RU2497820C2 - Кристаллические формы и две сольватные формы солей молочной кислоты 4-амино-5-фтор-3-[5-(4-метилпиперазин-1-ил)-1 - н-бензимидазол-2-ил]хинолин-2(1н)она - Google Patents
Кристаллические формы и две сольватные формы солей молочной кислоты 4-амино-5-фтор-3-[5-(4-метилпиперазин-1-ил)-1 - н-бензимидазол-2-ил]хинолин-2(1н)она Download PDFInfo
- Publication number
- RU2497820C2 RU2497820C2 RU2010142396/04A RU2010142396A RU2497820C2 RU 2497820 C2 RU2497820 C2 RU 2497820C2 RU 2010142396/04 A RU2010142396/04 A RU 2010142396/04A RU 2010142396 A RU2010142396 A RU 2010142396A RU 2497820 C2 RU2497820 C2 RU 2497820C2
- Authority
- RU
- Russia
- Prior art keywords
- methylpiperazin
- amino
- lactic acid
- benzimidazol
- cancer
- Prior art date
Links
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical class CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 title claims abstract description 41
- 239000012453 solvate Substances 0.000 title description 27
- IRFHMTUHTBSEBK-QGZVFWFLSA-N tert-butyl n-[(2s)-2-(2,5-difluorophenyl)-3-quinolin-3-ylpropyl]carbamate Chemical compound C1([C@H](CC=2C=C3C=CC=CC3=NC=2)CNC(=O)OC(C)(C)C)=CC(F)=CC=C1F IRFHMTUHTBSEBK-QGZVFWFLSA-N 0.000 title 1
- 230000000694 effects Effects 0.000 claims abstract description 13
- 238000000034 method Methods 0.000 claims abstract description 12
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 8
- 101000851007 Homo sapiens Vascular endothelial growth factor receptor 2 Proteins 0.000 claims abstract description 7
- 206010028980 Neoplasm Diseases 0.000 claims abstract 2
- 201000011510 cancer Diseases 0.000 claims abstract 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical group CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 53
- 239000000203 mixture Substances 0.000 claims description 19
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 claims description 15
- PIQCTGMSNWUMAF-UHFFFAOYSA-N chembl522892 Chemical compound C1CN(C)CCN1C1=CC=C(NC(=N2)C=3C(NC4=CC=CC(F)=C4C=3N)=O)C2=C1 PIQCTGMSNWUMAF-UHFFFAOYSA-N 0.000 claims description 15
- ZRHDKBOBHHFLBW-UHFFFAOYSA-N 4-amino-5-fluoro-3-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-quinolin-2-one 2-hydroxypropanoic acid Chemical compound CC(O)C(O)=O.C1CN(C)CCN1C1=CC=C(NC(=N2)C=3C(NC4=CC=CC(F)=C4C=3N)=O)C2=C1 ZRHDKBOBHHFLBW-UHFFFAOYSA-N 0.000 claims description 13
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 claims description 10
- 239000000843 powder Substances 0.000 claims description 10
- 239000004310 lactic acid Substances 0.000 claims description 9
- 235000014655 lactic acid Nutrition 0.000 claims description 9
- 150000003839 salts Chemical class 0.000 claims description 9
- 239000000725 suspension Substances 0.000 claims description 9
- 201000010099 disease Diseases 0.000 claims description 8
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 8
- 102100027842 Fibroblast growth factor receptor 3 Human genes 0.000 claims description 6
- 101710182396 Fibroblast growth factor receptor 3 Proteins 0.000 claims description 6
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 claims description 6
- 239000002904 solvent Substances 0.000 claims description 6
- 230000005764 inhibitory process Effects 0.000 claims description 5
- 239000003085 diluting agent Substances 0.000 claims description 4
- 239000003937 drug carrier Substances 0.000 claims description 4
- 239000003960 organic solvent Substances 0.000 claims description 4
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 4
- 208000032839 leukemia Diseases 0.000 claims description 3
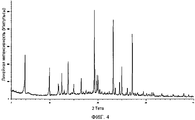
- 238000000634 powder X-ray diffraction Methods 0.000 claims description 3
- 238000003756 stirring Methods 0.000 claims description 3
- 208000031261 Acute myeloid leukaemia Diseases 0.000 claims description 2
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 claims description 2
- 206010006187 Breast cancer Diseases 0.000 claims description 2
- 208000026310 Breast neoplasm Diseases 0.000 claims description 2
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 claims description 2
- 206010009944 Colon cancer Diseases 0.000 claims description 2
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 2
- 206010051066 Gastrointestinal stromal tumour Diseases 0.000 claims description 2
- 208000032612 Glial tumor Diseases 0.000 claims description 2
- 206010018338 Glioma Diseases 0.000 claims description 2
- 208000008839 Kidney Neoplasms Diseases 0.000 claims description 2
- 206010025323 Lymphomas Diseases 0.000 claims description 2
- 208000034578 Multiple myelomas Diseases 0.000 claims description 2
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 claims description 2
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 claims description 2
- 208000014767 Myeloproliferative disease Diseases 0.000 claims description 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 2
- 206010060862 Prostate cancer Diseases 0.000 claims description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 2
- 206010038389 Renal cancer Diseases 0.000 claims description 2
- 206010039491 Sarcoma Diseases 0.000 claims description 2
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 2
- 208000024770 Thyroid neoplasm Diseases 0.000 claims description 2
- 208000006990 cholangiocarcinoma Diseases 0.000 claims description 2
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 claims description 2
- 206010017758 gastric cancer Diseases 0.000 claims description 2
- 201000011243 gastrointestinal stromal tumor Diseases 0.000 claims description 2
- 201000010982 kidney cancer Diseases 0.000 claims description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 2
- 201000001441 melanoma Diseases 0.000 claims description 2
- 201000002120 neuroendocrine carcinoma Diseases 0.000 claims description 2
- 201000002528 pancreatic cancer Diseases 0.000 claims description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 2
- 208000000649 small cell carcinoma Diseases 0.000 claims description 2
- 201000011549 stomach cancer Diseases 0.000 claims description 2
- 201000002510 thyroid cancer Diseases 0.000 claims description 2
- 208000003174 Brain Neoplasms Diseases 0.000 claims 1
- 230000002485 urinary effect Effects 0.000 claims 1
- 239000003814 drug Substances 0.000 abstract description 8
- 230000002401 inhibitory effect Effects 0.000 abstract description 3
- 239000000126 substance Substances 0.000 abstract description 3
- 150000001875 compounds Chemical class 0.000 abstract description 2
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 30
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 17
- 125000004195 4-methylpiperazin-1-yl group Chemical group [H]C([H])([H])N1C([H])([H])C([H])([H])N(*)C([H])([H])C1([H])[H] 0.000 description 11
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 9
- 238000004519 manufacturing process Methods 0.000 description 9
- 230000004048 modification Effects 0.000 description 9
- 238000012986 modification Methods 0.000 description 9
- LISFMEBWQUVKPJ-UHFFFAOYSA-N quinolin-2-ol Chemical compound C1=CC=C2NC(=O)C=CC2=C1 LISFMEBWQUVKPJ-UHFFFAOYSA-N 0.000 description 8
- 150000004682 monohydrates Chemical class 0.000 description 7
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 6
- 229940079593 drug Drugs 0.000 description 6
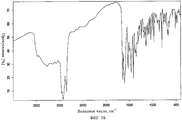
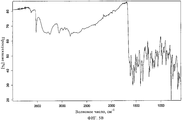
- 238000001237 Raman spectrum Methods 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 239000013078 crystal Substances 0.000 description 4
- 238000002425 crystallisation Methods 0.000 description 4
- 230000008025 crystallization Effects 0.000 description 4
- 238000001144 powder X-ray diffraction data Methods 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 238000004448 titration Methods 0.000 description 4
- 238000010438 heat treatment Methods 0.000 description 3
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 3
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 238000000113 differential scanning calorimetry Methods 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
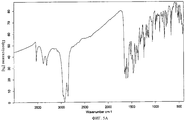
- 238000002329 infrared spectrum Methods 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- -1 methylpiperazin-1-yl Chemical group 0.000 description 2
- 239000002547 new drug Substances 0.000 description 2
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- VIDDDLSZDPUIHF-UHFFFAOYSA-N 5-fluoro-3-[6-(4-methylpiperazin-1-yl)-1h-benzimidazol-2-yl]quinolin-4-amine Chemical class C1CN(C)CCN1C1=CC=C(N=C(N2)C=3C(=C4C(F)=CC=CC4=NC=3)N)C2=C1 VIDDDLSZDPUIHF-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- FBWCJBXXXXZILY-UHFFFAOYSA-N C(CCC)O.C(C)(C)O.C(C)O.CC(=O)C Chemical compound C(CCC)O.C(C)(C)O.C(C)O.CC(=O)C FBWCJBXXXXZILY-UHFFFAOYSA-N 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- 238000005079 FT-Raman Methods 0.000 description 1
- 238000001157 Fourier transform infrared spectrum Methods 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 108090000873 Receptor Protein-Tyrosine Kinases Proteins 0.000 description 1
- 102000004278 Receptor Protein-Tyrosine Kinases Human genes 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- VJHCJDRQFCCTHL-UHFFFAOYSA-N acetic acid 2,3,4,5,6-pentahydroxyhexanal Chemical compound CC(O)=O.OCC(O)C(O)C(O)C(O)C=O VJHCJDRQFCCTHL-UHFFFAOYSA-N 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 229960005168 croscarmellose Drugs 0.000 description 1
- 229960000913 crospovidone Drugs 0.000 description 1
- 239000001767 crosslinked sodium carboxy methyl cellulose Substances 0.000 description 1
- 238000002447 crystallographic data Methods 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- 229940095079 dicalcium phosphate anhydrous Drugs 0.000 description 1
- UXGNZZKBCMGWAZ-UHFFFAOYSA-N dimethylformamide dmf Chemical compound CN(C)C=O.CN(C)C=O UXGNZZKBCMGWAZ-UHFFFAOYSA-N 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- MVPICKVDHDWCJQ-UHFFFAOYSA-N ethyl 3-pyrrolidin-1-ylpropanoate Chemical compound CCOC(=O)CCN1CCCC1 MVPICKVDHDWCJQ-UHFFFAOYSA-N 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 229960001375 lactose Drugs 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 1
- 150000004686 pentahydrates Chemical class 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229940069328 povidone Drugs 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940045902 sodium stearyl fumarate Drugs 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- WHRNULOCNSKMGB-UHFFFAOYSA-N tetrahydrofuran thf Chemical compound C1CCOC1.C1CCOC1 WHRNULOCNSKMGB-UHFFFAOYSA-N 0.000 description 1
- 238000002411 thermogravimetry Methods 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 231100000759 toxicological effect Toxicity 0.000 description 1
- 235000019731 tricalcium phosphate Nutrition 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Oncology (AREA)
- Hematology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US3774608P | 2008-03-19 | 2008-03-19 | |
| US61/037,746 | 2008-03-19 | ||
| PCT/EP2009/053222 WO2009115562A2 (en) | 2008-03-19 | 2009-03-18 | Crystalline forms and two solvated forms of 4-amino-5-fluoro-3-[5-(4-methylpiperazin-1-yl)-1h-benzimidazol-2-yl]quinolin-2(1h)-one lactic acid salts |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2010142396A RU2010142396A (ru) | 2012-04-27 |
| RU2497820C2 true RU2497820C2 (ru) | 2013-11-10 |
Family
ID=40622140
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2010142396/04A RU2497820C2 (ru) | 2008-03-19 | 2009-03-18 | Кристаллические формы и две сольватные формы солей молочной кислоты 4-амино-5-фтор-3-[5-(4-метилпиперазин-1-ил)-1 - н-бензимидазол-2-ил]хинолин-2(1н)она |
Country Status (24)
| Country | Link |
|---|---|
| US (1) | US8563556B2 (enExample) |
| EP (1) | EP2257544B1 (enExample) |
| JP (1) | JP2011515370A (enExample) |
| KR (1) | KR20100137517A (enExample) |
| CN (1) | CN101970425B (enExample) |
| AR (1) | AR070924A1 (enExample) |
| AU (1) | AU2009227003B2 (enExample) |
| BR (1) | BRPI0909762A2 (enExample) |
| CA (1) | CA2718076A1 (enExample) |
| CL (1) | CL2009000651A1 (enExample) |
| CO (1) | CO6321253A2 (enExample) |
| EC (1) | ECSP10010555A (enExample) |
| IL (1) | IL208020A0 (enExample) |
| MA (1) | MA32230B1 (enExample) |
| MX (1) | MX2010010152A (enExample) |
| MY (1) | MY150554A (enExample) |
| NZ (2) | NZ600887A (enExample) |
| PE (1) | PE20091628A1 (enExample) |
| PH (2) | PH12012502569A1 (enExample) |
| RU (1) | RU2497820C2 (enExample) |
| SG (1) | SG188919A1 (enExample) |
| TW (1) | TWI426072B (enExample) |
| WO (1) | WO2009115562A2 (enExample) |
| ZA (1) | ZA201006263B (enExample) |
Families Citing this family (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AR070924A1 (es) | 2008-03-19 | 2010-05-12 | Novartis Ag | Formas cristalinas y dos formas solvatadas de sales del acido lactico de 4- amino -5- fluoro-3-(5-(4-metilpiperazin-1-il ) -1h- bencimidazol-2-il) quinolin -2-(1h) - ona |
| WO2011128405A1 (en) | 2010-04-16 | 2011-10-20 | Novartis Ag | Combination of organic compounds |
| PL2558095T3 (pl) | 2010-04-16 | 2019-06-28 | Novartis Ag | Związek organiczny, przeznaczony do stosowania w leczeniu raka wątroby |
| AR081776A1 (es) | 2010-06-30 | 2012-10-17 | Novartis Ag | Composiciones farmaceuticas que comprenden monohidrato de lactato de 4-amino-5-fluoro-3-[6-(4-metil-piperazin-1-il)-1h-bencimidazol-2-il]-1h-quinolin-2-ona, proceso para la produccion de la composicion |
| AU2012229107A1 (en) | 2011-03-17 | 2013-09-19 | Novartis Ag | FGFR and ligands thereof as biomarkers for breast cancer in HR positive subjects |
| MX2013013437A (es) | 2011-05-19 | 2013-12-06 | Novartis Ag | 4-amino-5-fluoro-3- [6- (4-metil-piperazin-1-il) -1h-bencimidazol-2-il] -1h-quinolin-2-ona parea usarse en el tratamiento de un carcinoma adenoide quistico. |
| KR20140062485A (ko) | 2011-09-15 | 2014-05-23 | 노파르티스 아게 | 중등도 간 손상 환자의 암 치료에 있어서 4-아미노-5-플루오로-3-[6-(4-메틸피페라진-1-일)-1h-벤즈이미다졸-2-일]-1h-퀴놀린-2-온의 용도 |
| WO2013063003A1 (en) | 2011-10-28 | 2013-05-02 | Novartis Ag | Method of treating gastrointestinal stromal tumors |
| JP2015505562A (ja) | 2012-01-31 | 2015-02-23 | ノバルティス アーゲー | Rtk阻害剤と抗エストロゲン剤との組合せ、およびがん治療のためのその使用 |
| BR112015000349A2 (pt) | 2012-07-11 | 2017-06-27 | Novartis Ag | método de tratamento de tumores estromais gastrointestinais |
| WO2014058785A1 (en) | 2012-10-10 | 2014-04-17 | Novartis Ag | Combination therapy |
| EP2764866A1 (en) | 2013-02-07 | 2014-08-13 | IP Gesellschaft für Management mbH | Inhibitors of nedd8-activating enzyme |
| JOP20200094A1 (ar) | 2014-01-24 | 2017-06-16 | Dana Farber Cancer Inst Inc | جزيئات جسم مضاد لـ pd-1 واستخداماتها |
| JOP20200096A1 (ar) | 2014-01-31 | 2017-06-16 | Children’S Medical Center Corp | جزيئات جسم مضاد لـ tim-3 واستخداماتها |
| LT3116909T (lt) | 2014-03-14 | 2020-02-10 | Novartis Ag | Antikūno molekulės prieš lag-3 ir jų panaudojimas |
| MA41044A (fr) | 2014-10-08 | 2017-08-15 | Novartis Ag | Compositions et procédés d'utilisation pour une réponse immunitaire accrue et traitement contre le cancer |
| EP3206711B1 (en) | 2014-10-14 | 2023-05-31 | Novartis AG | Antibody molecules to pd-l1 and uses thereof |
| EP3233918A1 (en) | 2014-12-19 | 2017-10-25 | Novartis AG | Combination therapies |
| PE20171448A1 (es) | 2015-03-10 | 2017-10-02 | Aduro Biotech Inc | Composiciones y metodos para activar la senalizacion dependiente del estimulador del gen de interferon |
| WO2017019897A1 (en) | 2015-07-29 | 2017-02-02 | Novartis Ag | Combination therapies comprising antibody molecules to tim-3 |
| CN114272371A (zh) | 2015-07-29 | 2022-04-05 | 诺华股份有限公司 | 包含抗pd-1抗体分子的联合疗法 |
| EP3317301B1 (en) | 2015-07-29 | 2021-04-07 | Novartis AG | Combination therapies comprising antibody molecules to lag-3 |
| CA3004138A1 (en) | 2015-11-03 | 2017-05-11 | Janssen Biotech, Inc. | Antibodies specifically binding pd-1 and tim-3 and their uses |
| ES2986067T3 (es) | 2015-12-17 | 2024-11-08 | Novartis Ag | Moléculas de anticuerpos frente a PD-1 y usos de las mismas |
| WO2018009466A1 (en) | 2016-07-05 | 2018-01-11 | Aduro Biotech, Inc. | Locked nucleic acid cyclic dinucleotide compounds and uses thereof |
| UY37695A (es) | 2017-04-28 | 2018-11-30 | Novartis Ag | Compuesto dinucleótido cíclico bis 2’-5’-rr-(3’f-a)(3’f-a) y usos del mismo |
| US12398209B2 (en) | 2018-01-22 | 2025-08-26 | Janssen Biotech, Inc. | Methods of treating cancers with antagonistic anti-PD-1 antibodies |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ATE309996T1 (de) * | 2000-09-11 | 2005-12-15 | Chiron Corp | Chinolinonderivate als tyrosin-kinase inhibitoren |
| US20050256157A1 (en) * | 2002-08-23 | 2005-11-17 | Chiron Corporation | Combination therapy with CHK1 inhibitors |
| EP1539754A4 (en) * | 2002-08-23 | 2009-02-25 | Novartis Vaccines & Diagnostic | BENZIMIDAZOCHINOLINONE AND ITS USE |
| US7825132B2 (en) * | 2002-08-23 | 2010-11-02 | Novartis Vaccines And Diagnostics, Inc. | Inhibition of FGFR3 and treatment of multiple myeloma |
| US7838527B2 (en) * | 2002-11-13 | 2010-11-23 | Novartis Vaccines And Diagnostics, Inc. | Methods of treating cancer and related methods |
| CN100377709C (zh) * | 2002-11-13 | 2008-04-02 | 希龙公司 | 受体酪氨酸激酶抑制剂的制药用途及相关检测方法 |
| JP4890255B2 (ja) * | 2003-11-07 | 2012-03-07 | ノバルティス バクシンズ アンド ダイアグノスティックス,インコーポレーテッド | 改良された薬物特性を有するキノリノン化合物の薬学的に受容可能な塩 |
| RU2377988C2 (ru) * | 2004-02-20 | 2010-01-10 | Новартис Вэксинес Энд Дайэгностикс, Инк. | Модуляция воспалительных и метастатических процессов |
| SG173317A1 (en) * | 2005-01-27 | 2011-08-29 | Novartis Vaccines & Diagnostic | Treatment of metastasized tumors |
| AU2006249847B2 (en) * | 2005-05-23 | 2012-12-20 | Novartis Ag | Crystalline and other forms of 4-amino-5-fluoro-3-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]-1H-quinolin-2-one lactic acid salts |
| NZ567550A (en) | 2005-11-29 | 2011-08-26 | Novartis Ag | Formulations of lactic acid salts of 4-amino-5-fluoro-3-[6-(4-methyl-piperazin-1-yl)-1 H-benzimidazol-2-yl]1H-quinolin-2-one |
| AR070924A1 (es) | 2008-03-19 | 2010-05-12 | Novartis Ag | Formas cristalinas y dos formas solvatadas de sales del acido lactico de 4- amino -5- fluoro-3-(5-(4-metilpiperazin-1-il ) -1h- bencimidazol-2-il) quinolin -2-(1h) - ona |
| US8268834B2 (en) | 2008-03-19 | 2012-09-18 | Novartis Ag | Pyrazine derivatives that inhibit phosphatidylinositol 3-kinase enzyme |
-
2009
- 2009-03-17 AR ARP090100957A patent/AR070924A1/es unknown
- 2009-03-17 PE PE2009000395A patent/PE20091628A1/es not_active Application Discontinuation
- 2009-03-18 CA CA2718076A patent/CA2718076A1/en not_active Abandoned
- 2009-03-18 NZ NZ600887A patent/NZ600887A/xx not_active IP Right Cessation
- 2009-03-18 CN CN200980108402.0A patent/CN101970425B/zh not_active Expired - Fee Related
- 2009-03-18 SG SG2013020011A patent/SG188919A1/en unknown
- 2009-03-18 BR BRPI0909762A patent/BRPI0909762A2/pt not_active IP Right Cessation
- 2009-03-18 RU RU2010142396/04A patent/RU2497820C2/ru not_active IP Right Cessation
- 2009-03-18 AU AU2009227003A patent/AU2009227003B2/en not_active Ceased
- 2009-03-18 EP EP09721534.7A patent/EP2257544B1/en active Active
- 2009-03-18 MY MYPI20104156 patent/MY150554A/en unknown
- 2009-03-18 MX MX2010010152A patent/MX2010010152A/es not_active Application Discontinuation
- 2009-03-18 KR KR1020107023196A patent/KR20100137517A/ko not_active Ceased
- 2009-03-18 JP JP2011500221A patent/JP2011515370A/ja active Pending
- 2009-03-18 WO PCT/EP2009/053222 patent/WO2009115562A2/en not_active Ceased
- 2009-03-18 TW TW098108804A patent/TWI426072B/zh not_active IP Right Cessation
- 2009-03-18 CL CL2009000651A patent/CL2009000651A1/es unknown
- 2009-03-18 NZ NZ587829A patent/NZ587829A/xx not_active IP Right Cessation
- 2009-03-18 US US12/922,213 patent/US8563556B2/en not_active Expired - Fee Related
-
2010
- 2010-09-01 ZA ZA2010/06263A patent/ZA201006263B/en unknown
- 2010-09-06 IL IL208020A patent/IL208020A0/en not_active IP Right Cessation
- 2010-10-15 MA MA33250A patent/MA32230B1/fr unknown
- 2010-10-19 CO CO10129430A patent/CO6321253A2/es not_active Application Discontinuation
- 2010-10-19 EC EC2010010555A patent/ECSP10010555A/es unknown
-
2012
- 2012-12-26 PH PH12012502569A patent/PH12012502569A1/en unknown
- 2012-12-26 PH PH12012502568A patent/PH12012502568A1/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| AR070924A1 (es) | 2010-05-12 |
| US8563556B2 (en) | 2013-10-22 |
| EP2257544A2 (en) | 2010-12-08 |
| ZA201006263B (en) | 2011-05-25 |
| RU2010142396A (ru) | 2012-04-27 |
| CN101970425B (zh) | 2014-04-16 |
| MA32230B1 (fr) | 2011-04-01 |
| WO2009115562A2 (en) | 2009-09-24 |
| CL2009000651A1 (es) | 2010-07-19 |
| IL208020A0 (en) | 2010-12-30 |
| PH12012502568A1 (en) | 2014-10-27 |
| AU2009227003B2 (en) | 2013-03-14 |
| AU2009227003A1 (en) | 2009-09-24 |
| HK1147492A1 (en) | 2011-08-12 |
| CN101970425A (zh) | 2011-02-09 |
| KR20100137517A (ko) | 2010-12-30 |
| TWI426072B (zh) | 2014-02-11 |
| CO6321253A2 (es) | 2011-09-20 |
| MX2010010152A (es) | 2010-10-25 |
| BRPI0909762A2 (pt) | 2018-04-03 |
| ECSP10010555A (es) | 2010-11-30 |
| PH12012502569A1 (en) | 2015-09-21 |
| WO2009115562A3 (en) | 2009-11-26 |
| EP2257544B1 (en) | 2014-12-03 |
| CA2718076A1 (en) | 2009-09-24 |
| JP2011515370A (ja) | 2011-05-19 |
| US20110021536A1 (en) | 2011-01-27 |
| TW201000463A (en) | 2010-01-01 |
| SG188919A1 (en) | 2013-04-30 |
| NZ587829A (en) | 2012-08-31 |
| NZ600887A (en) | 2013-10-25 |
| MY150554A (en) | 2014-01-30 |
| PE20091628A1 (es) | 2009-11-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2497820C2 (ru) | Кристаллические формы и две сольватные формы солей молочной кислоты 4-амино-5-фтор-3-[5-(4-метилпиперазин-1-ил)-1 - н-бензимидазол-2-ил]хинолин-2(1н)она | |
| JP5714024B2 (ja) | 3−(2,6−ジクロロ−3,5−ジメトキシ−フェニル)−1−{6−[4−(4−エチル−ピペラジン−1−イル)−フェニルアミノ]−ピリミジン−4−イル}−1−メチル−尿素の結晶形態およびその塩 | |
| JP6317320B2 (ja) | 上皮成長因子受容体キナーゼ阻害剤の塩 | |
| JP2015017116A (ja) | 癌および他の疾患または障害の処置のための、4−[6−メトキシ−7−(3−ピペリジン−1−イル−プロポキシ)キナゾリン−4−イル]ピペラジン−1−カルボン酸(4−イソプロポキシフェニル)−アミドの乳酸塩およびその薬学的組成物 | |
| TW202327593A (zh) | Kras抑制劑的多晶型物及其製備方法和用途 | |
| JP6655608B2 (ja) | 6−((6,7−ジメトキシキナゾリン−4−イル)オキシ)−n,2−ジメチルベンゾフラン−3−カルボキサミドの結晶形 | |
| JP2020521003A (ja) | 重水素化azd9291の結晶形、製造方法および使用 | |
| KR20170032330A (ko) | C-Met 억제제의 결정질 유리 염기 또는 이의 결정질 산 염, 및 이들의 제조방법 및 용도 | |
| WO2011023146A1 (en) | Imatinib mesylate polymorphs generated by crystallization in aqueous inorganic salt solutions | |
| WO2016090257A1 (en) | Salts and crystalline forms of 6-acetyl-8-cyclopentyl-5-methyl-2((5-(piperazin-1-yl)pyridin-2-yl)amino)pyrido[2,3-d] pyrimidin-7(8h)-one (palbociclib) | |
| JP2025143319A (ja) | 化合物の塩及びその結晶形態 | |
| KR20230145030A (ko) | 테가비빈트의 결정형, 제조 방법, 및 이의 용도 | |
| EP4438600A1 (en) | Compound used as kinase inhibitor and use thereof | |
| JP6961348B2 (ja) | 置換されたイミダゾピリジニル−アミノピリジン化合物の塩および多型 | |
| JP7699054B2 (ja) | フロピリミジン化合物の酸付加塩の結晶形 | |
| JPWO2011152411A1 (ja) | チエノピリミジン誘導体の結晶 | |
| WO2022194265A1 (zh) | 一种喹唑啉类化合物、组合物及其应用 | |
| EP3941472A1 (en) | <smallcaps/>? ? ?n? ? ? ? ?crystalline and amorphous forms of-(5-((4-ethylpiperazin-1-yl)methyl)pyridine-2-yl)-5-fluoro-4-(3-isopropyl-2-methyl-2 <ns1:i>h</ns1:i>?-indazol-5-yl)pyrimidin-2-amine and its salts, and preparation methods and therapeutic uses thereof | |
| EA050369B1 (ru) | Кристаллическая форма трициклического производного соединения, способ его получения и содержащая его фармацевтическая композиция | |
| WO2022033471A1 (zh) | 含邻氨基吡啶炔基的化合物的盐及其制备方法和应用 | |
| HK40024929B (en) | Crystalline forms of a compound | |
| HK40019691A (en) | The salts of a compound and the crystalline forms thereof | |
| JP2020520384A (ja) | 化合物の塩及びその結晶形態 | |
| HK1147492B (en) | Crystalline forms and two solvated forms of 4-amino-5-fluoro-3-[5-(4-methylpiperazin-1-yl)-1h-benzimidazol-2-yl]quinolin-2(1h)-one lactic acid salts | |
| WO2016101912A1 (zh) | 一种表皮生长因子受体激酶抑制剂的盐的晶型及其制备方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20170319 |