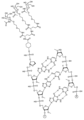
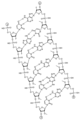
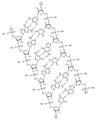
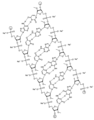
KR20240161202A - 아포지단백질 C-III (APOC3)의 발현을 억제하기 위한 RNAi 작용제 및 조성물 - Google Patents
아포지단백질 C-III (APOC3)의 발현을 억제하기 위한 RNAi 작용제 및 조성물 Download PDFInfo
- Publication number
- KR20240161202A KR20240161202A KR1020247035695A KR20247035695A KR20240161202A KR 20240161202 A KR20240161202 A KR 20240161202A KR 1020247035695 A KR1020247035695 A KR 1020247035695A KR 20247035695 A KR20247035695 A KR 20247035695A KR 20240161202 A KR20240161202 A KR 20240161202A
- Authority
- KR
- South Korea
- Prior art keywords
- apoc3
- rnai agent
- sequence
- antisense strand
- sense strand
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H3/00—Compounds containing only hydrogen atoms and saccharide radicals having only carbon, hydrogen, and oxygen atoms
- C07H3/02—Monosaccharides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7125—Nucleic acids or oligonucleotides having modified internucleoside linkage, i.e. other than 3'-5' phosphodiesters
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/11—Antisense
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering nucleic acids [NA]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/315—Phosphorothioates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/317—Chemical structure of the backbone with an inverted bond, e.g. a cap structure
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/321—2'-O-R Modification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/322—2'-R Modification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/323—Chemical structure of the sugar modified ring structure
- C12N2310/3233—Morpholino-type ring
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/33—Chemical structure of the base
- C12N2310/332—Abasic residue
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/343—Spatial arrangement of the modifications having patterns, e.g. ==--==--==--
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/346—Spatial arrangement of the modifications having a combination of backbone and sugar modifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/35—Nature of the modification
- C12N2310/351—Conjugate
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/35—Nature of the modification
- C12N2310/352—Nature of the modification linked to the nucleic acid via a carbon atom
- C12N2310/3521—Methyl
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/35—Nature of the modification
- C12N2310/353—Nature of the modification linked to the nucleic acid via an atom other than carbon
- C12N2310/3533—Halogen
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Physics & Mathematics (AREA)
- Heart & Thoracic Surgery (AREA)
- Child & Adolescent Psychology (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Cardiology (AREA)
- Vascular Medicine (AREA)
- Biophysics (AREA)
- Urology & Nephrology (AREA)
- Plant Pathology (AREA)
- Microbiology (AREA)
- Epidemiology (AREA)
- Dermatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762556818P | 2017-09-11 | 2017-09-11 | |
| US62/556,818 | 2017-09-11 | ||
| US201862643927P | 2018-03-16 | 2018-03-16 | |
| US62/643,927 | 2018-03-16 | ||
| US201862720434P | 2018-08-21 | 2018-08-21 | |
| US62/720,434 | 2018-08-21 | ||
| PCT/US2018/050248 WO2019051402A1 (en) | 2017-09-11 | 2018-09-10 | RNA INTERFERENCE AGENTS AND COMPOSITIONS FOR INHIBITING THE EXPRESSION OF APOLIPOPROTEIN C-III (APOC3) |
| KR1020207006708A KR102723833B1 (ko) | 2017-09-11 | 2018-09-10 | 아포지단백질 C-III (APOC3)의 발현을 억제하기 위한 RNAi 작용제 및 조성물 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207006708A Division KR102723833B1 (ko) | 2017-09-11 | 2018-09-10 | 아포지단백질 C-III (APOC3)의 발현을 억제하기 위한 RNAi 작용제 및 조성물 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20240161202A true KR20240161202A (ko) | 2024-11-12 |
Family
ID=65630632
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020247035695A Pending KR20240161202A (ko) | 2017-09-11 | 2018-09-10 | 아포지단백질 C-III (APOC3)의 발현을 억제하기 위한 RNAi 작용제 및 조성물 |
| KR1020207006708A Active KR102723833B1 (ko) | 2017-09-11 | 2018-09-10 | 아포지단백질 C-III (APOC3)의 발현을 억제하기 위한 RNAi 작용제 및 조성물 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207006708A Active KR102723833B1 (ko) | 2017-09-11 | 2018-09-10 | 아포지단백질 C-III (APOC3)의 발현을 억제하기 위한 RNAi 작용제 및 조성물 |
Country Status (24)
| Country | Link |
|---|---|
| US (4) | US10597657B2 (enExample) |
| EP (1) | EP3681516A4 (enExample) |
| JP (2) | JP7438103B2 (enExample) |
| KR (2) | KR20240161202A (enExample) |
| CN (2) | CN111107853B (enExample) |
| AU (1) | AU2018329190B2 (enExample) |
| BR (1) | BR112020002413A2 (enExample) |
| CA (1) | CA3074303A1 (enExample) |
| CL (2) | CL2020000593A1 (enExample) |
| CO (1) | CO2020002586A2 (enExample) |
| CR (1) | CR20200108A (enExample) |
| EC (1) | ECSP20017905A (enExample) |
| IL (2) | IL273172B1 (enExample) |
| JO (1) | JOP20200054A1 (enExample) |
| MX (1) | MX2020002648A (enExample) |
| MY (1) | MY202025A (enExample) |
| PE (1) | PE20201283A1 (enExample) |
| PH (1) | PH12020500332A1 (enExample) |
| SG (1) | SG11201912178VA (enExample) |
| TN (1) | TN2020000039A1 (enExample) |
| TW (2) | TW202415389A (enExample) |
| UY (1) | UY37871A (enExample) |
| WO (1) | WO2019051402A1 (enExample) |
| ZA (1) | ZA202100806B (enExample) |
Families Citing this family (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3221451A1 (en) | 2014-11-17 | 2017-09-27 | Alnylam Pharmaceuticals, Inc. | Apolipoprotein c3 (apoc3) irna compositions and methods of use thereof |
| CN109462981B (zh) * | 2016-09-02 | 2023-07-07 | 箭头药业股份有限公司 | 靶向配体 |
| LT3607069T (lt) | 2017-04-05 | 2023-01-10 | Silence Therapeutics Gmbh | Produktai ir kompozicijos |
| SG11201912178VA (en) * | 2017-09-11 | 2020-01-30 | Arrowhead Pharmaceuticals Inc | Rnai agents and compositions for inhibiting expression of apolipoprotein c-iii (apoc3) |
| JP7365052B2 (ja) | 2017-12-01 | 2023-10-19 | スーチョウ リボ ライフ サイエンス カンパニー、リミテッド | 核酸、当該核酸を含む組成物及び複合体ならびに調製方法と使用 |
| US11414661B2 (en) | 2017-12-01 | 2022-08-16 | Suzhou Ribo Life Science Co., Ltd. | Nucleic acid, composition and conjugate containing nucleic acid, preparation method therefor and use thereof |
| MX2022001460A (es) * | 2019-08-05 | 2022-02-22 | Arrowhead Pharmaceuticals Inc | Metodos para el tratamiento de enfermedades y trastornos relacionados con apoc3. |
| AU2021224778A1 (en) * | 2020-02-18 | 2022-09-29 | Alnylam Pharmaceuticals, Inc. | Apolipoprotein C3 (APOC3) iRNA compositions and methods of use thereof |
| CN114480382A (zh) * | 2020-11-12 | 2022-05-13 | 北京大学 | 用于抑制SURF4基因表达的RNAi试剂和组合物 |
| WO2022162155A1 (en) * | 2021-01-30 | 2022-08-04 | E-Therapeutics Plc | Nucleic acids containing abasic nucleotides |
| AU2022299514A1 (en) | 2021-06-24 | 2024-01-18 | Sirnaomics, Inc. | Products and compositions |
| CA3225015A1 (en) * | 2021-07-16 | 2023-01-19 | Zicai Liang | Nucleic acid, composition and conjugate containing same, and preparation method and use |
| JP2024537251A (ja) * | 2021-10-08 | 2024-10-10 | イー-セラピューティクス・ピーエルシー | 脱塩基ヌクレオシドを含有する核酸 |
| TW202330920A (zh) | 2021-12-01 | 2023-08-01 | 美商戴瑟納製藥股份有限公司 | 用於調節apoc3表現之組合物及方法 |
| WO2023138659A1 (zh) * | 2022-01-20 | 2023-07-27 | 上海拓界生物医药科技有限公司 | 一种dsRNA、其应用及制备方法 |
| TW202345865A (zh) * | 2022-01-24 | 2023-12-01 | 大陸商上海舶望製藥有限公司 | 抑制LPA(Apo(a))蛋白表達的組合物和方法 |
| CN116917477B (zh) | 2022-01-30 | 2024-04-09 | 大睿生物医药科技(上海)有限公司 | 含有n-乙酰半乳糖胺的靶向配体 |
| EP4541893A1 (en) | 2022-06-14 | 2025-04-23 | Rona Bioscience, Limited | Sirna molecule for regulating pcsk9 gene activity |
| AU2023299696A1 (en) * | 2022-06-27 | 2025-01-23 | Rona Bioscience, Limited | Sirna for inhibiting apolipoprotein c3 expression |
| EP4555088A1 (en) * | 2022-07-11 | 2025-05-21 | Sanegene Bio USA Inc. | Optimized 2'- modified ribose derivatives and methods of use |
| WO2024023254A1 (en) * | 2022-07-27 | 2024-02-01 | E-Therapeutics Plc | Nucleic acid compounds |
| US20250368992A1 (en) * | 2022-07-27 | 2025-12-04 | E-Therapeutics Plc | Nucleic acid compounds |
| AU2023313321A1 (en) * | 2022-07-27 | 2025-01-09 | E-Therapeutics Plc | Nucleic acid compounds |
| CN116814621B (zh) * | 2022-08-05 | 2025-09-09 | 厦门甘宝利生物医药有限公司 | 一种抑制apoc3基因表达的rna抑制剂及其应用 |
| WO2024032680A1 (zh) * | 2022-08-11 | 2024-02-15 | 益杰立科(上海)生物科技有限公司 | 一种表观编辑靶点的方法及用途 |
| WO2024165571A2 (en) * | 2023-02-06 | 2024-08-15 | E-Therapeutics Plc | Inhibitors of expression and/or function |
| AU2024218976A1 (en) * | 2023-02-06 | 2025-09-11 | E-Therapeutics Plc | Inhibitors of expression and/or function |
| WO2024188173A1 (zh) * | 2023-03-10 | 2024-09-19 | 苏州瑞博生物技术股份有限公司 | 一种核酸、含有该核酸的组合物与缀合物及其用途 |
| US20250243487A1 (en) | 2023-09-14 | 2025-07-31 | Ionis Pharmaceuticals, Inc. | Compounds and Methods for Reducing APOCIII Expression |
| WO2025087442A1 (zh) * | 2023-10-27 | 2025-05-01 | 北京安龙生物医药有限公司 | 靶向脂蛋白a的寡核苷酸及其用途 |
| WO2025125576A2 (en) * | 2023-12-15 | 2025-06-19 | E-Therapeutics Plc | Inhibitors of expression and/or function |
| WO2025133348A1 (en) * | 2023-12-22 | 2025-06-26 | E-Therapeutics Plc | Inhibitors of expression and/or function |
| WO2025184301A1 (en) * | 2024-02-29 | 2025-09-04 | Arrowhead Pharmaceuticals, Inc. | Rnai agents for dual inhibition of expression of apolipoprotein c-iii (apoc3) and proprotein convertase subtilisin kexin 9 (pcsk9), compositions thereof, and methods of use |
Family Cites Families (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4522811A (en) | 1982-07-08 | 1985-06-11 | Syntex (U.S.A.) Inc. | Serial injection of muramyldipeptides and liposomes enhances the anti-infective activity of muramyldipeptides |
| US5030453A (en) | 1983-03-24 | 1991-07-09 | The Liposome Company, Inc. | Stable plurilamellar vesicles |
| NL9201440A (nl) | 1992-08-11 | 1994-03-01 | Univ Leiden | Triantennaire clusterglycosiden, hun bereiding en toepassing. |
| US5998203A (en) | 1996-04-16 | 1999-12-07 | Ribozyme Pharmaceuticals, Inc. | Enzymatic nucleic acids containing 5'-and/or 3'-cap structures |
| US6977244B2 (en) | 1996-10-04 | 2005-12-20 | Board Of Regents, The University Of Texas Systems | Inhibition of Bcl-2 protein expression by liposomal antisense oligodeoxynucleotides |
| US5891467A (en) | 1997-01-31 | 1999-04-06 | Depotech Corporation | Method for utilizing neutral lipids to modify in vivo release from multivesicular liposomes |
| WO2000044914A1 (en) | 1999-01-28 | 2000-08-03 | Medical College Of Georgia Research Institute, Inc. | Composition and method for in vivo and in vitro attenuation of gene expression using double stranded rna |
| DE19956568A1 (de) | 1999-01-30 | 2000-08-17 | Roland Kreutzer | Verfahren und Medikament zur Hemmung der Expression eines vorgegebenen Gens |
| EP1159441B8 (en) | 1999-03-10 | 2008-10-29 | Marie Curie Cancer Care | Delivery of nucleic acids and proteins to cells |
| AU2001245793A1 (en) | 2000-03-16 | 2001-09-24 | Cold Spring Harbor Laboratory | Methods and compositions for rna interference |
| US6680068B2 (en) | 2000-07-06 | 2004-01-20 | The General Hospital Corporation | Drug delivery formulations and targeting |
| RU2322500C2 (ru) | 2000-12-01 | 2008-04-20 | Макс-Планк-Гезелльшафт Цур Фердерунг Дер Виссеншафтен Е.Ф. | Малые молекулы рнк, опосредующие интерференцию рнк |
| EP1399189A1 (en) | 2001-06-11 | 2004-03-24 | Universite De Montreal | Compositions and methods for enhancing nucleic acid transfer into cells |
| AU2002324723B2 (en) | 2001-08-16 | 2007-10-25 | The Trustees Of The University Of Pennsylvania | Synthesis and use of reagents for improved DNA lipofection and/or slow release pro-drug and drug therapies |
| US7901708B2 (en) | 2002-06-28 | 2011-03-08 | Protiva Biotherapeutics, Inc. | Liposomal apparatus and manufacturing methods |
| AU2003279004B2 (en) | 2002-09-28 | 2009-10-08 | Massachusetts Institute Of Technology | Influenza therapeutic |
| DK2284266T3 (da) | 2002-11-14 | 2014-01-13 | Thermo Fisher Scient Biosciences Inc | sIRNA-MOLEKYLE MOD TP53 |
| US20040204377A1 (en) | 2002-11-26 | 2004-10-14 | University Of Massachusetts | Delivery of siRNAs |
| US20040208921A1 (en) | 2003-01-14 | 2004-10-21 | Ho Rodney J. Y. | Lipid-drug formulations and methods for targeted delivery of lipid-drug complexes to lymphoid tissues |
| US7598227B2 (en) * | 2003-04-16 | 2009-10-06 | Isis Pharmaceuticals Inc. | Modulation of apolipoprotein C-III expression |
| SG190613A1 (en) | 2003-07-16 | 2013-06-28 | Protiva Biotherapeutics Inc | Lipid encapsulated interfering rna |
| CN1845993B (zh) | 2003-08-28 | 2010-06-23 | 诺瓦提斯公司 | 具有平端和3'修饰的干扰rna双链体 |
| AU2005248147A1 (en) | 2004-05-11 | 2005-12-08 | Alphagen Co., Ltd. | Polynucleotides for causing RNA interference and method for inhibiting gene expression using the same |
| US8101741B2 (en) | 2005-11-02 | 2012-01-24 | Protiva Biotherapeutics, Inc. | Modified siRNA molecules and uses thereof |
| WO2007107162A2 (en) | 2006-03-23 | 2007-09-27 | Santaris Pharma A/S | Small internally segmented interfering rna |
| GB0608838D0 (en) | 2006-05-04 | 2006-06-14 | Novartis Ag | Organic compounds |
| BRPI0715375A2 (pt) | 2006-08-18 | 2013-06-18 | Hoffmann La Roche | policonjugados para distribuiÇço in vivo de polinucleotÍdeos |
| BRPI0811170B8 (pt) | 2007-05-22 | 2021-05-25 | Arcturus Therapeutics Inc | oligonucleotídeos de rna e complexos de rna substituídos por hidroximetila |
| US7615374B2 (en) | 2007-09-25 | 2009-11-10 | Wisconsin Alumni Research Foundation | Generation of clonal mesenchymal progenitors and mesenchymal stem cell lines under serum-free conditions |
| CA2710713C (en) | 2007-12-27 | 2017-09-19 | Protiva Biotherapeutics, Inc. | Silencing of polo-like kinase expression using interfering rna |
| US8188060B2 (en) | 2008-02-11 | 2012-05-29 | Dharmacon, Inc. | Duplex oligonucleotides with enhanced functionality in gene regulation |
| WO2010083615A1 (en) * | 2009-01-26 | 2010-07-29 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing apolipoprotein c-iii expression |
| EP3721943A1 (en) | 2009-12-23 | 2020-10-14 | Novartis AG | Lipids, lipid compositions and methods of using them |
| ES2562817T3 (es) | 2010-02-24 | 2016-03-08 | Arrowhead Research Corporation | Composiciones para el suministro dirigido de ARNip |
| US8501930B2 (en) | 2010-12-17 | 2013-08-06 | Arrowhead Madison Inc. | Peptide-based in vivo siRNA delivery system |
| MX344807B (es) | 2011-06-21 | 2017-01-09 | Alnylam Pharmaceuticals Inc | Composiciones y metodos para inhibicion de genes para alipoproteina c-iii (apoc3). |
| MX2014001971A (es) | 2011-08-26 | 2014-03-31 | Arrowhead Res Corp | Polimeros de poli(vinilester) para suministro de acido nucleico in vivo. |
| MX2014001965A (es) | 2012-04-18 | 2014-03-31 | Arrowhead Res Corp | Polimeros de poli(acrilato) para suministro de acido nucleico in vivo. |
| EP3919620A1 (en) * | 2012-05-02 | 2021-12-08 | Sirna Therapeutics, Inc. | Short interfering nucleic acid (sina) compositions |
| AU2014284152B2 (en) * | 2013-06-21 | 2020-01-23 | Ionis Pharmaceuticals, Inc. | Compositions and methods for modulation of target nucleic acids |
| WO2015051366A2 (en) | 2013-10-04 | 2015-04-09 | Novartis Ag | Novel formats for organic compounds for use in rna interference |
| WO2016011123A1 (en) * | 2014-07-16 | 2016-01-21 | Arrowhead Research Corporation | Organic compositions to treat apoc3-related diseases |
| EP3221451A1 (en) * | 2014-11-17 | 2017-09-27 | Alnylam Pharmaceuticals, Inc. | Apolipoprotein c3 (apoc3) irna compositions and methods of use thereof |
| US20160272970A1 (en) * | 2015-03-17 | 2016-09-22 | Arrowhead Madison Inc. | RNA Interference Agents |
| JOP20210043A1 (ar) * | 2015-10-01 | 2017-06-16 | Arrowhead Pharmaceuticals Inc | تراكيب وأساليب لتثبيط تعبير جيني للـ lpa |
| CN109069529B (zh) | 2016-03-07 | 2021-08-20 | 箭头药业股份有限公司 | 用于治疗性化合物的靶向配体 |
| CN109462981B (zh) | 2016-09-02 | 2023-07-07 | 箭头药业股份有限公司 | 靶向配体 |
| WO2018223056A1 (en) * | 2017-06-02 | 2018-12-06 | Wave Life Sciences Ltd. | Oligonucleotide compositions and methods of use thereof |
| SG11201912178VA (en) * | 2017-09-11 | 2020-01-30 | Arrowhead Pharmaceuticals Inc | Rnai agents and compositions for inhibiting expression of apolipoprotein c-iii (apoc3) |
-
2018
- 2018-09-10 SG SG11201912178VA patent/SG11201912178VA/en unknown
- 2018-09-10 US US16/126,740 patent/US10597657B2/en active Active
- 2018-09-10 CN CN201880059004.3A patent/CN111107853B/zh active Active
- 2018-09-10 EP EP18853495.2A patent/EP3681516A4/en active Pending
- 2018-09-10 MX MX2020002648A patent/MX2020002648A/es unknown
- 2018-09-10 CR CR20200108A patent/CR20200108A/es unknown
- 2018-09-10 PE PE2020000340A patent/PE20201283A1/es unknown
- 2018-09-10 WO PCT/US2018/050248 patent/WO2019051402A1/en not_active Ceased
- 2018-09-10 JO JOP/2020/0054A patent/JOP20200054A1/ar unknown
- 2018-09-10 CN CN202410900721.1A patent/CN118879696A/zh active Pending
- 2018-09-10 JP JP2020514257A patent/JP7438103B2/ja active Active
- 2018-09-10 CA CA3074303A patent/CA3074303A1/en active Pending
- 2018-09-10 KR KR1020247035695A patent/KR20240161202A/ko active Pending
- 2018-09-10 AU AU2018329190A patent/AU2018329190B2/en active Active
- 2018-09-10 TN TNP/2020/000039A patent/TN2020000039A1/en unknown
- 2018-09-10 KR KR1020207006708A patent/KR102723833B1/ko active Active
- 2018-09-10 BR BR112020002413-9A patent/BR112020002413A2/pt unknown
- 2018-09-10 MY MYPI2020001153A patent/MY202025A/en unknown
- 2018-09-11 UY UY0001037871A patent/UY37871A/es active IP Right Grant
- 2018-09-11 TW TW112125927A patent/TW202415389A/zh unknown
- 2018-09-11 TW TW107131910A patent/TWI811238B/zh active
-
2020
- 2020-01-31 US US16/778,188 patent/US11214801B2/en active Active
- 2020-02-13 PH PH12020500332A patent/PH12020500332A1/en unknown
- 2020-03-04 CO CONC2020/0002586A patent/CO2020002586A2/es unknown
- 2020-03-09 CL CL2020000593A patent/CL2020000593A1/es unknown
- 2020-03-09 IL IL273172A patent/IL273172B1/en unknown
- 2020-03-11 EC ECSENADI202017905A patent/ECSP20017905A/es unknown
-
2021
- 2021-02-05 ZA ZA2021/00806A patent/ZA202100806B/en unknown
- 2021-11-18 US US17/529,364 patent/US20220064646A1/en not_active Abandoned
-
2022
- 2022-12-22 US US18/145,218 patent/US12365899B2/en active Active
-
2023
- 2023-03-28 JP JP2023051640A patent/JP2023073358A/ja active Pending
-
2024
- 2024-11-13 CL CL2024003470A patent/CL2024003470A1/es unknown
-
2025
- 2025-10-26 IL IL324223A patent/IL324223A/en unknown
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102723833B1 (ko) | 아포지단백질 C-III (APOC3)의 발현을 억제하기 위한 RNAi 작용제 및 조성물 | |
| JP7660154B2 (ja) | アンジオポエチン様3(ANGPTL3)の発現を阻害するためのRNAi剤および組成物、ならびに使用方法 | |
| JP7741927B2 (ja) | 17β-HSD13型(HSD17B13)の発現を阻害するためのRNAi剤、その組成物、および使用方法 | |
| JP2024086876A (ja) | アシアロ糖タンパク質受容体1の発現を阻害するためのRNAi剤および組成物 | |
| JP2025186315A (ja) | 17β-HSD13型(HSD17B13)の発現を阻害するためのRNAi剤、その組成物、および使用方法 | |
| TW202444904A (zh) | 用於抑制粒線體醯胺肟還原組分1(MARC1)之表現之RNAi藥劑、其醫藥組合物及使用方法 | |
| OA19513A (en) | RNAI agents and compositions for inhibiting expression of apolipoprotein C-lll (APOC3). | |
| EA047975B1 (ru) | РНКи АГЕНТЫ ДЛЯ ИНГИБИРОВАНИЯ ЭКСПРЕССИИ 17β-HSD ТИПА 13 (HSD17B13), ИХ КОМПОЗИЦИИ И СПОСОБЫ ПРИМЕНЕНИЯ | |
| EA048423B1 (ru) | РНКи-АГЕНТЫ И КОМПОЗИЦИИ ДЛЯ ИНГИБИРОВАНИЯ ЭКСПРЕССИИ АПОЛИПОПРОТЕИНА C-III (APOC3) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
St.27 status event code: A-0-1-A10-A18-div-PA0104 St.27 status event code: A-0-1-A10-A16-div-PA0104 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| A201 | Request for examination | ||
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |