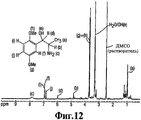
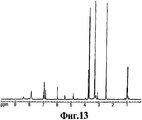
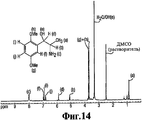
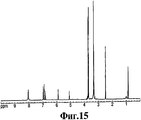
RU2332989C2 - Способ получения 1r,2s-метоксамина, его терапевтическое применение (варианты) и фармацевтическая композиция - Google Patents
Способ получения 1r,2s-метоксамина, его терапевтическое применение (варианты) и фармацевтическая композиция Download PDFInfo
- Publication number
- RU2332989C2 RU2332989C2 RU2004122639/15A RU2004122639A RU2332989C2 RU 2332989 C2 RU2332989 C2 RU 2332989C2 RU 2004122639/15 A RU2004122639/15 A RU 2004122639/15A RU 2004122639 A RU2004122639 A RU 2004122639A RU 2332989 C2 RU2332989 C2 RU 2332989C2
- Authority
- RU
- Russia
- Prior art keywords
- methoxamine
- pharmaceutical composition
- dose
- composition according
- acceptable salt
- Prior art date
Links
- 238000000034 method Methods 0.000 title claims abstract 27
- 239000008194 pharmaceutical composition Substances 0.000 title claims abstract 26
- 230000001225 therapeutic effect Effects 0.000 title 1
- 150000003839 salts Chemical class 0.000 claims abstract 15
- 239000000203 mixture Substances 0.000 claims abstract 5
- 230000000694 effects Effects 0.000 claims abstract 4
- 230000036772 blood pressure Effects 0.000 claims abstract 3
- 229940123955 Alpha adrenoreceptor agonist Drugs 0.000 claims abstract 2
- 239000003814 drug Substances 0.000 claims abstract 2
- 229960005192 methoxamine Drugs 0.000 claims 37
- WJAJPNHVVFWKKL-UHFFFAOYSA-N Methoxamine Chemical compound COC1=CC=C(OC)C(C(O)C(C)N)=C1 WJAJPNHVVFWKKL-UHFFFAOYSA-N 0.000 claims 7
- 210000002255 anal canal Anatomy 0.000 claims 6
- 210000000436 anus Anatomy 0.000 claims 6
- 230000000699 topical effect Effects 0.000 claims 6
- 125000003277 amino group Chemical group 0.000 claims 4
- 210000005070 sphincter Anatomy 0.000 claims 4
- 208000034347 Faecal incontinence Diseases 0.000 claims 3
- 229960003767 alanine Drugs 0.000 claims 3
- 150000001875 compounds Chemical class 0.000 claims 3
- VSMILISQVUOHED-ZETCQYMHSA-N (2s)-2-amino-1-(2,5-dimethoxyphenyl)propan-1-one Chemical compound COC1=CC=C(OC)C(C(=O)[C@H](C)N)=C1 VSMILISQVUOHED-ZETCQYMHSA-N 0.000 claims 2
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical group CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 claims 2
- MZRVEZGGRBJDDB-UHFFFAOYSA-N N-Butyllithium Chemical compound [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 claims 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 claims 2
- 239000004480 active ingredient Substances 0.000 claims 2
- 239000000695 adrenergic alpha-agonist Substances 0.000 claims 2
- 239000000443 aerosol Substances 0.000 claims 2
- 210000001217 buttock Anatomy 0.000 claims 2
- 238000006243 chemical reaction Methods 0.000 claims 2
- 210000001072 colon Anatomy 0.000 claims 2
- 239000006071 cream Substances 0.000 claims 2
- QKIUAMUSENSFQQ-UHFFFAOYSA-N dimethylazanide Chemical compound C[N-]C QKIUAMUSENSFQQ-UHFFFAOYSA-N 0.000 claims 2
- 239000002552 dosage form Substances 0.000 claims 2
- 239000000499 gel Substances 0.000 claims 2
- 210000005072 internal anal sphincter Anatomy 0.000 claims 2
- 239000002674 ointment Substances 0.000 claims 2
- WJAJPNHVVFWKKL-CPCISQLKSA-N (1r,2s)-2-amino-1-(2,5-dimethoxyphenyl)propan-1-ol Chemical compound COC1=CC=C(OC)C([C@@H](O)[C@H](C)N)=C1 WJAJPNHVVFWKKL-CPCISQLKSA-N 0.000 claims 1
- DWCGNRKFLRLWCJ-UHFFFAOYSA-N 2-bromo-1,4-dimethoxybenzene Chemical compound COC1=CC=C(OC)C(Br)=C1 DWCGNRKFLRLWCJ-UHFFFAOYSA-N 0.000 claims 1
- 241000894006 Bacteria Species 0.000 claims 1
- QNAYBMKLOCPYGJ-UHFFFAOYSA-N D-alpha-Ala Natural products CC([NH3+])C([O-])=O QNAYBMKLOCPYGJ-UHFFFAOYSA-N 0.000 claims 1
- QNAYBMKLOCPYGJ-UWTATZPHSA-N L-Alanine Natural products C[C@@H](N)C(O)=O QNAYBMKLOCPYGJ-UWTATZPHSA-N 0.000 claims 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 claims 1
- 241000124008 Mammalia Species 0.000 claims 1
- 239000004909 Moisturizer Substances 0.000 claims 1
- 239000013543 active substance Substances 0.000 claims 1
- 239000000853 adhesive Substances 0.000 claims 1
- 230000001070 adhesive effect Effects 0.000 claims 1
- 230000015556 catabolic process Effects 0.000 claims 1
- 239000003795 chemical substances by application Substances 0.000 claims 1
- 239000011248 coating agent Substances 0.000 claims 1
- 238000000576 coating method Methods 0.000 claims 1
- 238000001816 cooling Methods 0.000 claims 1
- 238000006731 degradation reaction Methods 0.000 claims 1
- OIKHZBFJHONJJB-UHFFFAOYSA-N dimethyl(phenyl)silicon Chemical compound C[Si](C)C1=CC=CC=C1 OIKHZBFJHONJJB-UHFFFAOYSA-N 0.000 claims 1
- 239000003937 drug carrier Substances 0.000 claims 1
- 239000006260 foam Substances 0.000 claims 1
- 238000011065 in-situ storage Methods 0.000 claims 1
- 230000007794 irritation Effects 0.000 claims 1
- 239000003589 local anesthetic agent Substances 0.000 claims 1
- 238000004519 manufacturing process Methods 0.000 claims 1
- XMJHPCRAQCTCFT-UHFFFAOYSA-N methyl chloroformate Chemical compound COC(Cl)=O XMJHPCRAQCTCFT-UHFFFAOYSA-N 0.000 claims 1
- 230000001333 moisturizer Effects 0.000 claims 1
- 238000007911 parenteral administration Methods 0.000 claims 1
- 239000006072 paste Substances 0.000 claims 1
- 239000003961 penetration enhancing agent Substances 0.000 claims 1
- 238000002360 preparation method Methods 0.000 claims 1
- 125000006239 protecting group Chemical group 0.000 claims 1
- 150000003431 steroids Chemical class 0.000 claims 1
- 239000000556 agonist Substances 0.000 abstract 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract 1
- 210000003608 fece Anatomy 0.000 abstract 1
- 208000013403 hyperactivity Diseases 0.000 abstract 1
- 239000000126 substance Substances 0.000 abstract 1
- 230000009897 systematic effect Effects 0.000 abstract 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/135—Amines having aromatic rings, e.g. ketamine, nortriptyline
- A61K31/137—Arylalkylamines, e.g. amphetamine, epinephrine, salbutamol, ephedrine or methadone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/12—Antidiarrhoeals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/02—Nasal agents, e.g. decongestants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/26—Psychostimulants, e.g. nicotine, cocaine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/08—Mydriatics or cycloplegics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/14—Decongestants or antiallergics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/02—Non-specific cardiovascular stimulants, e.g. drugs for syncope, antihypotensives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/04—Inotropic agents, i.e. stimulants of cardiac contraction; Drugs for heart failure
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C213/00—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C213/00—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton
- C07C213/10—Separation; Purification; Stabilisation; Use of additives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C217/00—Compounds containing amino and etherified hydroxy groups bound to the same carbon skeleton
- C07C217/54—Compounds containing amino and etherified hydroxy groups bound to the same carbon skeleton having etherified hydroxy groups bound to carbon atoms of at least one six-membered aromatic ring and amino groups bound to acyclic carbon atoms or to carbon atoms of rings other than six-membered aromatic rings of the same carbon skeleton
- C07C217/64—Compounds containing amino and etherified hydroxy groups bound to the same carbon skeleton having etherified hydroxy groups bound to carbon atoms of at least one six-membered aromatic ring and amino groups bound to acyclic carbon atoms or to carbon atoms of rings other than six-membered aromatic rings of the same carbon skeleton with amino groups linked to the six-membered aromatic ring, or to the condensed ring system containing that ring, by carbon chains further substituted by singly-bound oxygen atoms
- C07C217/66—Compounds containing amino and etherified hydroxy groups bound to the same carbon skeleton having etherified hydroxy groups bound to carbon atoms of at least one six-membered aromatic ring and amino groups bound to acyclic carbon atoms or to carbon atoms of rings other than six-membered aromatic rings of the same carbon skeleton with amino groups linked to the six-membered aromatic ring, or to the condensed ring system containing that ring, by carbon chains further substituted by singly-bound oxygen atoms with singly-bound oxygen atoms and six-membered aromatic rings bound to the same carbon atom of the carbon chain
- C07C217/70—Compounds containing amino and etherified hydroxy groups bound to the same carbon skeleton having etherified hydroxy groups bound to carbon atoms of at least one six-membered aromatic ring and amino groups bound to acyclic carbon atoms or to carbon atoms of rings other than six-membered aromatic rings of the same carbon skeleton with amino groups linked to the six-membered aromatic ring, or to the condensed ring system containing that ring, by carbon chains further substituted by singly-bound oxygen atoms with singly-bound oxygen atoms and six-membered aromatic rings bound to the same carbon atom of the carbon chain linked by carbon chains having two carbon atoms between the amino groups and the six-membered aromatic ring or the condensed ring system containing that ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/07—Optical isomers
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/55—Design of synthesis routes, e.g. reducing the use of auxiliary or protecting groups
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Ophthalmology & Optometry (AREA)
- Epidemiology (AREA)
- Emergency Medicine (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Hospice & Palliative Care (AREA)
- Psychiatry (AREA)
- Otolaryngology (AREA)
- Pulmonology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicinal Preparation (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0130763.6 | 2001-12-21 | ||
| GBGB0130763.6A GB0130763D0 (en) | 2001-12-21 | 2001-12-21 | Treatment methods |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2004122639A RU2004122639A (ru) | 2005-05-10 |
| RU2332989C2 true RU2332989C2 (ru) | 2008-09-10 |
Family
ID=9928258
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2004122639/15A RU2332989C2 (ru) | 2001-12-21 | 2002-12-20 | Способ получения 1r,2s-метоксамина, его терапевтическое применение (варианты) и фармацевтическая композиция |
Country Status (12)
| Country | Link |
|---|---|
| US (3) | US20050096397A1 (enExample) |
| EP (2) | EP1458370A1 (enExample) |
| JP (3) | JP5486143B2 (enExample) |
| CN (3) | CN101601665A (enExample) |
| AU (1) | AU2002353206B2 (enExample) |
| CA (1) | CA2471046C (enExample) |
| GB (1) | GB0130763D0 (enExample) |
| MX (1) | MXPA04006142A (enExample) |
| PL (1) | PL217404B1 (enExample) |
| RU (1) | RU2332989C2 (enExample) |
| WO (1) | WO2003055474A1 (enExample) |
| ZA (1) | ZA200404813B (enExample) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0130763D0 (en) * | 2001-12-21 | 2002-02-06 | Norgine Res Ltd | Treatment methods |
| GB0501580D0 (en) * | 2005-01-25 | 2005-03-02 | Norgine Europe Bv | Compounds for treating urinary incontinence |
| EP2473056A4 (en) | 2009-09-04 | 2013-02-13 | Glaxosmithkline Llc | CHEMICAL COMPOUNDS |
| ES2630102T3 (es) * | 2011-04-26 | 2017-08-18 | Rdd Pharma Ltd. | Oximetazolina para el tratamiento de trastornos ano-rectales |
| CN102976961B (zh) * | 2012-12-24 | 2014-09-24 | 武汉武药制药有限公司 | 盐酸甲氧明的制备方法 |
| US10708086B2 (en) * | 2016-01-19 | 2020-07-07 | National Instruments Corporation | Channel sounding techniques |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002041920A1 (en) * | 2000-11-23 | 2002-05-30 | Elan Corporation, Plc | Oral pharmaceutical compositions containing cyclodextrins as taste masking agent |
| WO2002080669A2 (en) * | 2001-04-03 | 2002-10-17 | University Of Florida | Use of alpha2-adrenergic receptor agonists and adrenergic inhibitors in reducing defoliation |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1593261A (en) * | 1976-07-23 | 1981-07-15 | Inveresk Res Int | Controlled release suppository |
| JPS60172953A (ja) * | 1984-02-17 | 1985-09-06 | Sagami Chem Res Center | 光学活性β−アミノアルコ−ルの製造方法 |
| FR2647014B1 (fr) * | 1989-05-16 | 1991-08-30 | Paris V Universite | Composition medicamenteuse pour le traitement et la prevention des symptomes de l'insuffisance cardiaque, renfermant un agent alpha-stimulant vasoconstricteur comme principe actif |
| DK339289D0 (da) | 1989-07-07 | 1989-07-07 | Dak Lab As | Farmaceutisk praeparat |
| DK469989D0 (da) * | 1989-09-22 | 1989-09-22 | Bukh Meditec | Farmaceutisk praeparat |
| NL9102142A (nl) * | 1991-12-20 | 1993-07-16 | Dagra Pharma Bv | Zetpillen met vertraagde afgifte en werkwijze voor de bereiding daarvan. |
| US6635678B1 (en) * | 1996-12-23 | 2003-10-21 | S.L.A. Pharma Ag | Pharmaceutical composition for treating fecal incontinence |
| WO1998038587A1 (en) * | 1997-02-26 | 1998-09-03 | Siebel Systems, Inc. | Method of using a cache to determine the visibility to a remote database client of a plurality of database transactions |
| US6539381B1 (en) * | 1999-04-21 | 2003-03-25 | Novell, Inc. | System and method for synchronizing database information |
| GB0130763D0 (en) | 2001-12-21 | 2002-02-06 | Norgine Res Ltd | Treatment methods |
| US7089228B2 (en) * | 2002-04-18 | 2006-08-08 | International Business Machines Corporation | Computer apparatus and method for caching results of a database query |
-
2001
- 2001-12-21 GB GBGB0130763.6A patent/GB0130763D0/en not_active Ceased
-
2002
- 2002-12-20 AU AU2002353206A patent/AU2002353206B2/en not_active Ceased
- 2002-12-20 MX MXPA04006142A patent/MXPA04006142A/es active IP Right Grant
- 2002-12-20 CN CNA2009101594203A patent/CN101601665A/zh active Pending
- 2002-12-20 CN CNA028253248A patent/CN1604773A/zh active Pending
- 2002-12-20 WO PCT/GB2002/005864 patent/WO2003055474A1/en not_active Ceased
- 2002-12-20 EP EP02788224A patent/EP1458370A1/en not_active Withdrawn
- 2002-12-20 CA CA2471046A patent/CA2471046C/en not_active Expired - Fee Related
- 2002-12-20 RU RU2004122639/15A patent/RU2332989C2/ru not_active IP Right Cessation
- 2002-12-20 JP JP2003556052A patent/JP5486143B2/ja not_active Expired - Fee Related
- 2002-12-20 CN CN2011104050612A patent/CN102516101A/zh active Pending
- 2002-12-20 EP EP10177730A patent/EP2275099A1/en not_active Withdrawn
- 2002-12-20 US US10/499,873 patent/US20050096397A1/en not_active Abandoned
- 2002-12-20 PL PL369828A patent/PL217404B1/pl unknown
-
2004
- 2004-06-17 ZA ZA2004/04813A patent/ZA200404813B/en unknown
-
2009
- 2009-09-30 US US12/586,973 patent/US8491931B2/en not_active Expired - Fee Related
-
2011
- 2011-02-28 JP JP2011041076A patent/JP2011105770A/ja active Pending
- 2011-11-04 JP JP2011241937A patent/JP5544535B2/ja not_active Expired - Fee Related
-
2012
- 2012-11-15 US US13/678,131 patent/US8613948B2/en not_active Expired - Fee Related
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002041920A1 (en) * | 2000-11-23 | 2002-05-30 | Elan Corporation, Plc | Oral pharmaceutical compositions containing cyclodextrins as taste masking agent |
| WO2002080669A2 (en) * | 2001-04-03 | 2002-10-17 | University Of Florida | Use of alpha2-adrenergic receptor agonists and adrenergic inhibitors in reducing defoliation |
Non-Patent Citations (2)
| Title |
|---|
| FUJITA et al. Erythro-directive reduction of alpha-substituted alkanones by means of hydrosilanes in acidic media. Journal of organic chemistry (1988), 53 (23), реферат. Patil et al. Steric aspects of adrenergic drugs. V. Beta adrenergic blocking effects of optical isomers of methoxamine and isopropylmethoxamine. J Pharmacol Exp Ther. 1967 Jun; 156(3):445-51. * |
| НИКОЛАЕВ М.П. Учебник фармакологии. - М.: Медгиз, 1948, с.36. * |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2003055474A1 (en) | 2003-07-10 |
| US20100022657A1 (en) | 2010-01-28 |
| GB0130763D0 (en) | 2002-02-06 |
| MXPA04006142A (es) | 2005-03-31 |
| PL369828A1 (en) | 2005-05-02 |
| US20050096397A1 (en) | 2005-05-05 |
| PL217404B1 (pl) | 2014-07-31 |
| JP2005519888A (ja) | 2005-07-07 |
| CA2471046A1 (en) | 2003-07-10 |
| US8613948B2 (en) | 2013-12-24 |
| JP5544535B2 (ja) | 2014-07-09 |
| JP2012025782A (ja) | 2012-02-09 |
| CN101601665A (zh) | 2009-12-16 |
| ZA200404813B (en) | 2005-10-26 |
| EP2275099A1 (en) | 2011-01-19 |
| JP2011105770A (ja) | 2011-06-02 |
| AU2002353206B2 (en) | 2008-09-04 |
| CA2471046C (en) | 2012-02-07 |
| RU2004122639A (ru) | 2005-05-10 |
| CN1604773A (zh) | 2005-04-06 |
| AU2002353206A1 (en) | 2003-07-15 |
| CN102516101A (zh) | 2012-06-27 |
| EP1458370A1 (en) | 2004-09-22 |
| US20130072567A1 (en) | 2013-03-21 |
| JP5486143B2 (ja) | 2014-05-07 |
| US8491931B2 (en) | 2013-07-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2009542657A5 (enExample) | ||
| US20040258740A1 (en) | Transdermal delivery composition | |
| AU2016270753B2 (en) | Compositions for treatment of atrophic vaginitis, peri-and post-menopausal dyspareunia, and/or oophorectomized females and treatment methods therewith | |
| JP2020500915A (ja) | 末梢神経障害性疼痛の治療に使用するための局所作用用フェニトイン | |
| JP2008156346A (ja) | 抗真菌医薬組成物 | |
| RU2332989C2 (ru) | Способ получения 1r,2s-метоксамина, его терапевтическое применение (варианты) и фармацевтическая композиция | |
| AU2003240113B2 (en) | Formulation of nefopam and its use in the treatment of pain | |
| JP2004521150A (ja) | 尿失禁治療用アリール(またはヘテロアリール)アゾリルカルビノール誘導体 | |
| US20080003275A1 (en) | Treatment of Premature Ejaculation | |
| WO2014176417A1 (en) | Topical preparation for bypassing gi tract, delivery of therapeutics, and trans-epithelial drug delivery system | |
| WO2023187116A1 (en) | Mirabegron formulation | |
| BE1009851A3 (fr) | Composition pharmaceutique pour le traitement des cephalees et son utilisation. | |
| CA2874961C (en) | Antispasmodic 1,2-diols and 1,2,3-triols | |
| JP4974526B2 (ja) | カンジダ症の予防又は治療用組成物 | |
| RU2830586C1 (ru) | Композиции на основе диклофенака для местного применения и связанные с ними способы | |
| KR20130040862A (ko) | 과민성 대장 증후군의 치료를 위한 방법 및 조성물 | |
| AU2005100183A4 (en) | Treatment of premature ejaculation | |
| JPS597115A (ja) | 消炎鎮痛外用剤 | |
| TWI284531B (en) | External preparation for skin diseases containing nitroimidazole | |
| WO2005007155A1 (ja) | 医薬組成物 | |
| KR20250068718A (ko) | 피부 방사선 손상의 예방 및 치료를 위한 조성물 및 방법 | |
| JP2006036687A (ja) | トラマドール含有外用医薬組成物 | |
| CN115279360A (zh) | 局部用双氯芬酸组合物和方法 | |
| HK40060739A (en) | Compositions and methods for potentiating derivatives of 4-aminophenols | |
| CN113747888A (zh) | 用于增强4-氨基苯酚衍生物的组合物和方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20141221 |