KR20070059161A - 도파민 수용체 효능제를 함유하는 장시간 작용 서방성 제제및 그 제조방법 - Google Patents
도파민 수용체 효능제를 함유하는 장시간 작용 서방성 제제및 그 제조방법 Download PDFInfo
- Publication number
- KR20070059161A KR20070059161A KR1020077008896A KR20077008896A KR20070059161A KR 20070059161 A KR20070059161 A KR 20070059161A KR 1020077008896 A KR1020077008896 A KR 1020077008896A KR 20077008896 A KR20077008896 A KR 20077008896A KR 20070059161 A KR20070059161 A KR 20070059161A
- Authority
- KR
- South Korea
- Prior art keywords
- pharmaceutically acceptable
- dopamine receptor
- acid
- receptor agonist
- rotigotine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000003136 dopamine receptor stimulating agent Substances 0.000 title claims abstract description 77
- 229940098778 Dopamine receptor agonist Drugs 0.000 title claims abstract description 55
- 239000003405 delayed action preparation Substances 0.000 title claims 11
- 238000002360 preparation method Methods 0.000 title description 6
- 238000013268 sustained release Methods 0.000 claims abstract description 91
- 239000012730 sustained-release form Substances 0.000 claims abstract description 91
- 239000000203 mixture Substances 0.000 claims abstract description 65
- 238000009472 formulation Methods 0.000 claims abstract description 55
- 208000018737 Parkinson disease Diseases 0.000 claims abstract description 19
- 239000004005 microsphere Substances 0.000 claims description 197
- KFQYTPMOWPVWEJ-INIZCTEOSA-N rotigotine Chemical group CCCN([C@@H]1CC2=CC=CC(O)=C2CC1)CCC1=CC=CS1 KFQYTPMOWPVWEJ-INIZCTEOSA-N 0.000 claims description 133
- 229960003179 rotigotine Drugs 0.000 claims description 133
- 150000003839 salts Chemical class 0.000 claims description 73
- 239000002245 particle Substances 0.000 claims description 60
- 238000000034 method Methods 0.000 claims description 54
- 239000002671 adjuvant Substances 0.000 claims description 42
- 239000007943 implant Substances 0.000 claims description 35
- 239000003960 organic solvent Substances 0.000 claims description 35
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical group ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 claims description 33
- 239000012458 free base Substances 0.000 claims description 28
- 239000004480 active ingredient Substances 0.000 claims description 27
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 26
- RKDVKSZUMVYZHH-UHFFFAOYSA-N 1,4-dioxane-2,5-dione Chemical compound O=C1COC(=O)CO1 RKDVKSZUMVYZHH-UHFFFAOYSA-N 0.000 claims description 24
- JJTUDXZGHPGLLC-UHFFFAOYSA-N lactide Chemical compound CC1OC(=O)C(C)OC1=O JJTUDXZGHPGLLC-UHFFFAOYSA-N 0.000 claims description 24
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 claims description 20
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 18
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 claims description 18
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 18
- 229920000642 polymer Polymers 0.000 claims description 18
- 229920002988 biodegradable polymer Polymers 0.000 claims description 16
- 239000004621 biodegradable polymer Substances 0.000 claims description 16
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims description 16
- JOAHPSVPXZTVEP-YXJHDRRASA-N Terguride Chemical compound C1=CC([C@H]2C[C@@H](CN(C)[C@@H]2C2)NC(=O)N(CC)CC)=C3C2=CNC3=C1 JOAHPSVPXZTVEP-YXJHDRRASA-N 0.000 claims description 15
- NQRIKTDKFHAOKC-UHFFFAOYSA-N 7-(4-methylpiperazin-1-yl)-3h-1,3-benzoxazol-2-one;hydrochloride Chemical compound Cl.C1CN(C)CCN1C1=CC=CC2=C1OC(=O)N2 NQRIKTDKFHAOKC-UHFFFAOYSA-N 0.000 claims description 14
- 229920000747 poly(lactic acid) Polymers 0.000 claims description 14
- 229960004558 terguride Drugs 0.000 claims description 14
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 claims description 13
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 claims description 13
- 239000000243 solution Substances 0.000 claims description 13
- 238000011282 treatment Methods 0.000 claims description 13
- MNIXOHSBCFNRSH-UQGUCNKVSA-N (6ar,9r,10ar)-7-methyl-9-(1,2,4-triazol-1-ylmethyl)-6,6a,8,9,10,10a-hexahydro-4h-indolo[4,3-fg]quinoline;(z)-but-2-enedioic acid Chemical compound OC(=O)\C=C/C(O)=O.C([C@H]1CN([C@H]2[C@@H](C=3C=CC=C4NC=C(C=34)C2)C1)C)N1C=NC=N1 MNIXOHSBCFNRSH-UQGUCNKVSA-N 0.000 claims description 12
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims description 12
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 claims description 12
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 claims description 12
- 229920002732 Polyanhydride Polymers 0.000 claims description 12
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 claims description 12
- 229960003089 pramipexole Drugs 0.000 claims description 12
- FASDKYOPVNHBLU-ZETCQYMHSA-N pramipexole Chemical compound C1[C@@H](NCCC)CCC2=C1SC(N)=N2 FASDKYOPVNHBLU-ZETCQYMHSA-N 0.000 claims description 12
- GDFGTRDCCWFXTG-SCTDSRPQSA-N (3r,4ar,10as)-3-(diethylsulfamoylamino)-6-hydroxy-1-propyl-3,4,4a,5,10,10a-hexahydro-2h-benzo[g]quinoline Chemical compound C1=CC=C2C[C@@H]3N(CCC)C[C@H](NS(=O)(=O)N(CC)CC)C[C@H]3CC2=C1O GDFGTRDCCWFXTG-SCTDSRPQSA-N 0.000 claims description 11
- KORNTPPJEAJQIU-KJXAQDMKSA-N Cabaser Chemical compound C1=CC([C@H]2C[C@H](CN(CC=C)[C@@H]2C2)C(=O)N(CCCN(C)C)C(=O)NCC)=C3C2=CNC3=C1 KORNTPPJEAJQIU-KJXAQDMKSA-N 0.000 claims description 11
- YEHCICAEULNIGD-MZMPZRCHSA-N pergolide Chemical compound C1=CC([C@H]2C[C@@H](CSC)CN([C@@H]2C2)CCC)=C3C2=CNC3=C1 YEHCICAEULNIGD-MZMPZRCHSA-N 0.000 claims description 11
- 229960000583 acetic acid Drugs 0.000 claims description 10
- KTEBZVJGMARTOQ-GCJKJVERSA-N adrogolide Chemical compound CC(=O)OC1=C(OC(C)=O)C=C2[C@H]3C(C=C(S4)CCC)=C4CN[C@@H]3CCC2=C1 KTEBZVJGMARTOQ-GCJKJVERSA-N 0.000 claims description 10
- 229960004596 cabergoline Drugs 0.000 claims description 10
- 229960004851 pergolide Drugs 0.000 claims description 10
- 229960000924 quinagolide Drugs 0.000 claims description 10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 9
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims description 9
- 229950007871 adrogolide Drugs 0.000 claims description 9
- 239000008346 aqueous phase Substances 0.000 claims description 9
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 9
- 239000006104 solid solution Substances 0.000 claims description 9
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 claims description 8
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 claims description 8
- -1 diapsoline Chemical compound 0.000 claims description 8
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 claims description 8
- 239000012071 phase Substances 0.000 claims description 8
- 239000002904 solvent Substances 0.000 claims description 8
- 238000001694 spray drying Methods 0.000 claims description 8
- 239000004626 polylactic acid Substances 0.000 claims description 7
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 claims description 6
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 claims description 6
- 102000015554 Dopamine receptor Human genes 0.000 claims description 6
- 108050004812 Dopamine receptor Proteins 0.000 claims description 6
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 claims description 6
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 claims description 6
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 claims description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 claims description 6
- 230000002378 acidificating effect Effects 0.000 claims description 6
- 229910000147 aluminium phosphate Inorganic materials 0.000 claims description 6
- 235000001014 amino acid Nutrition 0.000 claims description 6
- 150000001413 amino acids Chemical class 0.000 claims description 6
- 125000005251 aryl acyl group Chemical group 0.000 claims description 6
- 150000007522 mineralic acids Chemical class 0.000 claims description 6
- 150000007524 organic acids Chemical class 0.000 claims description 6
- 229920000151 polyglycol Polymers 0.000 claims description 6
- 239000010695 polyglycol Substances 0.000 claims description 6
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 claims description 6
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 claims description 5
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 claims description 5
- 239000004372 Polyvinyl alcohol Substances 0.000 claims description 5
- NPEZSCRKHFTLPE-MYXGOWFTSA-N abt-431 Chemical compound Cl.CC(=O)OC1=C(OC(C)=O)C=C2[C@H]3C(C=C(S4)CCC)=C4CN[C@@H]3CCC2=C1 NPEZSCRKHFTLPE-MYXGOWFTSA-N 0.000 claims description 5
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 claims description 5
- 239000012046 mixed solvent Substances 0.000 claims description 5
- 239000005015 poly(hydroxybutyrate) Substances 0.000 claims description 5
- 229920002451 polyvinyl alcohol Polymers 0.000 claims description 5
- 235000019422 polyvinyl alcohol Nutrition 0.000 claims description 5
- UHSKFQJFRQCDBE-UHFFFAOYSA-N ropinirole Chemical compound CCCN(CCC)CCC1=CC=CC2=C1CC(=O)N2 UHSKFQJFRQCDBE-UHFFFAOYSA-N 0.000 claims description 5
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 claims description 4
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 claims description 4
- 229920000331 Polyhydroxybutyrate Polymers 0.000 claims description 4
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 claims description 4
- 239000001768 carboxy methyl cellulose Substances 0.000 claims description 4
- 239000012362 glacial acetic acid Substances 0.000 claims description 4
- 239000008103 glucose Substances 0.000 claims description 4
- 229960004275 glycolic acid Drugs 0.000 claims description 4
- 239000004310 lactic acid Substances 0.000 claims description 4
- 235000014655 lactic acid Nutrition 0.000 claims description 4
- UQGPCEVQKLOLLM-UHFFFAOYSA-N pentaneperoxoic acid Chemical compound CCCCC(=O)OO UQGPCEVQKLOLLM-UHFFFAOYSA-N 0.000 claims description 4
- UWCVGPLTGZWHGS-ZORIOUSZSA-N pergolide mesylate Chemical compound CS(O)(=O)=O.C1=CC([C@H]2C[C@@H](CSC)CN([C@@H]2C2)CCC)=C3C2=CNC3=C1 UWCVGPLTGZWHGS-ZORIOUSZSA-N 0.000 claims description 4
- 229960001879 ropinirole Drugs 0.000 claims description 4
- 239000011734 sodium Substances 0.000 claims description 4
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 claims description 4
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 claims description 4
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 claims description 4
- DVLKVIJLALMCBQ-MSSRUXLCSA-N (3s,4as,10ar)-3-(diethylsulfamoylamino)-6-hydroxy-1-propyl-3,4,4a,5,10,10a-hexahydro-2h-benzo[g]quinoline;hydrochloride Chemical compound Cl.C1=CC=C2C[C@H]3N(CCC)C[C@@H](NS(=O)(=O)N(CC)CC)C[C@@H]3CC2=C1O DVLKVIJLALMCBQ-MSSRUXLCSA-N 0.000 claims description 3
- WHBMMWSBFZVSSR-UHFFFAOYSA-M 3-hydroxybutyrate Chemical compound CC(O)CC([O-])=O WHBMMWSBFZVSSR-UHFFFAOYSA-M 0.000 claims description 3
- 239000005711 Benzoic acid Substances 0.000 claims description 3
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 claims description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 3
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 claims description 3
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 claims description 3
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 claims description 3
- 229920000954 Polyglycolide Polymers 0.000 claims description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 claims description 3
- WHBMMWSBFZVSSR-UHFFFAOYSA-N R3HBA Natural products CC(O)CC(O)=O WHBMMWSBFZVSSR-UHFFFAOYSA-N 0.000 claims description 3
- 230000009471 action Effects 0.000 claims description 3
- 125000005257 alkyl acyl group Chemical group 0.000 claims description 3
- 125000000217 alkyl group Chemical group 0.000 claims description 3
- 235000003704 aspartic acid Nutrition 0.000 claims description 3
- 235000010233 benzoic acid Nutrition 0.000 claims description 3
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 claims description 3
- 229910052799 carbon Inorganic materials 0.000 claims description 3
- 125000004432 carbon atom Chemical group C* 0.000 claims description 3
- 239000001530 fumaric acid Substances 0.000 claims description 3
- 235000013922 glutamic acid Nutrition 0.000 claims description 3
- 239000004220 glutamic acid Substances 0.000 claims description 3
- 229940093915 gynecological organic acid Drugs 0.000 claims description 3
- 239000011976 maleic acid Substances 0.000 claims description 3
- 229940098779 methanesulfonic acid Drugs 0.000 claims description 3
- 229910017604 nitric acid Inorganic materials 0.000 claims description 3
- 229910052757 nitrogen Chemical group 0.000 claims description 3
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 3
- 235000005985 organic acids Nutrition 0.000 claims description 3
- 125000004430 oxygen atom Chemical group O* 0.000 claims description 3
- 239000003208 petroleum Substances 0.000 claims description 3
- 229920001495 poly(sodium acrylate) polymer Polymers 0.000 claims description 3
- 239000004633 polyglycolic acid Substances 0.000 claims description 3
- 238000006116 polymerization reaction Methods 0.000 claims description 3
- 229920000193 polymethacrylate Polymers 0.000 claims description 3
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 3
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 3
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 3
- 229960002652 pramipexole dihydrochloride Drugs 0.000 claims description 3
- 229960002765 quinagolide hydrochloride Drugs 0.000 claims description 3
- 229910052708 sodium Inorganic materials 0.000 claims description 3
- 235000015424 sodium Nutrition 0.000 claims description 3
- NNMHYFLPFNGQFZ-UHFFFAOYSA-M sodium polyacrylate Chemical compound [Na+].[O-]C(=O)C=C NNMHYFLPFNGQFZ-UHFFFAOYSA-M 0.000 claims description 3
- 229910052717 sulfur Chemical group 0.000 claims description 3
- 125000004434 sulfur atom Chemical group 0.000 claims description 3
- VQMNWIMYFHHFMC-UHFFFAOYSA-N tert-butyl 4-hydroxyindole-1-carboxylate Chemical compound C1=CC=C2N(C(=O)OC(C)(C)C)C=CC2=C1O VQMNWIMYFHHFMC-UHFFFAOYSA-N 0.000 claims description 3
- 229920003169 water-soluble polymer Polymers 0.000 claims description 3
- QCSYJICXNUHBML-CGCNXJRXSA-N (6ar,9r,10ar)-n-[3-(dimethylamino)propyl]-n-(ethylcarbamoyl)-7-prop-2-enyl-6,6a,8,9,10,10a-hexahydro-4h-indolo[4,3-fg]quinoline-9-carboxamide;phosphoric acid Chemical compound OP(O)(O)=O.OP(O)(O)=O.C1=CC([C@H]2C[C@H](CN(CC=C)[C@@H]2C2)C(=O)N(CCCN(C)C)C(=O)NCC)=C3C2=CNC3=C1 QCSYJICXNUHBML-CGCNXJRXSA-N 0.000 claims description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 claims description 2
- 150000001335 aliphatic alkanes Chemical class 0.000 claims description 2
- 238000001914 filtration Methods 0.000 claims description 2
- 239000003349 gelling agent Substances 0.000 claims description 2
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 claims description 2
- 229950011111 sumanirole Drugs 0.000 claims description 2
- RKZSNTNMEFVBDT-MRVPVSSYSA-N sumanirole Chemical compound C([C@H](C1)NC)C2=CC=CC3=C2N1C(=O)N3 RKZSNTNMEFVBDT-MRVPVSSYSA-N 0.000 claims description 2
- 239000000556 agonist Substances 0.000 claims 2
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical group N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 claims 1
- 239000003795 chemical substances by application Substances 0.000 claims 1
- 238000000386 microscopy Methods 0.000 claims 1
- PSZYNBSKGUBXEH-UHFFFAOYSA-N naphthalene-1-sulfonic acid Chemical compound C1=CC=C2C(S(=O)(=O)O)=CC=CC2=C1 PSZYNBSKGUBXEH-UHFFFAOYSA-N 0.000 claims 1
- 230000002035 prolonged effect Effects 0.000 claims 1
- 239000000546 pharmaceutical excipient Substances 0.000 abstract 2
- 229920003168 pharmaceutical polymer Polymers 0.000 abstract 2
- 239000003814 drug Substances 0.000 description 90
- 229940079593 drug Drugs 0.000 description 89
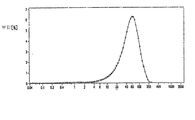
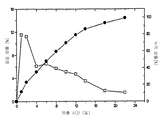
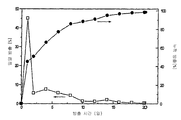
- 230000001186 cumulative effect Effects 0.000 description 37
- 239000000499 gel Substances 0.000 description 28
- 150000001875 compounds Chemical class 0.000 description 24
- 239000008280 blood Substances 0.000 description 23
- 210000004369 blood Anatomy 0.000 description 23
- 230000000694 effects Effects 0.000 description 21
- 238000012360 testing method Methods 0.000 description 20
- 238000001727 in vivo Methods 0.000 description 17
- 230000003278 mimic effect Effects 0.000 description 16
- 239000007788 liquid Substances 0.000 description 15
- 238000000338 in vitro Methods 0.000 description 12
- 239000007924 injection Substances 0.000 description 10
- 238000002347 injection Methods 0.000 description 10
- 239000012074 organic phase Substances 0.000 description 10
- 238000002474 experimental method Methods 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 8
- 238000010521 absorption reaction Methods 0.000 description 6
- 238000002844 melting Methods 0.000 description 6
- 230000008018 melting Effects 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 230000001225 therapeutic effect Effects 0.000 description 6
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 230000008859 change Effects 0.000 description 4
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 238000005070 sampling Methods 0.000 description 4
- 239000012064 sodium phosphate buffer Substances 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 238000004587 chromatography analysis Methods 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 238000000605 extraction Methods 0.000 description 3
- 238000010579 first pass effect Methods 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 210000004185 liver Anatomy 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- 239000002504 physiological saline solution Substances 0.000 description 3
- 229920003023 plastic Polymers 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 229920001610 polycaprolactone Polymers 0.000 description 3
- 239000004632 polycaprolactone Substances 0.000 description 3
- PVGBHEUCHKGFQP-UHFFFAOYSA-N sodium;n-[5-amino-2-(4-aminophenyl)sulfonylphenyl]sulfonylacetamide Chemical compound [Na+].CC(=O)NS(=O)(=O)C1=CC(N)=CC=C1S(=O)(=O)C1=CC=C(N)C=C1 PVGBHEUCHKGFQP-UHFFFAOYSA-N 0.000 description 3
- 238000000935 solvent evaporation Methods 0.000 description 3
- 239000007921 spray Substances 0.000 description 3
- 238000005303 weighing Methods 0.000 description 3
- KJCVRFUGPWSIIH-UHFFFAOYSA-N 1-naphthol Chemical compound C1=CC=C2C(O)=CC=CC2=C1 KJCVRFUGPWSIIH-UHFFFAOYSA-N 0.000 description 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 2
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 2
- 229940088007 benadryl Drugs 0.000 description 2
- 230000017531 blood circulation Effects 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- ZVYMSEWONAXFGO-UHFFFAOYSA-N decanedioic acid 1-propoxycyclohexa-3,5-diene-1,3-dicarboxylic acid Chemical compound OC(=O)CCCCCCCCC(O)=O.CCCOC1(C(O)=O)CC(C(O)=O)=CC=C1 ZVYMSEWONAXFGO-UHFFFAOYSA-N 0.000 description 2
- 238000004455 differential thermal analysis Methods 0.000 description 2
- PCHPORCSPXIHLZ-UHFFFAOYSA-N diphenhydramine hydrochloride Chemical compound [Cl-].C=1C=CC=CC=1C(OCC[NH+](C)C)C1=CC=CC=C1 PCHPORCSPXIHLZ-UHFFFAOYSA-N 0.000 description 2
- XQRLCLUYWUNEEH-UHFFFAOYSA-L diphosphonate(2-) Chemical compound [O-]P(=O)OP([O-])=O XQRLCLUYWUNEEH-UHFFFAOYSA-L 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- VYFYYTLLBUKUHU-UHFFFAOYSA-N dopamine Chemical compound NCCC1=CC=C(O)C(O)=C1 VYFYYTLLBUKUHU-UHFFFAOYSA-N 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 238000000589 high-performance liquid chromatography-mass spectrometry Methods 0.000 description 2
- 150000003840 hydrochlorides Chemical class 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 239000007927 intramuscular injection Substances 0.000 description 2
- 238000010255 intramuscular injection Methods 0.000 description 2
- GBMDVOWEEQVZKZ-UHFFFAOYSA-N methanol;hydrate Chemical compound O.OC GBMDVOWEEQVZKZ-UHFFFAOYSA-N 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 238000001878 scanning electron micrograph Methods 0.000 description 2
- 239000007929 subcutaneous injection Substances 0.000 description 2
- 238000010254 subcutaneous injection Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- DURPTKYDGMDSBL-UHFFFAOYSA-N 1-butoxybutane Chemical compound CCCCOCCCC DURPTKYDGMDSBL-UHFFFAOYSA-N 0.000 description 1
- CXBDYQVECUFKRK-UHFFFAOYSA-N 1-methoxybutane Chemical compound CCCCOC CXBDYQVECUFKRK-UHFFFAOYSA-N 0.000 description 1
- AIBHLZVVPXCTKY-UHFFFAOYSA-N 7-methyl-9-(1,2,4-triazol-1-ylmethyl)-6,6a,8,9,10,10a-hexahydro-4h-indolo[4,3-fg]quinoline Chemical compound C1C(C=2C=CC=C3NC=C(C=23)C2)C2N(C)CC1CN1C=NC=N1 AIBHLZVVPXCTKY-UHFFFAOYSA-N 0.000 description 1
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- XOBKSJJDNFUZPF-UHFFFAOYSA-N Methoxyethane Chemical compound CCOC XOBKSJJDNFUZPF-UHFFFAOYSA-N 0.000 description 1
- 206010034010 Parkinsonism Diseases 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- DQFAAIWCCHDCLO-UHFFFAOYSA-N acetonitrile;formic acid;hydrate Chemical compound O.CC#N.OC=O DQFAAIWCCHDCLO-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 238000009098 adjuvant therapy Methods 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000004397 blinking Effects 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 229920006237 degradable polymer Polymers 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- SYGWHBDCTWDQJT-UHFFFAOYSA-N dichloromethane;hexane;propan-2-ol Chemical compound ClCCl.CC(C)O.CCCCCC SYGWHBDCTWDQJT-UHFFFAOYSA-N 0.000 description 1
- 229910001873 dinitrogen Inorganic materials 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 229960003638 dopamine Drugs 0.000 description 1
- 239000002804 dopamine agent Substances 0.000 description 1
- 229940052760 dopamine agonists Drugs 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 210000003194 forelimb Anatomy 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- CEXBONHIOKGWNU-NTISSMGPSA-N hydron;(6s)-6-[propyl(2-thiophen-2-ylethyl)amino]-5,6,7,8-tetrahydronaphthalen-1-ol;chloride Chemical group [Cl-].CCC[NH+]([C@@H]1CC2=CC=CC(O)=C2CC1)CCC1=CC=CS1 CEXBONHIOKGWNU-NTISSMGPSA-N 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 229940102223 injectable solution Drugs 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 229960000448 lactic acid Drugs 0.000 description 1
- 238000012417 linear regression Methods 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000002075 main ingredient Substances 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 206010027175 memory impairment Diseases 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- TVMXDCGIABBOFY-UHFFFAOYSA-N octane Chemical compound CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 238000005191 phase separation Methods 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 229940044601 receptor agonist Drugs 0.000 description 1
- 239000000018 receptor agonist Substances 0.000 description 1
- 238000012429 release testing Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 239000013582 standard series solution Substances 0.000 description 1
- 239000012086 standard solution Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 230000001550 time effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 210000005239 tubule Anatomy 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 238000009777 vacuum freeze-drying Methods 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000008673 vomiting Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/34—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyesters, polyamino acids, polysiloxanes, polyphosphazines, copolymers of polyalkylene glycol or poloxamers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/38—Heterocyclic compounds having sulfur as a ring hetero atom
- A61K31/381—Heterocyclic compounds having sulfur as a ring hetero atom having five-membered rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0024—Solid, semi-solid or solidifying implants, which are implanted or injected in body tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1641—Organic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyethylene glycol, poloxamers
- A61K9/1647—Polyesters, e.g. poly(lactide-co-glycolide)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Dermatology (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Inorganic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Neurology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Psychology (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN200410077961.9 | 2004-09-21 | ||
| CN200410077961 | 2004-09-21 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20070059161A true KR20070059161A (ko) | 2007-06-11 |
Family
ID=36089849
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020077008896A Ceased KR20070059161A (ko) | 2004-09-21 | 2005-09-21 | 도파민 수용체 효능제를 함유하는 장시간 작용 서방성 제제및 그 제조방법 |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US8691277B2 (enExample) |
| EP (1) | EP1797871B1 (enExample) |
| JP (1) | JP5081622B2 (enExample) |
| KR (1) | KR20070059161A (enExample) |
| AU (1) | AU2005287743B2 (enExample) |
| CA (1) | CA2581143C (enExample) |
| ES (1) | ES2536460T3 (enExample) |
| PL (1) | PL1797871T3 (enExample) |
| WO (1) | WO2006032202A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101481643B1 (ko) * | 2010-11-25 | 2015-01-12 | 산동 루예 파마슈티칼 컴파니 리미티드 | 로티고틴, 이의 유도체 또는 로티고틴 또는 이의 유도체의 약제학적으로 허용가능한 염의 조성물 |
| WO2021133051A1 (ko) * | 2019-12-23 | 2021-07-01 | 환인제약 주식회사 | 로피니롤을 포함하는 마이크로스피어 및 이를 함유하는 주사제 조성물 |
Families Citing this family (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7435738B2 (en) | 2003-08-18 | 2008-10-14 | Solvay Pharmaceuticals, Inc. | Stable crystalline form of bifeprunox mesylate (7-[4-([1,1′-biphenyl]-3-ylmethyl)-1-piperazinyl]-2(3H)-benzoxazolone monomethanesulfonate) |
| US7405216B2 (en) | 2004-08-18 | 2008-07-29 | Solvay Pharmaceuticals, B.V. | Stable crystalline form of bifeprunox mesylate (7-[4-([1,1′-biphenyl]-3-ylmethyl)-1-piperazinyl]-2(3H)-benzoxazolone monomethanesulfonate) |
| US7964604B2 (en) | 2005-02-18 | 2011-06-21 | Solvay Pharmaceuticals B.V. | Bifeprunox mesylate maintenance dose compositions and methods for using the same |
| US7423040B2 (en) | 2005-02-18 | 2008-09-09 | Irene Eijgendaal | Stable crystalline form of bifeprunox mesylate, dosage forms thereof and methods for using same |
| US8475829B2 (en) * | 2006-04-06 | 2013-07-02 | Nupathe Inc. | Implants for the treatment of dopamine associated states |
| US7786126B2 (en) | 2006-06-16 | 2010-08-31 | Solvay Pharmaceuticals B.V. | Combination preparations comprising SLV308 and a dopamine agonist |
| CA2654719A1 (en) * | 2006-06-16 | 2007-12-21 | Solvay Pharmaceuticals B.V. | Combination preparations comprising slv308 and a l-dopa |
| US8106056B2 (en) | 2006-06-16 | 2012-01-31 | Solvay Pharmaceuticals B.V. | Combination preparations comprising bifeprunox and a dopamine agonist |
| TWI392670B (zh) | 2006-06-22 | 2013-04-11 | Ucb Pharma Gmbh | 經取代的2-胺基萘滿之於製造用於預防、減緩及/或治療各種類型疼痛之藥物上的用途 |
| WO2008009665A1 (en) * | 2006-07-19 | 2008-01-24 | Boehringer Ingelheim International Gmbh | Treatment of pain |
| US20090304794A1 (en) * | 2008-06-09 | 2009-12-10 | Supernus Pharmaceuticals, Inc. | Controlled release formulations of pramipexole |
| EP2452677A1 (en) | 2008-09-29 | 2012-05-16 | Wockhardt Limited | Extended release dosage form of ropinirole |
| FR2936710B1 (fr) * | 2008-10-07 | 2011-01-07 | Ceva Sante Animale | Composition veterinaire antiprolactinique destinee aux ruminants |
| AU2010266285A1 (en) * | 2009-07-02 | 2012-02-09 | Supernus Pharmaceuticals, Inc. | A method of treatment of a neurological disorder |
| WO2011012721A1 (en) | 2009-07-31 | 2011-02-03 | Ascendis Pharma As | Carrier linked pramipexole prodrugs |
| WO2011012723A1 (en) | 2009-07-31 | 2011-02-03 | Ascendis Pharma As | Injectable sustained release compositions comprising a pramipexole prodrug |
| DE102010014113A1 (de) * | 2010-04-07 | 2011-10-13 | Acino Ag | Freisetzungsverfahren für Implantate |
| EA201300607A8 (ru) * | 2010-12-02 | 2014-02-28 | Ратиофарм Гмбх | Ионная жидкость ротиготина |
| US9956201B2 (en) | 2014-07-21 | 2018-05-01 | Spriaso Llc | Compositions comprising bioreversible derivatives of hydroxy N-substituted-2-aminotetralins, and related dosage forms |
| WO2018096560A1 (en) | 2016-11-23 | 2018-05-31 | Cipla Limited | Long acting depot formulation for the continuous dopaminergic stimulation |
| WO2019234069A1 (en) | 2018-06-08 | 2019-12-12 | Boehringer Ingelheim Vetmedica Gmbh | Liquid pharmaceutical compositions comprising pergolide |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4166800A (en) * | 1977-08-25 | 1979-09-04 | Sandoz, Inc. | Processes for preparation of microspheres |
| JP2651320B2 (ja) | 1992-07-16 | 1997-09-10 | 田辺製薬株式会社 | 徐放性マイクロスフェア製剤の製造方法 |
| DE69734060T2 (de) * | 1996-05-24 | 2006-06-29 | Angiotech Pharmaceuticals, Inc., Vancouver | Zubereitungen und verfahren zur behandlung oder prävention von krankheiten der körperpassagewege |
| EP0930874A2 (en) | 1996-10-09 | 1999-07-28 | Takeda Chemical Industries, Ltd. | A method for producing a microparticle |
| JPH10167968A (ja) * | 1996-10-09 | 1998-06-23 | Takeda Chem Ind Ltd | マイクロパーティクルの製造法 |
| PT949905E (pt) * | 1996-12-20 | 2001-12-28 | Alza Corp | Composicao de gel injectavel de efeito retardado e processo para a sua preparacao |
| JP3663271B2 (ja) * | 1997-01-24 | 2005-06-22 | シュバルツ ファルマ アクチェンゲゼルシャフト | 新規の分枝鎖エステル及びその製法 |
| ATE428371T1 (de) * | 1998-07-17 | 2009-05-15 | Pacira Pharmaceuticals Inc | Biologisch abbaubare anordnungen zur kontrollierten freigabe eingeschlossener substanzen |
| US6455526B1 (en) * | 1998-12-16 | 2002-09-24 | Aventis Pharmaceuticals, Inc. | Biodegradable polymer encapsulated pharmaceutical compositions and method for preparing the same |
| MEP19408A (en) * | 1998-12-16 | 2010-06-10 | Aventis Pharma Inc | Biodegradable polymer encapsulated serotonin receptor antagonist and method for preparing the same |
| GB9828861D0 (en) * | 1998-12-31 | 1999-02-17 | Danbiosyst Uk | Compositions |
| JP4540280B2 (ja) | 1999-07-09 | 2010-09-08 | ベーテーエス ホールディング インターナショナル ベー ヴィ | 光学走査装置 |
| JP4287613B2 (ja) | 2000-04-28 | 2009-07-01 | 田辺三菱製薬株式会社 | マイクロスフェアの製法 |
| DE10041479A1 (de) * | 2000-08-24 | 2002-03-14 | Sanol Arznei Schwarz Gmbh | Neue pharmazeutische Zusammensetzung zur Verabreichung von N-0923 |
| DE10055742B4 (de) * | 2000-11-10 | 2006-05-11 | Schwarz Pharma Ag | Neue Polyester, Verfahren zu ihrer Herstellung und aus den Polyestern hergestellte Depot-Arzneiformen |
| KR20040005936A (ko) * | 2001-04-26 | 2004-01-16 | 컨트롤 딜리버리 시스템즈 인코포레이티드 | 공동약물을 함유하는 서방 약물 전달 시스템 |
| EP1572154B1 (en) * | 2002-11-18 | 2012-02-01 | Rutgers, The State University of New Jersey | Medical devices employing novel polymers |
| EP1610791B1 (en) * | 2003-03-31 | 2011-02-23 | Titan Pharmaceuticals, Inc. | Implantable polymeric device for sustained release of dopamine agonist |
| DE10334188B4 (de) * | 2003-07-26 | 2007-07-05 | Schwarz Pharma Ag | Verwendung von Rotigotin zur Behandlung von Depressionen |
-
2005
- 2005-09-21 EP EP05792179.3A patent/EP1797871B1/en not_active Expired - Lifetime
- 2005-09-21 KR KR1020077008896A patent/KR20070059161A/ko not_active Ceased
- 2005-09-21 AU AU2005287743A patent/AU2005287743B2/en not_active Ceased
- 2005-09-21 ES ES05792179.3T patent/ES2536460T3/es not_active Expired - Lifetime
- 2005-09-21 JP JP2007532749A patent/JP5081622B2/ja not_active Expired - Lifetime
- 2005-09-21 CA CA2581143A patent/CA2581143C/en not_active Expired - Fee Related
- 2005-09-21 WO PCT/CN2005/001521 patent/WO2006032202A1/zh not_active Ceased
- 2005-09-21 PL PL05792179T patent/PL1797871T3/pl unknown
- 2005-09-21 US US11/663,411 patent/US8691277B2/en active Active
-
2014
- 2014-03-17 US US14/182,652 patent/US9220782B2/en not_active Expired - Lifetime
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101481643B1 (ko) * | 2010-11-25 | 2015-01-12 | 산동 루예 파마슈티칼 컴파니 리미티드 | 로티고틴, 이의 유도체 또는 로티고틴 또는 이의 유도체의 약제학적으로 허용가능한 염의 조성물 |
| US9265835B2 (en) | 2010-11-25 | 2016-02-23 | Shan Dong Luye Pharmaceutical Co., Ltd. | Compositions of rotigotine, derivatives thereof, or pharmaceutically acceptable salts of rotigotine or its derivative |
| WO2021133051A1 (ko) * | 2019-12-23 | 2021-07-01 | 환인제약 주식회사 | 로피니롤을 포함하는 마이크로스피어 및 이를 함유하는 주사제 조성물 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1797871A4 (en) | 2012-11-28 |
| WO2006032202A1 (fr) | 2006-03-30 |
| CA2581143C (en) | 2015-03-31 |
| US20080260846A1 (en) | 2008-10-23 |
| US8691277B2 (en) | 2014-04-08 |
| ES2536460T3 (es) | 2015-05-25 |
| US9220782B2 (en) | 2015-12-29 |
| JP2008513524A (ja) | 2008-05-01 |
| CA2581143A1 (en) | 2006-03-30 |
| JP5081622B2 (ja) | 2012-11-28 |
| EP1797871A1 (en) | 2007-06-20 |
| HK1107012A1 (en) | 2008-03-28 |
| AU2005287743B2 (en) | 2011-09-29 |
| EP1797871B1 (en) | 2015-02-25 |
| PL1797871T3 (pl) | 2015-07-31 |
| AU2005287743A1 (en) | 2006-03-30 |
| US20140255488A1 (en) | 2014-09-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9220782B2 (en) | Long acting sustained-release formulation containing dopamine receptor agonist and the preparation method thereof | |
| EP2982367B1 (en) | Pharmaceutical composition for parenteral administration, containing donepezil | |
| US10406161B2 (en) | Risperidone sustained release microsphere composition | |
| JP6067803B2 (ja) | ロチゴチン、その誘導体、又はロチゴチン若しくはその誘導体の薬学的に許容可能な塩の組成物 | |
| US20040266813A1 (en) | Injectable sustained-release microspheres of huperzine a compoounds | |
| KR101663560B1 (ko) | 균일한 서방출성 미립구의 제조방법 | |
| WO2018108163A1 (zh) | 一种Talazoparib药物组合物及其应用 | |
| KR101663561B1 (ko) | 서방출성 미립구의 제조방법 | |
| KR20220170782A (ko) | 세마글루타이드 또는 이의 약학적으로 허용가능한 염을 포함하는 서방형 제제 조성물 | |
| CN1762495B (zh) | 含有多巴胺受体激动剂类药物的长效缓释制剂及其制备工艺 | |
| CN117530933B (zh) | 一种吡仑帕奈长效缓释微球、制备方法及缓释注射剂 | |
| CN102784111A (zh) | 一种多巴胺受体激动剂类药物的缓释制剂 | |
| US20180177777A1 (en) | Formulations for mitigating opioid overdose and methods of making and using the same | |
| CN113226288A (zh) | 纳曲酮注射型缓释制剂 | |
| HK1107012B (en) | Long acting sustained-release formulation containing dopamine receptor agonist and the preparation method thereof | |
| WO2006066509A1 (fr) | Préparation microsphérique à libération prolongée injectable de dérivés de 3,3-diphénylpropylamine servant d'antagonistes du récepteur muscarinique | |
| TR2022013222A1 (tr) | Naltrekson i̇çeren i̇n si̇tu jel oluşturan çözelti̇ler | |
| HK40047988A (en) | Naltrexone injectable sustained release formulation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20070419 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20070601 Comment text: Request for Examination of Application |
|
| PG1501 | Laying open of application | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20080417 Patent event code: PE09021S01D |
|
| AMND | Amendment | ||
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20090428 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20080417 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| J201 | Request for trial against refusal decision | ||
| PJ0201 | Trial against decision of rejection |
Patent event date: 20090528 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Patent event date: 20090428 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Appeal kind category: Appeal against decision to decline refusal Decision date: 20091230 Appeal identifier: 2009101004855 Request date: 20090528 |
|
| AMND | Amendment | ||
| PB0901 | Examination by re-examination before a trial |
Comment text: Amendment to Specification, etc. Patent event date: 20090629 Patent event code: PB09011R02I Comment text: Request for Trial against Decision on Refusal Patent event date: 20090528 Patent event code: PB09011R01I Comment text: Amendment to Specification, etc. Patent event date: 20090116 Patent event code: PB09011R02I |
|
| E801 | Decision on dismissal of amendment | ||
| PE0801 | Dismissal of amendment |
Patent event code: PE08012E01D Comment text: Decision on Dismissal of Amendment Patent event date: 20090709 Patent event code: PE08011R01I Comment text: Amendment to Specification, etc. Patent event date: 20090629 Patent event code: PE08011R01I Comment text: Amendment to Specification, etc. Patent event date: 20090116 |
|
| B601 | Maintenance of original decision after re-examination before a trial | ||
| PB0601 | Maintenance of original decision after re-examination before a trial | ||
| J301 | Trial decision |
Free format text: TRIAL DECISION FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL REQUESTED 20090528 Effective date: 20091230 |
|
| PJ1301 | Trial decision |
Patent event code: PJ13011S01D Patent event date: 20091230 Comment text: Trial Decision on Objection to Decision on Refusal Appeal kind category: Appeal against decision to decline refusal Request date: 20090528 Decision date: 20091230 Appeal identifier: 2009101004855 |
|
| J2X1 | Appeal (before the patent court) |
Free format text: APPEAL AGAINST DECISION TO DECLINE REFUSAL |
|
| PJ2001 | Appeal |
Patent event date: 20091230 Comment text: Trial Decision on Objection to Decision on Refusal Patent event code: PJ20011S01I Appeal kind category: Appeal against decision to decline refusal Decision date: 20101119 Appeal identifier: 2010201001244 Request date: 20100304 |
|
| J302 | Written judgement (patent court) |
Free format text: JUDGMENT (PATENT COURT) FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL REQUESTED 20100304 Effective date: 20101119 |
|
| PJ1302 | Judgment (patent court) |
Patent event date: 20101125 Comment text: Written Judgment (Patent Court) Patent event code: PJ13021S01D Request date: 20100304 Decision date: 20101119 Appeal identifier: 2010201001244 Appeal kind category: Appeal against decision to decline refusal |
|
| J2X2 | Appeal (before the supreme court) |
Free format text: APPEAL BEFORE THE SUPREME COURT FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL |
|
| PJ2002 | Appeal before the supreme court |
Comment text: Trial Decision on Objection to Decision on Refusal Patent event date: 20091230 Patent event code: PJ20021S01I Request date: 20101210 Appeal identifier: 2010301003561 Appeal kind category: Appeal against decision to decline refusal Decision date: 20110615 |
|
| J303 | Written judgement (supreme court) |
Free format text: JUDGMENT (SUPREME COURT) FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL REQUESTED 20101210 Effective date: 20110615 |
|
| PJ1303 | Judgment (supreme court) |
Comment text: Written Judgment (Supreme Court) Patent event date: 20110617 Patent event code: PJ13031S01D Decision date: 20110615 Appeal kind category: Appeal against decision to decline refusal Request date: 20101210 Appeal identifier: 2010301003561 |