KR20070103497A - 모노아민 산화효소 b 억제제로서의 순수한4-피롤리디노페닐벤질 에터 유도체의 제조 방법 - Google Patents
모노아민 산화효소 b 억제제로서의 순수한4-피롤리디노페닐벤질 에터 유도체의 제조 방법 Download PDFInfo
- Publication number
- KR20070103497A KR20070103497A KR1020077020985A KR20077020985A KR20070103497A KR 20070103497 A KR20070103497 A KR 20070103497A KR 1020077020985 A KR1020077020985 A KR 1020077020985A KR 20077020985 A KR20077020985 A KR 20077020985A KR 20070103497 A KR20070103497 A KR 20070103497A
- Authority
- KR
- South Korea
- Prior art keywords
- formula
- compound
- halogen
- salt
- racemate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/18—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having one double bond between ring members or between a ring member and a non-ring member
- C07D207/22—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having one double bond between ring members or between a ring member and a non-ring member with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/24—Oxygen or sulfur atoms
- C07D207/26—2-Pyrrolidones
- C07D207/273—2-Pyrrolidones with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to other ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/18—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having one double bond between ring members or between a ring member and a non-ring member
- C07D207/22—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having one double bond between ring members or between a ring member and a non-ring member with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/24—Oxygen or sulfur atoms
- C07D207/26—2-Pyrrolidones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/402—1-aryl substituted, e.g. piretanide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
- A61P25/32—Alcohol-abuse
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
- A61P25/34—Tobacco-abuse
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/18—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having one double bond between ring members or between a ring member and a non-ring member
- C07D207/22—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having one double bond between ring members or between a ring member and a non-ring member with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/24—Oxygen or sulfur atoms
- C07D207/26—2-Pyrrolidones
- C07D207/273—2-Pyrrolidones with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to other ring carbon atoms
- C07D207/277—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/07—Optical isomers
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Neurology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Psychiatry (AREA)
- Addiction (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Diabetes (AREA)
- Child & Adolescent Psychology (AREA)
- Hospice & Palliative Care (AREA)
- Pain & Pain Management (AREA)
- Psychology (AREA)
- Epidemiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Pyrrole Compounds (AREA)
Abstract
Description
Claims (23)
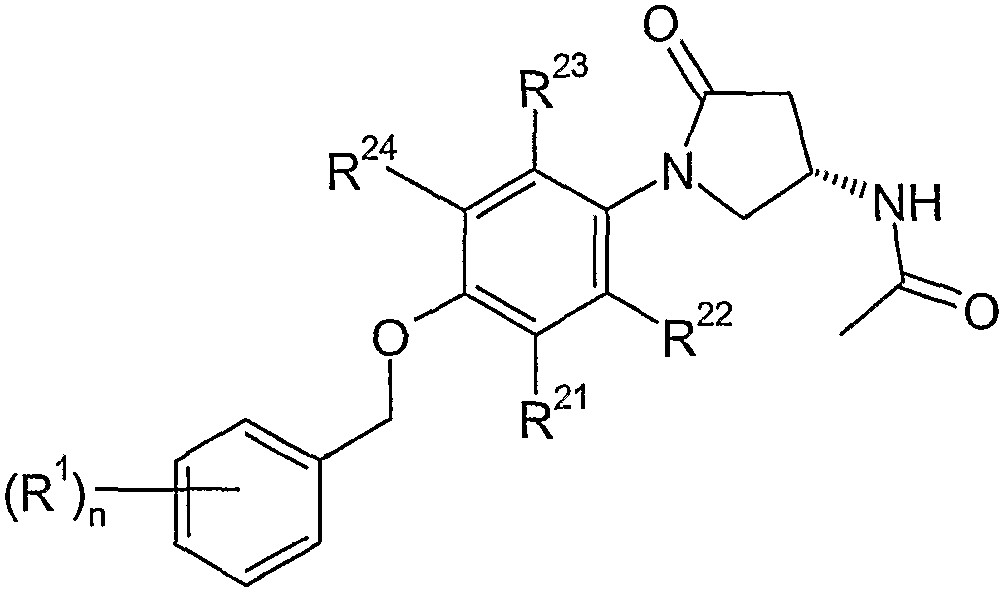
- a) 하기 화학식 II의 라세메이트를 분해제로 분해하여 하기 화학식 (S)-II의 (S)-에난티오머를 수득하는 단계;b) 화학식 (S)-II의 에난티오머를 하기 화학식 III의 상응하는 1차 아미드로 전환시키는 단계; 및c) 최소한 아세트산 및/또는 아세트산 무수물의 존재 하에서 하기 화학식 III의 화합물을 하기 화학식 IV의 화합물과 직접 반응시켜 하기 화학식 I의 화합물을 수득하는 단계, 또는d) 하기 화학식 III의 화합물을 하기 화학식 IV의 화합물과 반응시켜 하기 화학식 V의 화합물을 수득한 후, 화학식 V의 화합물을 아세틸화제와 반응시켜 화학식 I의 화합물을 수득하는 단계를 포함하는, 하기 화학식 I의 에난티오머 순수한 4-피롤리디노 페닐벤질 에터 유도체의 제조 방법:화학식 I화학식 II화학식 (S)-II화학식 III화학식 IVArI(OCOR)2화학식 V상기 식에서,Ar은 할로겐, 니트로, 시아노 및 C1-6-알킬로 구성된 군에서 선택되는 하나 이상의 치환체로 선택적으로 치환되는 아릴이고,R은 하나 이상의 할로겐으로 선택적으로 치환되는 C1 -6-알킬이고,R1은 할로겐, 할로겐-(C1-C6)-알킬, 시아노, (C1-C6)-알콕시 또는 할로겐-(C1-C6)-알콕시이고,R21, R22, R23 및 R24는 서로 독립적으로 수소 및 할로겐으로 구성된 군에서 선택되고,n은 0, 1, 2 또는 3이다.
- 제 1 항에 있어서,상기 분해제가 (R)-(-)-2-페닐글리신올, (S)-(+)-2-페닐글리신올, 신코니딘, D-페닐알라닌올, (+)-페닐에틸아민, (1S,2S)-(+)-티오미카민, (1S,2S)-(+)-2-아미 노-1-페닐-1,3-프로판다이올, (1S,2R)-(-)-시스-1-아미노-2-인단올, L-페닐에프린, (1S,2R)-(+)-N-메틸에페드린, L-프롤린올, (R)-(-)-2-아미노-1-부탄올 및 (R)-(+)-1-(나프틸)-에틸아민으로 구성된 군에서 선택되는 방법.
- 제 2 항에 있어서,상기 분해제가 (R)-(-)-2-페닐글리신올인 방법.
- 제 1 항 내지 제 3 항중 어느 한 항에 있어서,단계 a)에서 아세톤, 아이소프로판올, 아세토니트릴, 테트라하이드로푸란, 2-부탄온, 아이소프로판올 및 EtOH로 구성된 군에서 선택되는 용매가 사용되는 방법.
- 제 4 항에 있어서,상기 용매가 아세토니트릴인 방법.
- 제 1 항 내지 제 5 항중 어느 한 항에 있어서,단계 b)에서 화학식 (S)-II의 에난티오머의 상응하는 화학식 III의 1차 아미드로의 전환이 1,1'-카보닐다이이미다졸 및 암모니아의 공급원을 이용하여 수행되는 방법.
- 제 6 항에 있어서,상기 암모니아의 공급원이 수성 암모니아 또는 암모늄 아세테이트에서 선택되는 방법.
- 제 1 항 내지 제 6 항중 어느 한 항에 있어서,단계 b)에서 화학식 (S)-II의 에난티오머의 화학식 III의 상응하는 1차 아미드로의 전환이 N-메틸모폴린, 에틸 클로로포르메이트 및 암모니아의 공급원을 이용함으로써 수행되는 방법.
- 제 8 항에 있어서,상기 암모니아의 공급원이 기체 암모니아인 방법.
- 제 6 항 내지 제 9 항중 어느 한 항에 있어서,단계 b)에서 테트라하이드로푸란이 용매로서 추가로 이용되는 방법.
- 제 1 항 내지 제 10 항중 어느 한 항에 있어서,단계 c)에서 화학식 IV의 화합물이 (다이아세톡시요도)벤젠인 방법.
- 제 1 항 내지 제 11 항중 어느 한 항에 있어서,단계 d)에서 화학식 IV의 화합물이 (다이아세톡시요도)벤젠인 방법.
- 제 1 항 내지 제 12 항중 어느 한 항에 있어서,단계 d)에서 아세틸화제가 아세트산 무수물 또는 아세틸 클로라이드인 방법.
- 제 1 항 내지 제 13 항중 어느 한 항에 있어서,단계 d)에서 테트라하이드로푸란 및 물을 용매로서 1:1의 비로서 사용하는 방법.
- 제 1 항 내지 제 14 항중 어느 한 항에 있어서,화학식 II의 라세메이트가 (RS)-1-[4-(3-플루오로-벤질옥시)-페닐]-5-옥소-피롤리딘-3-카복실산이고, 화학식 (S)-II의 화합물이 (S)-1-[4-(3-플루오로-벤질옥시)-페닐]-5-옥소-피롤리딘-3-카복실산이고, 화학식 III의 화합물이 1,1'-카보닐다이이미다졸을 이용한 (S)-1-[4-(3-플루오로-벤질옥시)-페닐]-5-옥소-피롤리딘-3-카복실산 아미드이고, 화학식 V의 화합물이 (S)-4-아미노-1-[4-(3-플루오로-벤질옥시)-페닐]-피롤리딘-2-온이고, 화학식 I의 화합물이 (S)-N-{1-[4-(3-플루오로-벤질옥시)-페닐]-5-옥소-피롤리딘-3-일}-아세트아미드인 방법.
- 제 1 항 내지 제 15 항중 어느 한 항에 있어서,단계 a)가a1) 화학식 II의 라세메이트, 및 화학식 (S)-II의 화합물과 화학식 II의 라세메이트와의 염을 형성할 수 있는 분해제를 포함하는 반응 혼합물을 용매 중에서 제조하여 화학식 (S)-II의 염을 수득하는 단계;a2) 상기 반응 혼합물로부터 화학식 (S)-II의 화합물의 염을 단리하고, 이의 염으로부터 화학식 (S)-II의 화합물을 방출시키는 단계;a3) 단계 a1)의 반응 혼합물에 남아있는 (R)-II의 화합물을 단리시키는 단계;a4) 단리된 (R)-II의 화합물을 라세미화하여 재순환되는 라세메이트를 수득하는 단계;a5) 필요한 횟수만큼 단계 a1) 내지 a4)를 반복하고, 라세메이트를 재순환되는 라세메이트로 대체하는 단계를 포함하는 방법.
- 제 1 항 내지 제 15 항중 어느 한 항에 있어서,단계 a)가a1') 화학식 II의 라세메이트, 및 (R)-II의 화합물과 화학식 II의 라세메이트와의 염을 형성할 수 있는 분해제를 포함하는 반응 혼합물을 용매 중에서 제조하여 (R)-II의 염을 수득하는 단계;a2') 상기 반응 혼합물로부터 (R)-II의 화합물의 염을 단리하고, 이의 염으로부터 (R)-II의 화합물을 방출시키는 단계;a3') 단계 a1')의 반응 혼합물에 남아있는 화학식 (S)-II의 화합물을 단리시키는 단계;a4') 단리된 (R)-II의 화합물을 라세미화하여 재순환되는 라세메이트를 수득하는 단계;a5') 필요한 횟수만큼 단계 a1') 내지 a4')를 반복하고, 라세메이트를 재순환되는 라세메이트로 대체하는 단계를 포함하는 방법.
- 제 18 항에 있어서,R1이 3-플루오로이고, R21, R22, R23 및 R24가 수소이고, n이 1인 화학식 III의 중간체 화합물.
- 제 20 항에 있어서,R1이 3-플루오로이고, R21, R22, R23 및 R24가 수소이고, n이 1인 화학식 (S)-II의 중간체 화합물의 염.
- 제 20 항 또는 제 21 항에 있어서,(R)-(-)-2-페닐글리신올을 갖는 화학식 (S)-II의 중간체 화합물의 염.
- 본원에 개시된 바와 같은 발명.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP05102030.3 | 2005-03-15 | ||
| EP05102030 | 2005-03-15 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20070103497A true KR20070103497A (ko) | 2007-10-23 |
| KR100915737B1 KR100915737B1 (ko) | 2009-09-04 |
Family
ID=36465154
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020077020985A Expired - Fee Related KR100915737B1 (ko) | 2005-03-15 | 2006-03-14 | 모노아민 산화효소 b 억제제로서의 순수한4-피롤리디노페닐벤질 에터 유도체의 제조 방법 |
Country Status (14)
| Country | Link |
|---|---|
| US (2) | US7501528B2 (ko) |
| EP (1) | EP1861363B1 (ko) |
| JP (2) | JP5366192B2 (ko) |
| KR (1) | KR100915737B1 (ko) |
| CN (1) | CN101142182B (ko) |
| AT (1) | ATE526312T1 (ko) |
| AU (1) | AU2006224774B2 (ko) |
| BR (1) | BRPI0609061A2 (ko) |
| CA (1) | CA2600761C (ko) |
| ES (1) | ES2373738T3 (ko) |
| IL (1) | IL185545A (ko) |
| MX (1) | MX2007011155A (ko) |
| TW (1) | TW200714584A (ko) |
| WO (1) | WO2006097270A1 (ko) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7501528B2 (en) * | 2005-03-15 | 2009-03-10 | Hoffmann-La Roche Inc. | Method for preparing enantiomerically pure 4-pyrrolidino phenylbenzyl ether derivatives |
| ITMI20061297A1 (it) * | 2006-07-04 | 2008-01-05 | Laboratorio Chimico Int Spa | Procedimento per la preparazione della'acido (r)-(-)-3-(carbamoilmetil)-5-metilesanoico e del pregabalin e intermedi di sintesi |
| CN105085321B (zh) * | 2012-03-07 | 2017-11-24 | 浙江九洲药业股份有限公司 | 一种n‑甲氧羰基‑l‑叔亮氨酸的制备方法 |
| BR112015022202A2 (pt) | 2013-03-14 | 2017-07-18 | Dart Neuroscience Cayman Ltd | compostos substituídos de naftiridina e quinolina como inibidores da mao |
| BR112016003288A8 (pt) * | 2013-10-29 | 2020-02-04 | Hoffmann La Roche | processos para sintetizar n-[(3s)-1-[4-[(3-fluorofenil)metóxi]fenil]-5-oxo-pirrolidin-3-il]acetamida cristalina, e seu intermediário |
| EP3273946A1 (en) * | 2015-03-27 | 2018-01-31 | F. Hoffmann-La Roche AG | Pharmaceutical formulation comprising sembragiline |
| MA50371A (fr) | 2017-10-10 | 2021-03-31 | Biogen Inc | Procédé de préparation de dérivés spiro |
| US11225460B2 (en) | 2018-03-08 | 2022-01-18 | Sunshine Lake Pharma Co., Ltd. | Pyrrolidineamide derivatives and uses thereof |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6012339B2 (ja) * | 1976-08-04 | 1985-04-01 | 日本化薬株式会社 | 光学活性マンデル酸・フエニルグリシノ−ル塩及びその製造法 |
| US4348393A (en) * | 1978-06-09 | 1982-09-07 | Delalande S.A. | N-Aryl oxazolidinones, oxazolidinethiones, pyrrolidinones, pyrrolidines and thiazolidinones |
| JPH0395138A (ja) * | 1989-09-07 | 1991-04-19 | Nissan Chem Ind Ltd | 3―メチルヘプタン酸の光学分割法 |
| US5679715A (en) | 1995-06-07 | 1997-10-21 | Harris; Richard Y. | Method for treating multiple sclerosis |
| JP3258027B2 (ja) | 1996-03-15 | 2002-02-18 | サマーセット・ファーマシューティカルズ・インコーポレイテッド | セレジリン投与による末梢ニューロパシーの予防および治療方法 |
| AU1359801A (en) | 1999-11-05 | 2001-06-06 | Vela Pharmaceuticals Inc. | Methods and compositions for treating reward deficiency syndrome |
| TW504694B (en) * | 2000-01-12 | 2002-10-01 | Hitachi Ltd | Non-volatile semiconductor memory device and semiconductor disk device |
| PE20050077A1 (es) | 2002-09-20 | 2005-03-01 | Hoffmann La Roche | Derivados de 4-pirrolidino-fenil-bencil-eter |
| US7148362B2 (en) * | 2003-09-18 | 2006-12-12 | Hoffmann-La Roche Inc. | Process for the preparation of enantiopure pyrrolidin-2-one derivatives |
| AU2005268894B2 (en) * | 2004-08-02 | 2010-12-16 | F. Hoffmann-La Roche Ag | Benzyloxy derivatives as MAOB inhibitors |
| US7501528B2 (en) * | 2005-03-15 | 2009-03-10 | Hoffmann-La Roche Inc. | Method for preparing enantiomerically pure 4-pyrrolidino phenylbenzyl ether derivatives |
-
2006
- 2006-03-08 US US11/370,668 patent/US7501528B2/en not_active Expired - Fee Related
- 2006-03-13 TW TW095108434A patent/TW200714584A/zh unknown
- 2006-03-14 AT AT06707551T patent/ATE526312T1/de active
- 2006-03-14 AU AU2006224774A patent/AU2006224774B2/en not_active Ceased
- 2006-03-14 CA CA2600761A patent/CA2600761C/en not_active Expired - Fee Related
- 2006-03-14 WO PCT/EP2006/002314 patent/WO2006097270A1/en not_active Ceased
- 2006-03-14 MX MX2007011155A patent/MX2007011155A/es active IP Right Grant
- 2006-03-14 KR KR1020077020985A patent/KR100915737B1/ko not_active Expired - Fee Related
- 2006-03-14 ES ES06707551T patent/ES2373738T3/es active Active
- 2006-03-14 JP JP2008501217A patent/JP5366192B2/ja not_active Expired - Fee Related
- 2006-03-14 BR BRPI0609061-3A patent/BRPI0609061A2/pt not_active Application Discontinuation
- 2006-03-14 CN CN2006800082838A patent/CN101142182B/zh not_active Expired - Fee Related
- 2006-03-14 EP EP06707551A patent/EP1861363B1/en not_active Not-in-force
-
2007
- 2007-08-27 IL IL185545A patent/IL185545A/en not_active IP Right Cessation
-
2009
- 2009-01-26 US US12/359,356 patent/US8227505B2/en not_active Expired - Fee Related
-
2011
- 2011-11-18 JP JP2011252413A patent/JP2012067122A/ja not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| JP5366192B2 (ja) | 2013-12-11 |
| CA2600761C (en) | 2014-05-13 |
| MX2007011155A (es) | 2007-10-23 |
| CN101142182B (zh) | 2012-06-06 |
| WO2006097270A1 (en) | 2006-09-21 |
| BRPI0609061A2 (pt) | 2010-02-17 |
| US7501528B2 (en) | 2009-03-10 |
| JP2008533076A (ja) | 2008-08-21 |
| IL185545A0 (en) | 2008-01-06 |
| ATE526312T1 (de) | 2011-10-15 |
| EP1861363B1 (en) | 2011-09-28 |
| AU2006224774B2 (en) | 2011-08-18 |
| US20060211868A1 (en) | 2006-09-21 |
| ES2373738T3 (es) | 2012-02-08 |
| JP2012067122A (ja) | 2012-04-05 |
| CN101142182A (zh) | 2008-03-12 |
| CA2600761A1 (en) | 2006-09-21 |
| US20090171100A1 (en) | 2009-07-02 |
| TW200714584A (en) | 2007-04-16 |
| AU2006224774A1 (en) | 2006-09-21 |
| KR100915737B1 (ko) | 2009-09-04 |
| EP1861363A1 (en) | 2007-12-05 |
| US8227505B2 (en) | 2012-07-24 |
| IL185545A (en) | 2011-11-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR100681586B1 (ko) | 모노아민 산화효소 b 억제제로서 피롤리돈 유도체 | |
| US8227505B2 (en) | Method for preparing enantiomerically pure 4-pyrrolidino phenylbenzyl ether derivatives | |
| KR100915736B1 (ko) | 에난티오머 순수한 4-피롤리디노페닐, 벤질, 에터 유도체의제조 방법 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| R15-X000 | Change to inventor requested |
St.27 status event code: A-3-3-R10-R15-oth-X000 |
|
| R16-X000 | Change to inventor recorded |
St.27 status event code: A-3-3-R10-R16-oth-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
St.27 status event code: A-1-2-D10-D22-exm-PE0701 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
St.27 status event code: A-2-4-F10-F11-exm-PR0701 |
|
| PR1002 | Payment of registration fee |
St.27 status event code: A-2-2-U10-U12-oth-PR1002 Fee payment year number: 1 |
|
| PG1601 | Publication of registration |
St.27 status event code: A-4-4-Q10-Q13-nap-PG1601 |
|
| FPAY | Annual fee payment |
Payment date: 20120727 Year of fee payment: 4 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 4 |
|
| FPAY | Annual fee payment |
Payment date: 20130729 Year of fee payment: 5 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 5 |
|
| FPAY | Annual fee payment |
Payment date: 20140730 Year of fee payment: 6 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 6 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 7 |
|
| FPAY | Annual fee payment |
Payment date: 20160629 Year of fee payment: 8 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 8 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 9 |
|
| FPAY | Annual fee payment |
Payment date: 20180628 Year of fee payment: 10 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 10 |
|
| PC1903 | Unpaid annual fee |
St.27 status event code: A-4-4-U10-U13-oth-PC1903 Not in force date: 20190829 Payment event data comment text: Termination Category : DEFAULT_OF_REGISTRATION_FEE |
|
| PC1903 | Unpaid annual fee |
St.27 status event code: N-4-6-H10-H13-oth-PC1903 Ip right cessation event data comment text: Termination Category : DEFAULT_OF_REGISTRATION_FEE Not in force date: 20190829 |
|
| R18-X000 | Changes to party contact information recorded |
St.27 status event code: A-5-5-R10-R18-oth-X000 |