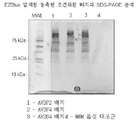
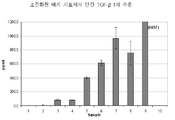
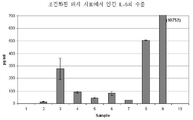
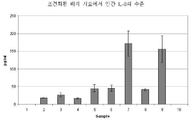
KR20120083407A - 줄기 세포 조건화된 배지 조성물 - Google Patents
줄기 세포 조건화된 배지 조성물 Download PDFInfo
- Publication number
- KR20120083407A KR20120083407A KR1020127009488A KR20127009488A KR20120083407A KR 20120083407 A KR20120083407 A KR 20120083407A KR 1020127009488 A KR1020127009488 A KR 1020127009488A KR 20127009488 A KR20127009488 A KR 20127009488A KR 20120083407 A KR20120083407 A KR 20120083407A
- Authority
- KR
- South Korea
- Prior art keywords
- cells
- cell
- medium
- protein
- growth
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 36
- 210000000130 stem cell Anatomy 0.000 title claims description 11
- 239000003636 conditioned culture medium Substances 0.000 title description 24
- 210000004027 cell Anatomy 0.000 claims abstract description 195
- 239000001963 growth medium Substances 0.000 claims abstract description 45
- 230000001143 conditioned effect Effects 0.000 claims abstract description 43
- 238000000034 method Methods 0.000 claims abstract description 39
- 239000006143 cell culture medium Substances 0.000 claims abstract description 34
- 230000002500 effect on skin Effects 0.000 claims abstract description 32
- 239000007640 basal medium Substances 0.000 claims abstract description 30
- 210000002950 fibroblast Anatomy 0.000 claims abstract description 25
- 230000010261 cell growth Effects 0.000 claims abstract description 21
- 230000029663 wound healing Effects 0.000 claims abstract description 21
- 210000004748 cultured cell Anatomy 0.000 claims abstract description 11
- 230000003833 cell viability Effects 0.000 claims abstract description 9
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 9
- 238000012258 culturing Methods 0.000 claims abstract description 6
- 201000008827 tuberculosis Diseases 0.000 claims abstract description 6
- 210000003527 eukaryotic cell Anatomy 0.000 claims abstract description 4
- 108090000623 proteins and genes Proteins 0.000 claims description 59
- 102000004169 proteins and genes Human genes 0.000 claims description 59
- 102000008186 Collagen Human genes 0.000 claims description 23
- 108010035532 Collagen Proteins 0.000 claims description 23
- 229920001436 collagen Polymers 0.000 claims description 21
- 108010022233 Plasminogen Activator Inhibitor 1 Proteins 0.000 claims description 15
- 108010067306 Fibronectins Proteins 0.000 claims description 11
- 102000004890 Interleukin-8 Human genes 0.000 claims description 10
- 108090001007 Interleukin-8 Proteins 0.000 claims description 10
- 230000008569 process Effects 0.000 claims description 10
- 108090001005 Interleukin-6 Proteins 0.000 claims description 9
- 102000004889 Interleukin-6 Human genes 0.000 claims description 8
- 101000713104 Homo sapiens C-C motif chemokine 1 Proteins 0.000 claims description 7
- 102100021669 Stromal cell-derived factor 1 Human genes 0.000 claims description 7
- 101710088580 Stromal cell-derived factor 1 Proteins 0.000 claims description 7
- 102100036841 C-C motif chemokine 1 Human genes 0.000 claims description 6
- 108010014419 Chemokine CXCL1 Proteins 0.000 claims description 6
- 102000016950 Chemokine CXCL1 Human genes 0.000 claims description 6
- 102000003816 Interleukin-13 Human genes 0.000 claims description 5
- 108090000176 Interleukin-13 Proteins 0.000 claims description 5
- 102000034655 MIF Human genes 0.000 claims description 5
- 108060004872 MIF Proteins 0.000 claims description 5
- 102000003974 Fibroblast growth factor 2 Human genes 0.000 claims description 3
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 claims description 3
- 108090001012 Transforming Growth Factor beta Proteins 0.000 claims description 3
- 102000004887 Transforming Growth Factor beta Human genes 0.000 claims description 3
- 102000009618 Transforming Growth Factors Human genes 0.000 claims description 2
- 108010009583 Transforming Growth Factors Proteins 0.000 claims description 2
- 210000005260 human cell Anatomy 0.000 claims description 2
- 210000002107 sheath cell Anatomy 0.000 claims description 2
- 102000012335 Plasminogen Activator Inhibitor 1 Human genes 0.000 claims 2
- 238000001035 drying Methods 0.000 claims 2
- 238000004108 freeze drying Methods 0.000 claims 2
- 238000007710 freezing Methods 0.000 claims 2
- 230000008014 freezing Effects 0.000 claims 2
- 238000000746 purification Methods 0.000 claims 2
- 102000016359 Fibronectins Human genes 0.000 claims 1
- -1 SPARC Proteins 0.000 claims 1
- 239000003814 drug Substances 0.000 claims 1
- 229940079593 drug Drugs 0.000 claims 1
- 235000018102 proteins Nutrition 0.000 description 56
- 238000004113 cell culture Methods 0.000 description 53
- 239000007758 minimum essential medium Substances 0.000 description 26
- 208000027418 Wounds and injury Diseases 0.000 description 24
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 22
- 206010052428 Wound Diseases 0.000 description 22
- 210000000270 basal cell Anatomy 0.000 description 22
- 239000012091 fetal bovine serum Substances 0.000 description 22
- 210000001519 tissue Anatomy 0.000 description 17
- 239000002609 medium Substances 0.000 description 16
- 210000004379 membrane Anatomy 0.000 description 16
- 239000012528 membrane Substances 0.000 description 16
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 15
- 239000002953 phosphate buffered saline Substances 0.000 description 15
- 102100039418 Plasminogen activator inhibitor 1 Human genes 0.000 description 13
- 238000011534 incubation Methods 0.000 description 12
- 238000004519 manufacturing process Methods 0.000 description 12
- 239000003102 growth factor Substances 0.000 description 11
- 102100037362 Fibronectin Human genes 0.000 description 10
- 108010010803 Gelatin Proteins 0.000 description 10
- 239000004472 Lysine Substances 0.000 description 10
- 229910002091 carbon monoxide Inorganic materials 0.000 description 10
- 239000008273 gelatin Substances 0.000 description 10
- 229920000159 gelatin Polymers 0.000 description 10
- 235000019322 gelatine Nutrition 0.000 description 10
- 235000011852 gelatine desserts Nutrition 0.000 description 10
- 230000012010 growth Effects 0.000 description 10
- 210000003470 mitochondria Anatomy 0.000 description 10
- 229910052760 oxygen Inorganic materials 0.000 description 10
- 102000002734 Collagen Type VI Human genes 0.000 description 9
- 108010043741 Collagen Type VI Proteins 0.000 description 9
- 102000004127 Cytokines Human genes 0.000 description 9
- 108090000695 Cytokines Proteins 0.000 description 9
- 230000028327 secretion Effects 0.000 description 9
- 239000000725 suspension Substances 0.000 description 9
- KPGXRSRHYNQIFN-UHFFFAOYSA-N 2-oxoglutaric acid Chemical compound OC(=O)CCC(=O)C(O)=O KPGXRSRHYNQIFN-UHFFFAOYSA-N 0.000 description 8
- 238000004458 analytical method Methods 0.000 description 8
- 239000002537 cosmetic Substances 0.000 description 8
- 210000000805 cytoplasm Anatomy 0.000 description 8
- 239000007789 gas Substances 0.000 description 8
- 102000014914 Carrier Proteins Human genes 0.000 description 7
- 101710088194 Dehydrogenase Proteins 0.000 description 7
- 108091008324 binding proteins Proteins 0.000 description 7
- 239000012228 culture supernatant Substances 0.000 description 7
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 7
- 230000001965 increasing effect Effects 0.000 description 7
- 239000002245 particle Substances 0.000 description 7
- 102000012432 Collagen Type V Human genes 0.000 description 6
- 108010022514 Collagen Type V Proteins 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 102000046299 Transforming Growth Factor beta1 Human genes 0.000 description 6
- 101800002279 Transforming growth factor beta-1 Proteins 0.000 description 6
- 230000001419 dependent effect Effects 0.000 description 6
- 230000014509 gene expression Effects 0.000 description 6
- 210000002966 serum Anatomy 0.000 description 6
- 238000010561 standard procedure Methods 0.000 description 6
- OAIGZYFGCNNVIE-ZPFDUUQYSA-N Ala-Val-Asp-Pro Chemical compound C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(O)=O OAIGZYFGCNNVIE-ZPFDUUQYSA-N 0.000 description 5
- DDPKBJZLAXLQGZ-KBIXCLLPSA-N Ala-Val-Asp-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O DDPKBJZLAXLQGZ-KBIXCLLPSA-N 0.000 description 5
- 108010067787 Proteoglycans Proteins 0.000 description 5
- 102000016611 Proteoglycans Human genes 0.000 description 5
- 102100037599 SPARC Human genes 0.000 description 5
- 101710100111 SPARC Proteins 0.000 description 5
- 239000000872 buffer Substances 0.000 description 5
- 238000000338 in vitro Methods 0.000 description 5
- 239000004615 ingredient Substances 0.000 description 5
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 5
- 239000011159 matrix material Substances 0.000 description 5
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 5
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 4
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 4
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 4
- 102000004266 Collagen Type IV Human genes 0.000 description 4
- 108010042086 Collagen Type IV Proteins 0.000 description 4
- 102100027869 Moesin Human genes 0.000 description 4
- 102000004316 Oxidoreductases Human genes 0.000 description 4
- 108090000854 Oxidoreductases Proteins 0.000 description 4
- 229920001213 Polysorbate 20 Polymers 0.000 description 4
- 238000009825 accumulation Methods 0.000 description 4
- 230000033115 angiogenesis Effects 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 230000000903 blocking effect Effects 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000018109 developmental process Effects 0.000 description 4
- 239000000499 gel Substances 0.000 description 4
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 4
- 230000014759 maintenance of location Effects 0.000 description 4
- 108010071525 moesin Proteins 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 4
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 108090000765 processed proteins & peptides Proteins 0.000 description 4
- 230000035755 proliferation Effects 0.000 description 4
- 238000007634 remodeling Methods 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 238000009331 sowing Methods 0.000 description 4
- 230000017423 tissue regeneration Effects 0.000 description 4
- 230000007838 tissue remodeling Effects 0.000 description 4
- WAAPEIZFCHNLKK-UFBFGSQYSA-N (2s,4s)-6-fluoro-2',5'-dioxospiro[2,3-dihydrochromene-4,4'-imidazolidine]-2-carboxamide Chemical compound C([C@H](OC1=CC=C(F)C=C11)C(=O)N)[C@@]21NC(=O)NC2=O WAAPEIZFCHNLKK-UFBFGSQYSA-N 0.000 description 3
- 102000017304 72kDa type IV collagenases Human genes 0.000 description 3
- 108050005269 72kDa type IV collagenases Proteins 0.000 description 3
- 102100033312 Alpha-2-macroglobulin Human genes 0.000 description 3
- 102100038910 Alpha-enolase Human genes 0.000 description 3
- 102100037917 CD109 antigen Human genes 0.000 description 3
- 101710157469 CD109 antigen Proteins 0.000 description 3
- 101710132601 Capsid protein Proteins 0.000 description 3
- 102000004225 Cathepsin B Human genes 0.000 description 3
- 108090000712 Cathepsin B Proteins 0.000 description 3
- 108010061117 Cathepsin Z Proteins 0.000 description 3
- 102000011937 Cathepsin Z Human genes 0.000 description 3
- 102000001187 Collagen Type III Human genes 0.000 description 3
- 108010069502 Collagen Type III Proteins 0.000 description 3
- 102000014870 Collagen Type XII Human genes 0.000 description 3
- 108010039001 Collagen Type XII Proteins 0.000 description 3
- 102100033601 Collagen alpha-1(I) chain Human genes 0.000 description 3
- 102100036213 Collagen alpha-2(I) chain Human genes 0.000 description 3
- 101710126238 Collagen alpha-2(I) chain Proteins 0.000 description 3
- 102100028067 EGF-containing fibulin-like extracellular matrix protein 2 Human genes 0.000 description 3
- 101710176518 EGF-containing fibulin-like extracellular matrix protein 2 Proteins 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 102100031509 Fibrillin-1 Human genes 0.000 description 3
- 108010030229 Fibrillin-1 Proteins 0.000 description 3
- 102100022277 Fructose-bisphosphate aldolase A Human genes 0.000 description 3
- 101710123627 Fructose-bisphosphate aldolase A Proteins 0.000 description 3
- 102000004878 Gelsolin Human genes 0.000 description 3
- 108090001064 Gelsolin Proteins 0.000 description 3
- 229930182566 Gentamicin Natural products 0.000 description 3
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 3
- 102100031132 Glucose-6-phosphate isomerase Human genes 0.000 description 3
- 108010070600 Glucose-6-phosphate isomerase Proteins 0.000 description 3
- 229920002971 Heparan sulfate Polymers 0.000 description 3
- 102000006479 Heterogeneous-Nuclear Ribonucleoproteins Human genes 0.000 description 3
- 108010019372 Heterogeneous-Nuclear Ribonucleoproteins Proteins 0.000 description 3
- 108090000144 Human Proteins Proteins 0.000 description 3
- 102000003839 Human Proteins Human genes 0.000 description 3
- 102000004369 Insulin-like growth factor-binding protein 4 Human genes 0.000 description 3
- 108090000969 Insulin-like growth factor-binding protein 4 Proteins 0.000 description 3
- 102000004883 Insulin-like growth factor-binding protein 6 Human genes 0.000 description 3
- 108090001014 Insulin-like growth factor-binding protein 6 Proteins 0.000 description 3
- 102100029228 Insulin-like growth factor-binding protein 7 Human genes 0.000 description 3
- 102100023481 Integrin beta-like protein 1 Human genes 0.000 description 3
- 108050004182 Integrin beta-like protein 1 Proteins 0.000 description 3
- 108010044467 Isoenzymes Proteins 0.000 description 3
- 101710115390 L-lactate dehydrogenase A chain Proteins 0.000 description 3
- 102100034671 L-lactate dehydrogenase A chain Human genes 0.000 description 3
- 102100032114 Lumican Human genes 0.000 description 3
- 108010076371 Lumican Proteins 0.000 description 3
- 102000000380 Matrix Metalloproteinase 1 Human genes 0.000 description 3
- 108010016113 Matrix Metalloproteinase 1 Proteins 0.000 description 3
- 102100040283 Peptidyl-prolyl cis-trans isomerase B Human genes 0.000 description 3
- 102000003992 Peroxidases Human genes 0.000 description 3
- 108010022181 Phosphopyruvate Hydratase Proteins 0.000 description 3
- 108010015078 Pregnancy-Associated alpha 2-Macroglobulins Proteins 0.000 description 3
- 101710087172 Procollagen C-endopeptidase enhancer 1 Proteins 0.000 description 3
- 102100041026 Procollagen C-endopeptidase enhancer 1 Human genes 0.000 description 3
- 102100032116 Putative nucleoside diphosphate kinase Human genes 0.000 description 3
- 101710205590 Putative nucleoside diphosphate kinase Proteins 0.000 description 3
- 102000013009 Pyruvate Kinase Human genes 0.000 description 3
- 108020005115 Pyruvate Kinase Proteins 0.000 description 3
- 102100034335 Rab GDP dissociation inhibitor alpha Human genes 0.000 description 3
- 101710102264 Rab GDP dissociation inhibitor alpha Proteins 0.000 description 3
- 102100034328 Rab GDP dissociation inhibitor beta Human genes 0.000 description 3
- 101710193720 Rab GDP dissociation inhibitor beta Proteins 0.000 description 3
- 102100037545 Semaphorin-7A Human genes 0.000 description 3
- 101710199489 Semaphorin-7A Proteins 0.000 description 3
- 102100025512 Serpin B6 Human genes 0.000 description 3
- 102100025521 Serpin B7 Human genes 0.000 description 3
- 101710156145 Serpin B7 Proteins 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 102100034371 Sulfhydryl oxidase 1 Human genes 0.000 description 3
- 101710159725 Sulfhydryl oxidase 1 Proteins 0.000 description 3
- 102100030269 Thioredoxin domain-containing protein 5 Human genes 0.000 description 3
- 101710154464 Thioredoxin domain-containing protein 5 Proteins 0.000 description 3
- 108020004530 Transaldolase Proteins 0.000 description 3
- 102100028601 Transaldolase Human genes 0.000 description 3
- 102000010208 Transforming growth factor-beta-induced protein ig-h3 Human genes 0.000 description 3
- 108050001894 Transforming growth factor-beta-induced protein ig-h3 Proteins 0.000 description 3
- 102000014701 Transketolase Human genes 0.000 description 3
- 108010043652 Transketolase Proteins 0.000 description 3
- 108090000848 Ubiquitin Proteins 0.000 description 3
- 102000044159 Ubiquitin Human genes 0.000 description 3
- 102100035071 Vimentin Human genes 0.000 description 3
- 108010065472 Vimentin Proteins 0.000 description 3
- 102100036551 WD repeat-containing protein 1 Human genes 0.000 description 3
- 101710083179 WD repeat-containing protein 1 Proteins 0.000 description 3
- 210000002469 basement membrane Anatomy 0.000 description 3
- 229920002988 biodegradable polymer Polymers 0.000 description 3
- 239000004621 biodegradable polymer Substances 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 230000008021 deposition Effects 0.000 description 3
- 238000011026 diafiltration Methods 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- 210000003754 fetus Anatomy 0.000 description 3
- 229960002518 gentamicin Drugs 0.000 description 3
- 239000000017 hydrogel Substances 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 230000002757 inflammatory effect Effects 0.000 description 3
- 208000014674 injury Diseases 0.000 description 3
- 108010008598 insulin-like growth factor binding protein-related protein 1 Proteins 0.000 description 3
- 210000002510 keratinocyte Anatomy 0.000 description 3
- 230000003902 lesion Effects 0.000 description 3
- 239000002207 metabolite Substances 0.000 description 3
- 239000003475 metalloproteinase inhibitor Substances 0.000 description 3
- 210000002894 multi-fate stem cell Anatomy 0.000 description 3
- 108010044156 peptidyl-prolyl cis-trans isomerase b Proteins 0.000 description 3
- 230000010412 perfusion Effects 0.000 description 3
- 108040007629 peroxidase activity proteins Proteins 0.000 description 3
- 102000030592 phosphoserine aminotransferase Human genes 0.000 description 3
- 108010088694 phosphoserine aminotransferase Proteins 0.000 description 3
- 239000000049 pigment Substances 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 238000001556 precipitation Methods 0.000 description 3
- 230000008439 repair process Effects 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 108010017282 serpin B6 Proteins 0.000 description 3
- 210000003491 skin Anatomy 0.000 description 3
- 235000002639 sodium chloride Nutrition 0.000 description 3
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 210000005048 vimentin Anatomy 0.000 description 3
- 101710157142 2-methylene-furan-3-one reductase Proteins 0.000 description 2
- HSTOKWSFWGCZMH-UHFFFAOYSA-N 3,3'-diaminobenzidine Chemical compound C1=C(N)C(N)=CC=C1C1=CC=C(N)C(N)=C1 HSTOKWSFWGCZMH-UHFFFAOYSA-N 0.000 description 2
- 229930183010 Amphotericin Natural products 0.000 description 2
- QGGFZZLFKABGNL-UHFFFAOYSA-N Amphotericin A Natural products OC1C(N)C(O)C(C)OC1OC1C=CC=CC=CC=CCCC=CC=CC(C)C(O)C(C)C(C)OC(=O)CC(O)CC(O)CCC(O)C(O)CC(O)CC(O)(CC(O)C2C(O)=O)OC2C1 QGGFZZLFKABGNL-UHFFFAOYSA-N 0.000 description 2
- 108010039206 Biotinidase Proteins 0.000 description 2
- 102100026044 Biotinidase Human genes 0.000 description 2
- 102100032528 C-type lectin domain family 11 member A Human genes 0.000 description 2
- 101710167766 C-type lectin domain family 11 member A Proteins 0.000 description 2
- 102100031168 CCN family member 2 Human genes 0.000 description 2
- 102100024210 CD166 antigen Human genes 0.000 description 2
- 101710164718 CD166 antigen Proteins 0.000 description 2
- ACTIUHUUMQJHFO-UHFFFAOYSA-N Coenzym Q10 Natural products COC1=C(OC)C(=O)C(CC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)C)=C(C)C1=O ACTIUHUUMQJHFO-UHFFFAOYSA-N 0.000 description 2
- 102000004510 Collagen Type VII Human genes 0.000 description 2
- 108010017377 Collagen Type VII Proteins 0.000 description 2
- 102000009736 Collagen Type XI Human genes 0.000 description 2
- 108010034789 Collagen Type XI Proteins 0.000 description 2
- 102100033781 Collagen alpha-2(IV) chain Human genes 0.000 description 2
- 101710179387 Collagen alpha-2(IV) chain Proteins 0.000 description 2
- 102100030149 Complement C1r subcomponent Human genes 0.000 description 2
- 108050005571 Complement C1r subcomponent Proteins 0.000 description 2
- 108010039419 Connective Tissue Growth Factor Proteins 0.000 description 2
- 102000012192 Cystatin C Human genes 0.000 description 2
- 108010061642 Cystatin C Proteins 0.000 description 2
- 102000016955 Erythrocyte Anion Exchange Protein 1 Human genes 0.000 description 2
- 108010014384 Erythrocyte Anion Exchange Protein 1 Proteins 0.000 description 2
- 108050001049 Extracellular proteins Proteins 0.000 description 2
- 102100020903 Ezrin Human genes 0.000 description 2
- 108010073385 Fibrin Proteins 0.000 description 2
- 102000009123 Fibrin Human genes 0.000 description 2
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 2
- 102100031812 Fibulin-1 Human genes 0.000 description 2
- 101710170731 Fibulin-1 Proteins 0.000 description 2
- 108010001498 Galectin 1 Proteins 0.000 description 2
- 102100021736 Galectin-1 Human genes 0.000 description 2
- 102100040510 Galectin-3-binding protein Human genes 0.000 description 2
- 101710197901 Galectin-3-binding protein Proteins 0.000 description 2
- 102100021023 Gamma-glutamyl hydrolase Human genes 0.000 description 2
- 102100033299 Glia-derived nexin Human genes 0.000 description 2
- 108010024636 Glutathione Proteins 0.000 description 2
- 102000005720 Glutathione transferase Human genes 0.000 description 2
- 108010070675 Glutathione transferase Proteins 0.000 description 2
- 102000003886 Glycoproteins Human genes 0.000 description 2
- 108090000288 Glycoproteins Proteins 0.000 description 2
- 108010045100 HSP27 Heat-Shock Proteins Proteins 0.000 description 2
- 102100039165 Heat shock protein beta-1 Human genes 0.000 description 2
- 108010004889 Heat-Shock Proteins Proteins 0.000 description 2
- 102000002812 Heat-Shock Proteins Human genes 0.000 description 2
- 108010026751 High-Temperature Requirement A Serine Peptidase 1 Proteins 0.000 description 2
- 102000018978 High-Temperature Requirement A Serine Peptidase 1 Human genes 0.000 description 2
- 101000802329 Homo sapiens Zinc finger protein 750 Proteins 0.000 description 2
- 108060003951 Immunoglobulin Proteins 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- 108010021099 Lamin Type A Proteins 0.000 description 2
- 102000008201 Lamin Type A Human genes 0.000 description 2
- 102100027448 Laminin subunit beta-1 Human genes 0.000 description 2
- 101710186246 Laminin subunit beta-1 Proteins 0.000 description 2
- 102100021948 Lysyl oxidase homolog 2 Human genes 0.000 description 2
- 101710183215 Lysyl oxidase homolog 2 Proteins 0.000 description 2
- 102100028762 Neuropilin-1 Human genes 0.000 description 2
- 108090000772 Neuropilin-1 Proteins 0.000 description 2
- 102100037369 Nidogen-1 Human genes 0.000 description 2
- 241000283973 Oryctolagus cuniculus Species 0.000 description 2
- 101710134970 Out at first protein homolog Proteins 0.000 description 2
- 102100034979 Out at first protein homolog Human genes 0.000 description 2
- 239000002033 PVDF binder Substances 0.000 description 2
- 102000035195 Peptidases Human genes 0.000 description 2
- 108091005804 Peptidases Proteins 0.000 description 2
- 102100037765 Periostin Human genes 0.000 description 2
- 101710199268 Periostin Proteins 0.000 description 2
- 102100028251 Phosphoglycerate kinase 1 Human genes 0.000 description 2
- 101710139464 Phosphoglycerate kinase 1 Proteins 0.000 description 2
- 102100037389 Phosphoglycerate mutase 1 Human genes 0.000 description 2
- 108050006040 Phosphoglycerate mutase 1 Proteins 0.000 description 2
- 102100030477 Plectin Human genes 0.000 description 2
- 108010054050 Plectin Proteins 0.000 description 2
- 102100022025 Pregnancy-specific beta-1-glycoprotein 5 Human genes 0.000 description 2
- 101710135783 Pregnancy-specific beta-1-glycoprotein 5 Proteins 0.000 description 2
- 102000000602 Prolyl 3-hydroxylase 1 Human genes 0.000 description 2
- 108050008031 Prolyl 3-hydroxylase 1 Proteins 0.000 description 2
- 239000004365 Protease Substances 0.000 description 2
- 102100037061 Protein disulfide-isomerase A6 Human genes 0.000 description 2
- 101710106306 Protein disulfide-isomerase A6 Proteins 0.000 description 2
- 101710189291 Quinone oxidoreductase Proteins 0.000 description 2
- 102100034576 Quinone oxidoreductase Human genes 0.000 description 2
- 102000012479 Serine Proteases Human genes 0.000 description 2
- 108010022999 Serine Proteases Proteins 0.000 description 2
- 206010072170 Skin wound Diseases 0.000 description 2
- 102000003932 Transgelin Human genes 0.000 description 2
- 108090000333 Transgelin Proteins 0.000 description 2
- 102100037236 Tyrosine-protein kinase receptor UFO Human genes 0.000 description 2
- 101710192735 Tyrosine-protein kinase receptor UFO Proteins 0.000 description 2
- 102000005918 Ubiquitin Thiolesterase Human genes 0.000 description 2
- 108010005656 Ubiquitin Thiolesterase Proteins 0.000 description 2
- 102100031358 Urokinase-type plasminogen activator Human genes 0.000 description 2
- 108090000435 Urokinase-type plasminogen activator Proteins 0.000 description 2
- 102000003970 Vinculin Human genes 0.000 description 2
- 108090000384 Vinculin Proteins 0.000 description 2
- 108010031318 Vitronectin Proteins 0.000 description 2
- 102100035140 Vitronectin Human genes 0.000 description 2
- 102100034644 Zinc finger protein 750 Human genes 0.000 description 2
- XJLXINKUBYWONI-DQQFMEOOSA-N [[(2r,3r,4r,5r)-5-(6-aminopurin-9-yl)-3-hydroxy-4-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2s,3r,4s,5s)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate Chemical compound NC(=O)C1=CC=C[N+]([C@@H]2[C@H]([C@@H](O)[C@H](COP([O-])(=O)OP(O)(=O)OC[C@@H]3[C@H]([C@@H](OP(O)(O)=O)[C@@H](O3)N3C4=NC=NC(N)=C4N=C3)O)O2)O)=C1 XJLXINKUBYWONI-DQQFMEOOSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000006978 adaptation Effects 0.000 description 2
- 229940009444 amphotericin Drugs 0.000 description 2
- APKFDSVGJQXUKY-INPOYWNPSA-N amphotericin B Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-INPOYWNPSA-N 0.000 description 2
- 210000004102 animal cell Anatomy 0.000 description 2
- 239000002518 antifoaming agent Substances 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000000975 bioactive effect Effects 0.000 description 2
- 239000001569 carbon dioxide Substances 0.000 description 2
- 229910002092 carbon dioxide Inorganic materials 0.000 description 2
- 238000011097 chromatography purification Methods 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 235000017471 coenzyme Q10 Nutrition 0.000 description 2
- ACTIUHUUMQJHFO-UPTCCGCDSA-N coenzyme Q10 Chemical compound COC1=C(OC)C(=O)C(C\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)=C(C)C1=O ACTIUHUUMQJHFO-UPTCCGCDSA-N 0.000 description 2
- 239000012141 concentrate Substances 0.000 description 2
- 210000002808 connective tissue Anatomy 0.000 description 2
- 210000004207 dermis Anatomy 0.000 description 2
- 230000002124 endocrine Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 108010055671 ezrin Proteins 0.000 description 2
- 229950003499 fibrin Drugs 0.000 description 2
- 108010062699 gamma-Glutamyl Hydrolase Proteins 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 229960003180 glutathione Drugs 0.000 description 2
- 102000006602 glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 2
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 2
- 230000037313 granulation tissue formation Effects 0.000 description 2
- 230000005484 gravity Effects 0.000 description 2
- 239000012510 hollow fiber Substances 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 229920002674 hyaluronan Polymers 0.000 description 2
- KIUKXJAPPMFGSW-MNSSHETKSA-N hyaluronan Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)C1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H](C(O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-MNSSHETKSA-N 0.000 description 2
- 229940099552 hyaluronan Drugs 0.000 description 2
- 102000018358 immunoglobulin Human genes 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 238000011031 large-scale manufacturing process Methods 0.000 description 2
- 239000010410 layer Substances 0.000 description 2
- 238000001294 liquid chromatography-tandem mass spectrometry Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 108010008217 nidogen Proteins 0.000 description 2
- 210000002445 nipple Anatomy 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- 230000003204 osmotic effect Effects 0.000 description 2
- 229920001184 polypeptide Polymers 0.000 description 2
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 2
- 230000000644 propagated effect Effects 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- NPCOQXAVBJJZBQ-UHFFFAOYSA-N reduced coenzyme Q9 Natural products COC1=C(O)C(C)=C(CC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)C)C(O)=C1OC NPCOQXAVBJJZBQ-UHFFFAOYSA-N 0.000 description 2
- 230000008929 regeneration Effects 0.000 description 2
- 238000011069 regeneration method Methods 0.000 description 2
- 239000012679 serum free medium Substances 0.000 description 2
- 239000004017 serum-free culture medium Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 238000013268 sustained release Methods 0.000 description 2
- 239000012730 sustained-release form Substances 0.000 description 2
- 229940035936 ubiquinone Drugs 0.000 description 2
- 238000000108 ultra-filtration Methods 0.000 description 2
- 239000011782 vitamin Substances 0.000 description 2
- 229940088594 vitamin Drugs 0.000 description 2
- 235000013343 vitamin Nutrition 0.000 description 2
- 229930003231 vitamin Natural products 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 238000001262 western blot Methods 0.000 description 2
- 230000006269 (delayed) early viral mRNA transcription Effects 0.000 description 1
- OZDAOHVKBFBBMZ-UHFFFAOYSA-N 2-aminopentanedioic acid;hydrate Chemical compound O.OC(=O)C(N)CCC(O)=O OZDAOHVKBFBBMZ-UHFFFAOYSA-N 0.000 description 1
- 102100022530 45 kDa calcium-binding protein Human genes 0.000 description 1
- 101710168918 45 kDa calcium-binding protein Proteins 0.000 description 1
- GHHWHXIJCGAUKG-UHFFFAOYSA-N 5,6-didehydropyrimidine Chemical compound C1=NC#CC=N1 GHHWHXIJCGAUKG-UHFFFAOYSA-N 0.000 description 1
- 102100029272 5-demethoxyubiquinone hydroxylase, mitochondrial Human genes 0.000 description 1
- 101710191936 70 kDa protein Proteins 0.000 description 1
- 101710169645 ATP synthase subunit beta Proteins 0.000 description 1
- 102100036601 Aggrecan core protein Human genes 0.000 description 1
- 101710192389 Aggrecan core protein Proteins 0.000 description 1
- 102000005751 Alcohol Oxidoreductases Human genes 0.000 description 1
- 108010031132 Alcohol Oxidoreductases Proteins 0.000 description 1
- 108010021809 Alcohol dehydrogenase Proteins 0.000 description 1
- 102000007698 Alcohol dehydrogenase Human genes 0.000 description 1
- 102100034561 Alpha-N-acetylglucosaminidase Human genes 0.000 description 1
- 101710181800 Aminopeptidase 1 Proteins 0.000 description 1
- 102000004145 Annexin A1 Human genes 0.000 description 1
- 108090000663 Annexin A1 Proteins 0.000 description 1
- 102100031491 Arylsulfatase B Human genes 0.000 description 1
- 102100035584 BRCA2 and CDKN1A-interacting protein Human genes 0.000 description 1
- 102100025351 C-type mannose receptor 2 Human genes 0.000 description 1
- 101710104922 C-type mannose receptor 2 Proteins 0.000 description 1
- 102100032912 CD44 antigen Human genes 0.000 description 1
- 108091016585 CD44 antigen Proteins 0.000 description 1
- 108700005376 Cartilage Oligomeric Matrix Proteins 0.000 description 1
- 102000055007 Cartilage Oligomeric Matrix Human genes 0.000 description 1
- 102100034667 Chloride intracellular channel protein 1 Human genes 0.000 description 1
- 108050001549 Chloride intracellular channel protein 1 Proteins 0.000 description 1
- 102100028757 Chondroitin sulfate proteoglycan 4 Human genes 0.000 description 1
- 102100039551 Collagen triple helix repeat-containing protein 1 Human genes 0.000 description 1
- 101710193823 Collagen triple helix repeat-containing protein 1 Proteins 0.000 description 1
- 102100025406 Complement C1s subcomponent Human genes 0.000 description 1
- 108050005572 Complement C1s subcomponent Proteins 0.000 description 1
- 108010060273 Cyclin A2 Proteins 0.000 description 1
- 102100025191 Cyclin-A2 Human genes 0.000 description 1
- 102100035784 Decorin Human genes 0.000 description 1
- 108090000738 Decorin Proteins 0.000 description 1
- 102100020750 Dipeptidyl peptidase 3 Human genes 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 102100025682 Dystroglycan 1 Human genes 0.000 description 1
- 108010071885 Dystroglycans Proteins 0.000 description 1
- 108700041152 Endoplasmic Reticulum Chaperone BiP Proteins 0.000 description 1
- 102100021451 Endoplasmic reticulum chaperone BiP Human genes 0.000 description 1
- 102100031857 Endoplasmic reticulum resident protein 29 Human genes 0.000 description 1
- 101710113964 Endoplasmic reticulum resident protein 29 Proteins 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 102100031758 Extracellular matrix protein 1 Human genes 0.000 description 1
- 101710127949 Extracellular matrix protein 1 Proteins 0.000 description 1
- 102100026671 F-actin-capping protein subunit alpha-1 Human genes 0.000 description 1
- 101710087984 F-actin-capping protein subunit alpha-1 Proteins 0.000 description 1
- 102100031510 Fibrillin-2 Human genes 0.000 description 1
- 108010030242 Fibrillin-2 Proteins 0.000 description 1
- 102100027269 Fructose-bisphosphate aldolase C Human genes 0.000 description 1
- 101710123709 Fructose-bisphosphate aldolase C Proteins 0.000 description 1
- 108091006027 G proteins Proteins 0.000 description 1
- 102000030782 GTP binding Human genes 0.000 description 1
- 108091000058 GTP-Binding Proteins 0.000 description 1
- 101710183811 Glia-derived nexin Proteins 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 102100028967 HLA class I histocompatibility antigen, alpha chain G Human genes 0.000 description 1
- 101710197836 HLA class I histocompatibility antigen, alpha chain G Proteins 0.000 description 1
- 102000004447 HSP40 Heat-Shock Proteins Human genes 0.000 description 1
- 108010042283 HSP40 Heat-Shock Proteins Proteins 0.000 description 1
- 102100031963 Heme-binding protein 2 Human genes 0.000 description 1
- 101710153908 Heme-binding protein 2 Proteins 0.000 description 1
- 101000770593 Homo sapiens 5-demethoxyubiquinone hydroxylase, mitochondrial Proteins 0.000 description 1
- 101000874304 Homo sapiens BRCA2 and CDKN1A-interacting protein Proteins 0.000 description 1
- 101000931862 Homo sapiens Dipeptidyl peptidase 3 Proteins 0.000 description 1
- 101000997803 Homo sapiens Glia-derived nexin Proteins 0.000 description 1
- 101001041393 Homo sapiens Serine protease HTRA1 Proteins 0.000 description 1
- 101000875296 Homo sapiens Serine/threonine-protein phosphatase CPPED1 Proteins 0.000 description 1
- 108010064711 Homoserine dehydrogenase Proteins 0.000 description 1
- 102000004157 Hydrolases Human genes 0.000 description 1
- 108090000604 Hydrolases Proteins 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- 108010050332 IQ motif containing GTPase activating protein 1 Proteins 0.000 description 1
- 102100027004 Inhibin beta A chain Human genes 0.000 description 1
- 102100023915 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000004372 Insulin-like growth factor binding protein 2 Human genes 0.000 description 1
- 108090000964 Insulin-like growth factor binding protein 2 Proteins 0.000 description 1
- 108090000965 Insulin-like growth factor binding protein 3 Proteins 0.000 description 1
- 102100022708 Insulin-like growth factor-binding protein 3 Human genes 0.000 description 1
- 239000007760 Iscove's Modified Dulbecco's Medium Substances 0.000 description 1
- 102000004195 Isomerases Human genes 0.000 description 1
- 108090000769 Isomerases Proteins 0.000 description 1
- 208000002260 Keloid Diseases 0.000 description 1
- 206010023330 Keloid scar Diseases 0.000 description 1
- 102100027929 Kinesin-like protein KIF7 Human genes 0.000 description 1
- 101710152646 Kinesin-like protein kif7 Proteins 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- 102100024580 L-lactate dehydrogenase B chain Human genes 0.000 description 1
- 101710133532 L-lactate dehydrogenase B chain Proteins 0.000 description 1
- 102000014017 Lactoylglutathione lyase Human genes 0.000 description 1
- 108010050765 Lactoylglutathione lyase Proteins 0.000 description 1
- 102000007547 Laminin Human genes 0.000 description 1
- 108010085895 Laminin Proteins 0.000 description 1
- 102100022743 Laminin subunit alpha-4 Human genes 0.000 description 1
- 101710200587 Laminin subunit alpha-4 Proteins 0.000 description 1
- 102100034710 Laminin subunit gamma-1 Human genes 0.000 description 1
- 101710095658 Laminin subunit gamma-1 Proteins 0.000 description 1
- 102000004317 Lyases Human genes 0.000 description 1
- 108090000856 Lyases Proteins 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 102100027998 Macrophage metalloelastase Human genes 0.000 description 1
- 108010076501 Matrix Metalloproteinase 12 Proteins 0.000 description 1
- 108010016160 Matrix Metalloproteinase 3 Proteins 0.000 description 1
- 102000002274 Matrix Metalloproteinases Human genes 0.000 description 1
- 108010000684 Matrix Metalloproteinases Proteins 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 101710165578 Mitochondrial fission factor homolog A Proteins 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 108010027520 N-Acetylgalactosamine-4-Sulfatase Proteins 0.000 description 1
- 102000018098 Nucleobindin-2 Human genes 0.000 description 1
- 108050007209 Nucleobindin-2 Proteins 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 102100040349 Peptidyl-prolyl cis-trans isomerase FKBP10 Human genes 0.000 description 1
- 101710111764 Peptidyl-prolyl cis-trans isomerase FKBP10 Proteins 0.000 description 1
- 102100038809 Peptidyl-prolyl cis-trans isomerase FKBP9 Human genes 0.000 description 1
- 101710147136 Peptidyl-prolyl cis-trans isomerase FKBP9 Proteins 0.000 description 1
- 102100028489 Phosphatidylethanolamine-binding protein 1 Human genes 0.000 description 1
- 101710204191 Phosphatidylethanolamine-binding protein 1 Proteins 0.000 description 1
- 101710081133 Plastin-3 Proteins 0.000 description 1
- 102100040682 Platelet-derived growth factor D Human genes 0.000 description 1
- 101710170209 Platelet-derived growth factor D Proteins 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 239000004236 Ponceau SX Substances 0.000 description 1
- 101710101148 Probable 6-oxopurine nucleoside phosphorylase Proteins 0.000 description 1
- 101800001065 Protein 2B Proteins 0.000 description 1
- 102100024647 Protein ABHD14B Human genes 0.000 description 1
- 101710173275 Protein ABHD14B Proteins 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 102100032421 Protein S100-A6 Human genes 0.000 description 1
- 101710156983 Protein S100-A6 Proteins 0.000 description 1
- 108010026552 Proteome Proteins 0.000 description 1
- 102000030764 Purine-nucleoside phosphorylase Human genes 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 102100034419 Ras GTPase-activating-like protein IQGAP1 Human genes 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 102100025642 Rho GDP-dissociation inhibitor 1 Human genes 0.000 description 1
- 206010039509 Scab Diseases 0.000 description 1
- 102100036209 Serine/threonine-protein phosphatase CPPED1 Human genes 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 102100030416 Stromelysin-1 Human genes 0.000 description 1
- 102000004338 Transferrin Human genes 0.000 description 1
- 108090000901 Transferrin Proteins 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 102100034396 Trypsin-3 Human genes 0.000 description 1
- 101710119642 Trypsin-3 Proteins 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000018472 Type I Keratins Human genes 0.000 description 1
- 108010091525 Type I Keratins Proteins 0.000 description 1
- 102100028437 Versican core protein Human genes 0.000 description 1
- 101710200894 Versican core protein Proteins 0.000 description 1
- 102100039662 Xaa-Pro dipeptidase Human genes 0.000 description 1
- 102100026335 Zinc finger protein 276 Human genes 0.000 description 1
- 101710143861 Zinc finger protein 276 Proteins 0.000 description 1
- FHKPLLOSJHHKNU-INIZCTEOSA-N [(3S)-3-[8-(1-ethyl-5-methylpyrazol-4-yl)-9-methylpurin-6-yl]oxypyrrolidin-1-yl]-(oxan-4-yl)methanone Chemical compound C(C)N1N=CC(=C1C)C=1N(C2=NC=NC(=C2N=1)O[C@@H]1CN(CC1)C(=O)C1CCOCC1)C FHKPLLOSJHHKNU-INIZCTEOSA-N 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 108060000200 adenylate cyclase Proteins 0.000 description 1
- 102000030621 adenylate cyclase Human genes 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 238000012382 advanced drug delivery Methods 0.000 description 1
- 108010009380 alpha-N-acetyl-D-glucosaminidase Proteins 0.000 description 1
- 229940024606 amino acid Drugs 0.000 description 1
- 235000001014 amino acid Nutrition 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 230000003698 anagen phase Effects 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000003367 anti-collagen effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 238000003491 array Methods 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 239000000090 biomarker Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- OWMVSZAMULFTJU-UHFFFAOYSA-N bis-tris Chemical compound OCCN(CCO)C(CO)(CO)CO OWMVSZAMULFTJU-UHFFFAOYSA-N 0.000 description 1
- UBAZGMLMVVQSCD-UHFFFAOYSA-N carbon dioxide;molecular oxygen Chemical compound O=O.O=C=O UBAZGMLMVVQSCD-UHFFFAOYSA-N 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 239000012876 carrier material Substances 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 239000013553 cell monolayer Substances 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 238000002038 chemiluminescence detection Methods 0.000 description 1
- 239000002975 chemoattractant Substances 0.000 description 1
- 108010039524 chondroitin sulfate proteoglycan 4 Proteins 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 230000037319 collagen production Effects 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 210000001608 connective tissue cell Anatomy 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 210000004292 cytoskeleton Anatomy 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 210000002257 embryonic structure Anatomy 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 210000002744 extracellular matrix Anatomy 0.000 description 1
- 230000004720 fertilization Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000001917 fluorescence detection Methods 0.000 description 1
- 238000005187 foaming Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000012737 fresh medium Substances 0.000 description 1
- 108091006104 gene-regulatory proteins Proteins 0.000 description 1
- 102000034356 gene-regulatory proteins Human genes 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 210000003780 hair follicle Anatomy 0.000 description 1
- 230000001969 hypertrophic effect Effects 0.000 description 1
- 230000001146 hypoxic effect Effects 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 108010019691 inhibin beta A subunit Proteins 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 210000001117 keloid Anatomy 0.000 description 1
- 230000029774 keratinocyte migration Effects 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 238000011177 media preparation Methods 0.000 description 1
- 210000002901 mesenchymal stem cell Anatomy 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 238000001471 micro-filtration Methods 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 229930027945 nicotinamide-adenine dinucleotide Natural products 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 125000003835 nucleoside group Chemical group 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 235000006180 nutrition needs Nutrition 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 108030002458 peroxiredoxin Proteins 0.000 description 1
- 210000002826 placenta Anatomy 0.000 description 1
- 229940012957 plasmin Drugs 0.000 description 1
- 210000001778 pluripotent stem cell Anatomy 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000035752 proliferative phase Effects 0.000 description 1
- 108010066823 proline dipeptidase Proteins 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 238000003498 protein array Methods 0.000 description 1
- 229940116540 protein supplement Drugs 0.000 description 1
- 235000005974 protein supplement Nutrition 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000008458 response to injury Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 108010084871 rho Guanine Nucleotide Dissociation Inhibitor alpha Proteins 0.000 description 1
- 239000012146 running buffer Substances 0.000 description 1
- 102000028528 s-formylglutathione hydrolase Human genes 0.000 description 1
- 108010093322 s-formylglutathione hydrolase Proteins 0.000 description 1
- 238000003118 sandwich ELISA Methods 0.000 description 1
- 102000014452 scavenger receptors Human genes 0.000 description 1
- 108010078070 scavenger receptors Proteins 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 210000002536 stromal cell Anatomy 0.000 description 1
- 238000013337 sub-cultivation Methods 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 238000004114 suspension culture Methods 0.000 description 1
- 238000004885 tandem mass spectrometry Methods 0.000 description 1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 239000012581 transferrin Substances 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 125000005454 tryptophanyl group Chemical group 0.000 description 1
- 102000003390 tumor necrosis factor Human genes 0.000 description 1
- DBESHHFMIFSNRV-RJYQSXAYSA-N ubiquinone-7 Chemical compound COC1=C(OC)C(=O)C(C\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)=C(C)C1=O DBESHHFMIFSNRV-RJYQSXAYSA-N 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 230000010388 wound contraction Effects 0.000 description 1
- 230000037314 wound repair Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0656—Adult fibroblasts
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/36—Skin; Hair; Nails; Sebaceous glands; Cerumen; Epidermis; Epithelial cells; Keratinocytes; Langerhans cells; Ectodermal cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A61K38/1709—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
- A61K38/1825—Fibroblast growth factor [FGF]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
- A61K38/1841—Transforming growth factor [TGF]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/195—Chemokines, e.g. RANTES
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/20—Interleukins [IL]
- A61K38/204—IL-6
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/20—Interleukins [IL]
- A61K38/2053—IL-8
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/20—Interleukins [IL]
- A61K38/2086—IL-13 to IL-16
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/39—Connective tissue peptides, e.g. collagen, elastin, laminin, fibronectin, vitronectin, cold insoluble globulin [CIG]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/55—Protease inhibitors
- A61K38/57—Protease inhibitors from animals; from humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N11/00—Carrier-bound or immobilised enzymes; Carrier-bound or immobilised microbial cells; Preparation thereof
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0625—Epidermal cells, skin cells; Cells of the oral mucosa
- C12N5/0627—Hair cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/148—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with compounds of unknown constitution, e.g. material from plants or animals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/19—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles lyophilised, i.e. freeze-dried, solutions or dispersions
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/13—Coculture with; Conditioned medium produced by connective tissue cells; generic mesenchyme cells, e.g. so-called "embryonic fibroblasts"
- C12N2502/1323—Adult fibroblasts
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/13—Coculture with; Conditioned medium produced by connective tissue cells; generic mesenchyme cells, e.g. so-called "embryonic fibroblasts"
- C12N2502/1394—Bone marrow stromal cells; whole marrow
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2531/00—Microcarriers
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Immunology (AREA)
- Epidemiology (AREA)
- Biomedical Technology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Organic Chemistry (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- Wood Science & Technology (AREA)
- Cell Biology (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Dermatology (AREA)
- Marine Sciences & Fisheries (AREA)
- Rheumatology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Developmental Biology & Embryology (AREA)
- Virology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Cosmetics (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB0916370.0A GB0916370D0 (en) | 2009-09-18 | 2009-09-18 | Compositions |
| GB0916370.0 | 2009-09-18 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20120083407A true KR20120083407A (ko) | 2012-07-25 |
Family
ID=41277906
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127009488A Withdrawn KR20120083407A (ko) | 2009-09-18 | 2010-09-16 | 줄기 세포 조건화된 배지 조성물 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US20120207705A1 (enExample) |
| EP (1) | EP2478091A1 (enExample) |
| JP (1) | JP2013505011A (enExample) |
| KR (1) | KR20120083407A (enExample) |
| CN (1) | CN102712899A (enExample) |
| GB (1) | GB0916370D0 (enExample) |
| IN (1) | IN2012DN02443A (enExample) |
| SG (1) | SG178914A1 (enExample) |
| WO (1) | WO2011033260A1 (enExample) |
Families Citing this family (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8728819B2 (en) | 2006-08-29 | 2014-05-20 | Fibrocell Technologies, Inc. | Methods for culturing minimally-passaged fibroblasts and uses thereof |
| US9446075B2 (en) * | 2011-05-06 | 2016-09-20 | Bioregenerative Sciences | Compositions derived from stem cell released molecules and methods for formulation thereof |
| KR20150009521A (ko) * | 2012-03-07 | 2015-01-26 | 파이브로셀 테크놀로지스, 인코퍼레이티드 | 국소피부제제 및 개인맞춤형 피부치료방법 |
| US9545370B2 (en) | 2012-05-08 | 2017-01-17 | BioRegenerative Sciences, Inc. | Bioactive compositions and methods for their preparation and use |
| JP6041199B2 (ja) * | 2012-09-14 | 2016-12-07 | 国立大学法人大阪大学 | 三次元細胞培養体の製造方法 |
| CN103333856B (zh) * | 2013-07-25 | 2015-08-26 | 张潇潇 | 人脐带间充质干细胞培养基 |
| US9585915B2 (en) * | 2014-01-23 | 2017-03-07 | Emory University | Polypeptide hydrogels and uses related thereto |
| JP6538081B2 (ja) * | 2014-07-07 | 2019-07-03 | メディポスト カンパニー リミテッド | 刺激を受けた幹細胞の馴化培地の発毛促進能およびそれらの使用 |
| RU2644650C2 (ru) | 2014-12-01 | 2018-02-13 | Общество с ограниченной ответственностью "Т-Хелпер Клеточные Технологии" | Материал стволовых клеток и способ его получения |
| US9944894B2 (en) | 2015-01-16 | 2018-04-17 | General Electric Company | Pluripotent stem cell expansion and passage using a rocking platform bioreactor |
| CN105250994A (zh) * | 2015-10-29 | 2016-01-20 | 广州赛莱拉干细胞科技股份有限公司 | 一种促进皮肤伤口愈合的制剂及其制备方法和应用 |
| KR101928488B1 (ko) * | 2016-01-15 | 2018-12-12 | 가톨릭대학교 산학협력단 | 페록시다신을 포함하는 무혈청 배지 첨가제 조성물 및 이의 이용 |
| RU2708329C2 (ru) | 2016-05-31 | 2019-12-05 | Общество с ограниченной ответственностью "Т-Хелпер Клеточные Технологии" | Материал стволовых клеток, композиции и способы применения |
| US11684574B2 (en) | 2016-06-30 | 2023-06-27 | University of Pittsburgh—of the Commonwealth System of Higher Education | Artificial cells and delivery devices for use in tissue engineering, and related methods |
| JP2018023343A (ja) * | 2016-08-12 | 2018-02-15 | 国立大学法人山口大学 | 細胞培養用培地、及び細胞培養方法 |
| WO2018179374A1 (ja) * | 2017-03-31 | 2018-10-04 | 株式会社セルバンク | 真皮線維芽細胞の培養上清液を含む化粧用組成物およびその製造方法 |
| CN110831694A (zh) * | 2017-03-31 | 2020-02-21 | 细尔琳生物医学公司 | 生物相容性调整细胞培养基组合物及其用途 |
| MY199879A (en) * | 2017-10-03 | 2023-11-27 | Univ Kebangsaan Malaysia | Dermal fibroblast conditioned sera based on fibroblast-specific medium for skin regeneration |
| EP3569698A1 (en) | 2018-05-16 | 2019-11-20 | Veterinärmedizinische Universität Wien | Products for therapy of a musculoskeletal condition and methods for their production |
| IL272145A (en) * | 2020-01-20 | 2021-07-29 | Stem Cell Medicine Ltd | Cosmetic preparations with protein concentrate from a conditioned growth medium of stem cells from adipose tissue |
| CN113396894A (zh) * | 2021-07-06 | 2021-09-17 | 南方医科大学南方医院 | 适用于单位毛囊保存的复合冻存液及其制备方法和应用 |
| CN114107188B (zh) * | 2021-11-30 | 2025-01-14 | 滨州医学院 | 一种干细胞成膜培养基及其应用 |
| BR102022016233A2 (pt) * | 2022-08-16 | 2024-02-27 | Omics Biotecnologia Animal Ltda | Concentrado de proteínas e peptídeos derivados de células estromais mesenquimais, método de obtenção e uso de concentrado |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9708692D0 (en) | 1997-04-30 | 1997-06-18 | Jahoda Colin A B | Dermal sheath tissue and/or cells derived therefrom in wound healing |
| US6372494B1 (en) | 1999-05-14 | 2002-04-16 | Advanced Tissue Sciences, Inc. | Methods of making conditioned cell culture medium compositions |
| NZ530481A (en) | 2001-06-07 | 2007-11-30 | Skinmedica Inc | Compositions comprising cell culture medium conditioned by cells grown in three-dimensional culture |
| CA2505409C (en) * | 2002-11-14 | 2017-04-04 | Intercytex Limited | Cultivation of hair inductive cells |
| HU227723B1 (en) * | 2004-07-09 | 2012-01-30 | Nagy Norbert Dr | Autologous keratinocytes, melanocytes and fibroblast culturing technique and serum-free medium for use in human therapy |
| WO2008020815A1 (en) | 2006-08-15 | 2008-02-21 | Agency For Science, Technology And Research | Mesenchymal stem cell conditioned medium |
| GB0814443D0 (en) * | 2008-08-07 | 2008-09-10 | Avecia Biolog Ltd | Process for cultivating cells |
-
2009
- 2009-09-18 GB GBGB0916370.0A patent/GB0916370D0/en not_active Ceased
-
2010
- 2010-09-16 US US13/395,779 patent/US20120207705A1/en not_active Abandoned
- 2010-09-16 KR KR1020127009488A patent/KR20120083407A/ko not_active Withdrawn
- 2010-09-16 JP JP2012529337A patent/JP2013505011A/ja not_active Withdrawn
- 2010-09-16 EP EP10755216A patent/EP2478091A1/en not_active Withdrawn
- 2010-09-16 WO PCT/GB2010/001739 patent/WO2011033260A1/en not_active Ceased
- 2010-09-16 SG SG2012014072A patent/SG178914A1/en unknown
- 2010-09-16 CN CN2010800520449A patent/CN102712899A/zh active Pending
-
2012
- 2012-03-21 IN IN2443DEN2012 patent/IN2012DN02443A/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| JP2013505011A (ja) | 2013-02-14 |
| US20120207705A1 (en) | 2012-08-16 |
| GB0916370D0 (en) | 2009-10-28 |
| WO2011033260A1 (en) | 2011-03-24 |
| CN102712899A (zh) | 2012-10-03 |
| SG178914A1 (en) | 2012-04-27 |
| EP2478091A1 (en) | 2012-07-25 |
| IN2012DN02443A (enExample) | 2015-08-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20120083407A (ko) | 줄기 세포 조건화된 배지 조성물 | |
| US20220313743A1 (en) | Compositions containing amniotic components and methods for preparation and use thereof | |
| US8871198B2 (en) | Methods related to wound healing | |
| US9051547B2 (en) | Use of PEDF-derived polypeptides for promoting stem cells proliferation and wound healing | |
| CN101366728A (zh) | 用于预防或者治疗皮肤缺损的组合物及其使用方法 | |
| US12491214B2 (en) | Amniotic fluid-derived extracellular vesicles and uses thereof for wound healing | |
| WO2010040302A1 (zh) | 从人或动物胚胎提取间质性干细胞及提取其分泌物的方法 | |
| CN110974944A (zh) | 间充质干细胞复合活性因子冻干粉及其制备方法和应用 | |
| Nagai et al. | Characteristics of a scaffold-free articular chondrocyte plate grown in rotational culture | |
| US20100221231A1 (en) | Skin replacement compositions and methods | |
| EP4012022A1 (en) | Method for producing skin-derived pluripotent progenitor cells | |
| CN114432427B (zh) | 抗衰老活性肽与骨髓间充质干细胞在制备抗衰老的药物中的用途 | |
| WO2003078607A2 (en) | Tissue specific signal-plexes to dedifferentiate and redifferentiate cells | |
| JP2022545311A (ja) | 髄核前駆細胞マスターレギュレーター転写因子を含む分化誘導剤、誘導髄核前駆細胞の製造方法、および誘導髄核前駆細胞の用途 | |
| JP5366353B2 (ja) | 細胞及び組織工学におけるlifの使用 | |
| Tanaka et al. | The synthetic peptide SVVYGLR promotes cell motility of myogenic cells and facilitates differentiation in skeletal muscle regeneration | |
| US20220235326A1 (en) | Enhancement of fibroblast therapeutic activity by rna | |
| CN117924426B (zh) | 干细胞外泌体的制备技术及其在药品和化妆品中的应用 | |
| Aphkhazava et al. | Integrated Cell Engineering and Data-Driven Regenerative Therapeutics: Convergence of Stem Cell Technologies, Exosome Biology, Aging Interventions, and Clinical Big Data Analytics | |
| US20250064859A1 (en) | Hpsc-derived articular chondrocyte compositions, systems and methods of use thereof | |
| JP2019081713A (ja) | 毛包幹細胞の未分化状態維持剤 | |
| Zeng et al. | Direct reprogramming of human fibroblasts into hair-inducing dermal papilla cell-like cells by a single small molecule | |
| US20220298474A1 (en) | Method for culturing dermal papilla cells | |
| Singhatanadgit et al. | Osteogenic potency of stem cell-based genetic engineering targeting Wnt3a and Wnt9a | |
| Minuth et al. | From the Renal Stem Cell Niche to Functional Parenchyme |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20120413 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |