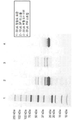
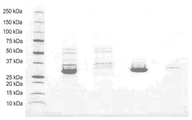
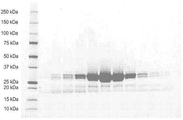
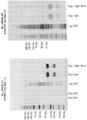
KR20180048731A - 재조합 폴리펩티드를 제조하기 위한 fkpa의 정제 및 그의 용도 - Google Patents
재조합 폴리펩티드를 제조하기 위한 fkpa의 정제 및 그의 용도 Download PDFInfo
- Publication number
- KR20180048731A KR20180048731A KR1020187007920A KR20187007920A KR20180048731A KR 20180048731 A KR20180048731 A KR 20180048731A KR 1020187007920 A KR1020187007920 A KR 1020187007920A KR 20187007920 A KR20187007920 A KR 20187007920A KR 20180048731 A KR20180048731 A KR 20180048731A
- Authority
- KR
- South Korea
- Prior art keywords
- fkpa
- antibody
- seq
- composition
- amino acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 428
- 102000004196 processed proteins & peptides Human genes 0.000 title claims abstract description 424
- 229920001184 polypeptide Polymers 0.000 title claims abstract description 423
- 238000000746 purification Methods 0.000 title claims description 36
- 238000000034 method Methods 0.000 claims abstract description 499
- 239000000203 mixture Substances 0.000 claims abstract description 288
- 238000004191 hydrophobic interaction chromatography Methods 0.000 claims abstract description 143
- 239000012535 impurity Substances 0.000 claims abstract description 89
- 238000012434 mixed-mode chromatography Methods 0.000 claims abstract description 36
- 238000003018 immunoassay Methods 0.000 claims abstract description 33
- 238000004519 manufacturing process Methods 0.000 claims abstract description 25
- 238000001042 affinity chromatography Methods 0.000 claims abstract description 23
- 241000894006 Bacteria Species 0.000 claims abstract description 15
- 108010006519 Molecular Chaperones Proteins 0.000 claims abstract description 15
- 102000005431 Molecular Chaperones Human genes 0.000 claims abstract description 8
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 335
- 230000027455 binding Effects 0.000 claims description 204
- 238000009739 binding Methods 0.000 claims description 203
- 210000004027 cell Anatomy 0.000 claims description 183
- 108090000623 proteins and genes Proteins 0.000 claims description 183
- 102000004169 proteins and genes Human genes 0.000 claims description 174
- 239000000463 material Substances 0.000 claims description 125
- 238000004587 chromatography analysis Methods 0.000 claims description 117
- 239000000427 antigen Substances 0.000 claims description 92
- 108091007433 antigens Proteins 0.000 claims description 91
- 102000036639 antigens Human genes 0.000 claims description 91
- 239000006167 equilibration buffer Substances 0.000 claims description 75
- 241000588724 Escherichia coli Species 0.000 claims description 71
- 238000005349 anion exchange Methods 0.000 claims description 69
- 239000012160 loading buffer Substances 0.000 claims description 68
- 239000000047 product Substances 0.000 claims description 68
- 150000007523 nucleic acids Chemical class 0.000 claims description 65
- 238000005341 cation exchange Methods 0.000 claims description 62
- 238000001542 size-exclusion chromatography Methods 0.000 claims description 62
- 238000010828 elution Methods 0.000 claims description 59
- 102000039446 nucleic acids Human genes 0.000 claims description 56
- 108020004707 nucleic acids Proteins 0.000 claims description 56
- 238000005571 anion exchange chromatography Methods 0.000 claims description 55
- 239000000872 buffer Substances 0.000 claims description 53
- 239000000523 sample Substances 0.000 claims description 52
- 239000011534 wash buffer Substances 0.000 claims description 52
- 229910052588 hydroxylapatite Inorganic materials 0.000 claims description 48
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 claims description 48
- 238000001514 detection method Methods 0.000 claims description 45
- 238000005277 cation exchange chromatography Methods 0.000 claims description 44
- 239000012149 elution buffer Substances 0.000 claims description 44
- 238000002360 preparation method Methods 0.000 claims description 41
- 241000282414 Homo sapiens Species 0.000 claims description 39
- 239000012634 fragment Substances 0.000 claims description 38
- 108090000176 Interleukin-13 Proteins 0.000 claims description 37
- 102000003816 Interleukin-13 Human genes 0.000 claims description 37
- 102000008394 Immunoglobulin Fragments Human genes 0.000 claims description 36
- 108010021625 Immunoglobulin Fragments Proteins 0.000 claims description 36
- 229920002684 Sepharose Polymers 0.000 claims description 36
- 238000003556 assay Methods 0.000 claims description 36
- -1 sulfopropyl group Chemical group 0.000 claims description 35
- 101000998146 Homo sapiens Interleukin-17A Proteins 0.000 claims description 33
- 102100033461 Interleukin-17A Human genes 0.000 claims description 33
- 235000002639 sodium chloride Nutrition 0.000 claims description 33
- 229920000936 Agarose Polymers 0.000 claims description 31
- 238000002156 mixing Methods 0.000 claims description 31
- 239000013592 cell lysate Substances 0.000 claims description 28
- 230000001580 bacterial effect Effects 0.000 claims description 27
- 230000014509 gene expression Effects 0.000 claims description 26
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 24
- 241001465754 Metazoa Species 0.000 claims description 22
- 238000011068 loading method Methods 0.000 claims description 22
- 230000002209 hydrophobic effect Effects 0.000 claims description 21
- 150000003839 salts Chemical class 0.000 claims description 21
- 239000003480 eluent Substances 0.000 claims description 20
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 claims description 18
- 239000001632 sodium acetate Substances 0.000 claims description 18
- 235000017281 sodium acetate Nutrition 0.000 claims description 18
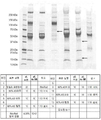
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 claims description 18
- 229940127121 immunoconjugate Drugs 0.000 claims description 17
- 239000012534 cell culture medium component Substances 0.000 claims description 16
- 230000003053 immunization Effects 0.000 claims description 16
- 230000003993 interaction Effects 0.000 claims description 15
- 210000002966 serum Anatomy 0.000 claims description 15
- 229920002873 Polyethylenimine Polymers 0.000 claims description 14
- 230000010412 perfusion Effects 0.000 claims description 14
- 238000004128 high performance liquid chromatography Methods 0.000 claims description 13
- 241000894007 species Species 0.000 claims description 13
- 238000000108 ultra-filtration Methods 0.000 claims description 13
- 241000283973 Oryctolagus cuniculus Species 0.000 claims description 12
- 239000007983 Tris buffer Substances 0.000 claims description 12
- 239000011780 sodium chloride Substances 0.000 claims description 12
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 claims description 12
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 claims description 11
- 239000008194 pharmaceutical composition Substances 0.000 claims description 11
- 230000008569 process Effects 0.000 claims description 11
- 239000000243 solution Substances 0.000 claims description 11
- 229920002307 Dextran Polymers 0.000 claims description 10
- 101100396718 Homo sapiens IL17A gene Proteins 0.000 claims description 10
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 10
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 10
- 239000012619 Butyl Sepharose® Substances 0.000 claims description 9
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 claims description 9
- 229910052921 ammonium sulfate Inorganic materials 0.000 claims description 9
- 235000011130 ammonium sulphate Nutrition 0.000 claims description 9
- 239000012501 chromatography medium Substances 0.000 claims description 9
- 230000001143 conditioned effect Effects 0.000 claims description 9
- 230000007717 exclusion Effects 0.000 claims description 9
- 230000001965 increasing effect Effects 0.000 claims description 9
- 238000000926 separation method Methods 0.000 claims description 9
- 239000001488 sodium phosphate Substances 0.000 claims description 9
- 229910000162 sodium phosphate Inorganic materials 0.000 claims description 9
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 claims description 9
- 101000998151 Homo sapiens Interleukin-17F Proteins 0.000 claims description 8
- 102100033454 Interleukin-17F Human genes 0.000 claims description 8
- 241000699666 Mus <mouse, genus> Species 0.000 claims description 8
- 239000008363 phosphate buffer Substances 0.000 claims description 8
- 239000012264 purified product Substances 0.000 claims description 8
- 238000001262 western blot Methods 0.000 claims description 8
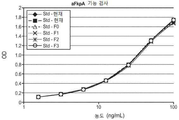
- 238000002965 ELISA Methods 0.000 claims description 7
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 claims description 7
- 241000700605 Viruses Species 0.000 claims description 7
- 150000001732 carboxylic acid derivatives Chemical group 0.000 claims description 7
- 150000001768 cations Chemical class 0.000 claims description 7
- 238000005119 centrifugation Methods 0.000 claims description 7
- 239000000919 ceramic Substances 0.000 claims description 7
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 claims description 7
- 229910052938 sodium sulfate Inorganic materials 0.000 claims description 7
- 235000011152 sodium sulphate Nutrition 0.000 claims description 7
- 125000000542 sulfonic acid group Chemical group 0.000 claims description 7
- 239000006228 supernatant Substances 0.000 claims description 7
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 6
- 239000004475 Arginine Substances 0.000 claims description 6
- 229930091371 Fructose Natural products 0.000 claims description 6
- 239000005715 Fructose Substances 0.000 claims description 6
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 claims description 6
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 claims description 6
- 239000003795 chemical substances by application Substances 0.000 claims description 6
- 238000011097 chromatography purification Methods 0.000 claims description 6
- 239000013628 high molecular weight specie Substances 0.000 claims description 6
- 239000006166 lysate Substances 0.000 claims description 6
- 229920000642 polymer Polymers 0.000 claims description 6
- 210000001236 prokaryotic cell Anatomy 0.000 claims description 6
- 238000012360 testing method Methods 0.000 claims description 6
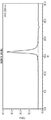
- 238000000954 titration curve Methods 0.000 claims description 6
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 claims description 5
- 241000700159 Rattus Species 0.000 claims description 5
- 230000002378 acidificating effect Effects 0.000 claims description 5
- 239000000539 dimer Substances 0.000 claims description 5
- 238000001502 gel electrophoresis Methods 0.000 claims description 5
- 239000013627 low molecular weight specie Substances 0.000 claims description 5
- SXGZJKUKBWWHRA-UHFFFAOYSA-N 2-(N-morpholiniumyl)ethanesulfonate Chemical compound [O-]S(=O)(=O)CC[NH+]1CCOCC1 SXGZJKUKBWWHRA-UHFFFAOYSA-N 0.000 claims description 4
- DVLFYONBTKHTER-UHFFFAOYSA-N 3-(N-morpholino)propanesulfonic acid Chemical compound OS(=O)(=O)CCCN1CCOCC1 DVLFYONBTKHTER-UHFFFAOYSA-N 0.000 claims description 4
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 4
- 241000700199 Cavia porcellus Species 0.000 claims description 4
- 241000699800 Cricetinae Species 0.000 claims description 4
- 241000283074 Equus asinus Species 0.000 claims description 4
- 241000287828 Gallus gallus Species 0.000 claims description 4
- 108010001336 Horseradish Peroxidase Proteins 0.000 claims description 4
- 229910019142 PO4 Inorganic materials 0.000 claims description 4
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 4
- 150000001875 compounds Chemical class 0.000 claims description 4
- 239000012516 mab select resin Substances 0.000 claims description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 4
- 239000010452 phosphate Substances 0.000 claims description 4
- 241000283707 Capra Species 0.000 claims description 3
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical class CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 claims description 3
- 239000008188 pellet Substances 0.000 claims description 3
- WOUANPHGFPAJCA-UHFFFAOYSA-N 2-[benzyl(methyl)amino]ethanol Chemical compound OCCN(C)CC1=CC=CC=C1 WOUANPHGFPAJCA-UHFFFAOYSA-N 0.000 claims description 2
- 208000002109 Argyria Diseases 0.000 claims description 2
- 101100286681 Homo sapiens IL13 gene Proteins 0.000 claims description 2
- 239000007993 MOPS buffer Substances 0.000 claims description 2
- 125000002091 cationic group Chemical group 0.000 claims description 2
- 238000011026 diafiltration Methods 0.000 claims description 2
- 238000012632 fluorescent imaging Methods 0.000 claims description 2
- 125000003976 glyceryl group Chemical group [H]C([*])([H])C(O[H])([H])C(O[H])([H])[H] 0.000 claims description 2
- 239000000155 melt Substances 0.000 claims description 2
- 239000005373 porous glass Substances 0.000 claims description 2
- 239000013641 positive control Substances 0.000 claims description 2
- 238000012163 sequencing technique Methods 0.000 claims description 2
- 238000012799 strong cation exchange Methods 0.000 claims description 2
- 150000001412 amines Chemical group 0.000 claims 4
- 150000001242 acetic acid derivatives Chemical class 0.000 claims 2
- 239000001166 ammonium sulphate Substances 0.000 claims 2
- 239000012472 biological sample Substances 0.000 claims 2
- 238000000275 quality assurance Methods 0.000 claims 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims 1
- 239000012564 Q sepharose fast flow resin Substances 0.000 claims 1
- 239000000460 chlorine Substances 0.000 claims 1
- 229910052801 chlorine Inorganic materials 0.000 claims 1
- 125000005395 methacrylic acid group Chemical group 0.000 claims 1
- 238000001303 quality assessment method Methods 0.000 claims 1
- 102000004195 Isomerases Human genes 0.000 abstract description 7
- 108090000769 Isomerases Proteins 0.000 abstract description 7
- 239000003124 biologic agent Substances 0.000 abstract 1
- 235000018102 proteins Nutrition 0.000 description 117
- 125000000539 amino acid group Chemical group 0.000 description 41
- 238000006467 substitution reaction Methods 0.000 description 36
- 108060003951 Immunoglobulin Proteins 0.000 description 31
- 102000018358 immunoglobulin Human genes 0.000 description 31
- 235000001014 amino acid Nutrition 0.000 description 30
- 239000013598 vector Substances 0.000 description 27
- 108020004414 DNA Proteins 0.000 description 25
- 229940024606 amino acid Drugs 0.000 description 25
- 150000001413 amino acids Chemical class 0.000 description 24
- 108091033319 polynucleotide Proteins 0.000 description 20
- 102000040430 polynucleotide Human genes 0.000 description 20
- 239000002157 polynucleotide Substances 0.000 description 20
- 239000012505 Superdex™ Substances 0.000 description 18
- 230000000694 effects Effects 0.000 description 18
- 239000004473 Threonine Substances 0.000 description 17
- 230000035772 mutation Effects 0.000 description 17
- 102000005962 receptors Human genes 0.000 description 17
- 108020003175 receptors Proteins 0.000 description 17
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 description 16
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 16
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 16
- 239000000306 component Substances 0.000 description 15
- 239000011347 resin Substances 0.000 description 15
- 229920005989 resin Polymers 0.000 description 15
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 14
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 14
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Chemical compound CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 14
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 14
- 230000004071 biological effect Effects 0.000 description 14
- 210000004408 hybridoma Anatomy 0.000 description 14
- 102000013691 Interleukin-17 Human genes 0.000 description 13
- 108050003558 Interleukin-17 Proteins 0.000 description 13
- 108010076504 Protein Sorting Signals Proteins 0.000 description 13
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 12
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 12
- 230000004927 fusion Effects 0.000 description 12
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 12
- 206010010356 Congenital anomaly Diseases 0.000 description 11
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 11
- 239000013604 expression vector Substances 0.000 description 11
- 239000000126 substance Substances 0.000 description 11
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 10
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 10
- 239000012615 aggregate Substances 0.000 description 10
- 235000004279 alanine Nutrition 0.000 description 10
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 10
- 230000006870 function Effects 0.000 description 10
- 238000000338 in vitro Methods 0.000 description 10
- 108010020062 Peptidylprolyl Isomerase Proteins 0.000 description 9
- 102000009658 Peptidylprolyl Isomerase Human genes 0.000 description 9
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 9
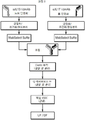
- 238000011210 chromatographic step Methods 0.000 description 9
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 9
- 230000013595 glycosylation Effects 0.000 description 9
- 238000006206 glycosylation reaction Methods 0.000 description 9
- 238000001727 in vivo Methods 0.000 description 9
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 8
- 102000004190 Enzymes Human genes 0.000 description 8
- 108090000790 Enzymes Proteins 0.000 description 8
- 108010087819 Fc receptors Proteins 0.000 description 8
- 102000009109 Fc receptors Human genes 0.000 description 8
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 8
- 108091028043 Nucleic acid sequence Proteins 0.000 description 8
- 206010035226 Plasma cell myeloma Diseases 0.000 description 8
- 235000018417 cysteine Nutrition 0.000 description 8
- 230000001086 cytosolic effect Effects 0.000 description 8
- 229940088598 enzyme Drugs 0.000 description 8
- 238000003780 insertion Methods 0.000 description 8
- 230000037431 insertion Effects 0.000 description 8
- 239000002609 medium Substances 0.000 description 8
- 230000004048 modification Effects 0.000 description 8
- 238000012986 modification Methods 0.000 description 8
- 201000000050 myeloid neoplasm Diseases 0.000 description 8
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 8
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 7
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 7
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 7
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 7
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 7
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 7
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 7
- 239000002253 acid Substances 0.000 description 7
- 125000003277 amino group Chemical group 0.000 description 7
- 150000001720 carbohydrates Chemical group 0.000 description 7
- 239000001963 growth medium Substances 0.000 description 7
- 230000001900 immune effect Effects 0.000 description 7
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 6
- 241001529936 Murinae Species 0.000 description 6
- 239000000556 agonist Substances 0.000 description 6
- 238000012870 ammonium sulfate precipitation Methods 0.000 description 6
- 230000002494 anti-cea effect Effects 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 238000004113 cell culture Methods 0.000 description 6
- 239000003153 chemical reaction reagent Substances 0.000 description 6
- 238000005516 engineering process Methods 0.000 description 6
- 238000000855 fermentation Methods 0.000 description 6
- 230000004151 fermentation Effects 0.000 description 6
- 239000000499 gel Substances 0.000 description 6
- 239000012528 membrane Substances 0.000 description 6
- 210000004379 membrane Anatomy 0.000 description 6
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 6
- YBYRMVIVWMBXKQ-UHFFFAOYSA-N phenylmethanesulfonyl fluoride Chemical compound FS(=O)(=O)CC1=CC=CC=C1 YBYRMVIVWMBXKQ-UHFFFAOYSA-N 0.000 description 6
- 238000010188 recombinant method Methods 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 238000003118 sandwich ELISA Methods 0.000 description 6
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 6
- 239000004474 valine Substances 0.000 description 6
- 108020004705 Codon Proteins 0.000 description 5
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 5
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 5
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 5
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 5
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 5
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 5
- 102000006010 Protein Disulfide-Isomerase Human genes 0.000 description 5
- 241000589516 Pseudomonas Species 0.000 description 5
- 239000005557 antagonist Substances 0.000 description 5
- 235000010633 broth Nutrition 0.000 description 5
- 230000000295 complement effect Effects 0.000 description 5
- 238000012217 deletion Methods 0.000 description 5
- 230000037430 deletion Effects 0.000 description 5
- 230000012010 growth Effects 0.000 description 5
- 238000002649 immunization Methods 0.000 description 5
- 230000016784 immunoglobulin production Effects 0.000 description 5
- 229940072221 immunoglobulins Drugs 0.000 description 5
- 239000003446 ligand Substances 0.000 description 5
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 5
- 239000002245 particle Substances 0.000 description 5
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 5
- 239000013612 plasmid Substances 0.000 description 5
- 238000003752 polymerase chain reaction Methods 0.000 description 5
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 5
- 108020003519 protein disulfide isomerase Proteins 0.000 description 5
- 230000012846 protein folding Effects 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 241000282836 Camelus dromedarius Species 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- 102000013463 Immunoglobulin Light Chains Human genes 0.000 description 4
- 108010065825 Immunoglobulin Light Chains Proteins 0.000 description 4
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 4
- 206010028980 Neoplasm Diseases 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 239000012616 hydrophobic interaction chromatography medium Substances 0.000 description 4
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 4
- 238000002703 mutagenesis Methods 0.000 description 4
- 231100000350 mutagenesis Toxicity 0.000 description 4
- 239000002773 nucleotide Substances 0.000 description 4
- 125000003729 nucleotide group Chemical group 0.000 description 4
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 4
- 239000002953 phosphate buffered saline Substances 0.000 description 4
- 230000035755 proliferation Effects 0.000 description 4
- 239000010979 ruby Substances 0.000 description 4
- 229910001750 ruby Inorganic materials 0.000 description 4
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 230000009466 transformation Effects 0.000 description 4
- 229910052720 vanadium Inorganic materials 0.000 description 4
- 241000251468 Actinopterygii Species 0.000 description 3
- 108091026890 Coding region Proteins 0.000 description 3
- 102000004127 Cytokines Human genes 0.000 description 3
- 108090000695 Cytokines Proteins 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- 239000012541 Fractogel® Substances 0.000 description 3
- 102000039989 IL-17 family Human genes 0.000 description 3
- 108091069193 IL-17 family Proteins 0.000 description 3
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 3
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 3
- 102000015696 Interleukins Human genes 0.000 description 3
- 108010063738 Interleukins Proteins 0.000 description 3
- 239000004472 Lysine Substances 0.000 description 3
- 108010025020 Nerve Growth Factor Proteins 0.000 description 3
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 3
- 108020004511 Recombinant DNA Proteins 0.000 description 3
- 241000607142 Salmonella Species 0.000 description 3
- 241000607768 Shigella Species 0.000 description 3
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 3
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 3
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 3
- 230000004913 activation Effects 0.000 description 3
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 3
- 229960000723 ampicillin Drugs 0.000 description 3
- 230000000890 antigenic effect Effects 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 210000004899 c-terminal region Anatomy 0.000 description 3
- 239000006143 cell culture medium Substances 0.000 description 3
- 230000002759 chromosomal effect Effects 0.000 description 3
- 238000004590 computer program Methods 0.000 description 3
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 3
- 210000000805 cytoplasm Anatomy 0.000 description 3
- 231100000433 cytotoxic Toxicity 0.000 description 3
- 230000001472 cytotoxic effect Effects 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 239000010432 diamond Substances 0.000 description 3
- 101150036810 eco gene Proteins 0.000 description 3
- 239000002158 endotoxin Substances 0.000 description 3
- 230000002255 enzymatic effect Effects 0.000 description 3
- 239000003102 growth factor Substances 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 238000013537 high throughput screening Methods 0.000 description 3
- 229940088597 hormone Drugs 0.000 description 3
- 239000005556 hormone Substances 0.000 description 3
- 229940047122 interleukins Drugs 0.000 description 3
- 150000002500 ions Chemical class 0.000 description 3
- 125000005647 linker group Chemical group 0.000 description 3
- 210000004698 lymphocyte Anatomy 0.000 description 3
- 230000000813 microbial effect Effects 0.000 description 3
- 238000012544 monitoring process Methods 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 235000015097 nutrients Nutrition 0.000 description 3
- 230000002018 overexpression Effects 0.000 description 3
- 238000010647 peptide synthesis reaction Methods 0.000 description 3
- 230000002093 peripheral effect Effects 0.000 description 3
- 235000021317 phosphate Nutrition 0.000 description 3
- 230000005180 public health Effects 0.000 description 3
- 238000011002 quantification Methods 0.000 description 3
- 238000003127 radioimmunoassay Methods 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 239000007790 solid phase Substances 0.000 description 3
- 238000010257 thawing Methods 0.000 description 3
- 238000013518 transcription Methods 0.000 description 3
- 230000035897 transcription Effects 0.000 description 3
- 230000014616 translation Effects 0.000 description 3
- 238000013519 translation Methods 0.000 description 3
- 125000000430 tryptophan group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12 0.000 description 3
- CHRJZRDFSQHIFI-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;styrene Chemical compound C=CC1=CC=CC=C1.C=CC1=CC=CC=C1C=C CHRJZRDFSQHIFI-UHFFFAOYSA-N 0.000 description 2
- 101150090724 3 gene Proteins 0.000 description 2
- 102100022749 Aminopeptidase N Human genes 0.000 description 2
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 2
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 2
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 2
- 235000014469 Bacillus subtilis Nutrition 0.000 description 2
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 2
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 2
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 2
- 229940045513 CTLA4 antagonist Drugs 0.000 description 2
- 241000589876 Campylobacter Species 0.000 description 2
- 241000282693 Cercopithecidae Species 0.000 description 2
- 241000251730 Chondrichthyes Species 0.000 description 2
- 102000007644 Colony-Stimulating Factors Human genes 0.000 description 2
- 108010071942 Colony-Stimulating Factors Proteins 0.000 description 2
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 description 2
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 description 2
- 229920002271 DEAE-Sepharose Polymers 0.000 description 2
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 2
- 102000001301 EGF receptor Human genes 0.000 description 2
- 108060006698 EGF receptor Proteins 0.000 description 2
- 241000305071 Enterobacterales Species 0.000 description 2
- 241000588722 Escherichia Species 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 2
- 229930182566 Gentamicin Natural products 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 108010031186 Glycoside Hydrolases Proteins 0.000 description 2
- 102000005744 Glycoside Hydrolases Human genes 0.000 description 2
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 description 2
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 2
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 2
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 description 2
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 2
- 101000716102 Homo sapiens T-cell surface glycoprotein CD4 Proteins 0.000 description 2
- 101000946843 Homo sapiens T-cell surface glycoprotein CD8 alpha chain Proteins 0.000 description 2
- 108010091358 Hypoxanthine Phosphoribosyltransferase Proteins 0.000 description 2
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 2
- 102100029098 Hypoxanthine-guanine phosphoribosyltransferase Human genes 0.000 description 2
- 108090001061 Insulin Proteins 0.000 description 2
- 102000004877 Insulin Human genes 0.000 description 2
- 108090000723 Insulin-Like Growth Factor I Proteins 0.000 description 2
- 102000004218 Insulin-Like Growth Factor I Human genes 0.000 description 2
- 108010008212 Integrin alpha4beta1 Proteins 0.000 description 2
- 102100025390 Integrin beta-2 Human genes 0.000 description 2
- 125000000510 L-tryptophano group Chemical group [H]C1=C([H])C([H])=C2N([H])C([H])=C(C([H])([H])[C@@]([H])(C(O[H])=O)N([H])[*])C2=C1[H] 0.000 description 2
- 239000012515 MabSelect SuRe Substances 0.000 description 2
- 241000699660 Mus musculus Species 0.000 description 2
- 101100335081 Mus musculus Flt3 gene Proteins 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- 241000588653 Neisseria Species 0.000 description 2
- 102000007072 Nerve Growth Factors Human genes 0.000 description 2
- 101100407828 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) ptr-3 gene Proteins 0.000 description 2
- 230000004989 O-glycosylation Effects 0.000 description 2
- 208000008589 Obesity Diseases 0.000 description 2
- 108020005187 Oligonucleotide Probes Proteins 0.000 description 2
- 102000004316 Oxidoreductases Human genes 0.000 description 2
- 108090000854 Oxidoreductases Proteins 0.000 description 2
- 108010038512 Platelet-Derived Growth Factor Proteins 0.000 description 2
- 102000010780 Platelet-Derived Growth Factor Human genes 0.000 description 2
- 239000004695 Polyether sulfone Substances 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 2
- 101800004937 Protein C Proteins 0.000 description 2
- 102000017975 Protein C Human genes 0.000 description 2
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 2
- 102100029986 Receptor tyrosine-protein kinase erbB-3 Human genes 0.000 description 2
- 101710100969 Receptor tyrosine-protein kinase erbB-3 Proteins 0.000 description 2
- 102100029981 Receptor tyrosine-protein kinase erbB-4 Human genes 0.000 description 2
- 101710100963 Receptor tyrosine-protein kinase erbB-4 Proteins 0.000 description 2
- 101800001700 Saposin-D Proteins 0.000 description 2
- 241000607720 Serratia Species 0.000 description 2
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 2
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 2
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 description 2
- 102100034922 T-cell surface glycoprotein CD8 alpha chain Human genes 0.000 description 2
- 210000001744 T-lymphocyte Anatomy 0.000 description 2
- 108010000499 Thromboplastin Proteins 0.000 description 2
- 102000002262 Thromboplastin Human genes 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- 102100033571 Tissue-type plasminogen activator Human genes 0.000 description 2
- 102400001320 Transforming growth factor alpha Human genes 0.000 description 2
- 101800004564 Transforming growth factor alpha Proteins 0.000 description 2
- 230000021736 acetylation Effects 0.000 description 2
- 238000006640 acetylation reaction Methods 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 239000012190 activator Substances 0.000 description 2
- 238000001261 affinity purification Methods 0.000 description 2
- 238000012867 alanine scanning Methods 0.000 description 2
- 230000000735 allogeneic effect Effects 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 230000003321 amplification Effects 0.000 description 2
- 210000000628 antibody-producing cell Anatomy 0.000 description 2
- 235000009582 asparagine Nutrition 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 description 2
- 235000003704 aspartic acid Nutrition 0.000 description 2
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 2
- 238000013357 binding ELISA Methods 0.000 description 2
- 229960000074 biopharmaceutical Drugs 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 230000006037 cell lysis Effects 0.000 description 2
- 238000010367 cloning Methods 0.000 description 2
- 229940047120 colony stimulating factors Drugs 0.000 description 2
- 230000024203 complement activation Effects 0.000 description 2
- 230000004540 complement-dependent cytotoxicity Effects 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 239000012531 culture fluid Substances 0.000 description 2
- 238000012258 culturing Methods 0.000 description 2
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 2
- 230000000593 degrading effect Effects 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 108020001507 fusion proteins Proteins 0.000 description 2
- 102000037865 fusion proteins Human genes 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- 229960002518 gentamicin Drugs 0.000 description 2
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 2
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 108091008042 inhibitory receptors Proteins 0.000 description 2
- 102000028416 insulin-like growth factor binding Human genes 0.000 description 2
- 108091022911 insulin-like growth factor binding Proteins 0.000 description 2
- 102000006495 integrins Human genes 0.000 description 2
- 108010044426 integrins Proteins 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 238000004811 liquid chromatography Methods 0.000 description 2
- 239000012139 lysis buffer Substances 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 239000003607 modifier Substances 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- 210000000822 natural killer cell Anatomy 0.000 description 2
- 239000003900 neurotrophic factor Substances 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 210000000440 neutrophil Anatomy 0.000 description 2
- 238000003199 nucleic acid amplification method Methods 0.000 description 2
- 235000020824 obesity Nutrition 0.000 description 2
- 239000002751 oligonucleotide probe Substances 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 2
- 210000001322 periplasm Anatomy 0.000 description 2
- 238000002823 phage display Methods 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 238000005424 photoluminescence Methods 0.000 description 2
- 239000013600 plasmid vector Substances 0.000 description 2
- 229920006393 polyether sulfone Polymers 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229960000856 protein c Drugs 0.000 description 2
- 239000008213 purified water Substances 0.000 description 2
- 230000002285 radioactive effect Effects 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 230000000392 somatic effect Effects 0.000 description 2
- 230000009870 specific binding Effects 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 239000013589 supplement Substances 0.000 description 2
- 125000003396 thiol group Chemical group [H]S* 0.000 description 2
- 230000014621 translational initiation Effects 0.000 description 2
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 2
- 241001515965 unidentified phage Species 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- UKAUYVFTDYCKQA-UHFFFAOYSA-N -2-Amino-4-hydroxybutanoic acid Natural products OC(=O)C(N)CCO UKAUYVFTDYCKQA-UHFFFAOYSA-N 0.000 description 1
- 125000003287 1H-imidazol-4-ylmethyl group Chemical group [H]N1C([H])=NC(C([H])([H])[*])=C1[H] 0.000 description 1
- 125000000979 2-amino-2-oxoethyl group Chemical group [H]C([*])([H])C(=O)N([H])[H] 0.000 description 1
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 1
- GUPXYSSGJWIURR-UHFFFAOYSA-N 3-octoxypropane-1,2-diol Chemical compound CCCCCCCCOCC(O)CO GUPXYSSGJWIURR-UHFFFAOYSA-N 0.000 description 1
- TVZGACDUOSZQKY-LBPRGKRZSA-N 4-aminofolic acid Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 TVZGACDUOSZQKY-LBPRGKRZSA-N 0.000 description 1
- 208000030507 AIDS Diseases 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- WQVFQXXBNHHPLX-ZKWXMUAHSA-N Ala-Ala-His Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O WQVFQXXBNHHPLX-ZKWXMUAHSA-N 0.000 description 1
- YYSWCHMLFJLLBJ-ZLUOBGJFSA-N Ala-Ala-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YYSWCHMLFJLLBJ-ZLUOBGJFSA-N 0.000 description 1
- YYAVDNKUWLAFCV-ACZMJKKPSA-N Ala-Ser-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O YYAVDNKUWLAFCV-ACZMJKKPSA-N 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 244000303258 Annona diversifolia Species 0.000 description 1
- 235000002198 Annona diversifolia Nutrition 0.000 description 1
- 108010032595 Antibody Binding Sites Proteins 0.000 description 1
- 241000203069 Archaea Species 0.000 description 1
- PTVGLOCPAVYPFG-CIUDSAMLSA-N Arg-Gln-Asp Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O PTVGLOCPAVYPFG-CIUDSAMLSA-N 0.000 description 1
- PTNFNTOBUDWHNZ-GUBZILKMSA-N Asn-Arg-Met Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(O)=O PTNFNTOBUDWHNZ-GUBZILKMSA-N 0.000 description 1
- MECFLTFREHAZLH-ACZMJKKPSA-N Asn-Glu-Cys Chemical compound C(CC(=O)O)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)N)N MECFLTFREHAZLH-ACZMJKKPSA-N 0.000 description 1
- KHCNTVRVAYCPQE-CIUDSAMLSA-N Asn-Lys-Asn Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O KHCNTVRVAYCPQE-CIUDSAMLSA-N 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 241000726110 Azoarcus Species 0.000 description 1
- 102100038080 B-cell receptor CD22 Human genes 0.000 description 1
- 241000193830 Bacillus <bacterium> Species 0.000 description 1
- 244000063299 Bacillus subtilis Species 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 102000015081 Blood Coagulation Factors Human genes 0.000 description 1
- 108010039209 Blood Coagulation Factors Proteins 0.000 description 1
- 241001453698 Buchnera <proteobacteria> Species 0.000 description 1
- 102100031092 C-C motif chemokine 3 Human genes 0.000 description 1
- 101710155856 C-C motif chemokine 3 Proteins 0.000 description 1
- 102100032367 C-C motif chemokine 5 Human genes 0.000 description 1
- 101150013553 CD40 gene Proteins 0.000 description 1
- 102400000113 Calcitonin Human genes 0.000 description 1
- 108060001064 Calcitonin Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 108010078791 Carrier Proteins Proteins 0.000 description 1
- 108010067225 Cell Adhesion Molecules Proteins 0.000 description 1
- 102000016289 Cell Adhesion Molecules Human genes 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 108010055166 Chemokine CCL5 Proteins 0.000 description 1
- 241000920610 Citrobacter youngae Species 0.000 description 1
- 102100022641 Coagulation factor IX Human genes 0.000 description 1
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 241000699802 Cricetulus griseus Species 0.000 description 1
- 108090000204 Dipeptidase 1 Proteins 0.000 description 1
- 241000255581 Drosophila <fruit fly, genus> Species 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 101001060743 Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) FK506-binding protein 1A Proteins 0.000 description 1
- 241000588914 Enterobacter Species 0.000 description 1
- 241000194033 Enterococcus Species 0.000 description 1
- 101710146739 Enterotoxin Proteins 0.000 description 1
- 241000588698 Erwinia Species 0.000 description 1
- 102000003951 Erythropoietin Human genes 0.000 description 1
- 108090000394 Erythropoietin Proteins 0.000 description 1
- 241001522878 Escherichia coli B Species 0.000 description 1
- 101000867232 Escherichia coli Heat-stable enterotoxin II Proteins 0.000 description 1
- 108010076282 Factor IX Proteins 0.000 description 1
- 108010054218 Factor VIII Proteins 0.000 description 1
- 102000001690 Factor VIII Human genes 0.000 description 1
- 108010021472 Fc gamma receptor IIB Proteins 0.000 description 1
- 102000018233 Fibroblast Growth Factor Human genes 0.000 description 1
- 108050007372 Fibroblast Growth Factor Proteins 0.000 description 1
- 108090000386 Fibroblast Growth Factor 1 Proteins 0.000 description 1
- 102100031706 Fibroblast growth factor 1 Human genes 0.000 description 1
- 102100024785 Fibroblast growth factor 2 Human genes 0.000 description 1
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 description 1
- 102000012673 Follicle Stimulating Hormone Human genes 0.000 description 1
- 108010079345 Follicle Stimulating Hormone Proteins 0.000 description 1
- 210000000712 G cell Anatomy 0.000 description 1
- 241001669573 Galeorhinus galeus Species 0.000 description 1
- 101001027134 Gibberella zeae (strain ATCC MYA-4620 / CBS 123657 / FGSC 9075 / NRRL 31084 / PH-1) FK506-binding protein 1 Proteins 0.000 description 1
- WQWMZOIPXWSZNE-WDSKDSINSA-N Gln-Asp-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O WQWMZOIPXWSZNE-WDSKDSINSA-N 0.000 description 1
- YYOBUPFZLKQUAX-FXQIFTODSA-N Glu-Asn-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O YYOBUPFZLKQUAX-FXQIFTODSA-N 0.000 description 1
- 102400000321 Glucagon Human genes 0.000 description 1
- 108060003199 Glucagon Proteins 0.000 description 1
- 108010024636 Glutathione Proteins 0.000 description 1
- 102000005720 Glutathione transferase Human genes 0.000 description 1
- 108010070675 Glutathione transferase Proteins 0.000 description 1
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000006771 Gonadotropins Human genes 0.000 description 1
- 108010086677 Gonadotropins Proteins 0.000 description 1
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 1
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 1
- 102000004457 Granulocyte-Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 108010051696 Growth Hormone Proteins 0.000 description 1
- 239000000095 Growth Hormone-Releasing Hormone Substances 0.000 description 1
- 241001107136 Gunnera Species 0.000 description 1
- 101100082540 Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd) pcp gene Proteins 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- 101000757160 Homo sapiens Aminopeptidase N Proteins 0.000 description 1
- 101000884305 Homo sapiens B-cell receptor CD22 Proteins 0.000 description 1
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 description 1
- 101000935040 Homo sapiens Integrin beta-2 Proteins 0.000 description 1
- 101000878605 Homo sapiens Low affinity immunoglobulin epsilon Fc receptor Proteins 0.000 description 1
- 101000914053 Homo sapiens Peptidyl-prolyl cis-trans isomerase FKBP2 Proteins 0.000 description 1
- 101000611023 Homo sapiens Tumor necrosis factor receptor superfamily member 6 Proteins 0.000 description 1
- 108010000521 Human Growth Hormone Proteins 0.000 description 1
- 102000002265 Human Growth Hormone Human genes 0.000 description 1
- 239000000854 Human Growth Hormone Substances 0.000 description 1
- 108091006905 Human Serum Albumin Proteins 0.000 description 1
- 102000008100 Human Serum Albumin Human genes 0.000 description 1
- 108010073807 IgG Receptors Proteins 0.000 description 1
- 102100026120 IgG receptor FcRn large subunit p51 Human genes 0.000 description 1
- 101710177940 IgG receptor FcRn large subunit p51 Proteins 0.000 description 1
- IOVUXUSIGXCREV-DKIMLUQUSA-N Ile-Leu-Phe Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 IOVUXUSIGXCREV-DKIMLUQUSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 108010009817 Immunoglobulin Constant Regions Proteins 0.000 description 1
- 102000009786 Immunoglobulin Constant Regions Human genes 0.000 description 1
- 108090001117 Insulin-Like Growth Factor II Proteins 0.000 description 1
- 102000048143 Insulin-Like Growth Factor II Human genes 0.000 description 1
- 102100022338 Integrin alpha-M Human genes 0.000 description 1
- 102100022297 Integrin alpha-X Human genes 0.000 description 1
- 108010064593 Intercellular Adhesion Molecule-1 Proteins 0.000 description 1
- 102100037877 Intercellular adhesion molecule 1 Human genes 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000003996 Interferon-beta Human genes 0.000 description 1
- 108090000467 Interferon-beta Proteins 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 102000003814 Interleukin-10 Human genes 0.000 description 1
- 108090000174 Interleukin-10 Proteins 0.000 description 1
- 102100020793 Interleukin-13 receptor subunit alpha-2 Human genes 0.000 description 1
- 241000588748 Klebsiella Species 0.000 description 1
- 241000588749 Klebsiella oxytoca Species 0.000 description 1
- SNDPXSYFESPGGJ-BYPYZUCNSA-N L-2-aminopentanoic acid Chemical compound CCC[C@H](N)C(O)=O SNDPXSYFESPGGJ-BYPYZUCNSA-N 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- UKAUYVFTDYCKQA-VKHMYHEASA-N L-homoserine Chemical compound OC(=O)[C@@H](N)CCO UKAUYVFTDYCKQA-VKHMYHEASA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- SNDPXSYFESPGGJ-UHFFFAOYSA-N L-norVal-OH Natural products CCCC(N)C(O)=O SNDPXSYFESPGGJ-UHFFFAOYSA-N 0.000 description 1
- VLJNHYLEOZPXFW-BYPYZUCNSA-N L-prolinamide Chemical compound NC(=O)[C@@H]1CCCN1 VLJNHYLEOZPXFW-BYPYZUCNSA-N 0.000 description 1
- 125000000174 L-prolyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(*)=O 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 235000019687 Lamb Nutrition 0.000 description 1
- 102100038007 Low affinity immunoglobulin epsilon Fc receptor Human genes 0.000 description 1
- 102100029205 Low affinity immunoglobulin gamma Fc region receptor II-b Human genes 0.000 description 1
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 description 1
- 241001625930 Luria Species 0.000 description 1
- 102000009151 Luteinizing Hormone Human genes 0.000 description 1
- 108010073521 Luteinizing Hormone Proteins 0.000 description 1
- 108010064548 Lymphocyte Function-Associated Antigen-1 Proteins 0.000 description 1
- 108090000542 Lymphotoxin-alpha Proteins 0.000 description 1
- 102000004083 Lymphotoxin-alpha Human genes 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- 108010046938 Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102000007651 Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 102000009571 Macrophage Inflammatory Proteins Human genes 0.000 description 1
- 108010009474 Macrophage Inflammatory Proteins Proteins 0.000 description 1
- 241001599018 Melanogaster Species 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 101100178822 Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) htrA1 gene Proteins 0.000 description 1
- OVRNDRQMDRJTHS-CBQIKETKSA-N N-Acetyl-D-Galactosamine Chemical compound CC(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@H](O)[C@@H]1O OVRNDRQMDRJTHS-CBQIKETKSA-N 0.000 description 1
- MBLBDJOUHNCFQT-UHFFFAOYSA-N N-acetyl-D-galactosamine Natural products CC(=O)NC(C=O)C(O)C(O)C(O)CO MBLBDJOUHNCFQT-UHFFFAOYSA-N 0.000 description 1
- 230000004988 N-glycosylation Effects 0.000 description 1
- 102000015336 Nerve Growth Factor Human genes 0.000 description 1
- 101000914065 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) FK506-binding protein 2 Proteins 0.000 description 1
- 102100029268 Neurotrophin-3 Human genes 0.000 description 1
- 108010079246 OMPA outer membrane proteins Proteins 0.000 description 1
- 241000238413 Octopus Species 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 241001504519 Papio ursinus Species 0.000 description 1
- 241001057811 Paracoccus <mealybug> Species 0.000 description 1
- 102000003982 Parathyroid hormone Human genes 0.000 description 1
- 108090000445 Parathyroid hormone Proteins 0.000 description 1
- 108010087702 Penicillinase Proteins 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102100026408 Peptidyl-prolyl cis-trans isomerase FKBP2 Human genes 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- WEMYTDDMDBLPMI-DKIMLUQUSA-N Phe-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N WEMYTDDMDBLPMI-DKIMLUQUSA-N 0.000 description 1
- KIQUCMUULDXTAZ-HJOGWXRNSA-N Phe-Tyr-Tyr Chemical compound N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O KIQUCMUULDXTAZ-HJOGWXRNSA-N 0.000 description 1
- 108010001014 Plasminogen Activators Proteins 0.000 description 1
- 102000001938 Plasminogen Activators Human genes 0.000 description 1
- 241000276498 Pollachius virens Species 0.000 description 1
- 108010076181 Proinsulin Proteins 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 241000588769 Proteus <enterobacteria> Species 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 108090000783 Renin Proteins 0.000 description 1
- 102100028255 Renin Human genes 0.000 description 1
- 108020005091 Replication Origin Proteins 0.000 description 1
- 108700008625 Reporter Genes Proteins 0.000 description 1
- 101100277437 Rhizobium meliloti (strain 1021) degP1 gene Proteins 0.000 description 1
- KJTLSVCANCCWHF-UHFFFAOYSA-N Ruthenium Chemical compound [Ru] KJTLSVCANCCWHF-UHFFFAOYSA-N 0.000 description 1
- 241001138501 Salmonella enterica Species 0.000 description 1
- 241000293869 Salmonella enterica subsp. enterica serovar Typhimurium Species 0.000 description 1
- 239000012506 Sephacryl® Substances 0.000 description 1
- 229920005654 Sephadex Polymers 0.000 description 1
- 239000012507 Sephadex™ Substances 0.000 description 1
- QMCDMHWAKMUGJE-IHRRRGAJSA-N Ser-Phe-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C(C)C)C(O)=O QMCDMHWAKMUGJE-IHRRRGAJSA-N 0.000 description 1
- DKGRNFUXVTYRAS-UBHSHLNASA-N Ser-Ser-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O DKGRNFUXVTYRAS-UBHSHLNASA-N 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 241000607766 Shigella boydii Species 0.000 description 1
- 102100022831 Somatoliberin Human genes 0.000 description 1
- 101710142969 Somatoliberin Proteins 0.000 description 1
- 102100038803 Somatotropin Human genes 0.000 description 1
- 108091081024 Start codon Proteins 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 102000019197 Superoxide Dismutase Human genes 0.000 description 1
- 108010012715 Superoxide dismutase Proteins 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- COYHRQWNJDJCNA-NUJDXYNKSA-N Thr-Thr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O COYHRQWNJDJCNA-NUJDXYNKSA-N 0.000 description 1
- 108090000190 Thrombin Proteins 0.000 description 1
- 102000011923 Thyrotropin Human genes 0.000 description 1
- 108010061174 Thyrotropin Proteins 0.000 description 1
- 108090000373 Tissue Plasminogen Activator Proteins 0.000 description 1
- 108050006955 Tissue-type plasminogen activator Proteins 0.000 description 1
- 102000004357 Transferases Human genes 0.000 description 1
- 108090000992 Transferases Proteins 0.000 description 1
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 1
- 102100040403 Tumor necrosis factor receptor superfamily member 6 Human genes 0.000 description 1
- KHPLUFDSWGDRHD-SLFFLAALSA-N Tyr-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N)C(=O)O KHPLUFDSWGDRHD-SLFFLAALSA-N 0.000 description 1
- 102000003990 Urokinase-type plasminogen activator Human genes 0.000 description 1
- 108090000435 Urokinase-type plasminogen activator Proteins 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 241000863000 Vitreoscilla Species 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 230000009824 affinity maturation Effects 0.000 description 1
- 108010050122 alpha 1-Antitrypsin Proteins 0.000 description 1
- 102000015395 alpha 1-Antitrypsin Human genes 0.000 description 1
- 229940024142 alpha 1-antitrypsin Drugs 0.000 description 1
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 1
- 230000009435 amidation Effects 0.000 description 1
- 238000007112 amidation reaction Methods 0.000 description 1
- 229960003896 aminopterin Drugs 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000003146 anticoagulant agent Substances 0.000 description 1
- 229940127219 anticoagulant drug Drugs 0.000 description 1
- 239000003418 antiprogestin Substances 0.000 description 1
- 229910052586 apatite Inorganic materials 0.000 description 1
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 1
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 230000001746 atrial effect Effects 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- 102000006635 beta-lactamase Human genes 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 238000002306 biochemical method Methods 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 238000005460 biophysical method Methods 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 239000003114 blood coagulation factor Substances 0.000 description 1
- 238000006664 bond formation reaction Methods 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 108010006025 bovine growth hormone Proteins 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- BBBFJLBPOGFECG-VJVYQDLKSA-N calcitonin Chemical compound N([C@H](C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(N)=O)C(C)C)C(=O)[C@@H]1CSSC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1 BBBFJLBPOGFECG-VJVYQDLKSA-N 0.000 description 1
- 229960004015 calcitonin Drugs 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 238000012219 cassette mutagenesis Methods 0.000 description 1
- 239000012930 cell culture fluid Substances 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 239000012560 cell impurity Substances 0.000 description 1
- 210000002421 cell wall Anatomy 0.000 description 1
- 238000012412 chemical coupling Methods 0.000 description 1
- 125000003636 chemical group Chemical group 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 238000007697 cis-trans-isomerization reaction Methods 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 230000004154 complement system Effects 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 229920006037 cross link polymer Polymers 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 230000001351 cycling effect Effects 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 230000006240 deamidation Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 101150018266 degP gene Proteins 0.000 description 1
- 108700001680 des-(1-3)- insulin-like growth factor 1 Proteins 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 125000002228 disulfide group Chemical group 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 229940030606 diuretics Drugs 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 229940088679 drug related substance Drugs 0.000 description 1
- 101150009558 dsbA gene Proteins 0.000 description 1
- 101150015101 dsbC gene Proteins 0.000 description 1
- 238000007812 electrochemical assay Methods 0.000 description 1
- 230000005670 electromagnetic radiation Effects 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 231100000655 enterotoxin Toxicity 0.000 description 1
- 239000000147 enterotoxin Substances 0.000 description 1
- 238000001976 enzyme digestion Methods 0.000 description 1
- 229940105423 erythropoietin Drugs 0.000 description 1
- 239000000328 estrogen antagonist Substances 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 229960004222 factor ix Drugs 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 210000003754 fetus Anatomy 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 101150027203 fkpA gene Proteins 0.000 description 1
- 238000007667 floating Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 108091006047 fluorescent proteins Proteins 0.000 description 1
- 102000034287 fluorescent proteins Human genes 0.000 description 1
- 229940028334 follicle stimulating hormone Drugs 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 238000002825 functional assay Methods 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 229930182830 galactose Natural products 0.000 description 1
- 239000010437 gem Substances 0.000 description 1
- 229910001751 gemstone Inorganic materials 0.000 description 1
- 102000034356 gene-regulatory proteins Human genes 0.000 description 1
- 108091006104 gene-regulatory proteins Proteins 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- MASNOZXLGMXCHN-ZLPAWPGGSA-N glucagon Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 MASNOZXLGMXCHN-ZLPAWPGGSA-N 0.000 description 1
- 229960004666 glucagon Drugs 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 125000000404 glutamine group Chemical group N[C@@H](CCC(N)=O)C(=O)* 0.000 description 1
- 229960003180 glutathione Drugs 0.000 description 1
- 125000003055 glycidyl group Chemical group C(C1CO1)* 0.000 description 1
- 239000002622 gonadotropin Substances 0.000 description 1
- 210000005256 gram-negative cell Anatomy 0.000 description 1
- 239000000122 growth hormone Substances 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 1
- 239000000833 heterodimer Substances 0.000 description 1
- 238000005734 heterodimerization reaction Methods 0.000 description 1
- 229920000140 heteropolymer Polymers 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 239000000710 homodimer Substances 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 125000002349 hydroxyamino group Chemical group [H]ON([H])[*] 0.000 description 1
- 230000033444 hydroxylation Effects 0.000 description 1
- 238000005805 hydroxylation reaction Methods 0.000 description 1
- 101150020087 ilvG gene Proteins 0.000 description 1
- 239000000677 immunologic agent Substances 0.000 description 1
- 238000001114 immunoprecipitation Methods 0.000 description 1
- 229940051026 immunotoxin Drugs 0.000 description 1
- 239000002596 immunotoxin Substances 0.000 description 1
- 230000002637 immunotoxin Effects 0.000 description 1
- 231100000608 immunotoxin Toxicity 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 229910052816 inorganic phosphate Inorganic materials 0.000 description 1
- 230000002608 insulinlike Effects 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 108040003607 interleukin-13 receptor activity proteins Proteins 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 230000037427 ion transport Effects 0.000 description 1
- 239000002085 irritant Substances 0.000 description 1
- 231100000021 irritant Toxicity 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 150000002614 leucines Chemical class 0.000 description 1
- 230000031700 light absorption Effects 0.000 description 1
- 230000029226 lipidation Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 101150074251 lpp gene Proteins 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 239000003580 lung surfactant Substances 0.000 description 1
- 229940040129 luteinizing hormone Drugs 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 239000000696 magnetic material Substances 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 230000008774 maternal effect Effects 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- TWXDDNPPQUTEOV-FVGYRXGTSA-N methamphetamine hydrochloride Chemical compound Cl.CN[C@@H](C)CC1=CC=CC=C1 TWXDDNPPQUTEOV-FVGYRXGTSA-N 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 231100000219 mutagenic Toxicity 0.000 description 1
- 230000003505 mutagenic effect Effects 0.000 description 1
- 229940053128 nerve growth factor Drugs 0.000 description 1
- MGFYIUFZLHCRTH-UHFFFAOYSA-N nitrilotriacetic acid Chemical compound OC(=O)CN(CC(O)=O)CC(O)=O MGFYIUFZLHCRTH-UHFFFAOYSA-N 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 230000002188 osteogenic effect Effects 0.000 description 1
- 229940043515 other immunoglobulins in atc Drugs 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 239000000199 parathyroid hormone Substances 0.000 description 1
- 229960001319 parathyroid hormone Drugs 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 229950009506 penicillinase Drugs 0.000 description 1
- VSIIXMUUUJUKCM-UHFFFAOYSA-D pentacalcium;fluoride;triphosphate Chemical compound [F-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O VSIIXMUUUJUKCM-UHFFFAOYSA-D 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 230000003094 perturbing effect Effects 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229940127126 plasminogen activator Drugs 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 108010054442 polyalanine Proteins 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 239000001103 potassium chloride Substances 0.000 description 1
- 235000011164 potassium chloride Nutrition 0.000 description 1
- OXCMYAYHXIHQOA-UHFFFAOYSA-N potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol Chemical compound [K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1 OXCMYAYHXIHQOA-UHFFFAOYSA-N 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000003623 progesteronic effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000001902 propagating effect Effects 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 238000010377 protein imaging Methods 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 230000003362 replicative effect Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 229910052707 ruthenium Inorganic materials 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000011896 sensitive detection Methods 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000007974 sodium acetate buffer Substances 0.000 description 1
- 239000012475 sodium chloride buffer Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 238000012956 testing procedure Methods 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 229960004072 thrombin Drugs 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 101150065732 tir gene Proteins 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 150000003668 tyrosines Chemical class 0.000 description 1
- 238000000870 ultraviolet spectroscopy Methods 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- 229960005356 urokinase Drugs 0.000 description 1
- 108700026220 vif Genes Proteins 0.000 description 1
- 239000011800 void material Substances 0.000 description 1
- 108010047303 von Willebrand Factor Proteins 0.000 description 1
- 102100036537 von Willebrand factor Human genes 0.000 description 1
- 229960001134 von willebrand factor Drugs 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 238000002424 x-ray crystallography Methods 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
- 229910052727 yttrium Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/16—Extraction; Separation; Purification by chromatography
- C07K1/165—Extraction; Separation; Purification by chromatography mixed-mode chromatography
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/16—Extraction; Separation; Purification by chromatography
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/16—Extraction; Separation; Purification by chromatography
- C07K1/18—Ion-exchange chromatography
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/16—Extraction; Separation; Purification by chromatography
- C07K1/20—Partition-, reverse-phase or hydrophobic interaction chromatography
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/16—Extraction; Separation; Purification by chromatography
- C07K1/22—Affinity chromatography or related techniques based upon selective absorption processes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/195—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria
- C07K14/24—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria from Enterobacteriaceae (F), e.g. Citrobacter, Serratia, Proteus, Providencia, Morganella, Yersinia
- C07K14/245—Escherichia (G)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/06—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies from serum
- C07K16/065—Purification, fragmentation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/12—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria
- C07K16/1203—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-negative bacteria
- C07K16/1228—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-negative bacteria from Enterobacteriaceae (F), e.g. Citrobacter, Serratia, Proteus, Providencia, Morganella, Yersinia
- C07K16/1232—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-negative bacteria from Enterobacteriaceae (F), e.g. Citrobacter, Serratia, Proteus, Providencia, Morganella, Yersinia from Escherichia (G)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/24—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against cytokines, lymphokines or interferons
- C07K16/244—Interleukins [IL]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/40—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against enzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/90—Isomerases (5.)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/563—Immunoassay; Biospecific binding assay; Materials therefor involving antibody fragments
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y502/00—Cis-trans-isomerases (5.2)
- C12Y502/01—Cis-trans-Isomerases (5.2.1)
- C12Y502/01008—Peptidylprolyl isomerase (5.2.1.8), i.e. cyclophilin
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/90—Enzymes; Proenzymes
- G01N2333/99—Isomerases (5.)
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Analytical Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- General Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Food Science & Technology (AREA)
- Cell Biology (AREA)
- Gastroenterology & Hepatology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Peptides Or Proteins (AREA)
- Enzymes And Modification Thereof (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562207910P | 2015-08-20 | 2015-08-20 | |
| US201562207908P | 2015-08-20 | 2015-08-20 | |
| US62/207,908 | 2015-08-20 | ||
| US62/207,910 | 2015-08-20 | ||
| US201562210378P | 2015-08-26 | 2015-08-26 | |
| US62/210,378 | 2015-08-26 | ||
| PCT/US2016/047912 WO2017031476A2 (en) | 2015-08-20 | 2016-08-19 | Purification of fkpa and uses thereof for producing recombinant polypeptides |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180048731A true KR20180048731A (ko) | 2018-05-10 |
Family
ID=58052052
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187007920A Ceased KR20180048731A (ko) | 2015-08-20 | 2016-08-19 | 재조합 폴리펩티드를 제조하기 위한 fkpa의 정제 및 그의 용도 |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US20180327446A1 (enExample) |
| EP (1) | EP3337819B1 (enExample) |
| JP (2) | JP7091238B2 (enExample) |
| KR (1) | KR20180048731A (enExample) |
| CN (1) | CN108473558A (enExample) |
| AU (1) | AU2016308383A1 (enExample) |
| BR (1) | BR112018003127A2 (enExample) |
| CA (1) | CA2995385A1 (enExample) |
| HK (1) | HK1259368A1 (enExample) |
| IL (2) | IL257400A (enExample) |
| MX (1) | MX2018002068A (enExample) |
| TW (1) | TW201718623A (enExample) |
| WO (1) | WO2017031476A2 (enExample) |
Families Citing this family (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9458450B2 (en) | 2012-03-15 | 2016-10-04 | Flodesign Sonics, Inc. | Acoustophoretic separation technology using multi-dimensional standing waves |
| US10704021B2 (en) | 2012-03-15 | 2020-07-07 | Flodesign Sonics, Inc. | Acoustic perfusion devices |
| CA2935960C (en) | 2014-01-08 | 2023-01-10 | Bart Lipkens | Acoustophoresis device with dual acoustophoretic chamber |
| US11708572B2 (en) | 2015-04-29 | 2023-07-25 | Flodesign Sonics, Inc. | Acoustic cell separation techniques and processes |
| US11377651B2 (en) | 2016-10-19 | 2022-07-05 | Flodesign Sonics, Inc. | Cell therapy processes utilizing acoustophoresis |
| US11459540B2 (en) | 2015-07-28 | 2022-10-04 | Flodesign Sonics, Inc. | Expanded bed affinity selection |
| US11474085B2 (en) | 2015-07-28 | 2022-10-18 | Flodesign Sonics, Inc. | Expanded bed affinity selection |
| US11085035B2 (en) | 2016-05-03 | 2021-08-10 | Flodesign Sonics, Inc. | Therapeutic cell washing, concentration, and separation utilizing acoustophoresis |
| US11214789B2 (en) | 2016-05-03 | 2022-01-04 | Flodesign Sonics, Inc. | Concentration and washing of particles with acoustics |
| KR20220066413A (ko) | 2017-12-14 | 2022-05-24 | 프로디자인 소닉스, 인크. | 음향 트랜스듀서 구동기 및 제어기 |
| CN111801120A (zh) * | 2017-12-29 | 2020-10-20 | 豪夫迈·罗氏有限公司 | 用于提供聚乙二醇化蛋白质组合物的方法 |
| CN111741770A (zh) | 2017-12-29 | 2020-10-02 | 豪夫迈·罗氏有限公司 | 用于提供聚乙二醇化蛋白质组合物的方法 |
| KR20240067985A (ko) * | 2018-02-27 | 2024-05-17 | 화이자 인코포레이티드 | 항체 정제 |
| MX2020009788A (es) * | 2018-03-21 | 2020-12-09 | Takeda Pharmaceuticals Co | Separación de factor de von willebrand (vwf) y propéptido de factor de von willebrand (vwf) por métodos cromatográficos. |
| US11453883B2 (en) | 2018-04-05 | 2022-09-27 | Bio-Rad Abd Serotec Gmbh | Display systems for proteins of interest |
| EP3856347A1 (en) * | 2018-09-25 | 2021-08-04 | Ichnos Sciences SA | Antibody quantification in biological samples |
| CN114409798A (zh) * | 2019-02-14 | 2022-04-29 | 美勒斯公司 | 制备包含两种或更多种抗体的组合物 |
| EP3942079A1 (en) * | 2019-03-18 | 2022-01-26 | Bio-Rad ABD Serotec GmbH | Protection of spytag-containing periplasmic fusion proteins from protease tsp and ompt degradation |
| EP3962924A1 (en) | 2019-05-03 | 2022-03-09 | Genentech, Inc. | Methods of reducing the enzymatic hydrolysis activity rate in a composition obtained from a purification platform |
| CN114829589A (zh) * | 2019-12-20 | 2022-07-29 | 默沙东有限公司 | 纯化的肠道病毒的组合物和使用谷胱甘肽亲和色谱的纯化方法 |
| AU2021224852A1 (en) * | 2020-02-21 | 2022-10-13 | Biogen Ma Inc. | Methods of preparing oligonucleotide compositions using ultrafiltration / diafiltration |
| EP4118093A4 (en) * | 2020-03-11 | 2024-04-17 | Dr. Reddy's Laboratories Ltd. | METHOD FOR PURIFYING A FC FUSION PROTEIN |
| CA3233419A1 (en) * | 2021-09-28 | 2023-04-06 | Kashiv Biosciences, Llc | An improved process for purification of protein |
| EP4437344A1 (en) * | 2021-11-25 | 2024-10-02 | F. Hoffmann-La Roche AG | Quantification of low amounts of antibody sideproducts |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE60135498D1 (de) * | 2000-12-14 | 2008-10-02 | Genentech Inc | Produktion von ganzen Antikörpern in prokaryontischen Zellen |
| KR20170023209A (ko) * | 2008-05-05 | 2017-03-02 | 노비뮨 에스 에이 | 항-il-17a/il-17f 교차-반응성 항체 및 그의 사용 방법 |
| KR20110091678A (ko) | 2008-10-20 | 2011-08-12 | 아보트 러보러터리즈 | 단백질 a 친화성 크로마토그래피를 이용한 항체의 분리 및 정제 |
| PL3037104T3 (pl) | 2009-10-20 | 2020-11-16 | Abbvie Inc. | Izolacja i oczyszczanie przeciwciał anty-il-13 z zastosowaniem chromatografii powinowactwa z białkiem a |
| WO2013028330A2 (en) | 2011-08-19 | 2013-02-28 | Emd Millipore Corporation | Methods of reducing level of one of more impurities in a sample during protein purification |
| JP6092892B2 (ja) | 2011-12-15 | 2017-03-08 | プレステージ バイオファーマ プライベート リミテッド | 抗体の精製方法 |
| WO2013102042A2 (en) * | 2011-12-30 | 2013-07-04 | Abbvie Inc. | Dual specific binding proteins directed against il-13 and/or il-17 |
| CN119979504A (zh) | 2013-01-09 | 2025-05-13 | 武田药品工业株式会社 | 含用于纯化芳基硫酸酯酶a的方法 |
| BR112015024553A2 (pt) * | 2013-04-05 | 2017-10-24 | Genentech Inc | anticorpo multiespecífico, anticorpo isolado, ácido nucleico isolado, célula hospedeira, método de produção de anticorpo, imunoconjugado, formulação farmacêutica, uso de anticorpo e método de tratamento de indivíduos com distúrbio |
| CA2909516C (en) * | 2013-04-19 | 2023-03-07 | Sutro Biopharma, Inc. | Expression of biologically active proteins in a bacterial cell-free synthesis system using cell extracts with elevated levels of exogenous chaperones |
| CN118271429A (zh) * | 2013-08-19 | 2024-07-02 | 豪夫迈·罗氏有限公司 | 用羟基磷灰石层析分离双特异性抗体和双特异性抗体生产副产物 |
| CN105722532A (zh) * | 2013-09-13 | 2016-06-29 | 豪夫迈·罗氏有限公司 | 包含纯化的重组多肽的方法和组合物 |
| EP3066123A1 (en) | 2013-11-07 | 2016-09-14 | AbbVie Inc. | Isolation and purification of antibodies |
| JP2017507939A (ja) * | 2014-02-21 | 2017-03-23 | ジェネンテック, インコーポレイテッド | 抗il−13/il−17二重特異性抗体及びその使用 |
-
2016
- 2016-08-19 KR KR1020187007920A patent/KR20180048731A/ko not_active Ceased
- 2016-08-19 WO PCT/US2016/047912 patent/WO2017031476A2/en not_active Ceased
- 2016-08-19 BR BR112018003127A patent/BR112018003127A2/pt not_active IP Right Cessation
- 2016-08-19 HK HK19101736.6A patent/HK1259368A1/zh unknown
- 2016-08-19 TW TW105126681A patent/TW201718623A/zh unknown
- 2016-08-19 CN CN201680061356.3A patent/CN108473558A/zh active Pending
- 2016-08-19 MX MX2018002068A patent/MX2018002068A/es unknown
- 2016-08-19 EP EP16758343.4A patent/EP3337819B1/en active Active
- 2016-08-19 CA CA2995385A patent/CA2995385A1/en not_active Abandoned
- 2016-08-19 JP JP2018509536A patent/JP7091238B2/ja active Active
- 2016-08-19 AU AU2016308383A patent/AU2016308383A1/en not_active Abandoned
-
2018
- 2018-02-07 IL IL257400A patent/IL257400A/en unknown
- 2018-02-16 US US15/932,222 patent/US20180327446A1/en not_active Abandoned
-
2021
- 2021-11-17 IL IL288205A patent/IL288205A/en unknown
-
2022
- 2022-01-13 JP JP2022003518A patent/JP2022062046A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| EP3337819A2 (en) | 2018-06-27 |
| BR112018003127A2 (pt) | 2018-09-25 |
| JP2018533355A (ja) | 2018-11-15 |
| IL257400A (en) | 2018-04-30 |
| TW201718623A (zh) | 2017-06-01 |
| MX2018002068A (es) | 2018-06-06 |
| CA2995385A1 (en) | 2017-02-23 |
| EP3337819B1 (en) | 2024-02-21 |
| AU2016308383A1 (en) | 2018-03-15 |
| JP2022062046A (ja) | 2022-04-19 |
| IL288205A (en) | 2022-01-01 |
| HK1259368A1 (zh) | 2019-11-29 |
| WO2017031476A3 (en) | 2017-04-13 |
| US20180327446A1 (en) | 2018-11-15 |
| JP7091238B2 (ja) | 2022-06-27 |
| CN108473558A (zh) | 2018-08-31 |
| WO2017031476A2 (en) | 2017-02-23 |
| EP3337819C0 (en) | 2024-02-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20180048731A (ko) | 재조합 폴리펩티드를 제조하기 위한 fkpa의 정제 및 그의 용도 | |
| JP7203928B2 (ja) | オーバーロード/溶出クロマトグラフィー | |
| US20220064209A1 (en) | Methods of purifying polypeptides | |
| JP7191132B2 (ja) | 超精製DsbA及びDsbC、ならびにそれらの作製及び使用方法 | |
| TWI870412B (zh) | 過載層析管柱之再生方法 | |
| JP2023547621A (ja) | 抗siglec-8抗体製剤 | |
| HK1240254B (zh) | 超载和洗脱层析 | |
| NZ624317B2 (en) | Overload and elute chromatography | |
| HK1170633A (en) | Concentrated polypeptide formulations with reduced viscosity |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20180320 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210819 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20231127 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20240206 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20231127 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |