KR20170078791A - 스피넬 재료의 제조 - Google Patents
스피넬 재료의 제조 Download PDFInfo
- Publication number
- KR20170078791A KR20170078791A KR1020177014766A KR20177014766A KR20170078791A KR 20170078791 A KR20170078791 A KR 20170078791A KR 1020177014766 A KR1020177014766 A KR 1020177014766A KR 20177014766 A KR20177014766 A KR 20177014766A KR 20170078791 A KR20170078791 A KR 20170078791A
- Authority
- KR
- South Korea
- Prior art keywords
- lmo
- lithium
- cathode
- manganese
- lmoa
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000000463 material Substances 0.000 title claims abstract description 82
- 239000011029 spinel Substances 0.000 title claims abstract description 56
- 229910052596 spinel Inorganic materials 0.000 title claims abstract description 56
- 238000004519 manufacturing process Methods 0.000 title claims abstract description 18
- VLXXBCXTUVRROQ-UHFFFAOYSA-N lithium;oxido-oxo-(oxomanganiooxy)manganese Chemical compound [Li+].[O-][Mn](=O)O[Mn]=O VLXXBCXTUVRROQ-UHFFFAOYSA-N 0.000 claims abstract description 153
- 229910002102 lithium manganese oxide Inorganic materials 0.000 claims abstract description 148
- 238000000034 method Methods 0.000 claims abstract description 37
- 238000000137 annealing Methods 0.000 claims abstract description 21
- 230000008569 process Effects 0.000 claims abstract description 17
- 238000005049 combustion synthesis Methods 0.000 claims abstract description 13
- 239000011572 manganese Substances 0.000 claims description 134
- 239000000843 powder Substances 0.000 claims description 39
- 238000006243 chemical reaction Methods 0.000 claims description 26
- 239000000243 solution Substances 0.000 claims description 26
- 239000003792 electrolyte Substances 0.000 claims description 25
- 229910052744 lithium Inorganic materials 0.000 claims description 21
- 229910052748 manganese Inorganic materials 0.000 claims description 20
- 229910052782 aluminium Inorganic materials 0.000 claims description 19
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 claims description 18
- 239000010406 cathode material Substances 0.000 claims description 17
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 claims description 14
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 11
- 239000002019 doping agent Substances 0.000 claims description 10
- 239000000376 reactant Substances 0.000 claims description 10
- IIPYXGDZVMZOAP-UHFFFAOYSA-N lithium nitrate Chemical compound [Li+].[O-][N+]([O-])=O IIPYXGDZVMZOAP-UHFFFAOYSA-N 0.000 claims description 8
- 239000000446 fuel Substances 0.000 claims description 7
- -1 aluminum compound Chemical class 0.000 claims description 6
- 238000007599 discharging Methods 0.000 claims description 6
- 239000002904 solvent Substances 0.000 claims description 6
- 239000012456 homogeneous solution Substances 0.000 claims description 4
- 150000002642 lithium compounds Chemical class 0.000 claims description 4
- 150000002697 manganese compounds Chemical class 0.000 claims description 4
- MIVBAHRSNUNMPP-UHFFFAOYSA-N manganese(2+);dinitrate Chemical compound [Mn+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O MIVBAHRSNUNMPP-UHFFFAOYSA-N 0.000 claims description 3
- 239000007864 aqueous solution Substances 0.000 claims description 2
- 238000011068 loading method Methods 0.000 claims description 2
- 230000000630 rising effect Effects 0.000 claims description 2
- 230000000977 initiatory effect Effects 0.000 claims 1
- XIXADJRWDQXREU-UHFFFAOYSA-M lithium acetate Chemical compound [Li+].CC([O-])=O XIXADJRWDQXREU-UHFFFAOYSA-M 0.000 claims 1
- XGZVUEUWXADBQD-UHFFFAOYSA-L lithium carbonate Chemical compound [Li+].[Li+].[O-]C([O-])=O XGZVUEUWXADBQD-UHFFFAOYSA-L 0.000 claims 1
- 229910052808 lithium carbonate Inorganic materials 0.000 claims 1
- INHCSSUBVCNVSK-UHFFFAOYSA-L lithium sulfate Inorganic materials [Li+].[Li+].[O-]S([O-])(=O)=O INHCSSUBVCNVSK-UHFFFAOYSA-L 0.000 claims 1
- 229940071125 manganese acetate Drugs 0.000 claims 1
- 229940099596 manganese sulfate Drugs 0.000 claims 1
- 235000007079 manganese sulphate Nutrition 0.000 claims 1
- 239000011702 manganese sulphate Substances 0.000 claims 1
- UOGMEBQRZBEZQT-UHFFFAOYSA-L manganese(2+);diacetate Chemical compound [Mn+2].CC([O-])=O.CC([O-])=O UOGMEBQRZBEZQT-UHFFFAOYSA-L 0.000 claims 1
- SQQMAOCOWKFBNP-UHFFFAOYSA-L manganese(II) sulfate Chemical compound [Mn+2].[O-]S([O-])(=O)=O SQQMAOCOWKFBNP-UHFFFAOYSA-L 0.000 claims 1
- 239000000758 substrate Substances 0.000 claims 1
- RBTVSNLYYIMMKS-UHFFFAOYSA-N tert-butyl 3-aminoazetidine-1-carboxylate;hydrochloride Chemical compound Cl.CC(C)(C)OC(=O)N1CC(N)C1 RBTVSNLYYIMMKS-UHFFFAOYSA-N 0.000 claims 1
- 239000002245 particle Substances 0.000 description 25
- 239000000523 sample Substances 0.000 description 24
- 229910001416 lithium ion Inorganic materials 0.000 description 23
- 150000002500 ions Chemical class 0.000 description 22
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 description 18
- 230000014759 maintenance of location Effects 0.000 description 14
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 13
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 10
- 229910010199 LiAl Inorganic materials 0.000 description 9
- 229910015643 LiMn 2 O 4 Inorganic materials 0.000 description 9
- 238000002441 X-ray diffraction Methods 0.000 description 9
- 230000001351 cycling effect Effects 0.000 description 9
- 230000015572 biosynthetic process Effects 0.000 description 8
- 238000009792 diffusion process Methods 0.000 description 7
- 238000003780 insertion Methods 0.000 description 7
- 230000037431 insertion Effects 0.000 description 7
- 229910018584 Mn 2-x O 4 Inorganic materials 0.000 description 6
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 6
- 239000004202 carbamide Substances 0.000 description 6
- 238000009826 distribution Methods 0.000 description 6
- 230000005855 radiation Effects 0.000 description 6
- 230000009467 reduction Effects 0.000 description 6
- 238000001228 spectrum Methods 0.000 description 6
- 238000003786 synthesis reaction Methods 0.000 description 6
- 229910015645 LiMn Inorganic materials 0.000 description 5
- 239000002033 PVDF binder Substances 0.000 description 5
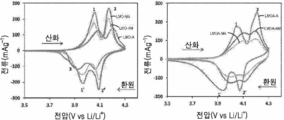
- 238000002484 cyclic voltammetry Methods 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 238000000157 electrochemical-induced impedance spectroscopy Methods 0.000 description 5
- 239000011888 foil Substances 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 5
- 241000894007 species Species 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 4
- OIFBSDVPJOWBCH-UHFFFAOYSA-N Diethyl carbonate Chemical compound CCOC(=O)OCC OIFBSDVPJOWBCH-UHFFFAOYSA-N 0.000 description 4
- KMTRUDSVKNLOMY-UHFFFAOYSA-N Ethylene carbonate Chemical compound O=C1OCCO1 KMTRUDSVKNLOMY-UHFFFAOYSA-N 0.000 description 4
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 description 4
- 238000001157 Fourier transform infrared spectrum Methods 0.000 description 4
- 230000005536 Jahn Teller effect Effects 0.000 description 4
- 229910013553 LiNO Inorganic materials 0.000 description 4
- 229910018663 Mn O Inorganic materials 0.000 description 4
- 229910003176 Mn-O Inorganic materials 0.000 description 4
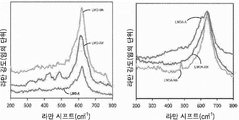
- 238000001237 Raman spectrum Methods 0.000 description 4
- 238000004833 X-ray photoelectron spectroscopy Methods 0.000 description 4
- 238000012512 characterization method Methods 0.000 description 4
- 239000013078 crystal Substances 0.000 description 4
- 238000004090 dissolution Methods 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 230000003647 oxidation Effects 0.000 description 4
- 238000007254 oxidation reaction Methods 0.000 description 4
- 238000001878 scanning electron micrograph Methods 0.000 description 4
- 238000001069 Raman spectroscopy Methods 0.000 description 3
- 238000003917 TEM image Methods 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- IEJIGPNLZYLLBP-UHFFFAOYSA-N dimethyl carbonate Chemical compound COC(=O)OC IEJIGPNLZYLLBP-UHFFFAOYSA-N 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000002848 electrochemical method Methods 0.000 description 3
- 238000002173 high-resolution transmission electron microscopy Methods 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 239000011164 primary particle Substances 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 101100317222 Borrelia hermsii vsp3 gene Proteins 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 229910013870 LiPF 6 Inorganic materials 0.000 description 2
- 229910006270 Li—Li Inorganic materials 0.000 description 2
- FBDMJGHBCPNRGF-UHFFFAOYSA-M [OH-].[Li+].[O-2].[Mn+2] Chemical compound [OH-].[Li+].[O-2].[Mn+2] FBDMJGHBCPNRGF-UHFFFAOYSA-M 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 239000011149 active material Substances 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 229910052786 argon Inorganic materials 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 239000006229 carbon black Substances 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 230000008602 contraction Effects 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 230000005518 electrochemistry Effects 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 238000000445 field-emission scanning electron microscopy Methods 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 238000000024 high-resolution transmission electron micrograph Methods 0.000 description 2
- 238000001453 impedance spectrum Methods 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 239000002105 nanoparticle Substances 0.000 description 2
- 239000011255 nonaqueous electrolyte Substances 0.000 description 2
- 230000010355 oscillation Effects 0.000 description 2
- 239000007800 oxidant agent Substances 0.000 description 2
- 230000003071 parasitic effect Effects 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 238000006479 redox reaction Methods 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 239000011163 secondary particle Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 230000003595 spectral effect Effects 0.000 description 2
- BNGXYYYYKUGPPF-UHFFFAOYSA-M (3-methylphenyl)methyl-triphenylphosphanium;chloride Chemical compound [Cl-].CC1=CC=CC(C[P+](C=2C=CC=CC=2)(C=2C=CC=CC=2)C=2C=CC=CC=2)=C1 BNGXYYYYKUGPPF-UHFFFAOYSA-M 0.000 description 1
- XNDZQQSKSQTQQD-UHFFFAOYSA-N 3-methylcyclohex-2-en-1-ol Chemical compound CC1=CC(O)CCC1 XNDZQQSKSQTQQD-UHFFFAOYSA-N 0.000 description 1
- 229910018516 Al—O Inorganic materials 0.000 description 1
- 229910016523 CuKa Inorganic materials 0.000 description 1
- 229910014078 LiMn1.9Al0.1O4 Inorganic materials 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 238000003841 Raman measurement Methods 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- 238000000026 X-ray photoelectron spectrum Methods 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 239000003738 black carbon Substances 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- RKTYLMNFRDHKIL-UHFFFAOYSA-N copper;5,10,15,20-tetraphenylporphyrin-22,24-diide Chemical compound [Cu+2].C1=CC(C(=C2C=CC([N-]2)=C(C=2C=CC=CC=2)C=2C=CC(N=2)=C(C=2C=CC=CC=2)C2=CC=C3[N-]2)C=2C=CC=CC=2)=NC1=C3C1=CC=CC=C1 RKTYLMNFRDHKIL-UHFFFAOYSA-N 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 238000002788 crimping Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 238000007606 doctor blade method Methods 0.000 description 1
- 238000005868 electrolysis reaction Methods 0.000 description 1
- 238000010894 electron beam technology Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 238000009830 intercalation Methods 0.000 description 1
- 238000005468 ion implantation Methods 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 229910001437 manganese ion Inorganic materials 0.000 description 1
- ALIMWUQMDCBYFM-UHFFFAOYSA-N manganese(2+);dinitrate;tetrahydrate Chemical compound O.O.O.O.[Mn+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O ALIMWUQMDCBYFM-UHFFFAOYSA-N 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- AHADSRNLHOHMQK-UHFFFAOYSA-N methylidenecopper Chemical compound [Cu].[C] AHADSRNLHOHMQK-UHFFFAOYSA-N 0.000 description 1
- 238000001000 micrograph Methods 0.000 description 1
- 238000007144 microwave assisted synthesis reaction Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000004570 mortar (masonry) Substances 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000012488 sample solution Substances 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 239000007784 solid electrolyte Substances 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G45/00—Compounds of manganese
- C01G45/12—Complex oxides containing manganese and at least one other metal element
- C01G45/1221—Manganates or manganites with trivalent manganese, tetravalent manganese or mixtures thereof
- C01G45/1242—Manganates or manganites with trivalent manganese, tetravalent manganese or mixtures thereof of the type (Mn2O4)-, e.g. LiMn2O4 or Li(MxMn2-x)O4
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/058—Construction or manufacture
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/50—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese
- H01M4/505—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese of mixed oxides or hydroxides containing manganese for inserting or intercalating light metals, e.g. LiMn2O4 or LiMn2OxFy
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/30—Three-dimensional structures
- C01P2002/32—Three-dimensional structures spinel-type (AB2O4)
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/50—Solid solutions
- C01P2002/52—Solid solutions containing elements as dopants
- C01P2002/54—Solid solutions containing elements as dopants one element only
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/70—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data
- C01P2002/72—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data by d-values or two theta-values, e.g. as X-ray diagram
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/80—Crystal-structural characteristics defined by measured data other than those specified in group C01P2002/70
- C01P2002/82—Crystal-structural characteristics defined by measured data other than those specified in group C01P2002/70 by IR- or Raman-data
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/80—Crystal-structural characteristics defined by measured data other than those specified in group C01P2002/70
- C01P2002/85—Crystal-structural characteristics defined by measured data other than those specified in group C01P2002/70 by XPS, EDX or EDAX data
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/01—Particle morphology depicted by an image
- C01P2004/03—Particle morphology depicted by an image obtained by SEM
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/01—Particle morphology depicted by an image
- C01P2004/04—Particle morphology depicted by an image obtained by TEM, STEM, STM or AFM
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/40—Electric properties
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M2004/026—Electrodes composed of, or comprising, active material characterised by the polarity
- H01M2004/028—Positive electrodes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Battery Electrode And Active Subsutance (AREA)
- Inorganic Compounds Of Heavy Metals (AREA)
- Secondary Cells (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ZA2014/07986 | 2014-10-31 | ||
| ZA201407986 | 2014-10-31 | ||
| PCT/ZA2015/050019 WO2016070205A2 (en) | 2014-10-31 | 2015-10-30 | Production of a spinel material |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170078791A true KR20170078791A (ko) | 2017-07-07 |
Family
ID=55802547
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177014766A Ceased KR20170078791A (ko) | 2014-10-31 | 2015-10-30 | 스피넬 재료의 제조 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US20180277844A1 (enExample) |
| EP (1) | EP3212578A2 (enExample) |
| JP (1) | JP2018500722A (enExample) |
| KR (1) | KR20170078791A (enExample) |
| CN (1) | CN107108260B (enExample) |
| AR (1) | AR102483A1 (enExample) |
| BR (1) | BR112017009040B1 (enExample) |
| CA (1) | CA2966361A1 (enExample) |
| WO (1) | WO2016070205A2 (enExample) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017109646A1 (en) * | 2015-12-22 | 2017-06-29 | Csir | Spinel material |
| CN109417191A (zh) * | 2017-02-22 | 2019-03-01 | 丰田自动车欧洲公司 | 锂离子蓄电池高温老化过程 |
| EP3412633A1 (en) * | 2017-06-08 | 2018-12-12 | Basf Se | Process for manufacturing an electrode active material |
| CL2017002221A1 (es) * | 2017-09-01 | 2018-01-19 | Univ Antofagasta | Espinela de manganeso dopada con magnesio, material catódico que la comprende, método de preparación, y batería de ion litio que la comprende |
| KR102671188B1 (ko) * | 2017-10-09 | 2024-06-03 | 씨에스아이알 | 음극 재료 |
| CN107887600B (zh) * | 2017-11-07 | 2020-05-05 | 哈尔滨工业大学 | 一种锂离子电池用预激活富锂锰基正极材料的制备方法 |
| KR102533794B1 (ko) * | 2018-11-30 | 2023-05-19 | 주식회사 엘지에너지솔루션 | 팔면체 구조의 리튬 망간계 양극 활물질, 이를 포함하는 양극 및 리튬 이차전지 |
| KR102394065B1 (ko) * | 2020-09-07 | 2022-05-09 | 한국에너지기술연구원 | 고결정성 리튬-금속 산화물 입자 및 이의 제조방법 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09320603A (ja) * | 1996-03-28 | 1997-12-12 | Aichi Steel Works Ltd | リチウム二次電池用粉末状活物質の製造方法 |
| DE19815611A1 (de) * | 1998-04-07 | 1999-10-14 | Riedel De Haen Gmbh | Verfahren zur Herstellung von Lithium-Metall-Oxiden |
| JP4360143B2 (ja) * | 2003-07-17 | 2009-11-11 | 株式会社豊田中央研究所 | リチウム二次電池用活物質の製造方法 |
| CN1249832C (zh) * | 2004-02-16 | 2006-04-05 | 厦门大学 | 一种锂锰氧化物的制备方法及其在电池中的应用 |
| CN102790203B (zh) * | 2011-05-19 | 2016-03-30 | 中国科学院宁波材料技术与工程研究所 | 一种锂离子电池正极材料的制备方法 |
| CN104703920A (zh) * | 2012-08-10 | 2015-06-10 | Csir公司 | 尖晶石材料的制造 |
| JP6081782B2 (ja) * | 2012-11-23 | 2017-02-15 | 日本ケミコン株式会社 | リチウムイオン二次電池用電極材料、リチウムイオン二次電池用電極材料の製造方法、及びリチウムイオン二次電池 |
-
2015
- 2015-10-30 EP EP15853628.4A patent/EP3212578A2/en not_active Withdrawn
- 2015-10-30 JP JP2017523515A patent/JP2018500722A/ja active Pending
- 2015-10-30 KR KR1020177014766A patent/KR20170078791A/ko not_active Ceased
- 2015-10-30 BR BR112017009040-6A patent/BR112017009040B1/pt not_active IP Right Cessation
- 2015-10-30 WO PCT/ZA2015/050019 patent/WO2016070205A2/en not_active Ceased
- 2015-10-30 CA CA2966361A patent/CA2966361A1/en not_active Abandoned
- 2015-10-30 US US15/522,979 patent/US20180277844A1/en not_active Abandoned
- 2015-10-30 AR ARP150103525A patent/AR102483A1/es active IP Right Grant
- 2015-10-30 CN CN201580071621.1A patent/CN107108260B/zh active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN107108260A (zh) | 2017-08-29 |
| JP2018500722A (ja) | 2018-01-11 |
| BR112017009040B1 (pt) | 2022-06-14 |
| WO2016070205A2 (en) | 2016-05-06 |
| BR112017009040A2 (pt) | 2018-07-03 |
| EP3212578A2 (en) | 2017-09-06 |
| US20180277844A1 (en) | 2018-09-27 |
| CN107108260B (zh) | 2019-08-23 |
| WO2016070205A3 (en) | 2016-09-09 |
| CA2966361A1 (en) | 2016-05-06 |
| AR102483A1 (es) | 2017-03-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11183692B2 (en) | Production of a layered lithium-manganese-nickel-cobalt oxide material | |
| JP7676851B2 (ja) | 遷移金属複合水酸化物粒子、遷移金属複合水酸化物粒子の製造方法、リチウムイオン二次電池用正極活物質、及びリチウムイオン二次電池 | |
| KR102402147B1 (ko) | 마그네슘으로 도핑된 망간 스피넬, 상기 망간 스피넬을 포함하는 캐소드 재료, 이들의 조제 방법 및 상기 스피넬을 포함하는 리튬 이온 배터리 | |
| CN107108260B (zh) | 尖晶石材料的制备 | |
| JP7412264B2 (ja) | リチウムイオン二次電池用正極活物質およびその製造方法 | |
| JP6316812B2 (ja) | スピネル材料の製造 | |
| KR20100060362A (ko) | 리튬 이차 전지용 양극 활물질, 이의 제조방법 및 이를 포함하는 리튬 이차 전지 | |
| JP7664896B2 (ja) | 高強度リチウムイオン二次電池用正極活物質、及び、該正極活物質を用いたリチウムイオン二次電池 | |
| CN114521300A (zh) | 锂离子二次电池用正极活性物质以及锂离子二次电池 | |
| JP2024086748A (ja) | 遷移金属複合水酸化物の粒子、リチウムイオン二次電池用正極活物質、およびリチウムイオン二次電池 | |
| Zhu et al. | A layered/spinel heterostructured cathode for Li-ion batteries prepared by ultrafast Joule heating | |
| CN111936425A (zh) | 锂离子二次电池用正极活性物质及其制造方法 | |
| Cui et al. | Laser-assisted surface lithium fluoride decoration of a cobalt-free high-voltage spinel LiNi0. 5Mn1. 5O4 cathode for long-life lithium-ion batteries | |
| Singh et al. | Role of impurity phases present in orthorhombic-Li2MnSiO4 towards the Li-reactivity and storage as LIB cathode | |
| Arof et al. | Electrochemical properties of LiMn2O4 prepared with tartaric acid chelating agent | |
| JP7474833B2 (ja) | リチウムイオン二次電池用正極活物質 | |
| Jiang et al. | Synergistic integration of internal gradient-doping and external coating for superior performance in lithium-rich Mn-based cathodes | |
| Phraewphiphat et al. | Electrochemical performance of Li2MnSiO4/C lithium-ion cathode materials with low-level doping of Ru in the Mn site | |
| Lee et al. | Omni-functional simultaneous interfacial treatment for enhancing stability and outgassing suppression of lithium-ion batteries | |
| CN108604673A (zh) | 尖晶石材料 | |
| KR20010081180A (ko) | 리튬 이차 전지용 양극 활물질의 제조 방법 | |
| Park et al. | Phase-and morphology-controlled MnO2: Its synthesis and influence on the electrochemical performance of spinel LiMn2O4 cathode materials | |
| Sathiyaraj et al. | H $ _ {\bm 2} $ O $ _ {\bm 2} $-Aided One-Pot Hydrothermal Synthesis of Nanocrystalline LiMn $ _ {\bm 2} $ O $ _ {\bm 4} $ Cathode for Lithium Batteries | |
| Wang et al. | Structural and electrochemical characterization of spinel LiMn2O4 synthesized from the phosphate-doped Mn3O4 | |
| Toghan et al. | Impact of Cs+ ion doping on the Physical, Microstructural and electrochemical characteristics of LiNi0. 5Mn1. 5O4 cathode for high voltage Li-ion batteries |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20170530 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20200925 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20220103 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20220407 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20220103 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |