RU2761953C2 - Лечение рака желудка с применением комбинационных видов терапии, содержащих липосомальный иринотекан, оксалиплатин, 5-фторурацил (и лейковорин) - Google Patents
Лечение рака желудка с применением комбинационных видов терапии, содержащих липосомальный иринотекан, оксалиплатин, 5-фторурацил (и лейковорин) Download PDFInfo
- Publication number
- RU2761953C2 RU2761953C2 RU2019114952A RU2019114952A RU2761953C2 RU 2761953 C2 RU2761953 C2 RU 2761953C2 RU 2019114952 A RU2019114952 A RU 2019114952A RU 2019114952 A RU2019114952 A RU 2019114952A RU 2761953 C2 RU2761953 C2 RU 2761953C2
- Authority
- RU
- Russia
- Prior art keywords
- dose
- yes
- oxaliplatin
- administered
- irinotecan
- Prior art date
Links
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 title claims abstract description 266
- 229960004768 irinotecan Drugs 0.000 title claims abstract description 256
- 229960002949 fluorouracil Drugs 0.000 title claims abstract description 228
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 title claims abstract description 226
- 229960001756 oxaliplatin Drugs 0.000 title claims abstract description 214
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 title claims abstract description 214
- 239000011672 folinic acid Substances 0.000 title claims abstract description 185
- 229960001691 leucovorin Drugs 0.000 title claims abstract description 185
- VVIAGPKUTFNRDU-UHFFFAOYSA-N 6S-folinic acid Natural products C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-UHFFFAOYSA-N 0.000 title claims abstract description 162
- 235000008191 folinic acid Nutrition 0.000 title claims abstract description 162
- VVIAGPKUTFNRDU-ABLWVSNPSA-N folinic acid Chemical compound C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-ABLWVSNPSA-N 0.000 title claims abstract description 162
- 238000011282 treatment Methods 0.000 title claims abstract description 104
- 208000005718 Stomach Neoplasms Diseases 0.000 title claims abstract description 29
- 206010017758 gastric cancer Diseases 0.000 title claims abstract description 29
- 201000011549 stomach cancer Diseases 0.000 title claims abstract description 29
- 238000002560 therapeutic procedure Methods 0.000 title claims description 41
- 238000000034 method Methods 0.000 claims description 63
- 238000001802 infusion Methods 0.000 claims description 56
- 238000011319 anticancer therapy Methods 0.000 claims description 51
- 230000000259 anti-tumor effect Effects 0.000 claims description 16
- 239000002502 liposome Substances 0.000 claims description 16
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims description 14
- 229920002505 N-(Carbonyl-Methoxypolyethylene Glycol 2000)-1,2-Distearoyl-Sn-Glycero-3-Phosphoethanolamine Polymers 0.000 claims description 8
- 239000002246 antineoplastic agent Substances 0.000 claims description 8
- 235000012000 cholesterol Nutrition 0.000 claims description 7
- NRJAVPSFFCBXDT-HUESYALOSA-N 1,2-distearoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCCCC NRJAVPSFFCBXDT-HUESYALOSA-N 0.000 claims description 6
- WEPNHBQBLCNOBB-FZJVNAOYSA-N sucrose octasulfate Chemical compound OS(=O)(=O)O[C@@H]1[C@H](OS(O)(=O)=O)[C@H](COS(=O)(=O)O)O[C@]1(COS(O)(=O)=O)O[C@@H]1[C@H](OS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@@H](COS(O)(=O)=O)O1 WEPNHBQBLCNOBB-FZJVNAOYSA-N 0.000 claims description 5
- 229940071238 n-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine Drugs 0.000 claims description 4
- 229940034982 antineoplastic agent Drugs 0.000 claims description 2
- SRLOHQKOADWDBV-NRONOFSHSA-M sodium;[(2r)-2,3-di(octadecanoyloxy)propyl] 2-(2-methoxyethoxycarbonylamino)ethyl phosphate Chemical compound [Na+].CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCCNC(=O)OCCOC)OC(=O)CCCCCCCCCCCCCCCCC SRLOHQKOADWDBV-NRONOFSHSA-M 0.000 claims 4
- 239000003814 drug Substances 0.000 abstract description 26
- 230000000694 effects Effects 0.000 abstract description 11
- 238000002648 combination therapy Methods 0.000 abstract description 9
- 238000002347 injection Methods 0.000 abstract description 9
- 239000007924 injection Substances 0.000 abstract description 9
- 238000003745 diagnosis Methods 0.000 abstract description 2
- 239000000126 substance Substances 0.000 abstract description 2
- 231100000419 toxicity Toxicity 0.000 description 56
- 230000001988 toxicity Effects 0.000 description 56
- 230000002829 reductive effect Effects 0.000 description 55
- 206010028980 Neoplasm Diseases 0.000 description 36
- 230000009467 reduction Effects 0.000 description 33
- 102100029152 UDP-glucuronosyltransferase 1A1 Human genes 0.000 description 32
- 101710205316 UDP-glucuronosyltransferase 1A1 Proteins 0.000 description 32
- 150000003839 salts Chemical class 0.000 description 32
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 28
- 206010012735 Diarrhoea Diseases 0.000 description 26
- 229940079593 drug Drugs 0.000 description 24
- 231100000226 haematotoxicity Toxicity 0.000 description 23
- 238000001990 intravenous administration Methods 0.000 description 23
- 230000004048 modification Effects 0.000 description 23
- 238000012986 modification Methods 0.000 description 23
- 230000004044 response Effects 0.000 description 23
- 230000002411 adverse Effects 0.000 description 21
- 239000012458 free base Substances 0.000 description 20
- 229950010538 irinotecan hydrochloride trihydrate Drugs 0.000 description 19
- 206010033553 Palmar-plantar erythrodysaesthesia syndrome Diseases 0.000 description 16
- 208000004235 neutropenia Diseases 0.000 description 16
- 238000004458 analytical method Methods 0.000 description 14
- 230000004083 survival effect Effects 0.000 description 14
- 201000010099 disease Diseases 0.000 description 13
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 13
- 201000011510 cancer Diseases 0.000 description 12
- 238000006243 chemical reaction Methods 0.000 description 12
- 206010047700 Vomiting Diseases 0.000 description 11
- 231100000371 dose-limiting toxicity Toxicity 0.000 description 11
- -1 for example Chemical compound 0.000 description 11
- 208000002633 Febrile Neutropenia Diseases 0.000 description 10
- 230000003474 anti-emetic effect Effects 0.000 description 10
- 239000002111 antiemetic agent Substances 0.000 description 10
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 10
- KLEAIHJJLUAXIQ-JDRGBKBRSA-N irinotecan hydrochloride hydrate Chemical compound O.O.O.Cl.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 KLEAIHJJLUAXIQ-JDRGBKBRSA-N 0.000 description 10
- 229940048191 onivyde Drugs 0.000 description 10
- 238000012360 testing method Methods 0.000 description 10
- 229940124650 anti-cancer therapies Drugs 0.000 description 9
- 238000011284 combination treatment Methods 0.000 description 9
- 230000003111 delayed effect Effects 0.000 description 9
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- 206010003549 asthenia Diseases 0.000 description 8
- 238000002512 chemotherapy Methods 0.000 description 8
- 239000000047 product Substances 0.000 description 8
- 108700028369 Alleles Proteins 0.000 description 7
- 206010048610 Cardiotoxicity Diseases 0.000 description 7
- 241000282412 Homo Species 0.000 description 7
- 208000022531 anorexia Diseases 0.000 description 7
- 230000000118 anti-neoplastic effect Effects 0.000 description 7
- 231100000259 cardiotoxicity Toxicity 0.000 description 7
- 210000004027 cell Anatomy 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 7
- 230000007423 decrease Effects 0.000 description 7
- 206010061428 decreased appetite Diseases 0.000 description 7
- 230000036541 health Effects 0.000 description 7
- GURKHSYORGJETM-WAQYZQTGSA-N irinotecan hydrochloride (anhydrous) Chemical class Cl.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 GURKHSYORGJETM-WAQYZQTGSA-N 0.000 description 7
- 238000011084 recovery Methods 0.000 description 7
- 238000009097 single-agent therapy Methods 0.000 description 7
- 239000011780 sodium chloride Substances 0.000 description 7
- 108010022394 Threonine synthase Proteins 0.000 description 6
- 230000037396 body weight Effects 0.000 description 6
- 230000008859 change Effects 0.000 description 6
- 229960003957 dexamethasone Drugs 0.000 description 6
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 6
- 238000011156 evaluation Methods 0.000 description 6
- 206010016256 fatigue Diseases 0.000 description 6
- 208000037819 metastatic cancer Diseases 0.000 description 6
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 6
- 206010061818 Disease progression Diseases 0.000 description 5
- 206010061188 Haematotoxicity Diseases 0.000 description 5
- 241000699670 Mus sp. Species 0.000 description 5
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 5
- 229940109239 creatinine Drugs 0.000 description 5
- 230000005750 disease progression Effects 0.000 description 5
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 5
- 201000002528 pancreatic cancer Diseases 0.000 description 5
- 208000008443 pancreatic carcinoma Diseases 0.000 description 5
- 230000036470 plasma concentration Effects 0.000 description 5
- 210000002966 serum Anatomy 0.000 description 5
- 239000007858 starting material Substances 0.000 description 5
- 206010043554 thrombocytopenia Diseases 0.000 description 5
- 230000008673 vomiting Effects 0.000 description 5
- 206010061968 Gastric neoplasm Diseases 0.000 description 4
- 206010020751 Hypersensitivity Diseases 0.000 description 4
- 206010028813 Nausea Diseases 0.000 description 4
- 208000002193 Pain Diseases 0.000 description 4
- 206010034620 Peripheral sensory neuropathy Diseases 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- 206010037660 Pyrexia Diseases 0.000 description 4
- 229940041216 diphenhydramine hydrochloride 50 mg Drugs 0.000 description 4
- 229940000406 drug candidate Drugs 0.000 description 4
- 230000007717 exclusion Effects 0.000 description 4
- 239000012530 fluid Substances 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 230000008693 nausea Effects 0.000 description 4
- 239000002547 new drug Substances 0.000 description 4
- 230000036407 pain Effects 0.000 description 4
- 229920001223 polyethylene glycol Polymers 0.000 description 4
- 201000005572 sensory peripheral neuropathy Diseases 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 230000003442 weekly effect Effects 0.000 description 4
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 3
- 108010082126 Alanine transaminase Proteins 0.000 description 3
- 208000009079 Bronchial Spasm Diseases 0.000 description 3
- 208000014181 Bronchial disease Diseases 0.000 description 3
- 206010006482 Bronchospasm Diseases 0.000 description 3
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 3
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 3
- 102000005497 Thymidylate Synthase Human genes 0.000 description 3
- 208000026935 allergic disease Diseases 0.000 description 3
- 208000003455 anaphylaxis Diseases 0.000 description 3
- 208000007502 anemia Diseases 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 239000000090 biomarker Substances 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 238000009096 combination chemotherapy Methods 0.000 description 3
- 229940127089 cytotoxic agent Drugs 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 102000004419 dihydrofolate reductase Human genes 0.000 description 3
- 238000002565 electrocardiography Methods 0.000 description 3
- 230000008030 elimination Effects 0.000 description 3
- 238000003379 elimination reaction Methods 0.000 description 3
- 229940014144 folate Drugs 0.000 description 3
- 235000019152 folic acid Nutrition 0.000 description 3
- 239000011724 folic acid Substances 0.000 description 3
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 210000001035 gastrointestinal tract Anatomy 0.000 description 3
- 229960005277 gemcitabine Drugs 0.000 description 3
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 3
- 230000002489 hematologic effect Effects 0.000 description 3
- 238000003384 imaging method Methods 0.000 description 3
- 230000002045 lasting effect Effects 0.000 description 3
- 230000003902 lesion Effects 0.000 description 3
- 206010061289 metastatic neoplasm Diseases 0.000 description 3
- 201000001119 neuropathy Diseases 0.000 description 3
- 230000007823 neuropathy Effects 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 208000033808 peripheral neuropathy Diseases 0.000 description 3
- 230000002085 persistent effect Effects 0.000 description 3
- 238000001959 radiotherapy Methods 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 238000011269 treatment regimen Methods 0.000 description 3
- CUGZEDSDRBMZMY-UHFFFAOYSA-N trihydrate;hydrochloride Chemical compound O.O.O.Cl CUGZEDSDRBMZMY-UHFFFAOYSA-N 0.000 description 3
- YXTKHLHCVFUPPT-YYFJYKOTSA-N (2s)-2-[[4-[(2-amino-5-formyl-4-oxo-1,6,7,8-tetrahydropteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid;(1r,2r)-1,2-dimethanidylcyclohexane;5-fluoro-1h-pyrimidine-2,4-dione;oxalic acid;platinum(2+) Chemical compound [Pt+2].OC(=O)C(O)=O.[CH2-][C@@H]1CCCC[C@H]1[CH2-].FC1=CNC(=O)NC1=O.C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 YXTKHLHCVFUPPT-YYFJYKOTSA-N 0.000 description 2
- 108010058566 130-nm albumin-bound paclitaxel Proteins 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 2
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 2
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 2
- 206010002198 Anaphylactic reaction Diseases 0.000 description 2
- 229930003347 Atropine Natural products 0.000 description 2
- BPYKTIZUTYGOLE-IFADSCNNSA-N Bilirubin Chemical compound N1C(=O)C(C)=C(C=C)\C1=C\C1=C(C)C(CCC(O)=O)=C(CC2=C(C(C)=C(\C=C/3C(=C(C=C)C(=O)N\3)C)N2)CCC(O)=O)N1 BPYKTIZUTYGOLE-IFADSCNNSA-N 0.000 description 2
- 229940123780 DNA topoisomerase I inhibitor Drugs 0.000 description 2
- 208000020401 Depressive disease Diseases 0.000 description 2
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 2
- RKUNBYITZUJHSG-UHFFFAOYSA-N Hyosciamin-hydrochlorid Natural products CN1C(C2)CCC1CC2OC(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-UHFFFAOYSA-N 0.000 description 2
- 239000000232 Lipid Bilayer Substances 0.000 description 2
- 241001529936 Murinae Species 0.000 description 2
- 101710183280 Topoisomerase Proteins 0.000 description 2
- 239000000365 Topoisomerase I Inhibitor Substances 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 229940086245 acetaminophen 650 mg Drugs 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 230000000172 allergic effect Effects 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- RKUNBYITZUJHSG-SPUOUPEWSA-N atropine Chemical compound O([C@H]1C[C@H]2CC[C@@H](C1)N2C)C(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-SPUOUPEWSA-N 0.000 description 2
- 229960000396 atropine Drugs 0.000 description 2
- 230000000740 bleeding effect Effects 0.000 description 2
- 229940124630 bronchodilator Drugs 0.000 description 2
- 239000000168 bronchodilator agent Substances 0.000 description 2
- 230000001149 cognitive effect Effects 0.000 description 2
- 210000001072 colon Anatomy 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 230000002354 daily effect Effects 0.000 description 2
- 230000006866 deterioration Effects 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 230000002996 emotional effect Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 238000009093 first-line therapy Methods 0.000 description 2
- JYEFSHLLTQIXIO-SMNQTINBSA-N folfiri regimen Chemical compound FC1=CNC(=O)NC1=O.C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 JYEFSHLLTQIXIO-SMNQTINBSA-N 0.000 description 2
- PJZDLZXMGBOJRF-CXOZILEQSA-L folfirinox Chemical compound [Pt+4].[O-]C(=O)C([O-])=O.[NH-][C@H]1CCCC[C@@H]1[NH-].FC1=CNC(=O)NC1=O.C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 PJZDLZXMGBOJRF-CXOZILEQSA-L 0.000 description 2
- 230000009610 hypersensitivity Effects 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 239000000411 inducer Substances 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 208000019423 liver disease Diseases 0.000 description 2
- 238000001325 log-rank test Methods 0.000 description 2
- 238000011866 long-term treatment Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 239000002207 metabolite Substances 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 229960001592 paclitaxel Drugs 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 238000009101 premedication Methods 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 150000003230 pyrimidines Chemical class 0.000 description 2
- 231100000279 safety data Toxicity 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 239000003369 serotonin 5-HT3 receptor antagonist Substances 0.000 description 2
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
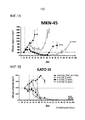
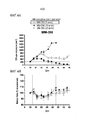
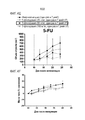
- 230000004614 tumor growth Effects 0.000 description 2
- 239000002691 unilamellar liposome Substances 0.000 description 2
- QHARDGMPGYZGKH-YCUNKVRLSA-N (19S)-10,19-diethyl-19-hydroxy-7-(1-piperidin-4-ylpiperidin-2-yl)-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaene-14,18-dione Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1C1CCCCN1C1CCNCC1 QHARDGMPGYZGKH-YCUNKVRLSA-N 0.000 description 1
- VVIAGPKUTFNRDU-STQMWFEESA-N (6S)-5-formyltetrahydrofolic acid Chemical compound C([C@H]1CNC=2N=C(NC(=O)C=2N1C=O)N)NC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-STQMWFEESA-N 0.000 description 1
- UCTWMZQNUQWSLP-VIFPVBQESA-N (R)-adrenaline Chemical compound CNC[C@H](O)C1=CC=C(O)C(O)=C1 UCTWMZQNUQWSLP-VIFPVBQESA-N 0.000 description 1
- 229930182837 (R)-adrenaline Natural products 0.000 description 1
- CIVCELMLGDGMKZ-UHFFFAOYSA-N 2,4-dichloro-6-methylpyridine-3-carboxylic acid Chemical compound CC1=CC(Cl)=C(C(O)=O)C(Cl)=N1 CIVCELMLGDGMKZ-UHFFFAOYSA-N 0.000 description 1
- MSTNYGQPCMXVAQ-KIYNQFGBSA-N 5,6,7,8-tetrahydrofolic acid Chemical compound N1C=2C(=O)NC(N)=NC=2NCC1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 MSTNYGQPCMXVAQ-KIYNQFGBSA-N 0.000 description 1
- 206010067484 Adverse reaction Diseases 0.000 description 1
- 201000004384 Alopecia Diseases 0.000 description 1
- 206010002388 Angina unstable Diseases 0.000 description 1
- 208000028185 Angioedema Diseases 0.000 description 1
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 1
- 102000013392 Carboxylesterase Human genes 0.000 description 1
- 108010051152 Carboxylesterase Proteins 0.000 description 1
- 206010007559 Cardiac failure congestive Diseases 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 206010010774 Constipation Diseases 0.000 description 1
- 108010000561 Cytochrome P-450 CYP2C8 Proteins 0.000 description 1
- 108010081668 Cytochrome P-450 CYP3A Proteins 0.000 description 1
- 102100029359 Cytochrome P450 2C8 Human genes 0.000 description 1
- 102100039205 Cytochrome P450 3A4 Human genes 0.000 description 1
- 230000000970 DNA cross-linking effect Effects 0.000 description 1
- 230000005778 DNA damage Effects 0.000 description 1
- 231100000277 DNA damage Toxicity 0.000 description 1
- 102000003844 DNA helicases Human genes 0.000 description 1
- 108090000133 DNA helicases Proteins 0.000 description 1
- 230000004543 DNA replication Effects 0.000 description 1
- 230000006820 DNA synthesis Effects 0.000 description 1
- 206010052805 Drug tolerance decreased Diseases 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 208000005189 Embolism Diseases 0.000 description 1
- 208000010201 Exanthema Diseases 0.000 description 1
- 208000018522 Gastrointestinal disease Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 206010019280 Heart failures Diseases 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- 208000005176 Hepatitis C Diseases 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- 208000001953 Hypotension Diseases 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 208000029523 Interstitial Lung disease Diseases 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 102100020870 La-related protein 6 Human genes 0.000 description 1
- 108050008265 La-related protein 6 Proteins 0.000 description 1
- 206010025476 Malabsorption Diseases 0.000 description 1
- 208000004155 Malabsorption Syndromes Diseases 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 206010027457 Metastases to liver Diseases 0.000 description 1
- 206010063916 Metastatic gastric cancer Diseases 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 230000006819 RNA synthesis Effects 0.000 description 1
- 208000013738 Sleep Initiation and Maintenance disease Diseases 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 208000001435 Thromboembolism Diseases 0.000 description 1
- 108090000340 Transaminases Proteins 0.000 description 1
- 102000003929 Transaminases Human genes 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 208000007814 Unstable Angina Diseases 0.000 description 1
- 208000024780 Urticaria Diseases 0.000 description 1
- 206010047281 Ventricular arrhythmia Diseases 0.000 description 1
- KYIKRXIYLAGAKQ-UHFFFAOYSA-N abcn Chemical compound C1CCCCC1(C#N)N=NC1(C#N)CCCCC1 KYIKRXIYLAGAKQ-UHFFFAOYSA-N 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000006838 adverse reaction Effects 0.000 description 1
- 229930013930 alkaloid Natural products 0.000 description 1
- 150000003797 alkaloid derivatives Chemical class 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 231100000360 alopecia Toxicity 0.000 description 1
- 230000036783 anaphylactic response Effects 0.000 description 1
- 229940035674 anesthetics Drugs 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 230000001142 anti-diarrhea Effects 0.000 description 1
- 229940125683 antiemetic agent Drugs 0.000 description 1
- 229940125715 antihistaminic agent Drugs 0.000 description 1
- 239000000739 antihistaminic agent Substances 0.000 description 1
- 230000004596 appetite loss Effects 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 229960000397 bevacizumab Drugs 0.000 description 1
- 208000034158 bleeding Diseases 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- 238000009534 blood test Methods 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000008366 buffered solution Substances 0.000 description 1
- 229940088954 camptosar Drugs 0.000 description 1
- 229940127093 camptothecin Drugs 0.000 description 1
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 230000022131 cell cycle Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 229960005395 cetuximab Drugs 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 230000000973 chemotherapeutic effect Effects 0.000 description 1
- 230000001713 cholinergic effect Effects 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 229940124301 concurrent medication Drugs 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- GYOZYWVXFNDGLU-XLPZGREQSA-N dTMP Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)C1 GYOZYWVXFNDGLU-XLPZGREQSA-N 0.000 description 1
- JSRLJPSBLDHEIO-SHYZEUOFSA-N dUMP Chemical compound O1[C@H](COP(O)(O)=O)[C@@H](O)C[C@@H]1N1C(=O)NC(=O)C=C1 JSRLJPSBLDHEIO-SHYZEUOFSA-N 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 239000005547 deoxyribonucleotide Substances 0.000 description 1
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 239000008355 dextrose injection Substances 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 208000010643 digestive system disease Diseases 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 229960000525 diphenhydramine hydrochloride Drugs 0.000 description 1
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 229960005139 epinephrine Drugs 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 201000005884 exanthem Diseases 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- ODKNJVUHOIMIIZ-RRKCRQDMSA-N floxuridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ODKNJVUHOIMIIZ-RRKCRQDMSA-N 0.000 description 1
- 229960000961 floxuridine Drugs 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 208000018685 gastrointestinal system disease Diseases 0.000 description 1
- 239000003193 general anesthetic agent Substances 0.000 description 1
- 230000023611 glucuronidation Effects 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- 230000003862 health status Effects 0.000 description 1
- 208000002672 hepatitis B Diseases 0.000 description 1
- 230000036543 hypotension Effects 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 206010022437 insomnia Diseases 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 201000004332 intermediate coronary syndrome Diseases 0.000 description 1
- 208000003243 intestinal obstruction Diseases 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 230000003907 kidney function Effects 0.000 description 1
- 229940008678 levoleucovorin Drugs 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 230000003908 liver function Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 229960001571 loperamide Drugs 0.000 description 1
- RDOIQAHITMMDAJ-UHFFFAOYSA-N loperamide Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(C(=O)N(C)C)CCN(CC1)CCC1(O)C1=CC=C(Cl)C=C1 RDOIQAHITMMDAJ-UHFFFAOYSA-N 0.000 description 1
- 208000019017 loss of appetite Diseases 0.000 description 1
- 235000021266 loss of appetite Nutrition 0.000 description 1
- 229940126601 medicinal product Drugs 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 208000011645 metastatic carcinoma Diseases 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 1
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 229940127084 other anti-cancer agent Drugs 0.000 description 1
- 229940078646 other antiemetics in atc Drugs 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- 230000002974 pharmacogenomic effect Effects 0.000 description 1
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 238000010837 poor prognosis Methods 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 238000009597 pregnancy test Methods 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 229940043274 prophylactic drug Drugs 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 239000003790 pyrimidine antagonist Substances 0.000 description 1
- 230000006824 pyrimidine synthesis Effects 0.000 description 1
- 238000012797 qualification Methods 0.000 description 1
- 239000002534 radiation-sensitizing agent Substances 0.000 description 1
- 206010037844 rash Diseases 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 208000020615 rectal carcinoma Diseases 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 229950003238 rilotumumab Drugs 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 208000013220 shortness of breath Diseases 0.000 description 1
- 208000020352 skin basal cell carcinoma Diseases 0.000 description 1
- 201000010106 skin squamous cell carcinoma Diseases 0.000 description 1
- 238000011255 standard chemotherapy Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 208000003265 stomatitis Diseases 0.000 description 1
- 238000013517 stratification Methods 0.000 description 1
- 238000002636 symptomatic treatment Methods 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000002626 targeted therapy Methods 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 208000007629 thrombocytopenia 3 Diseases 0.000 description 1
- 208000007631 thrombocytopenia 4 Diseases 0.000 description 1
- 230000009424 thromboembolic effect Effects 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 231100000440 toxicity profile Toxicity 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000006276 transfer reaction Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000000844 transformation Methods 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 238000013414 tumor xenograft model Methods 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/4353—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems
- A61K31/437—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems the heterocyclic ring system containing a five-membered ring having nitrogen as a ring hetero atom, e.g. indolizine, beta-carboline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4178—1,3-Diazoles not condensed 1,3-diazoles and containing further heterocyclic rings, e.g. pilocarpine, nitrofurantoin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4453—Non condensed piperidines, e.g. piperocaine only substituted in position 1, e.g. propipocaine, diperodon
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4738—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4745—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems condensed with ring systems having nitrogen as a ring hetero atom, e.g. phenantrolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/513—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim having oxo groups directly attached to the heterocyclic ring, e.g. cytosine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/555—Heterocyclic compounds containing heavy metals, e.g. hemin, hematin, melarsoprol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K33/00—Medicinal preparations containing inorganic active ingredients
- A61K33/24—Heavy metals; Compounds thereof
- A61K33/243—Platinum; Compounds thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Synthetic bilayered vehicles, e.g. liposomes or liposomes with cholesterol as the only non-phosphatidyl surfactant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Dermatology (AREA)
- Inorganic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662416317P | 2016-11-02 | 2016-11-02 | |
| US62/416,317 | 2016-11-02 | ||
| PCT/GB2017/053293 WO2018083470A1 (en) | 2016-11-02 | 2017-11-01 | Treating gastric cancer using combination therapies comprising liposomal irinotecan, oxaliplatin, 5-fluoruracil (and leucovorin) |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| RU2019114952A RU2019114952A (ru) | 2020-12-03 |
| RU2019114952A3 RU2019114952A3 (enExample) | 2021-03-03 |
| RU2761953C2 true RU2761953C2 (ru) | 2021-12-14 |
Family
ID=60569939
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2019114952A RU2761953C2 (ru) | 2016-11-02 | 2017-11-01 | Лечение рака желудка с применением комбинационных видов терапии, содержащих липосомальный иринотекан, оксалиплатин, 5-фторурацил (и лейковорин) |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US11071726B2 (enExample) |
| EP (1) | EP3535026A1 (enExample) |
| JP (2) | JP2019533684A (enExample) |
| KR (1) | KR20190077441A (enExample) |
| CN (1) | CN110402163A (enExample) |
| AU (1) | AU2017354903B2 (enExample) |
| BR (1) | BR112019007844A2 (enExample) |
| CA (1) | CA3040395A1 (enExample) |
| EA (1) | EA201990979A1 (enExample) |
| IL (2) | IL266187A (enExample) |
| MA (1) | MA46709A (enExample) |
| MX (1) | MX392408B (enExample) |
| MY (1) | MY202377A (enExample) |
| PH (1) | PH12019500892A1 (enExample) |
| RU (1) | RU2761953C2 (enExample) |
| SG (2) | SG11201903615WA (enExample) |
| TW (1) | TWI791467B (enExample) |
| WO (1) | WO2018083470A1 (enExample) |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2566007C (en) * | 2004-05-03 | 2013-09-24 | Hermes Biosciences, Inc. | Drug delivery liposomes containing anionic polyols or anionic sugars |
| US9717724B2 (en) | 2012-06-13 | 2017-08-01 | Ipsen Biopharm Ltd. | Methods for treating pancreatic cancer using combination therapies |
| AU2013202947B2 (en) | 2012-06-13 | 2016-06-02 | Ipsen Biopharm Ltd. | Methods for treating pancreatic cancer using combination therapies comprising liposomal irinotecan |
| US11318131B2 (en) | 2015-05-18 | 2022-05-03 | Ipsen Biopharm Ltd. | Nanoliposomal irinotecan for use in treating small cell lung cancer |
| EP3337467B1 (en) | 2015-08-20 | 2020-12-09 | Ipsen Biopharm Ltd. | Combination therapy using liposomal irinotecan and a parp inhibitor for cancer treatment |
| TW202243677A (zh) | 2015-08-21 | 2022-11-16 | 英商益普生生物製藥有限公司 | 使用包含微脂伊立替康(irinotecan)及奧沙利鉑(oxaliplatin)之組合療法治療轉移性胰臟癌的方法 |
| CN108366965B (zh) | 2015-10-16 | 2021-10-01 | 易普森生物制药有限公司 | 稳定喜树碱药物组合物 |
| AU2017354903B2 (en) | 2016-11-02 | 2023-04-13 | Ipsen Biopharm Ltd. | Treating gastric cancer using combination therapies comprising liposomal irinotecan, oxaliplatin, 5-fluoruracil (and leucovorin) |
| DK3603620T3 (da) | 2017-03-31 | 2025-12-01 | Fujifilm Corp | Liposomsammensætning og farmaceutisk sammensætning |
| EP3811931B1 (en) | 2018-06-20 | 2024-07-03 | FUJIFILM Corporation | Combination medication containing liposome composition encapsulating drug and immune checkpoint inhibitor |
| WO2020071349A1 (ja) * | 2018-10-01 | 2020-04-09 | 富士フイルム株式会社 | 薬物を内包するリポソーム組成物およびプラチナ製剤を含む組合せ医薬 |
| EP3866812A1 (en) * | 2018-10-17 | 2021-08-25 | BioLineRx Ltd. | Treatment of metastatic pancreatic adenocarcinoma |
| US20220072087A1 (en) * | 2019-01-17 | 2022-03-10 | Biolinerx Ltd. | Specific combination therapy for treatment of pancreatic cancer |
| WO2020148744A1 (en) * | 2019-01-17 | 2020-07-23 | Biolinerx Ltd. | Combination therapy for treatment of pancreatic cancer |
| WO2020170164A1 (en) * | 2019-02-22 | 2020-08-27 | Moshe Giladi | Treating gastric cancer using ttfields combined with xelox or folfox |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013188586A1 (en) * | 2012-06-13 | 2013-12-19 | Merrimack Pharmaceuticals, Inc. | Methods for treating pancreatic cancer using combination therapies comprising liposomal irinotecan |
Family Cites Families (85)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6019790A (ja) | 1983-07-14 | 1985-01-31 | Yakult Honsha Co Ltd | 新規なカンプトテシン誘導体 |
| US5077056A (en) | 1984-08-08 | 1991-12-31 | The Liposome Company, Inc. | Encapsulation of antineoplastic agents in liposomes |
| JPH0720857B2 (ja) | 1988-08-11 | 1995-03-08 | テルモ株式会社 | リポソームおよびその製法 |
| IL91664A (en) | 1988-09-28 | 1993-05-13 | Yissum Res Dev Co | Ammonium transmembrane gradient system for efficient loading of liposomes with amphipathic drugs and their controlled release |
| US5013556A (en) | 1989-10-20 | 1991-05-07 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5538954A (en) | 1994-06-24 | 1996-07-23 | A/S Dumex (Dumex Ltd.) | Salts of tetracyclines |
| US5783568A (en) | 1994-06-10 | 1998-07-21 | Sugen, Inc. | Methods for treating cancer and other cell proliferative diseases |
| US5543152A (en) | 1994-06-20 | 1996-08-06 | Inex Pharmaceuticals Corporation | Sphingosomes for enhanced drug delivery |
| US6214388B1 (en) | 1994-11-09 | 2001-04-10 | The Regents Of The University Of California | Immunoliposomes that optimize internalization into target cells |
| US5800833A (en) | 1995-02-27 | 1998-09-01 | University Of British Columbia | Method for loading lipid vesicles |
| DE19605024A1 (de) | 1996-01-31 | 1997-08-07 | Schering Ag | Neue selektive Taxane, Verfahren zu ihrer Herstellung und ihre pharmazeutische Verwendung |
| US5997899A (en) | 1996-10-01 | 1999-12-07 | Skyepharma Inc. | Method for producing liposomes with increased percent of compound encapsulated |
| WO1998017256A1 (en) | 1996-10-22 | 1998-04-30 | Dmitri Kirpotin | Compound-loaded liposomes and methods for their preparation |
| US6210707B1 (en) | 1996-11-12 | 2001-04-03 | The Regents Of The University Of California | Methods of forming protein-linked lipidic microparticles, and compositions thereof |
| US6787132B1 (en) | 1997-12-04 | 2004-09-07 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Combined chemo-immunotherapy with liposomal drugs and cytokines |
| US7244826B1 (en) | 1998-04-24 | 2007-07-17 | The Regents Of The University Of California | Internalizing ERB2 antibodies |
| US6726925B1 (en) | 1998-06-18 | 2004-04-27 | Duke University | Temperature-sensitive liposomal formulation |
| CA2346879A1 (en) | 1998-09-16 | 2000-04-27 | Alza Corporation | Liposome-entrapped topoisomerase inhibitors |
| US7311924B2 (en) | 1999-04-01 | 2007-12-25 | Hana Biosciences, Inc. | Compositions and methods for treating cancer |
| WO2000066125A1 (en) | 1999-04-29 | 2000-11-09 | Aventis Pharma S.A. | Method for treating cancer using camptothecin derivatives and 5-fluorouracil |
| US6720001B2 (en) | 1999-10-18 | 2004-04-13 | Lipocine, Inc. | Emulsion compositions for polyfunctional active ingredients |
| US6511676B1 (en) | 1999-11-05 | 2003-01-28 | Teni Boulikas | Therapy for human cancers using cisplatin and other drugs or genes encapsulated into liposomes |
| ES2272496T3 (es) | 2000-02-04 | 2007-05-01 | Lipoxen Technologies Limited | Procedimiento de deshidratacion/rehidritacion para la preparacion de lipososmas. |
| US6545010B2 (en) | 2000-03-17 | 2003-04-08 | Aventis Pharma S.A. | Composition comprising camptothecin or a camptothecin derivative and a platin derivative for the treatment of cancer |
| AU2001261473B2 (en) | 2000-05-15 | 2006-09-14 | Board Of Trustees Of The University Of Arkansas | Compositions and methods for the treatment of colorectal cancer |
| ES2253398T3 (es) | 2000-06-30 | 2006-06-01 | Inex Pharmaceuticals Corp. | Camptotecinas liposomales mejoradas y sus usos. |
| TWI283575B (en) | 2000-10-31 | 2007-07-11 | Eisai Co Ltd | Medicinal compositions for concomitant use as anticancer agent |
| EP1385479A4 (en) | 2001-03-26 | 2006-12-06 | Alza Corp | LIPOSOMIC COMPOSITION FOR IMPROVED INTRA-CELLULAR RELEASE OF A THERAPEUTIC AGENT |
| US7219016B2 (en) | 2001-04-20 | 2007-05-15 | Yale University | Systems and methods for automated analysis of cells and tissues |
| WO2003030864A1 (en) | 2001-05-29 | 2003-04-17 | Neopharm, Inc. | Liposomal formulation of irinotecan |
| AU2002331290A1 (en) | 2001-07-23 | 2003-02-24 | Epidauros Biotechnologie Ag | Methods for the treatment of cancer with irinotecan based on cyp3a5 |
| US7850990B2 (en) | 2001-10-03 | 2010-12-14 | Celator Pharmaceuticals, Inc. | Compositions for delivery of drug combinations |
| AU2003237864B2 (en) | 2002-05-15 | 2008-12-18 | California Pacific Medical Center | Delivery of nucleic acid-like compounds |
| EP1539207A4 (en) | 2002-05-31 | 2008-01-02 | Transmolecular Inc | TREATMENT OF CELL PROLIFERATION DISORDERS USING CHLOROTOXIN |
| AU2003296897A1 (en) | 2002-08-20 | 2004-05-04 | Neopharm, Inc. | Pharmaceutical formulations of camptothecine derivatives |
| RS20181002A1 (sr) | 2003-05-30 | 2018-12-31 | Genentech Inc | Tretman sa anti-vegf antitelima |
| CA2566007C (en) | 2004-05-03 | 2013-09-24 | Hermes Biosciences, Inc. | Drug delivery liposomes containing anionic polyols or anionic sugars |
| US8658203B2 (en) | 2004-05-03 | 2014-02-25 | Merrimack Pharmaceuticals, Inc. | Liposomes useful for drug delivery to the brain |
| GEP20094620B (en) * | 2004-06-01 | 2009-02-25 | Yakult Honsha Kk | Irinotecan preparation |
| JP2006248978A (ja) | 2005-03-10 | 2006-09-21 | Mebiopharm Co Ltd | 新規なリポソーム製剤 |
| TWI375673B (en) | 2005-04-11 | 2012-11-01 | Abbott Lab | 1h-benzimidazole-4-carboxamides substituted with a quaternary carbon at the 2-position are potent parp inhibitors |
| AU2006306108B2 (en) | 2005-10-25 | 2012-10-04 | Celator Pharmaceuticals, Inc. | Fixed ratio drug combination treatments for solid tumors |
| US20090148506A1 (en) | 2005-12-22 | 2009-06-11 | Awa Dicko | Liposomal formulations comprising secondary and tertiary amines and methods for preparing thereof |
| JP5289060B2 (ja) | 2006-01-17 | 2013-09-11 | アボット・ラボラトリーズ | Parpインヒビターとの組合せ療法 |
| EP2004175A4 (en) | 2006-03-16 | 2010-12-15 | Bionumerik Pharmaceuticals Inc | COMPOUNDS AND FORMULATIONS FOR INCREASING THE EFFECT OF CANCER AND METHOD FOR THEIR USE |
| JP5564266B2 (ja) | 2007-02-16 | 2014-07-30 | メリマック ファーマシューティカルズ インコーポレーティッド | Erbb3に対する抗体およびその使用 |
| US20120003160A1 (en) | 2007-06-29 | 2012-01-05 | Amag Pharmaceuticals, Inc. | Macrophage-Enhanced MRI (MEMRI) in a Single Imaging Session |
| ES2550759T3 (es) | 2007-08-17 | 2015-11-12 | Celator Pharmaceuticals, Inc. | Formulaciones farmacológicas de platino mejoradas |
| EP2197917A1 (en) | 2007-09-28 | 2010-06-23 | Universitätsspital Basel | Immunoliposomes for treatment of cancer |
| NZ586123A (en) | 2007-11-12 | 2012-12-21 | Bipar Sciences Inc | Treatment of ovarian cancer with 4-iodo-3-nitrobenzamide in combination with topoisomerase inhibitors |
| AU2008333786A1 (en) | 2007-12-07 | 2009-06-11 | Bipar Sciences, Inc. | Treatment of cancer with combinations of topoisomerase inhibitors and PARP inhibitors |
| MX2010010480A (es) | 2008-03-25 | 2010-10-15 | Schering Corp | Metodos de tratamiento o prevencion del cancer colorrectal. |
| US8067432B2 (en) | 2008-03-31 | 2011-11-29 | University Of Kentucky Research Foundation | Liposomal, ring-opened camptothecins with prolonged, site-specific delivery of active drug to solid tumors |
| ES2525065T3 (es) | 2008-04-11 | 2014-12-17 | Merrimack Pharmaceuticals, Inc. | Ligadores de seroalbúmina humana y sus conjugados |
| US8852630B2 (en) | 2008-05-13 | 2014-10-07 | Yale University | Chimeric small molecules for the recruitment of antibodies to cancer cells |
| KR20110112301A (ko) | 2008-11-18 | 2011-10-12 | 메리맥 파마슈티컬즈, 인크. | 인간 혈청 알부민 링커 및 그 콘쥬게이트 |
| JP2012525371A (ja) | 2009-05-01 | 2012-10-22 | オンコザイム・ファーマ・インコーポレイテッド | 癌を治療するためのペンタミジンの組み合わせ |
| PT2508170E (pt) | 2009-12-03 | 2015-10-16 | Shanghai Hengrui Pharm Co Ltd | Lipossoma de irinotecano ou o seu cloridrato e seu método de preparação |
| KR20180049180A (ko) | 2010-06-04 | 2018-05-10 | 아브락시스 바이오사이언스, 엘엘씨 | 췌장암의 치료 방법 |
| EP2595618A1 (en) | 2010-07-19 | 2013-05-29 | BiPar Sciences, Inc. | Methods of treating breast cancer using 4-iodo-3-nitrobenzamide in combination with anti-tumor agents |
| CA2806076A1 (en) | 2010-07-22 | 2012-01-26 | The Regents Of The University Of California | Anti-tumor antigen antibodies and methods of use |
| WO2012031293A1 (en) | 2010-09-03 | 2012-03-08 | Board Of Regents Of The University Of Nebraska | Compositions and methods for the treatment of cancer |
| KR20140041396A (ko) | 2010-12-06 | 2014-04-04 | 메리맥 파마슈티컬즈, 인크. | 안트라사이클린 화학요법제를 포함하는 erbb2-표적 면역리포좀으로의 치료에서 심장독성을 예방하기 위한 용량 및 투여 |
| JP5947807B2 (ja) | 2010-12-14 | 2016-07-06 | テクニカル ユニバーシティ オブ デンマークTechnical University Of Denmark | ナノ粒子組成物への放射性核種の封入 |
| CA2827118A1 (en) | 2011-02-15 | 2012-08-23 | Merrimack Pharmaceuticals, Inc. | Compositions and methods for delivering nucleic acid to a cell |
| MX346486B (es) | 2011-04-19 | 2017-03-22 | Merrimack Pharmaceuticals Inc | Anticuerpos anti-igf-1r y anti-erbb3 monoespecificos y biespecificos. |
| JO3283B1 (ar) | 2011-04-26 | 2018-09-16 | Sanofi Sa | تركيب يتضمن أفليبيرسيبت, حمض فولينيك, 5- فلورويوراسيل (5- Fu) وإرينوسيتان (FOLFIRI) |
| US8691231B2 (en) | 2011-06-03 | 2014-04-08 | Merrimack Pharmaceuticals, Inc. | Methods of treatment of tumors expressing predominantly high affinity EGFR ligands or tumors expressing predominantly low affinity EGFR ligands with monoclonal and oligoclonal anti-EGFR antibodies |
| WO2013138371A1 (en) * | 2012-03-12 | 2013-09-19 | Merrimack Pharmaceuticals, Inc. | Methods for treating pancreatic cancer using combination therapies comprising an anti-erbb3 antibody |
| KR20150050524A (ko) | 2012-04-17 | 2015-05-08 | 메리맥 파마슈티컬즈, 인크. | 비침습적 이미징을 위한 조성물 및 방법 |
| US9717724B2 (en) | 2012-06-13 | 2017-08-01 | Ipsen Biopharm Ltd. | Methods for treating pancreatic cancer using combination therapies |
| WO2014113167A1 (en) | 2012-12-14 | 2014-07-24 | Merrimack Pharmaceuticals, Inc. | Non-invasive imaging methods for patient selection for treatment with nanoparticulate therapeutic agents |
| HUE041687T2 (hu) | 2013-03-27 | 2019-05-28 | Taiho Pharmaceutical Co Ltd | Antitumor szer, amely tartalmaz kis dózisú irinotekan-hidroklorid-hidrát-ot |
| WO2015031536A1 (en) | 2013-08-27 | 2015-03-05 | Northeastern University | Nanoparticle drug delivery system and method of treating cancer and neurotrauma |
| WO2015061592A1 (en) | 2013-10-23 | 2015-04-30 | Merrimack Pharmaceuticals, Inc. | Liposomes for non-invasive imaging and drug delivery |
| JP7113619B2 (ja) | 2014-12-09 | 2022-08-05 | イプセン バイオファーム リミティド | リポソーマルイリノテカンによる乳がんの治療 |
| TW201701880A (zh) | 2015-04-14 | 2017-01-16 | 莫瑞麥克製藥公司 | 改善持續釋放藥物治療之藥物動力學及治療指數之方法 |
| EP3337467B1 (en) | 2015-08-20 | 2020-12-09 | Ipsen Biopharm Ltd. | Combination therapy using liposomal irinotecan and a parp inhibitor for cancer treatment |
| TW202243677A (zh) | 2015-08-21 | 2022-11-16 | 英商益普生生物製藥有限公司 | 使用包含微脂伊立替康(irinotecan)及奧沙利鉑(oxaliplatin)之組合療法治療轉移性胰臟癌的方法 |
| CN108366965B (zh) | 2015-10-16 | 2021-10-01 | 易普森生物制药有限公司 | 稳定喜树碱药物组合物 |
| US20170202840A1 (en) | 2016-01-14 | 2017-07-20 | Merrimack Pharmaceuticals, Inc. | Treatment of pancreatic cancer with liposomal irinotecan |
| WO2017172678A1 (en) * | 2016-03-30 | 2017-10-05 | Merrimack Pharmaceuticals, Inc. | Methods for treating cancer using combination therapies comprising an oligoclonal anti-egfr antibody preparation and lipsomal irinotecan |
| US20170333421A1 (en) | 2016-05-18 | 2017-11-23 | Ipsen Biopharm Ltd. | Population Pharmacokinetics of Liposomal Irinotecan |
| AU2017267449A1 (en) | 2016-05-18 | 2018-11-15 | Ipsen Biopharm Ltd. | Nanoliposomal irinotecan for use in treating small cell lung cancer |
| AU2017354903B2 (en) | 2016-11-02 | 2023-04-13 | Ipsen Biopharm Ltd. | Treating gastric cancer using combination therapies comprising liposomal irinotecan, oxaliplatin, 5-fluoruracil (and leucovorin) |
-
2017
- 2017-11-01 AU AU2017354903A patent/AU2017354903B2/en active Active
- 2017-11-01 EA EA201990979A patent/EA201990979A1/ru unknown
- 2017-11-01 US US16/346,436 patent/US11071726B2/en not_active Expired - Fee Related
- 2017-11-01 JP JP2019522381A patent/JP2019533684A/ja not_active Ceased
- 2017-11-01 MY MYPI2019002087A patent/MY202377A/en unknown
- 2017-11-01 WO PCT/GB2017/053293 patent/WO2018083470A1/en not_active Ceased
- 2017-11-01 RU RU2019114952A patent/RU2761953C2/ru active
- 2017-11-01 MA MA046709A patent/MA46709A/fr unknown
- 2017-11-01 CA CA3040395A patent/CA3040395A1/en active Pending
- 2017-11-01 TW TW106137766A patent/TWI791467B/zh active
- 2017-11-01 CN CN201780080584.XA patent/CN110402163A/zh active Pending
- 2017-11-01 SG SG11201903615WA patent/SG11201903615WA/en unknown
- 2017-11-01 KR KR1020197014970A patent/KR20190077441A/ko not_active Ceased
- 2017-11-01 BR BR112019007844A patent/BR112019007844A2/pt not_active IP Right Cessation
- 2017-11-01 EP EP17808557.7A patent/EP3535026A1/en not_active Ceased
- 2017-11-01 MX MX2019004783A patent/MX392408B/es unknown
- 2017-11-01 SG SG10201912338RA patent/SG10201912338RA/en unknown
-
2019
- 2019-04-23 IL IL266187A patent/IL266187A/en unknown
- 2019-04-24 PH PH12019500892A patent/PH12019500892A1/en unknown
-
2022
- 2022-03-24 IL IL291679A patent/IL291679A/en unknown
- 2022-09-15 JP JP2022146925A patent/JP2022171808A/ja active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013188586A1 (en) * | 2012-06-13 | 2013-12-19 | Merrimack Pharmaceuticals, Inc. | Methods for treating pancreatic cancer using combination therapies comprising liposomal irinotecan |
Non-Patent Citations (7)
| Title |
|---|
| Bouche, O., et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (Lv5fu2), Lv5fu2 plus cisplatin, or Lv5fu2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone De Cancerologie Digestive Group Study Ffcd 9803, J Clin Oncol. 2004 Nov 1;22(21):4319-28, найдено онлайн, найдено в Интернете: https://ascopubs.org/doi/10.1200/JCO.2004.01.140?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed;. * |
| Chiang, N.-J., et al., Development of nanoliposomal irinotecan (nal-IRI, MM-398, PEP02) in the management of metastatic pancreatic cancer. Expert Opinion on Pharmacotherapy, 17(10), 1413-1420, Published online: 17 May 2016, найдено онлайн, найдено в Интернет: https://www.tandfonline.com/doi/abs/10.1080/14656566.2016.1183646?journalCode=ieop20. * |
| Matteo Dalla Chiesa at al., Sequential chemotherapy with dose-dense docetaxel, cisplatin, folinic acid and 5-fluorouracil (TCF-dd) followed by combination of oxaliplatin, folinic acid, 5-fluorouracil and irinotecan (COFFI) in metastatic gastric cancer: results of a phase II trial, Cancer Chemother Pharmacol, Published online: 5 March 2010, 67 (1):41-48, the abstract, Patients and Methods, Results, Discussion, doi:10.1007/s00280-010-1281-5. * |
| Matteo Dalla Chiesa at al., Sequential chemotherapy with dose-dense docetaxel, cisplatin, folinic acid and 5-fluorouracil (TCF-dd) followed by combination of oxaliplatin, folinic acid, 5-fluorouracil and irinotecan (COFFI) in metastatic gastric cancer: results of a phase II trial, Cancer Chemother Pharmacol, Published online: 5 March 2010, 67 (1):41-48, the abstract, Patients and Methods, Results, Discussion, doi:10.1007/s00280-010-1281-5. STEFAN PEINERT ET AL, "Safety and efficacy of weekly 5-fluorouracil/ folinic acid/oxaliplatin/irinotecan in the first-line treatment of gastrointestinal cancer", Therapeutic Advances in Medical Oncology, Vol. 2 (3), 01 May 2010, page 161-174, the abstract, 'Patients and Methods', Results, Discussion', DOI: 10.1177/1758834010365061 external link. Bouche, O., et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (Lv5fu2), Lv5fu2 plus cisplatin, or Lv5fu2 plus irinotecan in patients with previously untreated m * |
| Min H Kang et al., Activity of MM-398, nanoliposomal irinotecan (nal-IRI), in Ewing's family tumor xenografts is associated with high exposure of tumor to drug and high SLFN11 expression Clin Cancer Res. 2015 Mar 1;21(5):1139-50. Найдено онлайн, найдено в Интернете: https://pubmed.ncbi.nlm.nih.gov/25733708/. * |
| STEFAN PEINERT ET AL, "Safety and efficacy of weekly 5-fluorouracil/ folinic acid/oxaliplatin/irinotecan in the first-line treatment of gastrointestinal cancer", Therapeutic Advances in Medical Oncology, Vol. 2 (3), 01 May 2010, page 161-174, the abstract, 'Patients and Methods', Results, Discussion', DOI: 10.1177/1758834010365061 external link. * |
| W. KOIZUMI at al., Phase I/II Study of Bi-weekly Irinotecan plus Cisplatin in the Treatment of Advanced Gastric Cancer, Anticancer Res. Mar-Apr 2005;25(2B):1257-62., найдено онлайн, найдено в Интернете: https://ar.iiarjournals.org/content/25/2B/1257. * |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2019533684A (ja) | 2019-11-21 |
| RU2019114952A3 (enExample) | 2021-03-03 |
| MX2019004783A (es) | 2019-08-12 |
| BR112019007844A2 (pt) | 2019-07-16 |
| PH12019500892A1 (en) | 2019-06-17 |
| SG10201912338RA (en) | 2020-02-27 |
| AU2017354903B2 (en) | 2023-04-13 |
| EA201990979A1 (ru) | 2019-09-30 |
| TWI791467B (zh) | 2023-02-11 |
| IL291679A (en) | 2022-05-01 |
| JP2022171808A (ja) | 2022-11-11 |
| MX392408B (es) | 2025-03-24 |
| MA46709A (fr) | 2019-09-11 |
| MY202377A (en) | 2024-04-24 |
| US11071726B2 (en) | 2021-07-27 |
| TW201825097A (zh) | 2018-07-16 |
| AU2017354903A1 (en) | 2019-05-02 |
| CA3040395A1 (en) | 2018-05-11 |
| IL266187A (en) | 2019-06-30 |
| US20200030302A1 (en) | 2020-01-30 |
| RU2019114952A (ru) | 2020-12-03 |
| WO2018083470A1 (en) | 2018-05-11 |
| CN110402163A (zh) | 2019-11-01 |
| KR20190077441A (ko) | 2019-07-03 |
| EP3535026A1 (en) | 2019-09-11 |
| SG11201903615WA (en) | 2019-05-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2761953C2 (ru) | Лечение рака желудка с применением комбинационных видов терапии, содержащих липосомальный иринотекан, оксалиплатин, 5-фторурацил (и лейковорин) | |
| US20250381191A1 (en) | Methods for Treating Metastatic Pancreatic Cancer Using Combination Therapies Comprising Liposomal Irinotecan and Oxaliplatin | |
| US12364691B2 (en) | Methods for treating pancreatic cancer using combination therapies | |
| CA2993451C (en) | Methods for treating metastatic pancreatic cancer using combination therapies comprising liposomal irinotecan and oxaliplatin | |
| HK40045473A (en) | Methods for treating metastatic pancreatic cancer using combination therapies comprising liposomal irinotecan and oxaliplatin | |
| HK1257250B (en) | Drug combination comprising liposomal irinotecan, oxaliplatin, 5-fluorouracil and leucovorin for treating metastatic pancreatic cancer | |
| HK40009222A (en) | Treating gastric cancer using combination therapies comprising liposomal irinotecan, oxaliplatin, 5-fluoruracil (and leucovorin) |