RU2423146C2 - Лекарственное средство для лечения эндометриоза - Google Patents
Лекарственное средство для лечения эндометриоза Download PDFInfo
- Publication number
- RU2423146C2 RU2423146C2 RU2009129252/15A RU2009129252A RU2423146C2 RU 2423146 C2 RU2423146 C2 RU 2423146C2 RU 2009129252/15 A RU2009129252/15 A RU 2009129252/15A RU 2009129252 A RU2009129252 A RU 2009129252A RU 2423146 C2 RU2423146 C2 RU 2423146C2
- Authority
- RU
- Russia
- Prior art keywords
- dopamine agonist
- endometriosis
- use according
- dose
- day
- Prior art date
Links
- 201000009273 Endometriosis Diseases 0.000 title claims abstract description 50
- 239000003814 drug Substances 0.000 title claims abstract description 13
- 229940079593 drug Drugs 0.000 title claims abstract description 10
- 239000003136 dopamine receptor stimulating agent Substances 0.000 claims abstract description 70
- KORNTPPJEAJQIU-KJXAQDMKSA-N Cabaser Chemical compound C1=CC([C@H]2C[C@H](CN(CC=C)[C@@H]2C2)C(=O)N(CCCN(C)C)C(=O)NCC)=C3C2=CNC3=C1 KORNTPPJEAJQIU-KJXAQDMKSA-N 0.000 claims abstract description 47
- 229960004596 cabergoline Drugs 0.000 claims abstract description 46
- 230000002357 endometrial effect Effects 0.000 claims abstract description 19
- 210000004907 gland Anatomy 0.000 claims abstract description 13
- GDFGTRDCCWFXTG-SCTDSRPQSA-N (3r,4ar,10as)-3-(diethylsulfamoylamino)-6-hydroxy-1-propyl-3,4,4a,5,10,10a-hexahydro-2h-benzo[g]quinoline Chemical compound C1=CC=C2C[C@@H]3N(CCC)C[C@H](NS(=O)(=O)N(CC)CC)C[C@H]3CC2=C1O GDFGTRDCCWFXTG-SCTDSRPQSA-N 0.000 claims abstract description 12
- 230000000694 effects Effects 0.000 claims abstract description 12
- 229960000924 quinagolide Drugs 0.000 claims abstract description 12
- 229960002802 bromocriptine Drugs 0.000 claims abstract description 8
- OZVBMTJYIDMWIL-AYFBDAFISA-N bromocriptine Chemical compound C1=CC(C=2[C@H](N(C)C[C@@H](C=2)C(=O)N[C@]2(C(=O)N3[C@H](C(N4CCC[C@H]4[C@]3(O)O2)=O)CC(C)C)C(C)C)C2)=C3C2=C(Br)NC3=C1 OZVBMTJYIDMWIL-AYFBDAFISA-N 0.000 claims abstract description 8
- 230000002265 prevention Effects 0.000 claims abstract description 8
- 230000009467 reduction Effects 0.000 claims abstract description 6
- BKRGVLQUQGGVSM-KBXCAEBGSA-N Revanil Chemical compound C1=CC(C=2[C@H](N(C)C[C@H](C=2)NC(=O)N(CC)CC)C2)=C3C2=CNC3=C1 BKRGVLQUQGGVSM-KBXCAEBGSA-N 0.000 claims abstract description 3
- DKNWSYNQZKUICI-UHFFFAOYSA-N amantadine Chemical compound C1C(C2)CC3CC2CC1(N)C3 DKNWSYNQZKUICI-UHFFFAOYSA-N 0.000 claims abstract description 3
- 229960003805 amantadine Drugs 0.000 claims abstract description 3
- 229960003587 lisuride Drugs 0.000 claims abstract description 3
- 238000004519 manufacturing process Methods 0.000 claims abstract description 3
- 229960004851 pergolide Drugs 0.000 claims abstract description 3
- YEHCICAEULNIGD-MZMPZRCHSA-N pergolide Chemical compound C1=CC([C@H]2C[C@@H](CSC)CN([C@@H]2C2)CCC)=C3C2=CNC3=C1 YEHCICAEULNIGD-MZMPZRCHSA-N 0.000 claims abstract description 3
- 229960003089 pramipexole Drugs 0.000 claims abstract description 3
- FASDKYOPVNHBLU-ZETCQYMHSA-N pramipexole Chemical compound C1[C@@H](NCCC)CCC2=C1SC(N)=N2 FASDKYOPVNHBLU-ZETCQYMHSA-N 0.000 claims abstract description 3
- 229960001879 ropinirole Drugs 0.000 claims abstract description 3
- UHSKFQJFRQCDBE-UHFFFAOYSA-N ropinirole Chemical compound CCCN(CCC)CCC1=CC=CC2=C1CC(=O)N2 UHSKFQJFRQCDBE-UHFFFAOYSA-N 0.000 claims abstract description 3
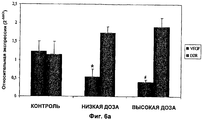
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 claims description 27
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 claims description 26
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 claims description 26
- 108010029741 Notch4 Receptor Proteins 0.000 claims description 9
- 102000001753 Notch4 Receptor Human genes 0.000 claims description 9
- 208000024891 symptom Diseases 0.000 claims description 8
- 239000000203 mixture Substances 0.000 claims description 7
- 239000002775 capsule Substances 0.000 claims description 4
- 229920002472 Starch Polymers 0.000 claims description 2
- 108091008605 VEGF receptors Proteins 0.000 claims description 2
- 102000009484 Vascular Endothelial Growth Factor Receptors Human genes 0.000 claims description 2
- 239000007937 lozenge Substances 0.000 claims description 2
- 239000008107 starch Substances 0.000 claims description 2
- 235000019698 starch Nutrition 0.000 claims description 2
- 239000006188 syrup Substances 0.000 claims description 2
- 235000020357 syrup Nutrition 0.000 claims description 2
- 239000003826 tablet Substances 0.000 claims description 2
- 235000012431 wafers Nutrition 0.000 claims 1
- 230000003412 degenerative effect Effects 0.000 abstract description 6
- 230000009471 action Effects 0.000 abstract description 3
- 239000000126 substance Substances 0.000 abstract description 2
- 108010053099 Vascular Endothelial Growth Factor Receptor-2 Proteins 0.000 description 25
- 210000004204 blood vessel Anatomy 0.000 description 25
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 description 23
- 230000014509 gene expression Effects 0.000 description 22
- 238000000034 method Methods 0.000 description 18
- 239000007943 implant Substances 0.000 description 17
- 241001465754 Metazoa Species 0.000 description 16
- BJHCYTJNPVGSBZ-YXSASFKJSA-N 1-[4-[6-amino-5-[(Z)-methoxyiminomethyl]pyrimidin-4-yl]oxy-2-chlorophenyl]-3-ethylurea Chemical compound CCNC(=O)Nc1ccc(Oc2ncnc(N)c2\C=N/OC)cc1Cl BJHCYTJNPVGSBZ-YXSASFKJSA-N 0.000 description 15
- 210000001519 tissue Anatomy 0.000 description 14
- 241000699670 Mus sp. Species 0.000 description 13
- 230000033115 angiogenesis Effects 0.000 description 12
- 108010047303 von Willebrand Factor Proteins 0.000 description 12
- 102100036537 von Willebrand factor Human genes 0.000 description 12
- 229960001134 von willebrand factor Drugs 0.000 description 12
- 210000004696 endometrium Anatomy 0.000 description 10
- 229940052760 dopamine agonists Drugs 0.000 description 9
- 210000004027 cell Anatomy 0.000 description 8
- 230000003902 lesion Effects 0.000 description 7
- 101100156752 Caenorhabditis elegans cwn-1 gene Proteins 0.000 description 6
- 102000015554 Dopamine receptor Human genes 0.000 description 6
- 108050004812 Dopamine receptor Proteins 0.000 description 6
- 102000052547 Wnt-1 Human genes 0.000 description 6
- 108700020987 Wnt-1 Proteins 0.000 description 6
- 238000011161 development Methods 0.000 description 6
- 230000018109 developmental process Effects 0.000 description 6
- VYFYYTLLBUKUHU-UHFFFAOYSA-N dopamine Chemical compound NCCC1=CC=C(O)C(O)=C1 VYFYYTLLBUKUHU-UHFFFAOYSA-N 0.000 description 6
- 238000001356 surgical procedure Methods 0.000 description 6
- 239000000579 Gonadotropin-Releasing Hormone Substances 0.000 description 5
- 101000857870 Squalus acanthias Gonadoliberin Proteins 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 210000002889 endothelial cell Anatomy 0.000 description 5
- XLXSAKCOAKORKW-AQJXLSMYSA-N gonadorelin Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 XLXSAKCOAKORKW-AQJXLSMYSA-N 0.000 description 5
- 229940035638 gonadotropin-releasing hormone Drugs 0.000 description 5
- 230000008520 organization Effects 0.000 description 5
- 238000003753 real-time PCR Methods 0.000 description 5
- 208000000450 Pelvic Pain Diseases 0.000 description 4
- 239000000556 agonist Substances 0.000 description 4
- POZRVZJJTULAOH-LHZXLZLDSA-N danazol Chemical compound C1[C@]2(C)[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=CC2=C1C=NO2 POZRVZJJTULAOH-LHZXLZLDSA-N 0.000 description 4
- 229960000766 danazol Drugs 0.000 description 4
- 238000002357 laparoscopic surgery Methods 0.000 description 4
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 4
- 239000013641 positive control Substances 0.000 description 4
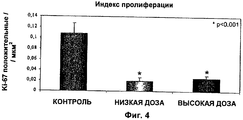
- 230000035755 proliferation Effects 0.000 description 4
- 108020004635 Complementary DNA Proteins 0.000 description 3
- 208000005171 Dysmenorrhea Diseases 0.000 description 3
- 206010013935 Dysmenorrhoea Diseases 0.000 description 3
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 3
- 230000001772 anti-angiogenic effect Effects 0.000 description 3
- 239000003886 aromatase inhibitor Substances 0.000 description 3
- 229940046844 aromatase inhibitors Drugs 0.000 description 3
- 238000010804 cDNA synthesis Methods 0.000 description 3
- 239000002299 complementary DNA Substances 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 229960003638 dopamine Drugs 0.000 description 3
- 229940088597 hormone Drugs 0.000 description 3
- 238000010166 immunofluorescence Methods 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 238000013425 morphometry Methods 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 210000000952 spleen Anatomy 0.000 description 3
- 238000010186 staining Methods 0.000 description 3
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 2
- 102000009088 Angiopoietin-1 Human genes 0.000 description 2
- 108010048154 Angiopoietin-1 Proteins 0.000 description 2
- 208000004483 Dyspareunia Diseases 0.000 description 2
- 206010033266 Ovarian Hyperstimulation Syndrome Diseases 0.000 description 2
- 208000002193 Pain Diseases 0.000 description 2
- 241000283984 Rodentia Species 0.000 description 2
- 239000004037 angiogenesis inhibitor Substances 0.000 description 2
- 230000002491 angiogenic effect Effects 0.000 description 2
- 230000003527 anti-angiogenesis Effects 0.000 description 2
- 238000011122 anti-angiogenic therapy Methods 0.000 description 2
- HSWPZIDYAHLZDD-UHFFFAOYSA-N atipamezole Chemical compound C1C2=CC=CC=C2CC1(CC)C1=CN=CN1 HSWPZIDYAHLZDD-UHFFFAOYSA-N 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 238000004624 confocal microscopy Methods 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 229940011871 estrogen Drugs 0.000 description 2
- 239000000262 estrogen Substances 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 230000000762 glandular Effects 0.000 description 2
- 229960003299 ketamine Drugs 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- HRLIOXLXPOHXTA-UHFFFAOYSA-N medetomidine Chemical compound C=1C=CC(C)=C(C)C=1C(C)C1=CN=C[N]1 HRLIOXLXPOHXTA-UHFFFAOYSA-N 0.000 description 2
- 229960002140 medetomidine Drugs 0.000 description 2
- 229960002985 medroxyprogesterone acetate Drugs 0.000 description 2
- PSGAAPLEWMOORI-PEINSRQWSA-N medroxyprogesterone acetate Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](OC(C)=O)(C(C)=O)CC[C@H]21 PSGAAPLEWMOORI-PEINSRQWSA-N 0.000 description 2
- 239000013642 negative control Substances 0.000 description 2
- 229940127234 oral contraceptive Drugs 0.000 description 2
- 239000003539 oral contraceptive agent Substances 0.000 description 2
- 230000027758 ovulation cycle Effects 0.000 description 2
- 230000036407 pain Effects 0.000 description 2
- 210000004303 peritoneum Anatomy 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 239000000825 pharmaceutical preparation Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 239000000583 progesterone congener Substances 0.000 description 2
- 230000002062 proliferating effect Effects 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 230000008521 reorganization Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 210000002460 smooth muscle Anatomy 0.000 description 2
- 238000007619 statistical method Methods 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- 210000004509 vascular smooth muscle cell Anatomy 0.000 description 2
- 230000003442 weekly effect Effects 0.000 description 2
- 108020004463 18S ribosomal RNA Proteins 0.000 description 1
- 101150028074 2 gene Proteins 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 101100381481 Caenorhabditis elegans baz-2 gene Proteins 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000808011 Homo sapiens Vascular endothelial growth factor A Proteins 0.000 description 1
- 208000037093 Menstruation Disturbances Diseases 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 208000034038 Pathologic Neovascularization Diseases 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 101100372762 Rattus norvegicus Flt1 gene Proteins 0.000 description 1
- 206010065951 Retrograde menstruation Diseases 0.000 description 1
- 208000006268 Sarcoma 180 Diseases 0.000 description 1
- JOAHPSVPXZTVEP-YXJHDRRASA-N Terguride Chemical compound C1=CC([C@H]2C[C@@H](CN(C)[C@@H]2C2)NC(=O)N(CC)CC)=C3C2=CNC3=C1 JOAHPSVPXZTVEP-YXJHDRRASA-N 0.000 description 1
- 102000009524 Vascular Endothelial Growth Factor A Human genes 0.000 description 1
- 108010053096 Vascular Endothelial Growth Factor Receptor-1 Proteins 0.000 description 1
- 102000016549 Vascular Endothelial Growth Factor Receptor-2 Human genes 0.000 description 1
- 102100033178 Vascular endothelial growth factor receptor 1 Human genes 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 229940109449 antisedan Drugs 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 229960003002 atipamezole Drugs 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 210000004190 broad ligament Anatomy 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007850 degeneration Effects 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 230000003291 dopaminomimetic effect Effects 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 230000000437 effect on angiogenesis Effects 0.000 description 1
- 201000002595 endometriosis of ovary Diseases 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 229960005309 estradiol Drugs 0.000 description 1
- RZTAMFZIAATZDJ-UHFFFAOYSA-N felodipine Chemical compound CCOC(=O)C1=C(C)NC(C)=C(C(=O)OC)C1C1=CC=CC(Cl)=C1Cl RZTAMFZIAATZDJ-UHFFFAOYSA-N 0.000 description 1
- 230000035558 fertility Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000003292 glue Substances 0.000 description 1
- 230000009931 harmful effect Effects 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000001744 histochemical effect Effects 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 102000058223 human VEGFA Human genes 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 238000003365 immunocytochemistry Methods 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 210000003041 ligament Anatomy 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 238000007491 morphometric analysis Methods 0.000 description 1
- 230000003562 morphometric effect Effects 0.000 description 1
- 230000014399 negative regulation of angiogenesis Effects 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000000399 optical microscopy Methods 0.000 description 1
- 239000007935 oral tablet Substances 0.000 description 1
- 229940096978 oral tablet Drugs 0.000 description 1
- 208000030747 ovarian endometriosis Diseases 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 229940023488 pill Drugs 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000001023 pro-angiogenic effect Effects 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000006884 regulation of angiogenesis Effects 0.000 description 1
- 239000003488 releasing hormone Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 108020004418 ribosomal RNA Proteins 0.000 description 1
- 238000011808 rodent model Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 229960004558 terguride Drugs 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 238000004627 transmission electron microscopy Methods 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 210000003606 umbilical vein Anatomy 0.000 description 1
- 210000000626 ureter Anatomy 0.000 description 1
- 210000001635 urinary tract Anatomy 0.000 description 1
- 210000004291 uterus Anatomy 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 210000003556 vascular endothelial cell Anatomy 0.000 description 1
- 230000008728 vascular permeability Effects 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/473—Quinolines; Isoquinolines ortho- or peri-condensed with carbocyclic ring systems, e.g. acridines, phenanthridines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/48—Ergoline derivatives, e.g. lysergic acid, ergotamine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0034—Urogenital system, e.g. vagina, uterus, cervix, penis, scrotum, urethra, bladder; Personal lubricants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/08—Drugs for genital or sexual disorders; Contraceptives for gonadal disorders or for enhancing fertility, e.g. inducers of ovulation or of spermatogenesis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Reproductive Health (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Gynecology & Obstetrics (AREA)
- Endocrinology (AREA)
- Urology & Nephrology (AREA)
- Pregnancy & Childbirth (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP07250429.3 | 2007-02-01 | ||
| EP07250429A EP1952813A1 (en) | 2007-02-01 | 2007-02-01 | Medicament for the treatment of endometriosis |
| US60/947,165 | 2007-06-29 | ||
| GB0712626.1 | 2007-06-29 | ||
| GB0712626A GB2450533A (en) | 2007-06-29 | 2007-06-29 | Treatment and prevention of endometriosis using dopamine agonists. |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2009129252A RU2009129252A (ru) | 2011-03-10 |
| RU2423146C2 true RU2423146C2 (ru) | 2011-07-10 |
Family
ID=39674574
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2009129252/15A RU2423146C2 (ru) | 2007-02-01 | 2008-02-01 | Лекарственное средство для лечения эндометриоза |
Country Status (20)
| Country | Link |
|---|---|
| US (2) | US8927568B2 (enExample) |
| EP (1) | EP2109449B1 (enExample) |
| JP (1) | JP5211073B2 (enExample) |
| AR (1) | AR065139A1 (enExample) |
| AT (1) | ATE516805T1 (enExample) |
| AU (1) | AU2008211633B2 (enExample) |
| BR (1) | BRPI0806944A2 (enExample) |
| CA (1) | CA2676910C (enExample) |
| DK (1) | DK2109449T3 (enExample) |
| IL (1) | IL200032A (enExample) |
| JO (1) | JO2730B1 (enExample) |
| MX (1) | MX2009008191A (enExample) |
| NZ (1) | NZ578569A (enExample) |
| PL (1) | PL2109449T3 (enExample) |
| PT (1) | PT2109449E (enExample) |
| RU (1) | RU2423146C2 (enExample) |
| SA (1) | SA08290041B1 (enExample) |
| SI (1) | SI2109449T1 (enExample) |
| TW (1) | TWI411436B (enExample) |
| WO (1) | WO2008093247A2 (enExample) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010150098A2 (en) * | 2009-06-26 | 2010-12-29 | Ferring International Center Sa | Treatment of endometriosis |
| EP3017809A1 (en) | 2014-11-07 | 2016-05-11 | Ferring B.V. | Drug-device unit containing quinagolide |
| WO2017210117A1 (en) * | 2016-06-02 | 2017-12-07 | Board Of Trustees, Southern Illinois University | Treatment of endometriosis and niclosamide derivatives |
| JP6562332B2 (ja) * | 2017-05-30 | 2019-08-21 | 有限会社イムノ | 活性化t細胞からのil−8産生を抑制するための組成物 |
| RU2732251C1 (ru) * | 2019-11-29 | 2020-09-14 | Федеральное государственное бюджетное научное учреждение "Научно-исследовательский институт акушерства, гинекологии и репродуктологии имени Д.О. Отта" | Способ лечения наружного генитального эндометриоза |
| US20240361338A1 (en) | 2021-08-31 | 2024-10-31 | Ferring B.V. | Diagnosis and treatment of ectopic endometriosis |
| WO2024153752A1 (en) | 2023-01-20 | 2024-07-25 | Ferring B.V. | Stereoselective synthesis of intermediates and synthesis of quinagolids |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1993003752A1 (en) * | 1991-08-19 | 1993-03-04 | The Arizona Board Of Regents On Behalf Of The University Of Arizona | Decapeptide having dopamine stimulating activity |
| US6359130B1 (en) * | 1998-06-05 | 2002-03-19 | Cephalon, Inc. | Bridged indenopyrrolocarbazoles |
| RU2273481C2 (ru) * | 2004-05-27 | 2006-04-10 | Варвара Анатольевна Волкова | Способ повышения эффективности лечения бесплодия, вызванного малыми формами внешнего генитального эндометриоза |
| WO2006117608A1 (en) * | 2005-04-29 | 2006-11-09 | Ferring International Center S.A. | Treatment or prevention of ovarian hyperstimulation syndrome (ohss) using a dopamine agonist |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0208417A3 (en) * | 1985-06-12 | 1989-09-06 | SPOFA Spojené Podniky Pro Zdravotnickou Vyrobu | Use of 1-(8-alpha-ergolinyl)-3,3-diethyl urea derivatives in the treatment of endometritis |
| US6572879B1 (en) | 1995-06-07 | 2003-06-03 | Alza Corporation | Formulations for transdermal delivery of pergolide |
| WO2010150098A2 (en) * | 2009-06-26 | 2010-12-29 | Ferring International Center Sa | Treatment of endometriosis |
-
2008
- 2008-01-30 SA SA08290041A patent/SA08290041B1/ar unknown
- 2008-02-01 DK DK08751001.2T patent/DK2109449T3/da active
- 2008-02-01 MX MX2009008191A patent/MX2009008191A/es active IP Right Grant
- 2008-02-01 PT PT08751001T patent/PT2109449E/pt unknown
- 2008-02-01 NZ NZ578569A patent/NZ578569A/en not_active IP Right Cessation
- 2008-02-01 PL PL08751001T patent/PL2109449T3/pl unknown
- 2008-02-01 RU RU2009129252/15A patent/RU2423146C2/ru active
- 2008-02-01 WO PCT/IB2008/001273 patent/WO2008093247A2/en not_active Ceased
- 2008-02-01 AU AU2008211633A patent/AU2008211633B2/en not_active Ceased
- 2008-02-01 CA CA2676910A patent/CA2676910C/en active Active
- 2008-02-01 AR ARP080100431A patent/AR065139A1/es not_active Application Discontinuation
- 2008-02-01 SI SI200830385T patent/SI2109449T1/sl unknown
- 2008-02-01 EP EP08751001A patent/EP2109449B1/en active Active
- 2008-02-01 TW TW097103971A patent/TWI411436B/zh not_active IP Right Cessation
- 2008-02-01 AT AT08751001T patent/ATE516805T1/de active
- 2008-02-01 BR BRPI0806944-1A2A patent/BRPI0806944A2/pt not_active Application Discontinuation
- 2008-02-01 US US12/525,342 patent/US8927568B2/en active Active
- 2008-02-01 JP JP2009547780A patent/JP5211073B2/ja active Active
- 2008-02-02 JO JO200833A patent/JO2730B1/en active
-
2009
- 2009-07-23 IL IL200032A patent/IL200032A/en active IP Right Grant
-
2014
- 2014-11-17 US US14/543,478 patent/US9023862B2/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1993003752A1 (en) * | 1991-08-19 | 1993-03-04 | The Arizona Board Of Regents On Behalf Of The University Of Arizona | Decapeptide having dopamine stimulating activity |
| US6359130B1 (en) * | 1998-06-05 | 2002-03-19 | Cephalon, Inc. | Bridged indenopyrrolocarbazoles |
| RU2273481C2 (ru) * | 2004-05-27 | 2006-04-10 | Варвара Анатольевна Волкова | Способ повышения эффективности лечения бесплодия, вызванного малыми формами внешнего генитального эндометриоза |
| WO2006117608A1 (en) * | 2005-04-29 | 2006-11-09 | Ferring International Center S.A. | Treatment or prevention of ovarian hyperstimulation syndrome (ohss) using a dopamine agonist |
Non-Patent Citations (2)
| Title |
|---|
| МАШКОВСКИЙ М.Д. Лекарственные средства. - М.: Медицина, 1993, 12-е изд-е, ч.1, с.342-343. ч.1, с.229-231, 173-177, 318-319. * |
| ЭНЦИКЛОПЕДИЯ ЛЕКАРСТВ. - М., РЛС, 2001 с.67, 156-157, 376-377, 696. Tsakok FH et al. The use of bromocriptine in hyperprolactinemia and galactorrhea in Singapore. Int J Gynaecol Obstet. 1985 Apr; 23 (2): 109-13. Реферат [он-лайн][найдено 2010-06-15]. Найдено из базы данных PubMed PMID: 2862070. * |
Also Published As
| Publication number | Publication date |
|---|---|
| HK1134911A1 (en) | 2010-05-20 |
| IL200032A0 (en) | 2010-04-15 |
| SI2109449T1 (sl) | 2011-11-30 |
| WO2008093247A2 (en) | 2008-08-07 |
| RU2009129252A (ru) | 2011-03-10 |
| US20150073010A1 (en) | 2015-03-12 |
| AU2008211633B2 (en) | 2011-04-21 |
| US9023862B2 (en) | 2015-05-05 |
| SA08290041B1 (ar) | 2012-06-05 |
| IL200032A (en) | 2015-06-30 |
| EP2109449B1 (en) | 2011-07-20 |
| JP5211073B2 (ja) | 2013-06-12 |
| PL2109449T3 (pl) | 2011-12-30 |
| AR065139A1 (es) | 2009-05-20 |
| AU2008211633A1 (en) | 2008-08-07 |
| JO2730B1 (en) | 2013-09-15 |
| PT2109449E (pt) | 2011-11-02 |
| US8927568B2 (en) | 2015-01-06 |
| BRPI0806944A2 (pt) | 2014-05-06 |
| CA2676910A1 (en) | 2008-08-07 |
| WO2008093247A3 (en) | 2008-12-04 |
| TW200845989A (en) | 2008-12-01 |
| ATE516805T1 (de) | 2011-08-15 |
| US20100113499A1 (en) | 2010-05-06 |
| DK2109449T3 (da) | 2011-09-12 |
| EP2109449A2 (en) | 2009-10-21 |
| MX2009008191A (es) | 2009-08-12 |
| NZ578569A (en) | 2011-08-26 |
| CA2676910C (en) | 2012-01-03 |
| JP2010517992A (ja) | 2010-05-27 |
| TWI411436B (zh) | 2013-10-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9023862B2 (en) | Medicament for the treatment of endometriosis | |
| JP5128743B2 (ja) | ホルモン置換療法用のドロスピレノン | |
| WO2021126320A1 (en) | Treatment of amyotrophic lateral sclerosis | |
| CN103037862A (zh) | 治疗或预防雌激素相关疾病的方法 | |
| US20250268876A1 (en) | Treatment of hidradenitis suppurativa with orismilast | |
| JP5968781B2 (ja) | 子宮内膜症の治療 | |
| US20240100034A1 (en) | Method for eradicating helicobacter pylori infection in patients regardless of body mass index | |
| JP7420735B2 (ja) | 注射用組成物 | |
| EP1952813A1 (en) | Medicament for the treatment of endometriosis | |
| US11357769B2 (en) | Drug combinations for reducing cell viability and/or cell proliferation | |
| KR101160225B1 (ko) | 자궁 내막증 치료용 약제 | |
| ES2370091T3 (es) | Medicamenteo para el tratamiento de la endometriosis. | |
| IL295807A (en) | A combination of alpelisib and 6-(2,4-dichlorophenyl)-5-[4-[(3s)-1(3-fluoropropyl)pyrrolidin-3-yl]oxyphenyl]-8,9-dihydro-7h-benzo[ 7]annulene-2-carboxylic acid | |
| HK1134911B (en) | Medicament for the treatment of endometriosis | |
| CN117460509B (zh) | 作为c-Met激酶抑制剂的化合物治疗I型神经纤维瘤病的用途 | |
| CN117794544A (zh) | 实体瘤治疗用医药组合物 | |
| CN120302974A (zh) | 喹诺林化合物治疗甲状腺癌的用途 | |
| US20210085711A1 (en) | Use of ferric citrate in prevention and/or treatment of iron-deficiency anemia in hypermenorrhea patient and/or patient suffering from hypermenorrhea-associated gynecologic disease | |
| NZ767891A (en) | Injectable composition |