KR20160148605A - 관능화된 시클로실록산의 제조 방법 - Google Patents
관능화된 시클로실록산의 제조 방법 Download PDFInfo
- Publication number
- KR20160148605A KR20160148605A KR1020167032298A KR20167032298A KR20160148605A KR 20160148605 A KR20160148605 A KR 20160148605A KR 1020167032298 A KR1020167032298 A KR 1020167032298A KR 20167032298 A KR20167032298 A KR 20167032298A KR 20160148605 A KR20160148605 A KR 20160148605A
- Authority
- KR
- South Korea
- Prior art keywords
- formula
- cyclosiloxane
- heptamethyl
- heteroatoms selected
- cyclotetrasiloxane
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
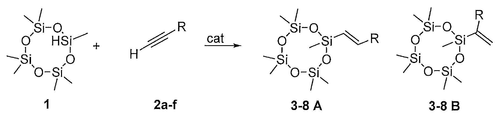
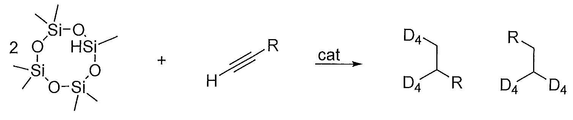
- 238000000034 method Methods 0.000 title claims abstract description 33
- 238000002360 preparation method Methods 0.000 title abstract description 7
- 239000003054 catalyst Substances 0.000 claims abstract description 32
- 238000006459 hydrosilylation reaction Methods 0.000 claims abstract description 11
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 claims description 39
- 229910052717 sulfur Inorganic materials 0.000 claims description 31
- -1 1-phenylvinyl Chemical group 0.000 claims description 30
- 125000005842 heteroatom Chemical group 0.000 claims description 30
- 229910052799 carbon Inorganic materials 0.000 claims description 23
- 229910052760 oxygen Inorganic materials 0.000 claims description 22
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical group [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 18
- 229910052736 halogen Inorganic materials 0.000 claims description 17
- 150000002367 halogens Chemical class 0.000 claims description 17
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 16
- 125000000217 alkyl group Chemical group 0.000 claims description 15
- 125000003118 aryl group Chemical group 0.000 claims description 14
- 238000006243 chemical reaction Methods 0.000 claims description 13
- 125000000623 heterocyclic group Chemical group 0.000 claims description 13
- 125000006652 (C3-C12) cycloalkyl group Chemical group 0.000 claims description 12
- 229910052739 hydrogen Inorganic materials 0.000 claims description 12
- 239000001257 hydrogen Substances 0.000 claims description 12
- 125000003710 aryl alkyl group Chemical group 0.000 claims description 11
- 125000000392 cycloalkenyl group Chemical group 0.000 claims description 11
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 claims description 11
- 125000002877 alkyl aryl group Chemical group 0.000 claims description 10
- 125000000304 alkynyl group Chemical group 0.000 claims description 10
- 125000004404 heteroalkyl group Chemical group 0.000 claims description 10
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 claims description 9
- 125000001072 heteroaryl group Chemical group 0.000 claims description 9
- 239000000377 silicon dioxide Substances 0.000 claims description 8
- 125000003342 alkenyl group Chemical group 0.000 claims description 7
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 7
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 6
- 239000012300 argon atmosphere Substances 0.000 claims description 6
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 6
- 238000001914 filtration Methods 0.000 claims description 6
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 6
- SNOOUWRIMMFWNE-UHFFFAOYSA-M sodium;6-[(3,4,5-trimethoxybenzoyl)amino]hexanoate Chemical compound [Na+].COC1=CC(C(=O)NCCCCCC([O-])=O)=CC(OC)=C1OC SNOOUWRIMMFWNE-UHFFFAOYSA-M 0.000 claims description 6
- RCNRJBWHLARWRP-UHFFFAOYSA-N ethenyl-[ethenyl(dimethyl)silyl]oxy-dimethylsilane;platinum Chemical group [Pt].C=C[Si](C)(C)O[Si](C)(C)C=C RCNRJBWHLARWRP-UHFFFAOYSA-N 0.000 claims description 5
- 229910052697 platinum Inorganic materials 0.000 claims description 5
- CFNAPNIXPUMJSP-UHFFFAOYSA-N 2,2,4,4,6,6,8-heptamethyl-8-(2-phenylethenyl)-1,3,5,7,2,4,6,8-tetraoxatetrasilocane Chemical compound O1[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O[Si]1(C)C=CC1=CC=CC=C1 CFNAPNIXPUMJSP-UHFFFAOYSA-N 0.000 claims description 4
- HCDVTAHLGOSFQN-UHFFFAOYSA-N 2-(1-phenylethenyl)-4-(2-phenylethenyl)-1,3,5,7,2,4,6,8-tetraoxatetrasilocane Chemical compound C1(=CC=CC=C1)C(=C)[SiH]1O[SiH](O[SiH2]O[SiH2]O1)C=CC1=CC=CC=C1 HCDVTAHLGOSFQN-UHFFFAOYSA-N 0.000 claims description 4
- FNGGZWAATUWAJZ-UHFFFAOYSA-N C[Si]1(O[Si](O[Si](O[Si](O1)(C(=C)C1=CC=CC=C1)C)(C(=C)C1=CC=CC=C1)C)(C(=C)C1=CC=CC=C1)C)C(=C)C1=CC=CC=C1 Chemical compound C[Si]1(O[Si](O[Si](O[Si](O1)(C(=C)C1=CC=CC=C1)C)(C(=C)C1=CC=CC=C1)C)(C(=C)C1=CC=CC=C1)C)C(=C)C1=CC=CC=C1 FNGGZWAATUWAJZ-UHFFFAOYSA-N 0.000 claims description 4
- ORLLOXPUFWBUKD-UHFFFAOYSA-N C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C(=C)C1=CC=C(C=C1)C(F)(F)F)C)C Chemical compound C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C(=C)C1=CC=C(C=C1)C(F)(F)F)C)C ORLLOXPUFWBUKD-UHFFFAOYSA-N 0.000 claims description 4
- FMYLXJJLWCRHJE-UHFFFAOYSA-N C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C(=C)C1=CC=CC=C1)C)C Chemical compound C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C(=C)C1=CC=CC=C1)C)C FMYLXJJLWCRHJE-UHFFFAOYSA-N 0.000 claims description 4
- JAUMDILTRARNGD-UHFFFAOYSA-N C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C(C(C)(O)C)=C Chemical compound C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C(C(C)(O)C)=C JAUMDILTRARNGD-UHFFFAOYSA-N 0.000 claims description 4
- NFEVXBSIQGLDPV-UHFFFAOYSA-N C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C(CCC(=O)O)=C Chemical compound C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C(CCC(=O)O)=C NFEVXBSIQGLDPV-UHFFFAOYSA-N 0.000 claims description 4
- GQWWFOMZTWQSBO-UHFFFAOYSA-N C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C(CCCO)=C Chemical compound C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C(CCCO)=C GQWWFOMZTWQSBO-UHFFFAOYSA-N 0.000 claims description 4
- MBWGUPIKYNCPKK-UHFFFAOYSA-N C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C=CC(C)(O)C Chemical compound C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C=CC(C)(O)C MBWGUPIKYNCPKK-UHFFFAOYSA-N 0.000 claims description 4
- KSWNLVJFDMCSOK-UHFFFAOYSA-N C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C=CCCC(=O)O Chemical compound C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C=CCCC(=O)O KSWNLVJFDMCSOK-UHFFFAOYSA-N 0.000 claims description 4
- IVISUCMNFVIESR-UHFFFAOYSA-N C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C=CCCCO Chemical compound C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C)C)C=CCCCO IVISUCMNFVIESR-UHFFFAOYSA-N 0.000 claims description 4
- JTLNXJOXFKTXCW-UHFFFAOYSA-N C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C=CC1=CC=C(C=C1)C(F)(F)F)C)C Chemical compound C[Si]1(O[Si](O[Si](O[Si](O1)(C)C)(C)C)(C=CC1=CC=C(C=C1)C(F)(F)F)C)C JTLNXJOXFKTXCW-UHFFFAOYSA-N 0.000 claims description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 4
- XYFCBTPGUUZFHI-UHFFFAOYSA-N Phosphine Chemical compound P XYFCBTPGUUZFHI-UHFFFAOYSA-N 0.000 claims description 4
- 239000003960 organic solvent Substances 0.000 claims description 4
- 239000010948 rhodium Substances 0.000 claims description 4
- 125000005741 alkyl alkenyl group Chemical group 0.000 claims description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 3
- 239000007983 Tris buffer Substances 0.000 claims description 2
- 150000001336 alkenes Chemical class 0.000 claims description 2
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 claims description 2
- 239000012298 atmosphere Substances 0.000 claims description 2
- 239000003610 charcoal Substances 0.000 claims description 2
- 239000000706 filtrate Substances 0.000 claims description 2
- 229910052741 iridium Inorganic materials 0.000 claims description 2
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims description 2
- 230000007935 neutral effect Effects 0.000 claims description 2
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 claims description 2
- 239000013110 organic ligand Substances 0.000 claims description 2
- 229910000073 phosphorus hydride Inorganic materials 0.000 claims description 2
- 230000035484 reaction time Effects 0.000 claims description 2
- 229910052703 rhodium Inorganic materials 0.000 claims description 2
- MHOVAHRLVXNVSD-UHFFFAOYSA-N rhodium atom Chemical compound [Rh] MHOVAHRLVXNVSD-UHFFFAOYSA-N 0.000 claims description 2
- 150000002431 hydrogen Chemical group 0.000 claims 3
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims 1
- UPIXZLGONUBZLK-UHFFFAOYSA-N platinum Chemical compound [Pt].[Pt] UPIXZLGONUBZLK-UHFFFAOYSA-N 0.000 claims 1
- 238000005406 washing Methods 0.000 claims 1
- SNYNNFDVNITLRQ-UHFFFAOYSA-N 2,2,4,4,6,6,8-heptamethyl-1,3,5,7,2,4,6,8$l^{3}-tetraoxatetrasilocane Chemical compound C[Si]1O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O1 SNYNNFDVNITLRQ-UHFFFAOYSA-N 0.000 description 15
- 239000000243 solution Substances 0.000 description 12
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 10
- 125000000524 functional group Chemical group 0.000 description 10
- 239000000203 mixture Substances 0.000 description 9
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 7
- 239000000047 product Substances 0.000 description 7
- 150000001875 compounds Chemical class 0.000 description 6
- 239000002904 solvent Substances 0.000 description 6
- 239000011865 Pt-based catalyst Substances 0.000 description 5
- 238000002390 rotary evaporation Methods 0.000 description 5
- UEXCJVNBTNXOEH-UHFFFAOYSA-N Ethynylbenzene Chemical group C#CC1=CC=CC=C1 UEXCJVNBTNXOEH-UHFFFAOYSA-N 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 3
- 238000004132 cross linking Methods 0.000 description 3
- 125000000753 cycloalkyl group Chemical group 0.000 description 3
- KPUWHANPEXNPJT-UHFFFAOYSA-N disiloxane Chemical class [SiH3]O[SiH3] KPUWHANPEXNPJT-UHFFFAOYSA-N 0.000 description 3
- FKRCODPIKNYEAC-UHFFFAOYSA-N ethyl propionate Chemical compound CCOC(=O)CC FKRCODPIKNYEAC-UHFFFAOYSA-N 0.000 description 3
- CLTMUXYRYKDGOK-UHFFFAOYSA-N 2,2,4,4,6,6,8-heptamethyl-1,3,5,7,2,4,6,8-tetraoxatetrasilocane Chemical compound C[SiH]1O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O1 CLTMUXYRYKDGOK-UHFFFAOYSA-N 0.000 description 2
- GICXPDMORPBXDD-UHFFFAOYSA-N 2-[1-(2,4,4,6,6,8,8-heptamethyl-1,3,5,7,2,4,6,8-tetraoxatetrasilocan-2-yl)ethyl]-2,4,4,6,6,8,8-heptamethyl-1,3,5,7,2,4,6,8-tetraoxatetrasilocane Chemical compound O1[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O[Si]1(C)C(C)[Si]1(C)O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O1 GICXPDMORPBXDD-UHFFFAOYSA-N 0.000 description 2
- LTIUDPOSFOYSKA-UHFFFAOYSA-N 2-ethenyl-2,4,4,6,6,8,8-heptamethyl-1,3,5,7,2,4,6,8-tetraoxatetrasilocane Chemical compound C[Si]1(C)O[Si](C)(C)O[Si](C)(C=C)O[Si](C)(C)O1 LTIUDPOSFOYSKA-UHFFFAOYSA-N 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical class C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 125000004432 carbon atom Chemical group C* 0.000 description 2
- CREMABGTGYGIQB-UHFFFAOYSA-N carbon carbon Chemical compound C.C CREMABGTGYGIQB-UHFFFAOYSA-N 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 229910052731 fluorine Inorganic materials 0.000 description 2
- 239000011737 fluorine Substances 0.000 description 2
- 150000002430 hydrocarbons Chemical group 0.000 description 2
- 125000004356 hydroxy functional group Chemical group O* 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 238000007151 ring opening polymerisation reaction Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 125000001424 substituent group Chemical group 0.000 description 2
- 238000010189 synthetic method Methods 0.000 description 2
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- DTGKSKDOIYIVQL-WEDXCCLWSA-N (+)-borneol Chemical group C1C[C@@]2(C)[C@@H](O)C[C@@H]1C2(C)C DTGKSKDOIYIVQL-WEDXCCLWSA-N 0.000 description 1
- 125000004765 (C1-C4) haloalkyl group Chemical group 0.000 description 1
- SKYKARIMXZGKOD-UHFFFAOYSA-N 2,2,4,4,6-pentamethyl-1,3,5,2,4,6-trioxatrisilinane Chemical group C[SiH]1O[Si](C)(C)O[Si](C)(C)O1 SKYKARIMXZGKOD-UHFFFAOYSA-N 0.000 description 1
- WZJUBBHODHNQPW-UHFFFAOYSA-N 2,4,6,8-tetramethyl-1,3,5,7,2$l^{3},4$l^{3},6$l^{3},8$l^{3}-tetraoxatetrasilocane Chemical compound C[Si]1O[Si](C)O[Si](C)O[Si](C)O1 WZJUBBHODHNQPW-UHFFFAOYSA-N 0.000 description 1
- MQKZXMQDBRILTR-UHFFFAOYSA-N 2-cyclopenta-2,4-dien-1-yl-2-methyl-1,3,5,7,2,4,6,8-tetraoxatetrasilocane Chemical compound C[Si]1(O[SiH2]O[SiH2]O[SiH2]O1)C1C=CC=C1 MQKZXMQDBRILTR-UHFFFAOYSA-N 0.000 description 1
- 125000000094 2-phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- XMSXQFUHVRWGNA-UHFFFAOYSA-N Decamethylcyclopentasiloxane Chemical compound C[Si]1(C)O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O1 XMSXQFUHVRWGNA-UHFFFAOYSA-N 0.000 description 1
- IUMSDRXLFWAGNT-UHFFFAOYSA-N Dodecamethylcyclohexasiloxane Chemical compound C[Si]1(C)O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O1 IUMSDRXLFWAGNT-UHFFFAOYSA-N 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 1
- 229910018557 Si O Inorganic materials 0.000 description 1
- BLRPTPMANUNPDV-UHFFFAOYSA-N Silane Chemical compound [SiH4] BLRPTPMANUNPDV-UHFFFAOYSA-N 0.000 description 1
- 239000006087 Silane Coupling Agent Substances 0.000 description 1
- 125000003670 adamantan-2-yl group Chemical group [H]C1([H])C(C2([H])[H])([H])C([H])([H])C3([H])C([*])([H])C1([H])C([H])([H])C2([H])C3([H])[H] 0.000 description 1
- 125000003172 aldehyde group Chemical group 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 150000001733 carboxylic acid esters Chemical class 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- DDJSWKLBKSLAAZ-UHFFFAOYSA-N cyclotetrasiloxane Chemical compound O1[SiH2]O[SiH2]O[SiH2]O[SiH2]1 DDJSWKLBKSLAAZ-UHFFFAOYSA-N 0.000 description 1
- JJRDHFIVAPVZJN-UHFFFAOYSA-N cyclotrisiloxane Chemical compound O1[SiH2]O[SiH2]O[SiH2]1 JJRDHFIVAPVZJN-UHFFFAOYSA-N 0.000 description 1
- 125000003493 decenyl group Chemical class [H]C([*])=C([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 239000004205 dimethyl polysiloxane Substances 0.000 description 1
- 125000005066 dodecenyl group Chemical class C(=CCCCCCCCCCC)* 0.000 description 1
- BITPLIXHRASDQB-UHFFFAOYSA-N ethenyl-[ethenyl(dimethyl)silyl]oxy-dimethylsilane Chemical compound C=C[Si](C)(C)O[Si](C)(C)C=C BITPLIXHRASDQB-UHFFFAOYSA-N 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid group Chemical group C(CCCCCC)(=O)O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000006038 hexenyl group Chemical class 0.000 description 1
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 1
- 239000013067 intermediate product Substances 0.000 description 1
- 150000002500 ions Chemical group 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 125000000468 ketone group Chemical group 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 125000005187 nonenyl group Chemical class C(=CCCCCCCC)* 0.000 description 1
- 125000004365 octenyl group Chemical class C(=CCCCCCC)* 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000000962 organic group Chemical group 0.000 description 1
- NFHFRUOZVGFOOS-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- XAKYZBMFCZISAU-UHFFFAOYSA-N platinum;triphenylphosphane Chemical compound [Pt].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 XAKYZBMFCZISAU-UHFFFAOYSA-N 0.000 description 1
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 125000006413 ring segment Chemical group 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 239000000565 sealant Substances 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 229910000077 silane Inorganic materials 0.000 description 1
- LIVNPJMFVYWSIS-UHFFFAOYSA-N silicon monoxide Inorganic materials [Si-]#[O+] LIVNPJMFVYWSIS-UHFFFAOYSA-N 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 125000005369 trialkoxysilyl group Chemical group 0.000 description 1
- PZJJKWKADRNWSW-UHFFFAOYSA-N trimethoxysilicon Chemical group CO[Si](OC)OC PZJJKWKADRNWSW-UHFFFAOYSA-N 0.000 description 1
- 229930195735 unsaturated hydrocarbon Natural products 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F7/00—Compounds containing elements of Groups 4 or 14 of the Periodic Table
- C07F7/02—Silicon compounds
- C07F7/21—Cyclic compounds having at least one ring containing silicon, but no carbon in the ring
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/38—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals
- B01J23/40—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals of the platinum group metals
- B01J23/42—Platinum
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J31/00—Catalysts comprising hydrides, coordination complexes or organic compounds
- B01J31/16—Catalysts comprising hydrides, coordination complexes or organic compounds containing coordination complexes
- B01J31/1616—Coordination complexes, e.g. organometallic complexes, immobilised on an inorganic support, e.g. ship-in-a-bottle type catalysts
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2231/00—Catalytic reactions performed with catalysts classified in B01J31/00
- B01J2231/30—Addition reactions at carbon centres, i.e. to either C-C or C-X multiple bonds
- B01J2231/32—Addition reactions to C=C or C-C triple bonds
- B01J2231/323—Hydrometalation, e.g. bor-, alumin-, silyl-, zirconation or analoguous reactions like carbometalation, hydrocarbation
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Inorganic Chemistry (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Silicon Polymers (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP14165544.9A EP2937353A1 (en) | 2014-04-23 | 2014-04-23 | Method for the preparation of functionalized cyclosiloxanes |
| EP14165544.9 | 2014-04-23 | ||
| PCT/EP2015/058638 WO2015162145A1 (en) | 2014-04-23 | 2015-04-22 | Method for the preparation of functionalized cyclosiloxanes |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160148605A true KR20160148605A (ko) | 2016-12-26 |
Family
ID=50513133
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167032298A Withdrawn KR20160148605A (ko) | 2014-04-23 | 2015-04-22 | 관능화된 시클로실록산의 제조 방법 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US10059728B2 (enExample) |
| EP (1) | EP2937353A1 (enExample) |
| JP (1) | JP6608844B2 (enExample) |
| KR (1) | KR20160148605A (enExample) |
| CN (1) | CN106414464A (enExample) |
| TW (1) | TWI672312B (enExample) |
| WO (1) | WO2015162145A1 (enExample) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112225852B (zh) * | 2020-09-28 | 2021-10-08 | 浙江大学 | 一种聚硅氧烷功能化乙烯-降冰片烯共聚物及其制备方法 |
| CN116789967A (zh) * | 2023-06-29 | 2023-09-22 | 中国科学院化学研究所 | 一种分子量可控的乙基硅氧烷聚合物的制备方法及其应用 |
| CN117003789A (zh) * | 2023-07-03 | 2023-11-07 | 衢州科峰新材料有限公司 | 一种甲基乙基环硅氧烷的制备方法 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2762829A (en) | 1952-01-02 | 1956-09-11 | Stanolind Oil & Gas Co | Process for converting olefins to oxygenated organic compounds |
| US2793222A (en) | 1954-05-11 | 1957-05-21 | Gen Electric | 1, 2-bis-heptamethylcyclotetrasiloxanylethane and polymeric derivatives thereof |
| US2785147A (en) | 1954-05-11 | 1957-03-12 | Gen Electric | Process for making octamethylcyclotetrasiloxane gels |
| US5352753A (en) * | 1991-04-25 | 1994-10-04 | Allergan, Inc. | Ultraviolet light absorbing compounds, silicone compositions and methods for making same |
| JPH0517486A (ja) * | 1991-07-04 | 1993-01-26 | Shin Etsu Chem Co Ltd | 有機ケイ素化合物及びその製造方法 |
| US7138108B2 (en) * | 2002-06-13 | 2006-11-21 | L'oreal | Photoprotective UV-screening compositions comprising (phenylsulfonyl) acrylonitrile-substituted silanes/siloxanes |
| JP4217870B2 (ja) * | 2002-07-15 | 2009-02-04 | 日本電気株式会社 | 有機シロキサン共重合体膜、その製造方法、成長装置、ならびに該共重合体膜を用いた半導体装置 |
| JP4296006B2 (ja) | 2003-02-27 | 2009-07-15 | 独立行政法人科学技術振興機構 | ビニルシランの製造方法 |
| US8501893B2 (en) | 2008-01-25 | 2013-08-06 | National Science And Technology Development Agency | Synthetic method for preparing dual curable silicone compositions |
| JP2011144272A (ja) * | 2010-01-15 | 2011-07-28 | Nippon Shokubai Co Ltd | ジルコニアナノ粒子を含むシリコーン樹脂組成物 |
-
2014
- 2014-04-23 EP EP14165544.9A patent/EP2937353A1/en not_active Withdrawn
-
2015
- 2015-04-10 TW TW104111543A patent/TWI672312B/zh not_active IP Right Cessation
- 2015-04-22 CN CN201580020913.2A patent/CN106414464A/zh active Pending
- 2015-04-22 WO PCT/EP2015/058638 patent/WO2015162145A1/en not_active Ceased
- 2015-04-22 KR KR1020167032298A patent/KR20160148605A/ko not_active Withdrawn
- 2015-04-22 JP JP2016563986A patent/JP6608844B2/ja not_active Expired - Fee Related
-
2016
- 2016-10-17 US US15/294,920 patent/US10059728B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| CN106414464A (zh) | 2017-02-15 |
| JP6608844B2 (ja) | 2019-11-20 |
| TWI672312B (zh) | 2019-09-21 |
| US20170096438A1 (en) | 2017-04-06 |
| US10059728B2 (en) | 2018-08-28 |
| TW201546084A (zh) | 2015-12-16 |
| JP2017517497A (ja) | 2017-06-29 |
| WO2015162145A1 (en) | 2015-10-29 |
| EP2937353A1 (en) | 2015-10-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JPS5931533B2 (ja) | アルケニル基及びSiの結合された水素を有するオルガノポリシロキサンを基礎とする物質の架橋方法 | |
| TWI712610B (zh) | 與聚矽氧相容之化合物 | |
| KR0147422B1 (ko) | 2작용성 말단실록산단위를 가진 오르가노폴리실록산 | |
| KR20120073259A (ko) | 플루오로실리콘과 그 유도체들의 합성 | |
| US20030139621A1 (en) | Method for preparing styryl-functionalized silanes | |
| KR20160148605A (ko) | 관능화된 시클로실록산의 제조 방법 | |
| CN103153966B (zh) | 用于制备2-羟苯基苯并三唑硅氧烷化合物的方法 | |
| CN107922442B (zh) | 具有(甲基)丙烯酸酯基团的有机硅化合物及用于制备其的方法 | |
| JP4889097B2 (ja) | ポリシクロオレフィン官能性ポリシロキサン | |
| JP6596075B2 (ja) | オルガノシリコン変性光開始剤およびその光硬化性接着剤組成物 | |
| JPH01132591A (ja) | 二量体化されたビニルビシクロヘプチル基含有ケイ素化合物およびその製造方法 | |
| JP2017517497A5 (enExample) | ||
| JP5190667B2 (ja) | かご型シルセスキオキサン骨格を含有する重合体を素材とする光学素子 | |
| CN110621680A (zh) | 具有烷氧基烷基的异氰脲酸衍生物及其制造方法 | |
| CN103288866A (zh) | 含氟马来酰亚胺化合物及其制造方法 | |
| WO2005073181A1 (en) | Vinylchloroformate-free synthesis of vinyl and allyl(thio)carbamates | |
| KR101972761B1 (ko) | 가교제용 실릴카보실록산 유도체와 이의 제조방법 | |
| CN114502618A (zh) | 用于由氢化硅化合物制备硅氧烷的方法 | |
| US5001247A (en) | Method of making organosilbutadiyne polymers | |
| JP7689416B2 (ja) | かご状シロキサン含有高分子、かご状シロキサン及びその製造方法 | |
| JPH0518834B2 (enExample) | ||
| JP2007045782A (ja) | ポリシクロオレフィン官能性ポリシロキサンの製造方法 | |
| JP6225888B2 (ja) | オルガノポリシロキサン化合物及びその製造方法 | |
| JP6574120B2 (ja) | 片末端(メタ)アクリルアミドシリコーンの製造方法 | |
| JP2018172498A (ja) | 液状ケイ素化合物及びその製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20161118 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination |