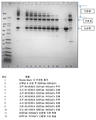
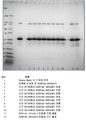
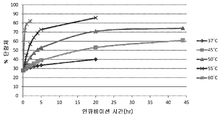
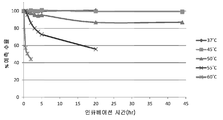
KR20170138551A - Fab-dsFv 다량체 종에서 단량체 항체의 백분율을 증가시키는 방법 - Google Patents
Fab-dsFv 다량체 종에서 단량체 항체의 백분율을 증가시키는 방법 Download PDFInfo
- Publication number
- KR20170138551A KR20170138551A KR1020177033852A KR20177033852A KR20170138551A KR 20170138551 A KR20170138551 A KR 20170138551A KR 1020177033852 A KR1020177033852 A KR 1020177033852A KR 20177033852 A KR20177033852 A KR 20177033852A KR 20170138551 A KR20170138551 A KR 20170138551A
- Authority
- KR
- South Korea
- Prior art keywords
- ser
- thr
- antibody
- gly
- leu
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000000034 method Methods 0.000 title claims abstract description 133
- 239000000178 monomer Substances 0.000 title claims abstract description 73
- 230000001965 increasing effect Effects 0.000 title claims abstract description 12
- 239000000203 mixture Substances 0.000 claims abstract description 75
- 239000003638 chemical reducing agent Substances 0.000 claims abstract description 68
- 239000000427 antigen Substances 0.000 claims abstract description 47
- 102000036639 antigens Human genes 0.000 claims abstract description 47
- 108091007433 antigens Proteins 0.000 claims abstract description 47
- 238000006243 chemical reaction Methods 0.000 claims abstract description 47
- 230000027455 binding Effects 0.000 claims description 72
- 150000001413 amino acids Chemical class 0.000 claims description 45
- 230000008569 process Effects 0.000 claims description 32
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 claims description 31
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims description 24
- 238000000746 purification Methods 0.000 claims description 22
- 238000011143 downstream manufacturing Methods 0.000 claims description 17
- 239000004472 Lysine Substances 0.000 claims description 16
- UFULAYFCSOUIOV-UHFFFAOYSA-N cysteamine Chemical compound NCCS UFULAYFCSOUIOV-UHFFFAOYSA-N 0.000 claims description 15
- 229960003151 mercaptamine Drugs 0.000 claims description 15
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 claims description 14
- 238000004587 chromatography analysis Methods 0.000 claims description 9
- 108091006905 Human Serum Albumin Proteins 0.000 claims description 8
- 102000008100 Human Serum Albumin Human genes 0.000 claims description 8
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 claims description 7
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 claims description 7
- 229960003180 glutathione Drugs 0.000 claims description 7
- 101000679851 Homo sapiens Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 claims description 6
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 claims description 6
- 238000004191 hydrophobic interaction chromatography Methods 0.000 claims description 6
- 239000004475 Arginine Substances 0.000 claims description 5
- 108010024636 Glutathione Proteins 0.000 claims description 5
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 claims description 5
- 238000011210 chromatographic step Methods 0.000 claims description 5
- 230000000295 complement effect Effects 0.000 claims description 5
- 102000050320 human TNFRSF4 Human genes 0.000 claims description 5
- 150000001875 compounds Chemical class 0.000 claims description 4
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 claims description 4
- 238000004255 ion exchange chromatography Methods 0.000 claims description 4
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 claims description 3
- 102000037865 fusion proteins Human genes 0.000 claims description 3
- 108020001507 fusion proteins Proteins 0.000 claims description 3
- 238000003756 stirring Methods 0.000 claims description 3
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 2
- NVJBFARDFTXOTO-UHFFFAOYSA-N diethyl sulfite Chemical compound CCOS(=O)OCC NVJBFARDFTXOTO-UHFFFAOYSA-N 0.000 claims 1
- 238000005215 recombination Methods 0.000 claims 1
- 230000006798 recombination Effects 0.000 claims 1
- 125000005647 linker group Chemical group 0.000 description 89
- 108090000623 proteins and genes Proteins 0.000 description 79
- 102000004169 proteins and genes Human genes 0.000 description 72
- 235000018102 proteins Nutrition 0.000 description 67
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 64
- 235000001014 amino acid Nutrition 0.000 description 44
- 229940024606 amino acid Drugs 0.000 description 43
- 239000012634 fragment Substances 0.000 description 33
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 29
- HKZAAJSTFUZYTO-LURJTMIESA-N (2s)-2-[[2-[[2-[[2-[(2-aminoacetyl)amino]acetyl]amino]acetyl]amino]acetyl]amino]-3-hydroxypropanoic acid Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O HKZAAJSTFUZYTO-LURJTMIESA-N 0.000 description 26
- 108010001064 glycyl-glycyl-glycyl-glycine Proteins 0.000 description 25
- 229920000642 polymer Polymers 0.000 description 23
- -1 2-mercaptoethanol) Chemical compound 0.000 description 22
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 22
- 239000000463 material Substances 0.000 description 20
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 19
- 239000000872 buffer Substances 0.000 description 18
- 239000012636 effector Substances 0.000 description 18
- 108090000765 processed proteins & peptides Proteins 0.000 description 18
- 241000894007 species Species 0.000 description 18
- 241000880493 Leptailurus serval Species 0.000 description 17
- QYSFWUIXDFJUDW-DCAQKATOSA-N Ser-Leu-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O QYSFWUIXDFJUDW-DCAQKATOSA-N 0.000 description 17
- 238000004458 analytical method Methods 0.000 description 17
- 239000006228 supernatant Substances 0.000 description 17
- 206010057190 Respiratory tract infections Diseases 0.000 description 15
- 238000002474 experimental method Methods 0.000 description 15
- 235000018977 lysine Nutrition 0.000 description 15
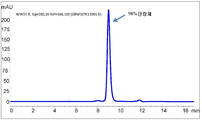
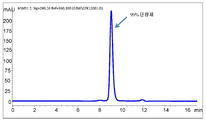
- 238000003998 size exclusion chromatography high performance liquid chromatography Methods 0.000 description 15
- 108010060199 cysteinylproline Proteins 0.000 description 14
- 108010020755 prolyl-glycyl-glycine Proteins 0.000 description 14
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 13
- ANHVRCNNGJMJNG-BZSNNMDCSA-N Tyr-Tyr-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CS)C(=O)O)N)O ANHVRCNNGJMJNG-BZSNNMDCSA-N 0.000 description 13
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 13
- 210000004027 cell Anatomy 0.000 description 13
- 239000000243 solution Substances 0.000 description 13
- 108020004414 DNA Proteins 0.000 description 12
- 108010008685 alanyl-glutamyl-aspartic acid Proteins 0.000 description 12
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 12
- 229960002433 cysteine Drugs 0.000 description 12
- 229910052720 vanadium Inorganic materials 0.000 description 12
- XYBJLTKSGFBLCS-QXEWZRGKSA-N Asp-Arg-Val Chemical compound NC(N)=NCCC[C@@H](C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC(O)=O XYBJLTKSGFBLCS-QXEWZRGKSA-N 0.000 description 11
- YQPFCZVKMUVZIN-AUTRQRHGSA-N Glu-Val-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQPFCZVKMUVZIN-AUTRQRHGSA-N 0.000 description 11
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 11
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 11
- SPSSJSICDYYTQN-HJGDQZAQSA-N Met-Thr-Gln Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O SPSSJSICDYYTQN-HJGDQZAQSA-N 0.000 description 11
- JZRYFUGREMECBH-XPUUQOCRSA-N Ser-Val-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O JZRYFUGREMECBH-XPUUQOCRSA-N 0.000 description 11
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 11
- GXUWHVZYDAHFSV-FLBSBUHZSA-N Thr-Ile-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GXUWHVZYDAHFSV-FLBSBUHZSA-N 0.000 description 11
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 11
- 229920001223 polyethylene glycol Polymers 0.000 description 11
- DONWIPDSZZJHHK-HJGDQZAQSA-N Asp-Lys-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N)O DONWIPDSZZJHHK-HJGDQZAQSA-N 0.000 description 10
- OYTPNWYZORARHL-XHNCKOQMSA-N Gln-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N OYTPNWYZORARHL-XHNCKOQMSA-N 0.000 description 10
- FQCILXROGNOZON-YUMQZZPRSA-N Gln-Pro-Gly Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O FQCILXROGNOZON-YUMQZZPRSA-N 0.000 description 10
- MIIVFRCYJABHTQ-ONGXEEELSA-N Gly-Leu-Val Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O MIIVFRCYJABHTQ-ONGXEEELSA-N 0.000 description 10
- 101001057612 Homo sapiens Dual specificity protein phosphatase 5 Proteins 0.000 description 10
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 10
- FEHQLKKBVJHSEC-SZMVWBNQSA-N Leu-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FEHQLKKBVJHSEC-SZMVWBNQSA-N 0.000 description 10
- KIZIOFNVSOSKJI-CIUDSAMLSA-N Leu-Ser-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N KIZIOFNVSOSKJI-CIUDSAMLSA-N 0.000 description 10
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 10
- BIBYEFRASCNLAA-CDMKHQONSA-N Thr-Phe-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=CC=C1 BIBYEFRASCNLAA-CDMKHQONSA-N 0.000 description 10
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 10
- 108010008355 arginyl-glutamine Proteins 0.000 description 10
- 102000043381 human DUSP5 Human genes 0.000 description 10
- 239000000546 pharmaceutical excipient Substances 0.000 description 10
- 238000011084 recovery Methods 0.000 description 10
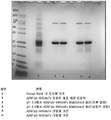
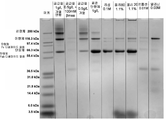
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 10
- 125000003396 thiol group Chemical group [H]S* 0.000 description 10
- UQJNXZSSGQIPIQ-FBCQKBJTSA-N Gly-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)CN UQJNXZSSGQIPIQ-FBCQKBJTSA-N 0.000 description 9
- HZWWOGWOBQBETJ-CUJWVEQBSA-N His-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N)O HZWWOGWOBQBETJ-CUJWVEQBSA-N 0.000 description 9
- NZGTYCMLUGYMCV-XUXIUFHCSA-N Ile-Lys-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N NZGTYCMLUGYMCV-XUXIUFHCSA-N 0.000 description 9
- UGCIQUYEJIEHKX-GVXVVHGQSA-N Lys-Val-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O UGCIQUYEJIEHKX-GVXVVHGQSA-N 0.000 description 9
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 9
- 235000018417 cysteine Nutrition 0.000 description 9
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 9
- 108010016616 cysteinylglycine Proteins 0.000 description 9
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 9
- 108010049041 glutamylalanine Proteins 0.000 description 9
- 239000002953 phosphate buffered saline Substances 0.000 description 9
- 239000000725 suspension Substances 0.000 description 9
- IFKQPMZRDQZSHI-GHCJXIJMSA-N Ala-Ile-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O IFKQPMZRDQZSHI-GHCJXIJMSA-N 0.000 description 8
- MMLHRUJLOUSRJX-CIUDSAMLSA-N Ala-Ser-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCCN MMLHRUJLOUSRJX-CIUDSAMLSA-N 0.000 description 8
- QNFRBNZGVVKBNJ-PEFMBERDSA-N Asp-Ile-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)O)N QNFRBNZGVVKBNJ-PEFMBERDSA-N 0.000 description 8
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 8
- KRRMJKMGWWXWDW-STQMWFEESA-N Gly-Arg-Phe Chemical compound NC(=N)NCCC[C@H](NC(=O)CN)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 KRRMJKMGWWXWDW-STQMWFEESA-N 0.000 description 8
- AAHSHTLISQUZJL-QSFUFRPTSA-N Gly-Ile-Ile Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O AAHSHTLISQUZJL-QSFUFRPTSA-N 0.000 description 8
- RMJWFINHACYKJI-SIUGBPQLSA-N Ile-Tyr-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N RMJWFINHACYKJI-SIUGBPQLSA-N 0.000 description 8
- CQGSYZCULZMEDE-UHFFFAOYSA-N Leu-Gln-Pro Natural products CC(C)CC(N)C(=O)NC(CCC(N)=O)C(=O)N1CCCC1C(O)=O CQGSYZCULZMEDE-UHFFFAOYSA-N 0.000 description 8
- ILDSIMPXNFWKLH-KATARQTJSA-N Leu-Thr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O ILDSIMPXNFWKLH-KATARQTJSA-N 0.000 description 8
- AIRZWUMAHCDDHR-KKUMJFAQSA-N Lys-Leu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O AIRZWUMAHCDDHR-KKUMJFAQSA-N 0.000 description 8
- 108010045023 alanyl-prolyl-tyrosine Proteins 0.000 description 8
- 238000009472 formulation Methods 0.000 description 8
- 239000007789 gas Substances 0.000 description 8
- 102000004196 processed proteins & peptides Human genes 0.000 description 8
- 239000000047 product Substances 0.000 description 8
- 108010048397 seryl-lysyl-leucine Proteins 0.000 description 8
- 230000001225 therapeutic effect Effects 0.000 description 8
- USNJAPJZSGTTPX-XVSYOHENSA-N Asp-Phe-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O USNJAPJZSGTTPX-XVSYOHENSA-N 0.000 description 7
- MNQMTYSEKZHIDF-GCJQMDKQSA-N Asp-Thr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O MNQMTYSEKZHIDF-GCJQMDKQSA-N 0.000 description 7
- HHABWQIFXZPZCK-ACZMJKKPSA-N Cys-Gln-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CS)N HHABWQIFXZPZCK-ACZMJKKPSA-N 0.000 description 7
- IXHKPDJKKCUKHS-GARJFASQSA-N Lys-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCCN)N IXHKPDJKKCUKHS-GARJFASQSA-N 0.000 description 7
- PDIDTSZKKFEDMB-UWVGGRQHSA-N Lys-Pro-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O PDIDTSZKKFEDMB-UWVGGRQHSA-N 0.000 description 7
- BKWJQWJPZMUWEG-LFSVMHDDSA-N Phe-Ala-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=CC=C1 BKWJQWJPZMUWEG-LFSVMHDDSA-N 0.000 description 7
- SFTZWNJFZYOLBD-ZDLURKLDSA-N Ser-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO SFTZWNJFZYOLBD-ZDLURKLDSA-N 0.000 description 7
- VGQVAVQWKJLIRM-FXQIFTODSA-N Ser-Ser-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O VGQVAVQWKJLIRM-FXQIFTODSA-N 0.000 description 7
- NADLKBTYNKUJEP-KATARQTJSA-N Ser-Thr-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O NADLKBTYNKUJEP-KATARQTJSA-N 0.000 description 7
- DEGUERSKQBRZMZ-FXQIFTODSA-N Val-Ser-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O DEGUERSKQBRZMZ-FXQIFTODSA-N 0.000 description 7
- 241000700605 Viruses Species 0.000 description 7
- 239000004480 active ingredient Substances 0.000 description 7
- 108010087924 alanylproline Proteins 0.000 description 7
- 238000010828 elution Methods 0.000 description 7
- 238000001914 filtration Methods 0.000 description 7
- 108010010096 glycyl-glycyl-tyrosine Proteins 0.000 description 7
- 108010015792 glycyllysine Proteins 0.000 description 7
- 238000010438 heat treatment Methods 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 108010064235 lysylglycine Proteins 0.000 description 7
- 108010090894 prolylleucine Proteins 0.000 description 7
- 150000003573 thiols Chemical class 0.000 description 7
- 108010009962 valyltyrosine Proteins 0.000 description 7
- KXEVYGKATAMXJJ-ACZMJKKPSA-N Ala-Glu-Asp Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O KXEVYGKATAMXJJ-ACZMJKKPSA-N 0.000 description 6
- REWSWYIDQIELBE-FXQIFTODSA-N Ala-Val-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O REWSWYIDQIELBE-FXQIFTODSA-N 0.000 description 6
- VKKYFICVTYKFIO-CIUDSAMLSA-N Arg-Ala-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N VKKYFICVTYKFIO-CIUDSAMLSA-N 0.000 description 6
- MKJBPDLENBUHQU-CIUDSAMLSA-N Asn-Ser-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O MKJBPDLENBUHQU-CIUDSAMLSA-N 0.000 description 6
- WNGZKSVJFDZICU-XIRDDKMYSA-N Asp-Leu-Trp Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CC(=O)O)N WNGZKSVJFDZICU-XIRDDKMYSA-N 0.000 description 6
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 6
- JXFLPKSDLDEOQK-JHEQGTHGSA-N Gln-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O JXFLPKSDLDEOQK-JHEQGTHGSA-N 0.000 description 6
- MSHXWFKYXJTLEZ-CIUDSAMLSA-N Gln-Met-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N MSHXWFKYXJTLEZ-CIUDSAMLSA-N 0.000 description 6
- UTYGDAHJBBDPBA-BYULHYEWSA-N Gly-Ile-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)CN UTYGDAHJBBDPBA-BYULHYEWSA-N 0.000 description 6
- PDUHNKAFQXQNLH-ZETCQYMHSA-N Gly-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)NCC(O)=O PDUHNKAFQXQNLH-ZETCQYMHSA-N 0.000 description 6
- TVTZEOHWHUVYCG-KYNKHSRBSA-N Gly-Thr-Thr Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O TVTZEOHWHUVYCG-KYNKHSRBSA-N 0.000 description 6
- SBVMXEZQJVUARN-XPUUQOCRSA-N Gly-Val-Ser Chemical compound NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O SBVMXEZQJVUARN-XPUUQOCRSA-N 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 108060003951 Immunoglobulin Proteins 0.000 description 6
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 6
- QNBVTHNJGCOVFA-AVGNSLFASA-N Leu-Leu-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCC(O)=O QNBVTHNJGCOVFA-AVGNSLFASA-N 0.000 description 6
- KZZCOWMDDXDKSS-CIUDSAMLSA-N Leu-Ser-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O KZZCOWMDDXDKSS-CIUDSAMLSA-N 0.000 description 6
- LJBVRCDPWOJOEK-PPCPHDFISA-N Leu-Thr-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LJBVRCDPWOJOEK-PPCPHDFISA-N 0.000 description 6
- QTDBZORPVYTRJU-KKXDTOCCSA-N Phe-Tyr-Ala Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O QTDBZORPVYTRJU-KKXDTOCCSA-N 0.000 description 6
- DYJTXTCEXMCPBF-UFYCRDLUSA-N Pro-Tyr-Phe Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CC3=CC=CC=C3)C(=O)O DYJTXTCEXMCPBF-UFYCRDLUSA-N 0.000 description 6
- RHAPJNVNWDBFQI-BQBZGAKWSA-N Ser-Pro-Gly Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O RHAPJNVNWDBFQI-BQBZGAKWSA-N 0.000 description 6
- BMKNXTJLHFIAAH-CIUDSAMLSA-N Ser-Ser-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O BMKNXTJLHFIAAH-CIUDSAMLSA-N 0.000 description 6
- XJDMUQCLVSCRSJ-VZFHVOOUSA-N Ser-Thr-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O XJDMUQCLVSCRSJ-VZFHVOOUSA-N 0.000 description 6
- XSLXHSYIVPGEER-KZVJFYERSA-N Thr-Ala-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O XSLXHSYIVPGEER-KZVJFYERSA-N 0.000 description 6
- SGAOHNPSEPVAFP-ZDLURKLDSA-N Thr-Ser-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SGAOHNPSEPVAFP-ZDLURKLDSA-N 0.000 description 6
- FBQHKSPOIAFUEI-OWLDWWDNSA-N Thr-Trp-Ala Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](C)C(O)=O FBQHKSPOIAFUEI-OWLDWWDNSA-N 0.000 description 6
- BPGDJSUFQKWUBK-KJEVXHAQSA-N Thr-Val-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 BPGDJSUFQKWUBK-KJEVXHAQSA-N 0.000 description 6
- AVYVKJMBNLPWRX-WFBYXXMGSA-N Trp-Ala-Ser Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O)=CNC2=C1 AVYVKJMBNLPWRX-WFBYXXMGSA-N 0.000 description 6
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 6
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 description 6
- MQGGXGKQSVEQHR-KKUMJFAQSA-N Tyr-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 MQGGXGKQSVEQHR-KKUMJFAQSA-N 0.000 description 6
- UMSZZGTXGKHTFJ-SRVKXCTJSA-N Tyr-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 UMSZZGTXGKHTFJ-SRVKXCTJSA-N 0.000 description 6
- OWFGFHQMSBTKLX-UFYCRDLUSA-N Val-Tyr-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N OWFGFHQMSBTKLX-UFYCRDLUSA-N 0.000 description 6
- 235000004279 alanine Nutrition 0.000 description 6
- 238000005571 anion exchange chromatography Methods 0.000 description 6
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 6
- 238000005277 cation exchange chromatography Methods 0.000 description 6
- 238000004113 cell culture Methods 0.000 description 6
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 6
- 239000003814 drug Substances 0.000 description 6
- 102000018358 immunoglobulin Human genes 0.000 description 6
- 230000002779 inactivation Effects 0.000 description 6
- 239000003112 inhibitor Substances 0.000 description 6
- 230000003647 oxidation Effects 0.000 description 6
- 238000007254 oxidation reaction Methods 0.000 description 6
- 108010070409 phenylalanyl-glycyl-glycine Proteins 0.000 description 6
- 229920001184 polypeptide Polymers 0.000 description 6
- 239000000523 sample Substances 0.000 description 6
- 239000011780 sodium chloride Substances 0.000 description 6
- 239000002904 solvent Substances 0.000 description 6
- 108010038745 tryptophylglycine Proteins 0.000 description 6
- 108010003137 tyrosyltyrosine Proteins 0.000 description 6
- CREYEAPXISDKSB-FQPOAREZSA-N Ala-Thr-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O CREYEAPXISDKSB-FQPOAREZSA-N 0.000 description 5
- PQWTZSNVWSOFFK-FXQIFTODSA-N Arg-Asp-Asn Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)CN=C(N)N PQWTZSNVWSOFFK-FXQIFTODSA-N 0.000 description 5
- XRNXPIGJPQHCPC-RCWTZXSCSA-N Arg-Thr-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CCCNC(N)=N)[C@@H](C)O)C(O)=O XRNXPIGJPQHCPC-RCWTZXSCSA-N 0.000 description 5
- HPNDKUOLNRVRAY-BIIVOSGPSA-N Asn-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CC(=O)N)N)C(=O)O HPNDKUOLNRVRAY-BIIVOSGPSA-N 0.000 description 5
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 5
- 102000004190 Enzymes Human genes 0.000 description 5
- 108090000790 Enzymes Proteins 0.000 description 5
- XZLLTYBONVKGLO-SDDRHHMPSA-N Gln-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(=O)N)N)C(=O)O XZLLTYBONVKGLO-SDDRHHMPSA-N 0.000 description 5
- PAQUJCSYVIBPLC-AVGNSLFASA-N Glu-Asp-Phe Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 PAQUJCSYVIBPLC-AVGNSLFASA-N 0.000 description 5
- NVTPVQLIZCOJFK-FOHZUACHSA-N Gly-Thr-Asp Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O NVTPVQLIZCOJFK-FOHZUACHSA-N 0.000 description 5
- 239000004471 Glycine Substances 0.000 description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 5
- 102000000589 Interleukin-1 Human genes 0.000 description 5
- 108010002352 Interleukin-1 Proteins 0.000 description 5
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 5
- LZDNBBYBDGBADK-UHFFFAOYSA-N L-valyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)C(C)C)C(O)=O)=CNC2=C1 LZDNBBYBDGBADK-UHFFFAOYSA-N 0.000 description 5
- AXZGZMGRBDQTEY-SRVKXCTJSA-N Leu-Gln-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O AXZGZMGRBDQTEY-SRVKXCTJSA-N 0.000 description 5
- QESXLSQLQHHTIX-RHYQMDGZSA-N Leu-Val-Thr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QESXLSQLQHHTIX-RHYQMDGZSA-N 0.000 description 5
- 108010066427 N-valyltryptophan Proteins 0.000 description 5
- YCCUXNNKXDGMAM-KKUMJFAQSA-N Phe-Leu-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YCCUXNNKXDGMAM-KKUMJFAQSA-N 0.000 description 5
- MGDFPGCFVJFITQ-CIUDSAMLSA-N Pro-Glu-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O MGDFPGCFVJFITQ-CIUDSAMLSA-N 0.000 description 5
- HAAQQNHQZBOWFO-LURJTMIESA-N Pro-Gly-Gly Chemical compound OC(=O)CNC(=O)CNC(=O)[C@@H]1CCCN1 HAAQQNHQZBOWFO-LURJTMIESA-N 0.000 description 5
- QEWBZBLXDKIQPS-STQMWFEESA-N Pro-Gly-Tyr Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O QEWBZBLXDKIQPS-STQMWFEESA-N 0.000 description 5
- HWLKHNDRXWTFTN-GUBZILKMSA-N Pro-Pro-Cys Chemical compound C1C[C@H](NC1)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CS)C(=O)O HWLKHNDRXWTFTN-GUBZILKMSA-N 0.000 description 5
- 108020004511 Recombinant DNA Proteins 0.000 description 5
- YQHZVYJAGWMHES-ZLUOBGJFSA-N Ser-Ala-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YQHZVYJAGWMHES-ZLUOBGJFSA-N 0.000 description 5
- BTPAWKABYQMKKN-LKXGYXEUSA-N Ser-Asp-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O BTPAWKABYQMKKN-LKXGYXEUSA-N 0.000 description 5
- UIGMAMGZOJVTDN-WHFBIAKZSA-N Ser-Gly-Ser Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O UIGMAMGZOJVTDN-WHFBIAKZSA-N 0.000 description 5
- GJFYFGOEWLDQGW-GUBZILKMSA-N Ser-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GJFYFGOEWLDQGW-GUBZILKMSA-N 0.000 description 5
- HDBOEVPDIDDEPC-CIUDSAMLSA-N Ser-Lys-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O HDBOEVPDIDDEPC-CIUDSAMLSA-N 0.000 description 5
- JURQXQBJKUHGJS-UHFFFAOYSA-N Ser-Ser-Ser-Ser Chemical compound OCC(N)C(=O)NC(CO)C(=O)NC(CO)C(=O)NC(CO)C(O)=O JURQXQBJKUHGJS-UHFFFAOYSA-N 0.000 description 5
- PZBFGYYEXUXCOF-UHFFFAOYSA-N TCEP Chemical compound OC(=O)CCP(CCC(O)=O)CCC(O)=O PZBFGYYEXUXCOF-UHFFFAOYSA-N 0.000 description 5
- 239000007983 Tris buffer Substances 0.000 description 5
- KBKTUNYBNJWFRL-UBHSHLNASA-N Trp-Ser-Asn Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O)=CNC2=C1 KBKTUNYBNJWFRL-UBHSHLNASA-N 0.000 description 5
- PKZIWSHDJYIPRH-JBACZVJFSA-N Trp-Tyr-Gln Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O PKZIWSHDJYIPRH-JBACZVJFSA-N 0.000 description 5
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 description 5
- DKKHULUSOSWGHS-UWJYBYFXSA-N Tyr-Asn-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CC=C(C=C1)O)N DKKHULUSOSWGHS-UWJYBYFXSA-N 0.000 description 5
- QUILOGWWLXMSAT-IHRRRGAJSA-N Tyr-Gln-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O QUILOGWWLXMSAT-IHRRRGAJSA-N 0.000 description 5
- 239000000443 aerosol Substances 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 229940088598 enzyme Drugs 0.000 description 5
- 239000000499 gel Substances 0.000 description 5
- 108010026364 glycyl-glycyl-leucine Proteins 0.000 description 5
- 108010037850 glycylvaline Proteins 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 229960000485 methotrexate Drugs 0.000 description 5
- 239000008194 pharmaceutical composition Substances 0.000 description 5
- 239000007858 starting material Substances 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 108010044292 tryptophyltyrosine Proteins 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- YYSWCHMLFJLLBJ-ZLUOBGJFSA-N Ala-Ala-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YYSWCHMLFJLLBJ-ZLUOBGJFSA-N 0.000 description 4
- XWFWAXPOLRTDFZ-FXQIFTODSA-N Ala-Pro-Ser Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O XWFWAXPOLRTDFZ-FXQIFTODSA-N 0.000 description 4
- DYXOFPBJBAHWFY-JBDRJPRFSA-N Ala-Ser-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)N DYXOFPBJBAHWFY-JBDRJPRFSA-N 0.000 description 4
- WNHNMKOFKCHKKD-BFHQHQDPSA-N Ala-Thr-Gly Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O WNHNMKOFKCHKKD-BFHQHQDPSA-N 0.000 description 4
- AOHKLEBWKMKITA-IHRRRGAJSA-N Arg-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N AOHKLEBWKMKITA-IHRRRGAJSA-N 0.000 description 4
- JBDLMLZNDRLDIX-HJGDQZAQSA-N Asn-Thr-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O JBDLMLZNDRLDIX-HJGDQZAQSA-N 0.000 description 4
- VPPXTHJNTYDNFJ-CIUDSAMLSA-N Asp-Ala-Lys Chemical compound C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)O)N VPPXTHJNTYDNFJ-CIUDSAMLSA-N 0.000 description 4
- YIDFBWRHIYOYAA-LKXGYXEUSA-N Asp-Ser-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YIDFBWRHIYOYAA-LKXGYXEUSA-N 0.000 description 4
- JSHWXQIZOCVWIA-ZKWXMUAHSA-N Asp-Ser-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O JSHWXQIZOCVWIA-ZKWXMUAHSA-N 0.000 description 4
- VHUKCUHLFMRHOD-MELADBBJSA-N Asp-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CC(=O)O)N)C(=O)O VHUKCUHLFMRHOD-MELADBBJSA-N 0.000 description 4
- SZQCDCKIGWQAQN-FXQIFTODSA-N Cys-Arg-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O SZQCDCKIGWQAQN-FXQIFTODSA-N 0.000 description 4
- OXOQBEVULIBOSH-ZDLURKLDSA-N Cys-Gly-Thr Chemical compound [H]N[C@@H](CS)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O OXOQBEVULIBOSH-ZDLURKLDSA-N 0.000 description 4
- KSMSFCBQBQPFAD-GUBZILKMSA-N Cys-Pro-Pro Chemical compound SC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 KSMSFCBQBQPFAD-GUBZILKMSA-N 0.000 description 4
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- HPBKQFJXDUVNQV-FHWLQOOXSA-N Gln-Tyr-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O HPBKQFJXDUVNQV-FHWLQOOXSA-N 0.000 description 4
- DENRBIYENOKSEX-PEXQALLHSA-N Gly-Ile-His Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 DENRBIYENOKSEX-PEXQALLHSA-N 0.000 description 4
- LHYJCVCQPWRMKZ-WEDXCCLWSA-N Gly-Leu-Thr Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LHYJCVCQPWRMKZ-WEDXCCLWSA-N 0.000 description 4
- VBOBNHSVQKKTOT-YUMQZZPRSA-N Gly-Lys-Ala Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O VBOBNHSVQKKTOT-YUMQZZPRSA-N 0.000 description 4
- VLIJYPMATZSOLL-YUMQZZPRSA-N Gly-Lys-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)CN VLIJYPMATZSOLL-YUMQZZPRSA-N 0.000 description 4
- WNZOCXUOGVYYBJ-CDMKHQONSA-N Gly-Phe-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)CN)O WNZOCXUOGVYYBJ-CDMKHQONSA-N 0.000 description 4
- ZZWUYQXMIFTIIY-WEDXCCLWSA-N Gly-Thr-Leu Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O ZZWUYQXMIFTIIY-WEDXCCLWSA-N 0.000 description 4
- MDOBWSFNSNPENN-PMVVWTBXSA-N His-Thr-Gly Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O MDOBWSFNSNPENN-PMVVWTBXSA-N 0.000 description 4
- FXJLRZFMKGHYJP-CFMVVWHZSA-N Ile-Tyr-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N FXJLRZFMKGHYJP-CFMVVWHZSA-N 0.000 description 4
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 4
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 4
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 4
- HXWALXSAVBLTPK-NUTKFTJISA-N Leu-Ala-Trp Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CC(C)C)N HXWALXSAVBLTPK-NUTKFTJISA-N 0.000 description 4
- IRMLZWSRWSGTOP-CIUDSAMLSA-N Leu-Ser-Ala Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O IRMLZWSRWSGTOP-CIUDSAMLSA-N 0.000 description 4
- AIMGJYMCTAABEN-GVXVVHGQSA-N Leu-Val-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AIMGJYMCTAABEN-GVXVVHGQSA-N 0.000 description 4
- GQZMPWBZQALKJO-UWVGGRQHSA-N Lys-Gly-Arg Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O GQZMPWBZQALKJO-UWVGGRQHSA-N 0.000 description 4
- CTJUSALVKAWFFU-CIUDSAMLSA-N Lys-Ser-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N CTJUSALVKAWFFU-CIUDSAMLSA-N 0.000 description 4
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 4
- CUMXHKAOHNWRFQ-BZSNNMDCSA-N Phe-Asp-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=CC=C1 CUMXHKAOHNWRFQ-BZSNNMDCSA-N 0.000 description 4
- JHSRGEODDALISP-XVSYOHENSA-N Phe-Thr-Asn Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(O)=O JHSRGEODDALISP-XVSYOHENSA-N 0.000 description 4
- FGWUALWGCZJQDJ-URLPEUOOSA-N Phe-Thr-Ile Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FGWUALWGCZJQDJ-URLPEUOOSA-N 0.000 description 4
- HFZNNDWPHBRNPV-KZVJFYERSA-N Pro-Ala-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O HFZNNDWPHBRNPV-KZVJFYERSA-N 0.000 description 4
- LGSANCBHSMDFDY-GARJFASQSA-N Pro-Glu-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCC(=O)O)C(=O)N2CCC[C@@H]2C(=O)O LGSANCBHSMDFDY-GARJFASQSA-N 0.000 description 4
- RMODQFBNDDENCP-IHRRRGAJSA-N Pro-Lys-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O RMODQFBNDDENCP-IHRRRGAJSA-N 0.000 description 4
- PRKWBYCXBBSLSK-GUBZILKMSA-N Pro-Ser-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O PRKWBYCXBBSLSK-GUBZILKMSA-N 0.000 description 4
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 4
- QEDMOZUJTGEIBF-FXQIFTODSA-N Ser-Arg-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O QEDMOZUJTGEIBF-FXQIFTODSA-N 0.000 description 4
- QGMLKFGTGXWAHF-IHRRRGAJSA-N Ser-Arg-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O QGMLKFGTGXWAHF-IHRRRGAJSA-N 0.000 description 4
- 108010071390 Serum Albumin Proteins 0.000 description 4
- 102000007562 Serum Albumin Human genes 0.000 description 4
- LIXBDERDAGNVAV-XKBZYTNZSA-N Thr-Gln-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O LIXBDERDAGNVAV-XKBZYTNZSA-N 0.000 description 4
- DXPURPNJDFCKKO-RHYQMDGZSA-N Thr-Lys-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)[C@@H](C)O)C(O)=O DXPURPNJDFCKKO-RHYQMDGZSA-N 0.000 description 4
- JVTHMUDOKPQBOT-NSHDSACASA-N Trp-Gly-Gly Chemical compound C1=CC=C2C(C[C@H]([NH3+])C(=O)NCC(=O)NCC([O-])=O)=CNC2=C1 JVTHMUDOKPQBOT-NSHDSACASA-N 0.000 description 4
- GQHAIUPYZPTADF-FDARSICLSA-N Trp-Ile-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 GQHAIUPYZPTADF-FDARSICLSA-N 0.000 description 4
- QOIKZODVIPOPDD-AVGNSLFASA-N Tyr-Cys-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(O)=O QOIKZODVIPOPDD-AVGNSLFASA-N 0.000 description 4
- GPLTZEMVOCZVAV-UFYCRDLUSA-N Tyr-Tyr-Arg Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)C1=CC=C(O)C=C1 GPLTZEMVOCZVAV-UFYCRDLUSA-N 0.000 description 4
- HGJRMXOWUWVUOA-GVXVVHGQSA-N Val-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N HGJRMXOWUWVUOA-GVXVVHGQSA-N 0.000 description 4
- 108010081404 acein-2 Proteins 0.000 description 4
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 4
- 125000003275 alpha amino acid group Chemical group 0.000 description 4
- 235000009697 arginine Nutrition 0.000 description 4
- 108010040443 aspartyl-aspartic acid Proteins 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 239000012228 culture supernatant Substances 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- DNJIEGIFACGWOD-UHFFFAOYSA-N ethyl mercaptane Natural products CCS DNJIEGIFACGWOD-UHFFFAOYSA-N 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 238000011835 investigation Methods 0.000 description 4
- 108010017391 lysylvaline Proteins 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 239000006199 nebulizer Substances 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- 229920001282 polysaccharide Polymers 0.000 description 4
- 239000005017 polysaccharide Substances 0.000 description 4
- 108010026333 seryl-proline Proteins 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- 229910052708 sodium Inorganic materials 0.000 description 4
- ISIJQEHRDSCQIU-UHFFFAOYSA-N tert-butyl 2,7-diazaspiro[4.5]decane-7-carboxylate Chemical compound C1N(C(=O)OC(C)(C)C)CCCC11CNCC1 ISIJQEHRDSCQIU-UHFFFAOYSA-N 0.000 description 4
- 108010058119 tryptophyl-glycyl-glycine Proteins 0.000 description 4
- 108010071635 tyrosyl-prolyl-arginine Proteins 0.000 description 4
- 238000004704 ultra performance liquid chromatography Methods 0.000 description 4
- 238000000108 ultra-filtration Methods 0.000 description 4
- 238000011100 viral filtration Methods 0.000 description 4
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical group COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 3
- QMOQBVOBWVNSNO-UHFFFAOYSA-N 2-[[2-[[2-[(2-azaniumylacetyl)amino]acetyl]amino]acetyl]amino]acetate Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(O)=O QMOQBVOBWVNSNO-UHFFFAOYSA-N 0.000 description 3
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- SBGXWWCLHIOABR-UHFFFAOYSA-N Ala Ala Gly Ala Chemical compound CC(N)C(=O)NC(C)C(=O)NCC(=O)NC(C)C(O)=O SBGXWWCLHIOABR-UHFFFAOYSA-N 0.000 description 3
- RLMISHABBKUNFO-WHFBIAKZSA-N Ala-Ala-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)NCC(O)=O RLMISHABBKUNFO-WHFBIAKZSA-N 0.000 description 3
- WCBVQNZTOKJWJS-ACZMJKKPSA-N Ala-Cys-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(O)=O WCBVQNZTOKJWJS-ACZMJKKPSA-N 0.000 description 3
- MNZHHDPWDWQJCQ-YUMQZZPRSA-N Ala-Leu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O MNZHHDPWDWQJCQ-YUMQZZPRSA-N 0.000 description 3
- 102000009027 Albumins Human genes 0.000 description 3
- 108010088751 Albumins Proteins 0.000 description 3
- RBOBTTLFPRSXKZ-BZSNNMDCSA-N Asn-Phe-Tyr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O RBOBTTLFPRSXKZ-BZSNNMDCSA-N 0.000 description 3
- YNQIDCRRTWGHJD-ZLUOBGJFSA-N Asp-Asn-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC(O)=O YNQIDCRRTWGHJD-ZLUOBGJFSA-N 0.000 description 3
- HSWYMWGDMPLTTH-FXQIFTODSA-N Asp-Glu-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HSWYMWGDMPLTTH-FXQIFTODSA-N 0.000 description 3
- NVFSJIXJZCDICF-SRVKXCTJSA-N Asp-Lys-Lys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)O)N NVFSJIXJZCDICF-SRVKXCTJSA-N 0.000 description 3
- VNXQRBXEQXLERQ-CIUDSAMLSA-N Asp-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(=O)O)N VNXQRBXEQXLERQ-CIUDSAMLSA-N 0.000 description 3
- SQIARYGNVQWOSB-BZSNNMDCSA-N Asp-Tyr-Phe Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O SQIARYGNVQWOSB-BZSNNMDCSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- AMRLSQGGERHDHJ-FXQIFTODSA-N Cys-Ala-Arg Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AMRLSQGGERHDHJ-FXQIFTODSA-N 0.000 description 3
- 108010092160 Dactinomycin Proteins 0.000 description 3
- 102100024746 Dihydrofolate reductase Human genes 0.000 description 3
- FGYPOQPQTUNESW-IUCAKERBSA-N Gln-Gly-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CCC(=O)N)N FGYPOQPQTUNESW-IUCAKERBSA-N 0.000 description 3
- XKPACHRGOWQHFH-IRIUXVKKSA-N Gln-Thr-Tyr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O XKPACHRGOWQHFH-IRIUXVKKSA-N 0.000 description 3
- DMYACXMQUABZIQ-NRPADANISA-N Glu-Ser-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O DMYACXMQUABZIQ-NRPADANISA-N 0.000 description 3
- VSVZIEVNUYDAFR-YUMQZZPRSA-N Gly-Ala-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN VSVZIEVNUYDAFR-YUMQZZPRSA-N 0.000 description 3
- XCLCVBYNGXEVDU-WHFBIAKZSA-N Gly-Asn-Ser Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O XCLCVBYNGXEVDU-WHFBIAKZSA-N 0.000 description 3
- BIRKKBCSAIHDDF-WDSKDSINSA-N Gly-Glu-Cys Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(O)=O BIRKKBCSAIHDDF-WDSKDSINSA-N 0.000 description 3
- YWAQATDNEKZFFK-BYPYZUCNSA-N Gly-Gly-Ser Chemical compound NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O YWAQATDNEKZFFK-BYPYZUCNSA-N 0.000 description 3
- HAOUOFNNJJLVNS-BQBZGAKWSA-N Gly-Pro-Ser Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O HAOUOFNNJJLVNS-BQBZGAKWSA-N 0.000 description 3
- WCORRBXVISTKQL-WHFBIAKZSA-N Gly-Ser-Ser Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WCORRBXVISTKQL-WHFBIAKZSA-N 0.000 description 3
- FFJQHWKSGAWSTJ-BFHQHQDPSA-N Gly-Thr-Ala Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O FFJQHWKSGAWSTJ-BFHQHQDPSA-N 0.000 description 3
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 3
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 3
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 3
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 3
- TVMNTHXFRSXZGR-IHRRRGAJSA-N His-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O TVMNTHXFRSXZGR-IHRRRGAJSA-N 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- FADXGVVLSPPEQY-GHCJXIJMSA-N Ile-Cys-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)N)C(=O)O)N FADXGVVLSPPEQY-GHCJXIJMSA-N 0.000 description 3
- 102000000588 Interleukin-2 Human genes 0.000 description 3
- 108010002350 Interleukin-2 Proteins 0.000 description 3
- UGTHTQWIQKEDEH-BQBZGAKWSA-N L-alanyl-L-prolylglycine zwitterion Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O UGTHTQWIQKEDEH-BQBZGAKWSA-N 0.000 description 3
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 3
- GPICTNQYKHHHTH-GUBZILKMSA-N Leu-Gln-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O GPICTNQYKHHHTH-GUBZILKMSA-N 0.000 description 3
- HYMLKESRWLZDBR-WEDXCCLWSA-N Leu-Gly-Thr Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O HYMLKESRWLZDBR-WEDXCCLWSA-N 0.000 description 3
- IAJFFZORSWOZPQ-SRVKXCTJSA-N Leu-Leu-Asn Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O IAJFFZORSWOZPQ-SRVKXCTJSA-N 0.000 description 3
- VCHVSKNMTXWIIP-SRVKXCTJSA-N Leu-Lys-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O VCHVSKNMTXWIIP-SRVKXCTJSA-N 0.000 description 3
- YQFZRHYZLARWDY-IHRRRGAJSA-N Leu-Val-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN YQFZRHYZLARWDY-IHRRRGAJSA-N 0.000 description 3
- MPGHETGWWWUHPY-CIUDSAMLSA-N Lys-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN MPGHETGWWWUHPY-CIUDSAMLSA-N 0.000 description 3
- YTJFXEDRUOQGSP-DCAQKATOSA-N Lys-Pro-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YTJFXEDRUOQGSP-DCAQKATOSA-N 0.000 description 3
- XABXVVSWUVCZST-GVXVVHGQSA-N Lys-Val-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN XABXVVSWUVCZST-GVXVVHGQSA-N 0.000 description 3
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 3
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 3
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 3
- 101100342977 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) leu-1 gene Proteins 0.000 description 3
- HXSUFWQYLPKEHF-IHRRRGAJSA-N Phe-Asn-Arg Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N HXSUFWQYLPKEHF-IHRRRGAJSA-N 0.000 description 3
- BWTKUQPNOMMKMA-FIRPJDEBSA-N Phe-Ile-Phe Chemical compound C([C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 BWTKUQPNOMMKMA-FIRPJDEBSA-N 0.000 description 3
- JLLJTMHNXQTMCK-UBHSHLNASA-N Phe-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC1=CC=CC=C1 JLLJTMHNXQTMCK-UBHSHLNASA-N 0.000 description 3
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 3
- IHCXPSYCHXFXKT-DCAQKATOSA-N Pro-Arg-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O IHCXPSYCHXFXKT-DCAQKATOSA-N 0.000 description 3
- FDMKYQQYJKYCLV-GUBZILKMSA-N Pro-Pro-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 FDMKYQQYJKYCLV-GUBZILKMSA-N 0.000 description 3
- GZFAWAQTEYDKII-YUMQZZPRSA-N Ser-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO GZFAWAQTEYDKII-YUMQZZPRSA-N 0.000 description 3
- FPCGZYMRFFIYIH-CIUDSAMLSA-N Ser-Lys-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O FPCGZYMRFFIYIH-CIUDSAMLSA-N 0.000 description 3
- HHJFMHQYEAAOBM-ZLUOBGJFSA-N Ser-Ser-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O HHJFMHQYEAAOBM-ZLUOBGJFSA-N 0.000 description 3
- CUXJENOFJXOSOZ-BIIVOSGPSA-N Ser-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CO)N)C(=O)O CUXJENOFJXOSOZ-BIIVOSGPSA-N 0.000 description 3
- PCJLFYBAQZQOFE-KATARQTJSA-N Ser-Thr-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N)O PCJLFYBAQZQOFE-KATARQTJSA-N 0.000 description 3
- AXKJPUBALUNJEO-UBHSHLNASA-N Ser-Trp-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(N)=O)C(O)=O AXKJPUBALUNJEO-UBHSHLNASA-N 0.000 description 3
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 3
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 3
- DWYAUVCQDTZIJI-VZFHVOOUSA-N Thr-Ala-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O DWYAUVCQDTZIJI-VZFHVOOUSA-N 0.000 description 3
- GKWNLDNXMMLRMC-GLLZPBPUSA-N Thr-Glu-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)O GKWNLDNXMMLRMC-GLLZPBPUSA-N 0.000 description 3
- DJDSEDOKJTZBAR-ZDLURKLDSA-N Thr-Gly-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O DJDSEDOKJTZBAR-ZDLURKLDSA-N 0.000 description 3
- YOOAQCZYZHGUAZ-KATARQTJSA-N Thr-Leu-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YOOAQCZYZHGUAZ-KATARQTJSA-N 0.000 description 3
- OGOYMQWIWHGTGH-KZVJFYERSA-N Thr-Val-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O OGOYMQWIWHGTGH-KZVJFYERSA-N 0.000 description 3
- NLWCSMOXNKBRLC-WDSOQIARSA-N Trp-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O NLWCSMOXNKBRLC-WDSOQIARSA-N 0.000 description 3
- SLCSPPCQWUHPPO-JYJNAYRXSA-N Tyr-Glu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 SLCSPPCQWUHPPO-JYJNAYRXSA-N 0.000 description 3
- GNWUWQAVVJQREM-NHCYSSNCSA-N Val-Asn-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N GNWUWQAVVJQREM-NHCYSSNCSA-N 0.000 description 3
- WDIGUPHXPBMODF-UMNHJUIQSA-N Val-Glu-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N WDIGUPHXPBMODF-UMNHJUIQSA-N 0.000 description 3
- ZIGZPYJXIWLQFC-QTKMDUPCSA-N Val-His-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](C(C)C)N)O ZIGZPYJXIWLQFC-QTKMDUPCSA-N 0.000 description 3
- VCIYTVOBLZHFSC-XHSDSOJGSA-N Val-Phe-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N VCIYTVOBLZHFSC-XHSDSOJGSA-N 0.000 description 3
- TVGWMCTYUFBXAP-QTKMDUPCSA-N Val-Thr-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N)O TVGWMCTYUFBXAP-QTKMDUPCSA-N 0.000 description 3
- GVNLOVJNNDZUHS-RHYQMDGZSA-N Val-Thr-Lys Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(O)=O GVNLOVJNNDZUHS-RHYQMDGZSA-N 0.000 description 3
- ZHWZDZFWBXWPDW-GUBZILKMSA-N Val-Val-Cys Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(O)=O ZHWZDZFWBXWPDW-GUBZILKMSA-N 0.000 description 3
- 108010024078 alanyl-glycyl-serine Proteins 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- 231100000599 cytotoxic agent Toxicity 0.000 description 3
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 3
- 108020001096 dihydrofolate reductase Proteins 0.000 description 3
- 239000000539 dimer Substances 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 239000012149 elution buffer Substances 0.000 description 3
- 210000001035 gastrointestinal tract Anatomy 0.000 description 3
- 150000004676 glycans Chemical class 0.000 description 3
- 108010000434 glycyl-alanyl-leucine Proteins 0.000 description 3
- 108010050475 glycyl-leucyl-tyrosine Proteins 0.000 description 3
- 108010085325 histidylproline Proteins 0.000 description 3
- 210000004408 hybridoma Anatomy 0.000 description 3
- 230000001900 immune effect Effects 0.000 description 3
- 229940072221 immunoglobulins Drugs 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 239000008101 lactose Substances 0.000 description 3
- 239000002502 liposome Substances 0.000 description 3
- 239000011777 magnesium Substances 0.000 description 3
- 229910052749 magnesium Inorganic materials 0.000 description 3
- 210000004962 mammalian cell Anatomy 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- MXHCPCSDRGLRER-UHFFFAOYSA-N pentaglycine Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(O)=O MXHCPCSDRGLRER-UHFFFAOYSA-N 0.000 description 3
- 238000002823 phage display Methods 0.000 description 3
- 108010024654 phenylalanyl-prolyl-alanine Proteins 0.000 description 3
- 108010051242 phenylalanylserine Proteins 0.000 description 3
- 239000011591 potassium Substances 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 108010053725 prolylvaline Proteins 0.000 description 3
- 230000002285 radioactive effect Effects 0.000 description 3
- 102000005962 receptors Human genes 0.000 description 3
- 108020003175 receptors Proteins 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 108010069117 seryl-lysyl-aspartic acid Proteins 0.000 description 3
- 239000001509 sodium citrate Substances 0.000 description 3
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 3
- 108010061238 threonyl-glycine Proteins 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 239000003053 toxin Substances 0.000 description 3
- 231100000765 toxin Toxicity 0.000 description 3
- 108700012359 toxins Proteins 0.000 description 3
- 238000012384 transportation and delivery Methods 0.000 description 3
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 2
- PCDUALPXEOKZPE-DXCABUDRSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-amino-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoic acid Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O PCDUALPXEOKZPE-DXCABUDRSA-N 0.000 description 2
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 description 2
- LVGUZGTVOIAKKC-UHFFFAOYSA-N 1,1,1,2-tetrafluoroethane Chemical compound FCC(F)(F)F LVGUZGTVOIAKKC-UHFFFAOYSA-N 0.000 description 2
- XJFPXLWGZWAWRQ-UHFFFAOYSA-N 2-[[2-[[2-[[2-[[2-[(2-azaniumylacetyl)amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetate Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(O)=O XJFPXLWGZWAWRQ-UHFFFAOYSA-N 0.000 description 2
- AXAVXPMQTGXXJZ-UHFFFAOYSA-N 2-aminoacetic acid;2-amino-2-(hydroxymethyl)propane-1,3-diol Chemical compound NCC(O)=O.OCC(N)(CO)CO AXAVXPMQTGXXJZ-UHFFFAOYSA-N 0.000 description 2
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 2
- IPZQNYYAYVRKKK-FXQIFTODSA-N Ala-Pro-Ala Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O IPZQNYYAYVRKKK-FXQIFTODSA-N 0.000 description 2
- RTZCUEHYUQZIDE-WHFBIAKZSA-N Ala-Ser-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O RTZCUEHYUQZIDE-WHFBIAKZSA-N 0.000 description 2
- KUFVXLQLDHJVOG-SHGPDSBTSA-N Ala-Thr-Thr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](C)N)O KUFVXLQLDHJVOG-SHGPDSBTSA-N 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- HZZIFFOVHLWGCS-KKUMJFAQSA-N Asn-Phe-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(O)=O HZZIFFOVHLWGCS-KKUMJFAQSA-N 0.000 description 2
- 102100021277 Beta-secretase 2 Human genes 0.000 description 2
- 101710150190 Beta-secretase 2 Proteins 0.000 description 2
- 238000009010 Bradford assay Methods 0.000 description 2
- 101150013553 CD40 gene Proteins 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- SMEYEQDCCBHTEF-FXQIFTODSA-N Cys-Pro-Ala Chemical compound [H]N[C@@H](CS)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O SMEYEQDCCBHTEF-FXQIFTODSA-N 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- YVGGHNCTFXOJCH-UHFFFAOYSA-N DDT Chemical compound C1=CC(Cl)=CC=C1C(C(Cl)(Cl)Cl)C1=CC=C(Cl)C=C1 YVGGHNCTFXOJCH-UHFFFAOYSA-N 0.000 description 2
- 229920002307 Dextran Polymers 0.000 description 2
- YCAGGFXSFQFVQL-UHFFFAOYSA-N Endothion Chemical compound COC1=COC(CSP(=O)(OC)OC)=CC1=O YCAGGFXSFQFVQL-UHFFFAOYSA-N 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- HMIXCETWRYDVMO-GUBZILKMSA-N Gln-Pro-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O HMIXCETWRYDVMO-GUBZILKMSA-N 0.000 description 2
- XTZDZAXYPDISRR-MNXVOIDGSA-N Glu-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N XTZDZAXYPDISRR-MNXVOIDGSA-N 0.000 description 2
- JVZLZVJTIXVIHK-SXNHZJKMSA-N Glu-Trp-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CCC(=O)O)N JVZLZVJTIXVIHK-SXNHZJKMSA-N 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- LJPIRKICOISLKN-WHFBIAKZSA-N Gly-Ala-Ser Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O LJPIRKICOISLKN-WHFBIAKZSA-N 0.000 description 2
- SSFWXSNOKDZNHY-QXEWZRGKSA-N Gly-Pro-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CN SSFWXSNOKDZNHY-QXEWZRGKSA-N 0.000 description 2
- DNAZKGFYFRGZIH-QWRGUYRKSA-N Gly-Tyr-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CC1=CC=C(O)C=C1 DNAZKGFYFRGZIH-QWRGUYRKSA-N 0.000 description 2
- 108010093488 His-His-His-His-His-His Proteins 0.000 description 2
- RGSOCXHDOPQREB-ZPFDUUQYSA-N Ile-Asp-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(C)C)C(=O)O)N RGSOCXHDOPQREB-ZPFDUUQYSA-N 0.000 description 2
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 2
- JZNVOBUNTWNZPW-GHCJXIJMSA-N Ile-Ser-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(=O)O)C(=O)O)N JZNVOBUNTWNZPW-GHCJXIJMSA-N 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 2
- WQWSMEOYXJTFRU-GUBZILKMSA-N Leu-Glu-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O WQWSMEOYXJTFRU-GUBZILKMSA-N 0.000 description 2
- MVJRBCJCRYGCKV-GVXVVHGQSA-N Leu-Val-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O MVJRBCJCRYGCKV-GVXVVHGQSA-N 0.000 description 2
- SSYOBDBNBQBSQE-SRVKXCTJSA-N Lys-Cys-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O SSYOBDBNBQBSQE-SRVKXCTJSA-N 0.000 description 2
- LOGFVTREOLYCPF-RHYQMDGZSA-N Lys-Pro-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCCN LOGFVTREOLYCPF-RHYQMDGZSA-N 0.000 description 2
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- 102000018697 Membrane Proteins Human genes 0.000 description 2
- 108010052285 Membrane Proteins Proteins 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- 206010028980 Neoplasm Diseases 0.000 description 2
- 108010079246 OMPA outer membrane proteins Proteins 0.000 description 2
- 241000283973 Oryctolagus cuniculus Species 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 101150034459 Parpbp gene Proteins 0.000 description 2
- FELJDCNGZFDUNR-WDSKDSINSA-N Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1 FELJDCNGZFDUNR-WDSKDSINSA-N 0.000 description 2
- KIZQGKLMXKGDIV-BQBZGAKWSA-N Pro-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1 KIZQGKLMXKGDIV-BQBZGAKWSA-N 0.000 description 2
- SBVPYBFMIGDIDX-SRVKXCTJSA-N Pro-Pro-Pro Chemical compound OC(=O)[C@@H]1CCCN1C(=O)[C@H]1N(C(=O)[C@H]2NCCC2)CCC1 SBVPYBFMIGDIDX-SRVKXCTJSA-N 0.000 description 2
- GOMUXSCOIWIJFP-GUBZILKMSA-N Pro-Ser-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O GOMUXSCOIWIJFP-GUBZILKMSA-N 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 2
- 239000012722 SDS sample buffer Substances 0.000 description 2
- ICHZYBVODUVUKN-SRVKXCTJSA-N Ser-Asn-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ICHZYBVODUVUKN-SRVKXCTJSA-N 0.000 description 2
- QPFJSHSJFIYDJZ-GHCJXIJMSA-N Ser-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO QPFJSHSJFIYDJZ-GHCJXIJMSA-N 0.000 description 2
- KCFKKAQKRZBWJB-ZLUOBGJFSA-N Ser-Cys-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(O)=O KCFKKAQKRZBWJB-ZLUOBGJFSA-N 0.000 description 2
- UIPXCLNLUUAMJU-JBDRJPRFSA-N Ser-Ile-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O UIPXCLNLUUAMJU-JBDRJPRFSA-N 0.000 description 2
- KCGIREHVWRXNDH-GARJFASQSA-N Ser-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CO)N KCGIREHVWRXNDH-GARJFASQSA-N 0.000 description 2
- MQUZANJDFOQOBX-SRVKXCTJSA-N Ser-Phe-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(O)=O MQUZANJDFOQOBX-SRVKXCTJSA-N 0.000 description 2
- ADJDNJCSPNFFPI-FXQIFTODSA-N Ser-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO ADJDNJCSPNFFPI-FXQIFTODSA-N 0.000 description 2
- AZWNCEBQZXELEZ-FXQIFTODSA-N Ser-Pro-Ser Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O AZWNCEBQZXELEZ-FXQIFTODSA-N 0.000 description 2
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 2
- XSTGOZBBXFKGHA-YJRXYDGGSA-N Thr-His-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N)O XSTGOZBBXFKGHA-YJRXYDGGSA-N 0.000 description 2
- KZSYAEWQMJEGRZ-RHYQMDGZSA-N Thr-Leu-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O KZSYAEWQMJEGRZ-RHYQMDGZSA-N 0.000 description 2
- MROIJTGJGIDEEJ-RCWTZXSCSA-N Thr-Pro-Pro Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 MROIJTGJGIDEEJ-RCWTZXSCSA-N 0.000 description 2
- QJIODPFLAASXJC-JHYOHUSXSA-N Thr-Thr-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N)O QJIODPFLAASXJC-JHYOHUSXSA-N 0.000 description 2
- QNXZCKMXHPULME-ZNSHCXBVSA-N Thr-Val-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N)O QNXZCKMXHPULME-ZNSHCXBVSA-N 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- HYVLNORXQGKONN-NUTKFTJISA-N Trp-Ala-Lys Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O)=CNC2=C1 HYVLNORXQGKONN-NUTKFTJISA-N 0.000 description 2
- DQDXHYIEITXNJY-BPUTZDHNSA-N Trp-Gln-Gln Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N DQDXHYIEITXNJY-BPUTZDHNSA-N 0.000 description 2
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 2
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 2
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 2
- NOXKHHXSHQFSGJ-FQPOAREZSA-N Tyr-Ala-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NOXKHHXSHQFSGJ-FQPOAREZSA-N 0.000 description 2
- VGQOVCHZGQWAOI-UHFFFAOYSA-N UNPD55612 Natural products N1C(O)C2CC(C=CC(N)=O)=CN2C(=O)C2=CC=C(C)C(O)=C12 VGQOVCHZGQWAOI-UHFFFAOYSA-N 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- JIODCDXKCJRMEH-NHCYSSNCSA-N Val-Arg-Gln Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N JIODCDXKCJRMEH-NHCYSSNCSA-N 0.000 description 2
- SJRUJQFQVLMZFW-WPRPVWTQSA-N Val-Pro-Gly Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O SJRUJQFQVLMZFW-WPRPVWTQSA-N 0.000 description 2
- UGFMVXRXULGLNO-XPUUQOCRSA-N Val-Ser-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O UGFMVXRXULGLNO-XPUUQOCRSA-N 0.000 description 2
- SVLAAUGFIHSJPK-JYJNAYRXSA-N Val-Trp-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CO)C(=O)O)N SVLAAUGFIHSJPK-JYJNAYRXSA-N 0.000 description 2
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 2
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- VGQOVCHZGQWAOI-HYUHUPJXSA-N anthramycin Chemical compound N1[C@@H](O)[C@@H]2CC(\C=C\C(N)=O)=CN2C(=O)C2=CC=C(C)C(O)=C12 VGQOVCHZGQWAOI-HYUHUPJXSA-N 0.000 description 2
- 108010009111 arginyl-glycyl-glutamic acid Proteins 0.000 description 2
- 108010060035 arginylproline Proteins 0.000 description 2
- 108010068265 aspartyltyrosine Proteins 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 238000012219 cassette mutagenesis Methods 0.000 description 2
- 239000012930 cell culture fluid Substances 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 238000010367 cloning Methods 0.000 description 2
- 230000001268 conjugating effect Effects 0.000 description 2
- 239000002619 cytotoxin Substances 0.000 description 2
- 229960000640 dactinomycin Drugs 0.000 description 2
- 229960000975 daunorubicin Drugs 0.000 description 2
- 238000000502 dialysis Methods 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- FSXRLASFHBWESK-UHFFFAOYSA-N dipeptide phenylalanyl-tyrosine Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FSXRLASFHBWESK-UHFFFAOYSA-N 0.000 description 2
- 150000002016 disaccharides Chemical class 0.000 description 2
- 229960004679 doxorubicin Drugs 0.000 description 2
- 239000013020 final formulation Substances 0.000 description 2
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 2
- 108010077515 glycylproline Proteins 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 108010044426 integrins Proteins 0.000 description 2
- 102000006495 integrins Human genes 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- NNPPMTNAJDCUHE-UHFFFAOYSA-N isobutane Chemical compound CC(C)C NNPPMTNAJDCUHE-UHFFFAOYSA-N 0.000 description 2
- 108010057821 leucylproline Proteins 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 229920001427 mPEG Polymers 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- 229930182817 methionine Natural products 0.000 description 2
- 229960004857 mitomycin Drugs 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 150000002772 monosaccharides Chemical class 0.000 description 2
- 238000002703 mutagenesis Methods 0.000 description 2
- 231100000350 mutagenesis Toxicity 0.000 description 2
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 108010089198 phenylalanyl-prolyl-arginine Proteins 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 229920001281 polyalkylene Polymers 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 229950008882 polysorbate Drugs 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- AQHHHDLHHXJYJD-UHFFFAOYSA-N propranolol Chemical compound C1=CC=C2C(OCC(O)CNC(C)C)=CC=CC2=C1 AQHHHDLHHXJYJD-UHFFFAOYSA-N 0.000 description 2
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 230000000241 respiratory effect Effects 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 108010048818 seryl-histidine Proteins 0.000 description 2
- 238000001542 size-exclusion chromatography Methods 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 235000010356 sorbitol Nutrition 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 230000001954 sterilising effect Effects 0.000 description 2
- 238000004659 sterilization and disinfection Methods 0.000 description 2
- 239000012536 storage buffer Substances 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 229920001059 synthetic polymer Polymers 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 2
- 229960003048 vinblastine Drugs 0.000 description 2
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 2
- 229960004528 vincristine Drugs 0.000 description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- XVZCXCTYGHPNEM-IHRRRGAJSA-N (2s)-1-[(2s)-2-[[(2s)-2-amino-4-methylpentanoyl]amino]-4-methylpentanoyl]pyrrolidine-2-carboxylic acid Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(O)=O XVZCXCTYGHPNEM-IHRRRGAJSA-N 0.000 description 1
- CWFMWBHMIMNZLN-NAKRPEOUSA-N (2s)-1-[(2s)-2-[[(2s,3s)-2-amino-3-methylpentanoyl]amino]propanoyl]pyrrolidine-2-carboxylic acid Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O CWFMWBHMIMNZLN-NAKRPEOUSA-N 0.000 description 1
- YFMFNYKEUDLDTL-UHFFFAOYSA-N 1,1,1,2,3,3,3-heptafluoropropane Chemical compound FC(F)(F)C(F)C(F)(F)F YFMFNYKEUDLDTL-UHFFFAOYSA-N 0.000 description 1
- WDXYVJKNSMILOQ-UHFFFAOYSA-N 1,3,2-dioxathiolane 2-oxide Chemical compound O=S1OCCO1 WDXYVJKNSMILOQ-UHFFFAOYSA-N 0.000 description 1
- YIWGJFPJRAEKMK-UHFFFAOYSA-N 1-(2H-benzotriazol-5-yl)-3-methyl-8-[2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidine-5-carbonyl]-1,3,8-triazaspiro[4.5]decane-2,4-dione Chemical compound CN1C(=O)N(c2ccc3n[nH]nc3c2)C2(CCN(CC2)C(=O)c2cnc(NCc3cccc(OC(F)(F)F)c3)nc2)C1=O YIWGJFPJRAEKMK-UHFFFAOYSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- WUAPFZMCVAUBPE-NJFSPNSNSA-N 188Re Chemical compound [188Re] WUAPFZMCVAUBPE-NJFSPNSNSA-N 0.000 description 1
- WQNHWIYLCRZRLR-UHFFFAOYSA-N 2-(3-hydroxy-2,5-dioxooxolan-3-yl)acetic acid Chemical compound OC(=O)CC1(O)CC(=O)OC1=O WQNHWIYLCRZRLR-UHFFFAOYSA-N 0.000 description 1
- DQVAZKGVGKHQDS-UHFFFAOYSA-N 2-[[1-[2-[(2-amino-4-methylpentanoyl)amino]-4-methylpentanoyl]pyrrolidine-2-carbonyl]amino]-4-methylpentanoic acid Chemical compound CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(=O)NC(CC(C)C)C(O)=O DQVAZKGVGKHQDS-UHFFFAOYSA-N 0.000 description 1
- SCPRYBYMKVYVND-UHFFFAOYSA-N 2-[[2-[[1-(2-amino-4-methylpentanoyl)pyrrolidine-2-carbonyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoic acid Chemical compound CC(C)CC(N)C(=O)N1CCCC1C(=O)NC(CC(C)C)C(=O)NC(CC(C)C)C(O)=O SCPRYBYMKVYVND-UHFFFAOYSA-N 0.000 description 1
- QWTLUPDHBKBULE-UHFFFAOYSA-N 2-[[2-[[2-[[2-[[2-[[2-[[2-[[2-[[2-[(2-aminoacetyl)amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetyl]amino]acetic acid Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(=O)NCC(O)=O QWTLUPDHBKBULE-UHFFFAOYSA-N 0.000 description 1
- GKYQGGAVQQOZQC-UHFFFAOYSA-N 3-phosphanylpropane-1,1,1-triol Chemical compound OC(O)(O)CCP GKYQGGAVQQOZQC-UHFFFAOYSA-N 0.000 description 1
- MCNQUWLLXZZZAC-UHFFFAOYSA-N 4-cyano-1-(2,4-dichlorophenyl)-5-(4-methoxyphenyl)-n-piperidin-1-ylpyrazole-3-carboxamide Chemical compound C1=CC(OC)=CC=C1C1=C(C#N)C(C(=O)NN2CCCCC2)=NN1C1=CC=C(Cl)C=C1Cl MCNQUWLLXZZZAC-UHFFFAOYSA-N 0.000 description 1
- 102100023990 60S ribosomal protein L17 Human genes 0.000 description 1
- CJIJXIFQYOPWTF-UHFFFAOYSA-N 7-hydroxycoumarin Natural products O1C(=O)C=CC2=CC(O)=CC=C21 CJIJXIFQYOPWTF-UHFFFAOYSA-N 0.000 description 1
- 102100031585 ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Human genes 0.000 description 1
- 108010066676 Abrin Proteins 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 102000012440 Acetylcholinesterase Human genes 0.000 description 1
- 108010022752 Acetylcholinesterase Proteins 0.000 description 1
- CXRCVCURMBFFOL-FXQIFTODSA-N Ala-Ala-Pro Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O CXRCVCURMBFFOL-FXQIFTODSA-N 0.000 description 1
- JAMAWBXXKFGFGX-KZVJFYERSA-N Ala-Arg-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JAMAWBXXKFGFGX-KZVJFYERSA-N 0.000 description 1
- FAJIYNONGXEXAI-CQDKDKBSSA-N Ala-His-Phe Chemical compound C([C@H](NC(=O)[C@@H](N)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CNC=N1 FAJIYNONGXEXAI-CQDKDKBSSA-N 0.000 description 1
- HJGZVLLLBJLXFC-LSJOCFKGSA-N Ala-His-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](C(C)C)C(O)=O HJGZVLLLBJLXFC-LSJOCFKGSA-N 0.000 description 1
- WPWUFUBLGADILS-WDSKDSINSA-N Ala-Pro Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(O)=O WPWUFUBLGADILS-WDSKDSINSA-N 0.000 description 1
- CQJHFKKGZXKZBC-BPNCWPANSA-N Ala-Pro-Tyr Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 CQJHFKKGZXKZBC-BPNCWPANSA-N 0.000 description 1
- YHBDGLZYNIARKJ-GUBZILKMSA-N Ala-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N YHBDGLZYNIARKJ-GUBZILKMSA-N 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 229920000856 Amylose Polymers 0.000 description 1
- 102400000068 Angiostatin Human genes 0.000 description 1
- 108010079709 Angiostatins Proteins 0.000 description 1
- 235000002198 Annona diversifolia Nutrition 0.000 description 1
- 102100031930 Anterior gradient protein 3 Human genes 0.000 description 1
- 101710099705 Anti-lipopolysaccharide factor Proteins 0.000 description 1
- 241000239290 Araneae Species 0.000 description 1
- OTOXOKCIIQLMFH-KZVJFYERSA-N Arg-Ala-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N OTOXOKCIIQLMFH-KZVJFYERSA-N 0.000 description 1
- JGDGLDNAQJJGJI-AVGNSLFASA-N Arg-Arg-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)N JGDGLDNAQJJGJI-AVGNSLFASA-N 0.000 description 1
- HKRXJBBCQBAGIM-FXQIFTODSA-N Arg-Asp-Ser Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CO)C(=O)O)N)CN=C(N)N HKRXJBBCQBAGIM-FXQIFTODSA-N 0.000 description 1
- YWENWUYXQUWRHQ-LPEHRKFASA-N Arg-Cys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CS)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O YWENWUYXQUWRHQ-LPEHRKFASA-N 0.000 description 1
- COXMUHNBYCVVRG-DCAQKATOSA-N Arg-Leu-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O COXMUHNBYCVVRG-DCAQKATOSA-N 0.000 description 1
- KZXPVYVSHUJCEO-ULQDDVLXSA-N Arg-Phe-Lys Chemical compound NC(=N)NCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CCCCN)C(O)=O)CC1=CC=CC=C1 KZXPVYVSHUJCEO-ULQDDVLXSA-N 0.000 description 1
- LQJAALCCPOTJGB-YUMQZZPRSA-N Arg-Pro Chemical compound NC(N)=NCCC[C@H](N)C(=O)N1CCC[C@H]1C(O)=O LQJAALCCPOTJGB-YUMQZZPRSA-N 0.000 description 1
- NGYHSXDNNOFHNE-AVGNSLFASA-N Arg-Pro-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O NGYHSXDNNOFHNE-AVGNSLFASA-N 0.000 description 1
- VUGWHBXPMAHEGZ-SRVKXCTJSA-N Arg-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCN=C(N)N VUGWHBXPMAHEGZ-SRVKXCTJSA-N 0.000 description 1
- KXOPYFNQLVUOAQ-FXQIFTODSA-N Arg-Ser-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O KXOPYFNQLVUOAQ-FXQIFTODSA-N 0.000 description 1
- KMFPQTITXUKJOV-DCAQKATOSA-N Arg-Ser-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O KMFPQTITXUKJOV-DCAQKATOSA-N 0.000 description 1
- XNSKSTRGQIPTSE-ACZMJKKPSA-N Arg-Thr Chemical compound C[C@@H]([C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O XNSKSTRGQIPTSE-ACZMJKKPSA-N 0.000 description 1
- INOIAEUXVVNJKA-XGEHTFHBSA-N Arg-Thr-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O INOIAEUXVVNJKA-XGEHTFHBSA-N 0.000 description 1
- ISVACHFCVRKIDG-SRVKXCTJSA-N Arg-Val-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O ISVACHFCVRKIDG-SRVKXCTJSA-N 0.000 description 1
- QLSRIZIDQXDQHK-RCWTZXSCSA-N Arg-Val-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QLSRIZIDQXDQHK-RCWTZXSCSA-N 0.000 description 1
- SWLOHUMCUDRTCL-ZLUOBGJFSA-N Asn-Ala-Asn Chemical compound C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N SWLOHUMCUDRTCL-ZLUOBGJFSA-N 0.000 description 1
- GXMSVVBIAMWMKO-BQBZGAKWSA-N Asn-Arg-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CCCN=C(N)N GXMSVVBIAMWMKO-BQBZGAKWSA-N 0.000 description 1
- BZWRLDPIWKOVKB-ZPFDUUQYSA-N Asn-Leu-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O BZWRLDPIWKOVKB-ZPFDUUQYSA-N 0.000 description 1
- GMUOCGCDOYYWPD-FXQIFTODSA-N Asn-Pro-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O GMUOCGCDOYYWPD-FXQIFTODSA-N 0.000 description 1
- XHTUGJCAEYOZOR-UBHSHLNASA-N Asn-Ser-Trp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O XHTUGJCAEYOZOR-UBHSHLNASA-N 0.000 description 1
- YSYTWUMRHSFODC-QWRGUYRKSA-N Asn-Tyr-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(O)=O YSYTWUMRHSFODC-QWRGUYRKSA-N 0.000 description 1
- KNMRXHIAVXHCLW-ZLUOBGJFSA-N Asp-Asn-Ser Chemical compound C([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CO)C(=O)O)N)C(=O)O KNMRXHIAVXHCLW-ZLUOBGJFSA-N 0.000 description 1
- KYQNAIMCTRZLNP-QSFUFRPTSA-N Asp-Ile-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(O)=O KYQNAIMCTRZLNP-QSFUFRPTSA-N 0.000 description 1
- RSMZEHCMIOKNMW-GSSVUCPTSA-N Asp-Thr-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O RSMZEHCMIOKNMW-GSSVUCPTSA-N 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 102100026189 Beta-galactosidase Human genes 0.000 description 1
- 102100023995 Beta-nerve growth factor Human genes 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical class OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 244000056139 Brassica cretica Species 0.000 description 1
- 235000003351 Brassica cretica Nutrition 0.000 description 1
- 235000003343 Brassica rupestris Nutrition 0.000 description 1
- 102100024217 CAMPATH-1 antigen Human genes 0.000 description 1
- 102100031173 CCN family member 4 Human genes 0.000 description 1
- 101710137353 CCN family member 4 Proteins 0.000 description 1
- 108010046080 CD27 Ligand Proteins 0.000 description 1
- 108010029697 CD40 Ligand Proteins 0.000 description 1
- 102100032937 CD40 ligand Human genes 0.000 description 1
- 102100025221 CD70 antigen Human genes 0.000 description 1
- 108091011896 CSF1 Proteins 0.000 description 1
- 102100035350 CUB domain-containing protein 1 Human genes 0.000 description 1
- 102100024153 Cadherin-15 Human genes 0.000 description 1
- 102100036364 Cadherin-2 Human genes 0.000 description 1
- 241000282832 Camelidae Species 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 102000005367 Carboxypeptidases Human genes 0.000 description 1
- 108010006303 Carboxypeptidases Proteins 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 102100031162 Collagen alpha-1(XVIII) chain Human genes 0.000 description 1
- 108010071942 Colony-Stimulating Factors Proteins 0.000 description 1
- 108020004635 Complementary DNA Proteins 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- PMPVIKIVABFJJI-UHFFFAOYSA-N Cyclobutane Chemical compound C1CCC1 PMPVIKIVABFJJI-UHFFFAOYSA-N 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- LVZWSLJZHVFIQJ-UHFFFAOYSA-N Cyclopropane Chemical compound C1CC1 LVZWSLJZHVFIQJ-UHFFFAOYSA-N 0.000 description 1
- SBMGKDLRJLYZCU-BIIVOSGPSA-N Cys-Asn-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)N)NC(=O)[C@H](CS)N)C(=O)O SBMGKDLRJLYZCU-BIIVOSGPSA-N 0.000 description 1
- BMHBJCVEXUBGFI-BIIVOSGPSA-N Cys-Cys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CS)NC(=O)[C@H](CS)N)C(=O)O BMHBJCVEXUBGFI-BIIVOSGPSA-N 0.000 description 1
- VIOQRFNAZDMVLO-NRPADANISA-N Cys-Val-Glu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O VIOQRFNAZDMVLO-NRPADANISA-N 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- 241000701022 Cytomegalovirus Species 0.000 description 1
- IGXWBGJHJZYPQS-SSDOTTSWSA-N D-Luciferin Chemical compound OC(=O)[C@H]1CSC(C=2SC3=CC=C(O)C=C3N=2)=N1 IGXWBGJHJZYPQS-SSDOTTSWSA-N 0.000 description 1
- 102100029010 D-aminoacyl-tRNA deacylase 1 Human genes 0.000 description 1
- 108010037897 DC-specific ICAM-3 grabbing nonintegrin Proteins 0.000 description 1
- 229940123780 DNA topoisomerase I inhibitor Drugs 0.000 description 1
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 1
- CYCGRDQQIOGCKX-UHFFFAOYSA-N Dehydro-luciferin Natural products OC(=O)C1=CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 CYCGRDQQIOGCKX-UHFFFAOYSA-N 0.000 description 1
- 102000016607 Diphtheria Toxin Human genes 0.000 description 1
- 108010053187 Diphtheria Toxin Proteins 0.000 description 1
- 239000012591 Dulbecco’s Phosphate Buffered Saline Substances 0.000 description 1
- 108010024212 E-Selectin Proteins 0.000 description 1
- 102100023471 E-selectin Human genes 0.000 description 1
- OVBJJZOQPCKUOR-UHFFFAOYSA-L EDTA disodium salt dihydrate Chemical compound O.O.[Na+].[Na+].[O-]C(=O)C[NH+](CC([O-])=O)CC[NH+](CC([O-])=O)CC([O-])=O OVBJJZOQPCKUOR-UHFFFAOYSA-L 0.000 description 1
- 102100025137 Early activation antigen CD69 Human genes 0.000 description 1
- MBYXEBXZARTUSS-QLWBXOBMSA-N Emetamine Natural products O(C)c1c(OC)cc2c(c(C[C@@H]3[C@H](CC)CN4[C@H](c5c(cc(OC)c(OC)c5)CC4)C3)ncc2)c1 MBYXEBXZARTUSS-QLWBXOBMSA-N 0.000 description 1
- 108010079505 Endostatins Proteins 0.000 description 1
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 1
- BJGNCJDXODQBOB-UHFFFAOYSA-N Fivefly Luciferin Natural products OC(=O)C1CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 BJGNCJDXODQBOB-UHFFFAOYSA-N 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- LOJYQMFIIJVETK-WDSKDSINSA-N Gln-Gln Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(O)=O LOJYQMFIIJVETK-WDSKDSINSA-N 0.000 description 1
- SXFPZRRVWSUYII-KBIXCLLPSA-N Gln-Ser-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N SXFPZRRVWSUYII-KBIXCLLPSA-N 0.000 description 1
- JILRMFFFCHUUTJ-ACZMJKKPSA-N Gln-Ser-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O JILRMFFFCHUUTJ-ACZMJKKPSA-N 0.000 description 1
- BIYNPVYAZOUVFQ-CIUDSAMLSA-N Glu-Pro-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O BIYNPVYAZOUVFQ-CIUDSAMLSA-N 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- QXPRJQPCFXMCIY-NKWVEPMBSA-N Gly-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN QXPRJQPCFXMCIY-NKWVEPMBSA-N 0.000 description 1
- CEXINUGNTZFNRY-BYPYZUCNSA-N Gly-Cys-Gly Chemical compound [NH3+]CC(=O)N[C@@H](CS)C(=O)NCC([O-])=O CEXINUGNTZFNRY-BYPYZUCNSA-N 0.000 description 1
- BYYNJRSNDARRBX-YFKPBYRVSA-N Gly-Gln-Gly Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O BYYNJRSNDARRBX-YFKPBYRVSA-N 0.000 description 1
- GDOZQTNZPCUARW-YFKPBYRVSA-N Gly-Gly-Glu Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CCC(O)=O GDOZQTNZPCUARW-YFKPBYRVSA-N 0.000 description 1
- INLIXXRWNUKVCF-JTQLQIEISA-N Gly-Gly-Tyr Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 INLIXXRWNUKVCF-JTQLQIEISA-N 0.000 description 1
- SWQALSGKVLYKDT-UHFFFAOYSA-N Gly-Ile-Ala Natural products NCC(=O)NC(C(C)CC)C(=O)NC(C)C(O)=O SWQALSGKVLYKDT-UHFFFAOYSA-N 0.000 description 1
- FCKPEGOCSVZPNC-WHOFXGATSA-N Gly-Ile-Phe Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 FCKPEGOCSVZPNC-WHOFXGATSA-N 0.000 description 1
- UHPAZODVFFYEEL-QWRGUYRKSA-N Gly-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)CN UHPAZODVFFYEEL-QWRGUYRKSA-N 0.000 description 1
- UUYBFNKHOCJCHT-VHSXEESVSA-N Gly-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN UUYBFNKHOCJCHT-VHSXEESVSA-N 0.000 description 1
- BMWFDYIYBAFROD-WPRPVWTQSA-N Gly-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CN BMWFDYIYBAFROD-WPRPVWTQSA-N 0.000 description 1
- FGPLUIQCSKGLTI-WDSKDSINSA-N Gly-Ser-Glu Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O FGPLUIQCSKGLTI-WDSKDSINSA-N 0.000 description 1
- ZKJZBRHRWKLVSJ-ZDLURKLDSA-N Gly-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)CN)O ZKJZBRHRWKLVSJ-ZDLURKLDSA-N 0.000 description 1
- XHVONGZZVUUORG-WEDXCCLWSA-N Gly-Thr-Lys Chemical compound NCC(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCCN XHVONGZZVUUORG-WEDXCCLWSA-N 0.000 description 1
- CUVBTVWFVIIDOC-YEPSODPASA-N Gly-Thr-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)CN CUVBTVWFVIIDOC-YEPSODPASA-N 0.000 description 1
- 229920002527 Glycogen Polymers 0.000 description 1
- VPZXBVLAVMBEQI-VKHMYHEASA-N Glycyl-alanine Chemical compound OC(=O)[C@H](C)NC(=O)CN VPZXBVLAVMBEQI-VKHMYHEASA-N 0.000 description 1
- 102000004457 Granulocyte-Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- JBCLFWXMTIKCCB-UHFFFAOYSA-N H-Gly-Phe-OH Natural products NCC(=O)NC(C(O)=O)CC1=CC=CC=C1 JBCLFWXMTIKCCB-UHFFFAOYSA-N 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- AWHJQEYGWRKPHE-LSJOCFKGSA-N His-Ala-Arg Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AWHJQEYGWRKPHE-LSJOCFKGSA-N 0.000 description 1
- LSQHWKPPOFDHHZ-YUMQZZPRSA-N His-Asp-Gly Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)NCC(=O)O)N LSQHWKPPOFDHHZ-YUMQZZPRSA-N 0.000 description 1
- MPXGJGBXCRQQJE-MXAVVETBSA-N His-Ile-Leu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O MPXGJGBXCRQQJE-MXAVVETBSA-N 0.000 description 1
- LVXFNTIIGOQBMD-SRVKXCTJSA-N His-Leu-Ser Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O LVXFNTIIGOQBMD-SRVKXCTJSA-N 0.000 description 1
- UWSMZKRTOZEGDD-CUJWVEQBSA-N His-Thr-Ser Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O UWSMZKRTOZEGDD-CUJWVEQBSA-N 0.000 description 1
- 101000777636 Homo sapiens ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Proteins 0.000 description 1
- 101000775037 Homo sapiens Anterior gradient protein 3 Proteins 0.000 description 1
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101000980814 Homo sapiens CAMPATH-1 antigen Proteins 0.000 description 1
- 101000737742 Homo sapiens CUB domain-containing protein 1 Proteins 0.000 description 1
- 101000762242 Homo sapiens Cadherin-15 Proteins 0.000 description 1
- 101000714537 Homo sapiens Cadherin-2 Proteins 0.000 description 1
- 101000714553 Homo sapiens Cadherin-3 Proteins 0.000 description 1
- 101000838688 Homo sapiens D-aminoacyl-tRNA deacylase 1 Proteins 0.000 description 1
- 101000934374 Homo sapiens Early activation antigen CD69 Proteins 0.000 description 1
- 101000606465 Homo sapiens Inactive tyrosine-protein kinase 7 Proteins 0.000 description 1
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 description 1
- 101000935040 Homo sapiens Integrin beta-2 Proteins 0.000 description 1
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 1
- 101000998146 Homo sapiens Interleukin-17A Proteins 0.000 description 1
- 101000998151 Homo sapiens Interleukin-17F Proteins 0.000 description 1
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 1
- 101000878605 Homo sapiens Low affinity immunoglobulin epsilon Fc receptor Proteins 0.000 description 1
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 description 1
- 101001017783 Homo sapiens Protein LRATD2 Proteins 0.000 description 1
- 101000693049 Homo sapiens Protein S100-A14 Proteins 0.000 description 1
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 1
- 101001100101 Homo sapiens Retinoic acid-induced protein 3 Proteins 0.000 description 1
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 1
- 101000679903 Homo sapiens Tumor necrosis factor receptor superfamily member 25 Proteins 0.000 description 1
- 101000851370 Homo sapiens Tumor necrosis factor receptor superfamily member 9 Proteins 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 102000004157 Hydrolases Human genes 0.000 description 1
- 108090000604 Hydrolases Proteins 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- QQFSKBMCAKWHLG-UHFFFAOYSA-N Ile-Phe-Pro-Pro Chemical compound C1CCC(C(=O)N2C(CCC2)C(O)=O)N1C(=O)C(NC(=O)C(N)C(C)CC)CC1=CC=CC=C1 QQFSKBMCAKWHLG-UHFFFAOYSA-N 0.000 description 1
- SVZFKLBRCYCIIY-CYDGBPFRSA-N Ile-Pro-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SVZFKLBRCYCIIY-CYDGBPFRSA-N 0.000 description 1
- TWVKGYNQQAUNRN-ACZMJKKPSA-N Ile-Ser Chemical compound CC[C@H](C)[C@H]([NH3+])C(=O)N[C@@H](CO)C([O-])=O TWVKGYNQQAUNRN-ACZMJKKPSA-N 0.000 description 1
- PXKACEXYLPBMAD-JBDRJPRFSA-N Ile-Ser-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PXKACEXYLPBMAD-JBDRJPRFSA-N 0.000 description 1
- RKQAYOWLSFLJEE-SVSWQMSJSA-N Ile-Thr-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)O)N RKQAYOWLSFLJEE-SVSWQMSJSA-N 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 102100039813 Inactive tyrosine-protein kinase 7 Human genes 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 102100022338 Integrin alpha-M Human genes 0.000 description 1
- 108010008212 Integrin alpha4beta1 Proteins 0.000 description 1
- 102100025390 Integrin beta-2 Human genes 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 108090000467 Interferon-beta Proteins 0.000 description 1
- 102000003996 Interferon-beta Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 102100027268 Interferon-stimulated gene 20 kDa protein Human genes 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 102000013462 Interleukin-12 Human genes 0.000 description 1
- 108010065805 Interleukin-12 Proteins 0.000 description 1
- 102000003816 Interleukin-13 Human genes 0.000 description 1
- 108090000176 Interleukin-13 Proteins 0.000 description 1
- 102000013691 Interleukin-17 Human genes 0.000 description 1
- 108050003558 Interleukin-17 Proteins 0.000 description 1
- 102100033461 Interleukin-17A Human genes 0.000 description 1
- 102100033454 Interleukin-17F Human genes 0.000 description 1
- 108010002386 Interleukin-3 Proteins 0.000 description 1
- 102000000646 Interleukin-3 Human genes 0.000 description 1
- 102000004388 Interleukin-4 Human genes 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 108010002616 Interleukin-5 Proteins 0.000 description 1
- 102100039897 Interleukin-5 Human genes 0.000 description 1
- 102000004889 Interleukin-6 Human genes 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 108010038498 Interleukin-7 Receptors Proteins 0.000 description 1
- 102000010782 Interleukin-7 Receptors Human genes 0.000 description 1
- 108090001007 Interleukin-8 Proteins 0.000 description 1
- 102000004890 Interleukin-8 Human genes 0.000 description 1
- 102000004195 Isomerases Human genes 0.000 description 1
- 108090000769 Isomerases Proteins 0.000 description 1
- IBMVEYRWAWIOTN-UHFFFAOYSA-N L-Leucyl-L-Arginyl-L-Proline Natural products CC(C)CC(N)C(=O)NC(CCCN=C(N)N)C(=O)N1CCCC1C(O)=O IBMVEYRWAWIOTN-UHFFFAOYSA-N 0.000 description 1
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 1
- 108010092694 L-Selectin Proteins 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- KFKWRHQBZQICHA-STQMWFEESA-N L-leucyl-L-phenylalanine Natural products CC(C)C[C@H](N)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 KFKWRHQBZQICHA-STQMWFEESA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- 102100033467 L-selectin Human genes 0.000 description 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 1
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 241000282838 Lama Species 0.000 description 1
- IBMVEYRWAWIOTN-RWMBFGLXSA-N Leu-Arg-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(O)=O IBMVEYRWAWIOTN-RWMBFGLXSA-N 0.000 description 1
- WGNOPSQMIQERPK-GARJFASQSA-N Leu-Asn-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N1CCC[C@@H]1C(=O)O)N WGNOPSQMIQERPK-GARJFASQSA-N 0.000 description 1
- WGNOPSQMIQERPK-UHFFFAOYSA-N Leu-Asn-Pro Natural products CC(C)CC(N)C(=O)NC(CC(=O)N)C(=O)N1CCCC1C(=O)O WGNOPSQMIQERPK-UHFFFAOYSA-N 0.000 description 1
- CQGSYZCULZMEDE-SRVKXCTJSA-N Leu-Gln-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(O)=O CQGSYZCULZMEDE-SRVKXCTJSA-N 0.000 description 1
- VZBIUJURDLFFOE-IHRRRGAJSA-N Leu-His-Arg Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O VZBIUJURDLFFOE-IHRRRGAJSA-N 0.000 description 1
- XVZCXCTYGHPNEM-UHFFFAOYSA-N Leu-Leu-Pro Natural products CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O XVZCXCTYGHPNEM-UHFFFAOYSA-N 0.000 description 1
- JLYUZRKPDKHUTC-WDSOQIARSA-N Leu-Pro-Trp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O JLYUZRKPDKHUTC-WDSOQIARSA-N 0.000 description 1
- RGUXWMDNCPMQFB-YUMQZZPRSA-N Leu-Ser-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O RGUXWMDNCPMQFB-YUMQZZPRSA-N 0.000 description 1
- SBANPBVRHYIMRR-GARJFASQSA-N Leu-Ser-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N SBANPBVRHYIMRR-GARJFASQSA-N 0.000 description 1
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 1
- HWMQRQIFVGEAPH-XIRDDKMYSA-N Leu-Ser-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 HWMQRQIFVGEAPH-XIRDDKMYSA-N 0.000 description 1
- QWWPYKKLXWOITQ-VOAKCMCISA-N Leu-Thr-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(C)C QWWPYKKLXWOITQ-VOAKCMCISA-N 0.000 description 1
- HGLKOTPFWOMPOB-MEYUZBJRSA-N Leu-Thr-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HGLKOTPFWOMPOB-MEYUZBJRSA-N 0.000 description 1
- HOMFINRJHIIZNJ-HOCLYGCPSA-N Leu-Trp-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)NCC(O)=O HOMFINRJHIIZNJ-HOCLYGCPSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 1
- 102100038007 Low affinity immunoglobulin epsilon Fc receptor Human genes 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- DDWFXDSYGUXRAY-UHFFFAOYSA-N Luciferin Natural products CCc1c(C)c(CC2NC(=O)C(=C2C=C)C)[nH]c1Cc3[nH]c4C(=C5/NC(CC(=O)O)C(C)C5CC(=O)O)CC(=O)c4c3C DDWFXDSYGUXRAY-UHFFFAOYSA-N 0.000 description 1
- 108090000856 Lyases Proteins 0.000 description 1
- 102000004317 Lyases Human genes 0.000 description 1
- 102000004083 Lymphotoxin-alpha Human genes 0.000 description 1
- 108090000542 Lymphotoxin-alpha Proteins 0.000 description 1
- QUCDKEKDPYISNX-HJGDQZAQSA-N Lys-Asn-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QUCDKEKDPYISNX-HJGDQZAQSA-N 0.000 description 1
- JOSAKOKSPXROGQ-BJDJZHNGSA-N Lys-Ser-Ile Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O JOSAKOKSPXROGQ-BJDJZHNGSA-N 0.000 description 1
- 102100028123 Macrophage colony-stimulating factor 1 Human genes 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 102000047724 Member 2 Solute Carrier Family 12 Human genes 0.000 description 1
- ONGCSGVHCSAATF-CIUDSAMLSA-N Met-Ala-Glu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(O)=O ONGCSGVHCSAATF-CIUDSAMLSA-N 0.000 description 1
- CGUYGMFQZCYJSG-DCAQKATOSA-N Met-Lys-Ser Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O CGUYGMFQZCYJSG-DCAQKATOSA-N 0.000 description 1
- NHXXGBXJTLRGJI-GUBZILKMSA-N Met-Pro-Ser Chemical compound [H]N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O NHXXGBXJTLRGJI-GUBZILKMSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- VFKZTMPDYBFSTM-KVTDHHQDSA-N Mitobronitol Chemical compound BrC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-KVTDHHQDSA-N 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 description 1
- 108010079364 N-glycylalanine Proteins 0.000 description 1
- 238000005481 NMR spectroscopy Methods 0.000 description 1
- BQVUABVGYYSDCJ-UHFFFAOYSA-N Nalpha-L-Leucyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)CC(C)C)C(O)=O)=CNC2=C1 BQVUABVGYYSDCJ-UHFFFAOYSA-N 0.000 description 1
- 108010025020 Nerve Growth Factor Proteins 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 108010035766 P-Selectin Proteins 0.000 description 1
- 102100023472 P-selectin Human genes 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000003992 Peroxidases Human genes 0.000 description 1
- ULECEJGNDHWSKD-QEJZJMRPSA-N Phe-Ala-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=CC=C1 ULECEJGNDHWSKD-QEJZJMRPSA-N 0.000 description 1
- BXNGIHFNNNSEOS-UWVGGRQHSA-N Phe-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@@H](N)CC1=CC=CC=C1 BXNGIHFNNNSEOS-UWVGGRQHSA-N 0.000 description 1
- RIYZXJVARWJLKS-KKUMJFAQSA-N Phe-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC1=CC=CC=C1 RIYZXJVARWJLKS-KKUMJFAQSA-N 0.000 description 1
- GLUBLISJVJFHQS-VIFPVBQESA-N Phe-Gly Chemical compound OC(=O)CNC(=O)[C@@H](N)CC1=CC=CC=C1 GLUBLISJVJFHQS-VIFPVBQESA-N 0.000 description 1
- MMYUOSCXBJFUNV-QWRGUYRKSA-N Phe-Gly-Cys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)NCC(=O)N[C@@H](CS)C(=O)O)N MMYUOSCXBJFUNV-QWRGUYRKSA-N 0.000 description 1
- METZZBCMDXHFMK-BZSNNMDCSA-N Phe-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC2=CC=CC=C2)N METZZBCMDXHFMK-BZSNNMDCSA-N 0.000 description 1
- WEQJQNWXCSUVMA-RYUDHWBXSA-N Phe-Pro Chemical compound C([C@H]([NH3+])C(=O)N1[C@@H](CCC1)C([O-])=O)C1=CC=CC=C1 WEQJQNWXCSUVMA-RYUDHWBXSA-N 0.000 description 1
- NJJBATPLUQHRBM-IHRRRGAJSA-N Phe-Pro-Ser Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)N)C(=O)N[C@@H](CO)C(=O)O NJJBATPLUQHRBM-IHRRRGAJSA-N 0.000 description 1
- BPCLGWHVPVTTFM-QWRGUYRKSA-N Phe-Ser-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)NCC(O)=O BPCLGWHVPVTTFM-QWRGUYRKSA-N 0.000 description 1
- KLYYKKGCPOGDPE-OEAJRASXSA-N Phe-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O KLYYKKGCPOGDPE-OEAJRASXSA-N 0.000 description 1
- 241000276498 Pollachius virens Species 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- OCSACVPBMIYNJE-GUBZILKMSA-N Pro-Arg-Asn Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(O)=O OCSACVPBMIYNJE-GUBZILKMSA-N 0.000 description 1
- OLHDPZMYUSBGDE-GUBZILKMSA-N Pro-Arg-Cys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CS)C(=O)O OLHDPZMYUSBGDE-GUBZILKMSA-N 0.000 description 1
- CYQQWUPHIZVCNY-GUBZILKMSA-N Pro-Arg-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O CYQQWUPHIZVCNY-GUBZILKMSA-N 0.000 description 1
- AHXPYZRZRMQOAU-QXEWZRGKSA-N Pro-Asn-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H]1CCCN1)C(O)=O AHXPYZRZRMQOAU-QXEWZRGKSA-N 0.000 description 1
- TUYWCHPXKQTISF-LPEHRKFASA-N Pro-Cys-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CS)C(=O)N2CCC[C@@H]2C(=O)O TUYWCHPXKQTISF-LPEHRKFASA-N 0.000 description 1
- XYSXOCIWCPFOCG-IHRRRGAJSA-N Pro-Leu-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O XYSXOCIWCPFOCG-IHRRRGAJSA-N 0.000 description 1
- VTFXTWDFPTWNJY-RHYQMDGZSA-N Pro-Leu-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VTFXTWDFPTWNJY-RHYQMDGZSA-N 0.000 description 1
- AJBQTGZIZQXBLT-STQMWFEESA-N Pro-Phe-Gly Chemical compound C([C@@H](C(=O)NCC(=O)O)NC(=O)[C@H]1NCCC1)C1=CC=CC=C1 AJBQTGZIZQXBLT-STQMWFEESA-N 0.000 description 1
- RWCOTTLHDJWHRS-YUMQZZPRSA-N Pro-Pro Chemical compound OC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 RWCOTTLHDJWHRS-YUMQZZPRSA-N 0.000 description 1
- SVXXJYJCRNKDDE-AVGNSLFASA-N Pro-Pro-His Chemical compound C([C@@H](C(=O)O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H]1NCCC1)C1=CN=CN1 SVXXJYJCRNKDDE-AVGNSLFASA-N 0.000 description 1
- CGSOWZUPLOKYOR-AVGNSLFASA-N Pro-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 CGSOWZUPLOKYOR-AVGNSLFASA-N 0.000 description 1
- NAIPAPCKKRCMBL-JYJNAYRXSA-N Pro-Pro-Phe Chemical compound C([C@@H](C(=O)O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H]1NCCC1)C1=CC=CC=C1 NAIPAPCKKRCMBL-JYJNAYRXSA-N 0.000 description 1
- POQFNPILEQEODH-FXQIFTODSA-N Pro-Ser-Ala Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O POQFNPILEQEODH-FXQIFTODSA-N 0.000 description 1
- CZCCVJUUWBMISW-FXQIFTODSA-N Pro-Ser-Cys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O CZCCVJUUWBMISW-FXQIFTODSA-N 0.000 description 1
- RNEFESSBTOQSAC-DCAQKATOSA-N Pro-Ser-His Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O RNEFESSBTOQSAC-DCAQKATOSA-N 0.000 description 1
- SXJOPONICMGFCR-DCAQKATOSA-N Pro-Ser-Lys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O SXJOPONICMGFCR-DCAQKATOSA-N 0.000 description 1
- QKDIHFHGHBYTKB-IHRRRGAJSA-N Pro-Ser-Phe Chemical compound N([C@@H](CO)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C(=O)[C@@H]1CCCN1 QKDIHFHGHBYTKB-IHRRRGAJSA-N 0.000 description 1
- MKGIILKDUGDRRO-FXQIFTODSA-N Pro-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 MKGIILKDUGDRRO-FXQIFTODSA-N 0.000 description 1
- UGDMQJSXSSZUKL-IHRRRGAJSA-N Pro-Ser-Tyr Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O UGDMQJSXSSZUKL-IHRRRGAJSA-N 0.000 description 1
- HRIXMVRZRGFKNQ-HJGDQZAQSA-N Pro-Thr-Gln Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HRIXMVRZRGFKNQ-HJGDQZAQSA-N 0.000 description 1
- OIDKVWTWGDWMHY-RYUDHWBXSA-N Pro-Tyr Chemical compound C([C@@H](C(=O)O)NC(=O)[C@H]1NCCC1)C1=CC=C(O)C=C1 OIDKVWTWGDWMHY-RYUDHWBXSA-N 0.000 description 1
- 241001415846 Procellariidae Species 0.000 description 1
- 101710089372 Programmed cell death protein 1 Proteins 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 102100033355 Protein LRATD2 Human genes 0.000 description 1
- 102100026298 Protein S100-A14 Human genes 0.000 description 1
- 101000762949 Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) Exotoxin A Proteins 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 102100038453 Retinoic acid-induced protein 3 Human genes 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- AUVVAXYIELKVAI-UHFFFAOYSA-N SJ000285215 Natural products N1CCC2=CC(OC)=C(OC)C=C2C1CC1CC2C3=CC(OC)=C(OC)C=C3CCN2CC1CC AUVVAXYIELKVAI-UHFFFAOYSA-N 0.000 description 1
- 108091006620 SLC12A2 Proteins 0.000 description 1
- 101100420762 Schizophyllum commune SC6 gene Proteins 0.000 description 1
- QVOGDCQNGLBNCR-FXQIFTODSA-N Ser-Arg-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O QVOGDCQNGLBNCR-FXQIFTODSA-N 0.000 description 1
- KAAPNMOKUUPKOE-SRVKXCTJSA-N Ser-Asn-Phe Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 KAAPNMOKUUPKOE-SRVKXCTJSA-N 0.000 description 1
- OHKFXGKHSJKKAL-NRPADANISA-N Ser-Glu-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O OHKFXGKHSJKKAL-NRPADANISA-N 0.000 description 1
- UQFYNFTYDHUIMI-WHFBIAKZSA-N Ser-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CO UQFYNFTYDHUIMI-WHFBIAKZSA-N 0.000 description 1
- CLKKNZQUQMZDGD-SRVKXCTJSA-N Ser-His-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)CC1=CN=CN1 CLKKNZQUQMZDGD-SRVKXCTJSA-N 0.000 description 1
- WEQAYODCJHZSJZ-KKUMJFAQSA-N Ser-His-Tyr Chemical compound C([C@H](NC(=O)[C@H](CO)N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CN=CN1 WEQAYODCJHZSJZ-KKUMJFAQSA-N 0.000 description 1
- BEAFYHFQTOTVFS-VGDYDELISA-N Ser-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CO)N BEAFYHFQTOTVFS-VGDYDELISA-N 0.000 description 1
- MQQBBLVOUUJKLH-HJPIBITLSA-N Ser-Ile-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O MQQBBLVOUUJKLH-HJPIBITLSA-N 0.000 description 1
- NFDYGNFETJVMSE-BQBZGAKWSA-N Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](N)CO NFDYGNFETJVMSE-BQBZGAKWSA-N 0.000 description 1
- ASGYVPAVFNDZMA-GUBZILKMSA-N Ser-Met-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CO)N ASGYVPAVFNDZMA-GUBZILKMSA-N 0.000 description 1
- WNDUPCKKKGSKIQ-CIUDSAMLSA-N Ser-Pro-Gln Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(O)=O WNDUPCKKKGSKIQ-CIUDSAMLSA-N 0.000 description 1
- QUGRFWPMPVIAPW-IHRRRGAJSA-N Ser-Pro-Phe Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 QUGRFWPMPVIAPW-IHRRRGAJSA-N 0.000 description 1
- JCLAFVNDBJMLBC-JBDRJPRFSA-N Ser-Ser-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O JCLAFVNDBJMLBC-JBDRJPRFSA-N 0.000 description 1
- FLMYSKVSDVHLEW-SVSWQMSJSA-N Ser-Thr-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FLMYSKVSDVHLEW-SVSWQMSJSA-N 0.000 description 1
- FGBLCMLXHRPVOF-IHRRRGAJSA-N Ser-Tyr-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O FGBLCMLXHRPVOF-IHRRRGAJSA-N 0.000 description 1
- OQSQCUWQOIHECT-YJRXYDGGSA-N Ser-Tyr-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OQSQCUWQOIHECT-YJRXYDGGSA-N 0.000 description 1
- LGIMRDKGABDMBN-DCAQKATOSA-N Ser-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N LGIMRDKGABDMBN-DCAQKATOSA-N 0.000 description 1
- HNDMFDBQXYZSRM-IHRRRGAJSA-N Ser-Val-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HNDMFDBQXYZSRM-IHRRRGAJSA-N 0.000 description 1
- LSHUNRICNSEEAN-BPUTZDHNSA-N Ser-Val-Trp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CO)N LSHUNRICNSEEAN-BPUTZDHNSA-N 0.000 description 1
- HSWXBJCBYSWBPT-GUBZILKMSA-N Ser-Val-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CO)C(C)C)C(O)=O HSWXBJCBYSWBPT-GUBZILKMSA-N 0.000 description 1
- GIIZNNXWQWCKIB-UHFFFAOYSA-N Serevent Chemical compound C1=C(O)C(CO)=CC(C(O)CNCCCCCCOCCCCC=2C=CC=CC=2)=C1 GIIZNNXWQWCKIB-UHFFFAOYSA-N 0.000 description 1
- 241000270295 Serpentes Species 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- ZSJLQEPLLKMAKR-UHFFFAOYSA-N Streptozotocin Natural products O=NN(C)C(=O)NC1C(O)OC(CO)C(O)C1O ZSJLQEPLLKMAKR-UHFFFAOYSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 108700012920 TNF Proteins 0.000 description 1
- LVHHEVGYAZGXDE-KDXUFGMBSA-N Thr-Ala-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)N1CCC[C@@H]1C(=O)O)N)O LVHHEVGYAZGXDE-KDXUFGMBSA-N 0.000 description 1
- XYEXCEPTALHNEV-RCWTZXSCSA-N Thr-Arg-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O XYEXCEPTALHNEV-RCWTZXSCSA-N 0.000 description 1
- VEWZSFGRQDUAJM-YJRXYDGGSA-N Thr-Cys-Tyr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N)O VEWZSFGRQDUAJM-YJRXYDGGSA-N 0.000 description 1
- XYFISNXATOERFZ-OSUNSFLBSA-N Thr-Ile-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N XYFISNXATOERFZ-OSUNSFLBSA-N 0.000 description 1
- XIULAFZYEKSGAJ-IXOXFDKPSA-N Thr-Leu-His Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CNC=N1 XIULAFZYEKSGAJ-IXOXFDKPSA-N 0.000 description 1
- JMBRNXUOLJFURW-BEAPCOKYSA-N Thr-Phe-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N)O JMBRNXUOLJFURW-BEAPCOKYSA-N 0.000 description 1
- KERCOYANYUPLHJ-XGEHTFHBSA-N Thr-Pro-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O KERCOYANYUPLHJ-XGEHTFHBSA-N 0.000 description 1
- DSGIVWSDDRDJIO-ZXXMMSQZSA-N Thr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O DSGIVWSDDRDJIO-ZXXMMSQZSA-N 0.000 description 1
- COYHRQWNJDJCNA-NUJDXYNKSA-N Thr-Thr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O COYHRQWNJDJCNA-NUJDXYNKSA-N 0.000 description 1
- LECUEEHKUFYOOV-ZJDVBMNYSA-N Thr-Thr-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](N)[C@@H](C)O LECUEEHKUFYOOV-ZJDVBMNYSA-N 0.000 description 1
- MNYNCKZAEIAONY-XGEHTFHBSA-N Thr-Val-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O MNYNCKZAEIAONY-XGEHTFHBSA-N 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 108010022394 Threonine synthase Proteins 0.000 description 1
- 239000000365 Topoisomerase I Inhibitor Substances 0.000 description 1
- 108090000992 Transferases Proteins 0.000 description 1
- 102000004357 Transferases Human genes 0.000 description 1
- IQGJAHMZWBTRIF-UBHSHLNASA-N Trp-Asp-Asn Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N IQGJAHMZWBTRIF-UBHSHLNASA-N 0.000 description 1
- SVGAWGVHFIYAEE-JSGCOSHPSA-N Trp-Gly-Gln Chemical compound C1=CC=C2C(C[C@H](N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O)=CNC2=C1 SVGAWGVHFIYAEE-JSGCOSHPSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 102100040247 Tumor necrosis factor Human genes 0.000 description 1
- 102100024587 Tumor necrosis factor ligand superfamily member 15 Human genes 0.000 description 1
- 108090000138 Tumor necrosis factor ligand superfamily member 15 Proteins 0.000 description 1
- 102100022203 Tumor necrosis factor receptor superfamily member 25 Human genes 0.000 description 1
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 description 1
- MICSYKFECRFCTJ-IHRRRGAJSA-N Tyr-Arg-Asp Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N)O MICSYKFECRFCTJ-IHRRRGAJSA-N 0.000 description 1
- SCCKSNREWHMKOJ-SRVKXCTJSA-N Tyr-Asn-Ser Chemical compound N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O SCCKSNREWHMKOJ-SRVKXCTJSA-N 0.000 description 1
- FQNUWOHNGJWNLM-QWRGUYRKSA-N Tyr-Cys-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)NCC(O)=O FQNUWOHNGJWNLM-QWRGUYRKSA-N 0.000 description 1
- NKUGCYDFQKFVOJ-JYJNAYRXSA-N Tyr-Leu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NKUGCYDFQKFVOJ-JYJNAYRXSA-N 0.000 description 1
- LMKKMCGTDANZTR-BZSNNMDCSA-N Tyr-Phe-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=C(O)C=C1 LMKKMCGTDANZTR-BZSNNMDCSA-N 0.000 description 1
- AUZADXNWQMBZOO-JYJNAYRXSA-N Tyr-Pro-Arg Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)C1=CC=C(O)C=C1 AUZADXNWQMBZOO-JYJNAYRXSA-N 0.000 description 1
- BIWVVOHTKDLRMP-ULQDDVLXSA-N Tyr-Pro-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O BIWVVOHTKDLRMP-ULQDDVLXSA-N 0.000 description 1
- YYLHVUCSTXXKBS-IHRRRGAJSA-N Tyr-Pro-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YYLHVUCSTXXKBS-IHRRRGAJSA-N 0.000 description 1
- LUMQYLVYUIRHHU-YJRXYDGGSA-N Tyr-Ser-Thr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LUMQYLVYUIRHHU-YJRXYDGGSA-N 0.000 description 1
- OJCISMMNNUNNJA-BZSNNMDCSA-N Tyr-Tyr-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=C(O)C=C1 OJCISMMNNUNNJA-BZSNNMDCSA-N 0.000 description 1
- ZLFHAAGHGQBQQN-AEJSXWLSSA-N Val-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](C(C)C)N ZLFHAAGHGQBQQN-AEJSXWLSSA-N 0.000 description 1
- ZLFHAAGHGQBQQN-GUBZILKMSA-N Val-Ala-Pro Natural products CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O ZLFHAAGHGQBQQN-GUBZILKMSA-N 0.000 description 1
- DNOOLPROHJWCSQ-RCWTZXSCSA-N Val-Arg-Thr Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O DNOOLPROHJWCSQ-RCWTZXSCSA-N 0.000 description 1
- WKWJJQZZZBBWKV-JYJNAYRXSA-N Val-Arg-Tyr Chemical compound NC(N)=NCCC[C@H](NC(=O)[C@@H](N)C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 WKWJJQZZZBBWKV-JYJNAYRXSA-N 0.000 description 1
- DBOXBUDEAJVKRE-LSJOCFKGSA-N Val-Asn-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](C(C)C)C(=O)O)N DBOXBUDEAJVKRE-LSJOCFKGSA-N 0.000 description 1
- QHDXUYOYTPWCSK-RCOVLWMOSA-N Val-Asp-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)NCC(=O)O)N QHDXUYOYTPWCSK-RCOVLWMOSA-N 0.000 description 1
- VCAWFLIWYNMHQP-UKJIMTQDSA-N Val-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](C(C)C)N VCAWFLIWYNMHQP-UKJIMTQDSA-N 0.000 description 1
- DJEVQCWNMQOABE-RCOVLWMOSA-N Val-Gly-Asp Chemical compound CC(C)[C@@H](C(=O)NCC(=O)N[C@@H](CC(=O)O)C(=O)O)N DJEVQCWNMQOABE-RCOVLWMOSA-N 0.000 description 1
- YDVDTCJGBBJGRT-GUBZILKMSA-N Val-Met-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CO)C(=O)O)N YDVDTCJGBBJGRT-GUBZILKMSA-N 0.000 description 1
- DOFAQXCYFQKSHT-SRVKXCTJSA-N Val-Pro-Pro Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DOFAQXCYFQKSHT-SRVKXCTJSA-N 0.000 description 1
- SSYBNWFXCFNRFN-GUBZILKMSA-N Val-Pro-Ser Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O SSYBNWFXCFNRFN-GUBZILKMSA-N 0.000 description 1
- VHIZXDZMTDVFGX-DCAQKATOSA-N Val-Ser-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)N VHIZXDZMTDVFGX-DCAQKATOSA-N 0.000 description 1
- GBIUHAYJGWVNLN-UHFFFAOYSA-N Val-Ser-Pro Natural products CC(C)C(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O GBIUHAYJGWVNLN-UHFFFAOYSA-N 0.000 description 1
- JXWGBRRVTRAZQA-ULQDDVLXSA-N Val-Tyr-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](C(C)C)N JXWGBRRVTRAZQA-ULQDDVLXSA-N 0.000 description 1
- JSOXWWFKRJKTMT-WOPDTQHZSA-N Val-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N JSOXWWFKRJKTMT-WOPDTQHZSA-N 0.000 description 1
- 108010053099 Vascular Endothelial Growth Factor Receptor-2 Proteins 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 229940022698 acetylcholinesterase Drugs 0.000 description 1
- 229930183665 actinomycin Natural products 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 238000012382 advanced drug delivery Methods 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 108010047495 alanylglycine Proteins 0.000 description 1
- 108010070944 alanylhistidine Proteins 0.000 description 1
- NDAUXUAQIAJITI-UHFFFAOYSA-N albuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-UHFFFAOYSA-N 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 150000001447 alkali salts Chemical class 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 150000003978 alpha-halocarboxylic acids Chemical class 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 1
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 1
- 235000011130 ammonium sulphate Nutrition 0.000 description 1
- 229960003473 androstanolone Drugs 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 229940045799 anthracyclines and related substance Drugs 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 229940034982 antineoplastic agent Drugs 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- 108010029539 arginyl-prolyl-proline Proteins 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 238000011190 asparagine deamidation Methods 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- FZCSTZYAHCUGEM-UHFFFAOYSA-N aspergillomarasmine B Natural products OC(=O)CNC(C(O)=O)CNC(C(O)=O)CC(O)=O FZCSTZYAHCUGEM-UHFFFAOYSA-N 0.000 description 1
- 210000004082 barrier epithelial cell Anatomy 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- QKSKPIVNLNLAAV-UHFFFAOYSA-N bis(2-chloroethyl) sulfide Chemical compound ClCCSCCCl QKSKPIVNLNLAAV-UHFFFAOYSA-N 0.000 description 1
- JCXGWMGPZLAOME-RNFDNDRNSA-N bismuth-213 Chemical compound [213Bi] JCXGWMGPZLAOME-RNFDNDRNSA-N 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 229930195731 calicheamicin Natural products 0.000 description 1
- HXCHCVDVKSCDHU-LULTVBGHSA-N calicheamicin Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-LULTVBGHSA-N 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 235000013877 carbamide Nutrition 0.000 description 1
- 150000005323 carbonate salts Chemical class 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical group OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- CZTQZXZIADLWOZ-CRAIPNDOSA-N cefaloridine Chemical compound O=C([C@@H](NC(=O)CC=1SC=CC=1)[C@H]1SC2)N1C(C(=O)[O-])=C2C[N+]1=CC=CC=C1 CZTQZXZIADLWOZ-CRAIPNDOSA-N 0.000 description 1
- 230000007910 cell fusion Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 229960005091 chloramphenicol Drugs 0.000 description 1
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical class OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 1
- 229960001338 colchicine Drugs 0.000 description 1
- 239000000599 controlled substance Substances 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 230000001054 cortical effect Effects 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 239000007822 coupling agent Substances 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 150000001944 cysteine derivatives Chemical class 0.000 description 1
- 108010004073 cysteinylcysteine Proteins 0.000 description 1
- 108010069495 cysteinyltyrosine Proteins 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 108010025198 decaglycine Proteins 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000011026 diafiltration Methods 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 235000013681 dietary sucrose Nutrition 0.000 description 1
- 102000004419 dihydrofolate reductase Human genes 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000006471 dimerization reaction Methods 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- AFOSIXZFDONLBT-UHFFFAOYSA-N divinyl sulfone Chemical compound C=CS(=O)(=O)C=C AFOSIXZFDONLBT-UHFFFAOYSA-N 0.000 description 1
- AMRJKAQTDDKMCE-UHFFFAOYSA-N dolastatin Chemical compound CC(C)C(N(C)C)C(=O)NC(C(C)C)C(=O)N(C)C(C(C)C)C(OC)CC(=O)N1CCCC1C(OC)C(C)C(=O)NC(C=1SC=CN=1)CC1=CC=CC=C1 AMRJKAQTDDKMCE-UHFFFAOYSA-N 0.000 description 1
- 229930188854 dolastatin Natural products 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 239000000890 drug combination Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 229940124274 edetate disodium Drugs 0.000 description 1
- 238000000804 electron spin resonance spectroscopy Methods 0.000 description 1
- 229960002694 emetine Drugs 0.000 description 1
- AUVVAXYIELKVAI-CKBKHPSWSA-N emetine Chemical compound N1CCC2=CC(OC)=C(OC)C=C2[C@H]1C[C@H]1C[C@H]2C3=CC(OC)=C(OC)C=C3CCN2C[C@@H]1CC AUVVAXYIELKVAI-CKBKHPSWSA-N 0.000 description 1
- AUVVAXYIELKVAI-UWBTVBNJSA-N emetine Natural products N1CCC2=CC(OC)=C(OC)C=C2[C@H]1C[C@H]1C[C@H]2C3=CC(OC)=C(OC)C=C3CCN2C[C@H]1CC AUVVAXYIELKVAI-UWBTVBNJSA-N 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- HKSZLNNOFSGOKW-UHFFFAOYSA-N ent-staurosporine Natural products C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1C1CC(NC)C(OC)C4(C)O1 HKSZLNNOFSGOKW-UHFFFAOYSA-N 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 230000004890 epithelial barrier function Effects 0.000 description 1
- 229930013356 epothilone Natural products 0.000 description 1
- 150000003883 epothilone derivatives Chemical class 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 235000019441 ethanol Nutrition 0.000 description 1
- 229960005542 ethidium bromide Drugs 0.000 description 1
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 229960002848 formoterol Drugs 0.000 description 1
- BPZSYCZIITTYBL-UHFFFAOYSA-N formoterol Chemical compound C1=CC(OC)=CC=C1CC(C)NCC(O)C1=CC=C(O)C(NC=O)=C1 BPZSYCZIITTYBL-UHFFFAOYSA-N 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 108010078144 glutaminyl-glycine Proteins 0.000 description 1
- 229940096919 glycogen Drugs 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 1
- 108010089804 glycyl-threonine Proteins 0.000 description 1
- 108010081551 glycylphenylalanine Proteins 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 150000008282 halocarbons Chemical class 0.000 description 1
- 150000005826 halohydrocarbons Chemical class 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- 229920006158 high molecular weight polymer Polymers 0.000 description 1
- 239000013628 high molecular weight specie Substances 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 108010092114 histidylphenylalanine Proteins 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 150000003949 imides Chemical class 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 238000010949 in-process test method Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 206010022000 influenza Diseases 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 229960001388 interferon-beta Drugs 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 150000004694 iodide salts Chemical class 0.000 description 1
- PGLTVOMIXTUURA-UHFFFAOYSA-N iodoacetamide Chemical compound NC(=O)CI PGLTVOMIXTUURA-UHFFFAOYSA-N 0.000 description 1
- GKOZUEZYRPOHIO-IGMARMGPSA-N iridium-192 Chemical compound [192Ir] GKOZUEZYRPOHIO-IGMARMGPSA-N 0.000 description 1
- 239000001282 iso-butane Substances 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 1
- 238000006317 isomerization reaction Methods 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 108010044056 leucyl-phenylalanine Proteins 0.000 description 1
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 1
- 229960004194 lidocaine Drugs 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 238000012417 linear regression Methods 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 239000006194 liquid suspension Substances 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- HWYHZTIRURJOHG-UHFFFAOYSA-N luminol Chemical compound O=C1NNC(=O)C2=C1C(N)=CC=C2 HWYHZTIRURJOHG-UHFFFAOYSA-N 0.000 description 1
- 108010009298 lysylglutamic acid Proteins 0.000 description 1
- 239000012516 mab select resin Substances 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 125000005439 maleimidyl group Chemical group C1(C=CC(N1*)=O)=O 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical compound ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- CFCUWKMKBJTWLW-BKHRDMLASA-N mithramycin Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1C)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](O[C@@H]2O[C@H](C)[C@H](O)[C@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@@](C)(O)C3)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@@H](O)[C@H](O)[C@@H](C)O1 CFCUWKMKBJTWLW-BKHRDMLASA-N 0.000 description 1
- 229960005485 mitobronitol Drugs 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- 238000012434 mixed-mode chromatography Methods 0.000 description 1
- 238000001823 molecular biology technique Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 235000010460 mustard Nutrition 0.000 description 1
- 231100000219 mutagenic Toxicity 0.000 description 1
- 230000003505 mutagenic effect Effects 0.000 description 1
- ZTLGJPIZUOVDMT-UHFFFAOYSA-N n,n-dichlorotriazin-4-amine Chemical compound ClN(Cl)C1=CC=NN=N1 ZTLGJPIZUOVDMT-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 229940053128 nerve growth factor Drugs 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000002482 oligosaccharides Chemical class 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 230000005298 paramagnetic effect Effects 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- BXRNXXXXHLBUKK-UHFFFAOYSA-N piperazine-2,5-dione Chemical compound O=C1CNC(=O)CN1 BXRNXXXXHLBUKK-UHFFFAOYSA-N 0.000 description 1
- 239000013600 plasmid vector Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229960003171 plicamycin Drugs 0.000 description 1
- 231100000614 poison Toxicity 0.000 description 1
- 239000002574 poison Substances 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229920002859 polyalkenylene Polymers 0.000 description 1
- 239000004633 polyglycolic acid Substances 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 229940068977 polysorbate 20 Drugs 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 238000002600 positron emission tomography Methods 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 230000001323 posttranslational effect Effects 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 108010014614 prolyl-glycyl-proline Proteins 0.000 description 1
- 108010077112 prolyl-proline Proteins 0.000 description 1
- 108010031719 prolyl-serine Proteins 0.000 description 1
- 108010079317 prolyl-tyrosine Proteins 0.000 description 1
- 108010070643 prolylglutamic acid Proteins 0.000 description 1
- 239000001294 propane Substances 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 229960003712 propranolol Drugs 0.000 description 1
- 239000011546 protein dye Substances 0.000 description 1
- 239000013014 purified material Substances 0.000 description 1
- 229950010131 puromycin Drugs 0.000 description 1
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 125000006853 reporter group Chemical group 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- BPRHUIZQVSMCRT-VEUZHWNKSA-N rosuvastatin Chemical compound CC(C)C1=NC(N(C)S(C)(=O)=O)=NC(C=2C=CC(F)=CC=2)=C1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O BPRHUIZQVSMCRT-VEUZHWNKSA-N 0.000 description 1
- 229960000672 rosuvastatin Drugs 0.000 description 1
- 229960002052 salbutamol Drugs 0.000 description 1
- 229960004017 salmeterol Drugs 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 108010071207 serylmethionine Proteins 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 229960002930 sirolimus Drugs 0.000 description 1
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 150000003384 small molecules Chemical group 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 238000012289 standard assay Methods 0.000 description 1
- HKSZLNNOFSGOKW-FYTWVXJKSA-N staurosporine Chemical compound C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1[C@H]1C[C@@H](NC)[C@@H](OC)[C@]4(C)O1 HKSZLNNOFSGOKW-FYTWVXJKSA-N 0.000 description 1
- CGPUWJWCVCFERF-UHFFFAOYSA-N staurosporine Natural products C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1C1CC(NC)C(OC)C4(OC)O1 CGPUWJWCVCFERF-UHFFFAOYSA-N 0.000 description 1
- 238000012027 sterile manufacturing Methods 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 229910021653 sulphate ion Inorganic materials 0.000 description 1
- FIAFUQMPZJWCLV-UHFFFAOYSA-N suramin Chemical compound OS(=O)(=O)C1=CC(S(O)(=O)=O)=C2C(NC(=O)C3=CC=C(C(=C3)NC(=O)C=3C=C(NC(=O)NC=4C=C(C=CC=4)C(=O)NC=4C(=CC=C(C=4)C(=O)NC=4C5=C(C=C(C=C5C(=CC=4)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C)C=CC=3)C)=CC=C(S(O)(=O)=O)C2=C1 FIAFUQMPZJWCLV-UHFFFAOYSA-N 0.000 description 1
- 229960005314 suramin Drugs 0.000 description 1
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 229960002372 tetracaine Drugs 0.000 description 1
- GKCBAIGFKIBETG-UHFFFAOYSA-N tetracaine Chemical compound CCCCNC1=CC=C(C(=O)OCCN(C)C)C=C1 GKCBAIGFKIBETG-UHFFFAOYSA-N 0.000 description 1
- 229940126622 therapeutic monoclonal antibody Drugs 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 239000012049 topical pharmaceutical composition Substances 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 239000013638 trimer Substances 0.000 description 1
- 108010080629 tryptophan-leucine Proteins 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- WFKWXMTUELFFGS-RNFDNDRNSA-N tungsten-188 Chemical compound [188W] WFKWXMTUELFFGS-RNFDNDRNSA-N 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- ORHBXUUXSCNDEV-UHFFFAOYSA-N umbelliferone Chemical compound C1=CC(=O)OC2=CC(O)=CC=C21 ORHBXUUXSCNDEV-UHFFFAOYSA-N 0.000 description 1
- HFTAFOQKODTIJY-UHFFFAOYSA-N umbelliferone Natural products Cc1cc2C=CC(=O)Oc2cc1OCC=CC(C)(C)O HFTAFOQKODTIJY-UHFFFAOYSA-N 0.000 description 1
- 108010015385 valyl-prolyl-proline Proteins 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/46—Hybrid immunoglobulins
- C07K16/468—Immunoglobulins having two or more different antigen binding sites, e.g. multifunctional antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/10—Immunoglobulins specific features characterized by their source of isolation or production
- C07K2317/14—Specific host cells or culture conditions, e.g. components, pH or temperature
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/624—Disulfide-stabilized antibody (dsFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/66—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising a swap of domains, e.g. CH3-CH2, VH-CL or VL-CH1
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Life Sciences & Earth Sciences (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB1506869.5A GB201506869D0 (en) | 2015-04-22 | 2015-04-22 | Method |
| GB1506869.5 | 2015-04-22 | ||
| PCT/EP2016/059050 WO2016170137A1 (en) | 2015-04-22 | 2016-04-22 | Method for increasing the percentage of monomeric antibody fab-dsfv multimeric species |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170138551A true KR20170138551A (ko) | 2017-12-15 |
Family
ID=53299018
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177033852A Abandoned KR20170138551A (ko) | 2015-04-22 | 2016-04-22 | Fab-dsFv 다량체 종에서 단량체 항체의 백분율을 증가시키는 방법 |
Country Status (16)
| Country | Link |
|---|---|
| US (2) | US10829565B2 (enExample) |
| EP (1) | EP3286220A1 (enExample) |
| JP (1) | JP6901402B2 (enExample) |
| KR (1) | KR20170138551A (enExample) |
| CN (1) | CN107683290B (enExample) |
| AU (1) | AU2016251960A1 (enExample) |
| BR (1) | BR112017022472A2 (enExample) |
| CA (1) | CA2983537A1 (enExample) |
| CL (1) | CL2017002677A1 (enExample) |
| CO (1) | CO2017010845A2 (enExample) |
| EA (1) | EA201792325A1 (enExample) |
| GB (1) | GB201506869D0 (enExample) |
| IL (1) | IL255094A0 (enExample) |
| MX (1) | MX2017013419A (enExample) |
| SG (1) | SG11201708430VA (enExample) |
| WO (1) | WO2016170137A1 (enExample) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB201223276D0 (en) * | 2012-12-21 | 2013-02-06 | Ucb Pharma Sa | Antibodies and methods of producing same |
| GB201411320D0 (en) | 2014-06-25 | 2014-08-06 | Ucb Biopharma Sprl | Antibody construct |
| GB201506868D0 (en) | 2015-04-22 | 2015-06-03 | Ucb Biopharma Sprl | Method for protein purification |
| GB201506869D0 (en) * | 2015-04-22 | 2015-06-03 | Ucb Biopharma Sprl | Method |
| GB201506870D0 (en) | 2015-04-22 | 2015-06-03 | Ucb Biopharma Sprl | Method |
| RU2769352C2 (ru) * | 2016-12-09 | 2022-03-30 | Осе Иммьюнотерапьютикс | Антитела и полипептиды, направленные против cd127 |
Family Cites Families (82)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4741900A (en) | 1982-11-16 | 1988-05-03 | Cytogen Corporation | Antibody-metal ion complexes |
| GB8308235D0 (en) | 1983-03-25 | 1983-05-05 | Celltech Ltd | Polypeptides |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| GB8422238D0 (en) | 1984-09-03 | 1984-10-10 | Neuberger M S | Chimeric proteins |
| DK336987D0 (da) | 1987-07-01 | 1987-07-01 | Novo Industri As | Immobiliseringsmetode |
| US4877868A (en) * | 1986-03-12 | 1989-10-31 | Neorx Corporation | Radionuclide antibody coupling |
| GB8719042D0 (en) | 1987-08-12 | 1987-09-16 | Parker D | Conjugate compounds |
| GB8720833D0 (en) | 1987-09-04 | 1987-10-14 | Celltech Ltd | Recombinant dna product |
| US5223409A (en) | 1988-09-02 | 1993-06-29 | Protein Engineering Corp. | Directed evolution of novel binding proteins |
| AU4308689A (en) | 1988-09-02 | 1990-04-02 | Protein Engineering Corporation | Generation and selection of recombinant varied binding proteins |
| GB8823869D0 (en) | 1988-10-12 | 1988-11-16 | Medical Res Council | Production of antibodies |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| GB8907617D0 (en) | 1989-04-05 | 1989-05-17 | Celltech Ltd | Drug delivery system |
| GB8928874D0 (en) | 1989-12-21 | 1990-02-28 | Celltech Ltd | Humanised antibodies |
| US5780225A (en) | 1990-01-12 | 1998-07-14 | Stratagene | Method for generating libaries of antibody genes comprising amplification of diverse antibody DNAs and methods for using these libraries for the production of diverse antigen combining molecules |
| AU7247191A (en) | 1990-01-11 | 1991-08-05 | Molecular Affinities Corporation | Production of antibodies using gene libraries |
| ATE356869T1 (de) | 1990-01-12 | 2007-04-15 | Amgen Fremont Inc | Bildung von xenogenen antikörpern |
| US5427908A (en) | 1990-05-01 | 1995-06-27 | Affymax Technologies N.V. | Recombinant library screening methods |
| GB9015198D0 (en) | 1990-07-10 | 1990-08-29 | Brien Caroline J O | Binding substance |
| EP0542810A1 (en) | 1990-08-02 | 1993-05-26 | B.R. Centre Limited | Methods for the production of proteins with a desired function |
| CA2089661C (en) | 1990-08-29 | 2007-04-03 | Nils Lonberg | Transgenic non-human animals capable of producing heterologous antibodies |
| US5770429A (en) | 1990-08-29 | 1998-06-23 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5661016A (en) | 1990-08-29 | 1997-08-26 | Genpharm International Inc. | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| US5625126A (en) | 1990-08-29 | 1997-04-29 | Genpharm International, Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5633425A (en) | 1990-08-29 | 1997-05-27 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US5698426A (en) | 1990-09-28 | 1997-12-16 | Ixsys, Incorporated | Surface expression libraries of heteromeric receptors |
| WO1992009690A2 (en) | 1990-12-03 | 1992-06-11 | Genentech, Inc. | Enrichment method for variant proteins with altered binding properties |
| ATE414768T1 (de) | 1991-04-10 | 2008-12-15 | Scripps Research Inst | Bibliotheken heterodimerer rezeptoren mittels phagemiden |
| GB9112536D0 (en) | 1991-06-11 | 1991-07-31 | Celltech Ltd | Chemical compounds |
| GB9120467D0 (en) | 1991-09-26 | 1991-11-06 | Celltech Ltd | Anti-hmfg antibodies and process for their production |
| ES2227512T3 (es) | 1991-12-02 | 2005-04-01 | Medical Research Council | Produccion de anticuerpos contra auto-antigenos a partir de repertorios de segmentos de anticuerpos fijados en un fago. |
| US5733743A (en) | 1992-03-24 | 1998-03-31 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| JPH09506262A (ja) | 1993-12-08 | 1997-06-24 | ジェンザイム・コーポレイション | 特異的抗体の製造方法 |
| PT1231268E (pt) | 1994-01-31 | 2005-11-30 | Univ Boston | Bancos de anticorpos policlonais |
| FR2716640B1 (fr) | 1994-02-28 | 1996-05-03 | Procedes Machines Speciales | Dispositif de centrage et de blocage d'une pièce en vue de son rodage à l'aide d'un rodoir à expansion. |
| US5516637A (en) | 1994-06-10 | 1996-05-14 | Dade International Inc. | Method involving display of protein binding pairs on the surface of bacterial pili and bacteriophage |
| JP2978435B2 (ja) | 1996-01-24 | 1999-11-15 | チッソ株式会社 | アクリロキシプロピルシランの製造方法 |
| US5980898A (en) | 1996-11-14 | 1999-11-09 | The United States Of America As Represented By The U.S. Army Medical Research & Material Command | Adjuvant for transcutaneous immunization |
| GB9625640D0 (en) | 1996-12-10 | 1997-01-29 | Celltech Therapeutics Ltd | Biological products |
| US6908963B2 (en) | 2001-10-09 | 2005-06-21 | Nektar Therapeutics Al, Corporation | Thioester polymer derivatives and method of modifying the N-terminus of a polypeptide therewith |
| GB0202633D0 (en) * | 2002-02-05 | 2002-03-20 | Delta Biotechnology Ltd | Stabilization of protein preparations |
| JP4603894B2 (ja) | 2002-12-03 | 2010-12-22 | ユセベ ファルマ ソシエテ アノニム | 抗体産生細胞を同定するためのアッセイ |
| GB0312481D0 (en) | 2003-05-30 | 2003-07-09 | Celltech R&D Ltd | Antibodies |
| GB0315450D0 (en) * | 2003-07-01 | 2003-08-06 | Celltech R&D Ltd | Biological products |
| DK1644412T4 (en) | 2003-07-01 | 2018-11-12 | Ucb Biopharma Sprl | MODIFIED ANTIBODY-FAB FRAGMENTS |
| GB0315457D0 (en) | 2003-07-01 | 2003-08-06 | Celltech R&D Ltd | Biological products |
| GB0412181D0 (en) | 2004-06-01 | 2004-06-30 | Celltech R&D Ltd | Biological products |
| EP1805320B1 (en) | 2004-10-22 | 2014-09-03 | Amgen Inc. | Methods for refolding of recombinant antibodies |
| GB0425534D0 (en) * | 2004-11-19 | 2004-12-22 | Celltech R&D Ltd | Process for obtaining antibodies |
| US20070191272A1 (en) | 2005-09-27 | 2007-08-16 | Stemmer Willem P | Proteinaceous pharmaceuticals and uses thereof |
| CN101583370A (zh) * | 2005-09-27 | 2009-11-18 | 阿穆尼克斯公司 | 蛋白质药物及其用途 |
| GB0619291D0 (en) | 2006-09-29 | 2006-11-08 | Ucb Sa | Altered antibodies |
| EP2014680A1 (en) | 2007-07-10 | 2009-01-14 | Friedrich-Alexander-Universität Erlangen-Nürnberg | Recombinant, single-chain, trivalent tri-specific or bi-specific antibody derivatives |
| CA2700714C (en) | 2007-09-26 | 2018-09-11 | Ucb Pharma S.A. | Dual specificity antibody fusions |
| US20090269343A1 (en) | 2008-04-11 | 2009-10-29 | Duke University | Dual Specific Immunotoxin for Brain Tumor Therapy |
| EP2321338A1 (en) | 2008-08-14 | 2011-05-18 | Merck Sharp & Dohme Corp. | Methods for purifying antibodies using protein a affinity chromatography |
| NZ591651A (en) * | 2008-09-26 | 2012-12-21 | Merck Sharp & Dohme | High titer antibody production and culture media comprising glucose, soy or wheat hydrolsyate, amino acids, and other chemical compounds |
| CA2737241C (en) * | 2008-09-26 | 2017-08-29 | Ucb Pharma S.A. | Multivalent antibody fusion proteins |
| PT2424889E (pt) * | 2009-04-30 | 2015-11-12 | Ablynx Nv | Método de produção de anticorpos de domínio |
| EP2475682B1 (en) | 2009-09-10 | 2018-01-31 | UCB Biopharma SPRL | Multivalent antibodies |
| GB201005063D0 (en) * | 2010-03-25 | 2010-05-12 | Ucb Pharma Sa | Biological products |
| GB0920127D0 (en) * | 2009-11-17 | 2009-12-30 | Ucb Pharma Sa | Antibodies |
| GB201000467D0 (en) | 2010-01-12 | 2010-02-24 | Ucb Pharma Sa | Antibodies |
| GB201001791D0 (en) * | 2010-02-03 | 2010-03-24 | Ucb Pharma Sa | Process for obtaining antibodies |
| GB201005064D0 (en) | 2010-03-25 | 2010-05-12 | Ucb Pharma Sa | Biological products |
| TR201903279T4 (tr) | 2010-03-25 | 2019-03-21 | Ucb Biopharma Sprl | Disülfür stabilize edilmiş DVD-IG molekülleri. |
| PL2560993T3 (pl) * | 2010-04-20 | 2024-11-04 | Genmab A/S | Heterodimeryczne białka zawierające region FC przeciwciała i sposoby ich wytwarzania |
| KR101860963B1 (ko) * | 2010-04-23 | 2018-05-24 | 제넨테크, 인크. | 이종다량체 단백질의 생산 |
| WO2012140214A1 (en) | 2011-04-13 | 2012-10-18 | Biomay Ag | IMMUNOAFFINITY SEPARATION MATERIALS COMPRISING ANTI-IgE ANTIBODY DERIVATIVES |
| KR102492792B1 (ko) | 2011-10-11 | 2023-01-30 | 제넨테크, 인크. | 이중특이적 항체의 개선된 어셈블리 |
| AU2012335496B2 (en) * | 2011-11-11 | 2017-05-11 | Ucb Biopharma Sprl | Albumin binding antibodies and binding fragments thereof |
| UA112203C2 (uk) | 2011-11-11 | 2016-08-10 | Юсб Фарма С.А. | Злитий білок біоспецифічного антитіла, який зв'язується з ox40 людини та сироватковим альбуміном людини |
| EP2822597A1 (en) | 2012-03-09 | 2015-01-14 | UCL Business Plc. | Chemical modification of antibodies |
| GB201208370D0 (en) | 2012-05-14 | 2012-06-27 | Ucb Pharma Sa | Antibodies |
| GB201223276D0 (en) * | 2012-12-21 | 2013-02-06 | Ucb Pharma Sa | Antibodies and methods of producing same |
| MX382731B (es) * | 2013-09-27 | 2025-03-13 | Chugai Pharmaceutical Co Ltd | Metodo para producir heteromultimeros de polipeptidos. |
| GB201411320D0 (en) | 2014-06-25 | 2014-08-06 | Ucb Biopharma Sprl | Antibody construct |
| GB201506868D0 (en) | 2015-04-22 | 2015-06-03 | Ucb Biopharma Sprl | Method for protein purification |
| GB201506869D0 (en) * | 2015-04-22 | 2015-06-03 | Ucb Biopharma Sprl | Method |
| GB201506870D0 (en) | 2015-04-22 | 2015-06-03 | Ucb Biopharma Sprl | Method |
| WO2017029620A1 (en) | 2015-08-17 | 2017-02-23 | Lupin Limited | An improved refolding process for antibody's fragments |
-
2015
- 2015-04-22 GB GBGB1506869.5A patent/GB201506869D0/en not_active Ceased
-
2016
- 2016-04-22 SG SG11201708430VA patent/SG11201708430VA/en unknown
- 2016-04-22 US US15/568,018 patent/US10829565B2/en active Active
- 2016-04-22 AU AU2016251960A patent/AU2016251960A1/en not_active Abandoned
- 2016-04-22 EP EP16721103.6A patent/EP3286220A1/en active Pending
- 2016-04-22 BR BR112017022472A patent/BR112017022472A2/pt not_active Application Discontinuation
- 2016-04-22 KR KR1020177033852A patent/KR20170138551A/ko not_active Abandoned
- 2016-04-22 WO PCT/EP2016/059050 patent/WO2016170137A1/en not_active Ceased
- 2016-04-22 MX MX2017013419A patent/MX2017013419A/es unknown
- 2016-04-22 CN CN201680031354.XA patent/CN107683290B/zh active Active
- 2016-04-22 JP JP2017555395A patent/JP6901402B2/ja active Active
- 2016-04-22 EA EA201792325A patent/EA201792325A1/ru unknown
- 2016-04-22 CA CA2983537A patent/CA2983537A1/en active Pending
-
2017
- 2017-10-17 IL IL255094A patent/IL255094A0/en unknown
- 2017-10-20 CL CL2017002677A patent/CL2017002677A1/es unknown
- 2017-10-25 CO CONC2017/0010845A patent/CO2017010845A2/es unknown
-
2020
- 2020-10-23 US US17/078,130 patent/US11834514B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CA2983537A1 (en) | 2016-10-27 |
| US11834514B2 (en) | 2023-12-05 |
| US10829565B2 (en) | 2020-11-10 |
| MX2017013419A (es) | 2018-02-09 |
| AU2016251960A1 (en) | 2017-11-02 |
| GB201506869D0 (en) | 2015-06-03 |
| EA201792325A1 (ru) | 2018-04-30 |
| JP2018515453A (ja) | 2018-06-14 |
| CO2017010845A2 (es) | 2018-03-20 |
| JP6901402B2 (ja) | 2021-07-14 |
| CN107683290A (zh) | 2018-02-09 |
| EP3286220A1 (en) | 2018-02-28 |
| CL2017002677A1 (es) | 2018-03-23 |
| SG11201708430VA (en) | 2017-11-29 |
| US20180142039A1 (en) | 2018-05-24 |
| BR112017022472A2 (pt) | 2018-07-17 |
| WO2016170137A1 (en) | 2016-10-27 |
| US20210079120A1 (en) | 2021-03-18 |
| IL255094A0 (en) | 2017-12-31 |
| CN107683290B (zh) | 2022-01-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7727387B2 (ja) | 新規の抗cd39抗体 | |
| US10844122B2 (en) | Prostate specific membrane antigen (PSMA) bispecific binding agents and uses thereof | |
| TWI772258B (zh) | Cdh3與cd3之雙特異性抗體構築體 | |
| KR102271204B1 (ko) | 다중특이적 항체 작제물 | |
| CN107849136B (zh) | 抗TfR抗体及其在治疗增殖性和炎性疾病中的用途 | |
| US20180194862A1 (en) | Bispecific binding proteins | |
| US11786593B2 (en) | Method of monomerisation of recombinant antibody molecules | |
| US11834514B2 (en) | Method for increasing the percentage of monomeric antibody Fab-dsFv multimeric species | |
| HK1247214B (zh) | 一种pdl-1抗体、其药物组合物及其用途 | |
| CN113301919A (zh) | 激活免疫细胞的双特异性抗体 | |
| KR20160077155A (ko) | 인간 암을 치료하기 위한 특이적 항-cd38 항체 | |
| JP2019502380A (ja) | TNF−α、IL−17A及びIL−17Fに特異性を有する多重特異性抗体分子 | |
| CN117355540A (zh) | 抗cd137抗体和使用方法 | |
| WO2022224997A1 (ja) | 抗cldn4-抗cd137二重特異性抗体 | |
| KR20180089521A (ko) | Tnf 알파에 결합하는 항체 분자 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20171122 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210315 Comment text: Request for Examination of Application |
|
| PC1902 | Submission of document of abandonment before decision of registration |