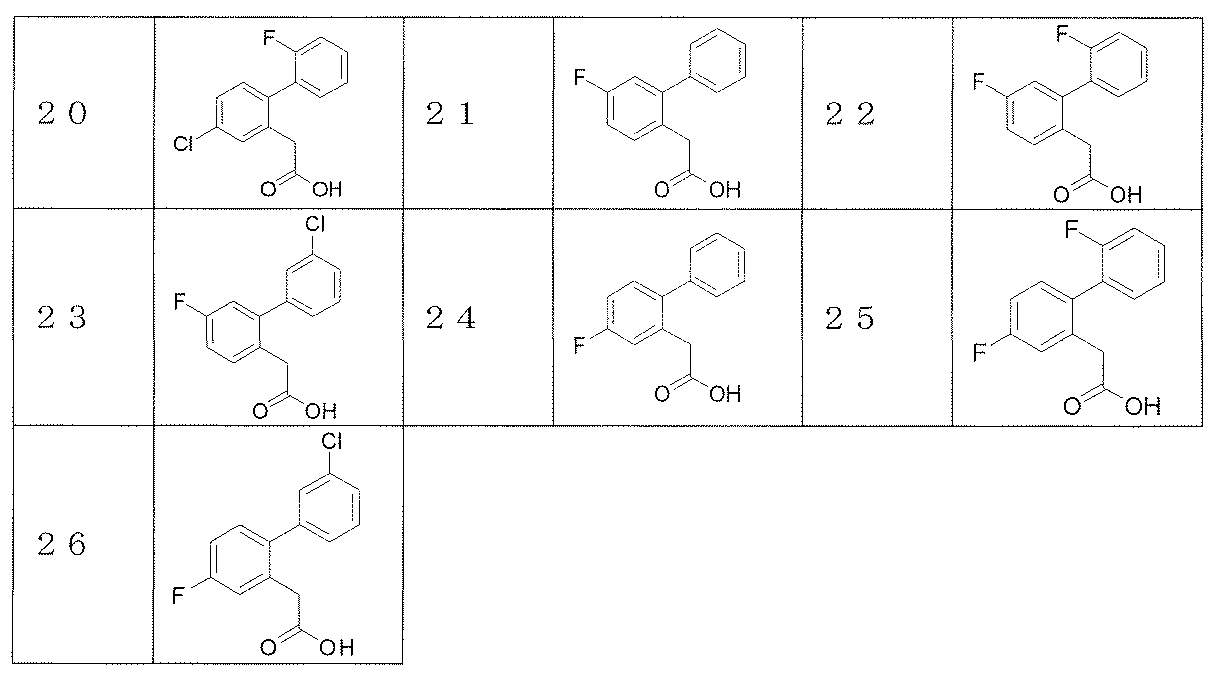
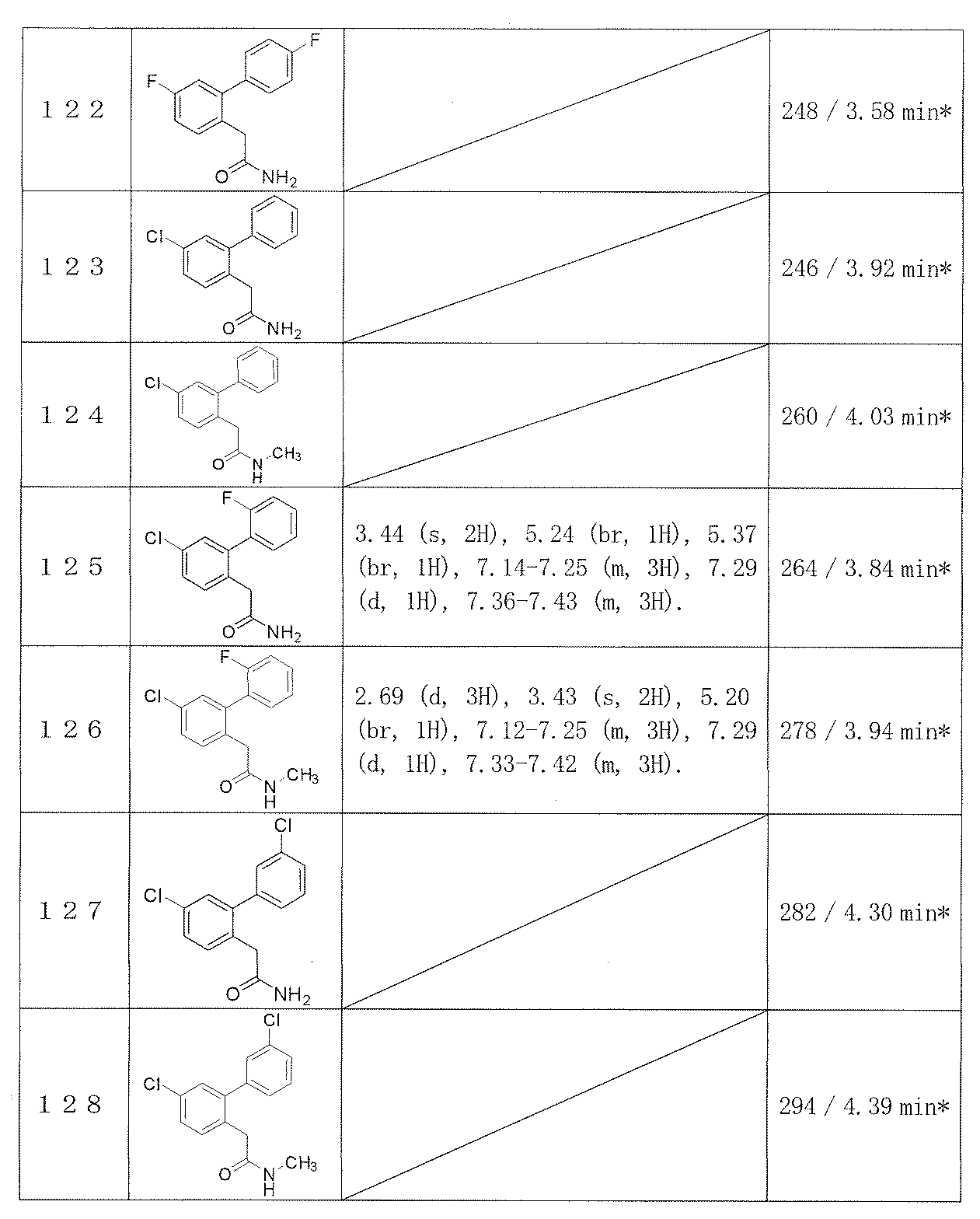
- Reference Example 2-26 The corresponding starting materials were used and reacted and treated in the same manner as described in Reference Example 1 to obtain the compounds shown in Table 1.
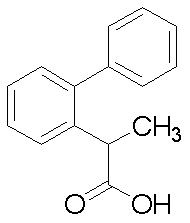
- Step 2 Ethyl 2- (biphenyl-2-yl) propanoate (1.20 g) in THF (5.0 ml) was added to lithium hydroxide monohydrate (0.24 g) in water (5.0 ml). ) Solution and methanol (5.0 ml) were added and stirred at room temperature for 12 hours. AMBERLITE (registered trademark) IR-120 PLUS (H) was added to adjust the solution to pH 4, followed by filtration, and the filtrate was concentrated. The precipitated crystals were collected by filtration to give 2- (biphenyl-2-yl) propanoic acid (1.03 g) as crystals.
- 1 H-NMR (CDCl 3 ) ⁇ : 1.38 (d, 3H), 3.93 (q, 1H), 7.24-7.28 (m, 2H), 7.29 (dd, 1H), 7.34-7.45 (m, 7H).
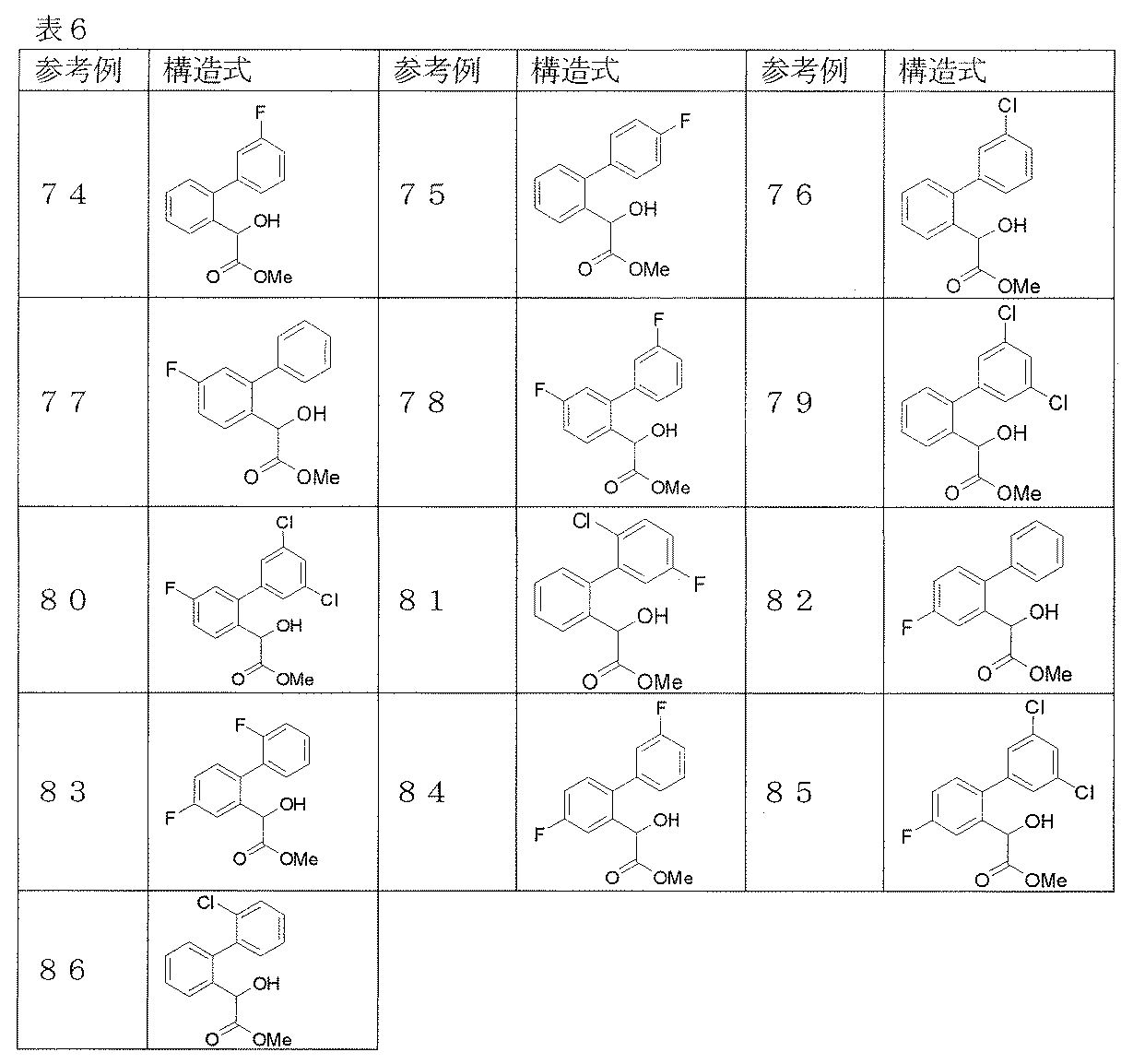
- Step 1 (2-Bromophenyl) (hydroxy) acetic acid (7.00 g) is dissolved in a mixture of toluene (20 ml) and methanol (20 ml), and trimethylsilyldiazomethane (2 mol / l hexane solution) (23 ml) is added dropwise. Then, it stirred at room temperature for 1 hour. After evaporating the solvent under reduced pressure, the residue was purified by silica gel column chromatography to obtain methyl (2-bromophenyl) (hydroxy) acetate (4.12 g) as an oil.
- Step 2 Methyl biphenyl-2-yl (methoxy) acetate (1.61 g) in THF (10 ml) solution, lithium hydroxide monohydrate (0.49 g) in water (10 ml) and methanol ( 10 ml) was added and stirred at room temperature overnight. A 10% aqueous citric acid solution was added to adjust the pH of the reaction solution to 3, followed by extraction with ethyl acetate. The extract was washed with saturated brine and dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give biphenyl-2-yl (methoxy) acetic acid (1.52 g) as an oil.
- 1 H-NMR (CDCl 3 ) ⁇ : 3.19 (s, 3H), 4.94 (s, 1H), 7.31-7.35 (m, 1H), 7.40-7.51 (m , 8H).
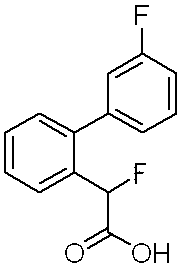
- Step 2 Ethyl fluoro (3′-fluorobiphenyl-2-yl) acetate (1.46 g) was dissolved in tetrahydrofuran (10 ml), and lithium hydroxide monohydrate (0.67 g) in water (10 ml) ) Solution and methanol (10 ml) were added and stirred at room temperature for 6 hours. A 10% aqueous citric acid solution was added to adjust the reaction solution to pH 3, followed by extraction with ethyl acetate.
- Step 1 Methyl (2′-fluorobiphenyl-2-yl) hydroxyacetate (1.70 g) was dissolved in dichloromethane (20 ml), and [bis (2-methoxyethyl) -amino] sulfur trifluoro under ice-cooling. Lido (2.0 ml) was added and stirred at room temperature overnight. After washing with ice water and drying over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure.
- Step 2 Methyl fluoro (2′-fluorobiphenyl-2-yl) acetate (1.33 g) was dissolved in tetrahydrofuran (10 ml), and lithium hydroxide monohydrate (0.64 g) in water (10 ml) ) Solution and methanol (10 ml) were added and stirred at room temperature for 6 hours. 2N-hydrochloric acid was added to adjust to pH 3, followed by extraction with ethyl acetate.
- Step 2 Ethyl 2-bromophenyl-fluoroacetate (1.20 g) and 3-chlorophenylboronic acid (1.08 g) are dissolved in 1,4-dioxane (20 ml), and tetrakis (triphenylphosphine) palladium ( 0.27 g), 3-chlorophenylboronic acid (1.08 g), potassium carbonate (1.91 g) and water (2 ml) were added, and the atmosphere was replaced with nitrogen, followed by stirring overnight at 80 ° C. with heating. Water was added, the mixture was extracted with ethyl acetate, washed with saturated brine, and dried over anhydrous magnesium sulfate.
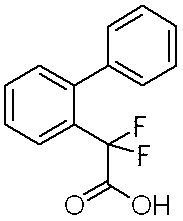
- Step 1 Ethyl biphenyl-2-yl (difluoro) acetic acid
- Step 1 Ethyl biphenyl-2-yl (difluoro) acetate (1.59 g) is dissolved in tetrahydrofuran (10 ml), a solution of lithium hydroxide monohydrate (0.72 g) in water (10 ml) and methanol (10 ml) are added. Stir overnight at room temperature. A 10% aqueous citric acid solution was added to adjust the reaction solution to pH 3, followed by extraction with ethyl acetate.
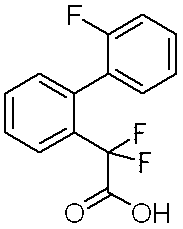
- Step 1 (2′-Fluorobiphenyl-2-yl) difluoroacetic acid
- Step 1 Ethyl (2′-fluorobiphenyl-2-yl) acetate (1.5 g) was dissolved in tetrahydrofuran (20 ml) under a nitrogen atmosphere, cooled to ⁇ 78 ° C., and then sodium hexamethyldisilazide. (1.06 M toluene solution) (12.1 ml) was added and stirred at that temperature for 20 minutes.
- Step 2 Ethyl (2′-fluorobiphenyl-2-yl) difluoroacetate (973 mg) was dissolved in tetrahydrofuran (5 ml), a solution of lithium hydroxide monohydrate (416 mg) in water (5 ml), methanol ( 5 ml) was added and stirred at room temperature for 6 hours. A 10% aqueous citric acid solution was added to adjust the pH to 3, followed by extraction with ethyl acetate. The extract was washed with saturated brine and dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give (2′-fluorobiphenyl-2-yl) difluoroacetic acid (792 mg) as white crystals.
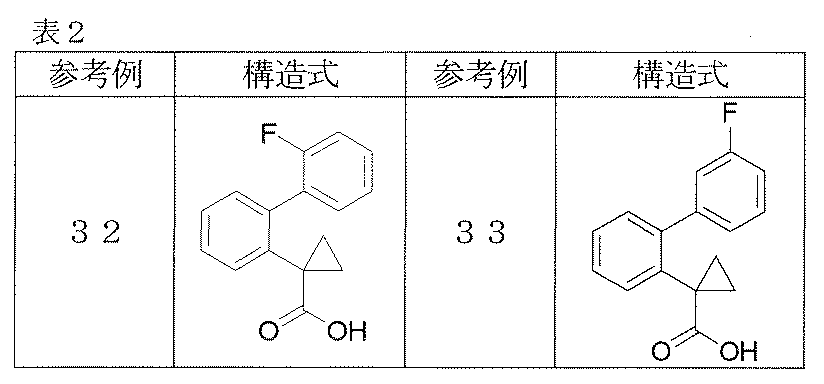
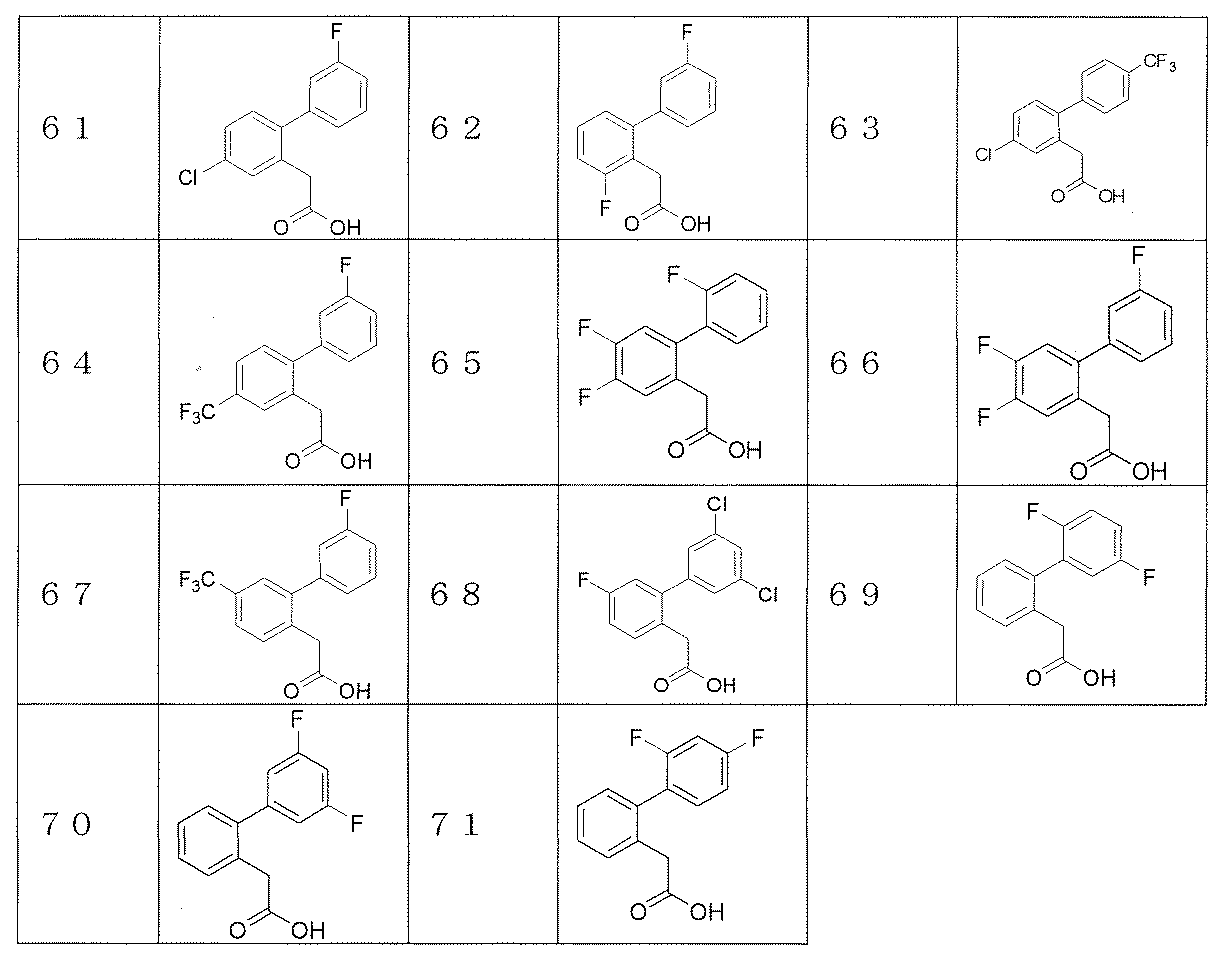
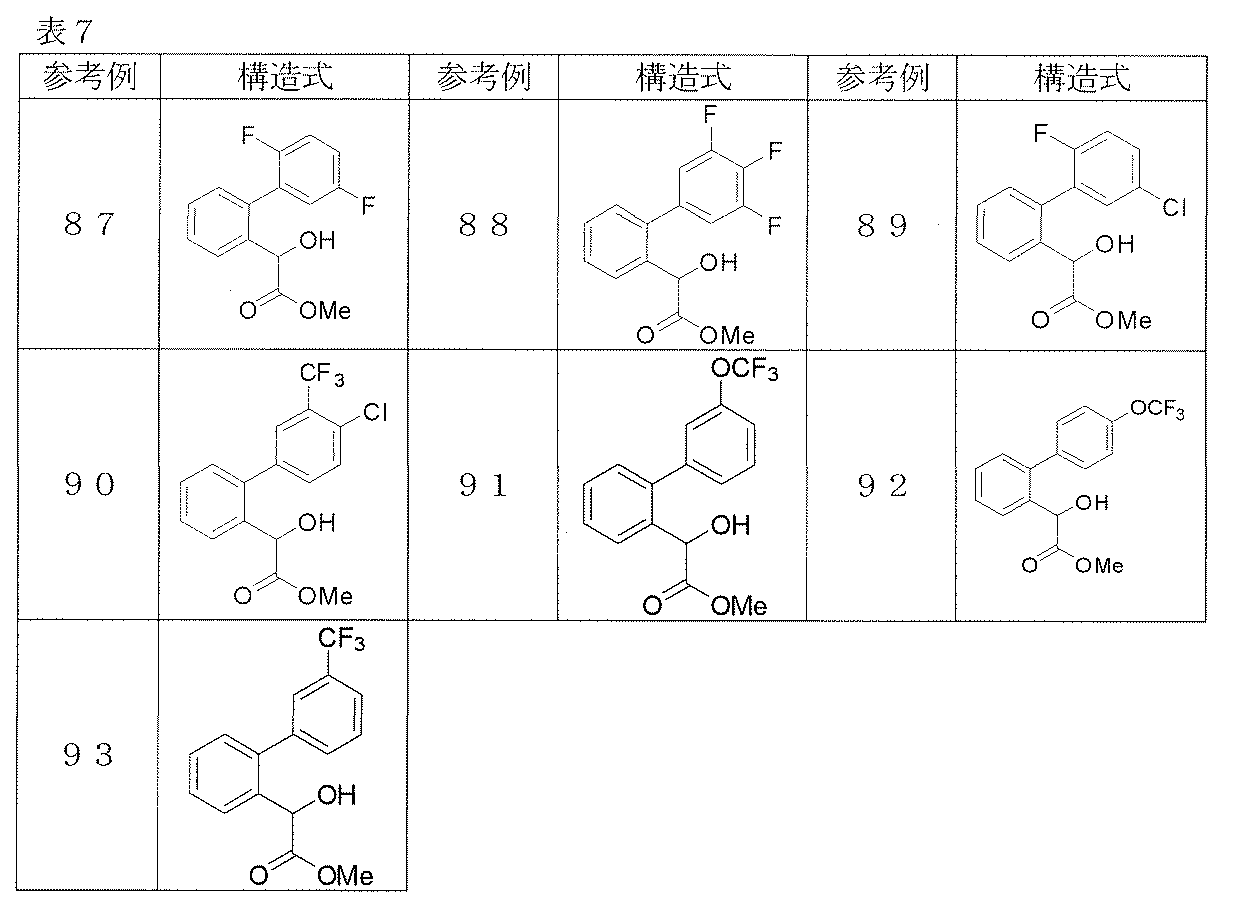
- Reference examples 41 and 42 The corresponding starting materials were used and reacted and treated in the same manner as in Reference Example 40 to obtain the compounds shown in Table 3.
- Example 1 2- (2′-Fluorobiphenyl-2-yl) acetamide (2′-Fluorobiphenyl-2-yl) acetic acid (0.96 g) obtained in Reference Example 1 was suspended in dichloromethane (8.0 ml), and DMF (0.05 ml) and oxalyl chloride (0.43 ml) were added. After the addition, the mixture was stirred at room temperature for 1 hour and concentrated. The obtained pale yellow crystals were dissolved in THF (3.0 ml), 28% aqueous ammonia solution (2.0 ml) was added, and the mixture was stirred at room temperature overnight.
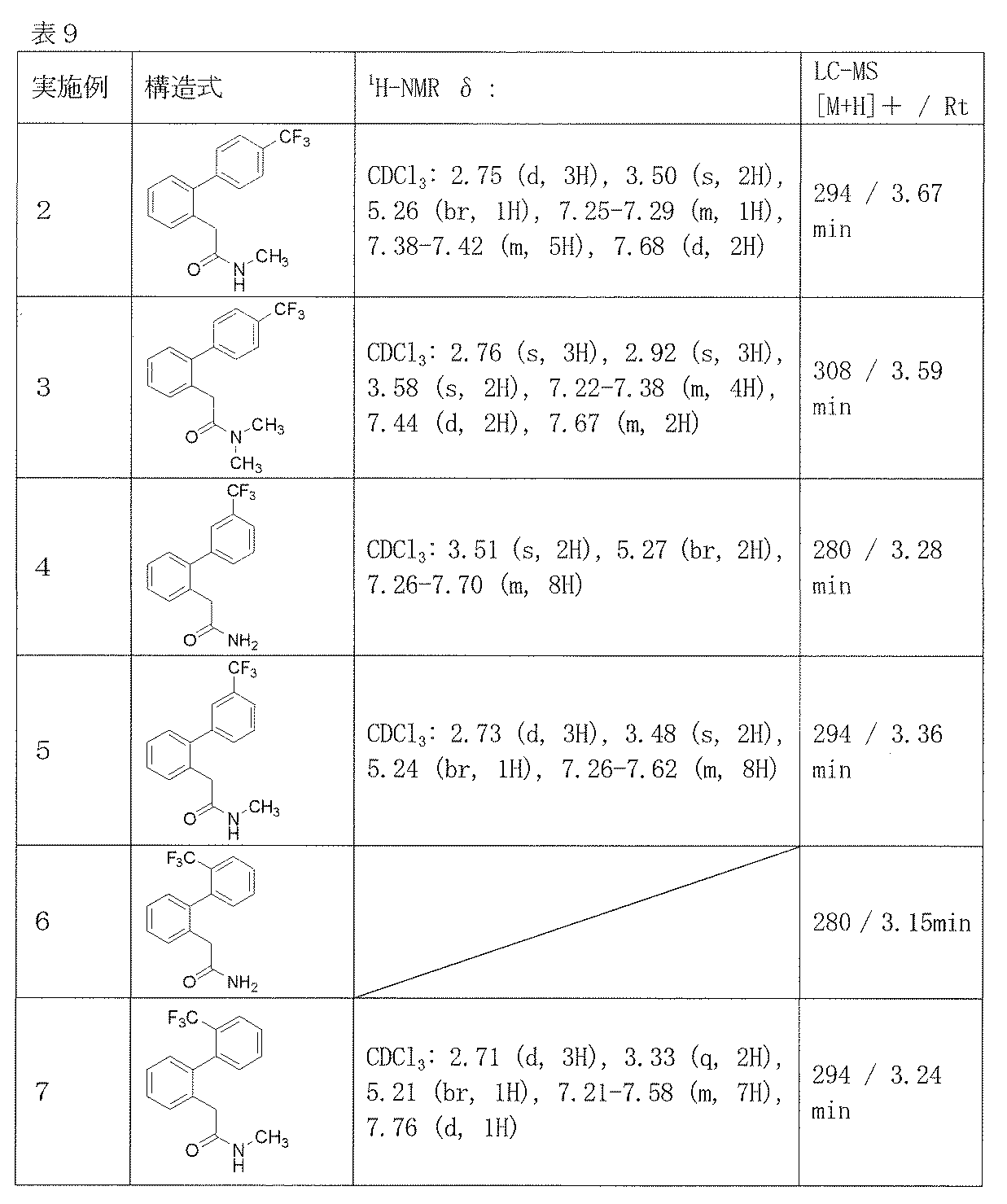
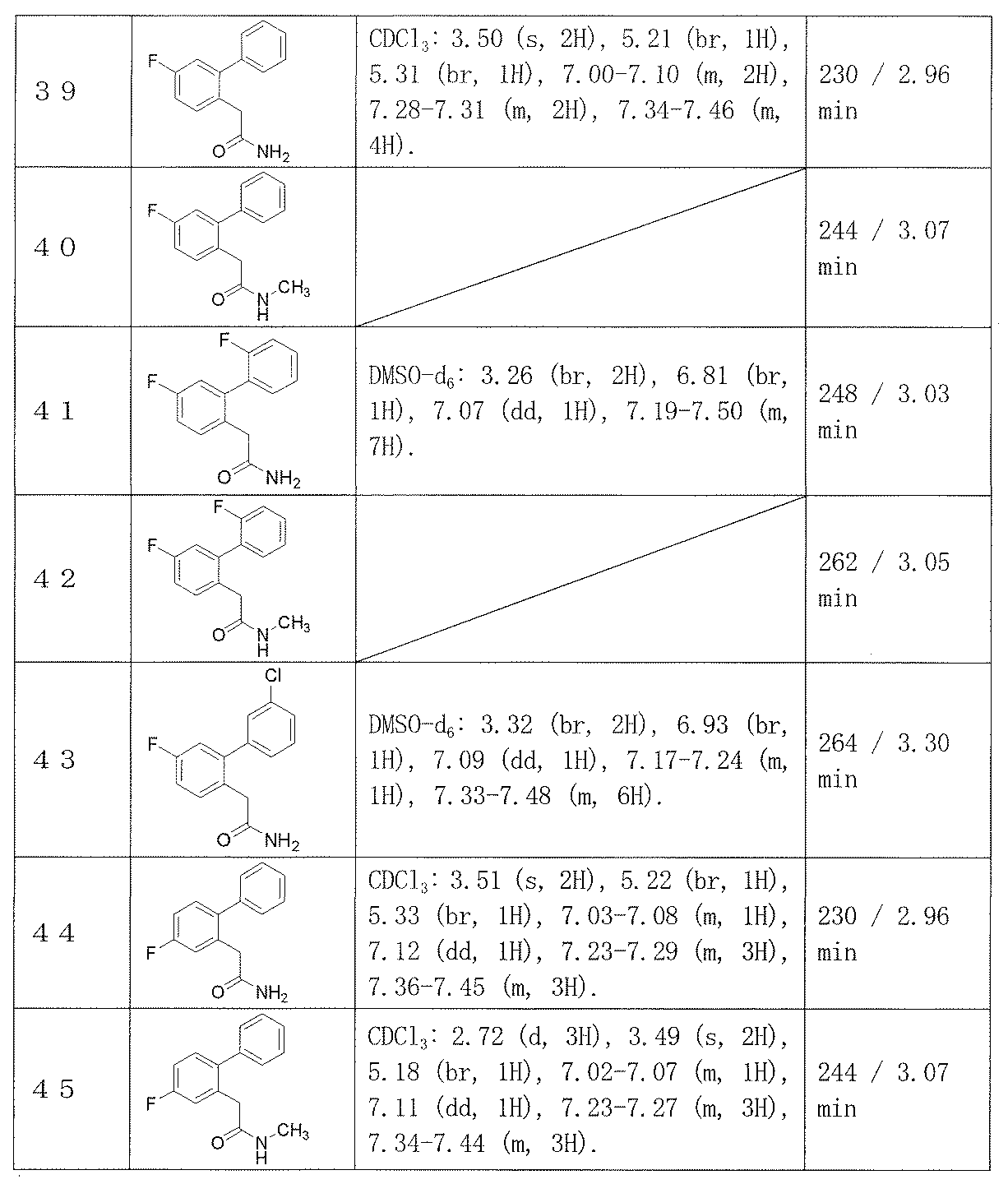
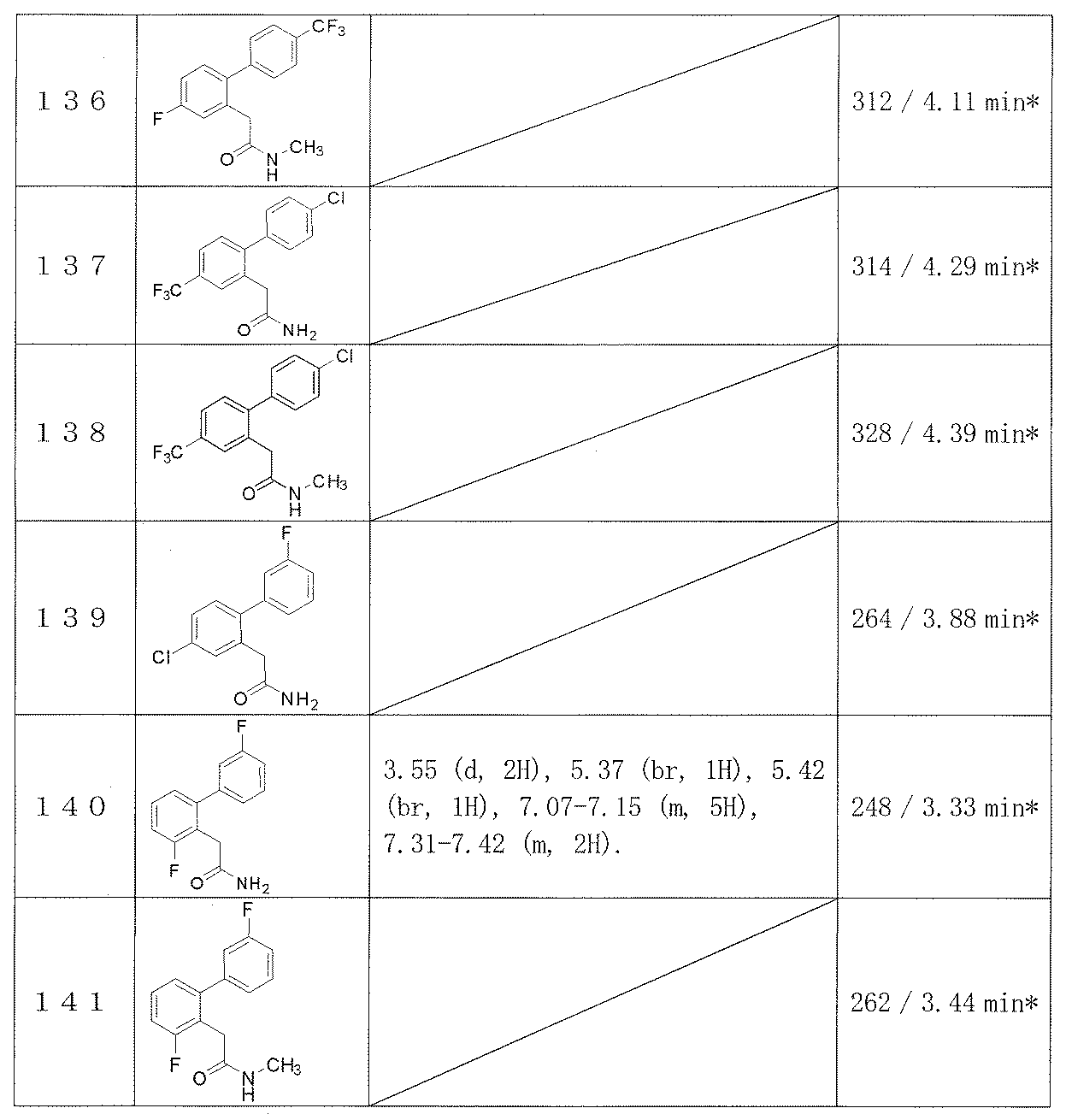
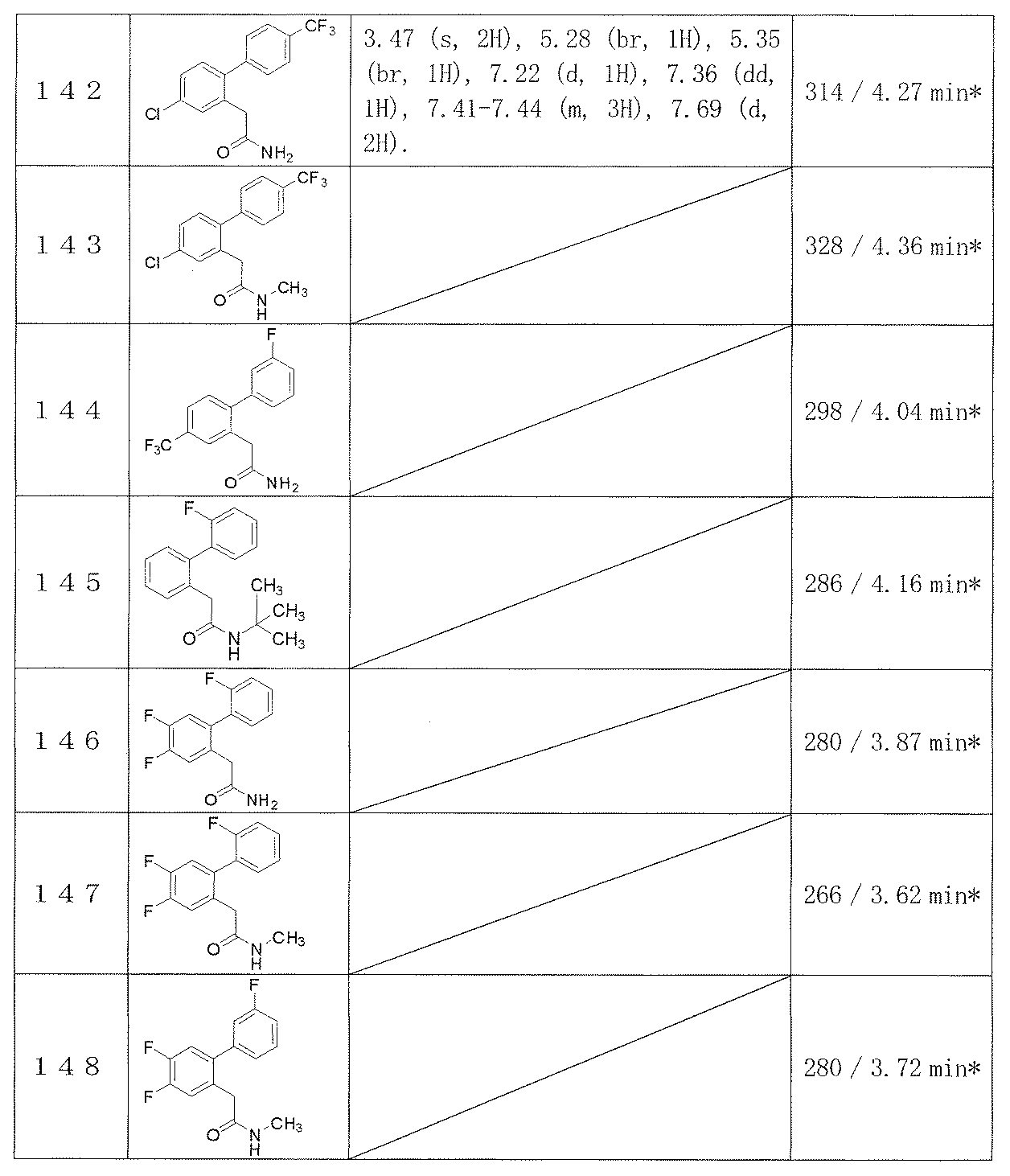
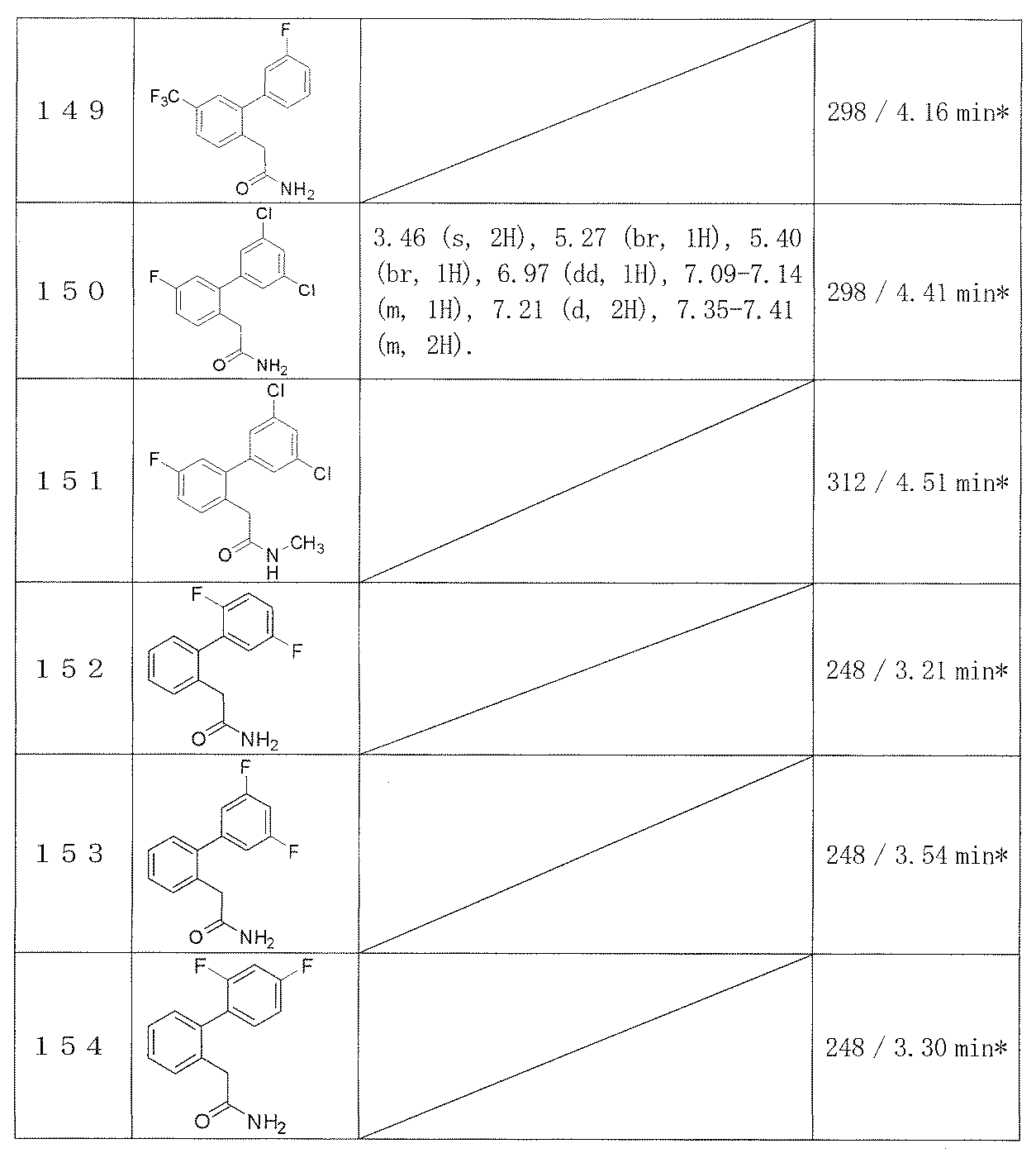
- Example 2-48 The corresponding starting materials were used and reacted in the same manner as in Example 1 to obtain the compounds shown in Table 9.
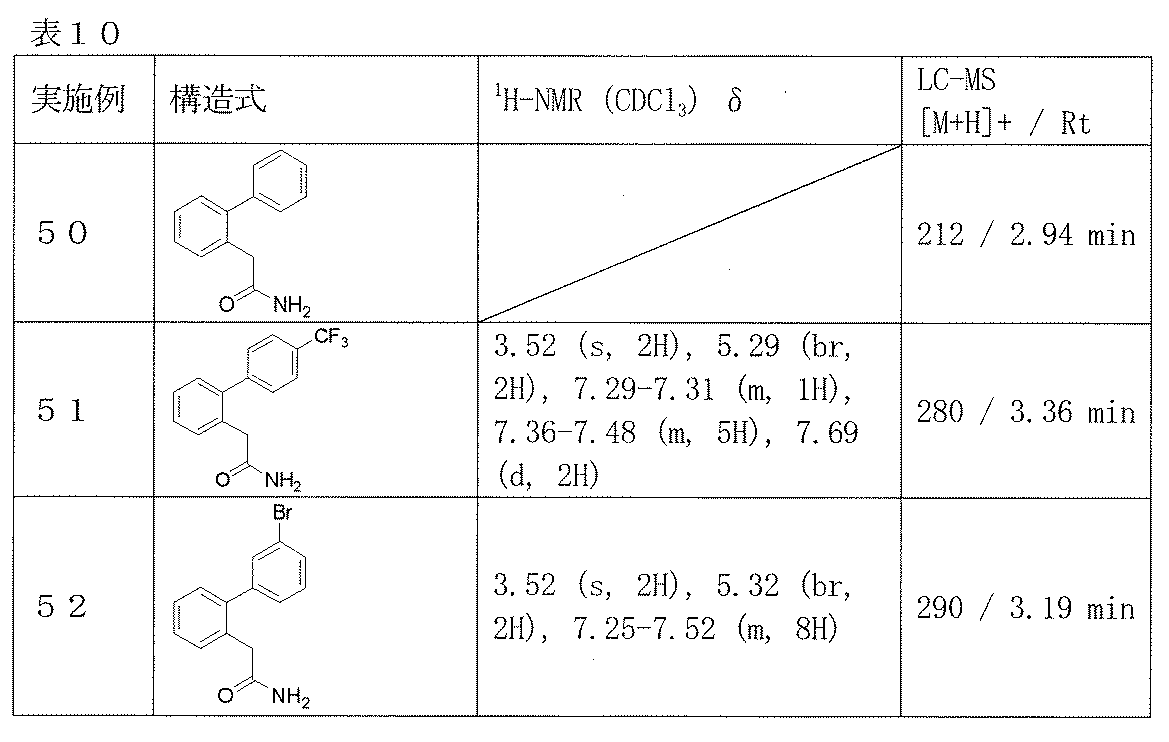
- Examples 50-52 Reaction and treatment were carried out in the same manner as in Example 49 using the corresponding starting compounds, and the compounds shown in Table 10 were obtained.
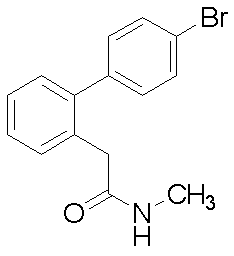
- Example 53 2- (4′-Bromobiphenyl-2-yl) -N-methylacetamide 2- (4′-bromobiphenyl-2-yl) acetic acid (391 mg), 1-ethyl-3- (3-dimethylaminopropylcarbodiimide) hydrochloride (515 mg), 1-hydroxybenzotriazole obtained in Reference Example 15 A solution of monohydrate (363 mg), methylamine hydrochloride (272 mg), N, N-diisopropylethylamine (0.70 ml) in DMF (10 ml) was stirred at room temperature overnight.
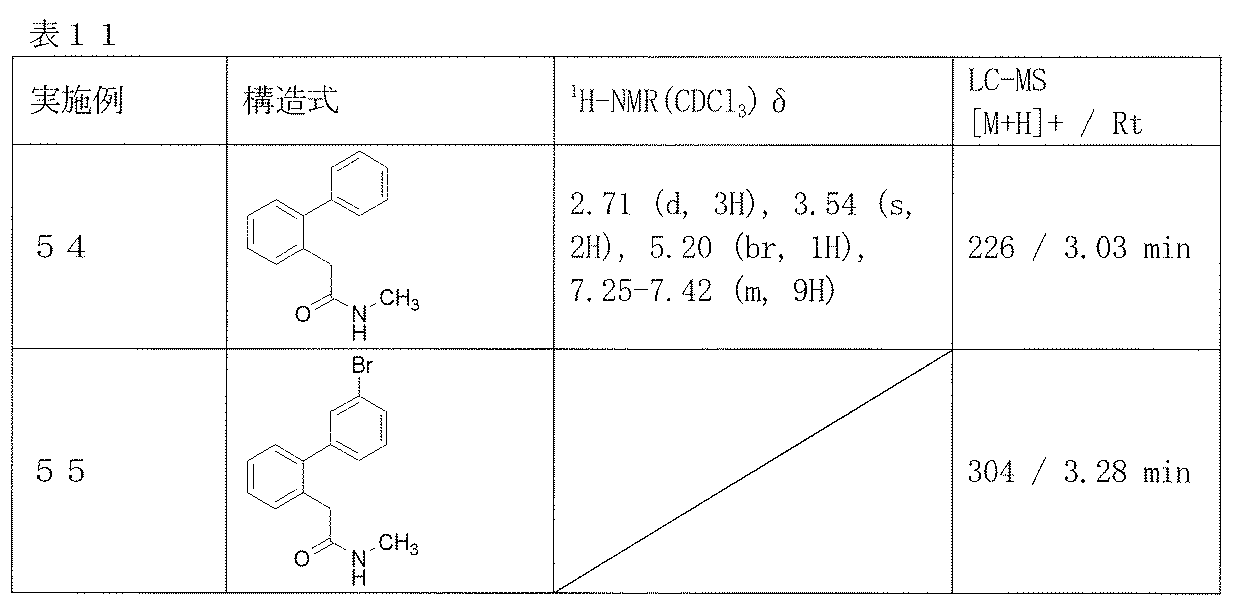
- Examples 54-55 The corresponding starting materials were used and reacted in the same manner as in Example 53 to obtain the compounds shown in Table 11.
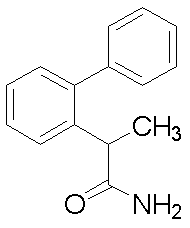
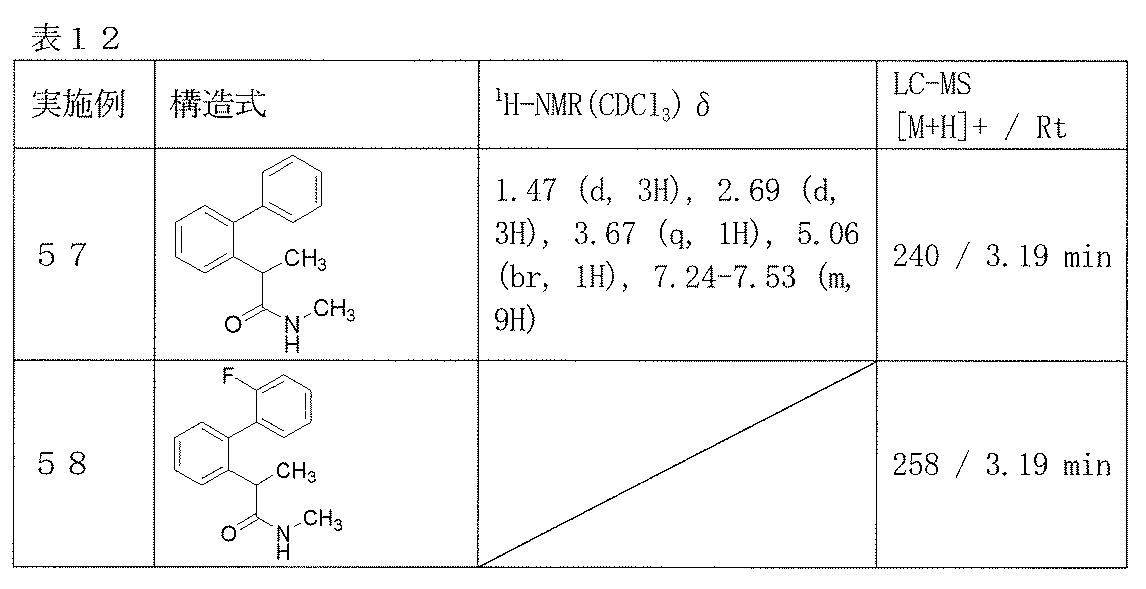
- Example 56 2- (Biphenyl-2-yl) propanamide
- 2- (biphenyl-2-yl) propanoic acid (1.03 g) obtained in Reference Example 27 was suspended in dichloromethane (8.0 ml), and DMF (0.05 ml) and oxalyl chloride (0.51 ml) were added. After that, the mixture was stirred at room temperature for 1 hour and concentrated. The obtained pale yellow crystals were dissolved in THF (2.0 ml), 28% aqueous ammonia solution (1.5 ml) was added, and the mixture was stirred at room temperature overnight. The resulting white solid was filtered and dried to give 2- (biphenyl-2-yl) propanamide (0.31 g) as crystals.
- Examples 57-58 Reaction and treatment were carried out in the same manner as in Example 56 using the corresponding starting materials, and the compounds shown in Table 12 were obtained.
- Example 59 2- (Biphenyl-2-yl) -2-hydroxyacetamide Methyl biphenyl-2-yl (hydroxy) acetate (190 mg) obtained in Reference Example 28 was dissolved in a 7 mol / l methanol solution (10 ml) of ammonia and stirred at room temperature for 60 hours. Water (20 ml) was added to the reaction solution, and methanol was distilled off under reduced pressure. The precipitated crystals were collected by filtration, washed with water and dried to give 2- (biphenyl-2-yl) -2-hydroxyacetamide (86 mg) as crystals.
- Example 60 The starting compound obtained in 2- (2′-fluorobiphenyl-2-yl) -2-hydroxyacetamide Reference Example 29 was reacted and treated in the same manner as in Example 59 to obtain the following compounds.
- MS (m / z) 246 (MH ⁇ +> ), Rt 2.56 min.
- Example 61 2- (Biphenyl-2-yl) -2-methoxyacetamide Biphenyl-2-yl (methoxy) acetic acid (506 mg) obtained in Reference Example 30 was dissolved in dichloromethane (10 ml), and DMF (0.05 ml) and 2M oxalyl chloride in dichloromethane (2.1 ml) were added. Stir at room temperature for 1 hour and concentrate. The obtained brown crystals were dissolved in THF (10 ml), 28% aqueous ammonia solution (2 ml) was added, and the mixture was stirred at room temperature for 2 days.
- Examples 62-63 Reaction and treatment were carried out in the same manner as in Example 61 using the corresponding starting compounds, and the compounds shown in Table 13 were obtained.
- Example 64 2- (2 ′, 3 ′, 5′-trichlorobiphenyl-2-yl) acetamide 2-bromoacetamide (3.84 g), 2,3,5-trichlorophenylboronic acid (6.04 g), tetrakis (triphenylphosphine) palladium (4.12 g), 2N aqueous sodium carbonate solution (27 ml), barium hydroxide -Octahydrate (1.12 g) and ethanol (24 ml) were added to toluene (84 ml), and the mixture was purged with nitrogen, followed by heating under reflux for 20 hours.
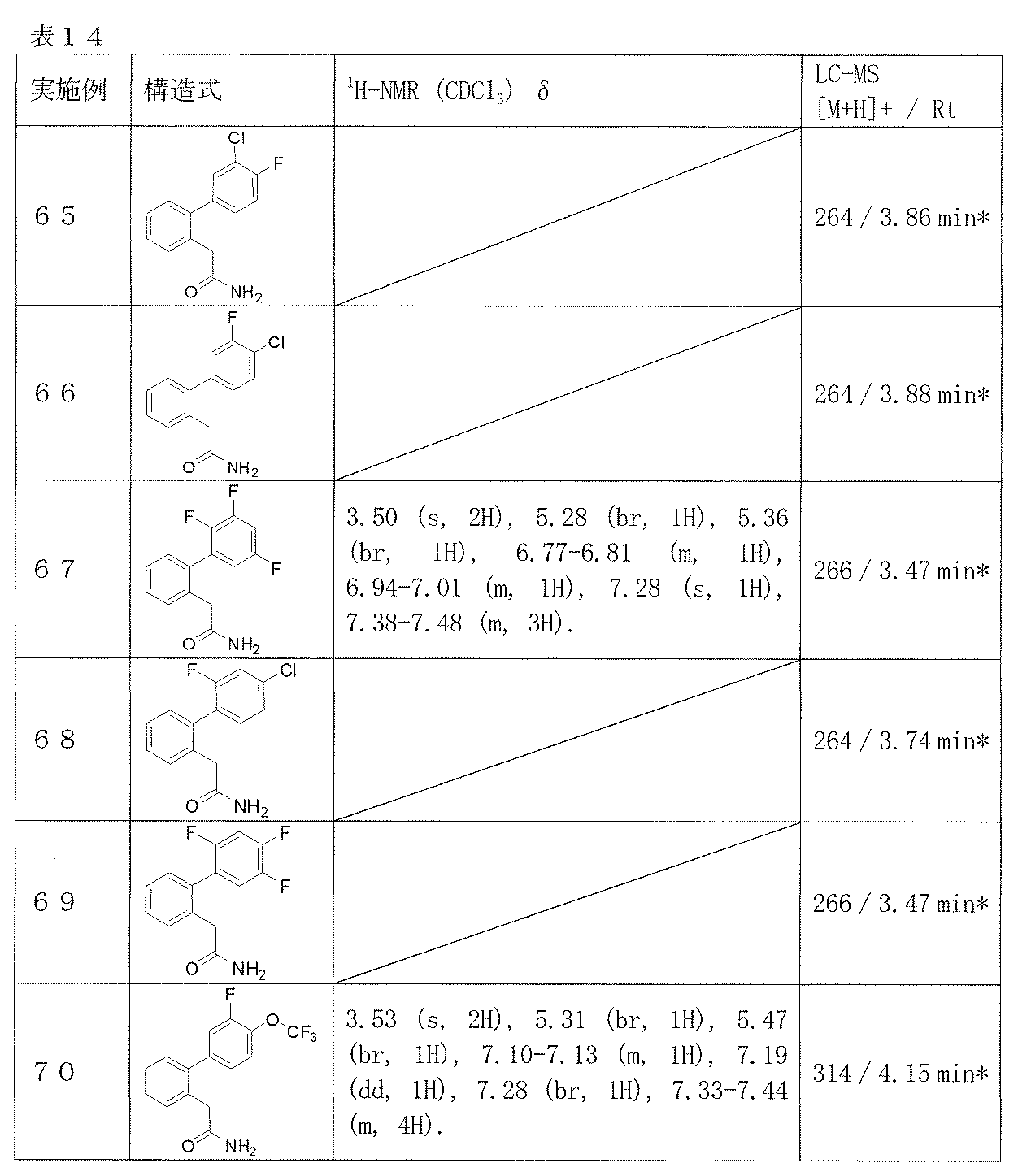
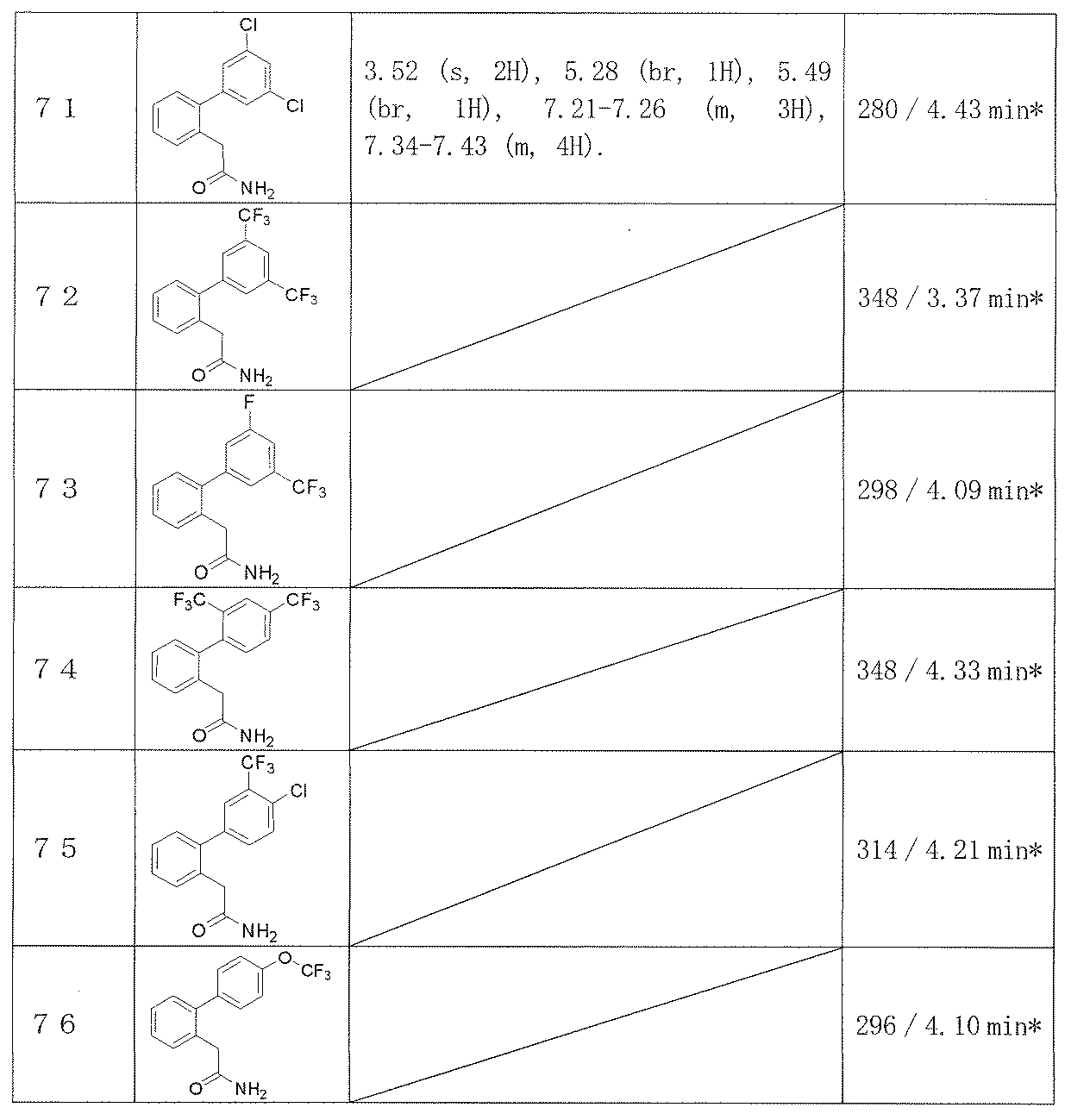
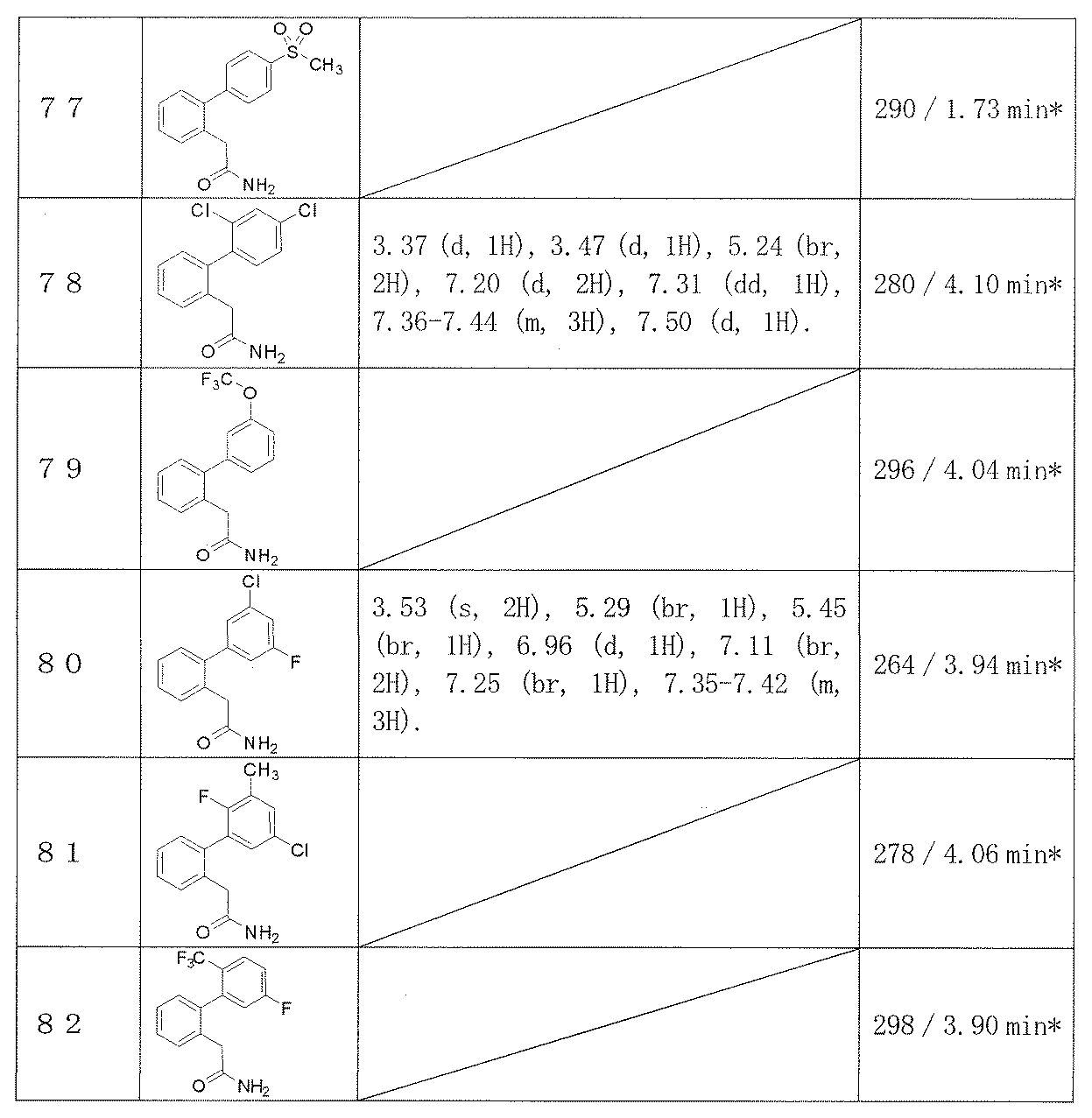
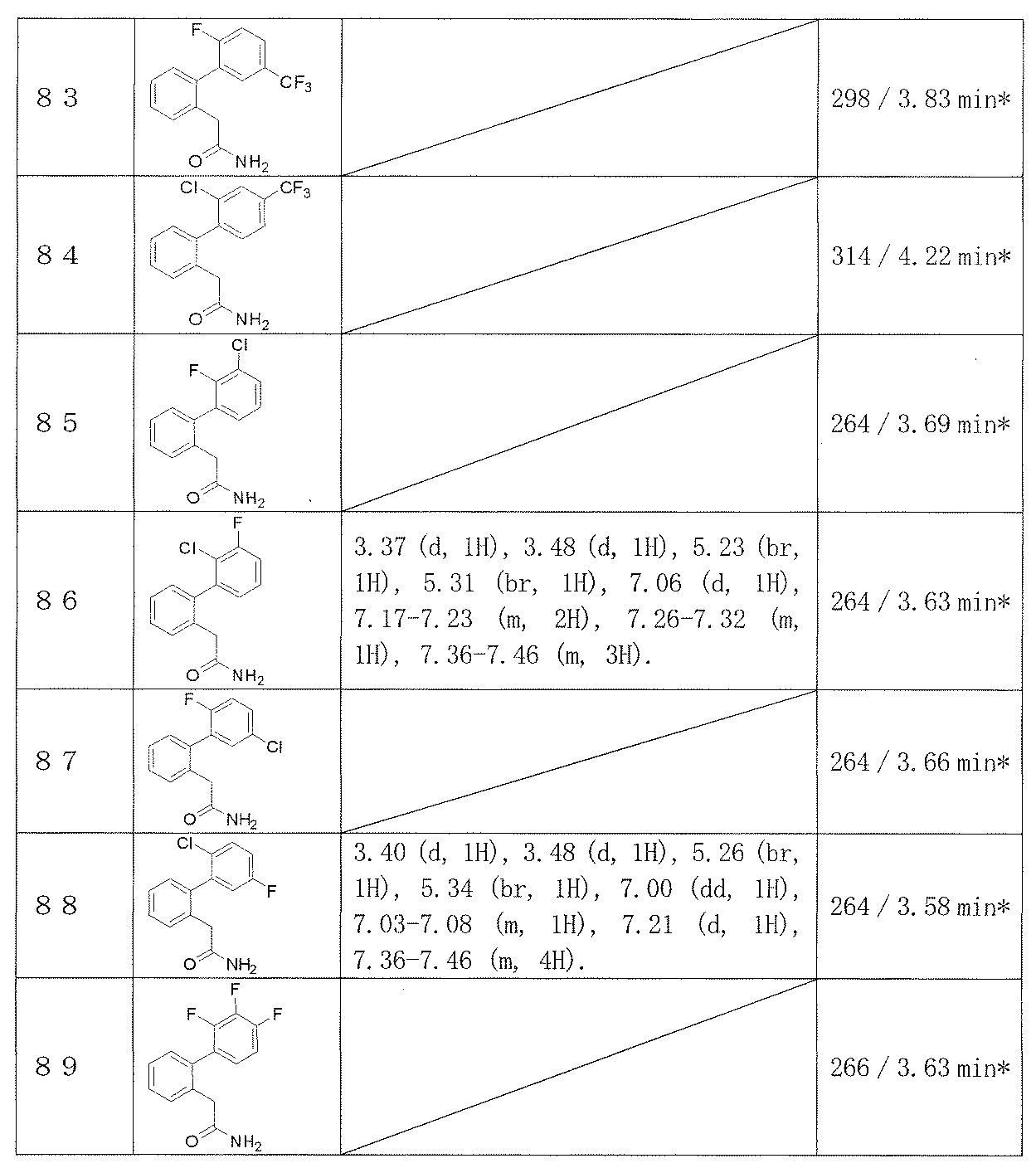
- Examples 65-108 Reaction and treatment were performed in the same manner as in Example 64 using the corresponding starting compounds, and the compounds shown in Table 14 were obtained.
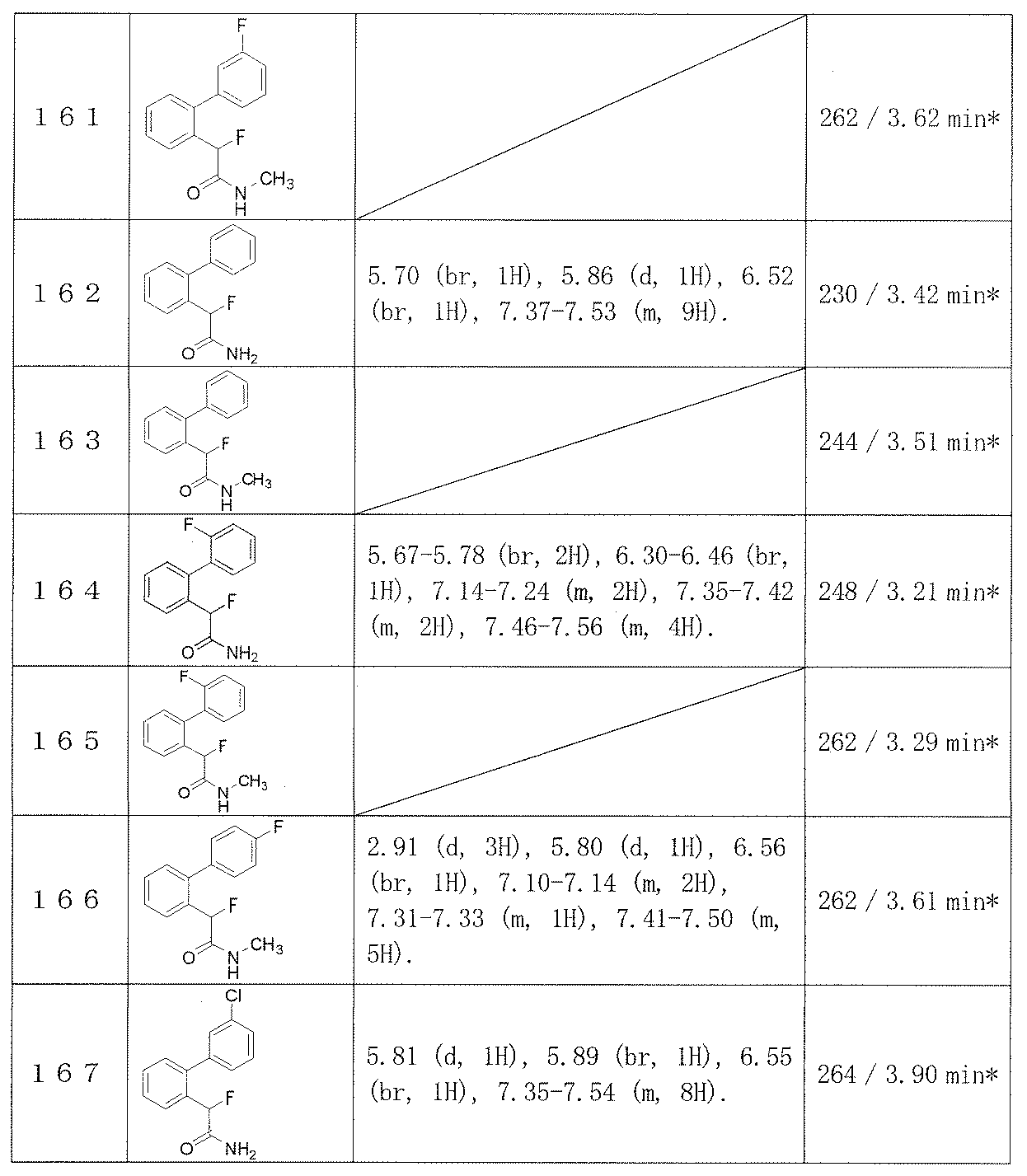
- Examples 109-173 The corresponding starting materials were used and reacted in the same manner as in Example 1 to obtain the compounds shown in Table 15.
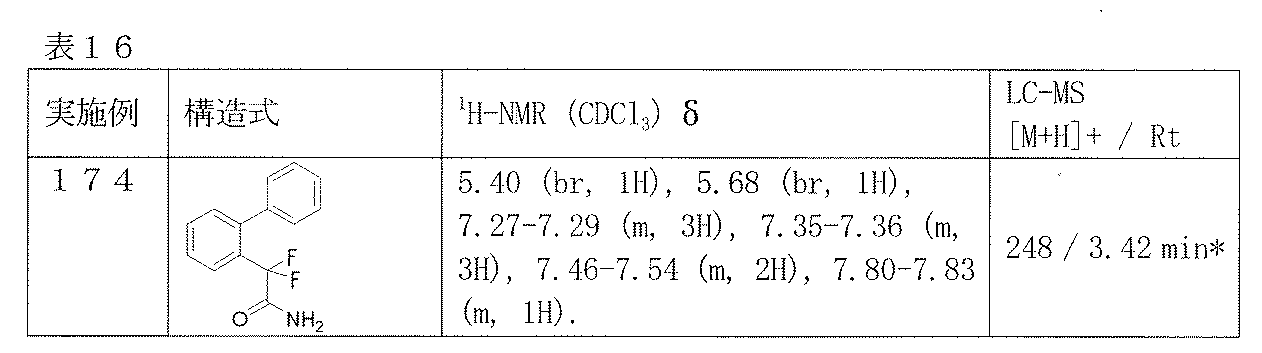
- Example 174 The corresponding starting materials were used and reacted in the same manner as in Example 49 to obtain the compounds shown in Table 16.
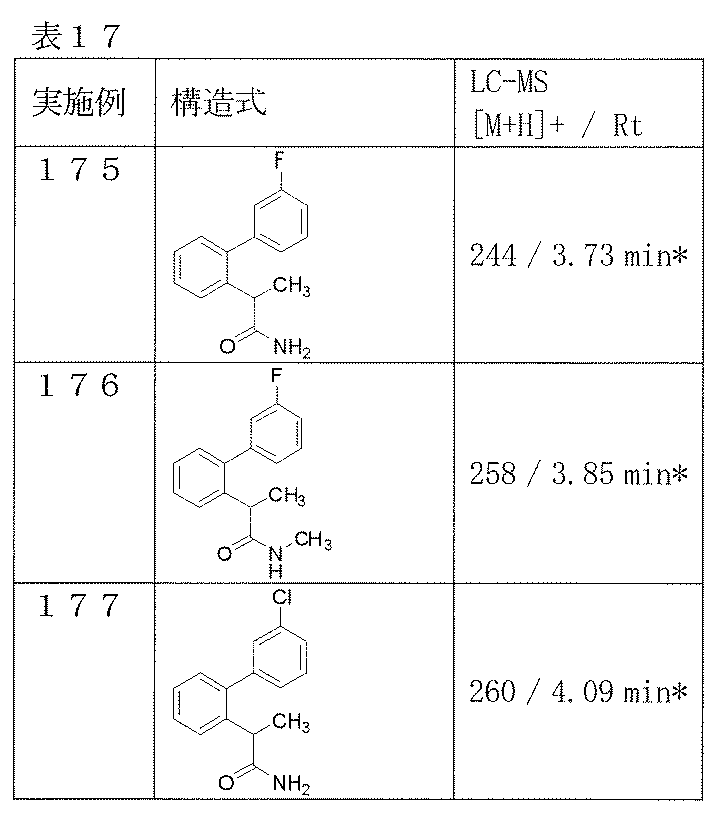
- Examples 175-177 Reaction and treatment were carried out in the same manner as in Example 56 using the corresponding starting compounds, and the compounds shown in Table 17 were obtained.
- Examples 178-198 Reaction and treatment were carried out in the same manner as in Example 59 using the corresponding starting compounds, and the compounds shown in Table 18 and Table 19 were obtained.
- Examples 199-206 Reaction and treatment were carried out in the same manner as in Example 61 using the corresponding starting compounds, and the compounds shown in Table 20 were obtained.
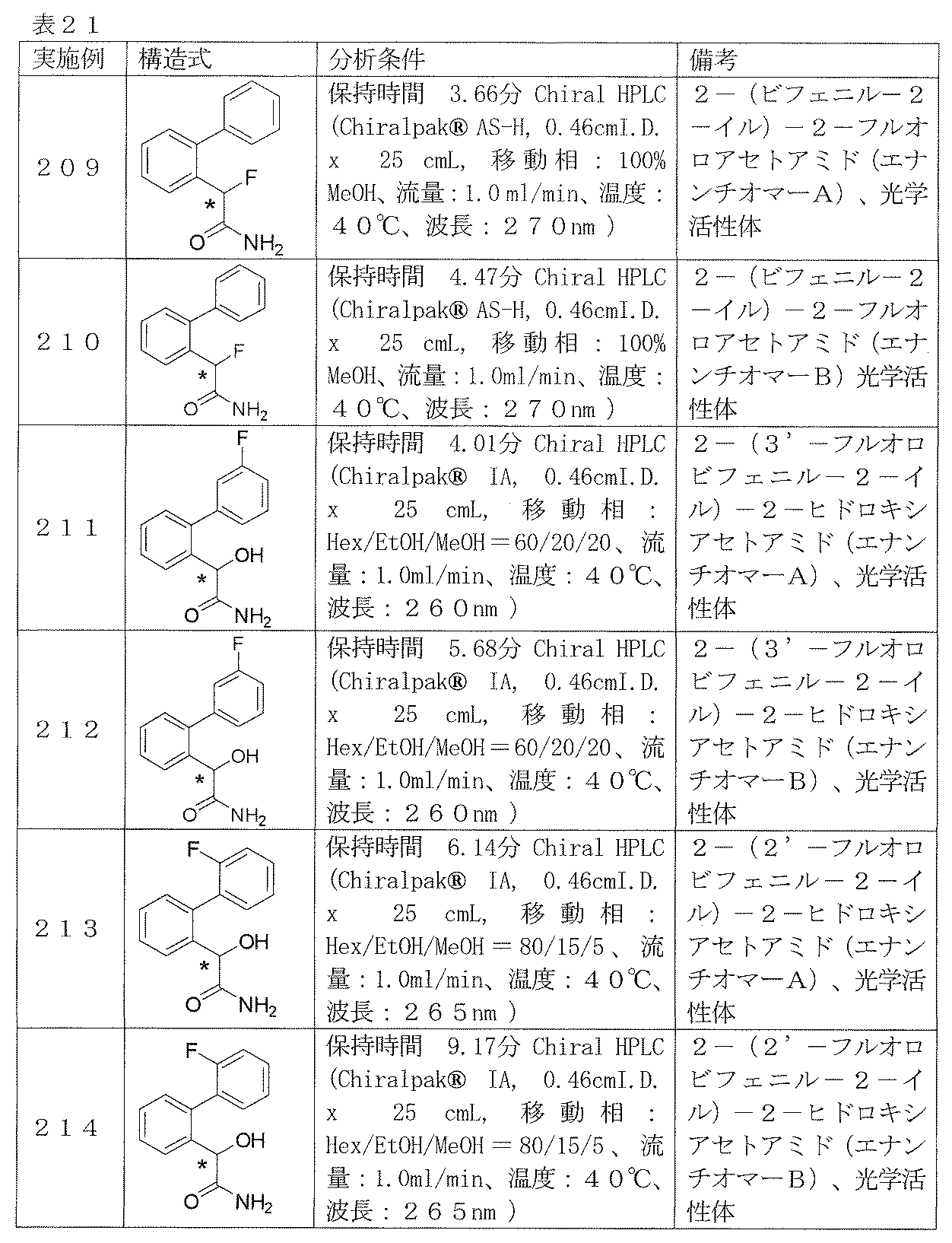
- Examples 207 and 208 2- (Biphenyl-2-yl) -2R-hydroxyacetamide and 2- (biphenyl-2-yl) -2S-hydroxyacetamide
- the compound of Example 59 was fractionated with CHIRALPAK IA (mobile phase: Hex-EtOH-MeOH) manufactured by Daicel to obtain a front peak (enantiomer A) and a rear peak (enantiomer B).
- Examples 209-214 Reaction and treatment were carried out in the same manner as in Examples 207 and 208 using the corresponding starting materials, and the compounds shown in Table 21 were obtained.
- Examples 215 and 216 2- (3 ′, 5′-dichlorobiphenyl-2-yl) -2R-hydroxyacetamide and 2- (3 ′, 5′-dichlorobiphenyl-2-yl) -2S-hydroxyacetamide
- Step 1 Example 183 (2.62 g) was dissolved in 34 ml of toluene, pyridinium p-toluenesulfonic acid (22 mg), (S) -allyl-2-oxabicyclo [3,3,0] oct-8 -Ene (2.66 g) was added and stirred at room temperature for 1 hour.
- Step 2 Ether (1.74 g) as the first eluate was dissolved in methanol (20 ml), p-toluenesulfonic acid monohydrate (74 mg) was added, and the mixture was stirred at room temperature for 8 hours. A saturated aqueous sodium hydrogen carbonate solution was added to terminate the reaction, and the mixture was extracted with chloroform. After drying over anhydrous magnesium sulfate and evaporating the solvent under reduced pressure, the residue was purified by silica gel column chromatography to obtain alcohol (896 mg) as a white amorphous.
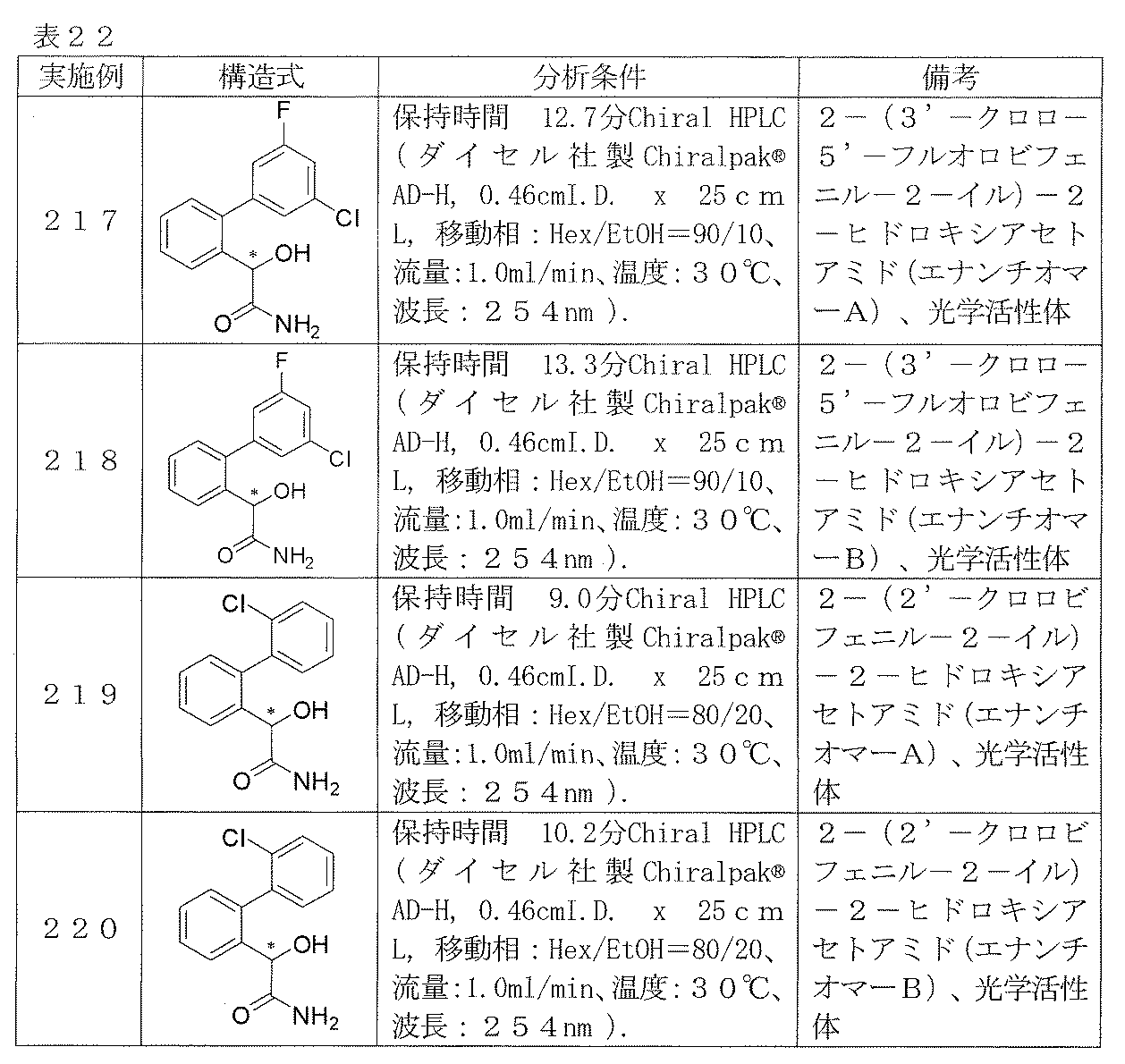
- Examples 217-220 Reaction and treatment were performed in the same manner as in Examples 215 and 216 using the corresponding starting compounds, and the compounds shown in Table 22 were obtained.
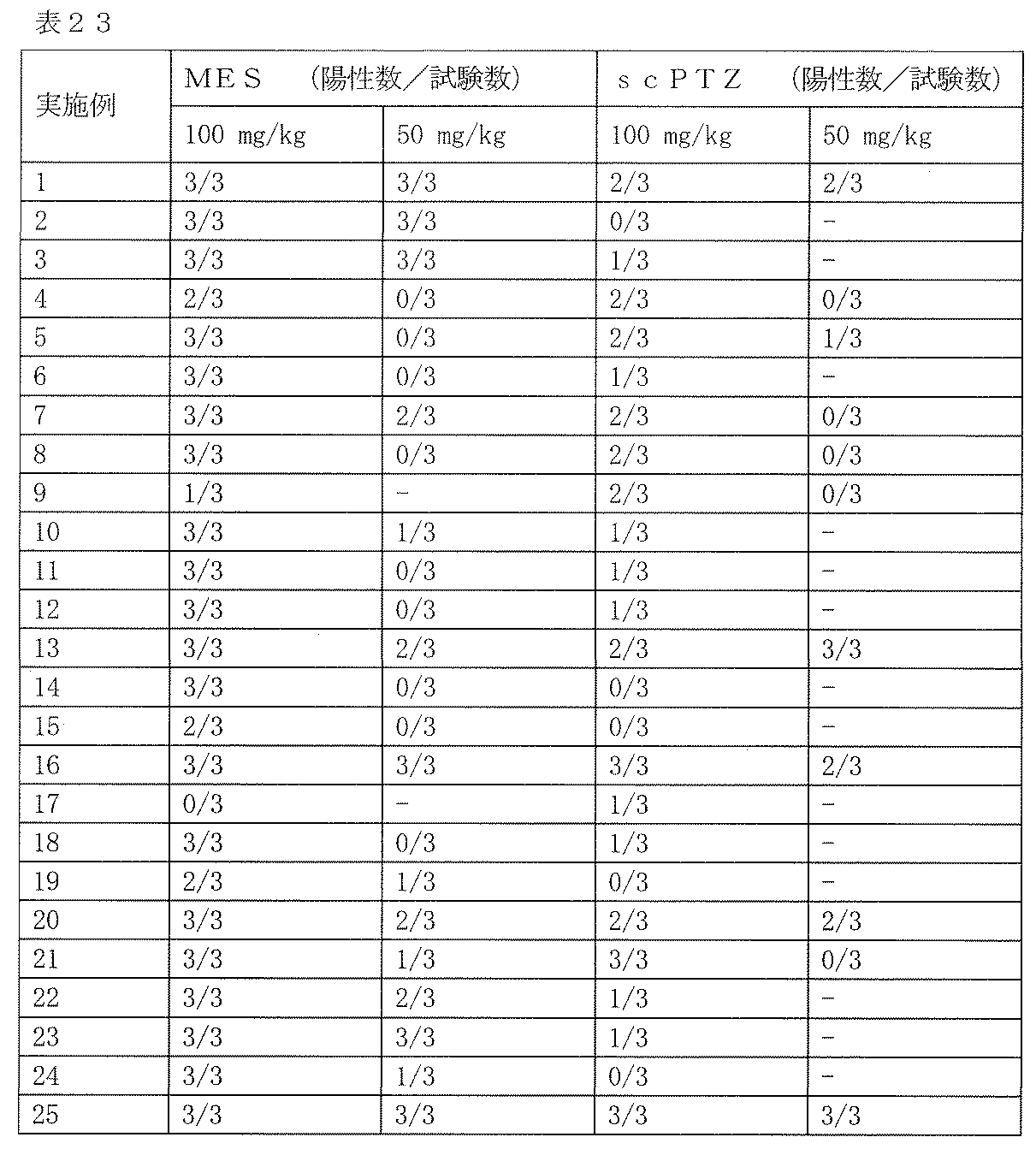
- MES maximum electric shock convulsion model
- scPTZ subcutaneous pentetrazole model
- rat amygdala kindling model evaluation which are typically highly clinically predictable.
- a compound showing anticonvulsant activity in any of these models is expected as a drug showing reliable antiepileptic activity in clinical practice.
- a compound showing an equivalent anticonvulsant activity in both MES evaluation and scPTZ evaluation is expected to have a broad spectrum of antiepileptic spectrum that is widely effective against partial seizures and general seizures like valproic acid.
- a 6 Hz psychomotor seizure model that is resistant to existing antiepileptic drugs has been used for the evaluation of new antiepileptic drugs.
- compounds that exhibit anticonvulsant activity are existing antiepileptic drugs. It is expected to be effective for patients who are resistant to treatment.
- a compound that exhibits equivalent anti-MES activity, anti-scPTZ activity, and anti-6 Hz model activity can be a therapeutic agent that has rare clinical utility in existing agents.
- no valid animal model with high clinical predictability has been reported yet to evaluate the improvement effect on bipolar disorder, but the pathological conditions of the “disease state” and “depressed state” that are the background of this disease are captured.
- Test Example 1 Evaluation of Maximum Electric Shock Convulsion Model (MES) This test is a test for evaluating the anticonvulsant action of a drug.
- the animal model used in this study is an expression system for generalized tonic clonic seizures and secondary generalized partial seizures.
- Test compound 50 and 100 mg / kg were orally administered to Slc: ddY male mice (3 mice per group, body weight 20-30 g), and after 1 hour, electrical stimulation (60 Hz, 25 mA, 0.2 seconds) was given from the cornea.
- electrical stimulation 60 Hz, 25 mA, 0.2 seconds
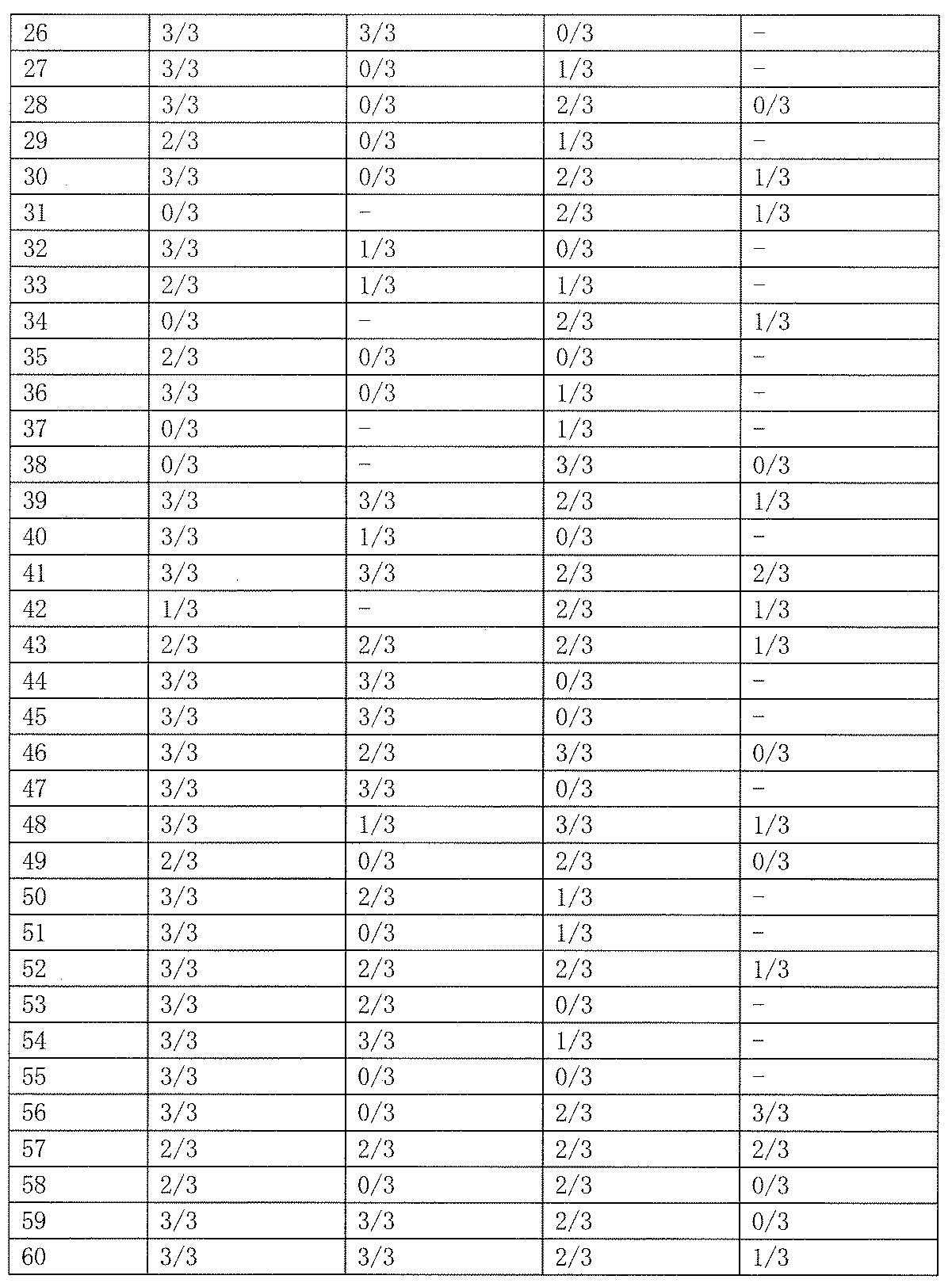
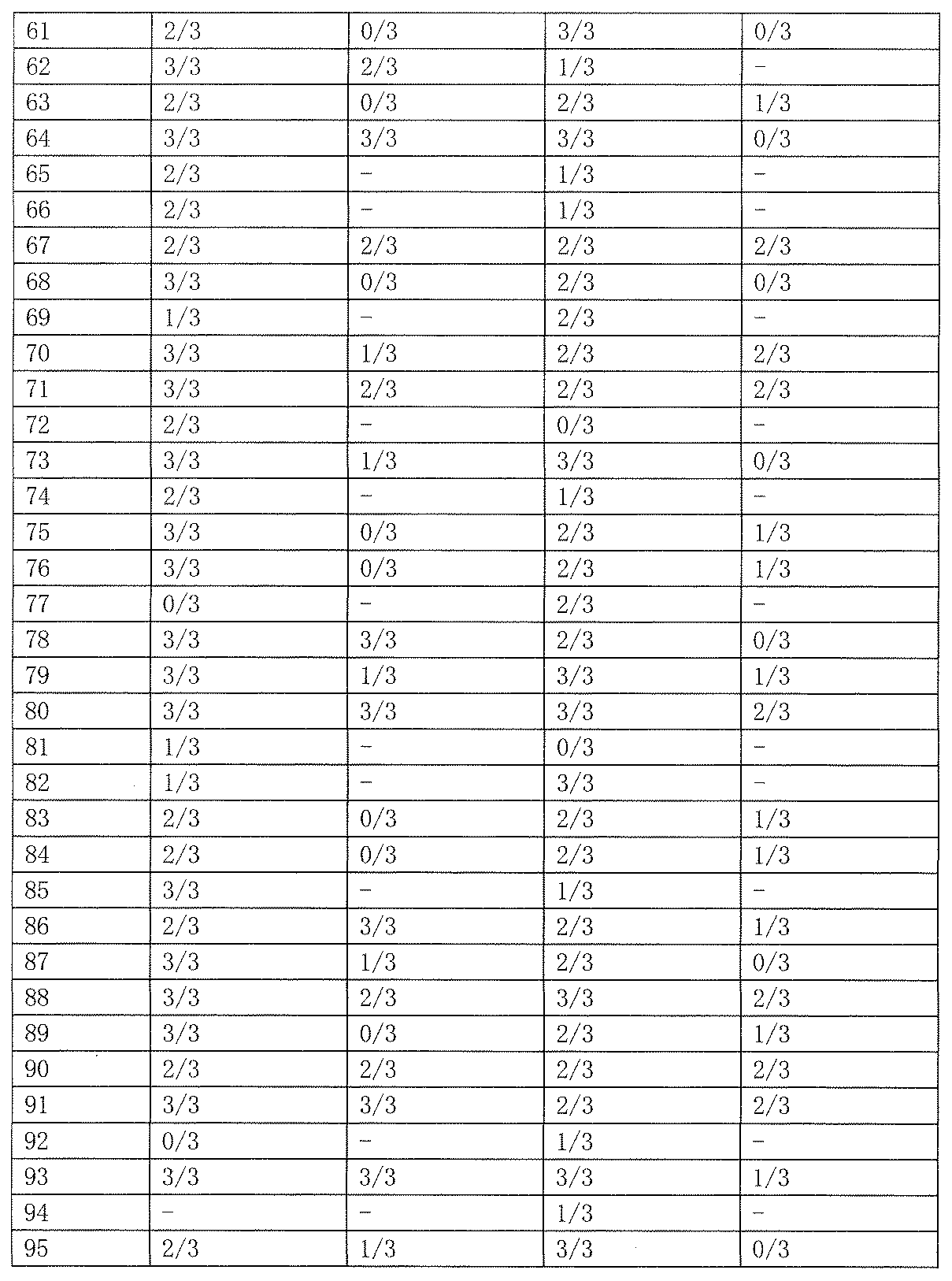
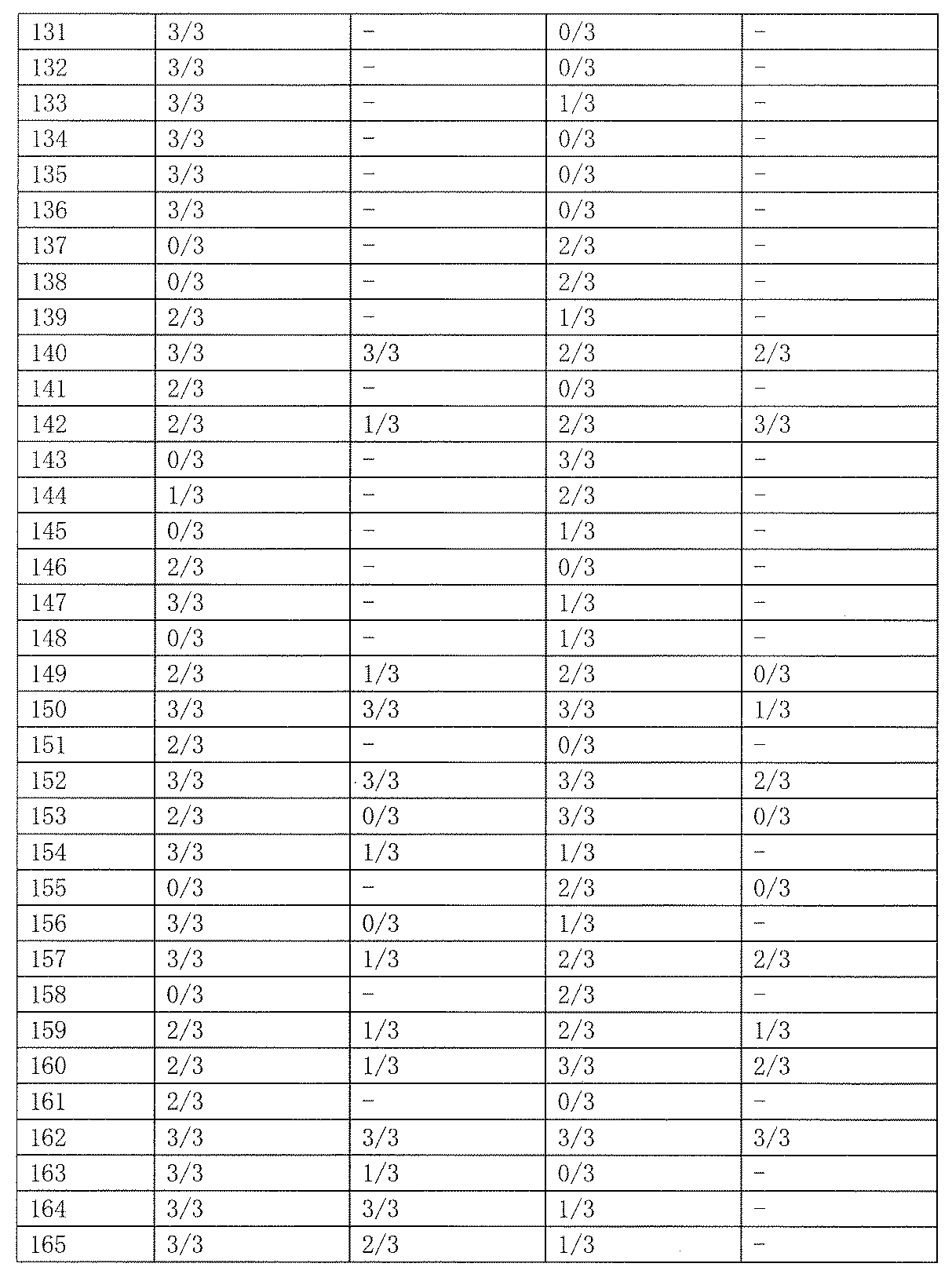
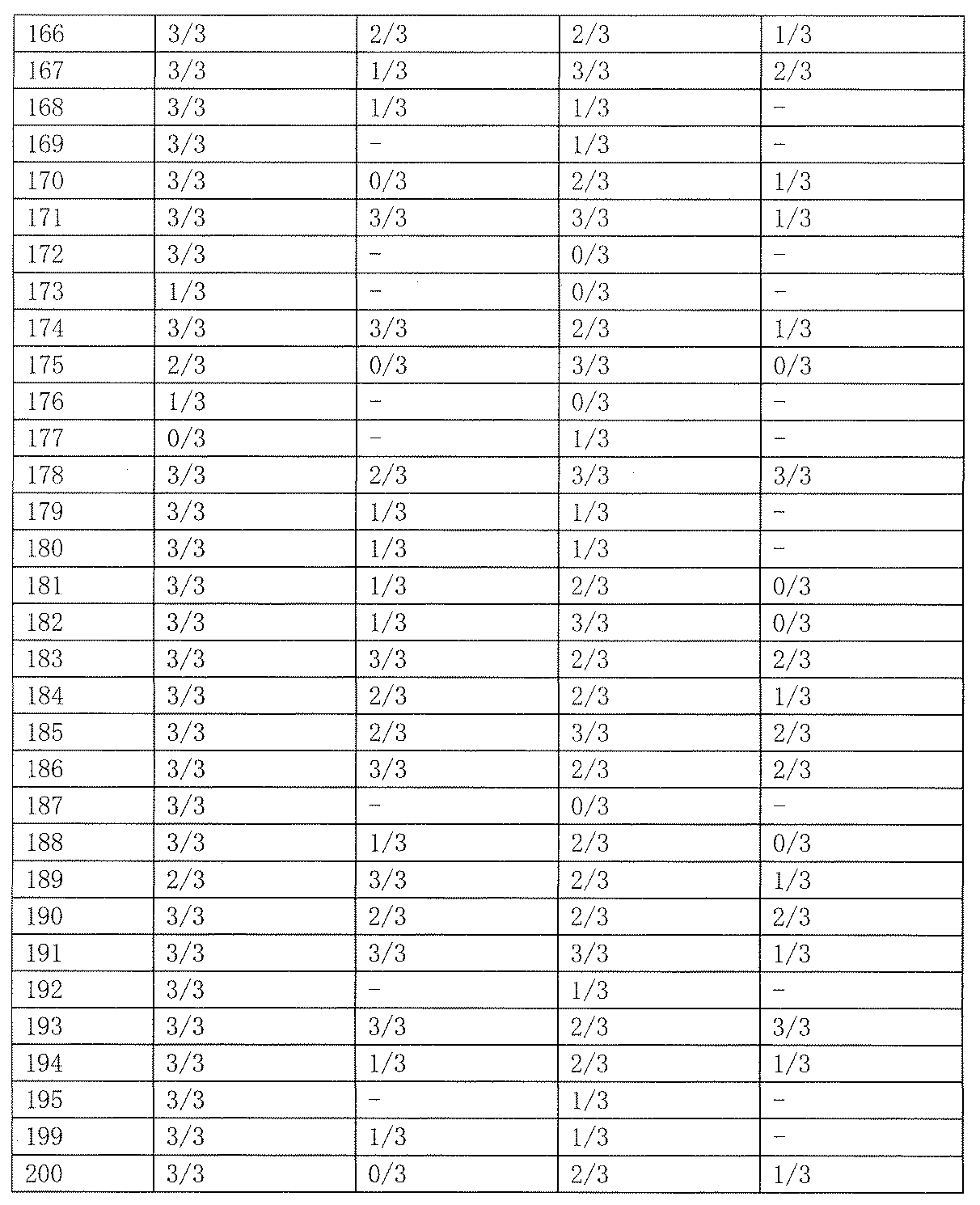
- As a control 0.5% tragacanth solution or 0.5% methylcellulose solution was administered. The results are shown in Table 19 below.
- Test Example 2 Subcutaneous Injection Pentetrazole Model (Minimum Spasm Model, scPTZ) Evaluation This test is a test for evaluating the anticonvulsant action of a drug in the same manner as Test Example 1. Unlike the expression system of Test Example 1, the animal model used in this test is an expression system for generalized absence seizures and myoclonic attacks. Test compounds 50 and 100 mg / kg were orally administered to Slc: ddY male mice (3 mice per group, body weight 20-30 g), and pentetrazole 85 mg / kg was subcutaneously administered 1 hour later. Thereafter, the presence or absence of onset of clonic convulsions in 30 minutes was observed. As a control, 0.5% tragacanth solution or 0.5% methylcellulose solution was administered. The results are shown in Table 23 below.
- the compound of the present invention exhibited an anticonvulsant effect by oral administration in the maximum electric shock convulsion model (MES) evaluation and / or the subcutaneous injection pentetrazole model (minimum convulsion model, scPTZ) evaluation.
- MES maximum electric shock convulsion model
- scPTZ subcutaneous injection pentetrazole model
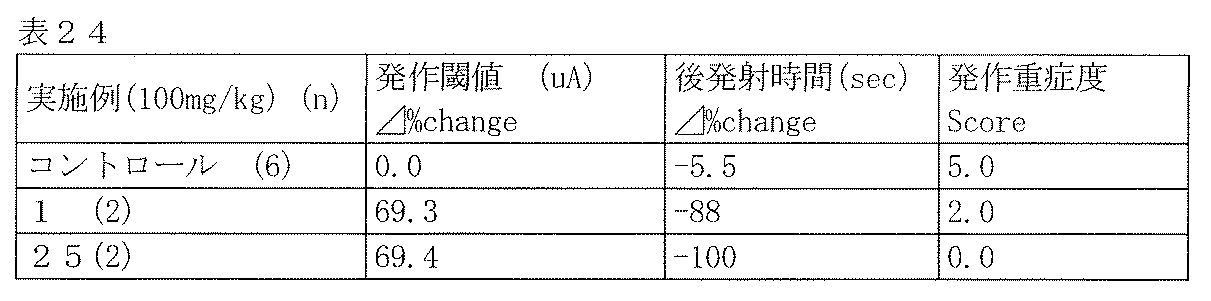
- Compounds 210, 211 and 212 showed strong anticonvulsant activity in both evaluations.
- Test Example 3 Rat Amygdaloid Kindling Model Evaluation This test is a test for evaluating the anticonvulsant action of drugs in rats in addition to mice.
- the animal model (kindling model) used in this study is a model similar to the clinical findings of focal simple partial attacks, complex partial attacks, and secondary generalized partial attacks, and is known to have extremely high clinical predictability.
- Slc A chronic electrode was placed in the cerebral cortex (front and back) and amygdala of Wistar male rats (body weight 250-300 g), and electrical stimulation was performed once a day for 2 weeks from the 1st week after surgery. (50 Hz, 400 ⁇ A, 1 second). Stimulation conditions were determined according to the method of Loscher et al. [Epilepsy Res.
- the compounds of Examples 1 and 25 showed high efficacy against each index in the rat amygdala kindling model by oral administration of 100 mg / kg.
- Test Example 4 Evaluation of 6 Hz psychomotor seizure model This test is a test for evaluating the anticonvulsant action of a drug in the same manner as Test Example 1 and Test Example 2. Unlike Test Example 1 and Test Example 2, the animal model used in this test is a seizure phenotype showing resistance to treatment with existing antiepileptic drugs.
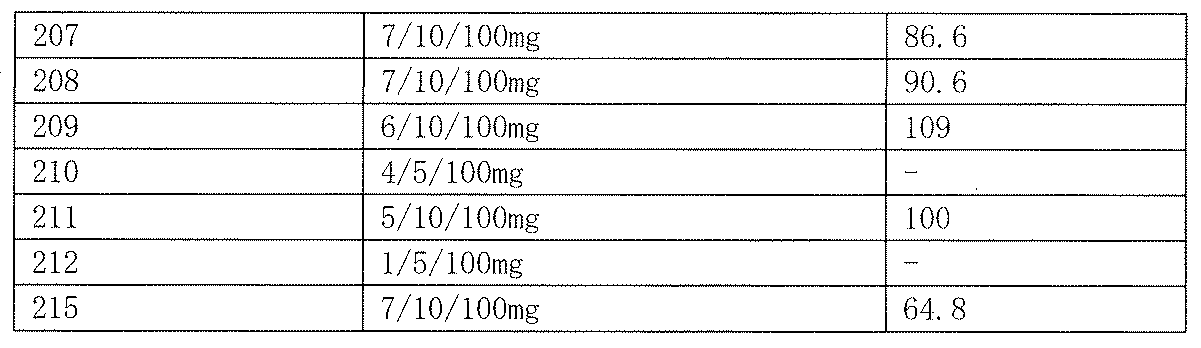
- Slc Ddy male mice (5 mice per group, body weight 20-30g) were orally administered with 100 mg / kg of the test compound, and after 30 minutes or 1 hour, electrical stimulation (6 Hz, 32 mA, 3 seconds) was applied from the cornea and induced We observed the presence or absence of the occurrence of clonic convulsions, tail reaction, and immobility in the forelimbs. As a control, 0.5% tragacanth solution, 0.5% methylcellulose solution, or olive oil was administered. The results are shown in Table 25.
- the compounds of Examples 1, 25, 91, 185, 190 and 207 showed high efficacy against the 6 Hz psychomotor seizure model by oral administration of 100 mg / kg.
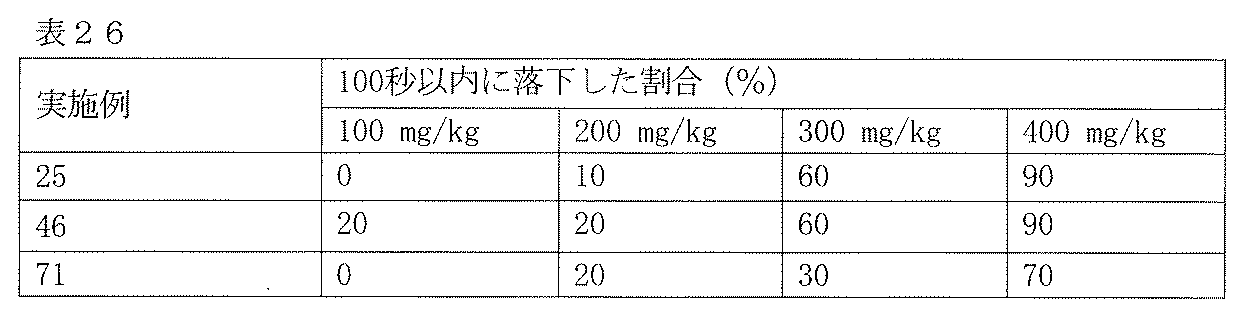
- Test Example 5 Rotor rod evaluation This test is a test for evaluating the coordinated motor ability inhibitory action of drugs.
- Slc ddy male mice (body weight 20-30 g) are trained on the day before the test so that they can walk without dropping for 3 minutes with a rotor rod device (a device that rotates a cylindrical rod with a diameter of 4 cm, 13 rpm).
- the test compound was orally administered to 10 mice per group, and after 1 hour, the test compound was placed on the rotor rod apparatus in the same manner as described above, and the walking state was observed for 100 seconds. Animals that fell due to coordination impairment within 100 seconds were counted as positive. As a control, 0.5% methylcellulose solution was administered. The results are shown in Table 26.
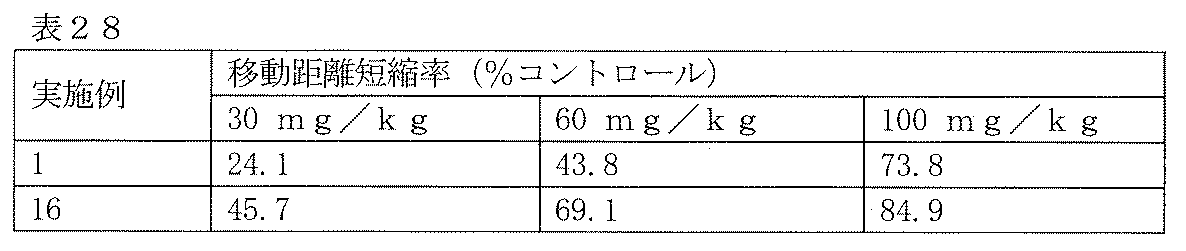
- Test Example 6 Evaluation of antidepressant This test is a representative test for evaluating the antidepressant action of a drug (Porsolt et al., Nature, 266: 730-732, 1977, Porsolt et al., Eur. J. Pharmacol., 47 : 379-391, 1978).
- Crl CD (SD) male rats (12 rats per group, body weight 200-250g) forcibly swim for 15 minutes in a 200mm diameter water tank with water accumulated to a depth (205mm) where the hind limbs cannot contact the bottom surface The test compound was orally administered 15 minutes after the end of swimming.
- Test Example 7 Antiepileptic Evaluation This test is a test for evaluating the antiepileptic effect of a drug.
- This evaluation system is a test system that can confirm the effectiveness of an antidepressant drug used clinically as a mood stabilizer different from the schizophrenia drug (Arban et al., Behav. Brain Res., 158: 123 -132, 2005, Foreman et al., Pharmacol. Biochem. Behav., 89: 523-534, 2008).
- the test compound was orally administered to Slc: C57BL / 6J male mice (8 per group, body weight 20 to 26 g), and methamphetamine (4 mg / kg) and chlordiazepoxide (10 mg / kg) were intraperitoneally administered 30 minutes later.
- Formulation Example 1 Manufacture of tablets The compound of Example 1 (250 g), corn starch (54 g), carboxymethylcellulose calcium (40 g), crystalline cellulose (50 g) and magnesium stearate (6 g) were mixed and granulated by a conventional method, and beaten at 400 mg per tablet. Lock and make 1000 tablets.
- Formulation Example 2 Preparation of powder After mixing the compound of Example 1 (500 g), lactose (470 g), hydroxypropylcellulose (25 g) and light anhydrous silicic acid (5 g), a powder is prepared.
- the biphenylacetamide derivative of the present invention has a maximum electric shock convulsion model (MES) evaluation, a subcutaneous injection pentetrazole model (minimum convulsion model, scPTZ) evaluation, and a 6 Hz spirit. Strong anticonvulsant activity in all motor seizure model evaluation and / or rat amygdala kindling model evaluation.
- MES electric shock convulsion model
- scPTZ subcutaneous injection pentetrazole model
- 6 Hz spirit 6 Hz spirit. Strong anticonvulsant activity in all motor seizure model evaluation and / or rat amygdala kindling model evaluation.
- the compounds of the present invention are antiepileptic drugs (e.g., partial seizures including simple partial seizures, complex partial seizures and persistent secondary generalized seizures with loss of consciousness, absence seizures, myoclonic seizures, clonic seizures, It is useful as a prophylactic and / or therapeutic drug for generalized seizures such as tonic seizures, tonic-clonic seizures, weakness seizures, West syndrome and Lennox-Gastaut syndrome.
- these group of compounds of the present invention are also expected as preventive and / or therapeutic agents for intractable epileptic seizures for which pharmacotherapy is not yet effective.
- the compound of the present invention is also useful as an antidepressant, an antidepressant and / or a therapeutic and / or preventive for bipolar disorder.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Biomedical Technology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Psychiatry (AREA)
- Anesthesiology (AREA)
- Pain & Pain Management (AREA)
- Epidemiology (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
R2は水素又はC1-3アルキルであり;Zは-(CH2)n-又は
該化合物は、ビフェニルとアミノ基が3以上の炭素原子を介して結合しており、後記式(I)で表される化合物と化学構造が異なる。
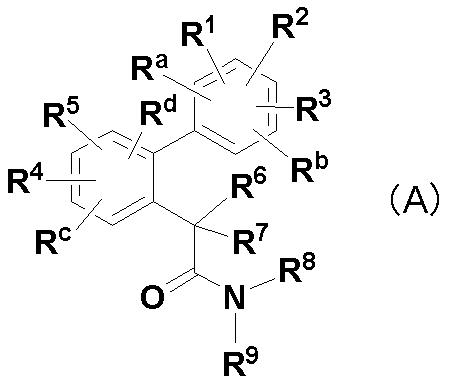
R1は水素原子、ヒドロキシ、シアノ、カルボキシ、ニトロ、ハロゲン、置換若しくは非置換の低級アルキル、-CONR7R8(式中、R7及びR8は同一又は異なって、水素原子、置換若しくは非置換の低級アルキル等)等を表し;R2は置換若しくは非置換の低級アルキル、置換若しくは非置換の低級アルケニル、置換若しくは非置換の低級アルキニル、置換若しくは非置換のアリール等を表し;R3及びR5は同一又は異なって、水素原子、置換若しくは非置換の低級アルキル等を表し;R4及びR6は同一又は異なって、水素原子、ヒドロキシ、ハロゲン、シアノ、置換若しくは非置換のアリール等を表す。]
該化合物は、ベンゼン環上の必須の置換基として水酸基あるいはアルコキシ基が存在するので、後記式(I)で表される化合物と化学構造が異なる。
R1、R2、R3、Ra及びRbは、それらが結合するベンゼン環上の置換可能な任意の炭素原子に1つずつ結合し、同一又は異なって、水素原子、フッ素原子、塩素原子、臭素原子、C1-6アルキル、1~3個のフッ素原子で置換されたC1-6アルコキシ、C1-6アルキル-S(O)n-又はシアノを表し、該アルキル又はアルキル-S(O)n-は、1~5個のフッ素原子で置換されていてもよく、ここにおいて、R1、R2、R3、Ra及びRbのうちいずれか2つの基が、2’-メチル及び3’-メチルのときは、残りの基のうちいずれか1つは水素原子以外の基であり、
R4、R5、Rc及びRdは、それらが結合するベンゼン環上の置換可能な任意の炭素原子に1つずつ結合し、同一又は異なって、水素原子、フッ素原子、塩素原子、C1-6アルキル、1~3個のフッ素原子で置換されたC1-6アルコキシ、C1-6アルキル-S(O)n-、シアノ又はニトロを表し、該アルキル又はアルキル-S(O)n-は、1~5個のフッ素原子で置換されていてもよく、
R6及びR7は、同一又は異なって、水素原子、フッ素原子、メチル、エチル、水酸基若しくはC1-6アルコキシを表すか(ただし、一方が水酸基の場合、他方はフッ素原子、水酸基またはC1-6アルコキシではない)、又はそれらに結合する炭素原子と一緒になってC3-6シクロアルキルを構成し、該メチル、エチル、アルコキシ及びシクロアルキルは、1~5個のフッ素原子で置換されていてもよく、
R8及びR9は、同一又は異なって、水素原子、C1-6アルキル、C3-7シクロアルキル-C1-3アルキル又はC3-6シクロアルキルを表し、該アルキル、シクロアルキル-アルキル及びシクロアルキルは、1~5個のフッ素原子で置換されていてもよく、
nは、0~2の整数を表すが、
ただし、R1、R2、R3、R4、R5、Ra、Rb、Rc及びRdがすべて水素原子であって、R6及びR7が共に水素原子のときは、R8及びR9のいずれか一方は水素原子であり、
R1、R2、R3、R4、R5、Ra、Rb、Rc及びRdがすべて水素原子であって、R6及びR7のいずれか一方がアルコキシのときは、R8及びR9は、同一又は異なって、水素原子、C1-3アルキル、C3-7シクロアルキル-C1-3アルキル又はC3-6シクロアルキルである。]
で表される化合物、またはその水和物もしくは溶媒和物。
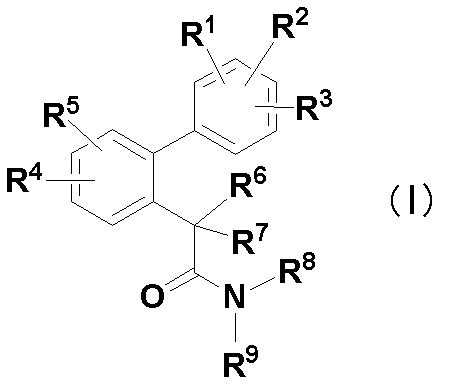
[項2]下記式(I):
R1、R2及びR3は、それらが結合するベンゼン環上の置換可能ないずれかの任意の炭素原子に1つずつ結合し、同一又は異なって、水素原子、フッ素原子、塩素原子、臭素原子、C1-6アルキル、1~3個のフッ素原子で置換されたC1-6アルコキシ、C1-6アルキル-S(O)n-又はシアノを表し、該アルキル又はアルキル-S(O)n-は、1~5個のフッ素原子で置換されていてもよく、ここにおいて、R1、R2及びR3のうちいずれか2つの基が、2’-メチル及び3’-メチルのときは、残りの基は水素原子以外の基であり、
R4及びR5は、それらが結合するベンゼン環上の置換可能ないずれかの任意の炭素原子に1つずつ結合し、同一又は異なって、水素原子、フッ素原子、塩素原子、C1-6アルキル、1~3個のフッ素原子で置換されたC1-6アルコキシ、C1-6アルキル-S(O)n-、シアノ又はニトロを表し、該アルキル又はアルキル-S(O)n-は、1~5個のフッ素原子で置換されていてもよく、
R6及びR7は、同一又は異なって、水素原子、フッ素原子、メチル、エチル、水酸基若しくはC1-6アルコキシを表すか(ただし、一方が水酸基の場合、他方はフッ素原子、水酸基またはC1-6アルコキシではない)、又はそれらに結合する炭素原子と一緒になってC3-6シクロアルキルを構成し、該メチル、エチル、アルコキシ及びシクロアルキルは、1~5個のフッ素原子で置換されていてもよく、
R8及びR9は、同一又は異なって、水素原子、C1-6アルキル、C3-7シクロアルキル-C1-3アルキル又はC3-6シクロアルキルを表し、該アルキル、シクロアルキル-アルキル及びシクロアルキルは、1~5個のフッ素原子で置換されていてもよく、
nは、0~2の整数を表すが、
ただし、R1、R2、R3、R4及びR5がすべて水素原子であって、R6及びR7が共に水素原子のときは、R8及びR9のいずれか一方は水素原子であり、
R1、R2、R3、R4及びR5がすべて水素原子であって、R6及びR7のいずれか一方がアルコキシのときは、R8及びR9は、同一又は異なって、水素原子、C1-3アルキル、C3-7シクロアルキル-C1-3アルキル又はC3-6シクロアルキルである。]
で表される化合物、またはその水和物もしくは溶媒和物。
項2に記載の化合物、またはその水和物もしくは溶媒和物。
項2又は項3に記載の化合物、またはその水和物もしくは溶媒和物。
項2又は項3に記載の化合物、またはその水和物もしくは溶媒和物。
項2~6のいずれかに記載の化合物、またはその水和物もしくは溶媒和物。
項2~7のいずれかに記載の化合物、またはその水和物もしくは溶媒和物。
R4及びR5が、同一又は異なって、水素原子、フッ素原子、塩素原子、トリフルオロメチル又はトリフルオロメトキシであり、
R6及びR7が、同一又は異なって、水素原子、メチル、水酸基又はメトキシであり(ただし、一方が水酸基の場合、他方は水酸基またはメトキシではない)、
R8が、水素原子、メチル又はエチルであり、
R9が水素原子である、
項2に記載の化合物、またはその水和物もしくは溶媒和物。
N,N-ジメチル-2-[4’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例3)、
2-[3’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例4)、
N-メチル-2-[3’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例5)、
N-メチル-2-[2’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例7)、
N-エチル-2-[2’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例8)、
2-(3’,4’-ジフルオロビフェニル-2-イル)アセトアミド(実施例21)、
2-(4’-フルオロビフェニル-2-イル)アセトアミド(実施例22)、
2-(4’-フルオロビフェニル-2-イル)-N-メチルアセトアミド(実施例23)、
2-(3’-フルオロビフェニル-2-イル)-N-メチルアセトアミド(実施例26)、
2-(2’-フルオロビフェニル-2-イル)-N-メチルアセトアミド(実施例28)、
2-(2’-フルオロビフェニル-2-イル)-N,N-ジメチルアセトアミド(実施例30)、
N-メチル-2-(3’-メチルビフェニル-2-イル)-アセトアミド(実施例31)、
2-(5-フルオロビフェニル-2-イル)アセトアミド(実施例39)、
2-(4-フルオロビフェニル-2-イル)-N-メチルアセトアミド(実施例45)、
2-(2’,4-ジフルオロビフェニル-2-イル)アセトアミド(実施例46)、
2-(2’,4-ジフルオロビフェニル-2-イル)-N-メチルアセトアミド(実施例47)、
2-(3’-クロロ-4-フルオロビフェニル-2-イル)アセトアミド(実施例48)、
2-(4’-ブロモビフェニル-2-イル)アセトアミド(実施例49)、
2-(ビフェニル-2-イル)アセトアミド(実施例50)、
2-(3’-ブロモビフェニル-2-イル)アセトアミド(実施例52)、
2-(4’-ブロモビフェニル-2-イル)-N-メチルアセトアミド(実施例53)、
2-(ビフェニル-2-イル)-N-メチルアセトアミド(実施例54)、
2-(ビフェニル-2-イル)プロパンアミド(実施例56)、
2-(2’-フルオロビフェニル-2-イル)-N-メチルプロパンアミド(実施例58)、
2-(ビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例59)、
2-(2’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例60)、
2-(ビフェニル-2-イル)-2-メトキシアセトアミド(実施例61)、
2-(ビフェニル-2-イル)-2-メトキシ-N-メチルアセトアミド(実施例62)、
2-(ビフェニル-2-イル)-2-メトキシ-N,N-ジメチルアセトアミド(実施例63)、
2-(2’,3’,5’-トリクロロビフェニル-2-イル)アセトアミド(実施例64)、
2-(4’-クロロ-2’-フルオロビフェニル-2-イル)アセトアミド(実施例68)、
2-[3’-フルオロ-4’-(トリフルオロメトキシ)ビフェニル-2-イル]アセトアミド(実施例70)、
2-[3’-フルオロ-5’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例73)、
2-[4’-クロロ-3’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例75)、
2-[4’-(トリフルオロメトキシ)ビフェニル-2-イル]アセトアミド(実施例76)、
2-(2’,4’-ジクロロビフェニル-2-イル)アセトアミド(実施例78)、
2-[3’-(トリフルオロメトキシ)ビフェニル-2-イル]アセトアミド(実施例79)、
2-[2’-フルオロ-5’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例83)、
2-[2’-クロロ-4’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例84)、
2-(2’-クロロ-3’-フルオロビフェニル-2-イル)アセトアミド(実施例86)、
2-(5’-クロロ-2’-フルオロビフェニル-2-イル)アセトアミド(実施例87)、
2-(2’,3’,4’-トリフルオロビフェニル-2-イル)アセトアミド(実施例89)、
2-[2’-フルオロ-5’-(トリフルオロメトキシ)ビフェニル-2-イル]アセトアミド(実施例93)、
2-(2’,5’-ジクロロビフェニル-2-イル)アセトアミド(実施例95)、
N-メチル-2-[3’-(トリフルオロメトキシ)ビフェニル-2-イル]アセトアミド(実施例99)、
2-(2’-フルオロ-5-ニトロビフェニル-2-イル)アセトアミド(実施例113)、
2-(3-フルオロビフェニル-2-イル)アセトアミド(実施例118)、
2-(3-フルオロビフェニル-2-イル)-N-メチルアセトアミド(実施例119)、
2-(5-クロロビフェニル-2-イル)-N-メチルアセトアミド(実施例124)、
2-(5-クロロ-2’-フルオロビフェニル-2-イル)アセトアミド(実施例125)、
2-(5-クロロ-2’-フルオロビフェニル-2-イル)-N-メチルアセトアミド(実施例126)、
2-[4-クロロ-4’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例142)、
2-[3’-フルオロ-5-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例149)、
2-(3’,5’-ジクロロ-5-フルオロビフェニル-2-イル)アセトアミド(実施例150)、
2-(3’,5’-ジフルオロビフェニル-2-イル)アセトアミド(実施例153)、
1-(2’-フルオロビフェニル-2-イル)-N-メチルシクロプロパンカルボキシアミド(実施例157)、
1-(3’-フルオロビフェニル-2-イル)-N-メチルシクロプロパンカルボキシアミド(実施例159)、
2-フルオロ-2-(3’-フルオロビフェニル-2-イル)アセトアミド(実施例160)、
2-フルオロ-2-(2’-フルオロビフェニル-2-イル)-N-メチルアセトアミド(実施例165)、
2-フルオロ-2-(4’-フルオロビフェニル-2-イル)-N-メチルアセトアミド(実施例166)、
2-(3’-クロロビフェニル-2-イル)-2-フルオロアセトアミド(実施例167)、
2,2-ジフルオロ-2-(3’-フルオロビフェニル-2-イル)アセトアミド(実施例170)、
2-(3’-クロロビフェニル-2-イル)-2,2-ジフルオロアセトアミド(実施例171)、
2-(ビフェニル-2-イル)-2,2-ジフルオロアセトアミド(実施例174)、
2-(3’-フルオロビフェニル-2-イル)プロパンアミド(実施例175)、
2-(5-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例181)、
2-(3’,5-ジフルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例182)、
2-(3’,5’-ジクロロ-5-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例184)、
2-(3’,4-ジフルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例188)、
2-(3’,5’-ジクロロ-4-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例189)、
2-(5’-クロロ-2’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例194)、
2-(2’-フルオロビフェニル-2-イル)-2-メトキシアセトアミド(実施例200)、
2-(2’-フルオロビフェニル-2-イル)-2-メトキシ―N―メチルアセトアミド(実施例201)、
2-(2’-フルオロビフェニル-2-イル)-2-メトキシ-N,N-ジメチルアセトアミド(実施例202)、
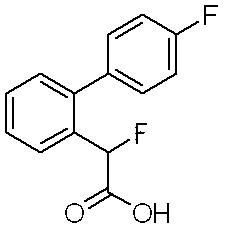
2-(4’-フルオロビフェニル-2-イル)-2-メトキシアセトアミド(実施例203)、
2-(4’-フルオロビフェニル-2-イル)-2-メトキシ-N,N-ジメチルアセトアミド(実施例205)、及び
2-(3’-クロロビフェニル-2-イル)-2-メトキシアセトアミド(実施例206)。
2-(2’-フルオロビフェニル-2-イル)アセトアミド(実施例1)、
N-メチル-2-[4’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例2)、
2-(3’-クロロビフェニル-2-イル)アセトアミド(実施例13)、
2-(2’-クロロビフェニル-2-イル)アセトアミド(実施例16)、
2-(2’,3’-ジフルオロビフェニル-2-イル)アセトアミド(実施例20)、
2-(3’-フルオロビフェニル-2-イル)アセトアミド(実施例25)、
2-(2’,5-ジフルオロビフェニル-2-イル)アセトアミド(実施例41)、
2-(4-フルオロビフェニル-2-イル)アセトアミド(実施例44)、
2-(ビフェニル-2-イル)-N-メチルプロパンアミド(実施例57)、
2-(ビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例59)、
2-(2’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例60)、
2-(2’,3’,5’-トリフルオロビフェニル-2-イル)アセトアミド(実施例67)、
2-(3’,5’-ジクロロビフェニル-2-イル)アセトアミド(実施例71)、
2-(3’-クロロ-5’-フルオロビフェニル-2-イル)アセトアミド(実施例80)、
2-(2’-クロロ-5’-フルオロビフェニル-2-イル)アセトアミド(実施例88)、
2-(3’,4’,5’-トリフルオロビフェニル-2-イル)アセトアミド(実施例90)、
2-[5’-クロロ-2’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド(実施例91)、
2-(4,4’-ジクロロビフェニル-2-イル)アセトアミド(実施例109)、
2-(4,4’-ジフルオロビフェニル-2-イル)アセトアミド(実施例114)、
2-(4,4’-ジフルオロビフェニル-2-イル)-N-メチルアセトアミド(実施例115)、
2-(3,3’-ジフルオロビフェニル-2-イル)アセトアミド(実施例140)、
2-(2’,5’-ジフルオロビフェニル-2-イル)アセトアミド(実施例152)、
2-(ビフェニル-2-イル)-2-フルオロアセトアミド(実施例162)、
2-フルオロ-2-(2’-フルオロビフェニル-2-イル)アセトアミド(実施例164)、
2-(3’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例178)、
2-(3’,5’-ジクロロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例183)、
2-(2’-クロロ-5’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例185)、
2-(4-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例186)、
2-(2’-クロロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例190)、
2-(3’-クロロ-5’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド(実施例191)、及び
2-ヒドロキシ-2-(3’,4’,5’-トリフルオロビフェニル-2-イル)アセトアミド(実施例193)。
R1~R3は、同一又は異なって、水素原子、フッ素原子、塩素原子、臭素原子、トリフルオロメチル又はトリフルオロメトキシが好ましく、更に好ましくは、同一又は異なって、水素原子、フッ素原子、塩素原子、トリフルオロメチル又はトリフルオロメトキシであり、あるいは同一又は異なって、水素原子、フッ素原子、または塩素原子である。
R4及びR5は、同一又は異なって、水素原子、フッ素原子、塩素原子、トリフルオロメチル又はトリフルオロメトキシが好ましく、更に好ましくは、同一又は異なって、水素原子、フッ素原子、又は塩素原子であり、あるいは両方が水素原子である。
R6及びR7は、同一又は異なって、水素原子、フッ素原子、メチル、水酸基又はメトキシが好ましく、更に好ましくは、同一又は異なって、水素原子、メチル、水酸基又はメトキシであり、あるいは同一又は異なって、水素原子、又は水酸基であり、あるいは両方が水素原子である。
R8は、水素原子、メチル又はエチルが好ましく、R9は、水素原子が好ましい。更に好ましくは、R8は、水素原子、又はメチルであり、R9は、水素原子である。
nは、2が好ましい。
上記R1~R9及びnの好ましい具体例は、それらの一つ又は任意の複数の組み合わせで、前記各項の化合物を限定してもよく、これらで限定された式(I)の化合物も本願発明の一つの態様になる。
本発明の式(1)の化合物におけるR1、R2及びR3の置換位置番号は、下記式(I)で表される。例えば、R1、R2及びR3のうちいずれか2つの基が、2’-メチル及び3’-メチルとは、下記式(I)の2’及び3’の位置に2つのメチルが置換していることを意味する。
「アルキル」とは、直鎖状又は分枝鎖状の飽和炭化水素を意味し、例えば、「C1-3アルキル」又は「C1-6アルキル」とは炭素原子数が1~3又は1~6の基をそれぞれ意味する。その具体例として、「C1-3アルキル」の場合には、メチル、エチル、プロピル、イソプロピル等が、「C1-6アルキル」の場合には、前記に加えて、ブチル、イソブチル、s-ブチル、t-ブチル、ペンチル、イソペンチル、ネオペンチル、ヘキシル等が挙げられる。
R6及びR7が水酸基以外の基である式(I)の化合物[下記式(Ia)の化合物]は、下記製造法により製造することができる。
化合物(II)をアミド化すると、化合物(Ia)が得られる。
R6及びR7の少なくとも一方が水酸基である式(I)の化合物[下記式(1b)の化合物]は、下記製造法により製造することができる。
化合物(III)とアンモニア又は各種アミンを反応すると、化合物(Ib)が得られる。
R6及びR7が水酸基以外の基である式(I)の化合物[下記式(Ic)の化合物]は、下記製造法により製造することができる。
化合物(VI)を各種置換フェニルボロン酸とカップリングすると、化合物(Ic)が得られる。
工程1のエステル化反応は、常法に従って行うことができる。例えば、この反応は適当な溶媒中で酸性又は塩基性条件下に化合物(IV)と各種アルコール又はハロゲン化アルキルとを接触させることにより行われる。又は、化合物(IV)とジアゾメタン、トリメチルシリルジアゾメタン等のジアゾ化合物とを反応させることによっても合成できる。溶媒の具体例としては、原料化合物の種類等に従って選択されるべきであるが、例えばTHF、ジオキサン、DME、アセトン、アセトニトリル、DMF、トルエン又はメタノール、エタノール、イソプロパノール等のアルコール類が挙げられ、単独あるいは混合溶媒として使用することができる。使用される酸の具体例としては、塩酸、硫酸及びフッ化水素酸等の鉱酸及びトリフルオロ酢酸、メタンスルホン酸、トリフルオロメタンスルホン酸及びp-トルエンスルホン酸等の有機酸が挙げられる。また、使用される塩基の具体例としては、水酸化ナトリウム、水酸化カリウム、水酸化リチウム等の水酸化アルカリ金属、t-ブトキシカリウム等のアルコキシアルカリ金属、炭酸ナトリウム、炭酸カリウム、炭酸リチウム等の炭酸アルカリ金属が挙げられる。反応温度は、用いられる原料化合物及び試薬の種類等によって異なるが、通常0~150℃、好ましくは20~100℃である。
工程3の加水分解反応は、常法に従って行うことができる。例えば、この反応は適当な溶媒中で酸性又は塩基性条件下に化合物(IIIa)と水とを接触させることにより行われる。溶媒の具体例としては、原料化合物の種類等に従って選択されるべきであるが、例えばTHF、ジオキサン、DME、アセトン、アセトニトリル、DMF、DMSO又はメタノール、エタノール、イソプロパノール等のアルコール類及び水等が挙げられ、単独あるいは混合溶媒として使用することができる。使用される酸の具体例としては、塩酸、硫酸等の鉱酸が挙げられる。また、使用される塩基の具体例としては、水酸化ナトリウム、水酸化カリウム、水酸化リチウム等の水酸化アルカリ金属、t-ブトキシカリウム等のアルコキシアルカリ金属、炭酸ナトリウム、炭酸カリウム、炭酸リチウム等の炭酸アルカリ金属が挙げられる。反応温度は、用いられる原料化合物及び試薬の種類等によって異なるが、通常0~150℃、好ましくは20~100℃である。
なお、本製造法において化合物(II)は、工程2及び工程3を経ずに化合物(IV)を工程1と同様の反応に付すことで製造することもできる。
化合物(IIIb)をアルキル化又はフッ素化すると、化合物(IIIc)が得られる。
検出機器:APIシリーズ用Agilent 1100シリーズ(applied Biosystems社製)
HPLC:API150EX LC/MS system (applied Biosystems社製)
Column:YMC CombiScreen ODS-A (S- 5μM, 12 nm, 4.6x50mm)
条件A(以下、実施例表中で特に記載のないものは、本条件での測定である。)
Solvent:A液:0.05%TFA/H2O、B液:0.035%TFA/MeCN
Gradient Condition:0.0-0.5min A 90%, 0.5-4.2min Linear gradient from A 90% to 1%, 4.2-4.4min Linear gradient from A 1% to 99%
Flow rate:3.5mL/min
UV:220nm
条件B(以下、実施例表中で*の記載のあるものは、本条件での測定である。)
Solvent:A液:0.05%TFA/H2O、B液:0.035%TFA/MeOH
Gradient Condition:0.0-1.0min A 60%, 1.0-4.7min Linear gradient from A 60% to 1%, 4.7-5.7min Linear gradient from A 1% to 60%
Flow rate:1.8mL/min
UV:220nm
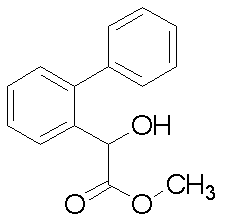
(2’-フルオロビフェニル-2-イル)酢酸
1H-NMR (CDCl3)δ:3.57 (s, 2H), 7.13 (t, 1H), 7.18 (t, 1H), 7.23-7.28 (m, 3H), 7.34-7.40 (m, 3H).
2-(ビフェニル-2-イル)プロパン酸
1H-NMR (CDCl3)δ:1.38 (d, 3H), 3.93 (q, 1H), 7.24-7.28 (m, 2H), 7.29 (dd, 1H), 7.34-7.45 (m, 7H).
メチル ビフェニル-2-イル(ヒドロキシ)アセテート
1H-NMR (CDCl3)δ:3.37 (d, 1H), 3.71 (s, 3H), 5.25 (d, 1H), 7.30- 7.34 (m, 1H), 7.39- 7.45 (m, 8H).
ビフェニル-2-イル(メトキシ)酢酸
1H-NMR (CDCl3)δ:3.19 (s, 3H), 4.94 (s, 1H), 7.31- 7.35 (m, 1H),7.40- 7.51(m, 8H).
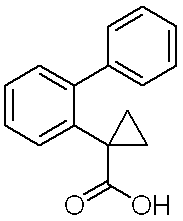
1-(ビフェニル-2-イル)シクロプロパンカルボン酸
1H-NMR (DMSO-d6)δ:0.77(br,2H),1.20(br,2H),7.19-7.21(m,1H),7.30-7.44(m,8H).
フルオロ(3’-フルオロビフェニル-2-イル)酢酸
1H-NMR (CDCl3)δ:1.25(t,3H),4.15-4.29(m,2H),5.81(d,1H),7.09-7.22(m,3H),7.34-7.48(m,4H),7.57-7.59(m,1H).
1H-NMR (CDCl3)δ:5.88(d,1H),7.09-7.17(m,2H),7.19-7.22(m,1H),7.34-7.53(m,4H),7.59-7.62(m,1H).
フルオロ(2’-フルオロビフェニル-2-イル)酢酸
1H-NMR (CDCl3)δ:3.69(s,3H),5.72(d,1H),7.16-7.25(m,2H),7.34-7.44(m,3H),7.47-7.49(m,2H),7.60(br,1H).
1H-NMR (CDCl3)δ:5.76(d,1H),7.15-7.23(m,2H),7.37(br,3H),7.49-7.50(m,2H),7.62(br,1H).
フルオロ(3’-クロロビフェニル-2-イル)酢酸
エチル 2-ブロモフェニル-ヒドロキシアセテート(1.74g)をジクロロメタン(20ml)に溶解し、氷冷下で[ビス(2-メトキシエチル)-アミノ]サルファー トリフルオリド(2.1ml)を加え、室温で1時間攪拌した。氷水で洗浄し無水硫酸マグネシウムで乾燥後、溶媒を減圧留去した。シリカゲルカラムクロマトグラフィーで精製し、エチル 2-ブロモフェニル-フルオロアセテート(1.20g)を無色油状物として得た。
1H-NMR (CDCl3)δ:1.27(t,3H),4.18-4.36(m,2H),6.20(d,1H),7.24-7.30(m,1H),7.35-7.40(m,1H),7.49-7.52(m,1H),7.60-7.64(m,1H).
1H-NMR (CDCl3)δ:5.86(d,1H),7.31-7.50(m,7H),7.59-7.61(m,1H).
ビフェニル-2-イル(ジフルオロ)酢酸
エチル ビフェニル-2-イル(ジフルオロ)アセテート(1.59g)をテトラヒドロフラン(10ml)に溶解し、水酸化リチウム・一水和物(0.72g)の水(10ml)溶液、メタノール(10ml)を加え室温で一晩攪拌した。10%クエン酸水溶液を加え反応液をpH 3に調整後、酢酸エチルで抽出した。飽和食塩水で洗浄し無水硫酸マグネシウムで乾燥後、溶媒を減圧留去してカルボン酸(1.53g)を無色油状物として得た。
1H-NMR (DMSO)δ:7.21-7.24(m,2H),7.28(d,1H),7.37-7.39(m,3H),7.54-7.62(m,2H),7.71-7.73(m,1H),14.5(br,1H).
(2’-フルオロビフェニル-2-イル)ジフルオロ酢酸
1H-NMR (CDCl3)δ:1.17(t,3H),3.98-4.06(m,2H),7.08-7.39(m,4H),7.35-7.41(m,1H),7.50-7.56(m,2H),7.80-7.84(m,1H).
1H-NMR (CDCl3)δ:7.00-7.10(m,2H),7.16-7.23(m,1H),7.28-7.32(m,2H),7.52-7.57(m,2H),7.79-7.80(m,1H).
2-(2’-フルオロビフェニル-2-イル)アセトアミド
1H-NMR (CDCl3)δ:3.50 (s, 2H), 5.24 (br, 2H), 7.13-7.44 (m, 8H)
MS(m/z)230(MH+),Rt= 2.88 min.
2-(4’-ブロモビフェニル-2-イル)アセトアミド
1H-NMR (CDCl3)δ:3.52 (s, 2H), 5.33 (br, 2H), 7.17-7.21 (m, 2H), 7.26-7.39 (m, 4H), 7.53-7.56 (m, 2H).
MS(m/z)290(MH+),Rt= 3.26 min.
2-(4’-ブロモビフェニル-2-イル)-N-メチルアセトアミド
1H-NMR (CDCl3)δ:2.73 (d, 3H), 3.50 (s, 2H), 5.25 (br, 1H), 7.13-7.16 (m, 2H), 7.25-7.36 (m, 4H), 7.52-7.55 (m, 2H).
MS(m/z)304(MH+),Rt= 3.32 min.
2-(ビフェニル-2-イル)プロパンアミド
1H-NMR (CDCl3)δ:1.47 (d, 3H), 3.74 (q, 1H), 5.17 (br, 2H), 7.25-7.53 (m, 9H)
MS(m/z)226(MH+),Rt= 3.09 min.
2-(ビフェニル-2-イル)-2-ヒドロキシアセトアミド
1H-NMR (DMSO-d6)δ:4.92 (d, 1H), 6.02 (d, 1H), 7.24-7.26 (m, 1H), 7.33-7.50 (m, 8H), 7.57-7.60 (m, 2H).
MS(m/z)228(MH+),Rt= 2.52 min.
2-(2’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド
参考例29で得られた原料化合物を用いて実施例59と同様に反応・処理し、下記に示す化合物を得た。
MS(m/z)246(MH+),Rt= 2.56 min.
2-(ビフェニル-2-イル)-2-メトキシアセトアミド
1H-NMR (CDCl3)δ:3.06 (s, 3H), 4.83 (s, 1H), 5.81 (br, 1H), 6.76 (br, 1H), 7.26-7.51 (m, 9H).
MS(m/z)242(MH+),Rt= 3.05 min.
2-(2’,3’,5’-トリクロロビフェニル-2-イル)アセトアミド
1H-NMR (CDCl3)δ:3.36 (d, 1H), 3.47 (d, 1H), 5.25 (br, 1H), 5.33 (br, 1H), 7.17-7.19 (m, 2H), 7.36-7.46 (m, 3H), 7.53 (d, 1H).
MS(m/z)314(MH+),Rt=4.41 min*.
2-(ビフェニル-2-イル)-2R-ヒドロキシアセトアミド及び2-(ビフェニル-2-イル)-2S-ヒドロキシアセトアミド
実施例207(エナンチオマーA):保持時間 4.13分 Chiral HPLC (Chiralpak IA, 0.46 cmI.D. x 25 cmL, 移動相:Hex/EtOH/MeOH=60/20/20、流量:1.0ml/min、温度:40℃、波長:260nm )、[α]D20.4 =+127.0°(c 0.521、CH3OH)
実施例208(エナンチオマーB):保持時間 6.99分 Chiral HPLC (Chiralpak IA, 0.46 cmI.D. x 25 cmL, 移動相:Hex/EtOH/MeOH=60/20/20、流量:1.0ml/min、温度:40℃、波長:260nm )、[α]D20.4 =-125.8°(c 0.985、CH3OH)
2-(3’,5’-ジクロロビフェニル-2-イル)-2R-ヒドロキシアセトアミド及び2-(3’,5’-ジクロロビフェニル-2-イル)-2S-ヒドロキシアセトアミド
第1溶出物:1H-NMR (CDCl3)δ:1.48-1.68(m,6H),1.75-1.81(m,1H),1.90-1.94(m,1H),2.12-2.17(m,1H),2.29-2.34(m,1H),3.04(dd,1H),3.50-3.56(m,1H),5.07-5.14(m,2H),5.20(s,1H),5.43(br,1H),5.78-5.88(m、1H),6.63(br,1H),7.18(dd,1H),7.29-7.40(m,3H),7.48(d,1H),7.53(d,2H).
第2溶出物:1H-NMR (CDCl3)δ:0.94-1.03(m,1H),1.37-1.52(m,5H),1.70-1.77(m,1H),1.93-1.98(m,1H),2.09-2.14(m,1H),2.21-2.26(m,1H),3.71-3.77(m,1H),3.82-3.87(m,1H),5.06-5.14(m,2H),5.30(s,1H),5.47(br,1H),5.75-5.85(m、1H),6.74(br,1H),7.20-7.22(m,1H),7.32-7.42(m,3H),7.48-7.51(m,1H),7.54(br,2H).
実施例215(エナンチオマーA):保持時間 13.3分 Chiral HPLC (ダイセル社製Chiralpak AD-H, 0.46cmI.D. x 25 cmL, 移動相:Hex/EtOH=90/10、流量:1.0ml/min、温度:30℃、波長:254nm ).
実施例216(エナンチオマーB):保持時間 14.2分 Chiral HPLC (ダイセル社製Chiralpak AD-H, 0.46cmI.D. x 25 cmL, 移動相:Hex/EtOH=90/10、流量:1.0ml/min、温度:30℃、波長:254nm ).
以下に、本発明の代表的化合物の薬理試験結果を示し、該化合物についての薬理作用を説明するが、本発明はこれらの試験例に限定されるものではない。
また、双極性障害への改善効果を評価するに際し臨床予測性の高い妥当な動物モデルは未だ報告されていないが、本疾患の背景にある「躁状態」、「うつ状態」それぞれの病態を捉えた動物モデルは幾つか報告されており、それぞれの代表的モデルとしてメタンフェタミンとクロルジアゼポキシドを併用投与した際に認められる過剰な運動亢進状態(躁状態)、強制水泳時に見られる無動状態(うつ状態)を指標にした改善評価が一般的に用いられている。これら両モデルに対し改善活性を示す化合物は本病態の躁うつ分裂的な気分変動を安定化させる効果として期待できる。
本試験は、薬物の抗痙攣作用を評価する試験である。この試験で用いる動物モデルは、全般性強直間代発作や二次性全般化部分発作の表現系である。Slc:ddY系雄性マウス(一群3匹、体重20~30g)に被験化合物50及び100 mg/kgを経口投与し、1時間後に角膜より電気刺激(60Hz,25mA,0.2秒間)を与え、誘発される後肢の強直性伸展痙攣の発現抑制を観察した。なお、コントロールは0.5%トラガント液あるいは0.5%メチルセルロース液を投与した。結果を以下の表19に示す。
本試験は、試験例1と同様に薬物の抗痙攣作用を評価する試験である。この試験で用いる動物モデルは、試験例1の表現系とは異なり、全般性の欠神発作やミオクロニー発作の表現系である。Slc:ddY系雄性マウス(一群3匹、体重20~30g)に被験化合物50及び100 mg/kgを経口投与し、1時間後にペンテトラゾール85 mg/kgを皮下投与した。その後、30分間における間代性痙攣の発現の有無を観察した。なお、コントロールは0.5%トラガント液あるいは0.5%メチルセルロース液を投与した。結果を以下の表23に示す。
本試験は、マウスに加え、ラットにおける薬物の抗痙攣作用を評価する試験である。この試験で用いる動物モデル(キンドリングモデル)は、焦点性の単純部分発作、複雑部分発作及び二次性全般化部分発作の臨床所見に類似するモデルであり、極めて臨床予測性が高いことが知られている。Slc:Wistar系雄性ラット(体重250~300g)の大脳皮質(前、後)ならびに扁桃核に慢性電極を留置し、術後1週間目より扁桃核へ1日1回、2週間前後にわたり電気刺激(50Hz,400μA,1秒)を与えた。刺激条件は、Loscherらの方法[Epilepsy Res., 40:63-77(2000)]に従った。また、Racineらの方法[Motor seizure. Electroenceph. Clin. Neurophysiol., 32:281-294(1972)]に基づいた発作重症度においてstage5の発作が連続10回発現するまで刺激を継続し、キンドリングモデルを完成させた。完成したモデルに、試験化合物を各々100mg/kgで経口投与し、投与1時間後に、発作が誘発される閾値刺激電流(発作閾値)、脳波上に認められる後発射の持続時間(後発射時間)及び発作重症度(stage score 1~5)を指標に評価した。発作閾値ならびに後発射時間は試験化合物投与前の各値に対する変化率を平均で表した。なお、コントロールは0.5%トラガント液を投与した。結果を表24に示す。マイナスは減少あるいは短縮を意味する。
本試験は、試験例1ならびに試験例2と同様に薬物の抗痙攣作用を評価する試験である。この試験で用いる動物モデルは、試験例1ならびに試験例2とは異なり、既存の抗てんかん薬に治療抵抗性を示す発作の表現系である。Slc:ddy系雄性マウス(一群5匹、体重20~30g)に被験化合物100 mg/kgを経口投与し、30分あるいは1時間後に角膜より電気刺激(6Hz,32mA,3秒間)を与え、誘発される前肢の間代性痙攣、挙尾反応、無動状態の発現の有無を観察した。なお、コントロールは0.5%トラガント液、0.5%メチルセルロース液、或いは、Olive oilを投与した。結果を表25に示す。
本試験は、薬物の協調運動能抑制作用を評価する試験である。Slc:ddy系雄性マウス(体重20~30g)を試験前日にローターロッド装置(直径4cmの円柱棒を回転させる装置、13回転/分)で3分間落下しないで歩行できるように訓練する。一群10匹に被験化合物を経口投与し、1時間後に上記同様にローターロッド装置に乗せ、100秒間歩行状態を観察した。100秒以内に協調運動障害にて落下した動物を陽性としてカウントした。なお、コントロールは0.5%メチルセルロース液を投与した。結果を表26に示す。
本試験は、薬物の抗うつ作用を評価する代表的な試験である(Porsoltら、Nature,266:730-732、1977,Porsolt ら、 Eur . J. Pharmacol .,47 : 379- 391 、 1978)。Crl:CD(SD)系雄性ラット(1群12匹、体重200~250g)を後肢が底面に接触できない深さ(205 mm)まで水を溜めた直径200 mmの水槽で15分間強制的に水泳を行わせ、水泳終了15分後に被験化合物を経口投与した。翌日、同用量の被験化合物を投与し、前日と同時刻に5分間水槽に移して、水泳行動を行わない無動時間を測定した。コントロール群に対する無動時間の短縮度合いにより抗うつ作用を評価した。コントロール群は0.5%メチルセルロース液を投与した。なお、上記条件において双極性障害に適応を持つLamotrigineは用量依存的に無動時間を短縮した。結果を表27に示す。
本試験は、薬物の抗躁作用を評価する試験である。本評価系は、統合失調症治療薬とは異なる気分安定化薬として臨床で使用されている抗躁病薬で有効性が確認できる試験系である(Arbanら、Behav. Brain Res.,158:123-132、2005、Foreman ら、 Pharmacol. Biochem. Behav. ,89 : 523-534 、 2008)。Slc:C57BL/6J 系雄性マウス(1群8匹、体重20~26g)に被験化合物を経口投与し、30分後にメタンフェタミン(4mg/kg)及びクロルジアゼポキシド(10mg/kg)を腹腔内投与した。その後90分間アクリル製ケージ(内寸:W360mm×D230mm×H310mm)の中で自由行動させ、動物の移動距離をEthovision software(Ver.3.1)を用いて測定した。新規環境馴化時間30分を除いた後半60分間の移動距離を測定値として用いた。コントロール群に対する短縮度合いにより抗躁作用を評価した。なおコントロール群は0.5%メチルセルロース液を投与した。なお、上記条件において、双極性障害に臨床適応を持つLamotrigineは用量依存的に移動距離を短縮した。結果を表28に示す。
実施例1の化合物(250g)、コーンスターチ(54g)、カルボキシメチルセルロースカルシウム(40g)、結晶セルロース(50g)及びステアリン酸マグネシウム(6g)を、常法により混合、造粒し、1錠あたり400mgで打錠し、1000錠を製する。
実施例1の化合物(500g)、乳糖(470g)、ヒドロキシプロピルセルロース(25g)及び軽質無水ケイ酸(5g)を、常法により混合した後、散剤に製する。
Claims (16)
- 下記式(A):
R1、R2、R3、Ra及びRbは、それらが結合するベンゼン環上の置換可能な任意の炭素原子に1つずつ結合し、同一又は異なって、水素原子、フッ素原子、塩素原子、臭素原子、C1-6アルキル、1~3個のフッ素原子で置換されたC1-6アルコキシ、C1-6アルキル-S(O)n-又はシアノを表し、該アルキル又はアルキル-S(O)n-は、1~5個のフッ素原子で置換されていてもよく、ここにおいて、R1、R2、R3、Ra及びRbのうちいずれか2つの基が、2’-メチル及び3’-メチルのときは、残りの基のうちいずれか1つは水素原子以外の基であり、
R4、R5、Rc及びRdは、それらが結合するベンゼン環上の置換可能な任意の炭素原子に1つずつ結合し、同一又は異なって、水素原子、フッ素原子、塩素原子、C1-6アルキル、1~3個のフッ素原子で置換されたC1-6アルコキシ、C1-6アルキル-S(O)n-、シアノ又はニトロを表し、該アルキル又はアルキル-S(O)n-は、1~5個のフッ素原子で置換されていてもよく、
R6及びR7は、同一又は異なって、水素原子、フッ素原子、メチル、エチル、水酸基若しくはC1-6アルコキシを表すか(ただし、一方が水酸基の場合、他方はフッ素原子、水酸基またはC1-6アルコキシではない)、又はそれらに結合する炭素原子と一緒になってC3-6シクロアルキルを構成し、該メチル、エチル、アルコキシ及びシクロアルキルは、1~5個のフッ素原子で置換されていてもよく、
R8及びR9は、同一又は異なって、水素原子、C1-6アルキル、C3-7シクロアルキル-C1-3アルキル又はC3-6シクロアルキルを表し、該アルキル、シクロアルキル-アルキル及びシクロアルキルは、1~5個のフッ素原子で置換されていてもよく、
nは、0~2の整数を表すが、
ただし、R1、R2、R3、R4、R5、Ra、Rb、Rc及びRdがすべて水素原子であって、R6及びR7が共に水素原子のときは、R8及びR9のいずれか一方は水素原子であり、
R1、R2、R3、R4、R5、Ra、Rb、Rc及びRdがすべて水素原子であって、R6及びR7のいずれか一方がアルコキシのときは、R8及びR9は、同一又は異なって、水素原子、C1-3アルキル、C3-7シクロアルキル-C1-3アルキル又はC3-6シクロアルキルである。]
で表される化合物、またはその水和物もしくは溶媒和物。 - Ra、Rb、RcおよびRdがいずれも水素原子である請求項1の化合物、またはその水和物もしくは溶媒和物。
- R8及びR9が、同一又は異なって、水素原子、C1-3アルキル、C3-7シクロアルキル-C1-3アルキル又はC3-6シクロアルキルであり、該アルキル、シクロアルキル-アルキル及びシクロアルキルは、1~5個のフッ素原子で置換されていてもよく、ここにおいて、R1、R2、R3、R4及びR5がすべて水素原子のときは、R8及びR9のいずれか一方は水素原子である、
請求項2に記載の化合物、またはその水和物もしくは溶媒和物。 - R1、R2及びR3が、同一又は異なって、水素原子、フッ素原子、塩素原子、臭素原子、トリフルオロメチル、トリフルオロメトキシ、C1-3アルキル-S(O)n-又はシアノであり、該アルキル-S(O)n-は、1~5個のフッ素原子で置換されていてもよい、
請求項2又は請求項3に記載の化合物、またはその水和物もしくは溶媒和物。 - R1、R2及びR3が、同一又は異なって、水素原子、フッ素原子、塩素原子、臭素原子、トリフルオロメチル、トリフルオロメトキシである、
請求項2又は請求項3に記載の化合物、またはその水和物もしくは溶媒和物。 - R9が、水素原子である、請求項2~5のいずれかに記載の化合物、またはその水和物もしくは溶媒和物。
- R4及びR5が、同一又は異なって、水素原子、フッ素原子、塩素原子、C1-3アルキル、トリフルオロメチル又はトリフルオロメトキシである、
請求項2~6のいずれかに記載の化合物、またはその水和物もしくは溶媒和物。 - R6及びR7が、同一又は異なって、水素原子、メチル、エチル、水酸基又はC1-3アルコキシである(ただし、一方が水酸基の場合、他方は水酸基またはC1-3アルコキシではない)、
請求項2~7のいずれかに記載の化合物、またはその水和物もしくは溶媒和物。 - R1、R2及びR3が、同一又は異なって、水素原子、フッ素原子、塩素原子、臭素原子、トリフルオロメチル又はトリフルオロメトキシであり、
R4及びR5が、同一又は異なって、水素原子、フッ素原子、塩素原子、トリフルオロメチル又はトリフルオロメトキシであり、
R6及びR7が、同一又は異なって、水素原子、メチル、水酸基又はメトキシであり(ただし、一方が水酸基の場合、他方は水酸基またはメトキシではない)、
R8が、水素原子、メチル又はエチルであり、
R9が水素原子である、
請求項2に記載の化合物、またはその水和物もしくは溶媒和物。 - R1、R2、R3、R4及びR5がすべて同時に水素原子ではない、請求項2又は請求項9に記載の化合物、またはその水和物もしくは溶媒和物。
- 以下の化合物から選択される、請求項2に記載の化合物、またはその水和物もしくは溶媒和物:
N,N-ジメチル-2-[4’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
2-[3’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
N-メチル-2-[3’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
N-メチル-2-[2’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
N-エチル-2-[2’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
2-(3’,4’-ジフルオロビフェニル-2-イル)アセトアミド、
2-(4’-フルオロビフェニル-2-イル)アセトアミド、
2-(4’-フルオロビフェニル-2-イル)-N-メチルアセトアミド、
2-(3’-フルオロビフェニル-2-イル)-N-メチルアセトアミド、
2-(2’-フルオロビフェニル-2-イル)-N-メチルアセトアミド、
2-(2’-フルオロビフェニル-2-イル)-N,N-ジメチルアセトアミド、
N-メチル-2-(3’-メチルビフェニル-2-イル)-アセトアミド、
2-(5-フルオロビフェニル-2-イル)アセトアミド、
2-(4-フルオロビフェニル-2-イル)-N-メチルアセトアミド、
2-(2’,4-ジフルオロビフェニル-2-イル)アセトアミド、
2-(2’,4-ジフルオロビフェニル-2-イル)-N-メチルアセトアミド、
2-(3’-クロロ-4-フルオロビフェニル-2-イル)アセトアミド、
2-(4’-ブロモビフェニル-2-イル)アセトアミド、
2-(ビフェニル-2-イル)アセトアミド、
2-(3’-ブロモビフェニル-2-イル)アセトアミド、
2-(4’-ブロモビフェニル-2-イル)-N-メチルアセトアミド、
2-(ビフェニル-2-イル)-N-メチルアセトアミド、
2-(ビフェニル-2-イル)プロパンアミド、
2-(2’-フルオロビフェニル-2-イル)-N-メチルプロパンアミド、
2-(ビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(2’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(ビフェニル-2-イル)-2-メトキシアセトアミド、
2-(ビフェニル-2-イル)-2-メトキシ-N-メチルアセトアミド、
2-(ビフェニル-2-イル)-2-メトキシ-N,N-ジメチルアセトアミド、
2-(2’,3’,5’-トリクロロビフェニル-2-イル)アセトアミド、
2-(4’-クロロ-2’-フルオロビフェニル-2-イル)アセトアミド、
2-[3’-フルオロ-4’-(トリフルオロメトキシ)ビフェニル-2-イル]アセトアミド、
2-[3’-フルオロ-5’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
2-[4’-クロロ-3’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
2-[4’-(トリフルオロメトキシ)ビフェニル-2-イル]アセトアミド、
2-(2’,4’-ジクロロビフェニル-2-イル)アセトアミド、
2-[3’-(トリフルオロメトキシ)ビフェニル-2-イル]アセトアミド、
2-[2’-フルオロ-5’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
2-[2’-クロロ-4’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
2-(2’-クロロ-3’-フルオロビフェニル-2-イル)アセトアミド、
2-(5’-クロロ-2’-フルオロビフェニル-2-イル)アセトアミド、
2-(2’,3’,4’-トリフルオロビフェニル-2-イル)アセトアミド、
2-[2’-フルオロ-5’-(トリフルオロメトキシ)ビフェニル-2-イル]アセトアミド、
2-(2’,5’-ジクロロビフェニル-2-イル)アセトアミド、
N-メチル-2-[3’-(トリフルオロメトキシ)ビフェニル-2-イル]アセトアミド、
2-(2’-フルオロ-5-ニトロビフェニル-2-イル)アセトアミド、
2-(3-フルオロビフェニル-2-イル)アセトアミド、
2-(3-フルオロビフェニル-2-イル)-N-メチルアセトアミド、
2-(5-クロロビフェニル-2-イル)-N-メチルアセトアミド、
2-(5-クロロ-2’-フルオロビフェニル-2-イル)アセトアミド、
2-(5-クロロ-2’-フルオロビフェニル-2-イル)-N-メチルアセトアミド、
2-[4-クロロ-4’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
2-[3’-フルオロ-5-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
2-(3’,5’-ジクロロ-5-フルオロビフェニル-2-イル)アセトアミド、
2-(3’,5’-ジフルオロビフェニル-2-イル)アセトアミド、
1-(2’-フルオロビフェニル-2-イル)-N-メチルシクロプロパンカルボキシアミド、
1-(3’-フルオロビフェニル-2-イル)-N-メチルシクロプロパンカルボキシアミド、
2-フルオロ-2-(3’-フルオロビフェニル-2-イル)アセトアミド、
2-フルオロ-2-(2’-フルオロビフェニル-2-イル)-N-メチルアセトアミド、
2-フルオロ-2-(4’-フルオロビフェニル-2-イル)-N-メチルアセトアミド、
2-(3’-クロロビフェニル-2-イル)-2-フルオロアセトアミド、
2,2-ジフルオロ-2-(3’-フルオロビフェニル-2-イル)アセトアミド、
2-(3’-クロロビフェニル-2-イル)-2,2-ジフルオロアセトアミド、
2-(ビフェニル-2-イル)-2,2-ジフルオロアセトアミド、
2-(3’-フルオロビフェニル-2-イル)プロパンアミド、
2-(5-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(3’,5-ジフルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(3’,5’-ジクロロ-5-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(3’,4-ジフルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(3’,5’-ジクロロ-4-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(5’-クロロ-2’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(2’-フルオロビフェニル-2-イル)-2-メトキシアセトアミド、
2-(2’-フルオロビフェニル-2-イル)-2-メトキシ―N―メチルアセトアミド、
2-(2’-フルオロビフェニル-2-イル)-2-メトキシ-N,N-ジメチルアセトアミド、
2-(4’-フルオロビフェニル-2-イル)-2-メトキシアセトアミド、
2-(4’-フルオロビフェニル-2-イル)-2-メトキシ-N,N-ジメチルアセトアミド、及び
2-(3’-クロロビフェニル-2-イル)-2-メトキシアセトアミド。 - 以下の化合物から選択される、請求項2に記載の化合物、またはその水和物もしくは溶媒和物:
2-(2’-フルオロビフェニル-2-イル)アセトアミド、
N-メチル-2-[4’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
2-(3’-クロロビフェニル-2-イル)アセトアミド、
2-(2’-クロロビフェニル-2-イル)アセトアミド、
2-(2’,3’-ジフルオロビフェニル-2-イル)アセトアミド、
2-(3’-フルオロビフェニル-2-イル)アセトアミド、
2-(2’,5-ジフルオロビフェニル-2-イル)アセトアミド、
2-(4-フルオロビフェニル-2-イル)アセトアミド、
2-(ビフェニル-2-イル)-N-メチルプロパンアミド、
2-(ビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(2’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(2’,3’,5’-トリフルオロビフェニル-2-イル)アセトアミド、
2-(3’,5’-ジクロロビフェニル-2-イル)アセトアミド、
2-(3’-クロロ-5’-フルオロビフェニル-2-イル)アセトアミド、
2-(2’-クロロ-5’-フルオロビフェニル-2-イル)アセトアミド、
2-(3’,4’,5’-トリフルオロビフェニル-2-イル)アセトアミド、
2-[5’-クロロ-2’-(トリフルオロメチル)ビフェニル-2-イル]アセトアミド、
2-(4,4’-ジクロロビフェニル-2-イル)アセトアミド、
2-(4,4’-ジフルオロビフェニル-2-イル)アセトアミド、
2-(4,4’-ジフルオロビフェニル-2-イル)-N-メチルアセトアミド、
2-(3,3’-ジフルオロビフェニル-2-イル)アセトアミド、
2-(2’,5’-ジフルオロビフェニル-2-イル)アセトアミド、
2-(ビフェニル-2-イル)-2-フルオロアセトアミド、
2-フルオロ-2-(2’-フルオロビフェニル-2-イル)アセトアミド、
2-(3’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(3’,5’-ジクロロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(2’-クロロ-5’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(4-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(2’-クロロビフェニル-2-イル)-2-ヒドロキシアセトアミド、
2-(3’-クロロ-5’-フルオロビフェニル-2-イル)-2-ヒドロキシアセトアミド、及び
2-ヒドロキシ-2-(3’,4’,5’-トリフルオロビフェニル-2-イル)アセトアミド。 - 請求項1~12のいずれか1項に記載の化合物またはその水和物もしくは溶媒和物、及び医薬的に許容される担体を含有する医薬組成物。
- 請求項1~12のいずれか1項に記載の化合物またはその水和物もしくは溶媒和物を有効成分とする抗てんかん薬。
- 請求項1~12のいずれか1項に記載の化合物またはその水和物もしくは溶媒和物を有効成分とする双極性障害に対する気分安定化薬。
- 抗てんかん薬を製造するための請求項1~12のいずれか1項に記載の化合物またはその水和物もしくは溶媒和物の使用。
Priority Applications (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2009314879A AU2009314879A1 (en) | 2008-11-14 | 2009-11-13 | Biphenylacetamide derivative |
| MX2011005098A MX2011005098A (es) | 2008-11-14 | 2009-11-13 | Derivados de bifenilacetamida. |
| CN2009801545934A CN102282124A (zh) | 2008-11-14 | 2009-11-13 | 联苯乙酰胺衍生物 |
| EP09826157A EP2374790A4 (en) | 2008-11-14 | 2009-11-13 | BIPHENYLACETAMIDE DERIVATIVE |
| JP2010537814A JPWO2010055911A1 (ja) | 2008-11-14 | 2009-11-13 | ビフェニルアセトアミド誘導体 |
| CA2742679A CA2742679A1 (en) | 2008-11-14 | 2009-11-13 | Biphenylacetamide derivatives |
| BRPI0921059-8A BRPI0921059A2 (ja) | 2008-11-14 | 2009-11-13 | Biphenyl acetamide derivative |
| US13/129,394 US20110224304A1 (en) | 2008-11-14 | 2009-11-13 | Biphenylacetamide derivative |
| RU2011123777/04A RU2011123777A (ru) | 2008-11-14 | 2009-11-13 | Бифенилацетамидные производные |
| IL212683A IL212683A0 (en) | 2008-11-14 | 2011-05-04 | Biphenylacetamide derivative |
| US13/368,325 US20120142771A1 (en) | 2008-11-14 | 2012-02-07 | Biphenylacetamide derivative |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008-292682 | 2008-11-14 | ||
| JP2008292682 | 2008-11-14 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/368,325 Continuation US20120142771A1 (en) | 2008-11-14 | 2012-02-07 | Biphenylacetamide derivative |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010055911A1 true WO2010055911A1 (ja) | 2010-05-20 |
Family
ID=42170041
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2009/069355 Ceased WO2010055911A1 (ja) | 2008-11-14 | 2009-11-13 | ビフェニルアセトアミド誘導体 |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US20110224304A1 (ja) |
| EP (1) | EP2374790A4 (ja) |
| JP (1) | JPWO2010055911A1 (ja) |
| KR (1) | KR20110086152A (ja) |
| CN (1) | CN102282124A (ja) |
| AU (1) | AU2009314879A1 (ja) |
| BR (1) | BRPI0921059A2 (ja) |
| CA (1) | CA2742679A1 (ja) |
| IL (1) | IL212683A0 (ja) |
| MX (1) | MX2011005098A (ja) |
| RU (1) | RU2011123777A (ja) |
| TW (1) | TW201023854A (ja) |
| WO (1) | WO2010055911A1 (ja) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2511273A1 (en) * | 2011-04-15 | 2012-10-17 | Laboratoire Biodim | Inhibitors of viral replication, their process of preparation and their therapeutical uses |
| WO2013062028A1 (ja) | 2011-10-25 | 2013-05-02 | 塩野義製薬株式会社 | Hiv複製阻害剤 |
| JPWO2021187486A1 (ja) * | 2020-03-17 | 2021-09-23 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101475115B1 (ko) * | 2012-06-15 | 2014-12-23 | 중앙대학교 산학협력단 | 탐식작용 억제 효과를 가지는 자가 면역질환의 예방 또는 치료용 화합물 |
| EP2821104A1 (en) | 2013-07-05 | 2015-01-07 | Laboratoire Biodim | Inhibitors of viral replication, their process of preparation and their therapeutical uses |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4277471A (en) | 1979-03-12 | 1981-07-07 | Eli Lilly And Company | 1,1-Biphenyl-2-yl alkylamines, formulations and antiarrhythmic treatment |
| US20040254166A1 (en) | 2003-04-10 | 2004-12-16 | Kreutter Kevin D. | Substituted phenyl acetamides and their use as protease inhibitors |
| WO2005063222A1 (ja) * | 2003-12-26 | 2005-07-14 | Kyowa Hakko Kogyo Co., Ltd. | Hsp90ファミリー蛋白質阻害剤 |
| WO2009013963A1 (ja) | 2007-07-23 | 2009-01-29 | Ntn Corporation | 動圧軸受装置 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3049564A (en) * | 1958-07-23 | 1962-08-14 | Ici Ltd | 2-phenylbenzamide derivatives and procedures for making same |
| WO1999029657A1 (en) * | 1997-12-10 | 1999-06-17 | Nps Pharmaceuticals, Inc. | Anticonvulsant and central nervous system-active bis(fluorophenyl)alkylamides |
-
2009
- 2009-11-13 WO PCT/JP2009/069355 patent/WO2010055911A1/ja not_active Ceased
- 2009-11-13 AU AU2009314879A patent/AU2009314879A1/en not_active Abandoned
- 2009-11-13 EP EP09826157A patent/EP2374790A4/en not_active Withdrawn
- 2009-11-13 MX MX2011005098A patent/MX2011005098A/es not_active Application Discontinuation
- 2009-11-13 BR BRPI0921059-8A patent/BRPI0921059A2/ja not_active IP Right Cessation
- 2009-11-13 KR KR1020117013390A patent/KR20110086152A/ko not_active Withdrawn
- 2009-11-13 JP JP2010537814A patent/JPWO2010055911A1/ja active Pending
- 2009-11-13 TW TW098138567A patent/TW201023854A/zh unknown
- 2009-11-13 RU RU2011123777/04A patent/RU2011123777A/ru not_active Application Discontinuation
- 2009-11-13 CN CN2009801545934A patent/CN102282124A/zh active Pending
- 2009-11-13 US US13/129,394 patent/US20110224304A1/en not_active Abandoned
- 2009-11-13 CA CA2742679A patent/CA2742679A1/en not_active Abandoned
-
2011
- 2011-05-04 IL IL212683A patent/IL212683A0/en unknown
-
2012
- 2012-02-07 US US13/368,325 patent/US20120142771A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4277471A (en) | 1979-03-12 | 1981-07-07 | Eli Lilly And Company | 1,1-Biphenyl-2-yl alkylamines, formulations and antiarrhythmic treatment |
| US20040254166A1 (en) | 2003-04-10 | 2004-12-16 | Kreutter Kevin D. | Substituted phenyl acetamides and their use as protease inhibitors |
| WO2005063222A1 (ja) * | 2003-12-26 | 2005-07-14 | Kyowa Hakko Kogyo Co., Ltd. | Hsp90ファミリー蛋白質阻害剤 |
| WO2009013963A1 (ja) | 2007-07-23 | 2009-01-29 | Ntn Corporation | 動圧軸受装置 |
Non-Patent Citations (12)
| Title |
|---|
| ARBAN ET AL., BEHAV. BRAIN RES., vol. 158, 2005, pages 123 - 132 |
| BRADSHER, C.K. ET AL.: "Aromatic cyclodehydration. XXVIII. 9, 10-Dialkylphenanthrenes by cyclization of ketones", JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, vol. 76, 1954, pages 4140 - 3 * |
| BRADSHER, C.K. ET AL.: "The cyclization of nitriles: a new route to some phenanthrylamines", JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, vol. 78, 1956, pages 2153 - 6 * |
| BRAMBILLA, P., BARRALE, F., SOARES, J.C.: "Perspectives on the use of anticonvulsants in the treatment of bipolar disorder", INTERNATIONAL JOURNAL OF NEUROPSYCHOPHARMACOLOGY, vol. 4, 2001, pages 421 - 446 |
| FOREMAN ET AL., PHARMACOL. BIOCHEM. BEHAV., vol. 89, 2008, pages 523 - 534 |
| LOSCHER ET AL., EPILEPSY RES., vol. 40, 2000, pages 63 - 77 |
| MERLIC, C.A. ET AL.: "Aminobenzannulation via metathesis of isonitriles using chromium carbene complexes", JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, vol. 114, no. 22, 1992, pages 8722 - 4 * |
| MOTOR SEIZURE. ELECTROENCEPH. CLIN. NEUROPHYSIOL., vol. 32, 1972, pages 281 - 294 |
| PAWEL, D.Z ET AL.: "Non-epilepsy use of antiepileptic drugs", PHARMACOLOGICAL REPORTS, vol. 58, 2006, pages 1 - 12 |
| PORSOLT ET AL., EUR. J. PHARMACOL., vol. 47, 1978, pages 379 - 391 |
| PORSOLT ET AL., NATURE, vol. 266, 1977, pages 730 - 732 |
| TETRAHEDRON LETTERS, vol. 27, 1986, pages 6103 - 6106 |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2511273A1 (en) * | 2011-04-15 | 2012-10-17 | Laboratoire Biodim | Inhibitors of viral replication, their process of preparation and their therapeutical uses |
| WO2012140243A1 (en) * | 2011-04-15 | 2012-10-18 | Laboratoire Biodim | Inhibitors of viral replication, their process of preparation and their therapeutical uses |
| US9604900B2 (en) | 2011-04-15 | 2017-03-28 | Laboratoire Biodim | Inhibitors of viral replication, their process of preparation and their therapeutical uses |
| WO2013062028A1 (ja) | 2011-10-25 | 2013-05-02 | 塩野義製薬株式会社 | Hiv複製阻害剤 |
| US20140249306A1 (en) * | 2011-10-25 | 2014-09-04 | Shionogi & Co., Ltd. | Hiv replication inhibitor |
| JPWO2013062028A1 (ja) * | 2011-10-25 | 2015-04-02 | 塩野義製薬株式会社 | Hiv複製阻害剤 |
| US9199959B2 (en) | 2011-10-25 | 2015-12-01 | Shionogi & Co., Ltd. | HIV replication inhibitor |
| JPWO2021187486A1 (ja) * | 2020-03-17 | 2021-09-23 | ||
| JP7637121B2 (ja) | 2020-03-17 | 2025-02-27 | 住友ファーマ株式会社 | オキサジアゾール誘導体 |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20110086152A (ko) | 2011-07-27 |
| TW201023854A (en) | 2010-07-01 |
| CN102282124A (zh) | 2011-12-14 |
| AU2009314879A1 (en) | 2011-06-30 |
| US20120142771A1 (en) | 2012-06-07 |
| MX2011005098A (es) | 2011-05-31 |
| BRPI0921059A2 (ja) | 2018-10-16 |
| RU2011123777A (ru) | 2012-12-20 |
| EP2374790A4 (en) | 2012-05-30 |
| JPWO2010055911A1 (ja) | 2012-04-12 |
| IL212683A0 (en) | 2011-07-31 |
| EP2374790A1 (en) | 2011-10-12 |
| CA2742679A1 (en) | 2010-05-20 |
| US20110224304A1 (en) | 2011-09-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN100369894C (zh) | 氧化氮合酶抑制剂磷酸盐 | |
| US8501814B2 (en) | Selective androgen receptor modulators | |
| WO2011145669A1 (ja) | アミド誘導体 | |
| TW201731817A (zh) | 經取代哌啶化合物及其用途 | |
| TW202136194A (zh) | 4-纈胺醯氧基丁酸的合成方法 | |
| KR101532570B1 (ko) | 벤즈아미드 화합물의 합성에 유용한 화합물 | |
| JP6348904B2 (ja) | 置換アルキルジアリール誘導体、その製造方法及び使用 | |
| WO2017214539A1 (en) | Fluorinated 2-amino-4-(substituted amino)phenyl carbamate derivatives | |
| KR100417670B1 (ko) | 비시클릭아미드유도체및근이완제로서의이의용도 | |
| CN102361557A (zh) | 制备阿格列汀的方法 | |
| WO2010055911A1 (ja) | ビフェニルアセトアミド誘導体 | |
| KR20100099134A (ko) | 항섬유증 제제의 할로겐화 유사체 | |
| US8835672B2 (en) | Manufacture of a triiodinated contrast agent | |
| WO1995030645A1 (en) | Amide derivatives and their therapeutic use | |
| JP3231775B2 (ja) | 心循環器系に作用する2−アミノ−1,2,3,4−テトラヒドロナフタレン誘導体、それらを製造する方法、及びそれらを含む医薬組成物 | |
| JP2012001537A (ja) | ビフェニルアセトアミド誘導体からなる医薬 | |
| CN110483417B (zh) | 一种DACOs类NNRTIs氨基酸酯衍生物、其制备方法、药物组合物及应用 | |
| CA2891932A1 (en) | Process for the preparation of (1s,4s,5s)-4-bromo-6-oxabicyclo[3.2.1] octan-7-one | |
| CN102001920B (zh) | 一种药物中间体的制备方法 | |
| HK1161584A (en) | Biphenylacetamide derivative | |
| US9951022B2 (en) | Method for producing 4,4,7-trifluoro-1,2,3,4-tetrahydro-5H-1-benzazepine compound and intermediate used in the method | |
| US6124284A (en) | Bicyclic amide derivatives and their use as muscle relaxants | |
| JPH06506916A (ja) | アミド誘導体およびそれらの治療への使用 | |
| CA2579230A1 (en) | Fibrate compounds having ppar agonist activity | |
| JPWO2017170859A1 (ja) | ビスアリール誘導体及びその医薬用途 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200980154593.4 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 09826157 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE2 | Request for preliminary examination filed before expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2742679 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 212683 Country of ref document: IL |
|
| ENP | Entry into the national phase |
Ref document number: 2010537814 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13129394 Country of ref document: US Ref document number: MX/A/2011/005098 Country of ref document: MX |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2009826157 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 4014/CHENP/2011 Country of ref document: IN |
|
| ENP | Entry into the national phase |
Ref document number: 20117013390 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011123777 Country of ref document: RU |
|
| ENP | Entry into the national phase |
Ref document number: 2009314879 Country of ref document: AU Date of ref document: 20091113 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: PI0921059 Country of ref document: BR Kind code of ref document: A2 Effective date: 20110513 |