WO2010041648A1 - チタン酸アルミニウム系セラミックス粉末の製造方法 - Google Patents
チタン酸アルミニウム系セラミックス粉末の製造方法 Download PDFInfo
- Publication number
- WO2010041648A1 WO2010041648A1 PCT/JP2009/067388 JP2009067388W WO2010041648A1 WO 2010041648 A1 WO2010041648 A1 WO 2010041648A1 JP 2009067388 W JP2009067388 W JP 2009067388W WO 2010041648 A1 WO2010041648 A1 WO 2010041648A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- powder
- aluminum
- aluminum titanate
- source powder
- particle size
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G23/00—Compounds of titanium
- C01G23/003—Titanates
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/01—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics
- C04B35/46—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics based on titanium oxides or titanates
- C04B35/462—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics based on titanium oxides or titanates based on titanates
- C04B35/478—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics based on titanium oxides or titanates based on titanates based on aluminium titanates
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/622—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/626—Preparing or treating the powders individually or as batches ; preparing or treating macroscopic reinforcing agents for ceramic products, e.g. fibres; mechanical aspects section B
- C04B35/62605—Treating the starting powders individually or as mixtures
- C04B35/6261—Milling
- C04B35/6262—Milling of calcined, sintered clinker or ceramics
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/54—Particles characterised by their aspect ratio, i.e. the ratio of sizes in the longest to the shortest dimension
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/60—Particles characterised by their size
- C01P2004/61—Micrometer sized, i.e. from 1-100 micrometer
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/60—Particles characterised by their size
- C01P2004/62—Submicrometer sized, i.e. from 0.1-1 micrometer
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/32—Metal oxides, mixed metal oxides, or oxide-forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3205—Alkaline earth oxides or oxide forming salts thereof, e.g. beryllium oxide
- C04B2235/3206—Magnesium oxides or oxide-forming salts thereof
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/32—Metal oxides, mixed metal oxides, or oxide-forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3217—Aluminum oxide or oxide forming salts thereof, e.g. bauxite, alpha-alumina
- C04B2235/3222—Aluminates other than alumino-silicates, e.g. spinel (MgAl2O4)
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/32—Metal oxides, mixed metal oxides, or oxide-forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3231—Refractory metal oxides, their mixed metal oxides, or oxide-forming salts thereof
- C04B2235/3232—Titanium oxides or titanates, e.g. rutile or anatase
- C04B2235/3234—Titanates, not containing zirconia
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/34—Non-metal oxides, non-metal mixed oxides, or salts thereof that form the non-metal oxides upon heating, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3418—Silicon oxide, silicic acids or oxide forming salts thereof, e.g. silica sol, fused silica, silica fume, cristobalite, quartz or flint
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/34—Non-metal oxides, non-metal mixed oxides, or salts thereof that form the non-metal oxides upon heating, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3427—Silicates other than clay, e.g. water glass
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/34—Non-metal oxides, non-metal mixed oxides, or salts thereof that form the non-metal oxides upon heating, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3427—Silicates other than clay, e.g. water glass
- C04B2235/3463—Alumino-silicates other than clay, e.g. mullite
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/38—Non-oxide ceramic constituents or additives
- C04B2235/3817—Carbides
- C04B2235/3826—Silicon carbides
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/38—Non-oxide ceramic constituents or additives
- C04B2235/3852—Nitrides, e.g. oxynitrides, carbonitrides, oxycarbonitrides, lithium nitride, magnesium nitride
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/38—Non-oxide ceramic constituents or additives
- C04B2235/3852—Nitrides, e.g. oxynitrides, carbonitrides, oxycarbonitrides, lithium nitride, magnesium nitride
- C04B2235/3873—Silicon nitrides, e.g. silicon carbonitride, silicon oxynitride
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/38—Non-oxide ceramic constituents or additives
- C04B2235/3852—Nitrides, e.g. oxynitrides, carbonitrides, oxycarbonitrides, lithium nitride, magnesium nitride
- C04B2235/3886—Refractory metal nitrides, e.g. vanadium nitride, tungsten nitride
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/40—Metallic constituents or additives not added as binding phase
- C04B2235/401—Alkaline earth metals
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/40—Metallic constituents or additives not added as binding phase
- C04B2235/402—Aluminium
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/40—Metallic constituents or additives not added as binding phase
- C04B2235/404—Refractory metals
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/44—Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate
- C04B2235/441—Alkoxides, e.g. methoxide, tert-butoxide
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/44—Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate
- C04B2235/442—Carbonates
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/44—Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate
- C04B2235/443—Nitrates or nitrites
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/44—Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate
- C04B2235/444—Halide containing anions, e.g. bromide, iodate, chlorite
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/44—Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate
- C04B2235/446—Sulfides, tellurides or selenides
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/44—Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate
- C04B2235/447—Phosphates or phosphites, e.g. orthophosphate or hypophosphite
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/44—Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate
- C04B2235/448—Sulphates or sulphites
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/44—Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate
- C04B2235/449—Organic acids, e.g. EDTA, citrate, acetate, oxalate
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/50—Constituents or additives of the starting mixture chosen for their shape or used because of their shape or their physical appearance
- C04B2235/54—Particle size related information
- C04B2235/5418—Particle size related information expressed by the size of the particles or aggregates thereof
- C04B2235/5436—Particle size related information expressed by the size of the particles or aggregates thereof micrometer sized, i.e. from 1 to 100 micron
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/50—Constituents or additives of the starting mixture chosen for their shape or used because of their shape or their physical appearance
- C04B2235/54—Particle size related information
- C04B2235/5463—Particle size distributions
- C04B2235/5481—Monomodal
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/65—Aspects relating to heat treatments of ceramic bodies such as green ceramics or pre-sintered ceramics, e.g. burning, sintering or melting processes
- C04B2235/656—Aspects relating to heat treatments of ceramic bodies such as green ceramics or pre-sintered ceramics, e.g. burning, sintering or melting processes characterised by specific heating conditions during heat treatment
- C04B2235/6562—Heating rate
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/29—Coated or structually defined flake, particle, cell, strand, strand portion, rod, filament, macroscopic fiber or mass thereof
- Y10T428/2982—Particulate matter [e.g., sphere, flake, etc.]
Definitions
- the present invention relates to a method for producing an aluminum titanate-based ceramic powder, and more specifically, a precursor mixture containing a titanium source powder, an aluminum source powder and a silicon source powder is fired, and pulverized and classified to obtain an aluminum titanate-based powder.
- the present invention relates to a method for producing ceramic powder.
- Patent Document 1 discloses a method of mixing a powdery titanium source and an aluminum source and firing the obtained precursor mixture. Yes.
- the aluminum titanate-based ceramics obtained by such a method are usually massive, but a powder can be obtained by pulverizing this.
- the obtained aluminum titanate-based ceramic powder can be made into a molded body by a method such as extrusion molding after adding a liquid component such as water to form a clay.
- the aluminum titanate-based ceramic powder obtained by pulverization contains many fine particle components. For this reason, the pulverized aluminum titanate ceramic powder is usually used for molding after removing fine and coarse components by classification such as sieving.
- the removed fine particle component closes the pores of the ceramic molded body obtained at the time of manufacturing the extruded product.
- the coarse particle component may clog the extruder, and there is a problem that a molded product having a thin wall surface cannot be obtained from the coarse particle component. Therefore, the removed fine and coarse components of the aluminum titanate-based ceramic powder cannot be used for forming as they are, and are all wasted.
- An object of the present invention is to provide a method capable of efficiently producing an aluminum titanate-based ceramic powder having a very sharp particle size distribution, in which the production of fine and coarse components is suppressed, and with a high yield. It is.
- the present invention includes a step of holding a precursor mixture containing a titanium source powder, an aluminum source powder and a silicon source powder in a temperature range of 1100 ° C. to 1350 ° C. for 3 hours or more, and raising the temperature to a temperature of 1400 ° C. or more. Firing the precursor mixture after holding at a temperature to obtain an aluminum titanate-based ceramic fired body, and crushing and classifying the aluminum titanate-based ceramic fired body, wherein: In the step of pulverizing and classifying the aluminum titanate ceramic fired body, the aluminum titanate ceramic fired body is impacted and pulverized, and then the obtained pulverized product is classified to obtain titanium having a predetermined particle size or less.
- the step (B) is preferably repeated twice or more.
- step (B) When the step (B) is repeated twice or more, the “remaining pulverized product” in the second and subsequent steps (B) is read as “remaining pulverized product obtained in the classification in the previous step (B)”. It is done.
- the aluminum titanate ceramic powder having a predetermined particle size or less generated by pulverization is immediately classified and discharged from the pulverization region, while continuing the pulverization with respect to the remainder of the pulverized material existing in the pulverization region, It is preferable to perform the step (A) and the step (B) continuously, and the step of pulverizing and classifying is preferably performed by a pulverizer with a built-in classification mechanism.
- the aluminum titanate-based ceramic powder having a predetermined particle size or less obtained in the steps (A) and (B) is an aluminum titanate-based ceramic powder having a maximum particle size of 110 ⁇ m or less.
- the precursor mixture may further contain a magnesium source powder.
- the amount of titania-converted titanium source powder used is 20 per 100 parts by mass of the total amount of titania-converted titanium source powder used, alumina-converted aluminum source powder, and magnesia-converted magnesium source powder. It is preferable that it is not less than 60 parts by mass.
- the amount of aluminum source powder converted into alumina is about 100 parts by mass of the total amount of titanium source powder converted into titania, the amount of aluminum source powder converted into alumina, and the amount of magnesium source powder converted into magnesia. 30 parts by mass or more and 70 parts by mass or less is preferable.
- the amount of magnesium source powder converted to magnesia is about 100 parts by mass of the total amount of titanium source powder converted to titania, the amount of aluminum source powder converted to alumina, and the amount of magnesium source powder converted to magnesia.
- the content is preferably 0.1 parts by mass or more and 10 parts by mass or less.
- the present invention also includes an aluminum titanate ceramic powder obtained by any of the above methods.
- the aluminum titanate-based ceramic powder preferably has a content of fine particles having a particle size of 10 ⁇ m or less and 20% by volume or less, and a content of coarse particles having a particle size of 70 ⁇ m or less is less than 10% by volume. .
- an aluminum titanate-based ceramic powder having a very sharp particle size distribution that does not contain fine and coarse components it is possible to efficiently produce an aluminum titanate-based ceramic powder having a very sharp particle size distribution that does not contain fine and coarse components. That is, according to the production method of the present invention, the generation of an unsuitable component as a molding powder such as a fine particle component and a coarse particle component is suppressed, and an aluminum titanate ceramic powder having a particle size distribution suitable as a molding powder is obtained. It can be manufactured with good yield.
- the manufacturing method of the aluminum titanate ceramic powder of the present invention includes the following steps. (1) A step of holding a precursor mixture containing a titanium source powder, an aluminum source powder and a silicon source powder in a temperature range of 1100 ° C. to 1350 ° C. for 3 hours or more (holding step). (2) A step of obtaining an aluminum titanate-based ceramic fired body by firing at a temperature of 1400 ° C. or higher and firing the retained precursor mixture at the same temperature (firing step). (3) A step of pulverizing and classifying the aluminum titanate-based ceramic fired body (pulverization / classification step).
- a precursor mixture containing a titanium source powder, an aluminum source powder and a silicon source powder is held at a temperature range of 1100 ° C. to 1350 ° C. for 3 hours or more.
- the precursor mixture can be obtained by mixing a titanium source powder, an aluminum source powder, and a silicon source powder. This precursor mixture is led to aluminum titanate ceramics by firing.
- the titanium source powder constituting the precursor mixture is a powder of a substance that becomes a titanium component constituting the aluminum titanate-based ceramics, and examples of such a substance include titanium oxide powder.
- examples of titanium oxide include titanium (IV) oxide, titanium (III) oxide, and titanium (II) oxide, and titanium (IV) oxide is preferably used.
- titanium oxide may be crystalline or amorphous.
- examples of the crystal type include anatase type, rutile type, brookite type, and the like, preferably anatase type and rutile type.
- the titanium source powder used in the present invention may be a powder of a substance that is led to titania (titanium oxide) by firing in air.
- titania titanium oxide
- examples of such substances include titanium salts, titanium alkoxides, titanium hydroxide, titanium nitride, titanium sulfide, and titanium.
- titanium salt examples include titanium trichloride, titanium tetrachloride, titanium sulfide (IV), titanium sulfide (VI), and titanium sulfate (IV).
- titanium alkoxide examples include titanium (IV) ethoxide, titanium (IV) methoxide, titanium (IV) tert-butoxide, titanium (IV) isobutoxide, titanium (IV) n-propoxide, titanium (IV) tetraiso Examples thereof include propoxide and chelates thereof.
- titanium oxide powder is preferably used, and titanium oxide (IV) powder is more preferable.
- the aluminum source powder is a powder of a substance that is an aluminum component constituting the aluminum titanate ceramic, and examples thereof include alumina (aluminum oxide) powder.
- Alumina may be crystalline or amorphous. When the alumina is crystalline, examples of the crystal type include ⁇ -type, ⁇ -type, ⁇ -type, ⁇ -type, etc., and ⁇ -type alumina is preferably used.
- the aluminum source powder used in the present invention may be a powder of a substance led to alumina by firing in air.
- a substance led to alumina by firing in air examples include an aluminum salt, aluminum alkoxide, aluminum hydroxide, and metal aluminum.
- the aluminum salt may be an inorganic acid salt (inorganic salt) or an organic acid salt (organic salt).
- the aluminum inorganic salt include nitrates such as aluminum nitrate and ammonium aluminum nitrate; carbonates such as ammonium aluminum carbonate and the like.
- the aluminum organic salt include aluminum oxalate, aluminum acetate, aluminum stearate, aluminum lactate, and aluminum laurate.
- aluminum alkoxide examples include aluminum isopropoxide, aluminum ethoxide, aluminum sec-butoxide, aluminum tert-butoxide, and the like.
- Aluminum hydroxide may be crystalline or amorphous.
- examples of the crystal form include a gibbsite type, a bayerite type, a norosotrandite type, a boehmite type, and a pseudoboehmite type.
- examples of the amorphous aluminum hydroxide include an aluminum hydrolyzate obtained by hydrolyzing an aqueous solution of a water-soluble aluminum compound such as an aluminum salt or an aluminum alkoxide.
- alumina powder is preferably used, and ⁇ -type alumina powder is more preferable.
- the silicon source powder is a powder of a substance that becomes a silicon component and is contained in the aluminum titanate-based ceramics.
- Examples thereof include silicon oxide (silica) powders such as silicon dioxide and silicon monoxide.
- the silicon source powder used in the present invention may be a powder of a substance guided to silica by firing in air.
- examples of such substances include silicic acid, silicon carbide, silicon nitride, silicon sulfide, silicon tetrachloride, silicon acetate, sodium silicate, sodium orthosilicate, glass frit, and the like, preferably easily available industrially. In this respect, it is a glass frit or the like.
- Glass frit refers to glass flakes or powder obtained by melting a raw material mixture composed of silica sand, feldspar, lime, etc., and rapidly cooling the resulting melt.
- the silicon source powder a powder of a substance that serves as both a silicon source and an aluminum source can be used.
- a substance that serves as both a silicon source and an aluminum source.
- examples of such a substance include feldspar such as alkali feldspar.
- the precursor mixture may contain a magnesium source powder.
- aluminum magnesium titanate can be obtained as the aluminum titanate ceramic.
- the magnesium source powder is a powder of a substance that becomes a magnesium component constituting the aluminum titanate-based ceramic, and examples thereof include magnesia (magnesium oxide) powder.
- the magnesium source powder may be a powder of a substance that is guided to magnesia by firing in air.
- a substance include magnesium salt, magnesium alkoxide, magnesium hydroxide, magnesium nitride, and magnesium metal.
- magnesium salts include magnesium chloride, magnesium perchlorate, magnesium phosphate, magnesium pyrophosphate, magnesium oxalate, magnesium nitrate, magnesium carbonate, magnesium acetate, magnesium sulfate, magnesium citrate, magnesium lactate, magnesium stearate, Examples include magnesium salicylate, magnesium myristate, magnesium gluconate, magnesium dimethacrylate, and magnesium benzoate.
- magnesium alkoxide examples include magnesium methoxide and magnesium ethoxide.
- the magnesium source powder a powder of a substance serving as both a magnesium source and an aluminum source can be used.
- a substance is magnesia spinel (MgAl 2 O 4 ).
- the usage amount of the titanium source powder, the usage amount of the aluminum source powder, the usage amount of the magnesium source powder, and the usage amount of the silicon source powder contain the same amount of Ti, Al, Mg, or Si as included in each. , Titania [TiO 2 ], alumina [Al 2 O 3 ], magnesia [MgO], and silica [SiO 2 ].
- the amount of titania-converted titanium source powder used is the total amount of titania-converted titanium source powder, alumina-converted aluminum source powder, and magnesia-converted magnesium source powder (hereinafter referred to as all titania-alumina).
- magnesia amount Usually, it is 20 to 60 parts by mass, preferably 30 to 50 parts by mass per 100 parts by mass.
- the amount of the aluminum source powder in terms of alumina is usually 30 to 70 parts by mass, preferably 40 to 60 parts by mass, per 100 parts by mass of the total titania / alumina / magnesia.
- the amount of silica-based silicon source powder used is usually 0.1 to 20 parts by mass, preferably 1 to 10 parts by mass per 100 parts by mass of the total titania / alumina / magnesia.
- the amount of magnesium source powder converted to magnesia is usually 0.1 to 10 parts by mass, preferably 0.5 to 5 parts by mass per 100 parts by mass of the total titania / alumina / magnesia.
- the pulverization container one made of a metal material such as stainless steel is usually used, and the inner surface may be coated with a fluorine resin, a silicone resin, a urethane resin, or the like.
- the grinding media include alumina beads and zirconia beads having a particle diameter of 1 mm to 100 mm, preferably 5 mm to 50 mm.
- Stirring can be performed, for example, by vibrating or rotating the pulverization container charged with the raw material powder and pulverization media.
- the raw material powder is agitated and mixed with the pulverization media and pulverized.
- a normal pulverizer such as a vibration mill, a ball mill, a planetary mill, etc.
- the vibration mill is easy to implement on an industrial scale.
- Mixing may be carried out continuously or batchwise, but is preferably carried out continuously because it is easy to implement on an industrial scale.
- the time required for mixing and grinding is usually 1 minute to 6 hours, preferably 1.5 minutes to 2 hours.
- additives such as a dispersant, a pulverization aid, and a peptizer may be added.
- the grinding aid include monohydric alcohols such as methanol, ethanol and propanol; dihydric alcohols such as propylene glycol, polypropylene glycol and ethylene glycol; amines such as triethanolamine; palmitic acid, stearic acid and olein. Higher fatty acids such as acids; carbon materials such as carbon black and graphite. These may be used alone or in combination of two or more.
- the total use amount thereof is generally 0. 0 relative to the total use amount of the raw material powder, that is, 100 parts by mass of the total use amount of the titanium source powder, aluminum source powder, silicon source powder and magnesium source powder. 1 to 10 parts by mass, preferably 0.5 to 5 parts by mass, and more preferably 0.75 to 2 parts by mass.
- the additive may be removed from the precursor mixture after mixing depending on its properties.
- the additive may be removed by heating and burning in the air.
- the heating temperature at this time is usually 500 ° C. or less.
- the precursor mixture as described above is held at a temperature range of 1100 ° C. to 1350 ° C. for 3 hours or more.
- a dense aluminum titanate ceramic fired body can be obtained, thereby suppressing the generation of fine particle components during pulverization of the aluminum titanate ceramic fired body. be able to.
- the reason why the temperature is maintained in the temperature range of 1100 to 1350 ° C. is to produce an aluminum titanate ceramic having a small thermal expansion coefficient at a firing temperature of less than 1500 ° C.
- the precursor mixture (a mixture of a titanium source powder, an aluminum source powder, a silicon source powder, and an optional magnesium source powder) is heated to a temperature range of 1100 ° C. to 1350 ° C. in the form of a powder, You may hold
- a method for forming the precursor mixture an ordinary method, for example, a method in which pressure is applied in a mold using a single screw press, a tableting machine, etc., a liquid component such as water is added to the precursor mixture, Examples of the method include molding using a granulator, an extruder, and the like, and drying.
- the time for holding the precursor mixture in the temperature range of 1100 ° C. to 1350 ° C. is 3 hours or more, preferably 4 hours or more, more preferably 6 hours or more.
- the holding time is usually 24 hours or less.
- the precursor mixture may be maintained at a constant temperature, gradually increased in temperature or gradually decreased unless the temperature range is deviated. Alternatively, the temperature increase and the temperature decrease may be alternately repeated.
- the temperature increase rate or temperature decrease rate when the temperature is increased or decreased is usually 100 ° C./hour or less, preferably 80 ° C./hour or less, more preferably 50 ° C./hour, in that the precursor mixture is easily maintained in the same temperature range. Below time.
- the atmosphere at the time of raising the temperature to the above temperature range and holding at the above temperature range is usually in the air, but the raw material powder used, that is, titanium source powder, aluminum source powder, silicon source powder and magnesium source powder. Depending on the type and usage ratio, it may be in an inert gas such as nitrogen gas or argon gas, or in a reducing gas such as carbon monoxide gas or hydrogen gas. Further, the temperature may be raised and maintained in an atmosphere with a reduced water vapor partial pressure.
- the temperature rise and maintenance to the above temperature range is usually a tubular electric furnace, box-type electric furnace, tunnel furnace, far-infrared furnace, microwave heating furnace, shaft furnace, reflection furnace, rotary furnace, roller hearth furnace, etc. It is performed using a heating furnace.
- the precursor mixture that has undergone the holding step is heated to a temperature of 1400 ° C or higher, usually less than 1500 ° C, and fired at the same temperature, thereby firing the aluminum titanate ceramic fired body. Get.
- the firing is usually performed by raising the temperature to the firing temperature subsequent to the holding step, and using the same heating furnace in the same atmosphere as the holding step.
- the time required for firing may be sufficient time for the precursor mixture that has undergone the holding step to transition to the aluminum titanate-based ceramics, such as the amount of the precursor mixture, the type of firing furnace, firing temperature, firing atmosphere, etc. Usually, it is 10 minutes to 24 hours, although it varies depending on the situation.
- an aluminum titanate ceramic fired body can be obtained.
- Such an aluminum titanate-based ceramic fired body is usually in the form of a lump when the precursor mixture is fired in powder form, and when the shaped body of the precursor mixture is fired, molding immediately after molding. It is a shape that almost maintains the shape of the body.
- the pulverization / classification step includes the following steps. (A) A process of obtaining an aluminum titanate ceramic powder having a predetermined particle size or less by classifying the obtained pulverized product after impacting and pulverizing the aluminum titanate ceramic fired body; and (B) A step of applying an impact to the remainder of the pulverized product and pulverizing it again, and then classifying the obtained pulverized product to obtain an aluminum titanate ceramic powder having a predetermined particle size or less.
- the target aluminum titanate ceramic powder is a combination of the aluminum titanate ceramic powder having a predetermined particle size or less obtained in the steps (A) and (B). According to the pulverization and classification provided with the above steps (A) and (B), only components having a predetermined particle size or less can be selected and recovered, so that an aluminum titanate ceramic powder not containing coarse components can be obtained. it can. Moreover, since the coarse components in step (A) are recovered to a component having a predetermined particle size or less by re-grinding and classification in step (B), the generation of coarse components that are inappropriate as molded powders is highly suppressed. Can do. In the present invention, the step (B) is preferably repeated twice or more. The larger the number of repetitions of the step (B), the higher the yield of the target aluminum titanate ceramic powder can be increased, and the amount of the coarse-grained component remaining finally can be reduced.
- step (B) When the step (B) is repeated twice or more, the “remaining pulverized product” in the second and subsequent steps (B) is read as “remaining pulverized product obtained in the classification in the previous step (B)”. It is done.
- the aluminum titanate ceramic powder having a predetermined particle size or less generated by the pulverization is immediately classified and discharged from the pulverization region, while continuing the pulverization for the remainder of the pulverized material existing in the pulverization region, It is preferable to perform a process (A) and a process (B) continuously.
- a process (A) and a process (B) continuously.
- the fine powder generated by pulverization stays in the pulverization region, it adversely affects the subsequent pulverization, and when the aluminum titanate-based ceramic powder having a predetermined particle size or less generated by pulverization is immediately discharged from the pulverization region, the process continues.
- the remaining pulverized material can be pulverized efficiently.
- the aluminum titanate-based ceramic fired body obtained through the holding step and the firing step used in the grinding / classification step is dense, fine components are hardly generated even by the grinding and classification. Therefore, according to the production method of the present invention, it is possible to obtain an aluminum titanate-based ceramic powder having a sharp particle size distribution that does not contain fine and coarse components.
- the aluminum titanate-based ceramic powder having a predetermined particle size or less recovered in the above steps (A) and (B) is easy to mold the aluminum titanate-based ceramic powder obtained according to the present invention and In view of quality and the like, a powder having a maximum particle size of 110 ⁇ m or less is preferable.
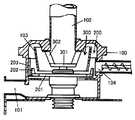
- the pulverizing apparatus shown in FIG. 1 includes a main body 100 having a gas inlet 101 on the lower side and a gas and powder outlet 102 on the upper side.
- the inside of the main body 100 is divided into an outer pulverization region 200 and an inner classification region 300 by a cylindrical body 103.
- the main body 100 is provided with an inlet 104 for supplying a substance to be pulverized and classified, which leads to the pulverization region 200.
- the crushing area 200 is provided with a crushing rotating body 201 having a crushing hammer 202 at the end for crushing. More specifically, a plurality of crushing hammers 202 are provided on the outer peripheral portion of the crushing rotating body 201 so as to be spaced from the liner 203 formed on the inner wall of the crushing region 200.
- the crushing rotating body 201 is rotatable about its central axis.
- the pulverization region 200 communicates with the gas introduction port 101 below the pulverization region 200.
- the classification area 300 is an area for classifying the powder that has been pulverized in the pulverization area 200 and moved along the gas stream introduced from the gas inlet 101 into fine powder and coarse powder, and allows only fine powder to pass through. Yes, and communicates with the discharge port 102.
- the classification region 300 includes a classification rotating body 301 that is rotatable about its central axis, and a plurality of classification fans 302 that are erected on the outer periphery of the classification rotating body 301 at, for example, an inclination angle of about 6 degrees.
- a classification mechanism consisting of The classification mechanism is arranged so that only the classified fine powder can pass through and can be discharged from the discharge port 102.
- the aluminum titanate-based ceramic fired body introduced from the inlet 104 is first introduced into the pulverization region 200 and pulverized.
- the crushing is performed by rotating the crushing rotating body 201 and applying an impact by a crushing hammer 202.
- a gas such as air or an inert gas is introduced into the main body 100 from the gas introduction port 101, and the gas passes from the bottom of the pulverization region 200 to the bottom of the classification region 300, and the classification fan.
- the classification area 302 flows through the classification area 300 from the bottom of the pulverization area 200 to the lower side of the classification area 300, flows from the upper side to the lower side of the classification area 300, crosses the classification fan 302, and goes to the outlet 102. Flowing. Therefore, the pulverized powder flows into the classification region 300 by the gas. In the classification region 300, the pulverized powder is separated into fine powder and coarse powder by the difference between the conveying force of the air flow from the classification region 300 toward the discharge port 102 and the centrifugal force applied by the classification rotating body 301. That is, the fine powder having a greater conveying force due to the airflow passes through the classification fan 302 and is discharged from the discharge port 102 and collected.
- the coarse powder on which the centrifugal force acts more greatly returns to the pulverization region 200 from below the cylindrical body 103 without passing through the classification fan 302.
- the coarse powder returned to the pulverization region 200 is pulverized again and then classified again in the classification region 300.
- an ACM pulverizer for example, ACM pulverizer ACM-10 manufactured by Hosokawa Micron Co., Ltd. can be cited, which can be suitably applied to the present invention.
- the aluminum titanate-based ceramic powder obtained according to the present invention has a sharp particle size distribution that does not contain fine and coarse components.
- the content of a fine particle component having a particle size of 10 ⁇ m or less is 20% by volume or less, preferably 10% by volume or less, and the content of a coarse particle component having a particle size of 70 ⁇ m or more. Is less than 10% by volume, and preferably less than 5% by volume.
- the average particle diameter (median diameter) of the aluminum titanate ceramic powder obtained by the present invention is preferably 20 ⁇ m or more, and the maximum particle diameter is preferably 110 ⁇ m or less.
- the holding process holding time, holding temperature, etc.
- the number of rotations of the pulverizing rotator and the classifying rotator in the classifier built-in type pulverizer By adjusting the conditions of the holding process (holding time, holding temperature, etc.), the number of rotations of the pulverizing rotator and the classifying rotator in the classifier built-in type pulverizer, the flow rate of the gas, etc. It is possible to control the particle size characteristics (particle size distribution, average particle size and maximum particle size).
- the aluminum titanate-based ceramic powder obtained by the present invention has a sharp particle size distribution that does not contain fine and coarse components, it can be suitably applied as a ceramic molded body material.
- the ceramic molded body for example, firing furnace jigs such as crucibles, setters, mortars, furnace materials, filters and catalyst carriers used for exhaust gas purification of internal combustion engines such as diesel engines and gasoline engines, parts of power generators, Examples include electronic components such as substrates and capacitors.
- Example 1 38.1 parts by mass of titanium (IV) oxide powder [DuPont Co., Ltd., “R-900”], 52.5 parts by mass of ⁇ -alumina powder (manufactured by Sumitomo Chemical Co., Ltd., “AES-12”), magnesia spinel powder 5.7 parts by mass, feldspar powder (Fukushima feldspar, SiO 2 equivalent silicon content is 72% by mass, Al 2 O 3 equivalent aluminum content is 15% by mass) and 3.7 parts by mass are mixed, and the precursor mixture Got.
- titanium (IV) oxide powder DuPont Co., Ltd., “R-900”
- ⁇ -alumina powder manufactured by Sumitomo Chemical Co., Ltd., “AES-12”
- magnesia spinel powder 5.7 parts by mass
- feldspar powder Frukushima feldspar, SiO 2 equivalent silicon content is 72% by mass
- Al 2 O 3 equivalent aluminum content is 15% by mass
- This precursor mixture is put in an alumina crucible, heated to 1100 ° C. at a rate of 300 ° C./hour in the atmosphere, held at 1100 to 1300 ° C. for 5 hours, and further increased to 1430 ° C. at a rate of 300 ° C./hour. The temperature was raised and the mixture was fired by holding at that temperature for 3.75 hours to obtain a fired aluminum magnesium titanate.
- the sintered body of aluminum magnesium titanate was rotated by using a grinding device with built-in classification mechanism (ACM Pulverizer ACM-10 manufactured by Hosokawa Micron Co., Ltd.) having the structure shown in FIG.
- ACM Pulverizer ACM-10 manufactured by Hosokawa Micron Co., Ltd.
- gas (air) flow rate 15 Nm 3 / min an aluminum magnesium titanate powder was obtained.
- the particle size characteristics of the obtained aluminum magnesium titanate powder are shown in Table 1.
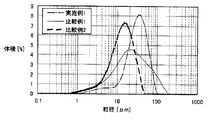
- the particle size distribution spectrum is shown in FIG.
- the particle size characteristics shown in Table 1 and the particle size distribution spectrum shown in FIG. 1 were measured using “Mastersizer 2000” manufactured by Sysmex Corporation.
- Example 2 An aluminum magnesium titanate powder was obtained in the same manner as in Example 1 except that the holding time at 1100 to 1300 ° C. was 6.7 hours. The particle size characteristics of the obtained aluminum magnesium titanate powder are shown in Table 1.
- Example 3 An aluminum magnesium titanate powder was obtained in the same manner as in Example 2 except that the rotational speed of the pulverizing rotating body was 6800 rpm. The particle size characteristics of the obtained aluminum magnesium titanate powder are shown in Table 1.
- Example 1 the aluminum magnesium titanate fired body was obtained in the same manner as in Example 2. Next, this aluminum magnesium titanate fired body was pulverized using a stone mill (Premax PR-200V manufactured by Chuo Kako Co., Ltd.) under the conditions of a tooth gap of 90 ⁇ m and a rotational speed of 2000 rpm. An aluminum magnesium acid powder was obtained. The particle size characteristics of the obtained aluminum magnesium titanate powder are shown in Table 1. The particle size distribution spectrum is shown in FIG.
- Example 2 First, the same precursor mixture as used in Example 1 was put in an alumina crucible, heated to 1100 ° C. at a rate of 300 ° C./hour in the atmosphere, held at 1100 to 1300 ° C. for 2 hours, and further raised. The temperature was increased to 1450 ° C. at a temperature rate of 300 ° C./hour, and firing was carried out by holding at that temperature for 4 hours to obtain a fired aluminum magnesium titanate.
- this magnesium aluminum titanate fired body was crushed with the above-mentioned classification mechanism built-in type pulverizer, the rotation speed of the rotator for grinding was 6800 rpm, the rotation speed of the rotator for classification was 2000 rpm, and the flow rate of gas (air) was 15 Nm 3.
- An aluminum magnesium titanate powder was obtained by pulverization and classification under the conditions of / min. The particle size characteristics of the obtained aluminum magnesium titanate powder are shown in Table 1. The particle size distribution spectrum is shown in FIG.
- Examples 1 to 3 satisfy all the requirements of the present invention, the production of fine and coarse components of the resulting aluminum titanate ceramic powder is suppressed. In addition, the tendency for a fine particle component to increase was seen with the increase in the rotation speed of the rotary body for grinding
- Comparative Examples 1 to 5 are examples that do not satisfy any of the requirements of the present invention.
- the holding process was appropriately controlled, but since the particles were not classified, the fine particle component and the coarse particle component increased.
- Comparative Examples 2 to 5 since the holding time in the holding process was short, the fine particle component increased.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Ceramic Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Structural Engineering (AREA)
- Inorganic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental & Geological Engineering (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- Compositions Of Oxide Ceramics (AREA)
- Inorganic Compounds Of Heavy Metals (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP09819182.8A EP2336087B1 (en) | 2008-10-07 | 2009-10-06 | Process for producing a powder of aluminum titanate-based ceramics |
| US13/119,661 US8920705B2 (en) | 2008-10-07 | 2009-10-06 | Process for producing a powder of aluminum titanate-based ceramics |
| CN2009801397449A CN102177096A (zh) | 2008-10-07 | 2009-10-06 | 钛酸铝系陶瓷粉末的制造方法 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008260240A JP4774564B2 (ja) | 2008-10-07 | 2008-10-07 | チタン酸アルミニウム系セラミックス粉末の製造方法 |
| JP2008-260240 | 2008-10-07 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010041648A1 true WO2010041648A1 (ja) | 2010-04-15 |
Family
ID=42100598
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2009/067388 Ceased WO2010041648A1 (ja) | 2008-10-07 | 2009-10-06 | チタン酸アルミニウム系セラミックス粉末の製造方法 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US8920705B2 (enExample) |
| EP (1) | EP2336087B1 (enExample) |
| JP (1) | JP4774564B2 (enExample) |
| KR (1) | KR101614181B1 (enExample) |
| CN (1) | CN102177096A (enExample) |
| PL (1) | PL2336087T3 (enExample) |
| TW (1) | TW201033154A (enExample) |
| WO (1) | WO2010041648A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015505801A (ja) * | 2011-11-30 | 2015-02-26 | ポスコ イーエス マテリアルス カンパニー リミテッドPOSCO ES Materials Co., Ltd. | 異種金属がドープされたリチウムチタン複合酸化物の製造方法、及びそれによって製造された異種金属がドープされたリチウムチタン複合酸化物 |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010116289A (ja) * | 2008-11-12 | 2010-05-27 | Sumitomo Chemical Co Ltd | チタン酸アルミニウム系セラミックスの製造方法 |
| CN102378746A (zh) | 2009-03-30 | 2012-03-14 | 住友化学株式会社 | 钛酸铝系陶瓷体的制造方法 |
| US9908260B2 (en) | 2013-05-20 | 2018-03-06 | Corning Incorporated | Porous ceramic article and method of manufacturing the same |
| US9623360B2 (en) | 2013-05-20 | 2017-04-18 | Corning Incorporated | Porous ceramic article and method of manufacturing the same |
| US9376347B2 (en) | 2013-05-20 | 2016-06-28 | Corning Incorporated | Porous ceramic article and method of manufacturing the same |
| JP6502495B2 (ja) * | 2014-11-19 | 2019-04-17 | コーニング インコーポレイテッド | 制御されたサイズ分布を有するセラミック粉末 |
| CN109219589B (zh) | 2016-05-31 | 2022-04-26 | 康宁股份有限公司 | 多孔制品及其制造方法 |
| CN111527055A (zh) | 2017-10-31 | 2020-08-11 | 康宁股份有限公司 | 包含预反应过的无机颗粒的批料组合物及由其制造生坯体的方法 |
| US20230101880A1 (en) * | 2020-03-20 | 2023-03-30 | Corning Incorporated | Aluminum titanate-containing particles, at-containing green and ceramic honeycomb bodies, batch mixtures, and methods of manufacture |
| CN111704390A (zh) * | 2020-06-28 | 2020-09-25 | 潮州市泥香陶瓷新材料有限公司 | 一种陶瓷保湿涂层制备装置及制备方法 |
| CN113244866B (zh) * | 2021-05-14 | 2022-05-06 | 昆明理工大学 | 一种微波辅助气体催化合成轻烃的装置及其方法 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63236759A (ja) * | 1987-03-24 | 1988-10-03 | 日本碍子株式会社 | 鋳ぐるみ用セラミツク材料 |
| JPH1160240A (ja) * | 1997-08-18 | 1999-03-02 | Tsutomu Fukuda | チタン酸アルミニウム粉体及びチタン酸アルミニウム焼結体の製造方法 |
| WO2004039747A1 (ja) * | 2002-11-01 | 2004-05-13 | Ohcera Co., Ltd. | チタン酸アルミニウムマグネシウム焼結体の製造方法 |
| WO2005105704A1 (ja) | 2004-04-28 | 2005-11-10 | Ohcera Co., Ltd. | チタン酸アルミニウムマグネシウム結晶構造物及びその製造方法 |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE29794C (de) | A. SÖDERSTRÖM in Stockholm | Neuerungen an Bohrhaltern und Bohrern. (Abhängig vom Patente Nr. 29046.) | ||
| DD29794A1 (de) * | 1962-01-16 | 1964-09-25 | Verfahren zur Herstellung von hochfeuerfesten oxydischen Werkstoffen mit sehr guter Temperaturwechselbeständigkeit | |
| US3825653A (en) * | 1972-09-11 | 1974-07-23 | Atomic Energy Commission | Process for preparing sinterable aluminum titanate powder |
| JPS6272522A (ja) * | 1985-09-27 | 1987-04-03 | Kureha Chem Ind Co Ltd | アルミナ−チタニア複合粉体及びその製造方法 |
| DE3852513T2 (de) | 1987-03-24 | 1995-07-13 | Ngk Insulators Ltd | Zu umgiessendes keramisches Material und keramische Kanalauskleidungen. |
| US5260116A (en) | 1987-03-24 | 1993-11-09 | Ngk Insulators, Ltd. | Ceramicm port liners |
| DE3827646A1 (de) * | 1988-08-16 | 1990-02-22 | Bayer Ag | Sinterbares rohstoffpulver auf basis von aluminiumtitanat, verfahren zu seiner herstellung sowie daraus hergestellte sinterformkoerper und deren verwendung |
| AT389693B (de) * | 1988-09-30 | 1990-01-10 | Andritz Ag Maschf | Verfahren zur herstellung von aluminium-titanat |
| JP3096814B1 (ja) * | 1999-11-08 | 2000-10-10 | 勉 福田 | チタン酸アルミニウム焼結体の製造方法 |
| US20060021308A1 (en) * | 2004-07-29 | 2006-02-02 | Merkel Gregory A | Mullite-aluminum titanate body and method for making same |
| US8974724B2 (en) * | 2006-11-29 | 2015-03-10 | Corning Incorporated | Aluminum titanate batch compositions and method |
| WO2009073082A1 (en) * | 2007-11-29 | 2009-06-11 | Corning Incorporated | System and method for forming ceramic precursor material for thin-walled ceramic honeycomb structures |
| EP2261192A4 (en) * | 2008-03-31 | 2011-08-31 | Ibiden Co Ltd | METHOD FOR PRODUCING A HONEYCOMB STRUCTURE |
| JP5345502B2 (ja) * | 2008-11-10 | 2013-11-20 | 日本碍子株式会社 | セラミックスハニカム構造体の製造方法およびセラミックスハニカム構造体用のコート材 |
| JP2011051854A (ja) * | 2009-09-03 | 2011-03-17 | Sumitomo Chemical Co Ltd | チタン酸アルミニウム系焼成体の製造方法およびセラミックス成形体 |
-
2008
- 2008-10-07 JP JP2008260240A patent/JP4774564B2/ja not_active Expired - Fee Related
-
2009
- 2009-10-06 KR KR1020117008593A patent/KR101614181B1/ko not_active Expired - Fee Related
- 2009-10-06 WO PCT/JP2009/067388 patent/WO2010041648A1/ja not_active Ceased
- 2009-10-06 US US13/119,661 patent/US8920705B2/en not_active Expired - Fee Related
- 2009-10-06 EP EP09819182.8A patent/EP2336087B1/en not_active Not-in-force
- 2009-10-06 PL PL09819182T patent/PL2336087T3/pl unknown
- 2009-10-06 CN CN2009801397449A patent/CN102177096A/zh active Pending
- 2009-10-07 TW TW098133963A patent/TW201033154A/zh unknown
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63236759A (ja) * | 1987-03-24 | 1988-10-03 | 日本碍子株式会社 | 鋳ぐるみ用セラミツク材料 |
| JPH1160240A (ja) * | 1997-08-18 | 1999-03-02 | Tsutomu Fukuda | チタン酸アルミニウム粉体及びチタン酸アルミニウム焼結体の製造方法 |
| WO2004039747A1 (ja) * | 2002-11-01 | 2004-05-13 | Ohcera Co., Ltd. | チタン酸アルミニウムマグネシウム焼結体の製造方法 |
| WO2005105704A1 (ja) | 2004-04-28 | 2005-11-10 | Ohcera Co., Ltd. | チタン酸アルミニウムマグネシウム結晶構造物及びその製造方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2336087A4 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015505801A (ja) * | 2011-11-30 | 2015-02-26 | ポスコ イーエス マテリアルス カンパニー リミテッドPOSCO ES Materials Co., Ltd. | 異種金属がドープされたリチウムチタン複合酸化物の製造方法、及びそれによって製造された異種金属がドープされたリチウムチタン複合酸化物 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP4774564B2 (ja) | 2011-09-14 |
| TW201033154A (en) | 2010-09-16 |
| US20110236688A1 (en) | 2011-09-29 |
| EP2336087A1 (en) | 2011-06-22 |
| EP2336087B1 (en) | 2016-11-30 |
| JP2010089981A (ja) | 2010-04-22 |
| EP2336087A4 (en) | 2013-05-01 |
| KR101614181B1 (ko) | 2016-04-20 |
| US8920705B2 (en) | 2014-12-30 |
| KR20110066938A (ko) | 2011-06-17 |
| CN102177096A (zh) | 2011-09-07 |
| PL2336087T3 (pl) | 2017-05-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4774564B2 (ja) | チタン酸アルミニウム系セラミックス粉末の製造方法 | |
| JP5502135B2 (ja) | チタン酸アルミニウム系セラミックスの製造方法 | |
| JP5122527B2 (ja) | チタン酸アルミニウムマグネシウムの製造方法 | |
| JP4903820B2 (ja) | チタン酸アルミニウムマグネシウムの製造方法 | |
| US20110248106A1 (en) | Process for producing aluminum titanate-based ceramics | |
| JP2010100510A (ja) | チタン酸アルミニウムセラミックスの製造方法 | |
| CN102471165A (zh) | 钛酸铝系陶瓷的制造方法和钛酸铝系陶瓷 | |
| TW201107268A (en) | Method for producing aluminum titanate ceramic body | |
| CN102143925A (zh) | 钛酸铝系陶瓷的制造方法 | |
| JP5133208B2 (ja) | チタン酸アルミニウム系セラミックスの製造方法 | |
| JP5133207B2 (ja) | チタン酸アルミニウム系セラミックスの製造方法 | |
| JP2010013293A (ja) | チタン酸アルミニウム系セラミックス粉末の製造方法 | |
| JP5537827B2 (ja) | チタン酸アルミニウム系セラミックスの製造方法およびチタン酸アルミニウム系セラミックス |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200980139744.9 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 09819182 Country of ref document: EP Kind code of ref document: A1 |
|
| REEP | Request for entry into the european phase |
Ref document number: 2009819182 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2009819182 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20117008593 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 3008/CHENP/2011 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13119661 Country of ref document: US |