RU2451689C2 - Новые антипролиферативные антитела - Google Patents
Новые антипролиферативные антитела Download PDFInfo
- Publication number
- RU2451689C2 RU2451689C2 RU2009123409/10A RU2009123409A RU2451689C2 RU 2451689 C2 RU2451689 C2 RU 2451689C2 RU 2009123409/10 A RU2009123409/10 A RU 2009123409/10A RU 2009123409 A RU2009123409 A RU 2009123409A RU 2451689 C2 RU2451689 C2 RU 2451689C2
- Authority
- RU
- Russia
- Prior art keywords
- antibody
- cdr
- seq
- functional fragment
- sequence
- Prior art date
Links
- 230000001028 anti-proliverative effect Effects 0.000 title 1
- 239000012634 fragment Substances 0.000 claims abstract 31
- 108010040082 Junctional Adhesion Molecule A Proteins 0.000 claims abstract 11
- 102100022304 Junctional adhesion molecule A Human genes 0.000 claims abstract 11
- 102000039446 nucleic acids Human genes 0.000 claims abstract 7
- 108020004707 nucleic acids Proteins 0.000 claims abstract 7
- 150000007523 nucleic acids Chemical class 0.000 claims abstract 7
- 230000035755 proliferation Effects 0.000 claims abstract 5
- 210000004881 tumor cell Anatomy 0.000 claims abstract 5
- 239000003814 drug Substances 0.000 claims abstract 4
- 210000004027 cell Anatomy 0.000 claims abstract 3
- 210000004408 hybridoma Anatomy 0.000 claims abstract 3
- 238000004519 manufacturing process Methods 0.000 claims abstract 3
- 238000011282 treatment Methods 0.000 claims abstract 3
- 239000013604 expression vector Substances 0.000 claims abstract 2
- 238000000034 method Methods 0.000 claims abstract 2
- 230000003248 secreting effect Effects 0.000 claims abstract 2
- 125000003275 alpha amino acid group Chemical group 0.000 claims 6
- 206010028980 Neoplasm Diseases 0.000 claims 5
- 201000011510 cancer Diseases 0.000 claims 5
- 230000002401 inhibitory effect Effects 0.000 claims 3
- 206010006187 Breast cancer Diseases 0.000 claims 2
- 208000026310 Breast neoplasm Diseases 0.000 claims 2
- 206010009944 Colon cancer Diseases 0.000 claims 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims 2
- 208000029742 colonic neoplasm Diseases 0.000 claims 2
- 230000003111 delayed effect Effects 0.000 claims 2
- 238000000338 in vitro Methods 0.000 claims 2
- 238000001727 in vivo Methods 0.000 claims 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims 2
- 201000002528 pancreatic cancer Diseases 0.000 claims 2
- 208000008443 pancreatic carcinoma Diseases 0.000 claims 2
- 241000894007 species Species 0.000 claims 2
- 206010014733 Endometrial cancer Diseases 0.000 claims 1
- 206010014759 Endometrial neoplasm Diseases 0.000 claims 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims 1
- 208000034578 Multiple myelomas Diseases 0.000 claims 1
- 241001529936 Murinae Species 0.000 claims 1
- 206010033128 Ovarian cancer Diseases 0.000 claims 1
- 206010061535 Ovarian neoplasm Diseases 0.000 claims 1
- 206010035226 Plasma cell myeloma Diseases 0.000 claims 1
- 206010060862 Prostate cancer Diseases 0.000 claims 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims 1
- 239000004480 active ingredient Substances 0.000 claims 1
- 239000000853 adhesive Substances 0.000 claims 1
- 230000001070 adhesive effect Effects 0.000 claims 1
- 230000000259 anti-tumor effect Effects 0.000 claims 1
- 239000002246 antineoplastic agent Substances 0.000 claims 1
- 229940041181 antineoplastic drug Drugs 0.000 claims 1
- 230000000295 complement effect Effects 0.000 claims 1
- 150000001875 compounds Chemical class 0.000 claims 1
- 210000004748 cultured cell Anatomy 0.000 claims 1
- 238000012258 culturing Methods 0.000 claims 1
- 239000000824 cytostatic agent Substances 0.000 claims 1
- 231100000433 cytotoxic Toxicity 0.000 claims 1
- 239000002254 cytotoxic agent Substances 0.000 claims 1
- 230000001472 cytotoxic effect Effects 0.000 claims 1
- 230000001419 dependent effect Effects 0.000 claims 1
- 201000010099 disease Diseases 0.000 claims 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims 1
- 229940011871 estrogen Drugs 0.000 claims 1
- 239000000262 estrogen Substances 0.000 claims 1
- 239000001963 growth medium Substances 0.000 claims 1
- 238000002955 isolation Methods 0.000 claims 1
- 201000005202 lung cancer Diseases 0.000 claims 1
- 208000020816 lung neoplasm Diseases 0.000 claims 1
- 239000002609 medium Substances 0.000 claims 1
- 244000005700 microbiome Species 0.000 claims 1
- LWGJTAZLEJHCPA-UHFFFAOYSA-N n-(2-chloroethyl)-n-nitrosomorpholine-4-carboxamide Chemical compound ClCCN(N=O)C(=O)N1CCOCC1 LWGJTAZLEJHCPA-UHFFFAOYSA-N 0.000 claims 1
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims 1
- 201000008968 osteosarcoma Diseases 0.000 claims 1
- 230000002265 prevention Effects 0.000 claims 1
- 238000011321 prophylaxis Methods 0.000 claims 1
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims 1
- 239000013598 vector Substances 0.000 claims 1
- 230000006806 disease prevention Effects 0.000 abstract 1
- 230000000694 effects Effects 0.000 abstract 1
- 239000000126 substance Substances 0.000 abstract 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/10—Cells modified by introduction of foreign genetic material
- C12N5/12—Fused cells, e.g. hybridomas
- C12N5/16—Animal cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/54—F(ab')2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Engineering & Computer Science (AREA)
- Public Health (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biophysics (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Microbiology (AREA)
- Wood Science & Technology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biomedical Technology (AREA)
- Cell Biology (AREA)
- Epidemiology (AREA)
- Mycology (AREA)
- Oncology (AREA)
- General Engineering & Computer Science (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0610329A FR2909092B1 (fr) | 2006-11-24 | 2006-11-24 | Nouveaux anticorps anti-proliferation |
| FR0610329 | 2006-11-24 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2009123409A RU2009123409A (ru) | 2010-12-27 |
| RU2451689C2 true RU2451689C2 (ru) | 2012-05-27 |
Family
ID=37903981
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2009123409/10A RU2451689C2 (ru) | 2006-11-24 | 2007-11-23 | Новые антипролиферативные антитела |
Country Status (27)
| Country | Link |
|---|---|
| US (2) | US8071730B2 (enExample) |
| EP (1) | EP2074148A1 (enExample) |
| JP (1) | JP2010509931A (enExample) |
| KR (1) | KR20090088878A (enExample) |
| CN (1) | CN101535344B (enExample) |
| AU (1) | AU2007324509B2 (enExample) |
| BR (1) | BRPI0719323A2 (enExample) |
| CA (1) | CA2670039A1 (enExample) |
| CL (1) | CL2007003357A1 (enExample) |
| CR (1) | CR10788A (enExample) |
| CU (1) | CU23792A3 (enExample) |
| EC (1) | ECSP099341A (enExample) |
| FR (1) | FR2909092B1 (enExample) |
| GE (1) | GEP20125629B (enExample) |
| GT (1) | GT200900126A (enExample) |
| IL (1) | IL198744A0 (enExample) |
| MA (1) | MA30891B1 (enExample) |
| MX (1) | MX2009005293A (enExample) |
| NI (1) | NI200900079A (enExample) |
| NO (1) | NO20092360L (enExample) |
| NZ (1) | NZ576174A (enExample) |
| RU (1) | RU2451689C2 (enExample) |
| SA (1) | SA07280637B1 (enExample) |
| TN (1) | TN2009000194A1 (enExample) |
| TW (1) | TW200829602A (enExample) |
| UA (1) | UA99602C2 (enExample) |
| WO (1) | WO2008062063A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10906987B2 (en) | 2016-03-01 | 2021-02-02 | Yissum Research Development Company Of The Hebrew University Of Jerusalem Ltd. | Antibodies specific to human poliovirus receptor (PVR) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JOP20080381B1 (ar) | 2007-08-23 | 2023-03-28 | Amgen Inc | بروتينات مرتبطة بمولدات مضادات تتفاعل مع بروبروتين كونفيرتاز سيتيليزين ككسين من النوع 9 (pcsk9) |
| EP2313435A4 (en) | 2008-07-01 | 2012-08-08 | Aveo Pharmaceuticals Inc | FIBROBLAST GROWTH FACTOR RECEPTOR 3 (FGFR3) BINDING PROTEINS |
| AU2010313381A1 (en) * | 2009-10-30 | 2012-04-12 | Merck Sharp & Dohme Corp. | AX1 and AX189 PCSK9 antagonists and variants |
| EP2632490A4 (en) * | 2010-10-29 | 2014-10-22 | Immunogen Inc | NOVEL MOLECULES BINDING TO THE EGF RECEPTOR AND IMMUNOCONJUGATES THEREOF |
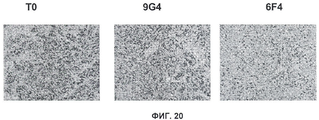
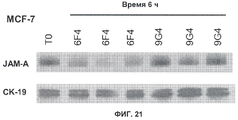
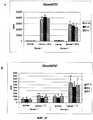
| EP2455403A1 (en) | 2010-11-23 | 2012-05-23 | Pierre Fabre Medicament | Homogeneous humanized antibodies against JAM-A that inhibit proliferation |
| JOP20200043A1 (ar) | 2011-05-10 | 2017-06-16 | Amgen Inc | طرق معالجة أو منع الاضطرابات المختصة بالكوليسترول |
| US20130287790A1 (en) * | 2012-03-30 | 2013-10-31 | The Board Of Trustees Of The Leland Standford Junior University | Use of jam-a in diagnosing and treating leukemia |
| MY181648A (en) | 2012-08-24 | 2020-12-30 | Univ California | Antibodies and vaccines for use in treating ror1 cancers and inhibiting metastasis |
| KR20150097304A (ko) | 2014-02-18 | 2015-08-26 | 삼성전자주식회사 | 항 EGFR DARPin을 포함하는 EGFR/HER2 이중 특이 항체 |
| KR20230047507A (ko) | 2016-06-27 | 2023-04-07 | 더 리젠츠 오브 더 유니버시티 오브 캘리포니아 | 암 치료 조합 |
| BR112019002127A2 (pt) * | 2016-08-03 | 2019-09-17 | Nextcure Inc | proteína de fusão, vetor, célula, composição farmacêutica, e, uso da proteína de fusão |
| CN109022366A (zh) * | 2018-08-16 | 2018-12-18 | 江南大学 | 一株分泌抗左旋咪唑单克隆抗体的杂交瘤细胞株及其应用 |
| CN114740108B (zh) * | 2022-03-28 | 2023-07-14 | 天津键凯科技有限公司 | 一种聚合物修饰抗体类药物的修饰度的测定方法 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2244748C2 (ru) * | 1999-03-11 | 2005-01-20 | РМФ Диктажен С.А. | Адгезивные молекулы сосудов и модуляция их функций |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SE9604470D0 (sv) * | 1996-12-04 | 1996-12-04 | Tamas Bartfai Konsulting Ab | Transmembrane component of tight junction |
| US6235883B1 (en) * | 1997-05-05 | 2001-05-22 | Abgenix, Inc. | Human monoclonal antibodies to epidermal growth factor receptor |
| US20030171568A1 (en) * | 1998-09-16 | 2003-09-11 | Avi Ashkenazi | Use of A33 antigens and JAM-IT |
| DK1069185T3 (da) * | 1998-04-03 | 2011-06-27 | Chugai Pharmaceutical Co Ltd | Humaniseret antistof mod human vævsfaktor (TF) og fremgangsmåde til at konstruere humaniseret antistof |
| EP1708961B1 (en) * | 2003-12-04 | 2011-03-09 | Abbott Biotherapeutics Corp. | Anti-ip-10 antibodies |
| WO2006008076A2 (en) * | 2004-07-16 | 2006-01-26 | Universita Degli Studi Di Milano | Methods and agents stimulating the immune response |
| WO2006121207A1 (en) * | 2005-05-12 | 2006-11-16 | Oncotherapy Science, Inc. | Methods for damaging cells using effector function of anti-dsc2 antibody |
| EP2021371A2 (en) * | 2006-04-28 | 2009-02-11 | Oregon Health and Science University | Monoclonal antibodies specific for pancreatic endocrine, exocrine or ductal cells |
-
2006
- 2006-11-24 FR FR0610329A patent/FR2909092B1/fr not_active Expired - Fee Related
-
2007
- 2007-11-23 UA UAA200906473A patent/UA99602C2/ru unknown
- 2007-11-23 CA CA002670039A patent/CA2670039A1/en not_active Abandoned
- 2007-11-23 BR BRPI0719323-8A patent/BRPI0719323A2/pt not_active IP Right Cessation
- 2007-11-23 KR KR1020097009675A patent/KR20090088878A/ko not_active Ceased
- 2007-11-23 AU AU2007324509A patent/AU2007324509B2/en not_active Ceased
- 2007-11-23 JP JP2009537651A patent/JP2010509931A/ja not_active Ceased
- 2007-11-23 NZ NZ576174A patent/NZ576174A/en not_active IP Right Cessation
- 2007-11-23 CN CN2007800405398A patent/CN101535344B/zh not_active Expired - Fee Related
- 2007-11-23 WO PCT/EP2007/062760 patent/WO2008062063A1/en not_active Ceased
- 2007-11-23 CL CL200703357A patent/CL2007003357A1/es unknown
- 2007-11-23 MX MX2009005293A patent/MX2009005293A/es active IP Right Grant
- 2007-11-23 RU RU2009123409/10A patent/RU2451689C2/ru not_active IP Right Cessation
- 2007-11-23 EP EP07847306A patent/EP2074148A1/en not_active Withdrawn
- 2007-11-23 GE GEAP200711322A patent/GEP20125629B/en unknown
- 2007-11-24 SA SA07280637A patent/SA07280637B1/ar unknown
- 2007-11-26 TW TW096144763A patent/TW200829602A/zh unknown
-
2008
- 2008-05-22 US US12/125,726 patent/US8071730B2/en not_active Expired - Fee Related
-
2009
- 2009-05-06 NI NI200900079A patent/NI200900079A/es unknown
- 2009-05-08 CR CR10788A patent/CR10788A/es unknown
- 2009-05-14 CU CU20090082A patent/CU23792A3/es not_active IP Right Cessation
- 2009-05-14 MA MA31875A patent/MA30891B1/fr unknown
- 2009-05-14 GT GT200900126A patent/GT200900126A/es unknown
- 2009-05-14 IL IL198744A patent/IL198744A0/en unknown
- 2009-05-18 EC EC2009009341A patent/ECSP099341A/es unknown
- 2009-05-18 TN TNP2009000194A patent/TN2009000194A1/fr unknown
- 2009-06-19 NO NO20092360A patent/NO20092360L/no not_active Application Discontinuation
-
2011
- 2011-12-05 US US13/310,840 patent/US20120156191A1/en not_active Abandoned
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2244748C2 (ru) * | 1999-03-11 | 2005-01-20 | РМФ Диктажен С.А. | Адгезивные молекулы сосудов и модуляция их функций |
Non-Patent Citations (1)
| Title |
|---|
| BAZZONI G. et al. Homophilic Interaction of Junctional Adhesion Molecule, J. Biological Chemistry (2000), 275(40): 30970-30976. NAIK M.U. and NAIK U.P. Junctional adhesion molecule-A-induced endothelial cell migration on vitronectin is integrin α v β 3 specific, Journal of Cell Science (2005), 119(3): 490-499. * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10906987B2 (en) | 2016-03-01 | 2021-02-02 | Yissum Research Development Company Of The Hebrew University Of Jerusalem Ltd. | Antibodies specific to human poliovirus receptor (PVR) |
| RU2756275C2 (ru) * | 2016-03-01 | 2021-09-29 | Юссум Рисёрч Девелопмент Компани Оф Зэ Хибру Юниверсити Оф Иерусалим Лтд. | Антитела, специфические к рецептору полиовируса (pvr) человека |
Also Published As
| Publication number | Publication date |
|---|---|
| MX2009005293A (es) | 2009-08-07 |
| ECSP099341A (es) | 2009-06-30 |
| RU2009123409A (ru) | 2010-12-27 |
| FR2909092A1 (fr) | 2008-05-30 |
| GT200900126A (es) | 2011-09-02 |
| US20120156191A1 (en) | 2012-06-21 |
| US8071730B2 (en) | 2011-12-06 |
| GEP20125629B (en) | 2012-09-10 |
| NZ576174A (en) | 2012-03-30 |
| NI200900079A (es) | 2010-11-10 |
| AU2007324509A1 (en) | 2008-05-29 |
| HK1132752A1 (en) | 2010-03-05 |
| NO20092360L (no) | 2009-08-11 |
| SA07280637B1 (ar) | 2012-04-11 |
| BRPI0719323A2 (pt) | 2014-02-04 |
| CN101535344A (zh) | 2009-09-16 |
| CR10788A (es) | 2009-06-23 |
| JP2010509931A (ja) | 2010-04-02 |
| WO2008062063A1 (en) | 2008-05-29 |
| US20100092455A1 (en) | 2010-04-15 |
| AU2007324509B2 (en) | 2013-01-17 |
| TW200829602A (en) | 2008-07-16 |
| CN101535344B (zh) | 2013-10-16 |
| UA99602C2 (ru) | 2012-09-10 |
| MA30891B1 (fr) | 2009-11-02 |
| FR2909092B1 (fr) | 2012-10-19 |
| KR20090088878A (ko) | 2009-08-20 |
| TN2009000194A1 (en) | 2010-10-18 |
| EP2074148A1 (en) | 2009-07-01 |
| CU23792A3 (es) | 2012-03-15 |
| CA2670039A1 (en) | 2008-05-29 |
| IL198744A0 (en) | 2010-02-17 |
| CL2007003357A1 (es) | 2008-04-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2451689C2 (ru) | Новые антипролиферативные антитела | |
| JP7425604B2 (ja) | 抗ctla4-抗pd-1二機能性抗体、その医薬組成物および使用 | |
| JP7264827B2 (ja) | TGF-β受容体含有融合タンパク質およびそれらの医薬的用途 | |
| CN107151269B (zh) | 一种pdl-1抗体、其药物组合物及其用途 | |
| TWI708788B (zh) | 雙特異性抗體 | |
| TWI769537B (zh) | 標靶bcma的抗體、雙專一性抗體及其用途 | |
| TW202126692A (zh) | 抗人Claudin18.2抗體及其應用 | |
| RU2011124751A (ru) | Антитело против смет | |
| ME02341B (me) | Nova antitijela za inhibiciju c-met dimerizacije i njihova upotreba | |
| HRP20140502T1 (hr) | Humanizirana anti-cxcr4 antitijela za lijeäśenje karcinoma | |
| RU2011115559A (ru) | Антитела к cxcr4 и их применение для лечения рака | |
| JP2010509931A5 (enExample) | ||
| WO2017197667A1 (zh) | 抗人pd-l1人源化单克隆抗体及其应用 | |
| JP2010532982A5 (enExample) | ||
| CN105777906A (zh) | 抗pd-l1全人抗体及其应用 | |
| RU2014103784A (ru) | Антитело, блокирующее agr2, и его применение | |
| RU2010145063A (ru) | Новые антитела, используемые для лечения рака | |
| JP7646768B2 (ja) | 抗-ヒトプログラム細胞死リガンド-1(pd-l1)の抗体及びその用途 | |
| KR20230132544A (ko) | 신규한 항-그렘린1 항체 | |
| KR20240099351A (ko) | Gprc5d에 결합하는 항체 및 그 용도 | |
| WO2021209495A1 (en) | Anti-flt3 antibodies and compositions | |
| Akbari et al. | Design, expression and evaluation of a novel humanized single chain antibody against epidermal growth factor receptor (EGFR) | |
| AU2021329456A1 (en) | Antibody specifically bound to glycosylated CEACAM5 | |
| CN114340737A (zh) | 激活性抗gal9结合分子 | |
| RU2016129198A (ru) | Новое антитело против adam 17 и его применения для лечения рака |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20151124 |