RU2389501C2 - Применение модифицированных циклоспоринов для лечения заболеваний, вызванных hcv - Google Patents
Применение модифицированных циклоспоринов для лечения заболеваний, вызванных hcv Download PDFInfo
- Publication number
- RU2389501C2 RU2389501C2 RU2006110533/15A RU2006110533A RU2389501C2 RU 2389501 C2 RU2389501 C2 RU 2389501C2 RU 2006110533/15 A RU2006110533/15 A RU 2006110533/15A RU 2006110533 A RU2006110533 A RU 2006110533A RU 2389501 C2 RU2389501 C2 RU 2389501C2
- Authority
- RU
- Russia
- Prior art keywords
- cyclosporin
- hcv
- cyclosporine
- pharmaceutical composition
- use according
- Prior art date
Links
- 229930182912 cyclosporin Natural products 0.000 title claims abstract description 65
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 title claims abstract description 10
- 108010036941 Cyclosporins Proteins 0.000 title description 19
- 201000010099 disease Diseases 0.000 title description 4
- 229960001265 ciclosporin Drugs 0.000 claims abstract description 74
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 claims abstract description 64
- 108010036949 Cyclosporine Proteins 0.000 claims abstract description 62
- 229930105110 Cyclosporin A Natural products 0.000 claims abstract description 47
- 102000001493 Cyclophilins Human genes 0.000 claims abstract description 29
- 108010068682 Cyclophilins Proteins 0.000 claims abstract description 29
- 150000001875 compounds Chemical class 0.000 claims abstract description 24
- 239000003795 chemical substances by application Substances 0.000 claims abstract description 23
- 230000000694 effects Effects 0.000 claims abstract description 23
- 238000002474 experimental method Methods 0.000 claims abstract description 15
- 238000011282 treatment Methods 0.000 claims abstract description 15
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 14
- 208000005176 Hepatitis C Diseases 0.000 claims abstract description 11
- 208000015181 infectious disease Diseases 0.000 claims abstract description 9
- 238000006243 chemical reaction Methods 0.000 claims abstract description 6
- 230000002265 prevention Effects 0.000 claims abstract description 6
- 210000004698 lymphocyte Anatomy 0.000 claims abstract description 5
- 238000002965 ELISA Methods 0.000 claims abstract description 4
- XJODGRWDFZVTKW-LURJTMIESA-N (2s)-4-methyl-2-(methylamino)pentanoic acid Chemical compound CN[C@H](C(O)=O)CC(C)C XJODGRWDFZVTKW-LURJTMIESA-N 0.000 claims description 15
- AKCRVYNORCOYQT-YFKPBYRVSA-N N-methyl-L-valine Chemical compound CN[C@@H](C(C)C)C(O)=O AKCRVYNORCOYQT-YFKPBYRVSA-N 0.000 claims description 12
- 230000010076 replication Effects 0.000 claims description 8
- 238000002360 preparation method Methods 0.000 claims description 7
- AHQFCPOIMVMDEZ-UNISNWAASA-N (e,2s,3r,4r)-3-hydroxy-4-methyl-2-(methylamino)oct-6-enoic acid Chemical group CN[C@H](C(O)=O)[C@H](O)[C@H](C)C\C=C\C AHQFCPOIMVMDEZ-UNISNWAASA-N 0.000 claims description 4
- 208000035415 Reinfection Diseases 0.000 claims description 4
- 239000000969 carrier Substances 0.000 claims description 4
- 239000003085 diluting agent Substances 0.000 claims description 4
- 230000002401 inhibitory effect Effects 0.000 claims description 4
- 239000000018 receptor agonist Substances 0.000 claims description 4
- 229940044601 receptor agonist Drugs 0.000 claims description 4
- 150000003839 salts Chemical class 0.000 claims description 4
- 230000003510 anti-fibrotic effect Effects 0.000 claims description 3
- 230000002860 competitive effect Effects 0.000 claims description 3
- 239000002955 immunomodulating agent Substances 0.000 claims description 3
- 229940121354 immunomodulator Drugs 0.000 claims description 3
- 230000002584 immunomodulator Effects 0.000 claims description 3
- FYCWLJLGIAUCCL-DMTCNVIQSA-N O-methyl-L-threonine Chemical compound CO[C@H](C)[C@H](N)C(O)=O FYCWLJLGIAUCCL-DMTCNVIQSA-N 0.000 claims description 2
- SNDPXSYFESPGGJ-UHFFFAOYSA-N 2-aminopentanoic acid Chemical compound CCCC(N)C(O)=O SNDPXSYFESPGGJ-UHFFFAOYSA-N 0.000 claims 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 claims 1
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Chemical compound CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 claims 1
- 239000003814 drug Substances 0.000 abstract description 8
- KSPIYJQBLVDRRI-WDSKDSINSA-N N-methyl-L-isoleucine Chemical compound CC[C@H](C)[C@H](NC)C(O)=O KSPIYJQBLVDRRI-WDSKDSINSA-N 0.000 abstract 1
- 238000012875 competitive assay Methods 0.000 abstract 1
- 210000000987 immune system Anatomy 0.000 abstract 1
- 239000000126 substance Substances 0.000 abstract 1
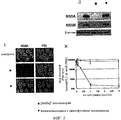
- 241000711549 Hepacivirus C Species 0.000 description 42
- 230000001506 immunosuppresive effect Effects 0.000 description 27
- 210000004027 cell Anatomy 0.000 description 23
- 108010050904 Interferons Proteins 0.000 description 19
- 102000014150 Interferons Human genes 0.000 description 19
- 238000000034 method Methods 0.000 description 18
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 15
- 229940079322 interferon Drugs 0.000 description 15
- -1 thio MeLeu Chemical group 0.000 description 12
- 238000004458 analytical method Methods 0.000 description 11
- RPJPZDVUUKWPGT-FOIHOXPVSA-N nim811 Chemical compound CC[C@H](C)[C@@H]1N(C)C(=O)CN(C)C(=O)[C@H](CC)NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC1=O RPJPZDVUUKWPGT-FOIHOXPVSA-N 0.000 description 9
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 6
- 108010082126 Alanine transaminase Proteins 0.000 description 6
- 229940079593 drug Drugs 0.000 description 6
- 229920000642 polymer Polymers 0.000 description 6
- 230000004044 response Effects 0.000 description 5
- 210000002966 serum Anatomy 0.000 description 5
- 102000004631 Calcineurin Human genes 0.000 description 4
- 108010042955 Calcineurin Proteins 0.000 description 4
- 102100040018 Interferon alpha-2 Human genes 0.000 description 4
- 108010079944 Interferon-alpha2b Proteins 0.000 description 4
- 101800001014 Non-structural protein 5A Proteins 0.000 description 4
- 238000003556 assay Methods 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- 208000006454 hepatitis Diseases 0.000 description 4
- 125000000623 heterocyclic group Chemical group 0.000 description 4
- 238000003119 immunoblot Methods 0.000 description 4
- 229940047124 interferons Drugs 0.000 description 4
- 238000003752 polymerase chain reaction Methods 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 206010008909 Chronic Hepatitis Diseases 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 241000699670 Mus sp. Species 0.000 description 3
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 3
- 238000000636 Northern blotting Methods 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- 102000006010 Protein Disulfide-Isomerase Human genes 0.000 description 3
- 101800001554 RNA-directed RNA polymerase Proteins 0.000 description 3
- 241000700605 Viruses Species 0.000 description 3
- 230000000890 antigenic effect Effects 0.000 description 3
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 238000011081 inoculation Methods 0.000 description 3
- 210000004185 liver Anatomy 0.000 description 3
- 208000019423 liver disease Diseases 0.000 description 3
- 229960000951 mycophenolic acid Drugs 0.000 description 3
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- 229940002612 prodrug Drugs 0.000 description 3
- 239000000651 prodrug Substances 0.000 description 3
- 108020003519 protein disulfide isomerase Proteins 0.000 description 3
- 235000018102 proteins Nutrition 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 108020004418 ribosomal RNA Proteins 0.000 description 3
- 239000000523 sample Substances 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 241000283690 Bos taurus Species 0.000 description 2
- 206010016654 Fibrosis Diseases 0.000 description 2
- 108010005716 Interferon beta-1a Proteins 0.000 description 2
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 2
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 2
- IWUCXVSUMQZMFG-AFCXAGJDSA-N Ribavirin Chemical compound N1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 IWUCXVSUMQZMFG-AFCXAGJDSA-N 0.000 description 2
- 102000011011 Sphingosine 1-phosphate receptors Human genes 0.000 description 2
- 108050001083 Sphingosine 1-phosphate receptors Proteins 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000000427 antigen Substances 0.000 description 2
- 102000036639 antigens Human genes 0.000 description 2
- 108091007433 antigens Proteins 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 208000019425 cirrhosis of liver Diseases 0.000 description 2
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 229960000556 fingolimod Drugs 0.000 description 2
- KKGQTZUTZRNORY-UHFFFAOYSA-N fingolimod Chemical compound CCCCCCCCC1=CC=C(CCC(N)(CO)CO)C=C1 KKGQTZUTZRNORY-UHFFFAOYSA-N 0.000 description 2
- 208000010710 hepatitis C virus infection Diseases 0.000 description 2
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 2
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 2
- 210000003494 hepatocyte Anatomy 0.000 description 2
- 125000005842 heteroatom Chemical group 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 238000010166 immunofluorescence Methods 0.000 description 2
- 238000010185 immunofluorescence analysis Methods 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- JTEGQNOMFQHVDC-NKWVEPMBSA-N lamivudine Chemical compound O=C1N=C(N)C=CN1[C@H]1O[C@@H](CO)SC1 JTEGQNOMFQHVDC-NKWVEPMBSA-N 0.000 description 2
- 229960001627 lamivudine Drugs 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 2
- 229960004857 mitomycin Drugs 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 230000017074 necrotic cell death Effects 0.000 description 2
- 125000004433 nitrogen atom Chemical group N* 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 239000002953 phosphate buffered saline Substances 0.000 description 2
- 125000005936 piperidyl group Chemical group 0.000 description 2
- 229920000233 poly(alkylene oxides) Polymers 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 230000009696 proliferative response Effects 0.000 description 2
- 125000004076 pyridyl group Chemical group 0.000 description 2
- 238000003753 real-time PCR Methods 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 229960000329 ribavirin Drugs 0.000 description 2
- HZCAHMRRMINHDJ-DBRKOABJSA-N ribavirin Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1N=CN=C1 HZCAHMRRMINHDJ-DBRKOABJSA-N 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 210000000952 spleen Anatomy 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 125000005942 tetrahydropyridyl group Chemical group 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 229920003169 water-soluble polymer Polymers 0.000 description 2
- GBIWPCXWDSDANU-JYFGSSRXSA-N (3s,6s,9s,12r,15s,18s,21s,24s,30s)-30-ethyl-33-[(e,1r,2r)-1-hydroxy-2-methylhex-4-enyl]-1,4,7,10,12,15,18,19,25,28-decamethyl-6,9,24-tris(2-methylpropyl)-3,21-di(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecazacyclotritriacontane-2,5,8,11,14,17,20,23,2 Chemical compound CC[C@@H]1NC(=O)C([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O GBIWPCXWDSDANU-JYFGSSRXSA-N 0.000 description 1
- OYHQOLUKZRVURQ-NTGFUMLPSA-N (9Z,12Z)-9,10,12,13-tetratritiooctadeca-9,12-dienoic acid Chemical compound C(CCCCCCC\C(=C(/C\C(=C(/CCCCC)\[3H])\[3H])\[3H])\[3H])(=O)O OYHQOLUKZRVURQ-NTGFUMLPSA-N 0.000 description 1
- 125000006656 (C2-C4) alkenyl group Chemical group 0.000 description 1
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 description 1
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 1
- 102000007445 2',5'-Oligoadenylate Synthetase Human genes 0.000 description 1
- 108010086241 2',5'-Oligoadenylate Synthetase Proteins 0.000 description 1
- 150000004046 2-(N-anilino)pyrimidines Chemical class 0.000 description 1
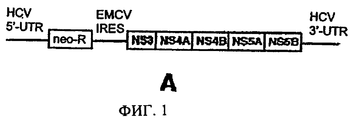
- 108020003589 5' Untranslated Regions Proteins 0.000 description 1
- 208000030507 AIDS Diseases 0.000 description 1
- 208000037859 AIDS-related disorder Diseases 0.000 description 1
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 1
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 1
- 101000729818 Bacillus licheniformis Glutamate racemase Proteins 0.000 description 1
- 101000921794 Caenorhabditis elegans Peptidyl-prolyl cis-trans isomerase 10 Proteins 0.000 description 1
- 208000006154 Chronic hepatitis C Diseases 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 1
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 238000003718 Dual-Luciferase Reporter Assay System Methods 0.000 description 1
- 102400001368 Epidermal growth factor Human genes 0.000 description 1
- 101800003838 Epidermal growth factor Proteins 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- 102000051325 Glucagon Human genes 0.000 description 1
- 108060003199 Glucagon Proteins 0.000 description 1
- 101000878213 Homo sapiens Inactive peptidyl-prolyl cis-trans isomerase FKBP6 Proteins 0.000 description 1
- 108091006905 Human Serum Albumin Proteins 0.000 description 1
- 102000008100 Human Serum Albumin Human genes 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 102100036984 Inactive peptidyl-prolyl cis-trans isomerase FKBP6 Human genes 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 108010005714 Interferon beta-1b Proteins 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 102000000588 Interleukin-2 Human genes 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 1
- 239000012097 Lipofectamine 2000 Substances 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 108060004795 Methyltransferase Proteins 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 108010018525 NFATC Transcription Factors Proteins 0.000 description 1
- 102000002673 NFATC Transcription Factors Human genes 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 102000003946 Prolactin Human genes 0.000 description 1
- 108010057464 Prolactin Proteins 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 102000001253 Protein Kinase Human genes 0.000 description 1
- 238000010240 RT-PCR analysis Methods 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 241000282849 Ruminantia Species 0.000 description 1
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 1
- 108091027544 Subgenomic mRNA Proteins 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 230000006044 T cell activation Effects 0.000 description 1
- 102000004338 Transferrin Human genes 0.000 description 1
- 108090000901 Transferrin Proteins 0.000 description 1
- 238000001994 activation Methods 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 108010080374 albuferon Proteins 0.000 description 1
- 230000000735 allogeneic effect Effects 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 229940003504 avonex Drugs 0.000 description 1
- 125000002393 azetidinyl group Chemical group 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 230000036983 biotransformation Effects 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 230000007882 cirrhosis Effects 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 210000004748 cultured cell Anatomy 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 125000002993 cycloalkylene group Chemical group 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 238000001212 derivatisation Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 230000035622 drinking Effects 0.000 description 1
- 210000002472 endoplasmic reticulum Anatomy 0.000 description 1
- 229940116977 epidermal growth factor Drugs 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 239000012737 fresh medium Substances 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- MASNOZXLGMXCHN-ZLPAWPGGSA-N glucagon Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 MASNOZXLGMXCHN-ZLPAWPGGSA-N 0.000 description 1
- 229960004666 glucagon Drugs 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000005802 health problem Effects 0.000 description 1
- 231100000283 hepatitis Toxicity 0.000 description 1
- 231100000805 hepatocellular lesion Toxicity 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 1
- 229960002411 imatinib Drugs 0.000 description 1
- MTNDZQHUAFNZQY-UHFFFAOYSA-N imidazoline Chemical compound C1CN=CN1 MTNDZQHUAFNZQY-UHFFFAOYSA-N 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000000122 inhibitory effect on hepatitis Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 210000005229 liver cell Anatomy 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 125000000896 monocarboxylic acid group Chemical group 0.000 description 1
- 229960004866 mycophenolate mofetil Drugs 0.000 description 1
- RTGDFNSFWBGLEC-SYZQJQIISA-N mycophenolate mofetil Chemical compound COC1=C(C)C=2COC(=O)C=2C(O)=C1C\C=C(/C)CCC(=O)OCCN1CCOCC1 RTGDFNSFWBGLEC-SYZQJQIISA-N 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 238000010606 normalization Methods 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Chemical group 0.000 description 1
- 229940002988 pegasys Drugs 0.000 description 1
- 108010092853 peginterferon alfa-2a Proteins 0.000 description 1
- 108010092851 peginterferon alfa-2b Proteins 0.000 description 1
- 229940106366 pegintron Drugs 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 125000004193 piperazinyl group Chemical group 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 210000003240 portal vein Anatomy 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 229940097325 prolactin Drugs 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 230000012846 protein folding Effects 0.000 description 1
- 108060006633 protein kinase Proteins 0.000 description 1
- 229940038850 rebif Drugs 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000010839 reverse transcription Methods 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 229910052717 sulfur Chemical group 0.000 description 1
- 239000011593 sulfur Chemical group 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 239000011885 synergistic combination Substances 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000005505 thiomorpholino group Chemical group 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 239000012096 transfection reagent Substances 0.000 description 1
- 239000012581 transferrin Substances 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/12—Cyclic peptides, e.g. bacitracins; Polymyxins; Gramicidins S, C; Tyrocidins A, B or C
- A61K38/13—Cyclosporins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Epidemiology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Virology (AREA)
- Molecular Biology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB0320638.0A GB0320638D0 (en) | 2003-09-03 | 2003-09-03 | Organic compounds |
| GB0320638.0 | 2003-09-03 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2006110533A RU2006110533A (ru) | 2007-10-10 |
| RU2389501C2 true RU2389501C2 (ru) | 2010-05-20 |
Family
ID=28686843
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2006110533/15A RU2389501C2 (ru) | 2003-09-03 | 2004-09-02 | Применение модифицированных циклоспоринов для лечения заболеваний, вызванных hcv |
Country Status (29)
| Country | Link |
|---|---|
| US (1) | US7968518B2 (enExample) |
| EP (2) | EP1797892B1 (enExample) |
| JP (1) | JP4688806B2 (enExample) |
| KR (1) | KR101116968B1 (enExample) |
| CN (1) | CN1842343A (enExample) |
| AU (2) | AU2004268382A1 (enExample) |
| BR (1) | BRPI0414062B8 (enExample) |
| CA (1) | CA2537137C (enExample) |
| CY (1) | CY1110506T1 (enExample) |
| DE (1) | DE602004022061D1 (enExample) |
| DK (1) | DK1663287T3 (enExample) |
| ES (1) | ES2328361T3 (enExample) |
| GB (1) | GB0320638D0 (enExample) |
| HR (1) | HRP20090535T1 (enExample) |
| IL (1) | IL173909A (enExample) |
| IS (1) | IS2917B (enExample) |
| MA (1) | MA28032A1 (enExample) |
| MX (1) | MXPA06002394A (enExample) |
| NO (1) | NO20061479L (enExample) |
| NZ (1) | NZ545495A (enExample) |
| PL (1) | PL1663287T3 (enExample) |
| PT (1) | PT1663287E (enExample) |
| RU (1) | RU2389501C2 (enExample) |
| SG (1) | SG149063A1 (enExample) |
| SI (1) | SI1663287T1 (enExample) |
| TN (1) | TNSN06071A1 (enExample) |
| TW (1) | TWI342782B (enExample) |
| WO (1) | WO2005021028A1 (enExample) |
| ZA (1) | ZA200601550B (enExample) |
Families Citing this family (59)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU759480B2 (en) | 1998-07-01 | 2003-04-17 | Debiopharm S.A. | Novel cyclosporin with improved activity profile |
| JP2006524232A (ja) | 2003-03-17 | 2006-10-26 | アルバニー モレキュラー リサーチ インコーポレーティッド | 新規サイクロスポリン |
| GB0320638D0 (en) | 2003-09-03 | 2003-10-01 | Novartis Ag | Organic compounds |
| GB0500020D0 (en) * | 2005-01-04 | 2005-02-09 | Novartis Ag | Organic compounds |
| US7671017B2 (en) | 2004-07-14 | 2010-03-02 | Novartis Ag | Use of a combination of cyclosporine and pegylated interferon for treating hepatitis C (HCV) |
| WO2006039164A2 (en) | 2004-09-29 | 2006-04-13 | Amr Technology, Inc. | Novel cyclosporin analogues and their pharmaceutical uses |
| EP1809656A4 (en) | 2004-09-29 | 2009-03-25 | Amr Technology Inc | CYCLOSPORINAL KINANALOGA AND ITS PHARMACEUTICAL APPLICATIONS |
| MX2007003387A (es) * | 2004-10-01 | 2007-05-23 | Debiopharm Sa | Uso de [d-meala]3-[etval]4-ciclosporina para el tratamiento de infeccion por hepatitis c y composicion farmaceutica que comprende la [d-meala]3-[etval]4-ciclosporina. |
| US7196161B2 (en) | 2004-10-01 | 2007-03-27 | Scynexis Inc. | 3-ether and 3-thioether substituted cyclosporin derivatives for the treatment and prevention of hepatitis C infection |
| CA2583494C (en) * | 2004-10-01 | 2014-01-21 | Scynexis, Inc. | 3-ether and 3-thioether substituted cyclosporin derivatives for the treatment and prevention of hepatitis c infection |
| US7361636B2 (en) | 2004-10-06 | 2008-04-22 | Amr Technology, Inc. | Cyclosporin alkynes and their utility as pharmaceutical agents |
| DE602005010607D1 (de) | 2004-11-22 | 2008-12-04 | Astellas Pharma Inc | Zyklosporinanalog |
| KR20070089954A (ko) * | 2004-12-23 | 2007-09-04 | 노파르티스 아게 | 플라비비리대 치료용 화합물 |
| RU2007128099A (ru) | 2004-12-23 | 2009-01-27 | Новартис АГ (CH) | Композиции для лечения гепатита с |
| MX2007016068A (es) | 2005-06-17 | 2008-03-10 | Novartis Ag | Uso de sanglifehrina en hcv. |
| CN101316606B (zh) * | 2005-09-30 | 2015-05-27 | 西尼克斯公司 | 治疗和预防丙型肝炎感染的方法和药物组合物 |
| JP5322647B2 (ja) * | 2005-09-30 | 2013-10-23 | スシネキス インク | ウイルス感染の治療及び予防のためのシクロスポリンaのアリールアルキル及びヘテロアリールアルキル誘導体 |
| JP5517454B2 (ja) * | 2005-09-30 | 2014-06-11 | スシネキス インク | C型肝炎感染の治療及び予防のための方法及び医薬組成物 |
| AU2006306974B2 (en) | 2005-10-26 | 2011-09-22 | Astellas Pharma Inc. | New cyclic peptide compounds |
| US7696166B2 (en) | 2006-03-28 | 2010-04-13 | Albany Molecular Research, Inc. | Use of cyclosporin alkyne/alkene analogues for preventing or treating viral-induced disorders |
| US7696165B2 (en) | 2006-03-28 | 2010-04-13 | Albany Molecular Research, Inc. | Use of cyclosporin alkyne analogues for preventing or treating viral-induced disorders |
| ATE502633T1 (de) | 2006-05-19 | 2011-04-15 | Scynexis Inc | Cyclosporins zur behandlung und vorbeugung von augenerkrankungen |
| CN101502196A (zh) * | 2006-06-02 | 2009-08-05 | 克洛德·安妮·佩里西恩 | 激活电子的管理 |
| CN101557819A (zh) * | 2006-10-12 | 2009-10-14 | 诺瓦提斯公司 | 修饰的环孢素的用途 |
| US7576057B2 (en) | 2006-11-20 | 2009-08-18 | Scynexis, Inc. | Cyclic peptides |
| US8450281B2 (en) * | 2007-01-04 | 2013-05-28 | Debiopharm S.A. | Non-immunosuppressive cyclosporin for treatment of Ullrich congenital muscular dystrophy |
| ES2396170T3 (es) * | 2007-05-02 | 2013-02-19 | Astellas Pharma Inc. | Nuevos compuestos péptídicos ciclicos |
| EP2193807A4 (en) * | 2007-08-24 | 2012-09-12 | Univ Sapporo Medical | BINDING PROTEIN AT CYCLOSPORIN A |
| US20090306033A1 (en) * | 2008-06-06 | 2009-12-10 | Keqiang Li | Novel cyclic peptides |
| CN102083852A (zh) * | 2008-06-06 | 2011-06-01 | 西尼克斯公司 | 环孢菌素类似物及其在治疗hcv感染中的应用 |
| JP5726733B2 (ja) * | 2008-07-30 | 2015-06-03 | シクロフィリン ファーマシューティカルズ コープ. | 非免疫抑制性シクロスポリン類似体分子 |
| CN102164947A (zh) * | 2008-09-24 | 2011-08-24 | 安斯泰来制药有限公司 | 肽化合物及其制备方法 |
| WO2010052559A1 (en) * | 2008-11-06 | 2010-05-14 | Debio Recherche Pharmaceutique S.A. | Cycloundecadepsipeptide compounds and use of said compounds as a medicament |
| JP5780969B2 (ja) * | 2008-12-31 | 2015-09-16 | サイネクシス,インコーポレーテッド | シクロスポリンaの誘導体 |
| US8367618B2 (en) * | 2009-01-30 | 2013-02-05 | Enanta Pharmaceuticals, Inc. | Cyclosporin analogues for preventing or treating hepatitis C infection |
| US8481483B2 (en) | 2009-02-19 | 2013-07-09 | Enanta Pharmaceuticals, Inc. | Cyclosporin analogues |
| US8349312B2 (en) | 2009-07-09 | 2013-01-08 | Enanta Pharmaceuticals, Inc. | Proline substituted cyclosporin analogues |
| US8685917B2 (en) | 2009-07-09 | 2014-04-01 | Enanta Pharmaceuticals, Inc. | Cyclosporin analogues |
| US8367053B2 (en) | 2009-07-09 | 2013-02-05 | Enanta Pharmaceuticals, Inc. | Cyclosporin analogues |
| US20110117055A1 (en) | 2009-11-19 | 2011-05-19 | Macdonald James E | Methods of Treating Hepatitis C Virus with Oxoacetamide Compounds |
| CN102869367A (zh) | 2009-12-09 | 2013-01-09 | 西尼克斯公司 | 新颖的环肽 |
| US8623814B2 (en) | 2010-02-23 | 2014-01-07 | Enanta Pharmaceuticals, Inc. | Antiviral agents |
| WO2012045704A1 (en) | 2010-10-05 | 2012-04-12 | Novartis Ag | New treatments of hepatitis c virus infection |
| US20130251678A1 (en) | 2010-11-30 | 2013-09-26 | Novartis Ag | Bid dosage regimen for deb025 |
| JO3337B1 (ar) | 2010-12-13 | 2019-03-13 | Debiopharm Sa | تركيبات صيدلية تشمل أليسبوريفير |
| HRP20190096T1 (hr) | 2010-12-15 | 2019-02-22 | Contravir Pharmaceuticals, Inc. | Molekule analoga ciklosporina modificirani na amino kiselini 1 i 3 |
| KR20140007927A (ko) | 2011-03-31 | 2014-01-20 | 노파르티스 아게 | C형 간염 바이러스 감염을 치료하기 위한 알리스포리비르 |
| BR112013024809A2 (pt) | 2011-04-01 | 2016-09-06 | Novartis Ag | tratamento para infecção com vírus de hepatite b sozinho ou em combinação com vírus de hepatite delta e doenças do fígado associadas |
| SG10201602184TA (en) | 2011-04-13 | 2016-04-28 | Novartis Ag | Treatment of hepatitis c virus infection with alisporivir |
| CA2850052A1 (en) | 2011-09-27 | 2013-04-04 | Novartis Ag | Alisporivr for treatment of hepatis c virus infection |
| WO2013169616A1 (en) | 2012-05-07 | 2013-11-14 | Novartis Ag | Pharmacokinetic modulation with alisporivir |
| JP2015519368A (ja) | 2012-06-01 | 2015-07-09 | アラーガン インコーポレイテッドAllergan,Incorporated | シクロスポリンa類似体 |
| US8906853B2 (en) | 2012-11-28 | 2014-12-09 | Enanta Pharmaceuticals, Inc. | [N-Me-4-hydroxyleucine]-9-cyclosporin analogues for treatment and prevention of hepatitis C infection |
| WO2015008223A1 (en) | 2013-07-17 | 2015-01-22 | Novartis Ag | Treatment of hepatitis c virus infection with alisporivir and ribavirin |
| WO2015031381A1 (en) | 2013-08-26 | 2015-03-05 | Enanta Pharmaceuticals, Inc. | Cyclosporin analogues for preventing or treating hepatitis c |
| WO2015136455A1 (en) | 2014-03-13 | 2015-09-17 | Novartis Ag | New treatments of hepatitis c virus infection |
| US9669095B2 (en) | 2014-11-03 | 2017-06-06 | Enanta Pharmaceuticals, Inc. | Cyclosporin analogues for preventing or treating hepatitis C infection |
| WO2016112321A1 (en) | 2015-01-08 | 2016-07-14 | Allergan, Inc. | Cyclosporin derivatives wherein the mebmt sidechain has been cyclized |
| WO2022043678A1 (en) * | 2020-08-24 | 2022-03-03 | Nanomerics Limited | Viral inhibitors |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5994299A (en) * | 1996-12-24 | 1999-11-30 | Rhone-Poulenc Rorer, S.A. | Cyclosporin compounds, their preparation and the pharmaceutical compositions which contain them |
| WO1999062540A1 (en) * | 1998-06-02 | 1999-12-09 | Novartis Ag | Use of cyclosporins in the treatment of inflammatory autoimmune diseases |
| WO2002032447A2 (en) * | 2000-10-19 | 2002-04-25 | Fujisawa Pharmaceutical Co., Ltd. | Cell damage inhibitor |
| US20020102279A1 (en) * | 1997-09-02 | 2002-08-01 | Kenji Chiba | Compositions and methods of using compositions with accelerated lymphocyte homing immunosuppressive properties |
Family Cites Families (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5639724A (en) * | 1984-07-24 | 1997-06-17 | Sandoz Ltd. | Cyclosporin galenic forms |
| US4766106A (en) | 1985-06-26 | 1988-08-23 | Cetus Corporation | Solubilization of proteins for pharmaceutical compositions using polymer conjugation |
| US4917888A (en) | 1985-06-26 | 1990-04-17 | Cetus Corporation | Solubilization of immunotoxins for pharmaceutical compositions using polymer conjugation |
| JP2514950B2 (ja) | 1986-03-10 | 1996-07-10 | エフ・ホフマン―ラ ロシユ アーゲー | 化学修飾蛋白質,その製造法および中間体 |
| KR0148748B1 (ko) | 1988-09-16 | 1998-08-17 | 장 크라메르, 한스 루돌프 하우스 | 사이클로스포린을 함유하는 약학조성물 |
| DE69124459T3 (de) * | 1990-11-02 | 2001-05-31 | Novartis Ag, Basel | Zyklosporine |
| US5595732A (en) | 1991-03-25 | 1997-01-21 | Hoffmann-La Roche Inc. | Polyethylene-protein conjugates |
| US5382657A (en) | 1992-08-26 | 1995-01-17 | Hoffmann-La Roche Inc. | Peg-interferon conjugates |
| US6174868B1 (en) * | 1992-09-10 | 2001-01-16 | Isis Pharmaceuticals, Inc. | Compositions and methods for treatment of hepatitis C virus-associated diseases |
| DK0627406T3 (da) | 1992-10-21 | 1999-07-12 | Taito Co | 2-Amino-1,3-propandiolforbindelser og immunundertrykkende midler |
| KR960705579A (ko) | 1993-11-10 | 1996-11-08 | 에릭에스. 딕커 | 개선된 인터페론 중합체 결합체(Improved interferon polymer conjugates) |
| KR100358922B1 (ko) | 1994-08-22 | 2003-01-24 | 미쯔비시 웰 파마 가부시키가이샤 | 벤젠화합물및그의의약으로서의용도 |
| US5824784A (en) | 1994-10-12 | 1998-10-20 | Amgen Inc. | N-terminally chemically modified protein compositions and methods |
| JP3786447B2 (ja) * | 1995-03-31 | 2006-06-14 | エーザイ株式会社 | C型肝炎の予防・治療剤 |
| US5908621A (en) | 1995-11-02 | 1999-06-01 | Schering Corporation | Polyethylene glycol modified interferon therapy |
| FR2757520B1 (fr) | 1996-12-24 | 1999-01-29 | Rhone Poulenc Rorer Sa | Derive de cyclosporine, sa preparation et les compositions pharmaceutiques qui le contiennent |
| FR2757522B1 (fr) | 1996-12-24 | 1999-01-29 | Rhone Poulenc Rorer Sa | Derives de cyclosporine, leur preparation et les compositions pharmaceutiques qui les contiennent |
| CN1290819C (zh) | 1997-04-04 | 2006-12-20 | 三菱制药株式会社 | 2-氨基丙烷-1,3-二醇化合物、其作为医药的用途及其合成中间体 |
| AU759480B2 (en) | 1998-07-01 | 2003-04-17 | Debiopharm S.A. | Novel cyclosporin with improved activity profile |
| BR0112484A (pt) | 2000-07-13 | 2003-09-23 | Sankyo Co | Composto ou um seu sal, éster ou outro derivado farmacologicamente aceitável, composição farmacêutica, uso de um composto ou de um seu sal, éster ou outro derivado farmacologicamente aceitável, e, processo para a preparação do mesmo |
| AU8533101A (en) | 2000-08-31 | 2002-03-13 | Merck & Co Inc | Phosphate derivatives as immunoregulatory agents |
| JP2002125683A (ja) * | 2000-10-27 | 2002-05-08 | Tokyoto Igaku Kenkyu Kiko | インターフェロンの有効性を予測する方法並びにそれに用いられるプライマー及びプローブ |
| JP4012823B2 (ja) | 2001-03-26 | 2007-11-21 | ノバルティス アクチエンゲゼルシャフト | 2−アミノ−誘導体 |
| JP2002316985A (ja) | 2001-04-20 | 2002-10-31 | Sankyo Co Ltd | ベンゾチオフェン誘導体 |
| BRPI0212894B8 (pt) | 2001-09-27 | 2021-05-25 | Kyorin Seiyaku Kk | composto ou sal do mesmo farmaceuticamente aceitável e composição farmacêutica |
| DE60235900D1 (de) | 2001-09-27 | 2010-05-20 | Kyorin Seiyaku Kk | Osuppressivum |
| AU2003207567B2 (en) | 2002-01-18 | 2008-01-24 | Merck Sharp & Dohme Corp. | Edg receptor agonists |
| US7351725B2 (en) | 2002-01-18 | 2008-04-01 | Merck & Co., Inc. | N-(benzyl)aminoalkylcarboxylates, phosphinates, phosphonates and tetrazoles as Edg receptor agonists |
| US20030213603A1 (en) * | 2002-05-14 | 2003-11-20 | Fisher David B. | Dual use extension apparatus for a tool |
| KR20050035194A (ko) | 2002-06-28 | 2005-04-15 | 이데닉스 (케이만) 리미티드 | 플라비비리다에 감염 치료용 2'-c-메틸-3'-o-l-발린에스테르 리보푸라노실 사이티딘 |
| GB0313444D0 (en) | 2003-06-11 | 2003-07-16 | Midland Medical Technologies L | Modular dysplasia cup |
| GB0320638D0 (en) | 2003-09-03 | 2003-10-01 | Novartis Ag | Organic compounds |
| MX2007003387A (es) | 2004-10-01 | 2007-05-23 | Debiopharm Sa | Uso de [d-meala]3-[etval]4-ciclosporina para el tratamiento de infeccion por hepatitis c y composicion farmaceutica que comprende la [d-meala]3-[etval]4-ciclosporina. |
| RU2007128099A (ru) | 2004-12-23 | 2009-01-27 | Новартис АГ (CH) | Композиции для лечения гепатита с |
-
2003
- 2003-09-03 GB GBGB0320638.0A patent/GB0320638D0/en not_active Ceased
-
2004
- 2004-09-02 TW TW093126578A patent/TWI342782B/zh not_active IP Right Cessation
- 2004-09-02 US US10/570,097 patent/US7968518B2/en active Active
- 2004-09-02 JP JP2006525712A patent/JP4688806B2/ja not_active Expired - Fee Related
- 2004-09-02 DK DK04764762T patent/DK1663287T3/da active
- 2004-09-02 NZ NZ545495A patent/NZ545495A/en not_active IP Right Cessation
- 2004-09-02 ES ES04764762T patent/ES2328361T3/es not_active Expired - Lifetime
- 2004-09-02 SG SG200809555-6A patent/SG149063A1/en unknown
- 2004-09-02 EP EP06125649.1A patent/EP1797892B1/en not_active Expired - Lifetime
- 2004-09-02 CA CA2537137A patent/CA2537137C/en not_active Expired - Fee Related
- 2004-09-02 PT PT04764762T patent/PT1663287E/pt unknown
- 2004-09-02 WO PCT/EP2004/009804 patent/WO2005021028A1/en not_active Ceased
- 2004-09-02 MX MXPA06002394A patent/MXPA06002394A/es active IP Right Grant
- 2004-09-02 RU RU2006110533/15A patent/RU2389501C2/ru not_active IP Right Cessation
- 2004-09-02 DE DE602004022061T patent/DE602004022061D1/de not_active Expired - Lifetime
- 2004-09-02 HR HR20090535T patent/HRP20090535T1/hr unknown
- 2004-09-02 SI SI200431200T patent/SI1663287T1/sl unknown
- 2004-09-02 PL PL04764762T patent/PL1663287T3/pl unknown
- 2004-09-02 EP EP04764762A patent/EP1663287B1/en not_active Expired - Lifetime
- 2004-09-02 CN CNA2004800246044A patent/CN1842343A/zh active Pending
- 2004-09-02 BR BRPI0414062A patent/BRPI0414062B8/pt not_active IP Right Cessation
- 2004-09-02 KR KR1020067004342A patent/KR101116968B1/ko not_active Expired - Fee Related
- 2004-09-02 AU AU2004268382A patent/AU2004268382A1/en not_active Abandoned
-
2006
- 2006-02-22 ZA ZA200601550A patent/ZA200601550B/en unknown
- 2006-02-23 IL IL173909A patent/IL173909A/en active IP Right Grant
- 2006-03-02 TN TNP2006000071A patent/TNSN06071A1/en unknown
- 2006-03-10 MA MA28865A patent/MA28032A1/fr unknown
- 2006-03-24 IS IS8371A patent/IS2917B/is unknown
- 2006-03-31 NO NO20061479A patent/NO20061479L/no not_active Application Discontinuation
-
2008
- 2008-07-09 AU AU2008203031A patent/AU2008203031B2/en not_active Ceased
-
2009
- 2009-09-16 CY CY20091100955T patent/CY1110506T1/el unknown
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5994299A (en) * | 1996-12-24 | 1999-11-30 | Rhone-Poulenc Rorer, S.A. | Cyclosporin compounds, their preparation and the pharmaceutical compositions which contain them |
| US20020102279A1 (en) * | 1997-09-02 | 2002-08-01 | Kenji Chiba | Compositions and methods of using compositions with accelerated lymphocyte homing immunosuppressive properties |
| WO1999062540A1 (en) * | 1998-06-02 | 1999-12-09 | Novartis Ag | Use of cyclosporins in the treatment of inflammatory autoimmune diseases |
| WO2002032447A2 (en) * | 2000-10-19 | 2002-04-25 | Fujisawa Pharmaceutical Co., Ltd. | Cell damage inhibitor |
Non-Patent Citations (2)
| Title |
|---|
| с.3-4. * |
| ЭНЦИКЛОПЕДИЯ ЛЕКАРСТВ. - М., 2001 РЛС с.758-759. ГРЭХАМ-СМИТ Д.Г. и др. Оксфордский справочник по клинической фармакологии и фармакотерапии. - М.: Медицина, 2000, с.25-26, 136-137 СЭНФОРД Дж. и др. Антимикробная терапия. - М.: Практика, 1996 с.142-150. * |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2389501C2 (ru) | Применение модифицированных циклоспоринов для лечения заболеваний, вызванных hcv | |
| KR20070087624A (ko) | Hcv 치료용 조성물 | |
| RS53911B1 (sr) | Tretman u infekciji virusom hepatitis b zasebno ili u kombinaciji sa hepatitis delta virusom i bolesti jetre koje ih prate | |
| RU2463071C2 (ru) | Применение модифицированных циклоспоринов | |
| JP2008525458A (ja) | フラビウイルス科処置用化合物 | |
| EP0666755B1 (en) | Inhibition of hiv-infection | |
| HK1091732B (en) | Use of modified cyclosporins for the treatment of hcv disorders | |
| NZ576523A (en) | Use of modified cyclosporins | |
| WO2013137869A1 (en) | Combination therapy for treating hcv infection in an hcv-hiv coinfected patient population |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20200903 |