JP7190822B2 - ヒト抗rankl抗体の製剤及びその使用方法 - Google Patents
ヒト抗rankl抗体の製剤及びその使用方法 Download PDFInfo
- Publication number
- JP7190822B2 JP7190822B2 JP2018086261A JP2018086261A JP7190822B2 JP 7190822 B2 JP7190822 B2 JP 7190822B2 JP 2018086261 A JP2018086261 A JP 2018086261A JP 2018086261 A JP2018086261 A JP 2018086261A JP 7190822 B2 JP7190822 B2 JP 7190822B2
- Authority
- JP
- Japan
- Prior art keywords
- formulation
- aqueous pharmaceutical
- pharmaceutical formulation
- antibody
- amino acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000000034 method Methods 0.000 title claims description 98
- 241000282414 Homo sapiens Species 0.000 title claims description 79
- 239000000203 mixture Substances 0.000 title description 587
- 238000009472 formulation Methods 0.000 title description 567
- 150000001413 amino acids Chemical group 0.000 claims description 221
- 239000013628 high molecular weight specie Substances 0.000 claims description 169
- 239000008194 pharmaceutical composition Substances 0.000 claims description 113
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 95
- 239000000872 buffer Substances 0.000 claims description 91
- 229960005190 phenylalanine Drugs 0.000 claims description 86
- 239000000427 antigen Substances 0.000 claims description 85
- 108091007433 antigens Proteins 0.000 claims description 85
- 102000036639 antigens Human genes 0.000 claims description 85
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical group CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 claims description 81
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 claims description 80
- 239000000600 sorbitol Substances 0.000 claims description 80
- 235000010356 sorbitol Nutrition 0.000 claims description 80
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical group OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 claims description 79
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 claims description 73
- 238000003860 storage Methods 0.000 claims description 66
- 229920001213 Polysorbate 20 Polymers 0.000 claims description 41
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 claims description 41
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 claims description 41
- 229940068977 polysorbate 20 Drugs 0.000 claims description 41
- 238000011282 treatment Methods 0.000 claims description 35
- 239000004094 surface-active agent Substances 0.000 claims description 29
- 210000000988 bone and bone Anatomy 0.000 claims description 26
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 claims description 24
- 102000014128 RANK Ligand Human genes 0.000 claims description 22
- 108010025832 RANK Ligand Proteins 0.000 claims description 22
- 239000003446 ligand Substances 0.000 claims description 21
- 229960004799 tryptophan Drugs 0.000 claims description 21
- 102000003945 NF-kappa B Human genes 0.000 claims description 20
- 108010057466 NF-kappa B Proteins 0.000 claims description 20
- 108091006084 receptor activators Proteins 0.000 claims description 20
- 206010017076 Fracture Diseases 0.000 claims description 19
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 claims description 18
- 208000001132 Osteoporosis Diseases 0.000 claims description 17
- 206010028980 Neoplasm Diseases 0.000 claims description 16
- 206010006187 Breast cancer Diseases 0.000 claims description 15
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 15
- 208000026310 Breast neoplasm Diseases 0.000 claims description 14
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 claims description 14
- 239000008181 tonicity modifier Substances 0.000 claims description 14
- 238000001195 ultra high performance liquid chromatography Methods 0.000 claims description 14
- 208000010392 Bone Fractures Diseases 0.000 claims description 13
- 229930195712 glutamate Natural products 0.000 claims description 13
- 208000002966 Giant Cell Tumor of Bone Diseases 0.000 claims description 10
- 201000011143 bone giant cell tumor Diseases 0.000 claims description 10
- 239000006172 buffering agent Substances 0.000 claims description 9
- 239000011521 glass Substances 0.000 claims description 9
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims description 8
- 239000011575 calcium Substances 0.000 claims description 8
- 229910052791 calcium Inorganic materials 0.000 claims description 8
- 238000002560 therapeutic procedure Methods 0.000 claims description 8
- 239000008351 acetate buffer Substances 0.000 claims description 7
- 238000004519 manufacturing process Methods 0.000 claims description 7
- 206010027452 Metastases to bone Diseases 0.000 claims description 6
- 208000034578 Multiple myelomas Diseases 0.000 claims description 6
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 6
- 229930003316 Vitamin D Natural products 0.000 claims description 6
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 claims description 6
- 239000002671 adjuvant Substances 0.000 claims description 6
- 229940071643 prefilled syringe Drugs 0.000 claims description 6
- 235000019166 vitamin D Nutrition 0.000 claims description 6
- 239000011710 vitamin D Substances 0.000 claims description 6
- 229940046008 vitamin d Drugs 0.000 claims description 6
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 claims description 5
- 229940122815 Aromatase inhibitor Drugs 0.000 claims description 5
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 claims description 5
- 229930195725 Mannitol Natural products 0.000 claims description 5
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 claims description 5
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 claims description 5
- 238000009167 androgen deprivation therapy Methods 0.000 claims description 5
- 239000003886 aromatase inhibitor Substances 0.000 claims description 5
- 201000011510 cancer Diseases 0.000 claims description 5
- 230000036210 malignancy Effects 0.000 claims description 5
- 239000000594 mannitol Substances 0.000 claims description 5
- 235000010355 mannitol Nutrition 0.000 claims description 5
- 208000010658 metastatic prostate carcinoma Diseases 0.000 claims description 5
- 230000000683 nonmetastatic effect Effects 0.000 claims description 5
- 150000003710 vitamin D derivatives Chemical class 0.000 claims description 5
- 229940122361 Bisphosphonate Drugs 0.000 claims description 4
- 208000037147 Hypercalcaemia Diseases 0.000 claims description 4
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims description 4
- 229930006000 Sucrose Natural products 0.000 claims description 4
- 150000004663 bisphosphonates Chemical class 0.000 claims description 4
- 230000000148 hypercalcaemia Effects 0.000 claims description 4
- 208000030915 hypercalcemia disease Diseases 0.000 claims description 4
- 238000002156 mixing Methods 0.000 claims description 4
- 238000002271 resection Methods 0.000 claims description 4
- 239000005720 sucrose Substances 0.000 claims description 4
- 239000003599 detergent Substances 0.000 claims description 3
- 208000007569 Giant Cell Tumors Diseases 0.000 claims 1
- 235000001014 amino acid Nutrition 0.000 description 240
- 229940024606 amino acid Drugs 0.000 description 239
- 229960001251 denosumab Drugs 0.000 description 155
- 230000002776 aggregation Effects 0.000 description 141
- 238000004220 aggregation Methods 0.000 description 141
- 239000003112 inhibitor Substances 0.000 description 105
- 239000004475 Arginine Substances 0.000 description 74
- 229960003121 arginine Drugs 0.000 description 74
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 70
- 235000009697 arginine Nutrition 0.000 description 70
- 230000006870 function Effects 0.000 description 61
- 230000015572 biosynthetic process Effects 0.000 description 56
- 235000018102 proteins Nutrition 0.000 description 53
- 108090000623 proteins and genes Proteins 0.000 description 53
- 102000004169 proteins and genes Human genes 0.000 description 53
- 125000003275 alpha amino acid group Chemical group 0.000 description 50
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 47
- 239000000243 solution Substances 0.000 description 46
- 238000011026 diafiltration Methods 0.000 description 38
- 229960003589 arginine hydrochloride Drugs 0.000 description 33
- 230000002209 hydrophobic effect Effects 0.000 description 30
- 230000003993 interaction Effects 0.000 description 28
- -1 aromatic amino acid Chemical class 0.000 description 26
- 230000007717 exclusion Effects 0.000 description 23
- 239000000047 product Substances 0.000 description 23
- 239000000654 additive Substances 0.000 description 22
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 21
- 108010016626 Dipeptides Proteins 0.000 description 19
- 102100035360 Cerebellar degeneration-related antigen 1 Human genes 0.000 description 18
- 230000000694 effects Effects 0.000 description 18
- 241000894007 species Species 0.000 description 17
- 229910052805 deuterium Inorganic materials 0.000 description 16
- 239000000463 material Substances 0.000 description 16
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 15
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 15
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 14
- 230000005764 inhibitory process Effects 0.000 description 14
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 13
- 239000012634 fragment Substances 0.000 description 13
- 102000040430 polynucleotide Human genes 0.000 description 13
- 108091033319 polynucleotide Proteins 0.000 description 13
- 239000002157 polynucleotide Substances 0.000 description 13
- 108020003175 receptors Proteins 0.000 description 13
- 102000005962 receptors Human genes 0.000 description 13
- 229930064664 L-arginine Natural products 0.000 description 12
- 235000014852 L-arginine Nutrition 0.000 description 12
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 12
- 230000000996 additive effect Effects 0.000 description 12
- 229940049906 glutamate Drugs 0.000 description 12
- 102000039446 nucleic acids Human genes 0.000 description 12
- 108020004707 nucleic acids Proteins 0.000 description 12
- 150000007523 nucleic acids Chemical class 0.000 description 12
- 229920000136 polysorbate Polymers 0.000 description 12
- 229950008882 polysorbate Drugs 0.000 description 12
- 230000002829 reductive effect Effects 0.000 description 12
- 125000000217 alkyl group Chemical group 0.000 description 11
- 239000003398 denaturant Substances 0.000 description 11
- 238000000502 dialysis Methods 0.000 description 11
- 230000009467 reduction Effects 0.000 description 11
- 230000000087 stabilizing effect Effects 0.000 description 11
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 10
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 10
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 10
- 239000003814 drug Substances 0.000 description 10
- 229960003136 leucine Drugs 0.000 description 10
- 101100112922 Candida albicans CDR3 gene Proteins 0.000 description 9
- 239000007864 aqueous solution Substances 0.000 description 9
- 238000010790 dilution Methods 0.000 description 9
- 239000012895 dilution Substances 0.000 description 9
- 239000013020 final formulation Substances 0.000 description 9
- 230000002265 prevention Effects 0.000 description 9
- 102100035361 Cerebellar degeneration-related protein 2 Human genes 0.000 description 8
- 101000737793 Homo sapiens Cerebellar degeneration-related antigen 1 Proteins 0.000 description 8
- 101000737796 Homo sapiens Cerebellar degeneration-related protein 2 Proteins 0.000 description 8
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 8
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 8
- 108010038807 Oligopeptides Proteins 0.000 description 8
- 102000015636 Oligopeptides Human genes 0.000 description 8
- 230000007423 decrease Effects 0.000 description 8
- 238000013467 fragmentation Methods 0.000 description 8
- 238000006062 fragmentation reaction Methods 0.000 description 8
- 235000014304 histidine Nutrition 0.000 description 8
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 8
- 229960002885 histidine Drugs 0.000 description 8
- 229960000310 isoleucine Drugs 0.000 description 8
- 150000003839 salts Chemical group 0.000 description 8
- 229960004295 valine Drugs 0.000 description 8
- 239000004472 Lysine Substances 0.000 description 7
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 7
- 238000007792 addition Methods 0.000 description 7
- 239000013011 aqueous formulation Substances 0.000 description 7
- 238000003508 chemical denaturation Methods 0.000 description 7
- 239000000539 dimer Substances 0.000 description 7
- 229960003646 lysine Drugs 0.000 description 7
- 235000018977 lysine Nutrition 0.000 description 7
- 239000002773 nucleotide Substances 0.000 description 7
- 125000003729 nucleotide group Chemical group 0.000 description 7
- 239000000546 pharmaceutical excipient Substances 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- 108090000765 processed proteins & peptides Proteins 0.000 description 7
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 6
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 6
- 210000001015 abdomen Anatomy 0.000 description 6
- 229960000106 biosimilars Drugs 0.000 description 6
- 229910052799 carbon Inorganic materials 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- 238000011156 evaluation Methods 0.000 description 6
- 230000007246 mechanism Effects 0.000 description 6
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 6
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 6
- 229920000053 polysorbate 80 Polymers 0.000 description 6
- 229940068968 polysorbate 80 Drugs 0.000 description 6
- 235000002639 sodium chloride Nutrition 0.000 description 6
- 239000003381 stabilizer Substances 0.000 description 6
- 210000000689 upper leg Anatomy 0.000 description 6
- 206010065687 Bone loss Diseases 0.000 description 5
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 5
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 5
- SNEIUMQYRCDYCH-LURJTMIESA-N N(alpha)-acetyl-L-arginine Chemical compound CC(=O)N[C@H](C(O)=O)CCCNC(N)=N SNEIUMQYRCDYCH-LURJTMIESA-N 0.000 description 5
- 206010060862 Prostate cancer Diseases 0.000 description 5
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 5
- 229960000074 biopharmaceutical Drugs 0.000 description 5
- 201000010099 disease Diseases 0.000 description 5
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 5
- 238000002474 experimental method Methods 0.000 description 5
- 239000012458 free base Substances 0.000 description 5
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 description 5
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 description 5
- 239000013627 low molecular weight specie Substances 0.000 description 5
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 5
- 229920001223 polyethylene glycol Polymers 0.000 description 5
- 238000007920 subcutaneous administration Methods 0.000 description 5
- 229940124597 therapeutic agent Drugs 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
- KWTQSFXGGICVPE-UHFFFAOYSA-N 2-amino-5-(diaminomethylideneamino)pentanoic acid;hydron;chloride Chemical compound Cl.OC(=O)C(N)CCCN=C(N)N KWTQSFXGGICVPE-UHFFFAOYSA-N 0.000 description 4
- KWTQSFXGGICVPE-WCCKRBBISA-N Arginine hydrochloride Chemical compound Cl.OC(=O)[C@@H](N)CCCN=C(N)N KWTQSFXGGICVPE-WCCKRBBISA-N 0.000 description 4
- 208000006386 Bone Resorption Diseases 0.000 description 4
- 229920000858 Cyclodextrin Polymers 0.000 description 4
- 101000830603 Homo sapiens Tumor necrosis factor ligand superfamily member 11 Proteins 0.000 description 4
- 206010020584 Hypercalcaemia of malignancy Diseases 0.000 description 4
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 description 4
- 102000037984 Inhibitory immune checkpoint proteins Human genes 0.000 description 4
- 108091008026 Inhibitory immune checkpoint proteins Proteins 0.000 description 4
- 229930182844 L-isoleucine Natural products 0.000 description 4
- 239000004395 L-leucine Substances 0.000 description 4
- 235000019454 L-leucine Nutrition 0.000 description 4
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 4
- 229940022663 acetate Drugs 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- 125000003118 aryl group Chemical group 0.000 description 4
- 230000024279 bone resorption Effects 0.000 description 4
- 239000007853 buffer solution Substances 0.000 description 4
- 230000000295 complement effect Effects 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 239000012141 concentrate Substances 0.000 description 4
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 4
- 239000002552 dosage form Substances 0.000 description 4
- 235000011187 glycerol Nutrition 0.000 description 4
- 102000053529 human TNFSF11 Human genes 0.000 description 4
- 208000008750 humoral hypercalcemia of malignancy Diseases 0.000 description 4
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 description 4
- 230000006872 improvement Effects 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 4
- 239000003550 marker Substances 0.000 description 4
- 229920001983 poloxamer Polymers 0.000 description 4
- 210000002966 serum Anatomy 0.000 description 4
- 239000011550 stock solution Substances 0.000 description 4
- 230000035882 stress Effects 0.000 description 4
- 235000000346 sugar Nutrition 0.000 description 4
- 150000008163 sugars Chemical class 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- 238000000108 ultra-filtration Methods 0.000 description 4
- 239000004474 valine Substances 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- QDGAVODICPCDMU-UHFFFAOYSA-N 2-amino-3-[3-[bis(2-chloroethyl)amino]phenyl]propanoic acid Chemical compound OC(=O)C(N)CC1=CC=CC(N(CCCl)CCCl)=C1 QDGAVODICPCDMU-UHFFFAOYSA-N 0.000 description 3
- PQBHGSGQZSOLIR-RYUDHWBXSA-N Arg-Phe Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 PQBHGSGQZSOLIR-RYUDHWBXSA-N 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- 108060003951 Immunoglobulin Proteins 0.000 description 3
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 3
- VEYYWZRYIYDQJM-ZETCQYMHSA-N N(2)-acetyl-L-lysine Chemical compound CC(=O)N[C@H](C([O-])=O)CCCC[NH3+] VEYYWZRYIYDQJM-ZETCQYMHSA-N 0.000 description 3
- 101710089372 Programmed cell death protein 1 Proteins 0.000 description 3
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 125000001931 aliphatic group Chemical group 0.000 description 3
- 125000000539 amino acid group Chemical group 0.000 description 3
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 3
- WHGYBXFWUBPSRW-FOUAGVGXSA-N beta-cyclodextrin Chemical compound OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO WHGYBXFWUBPSRW-FOUAGVGXSA-N 0.000 description 3
- 229960004853 betadex Drugs 0.000 description 3
- 210000004027 cell Anatomy 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 229940097362 cyclodextrins Drugs 0.000 description 3
- 238000004925 denaturation Methods 0.000 description 3
- 230000036425 denaturation Effects 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 239000012538 diafiltration buffer Substances 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 125000001165 hydrophobic group Chemical group 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 102000018358 immunoglobulin Human genes 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 229960005386 ipilimumab Drugs 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 201000005202 lung cancer Diseases 0.000 description 3
- 208000020816 lung neoplasm Diseases 0.000 description 3
- 229960003301 nivolumab Drugs 0.000 description 3
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 3
- 229960002621 pembrolizumab Drugs 0.000 description 3
- 229920001993 poloxamer 188 Polymers 0.000 description 3
- 239000003755 preservative agent Substances 0.000 description 3
- 102000004196 processed proteins & peptides Human genes 0.000 description 3
- 229940092597 prolia Drugs 0.000 description 3
- 229960002429 proline Drugs 0.000 description 3
- 230000004845 protein aggregation Effects 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 230000006641 stabilisation Effects 0.000 description 3
- 238000011105 stabilization Methods 0.000 description 3
- 238000010254 subcutaneous injection Methods 0.000 description 3
- 239000007929 subcutaneous injection Substances 0.000 description 3
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 3
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 3
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 description 2
- 102100023990 60S ribosomal protein L17 Human genes 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Natural products CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 2
- 102100029822 B- and T-lymphocyte attenuator Human genes 0.000 description 2
- 108010074708 B7-H1 Antigen Proteins 0.000 description 2
- 206010055113 Breast cancer metastatic Diseases 0.000 description 2
- 102100038078 CD276 antigen Human genes 0.000 description 2
- 101710185679 CD276 antigen Proteins 0.000 description 2
- 239000012275 CTLA-4 inhibitor Substances 0.000 description 2
- 229940045513 CTLA4 antagonist Drugs 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 241000283707 Capra Species 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- 241000699800 Cricetinae Species 0.000 description 2
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 description 2
- 241000287828 Gallus gallus Species 0.000 description 2
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 2
- 102100034458 Hepatitis A virus cellular receptor 2 Human genes 0.000 description 2
- 208000017604 Hodgkin disease Diseases 0.000 description 2
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 2
- 101000864344 Homo sapiens B- and T-lymphocyte attenuator Proteins 0.000 description 2
- 101000889276 Homo sapiens Cytotoxic T-lymphocyte protein 4 Proteins 0.000 description 2
- 101001068133 Homo sapiens Hepatitis A virus cellular receptor 2 Proteins 0.000 description 2
- 101001137987 Homo sapiens Lymphocyte activation gene 3 protein Proteins 0.000 description 2
- 101000955999 Homo sapiens V-set domain-containing T-cell activation inhibitor 1 Proteins 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 2
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 2
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 2
- 150000008575 L-amino acids Chemical class 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- BVHLGVCQOALMSV-JEDNCBNOSA-N L-lysine hydrochloride Chemical compound Cl.NCCCC[C@H](N)C(O)=O BVHLGVCQOALMSV-JEDNCBNOSA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- 102000017578 LAG3 Human genes 0.000 description 2
- 206010027476 Metastases Diseases 0.000 description 2
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 2
- 241000283973 Oryctolagus cuniculus Species 0.000 description 2
- 241000209094 Oryza Species 0.000 description 2
- 235000007164 Oryza sativa Nutrition 0.000 description 2
- 206010033128 Ovarian cancer Diseases 0.000 description 2
- 206010061535 Ovarian neoplasm Diseases 0.000 description 2
- 108090000526 Papain Proteins 0.000 description 2
- 206010034156 Pathological fracture Diseases 0.000 description 2
- 102000057297 Pepsin A Human genes 0.000 description 2
- 108090000284 Pepsin A Proteins 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- 229920001214 Polysorbate 60 Polymers 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 2
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 2
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 description 2
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 2
- 239000004365 Protease Substances 0.000 description 2
- 108010029485 Protein Isoforms Proteins 0.000 description 2
- 102000001708 Protein Isoforms Human genes 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 208000006265 Renal cell carcinoma Diseases 0.000 description 2
- 102000008115 Signaling Lymphocytic Activation Molecule Family Member 1 Human genes 0.000 description 2
- 108010074687 Signaling Lymphocytic Activation Molecule Family Member 1 Proteins 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 206010041549 Spinal cord compression Diseases 0.000 description 2
- 208000005250 Spontaneous Fractures Diseases 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 102100038929 V-set domain-containing T-cell activation inhibitor 1 Human genes 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 238000009098 adjuvant therapy Methods 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 235000004279 alanine Nutrition 0.000 description 2
- 229960003767 alanine Drugs 0.000 description 2
- 125000002877 alkyl aryl group Chemical group 0.000 description 2
- 235000003704 aspartic acid Nutrition 0.000 description 2
- 229960005261 aspartic acid Drugs 0.000 description 2
- 229960003852 atezolizumab Drugs 0.000 description 2
- 229950002916 avelumab Drugs 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 2
- 230000037182 bone density Effects 0.000 description 2
- 230000008416 bone turnover Effects 0.000 description 2
- 125000002837 carbocyclic group Chemical group 0.000 description 2
- 125000002579 carboxylato group Chemical group [O-]C(*)=O 0.000 description 2
- YCIMNLLNPGFGHC-UHFFFAOYSA-N catechol Chemical compound OC1=CC=CC=C1O YCIMNLLNPGFGHC-UHFFFAOYSA-N 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 238000002512 chemotherapy Methods 0.000 description 2
- 235000013330 chicken meat Nutrition 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 230000001054 cortical effect Effects 0.000 description 2
- 229940109239 creatinine Drugs 0.000 description 2
- 125000000753 cycloalkyl group Chemical group 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 229940014553 denosumab 70 mg/ml Drugs 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 210000002436 femur neck Anatomy 0.000 description 2
- 108020001507 fusion proteins Proteins 0.000 description 2
- 102000037865 fusion proteins Human genes 0.000 description 2
- 235000013922 glutamic acid Nutrition 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- ZRALSGWEFCBTJO-UHFFFAOYSA-O guanidinium Chemical compound NC(N)=[NH2+] ZRALSGWEFCBTJO-UHFFFAOYSA-O 0.000 description 2
- IXCSERBJSXMMFS-UHFFFAOYSA-N hcl hcl Chemical class Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 2
- 125000001072 heteroaryl group Chemical group 0.000 description 2
- 150000003840 hydrochlorides Chemical class 0.000 description 2
- 230000000121 hypercalcemic effect Effects 0.000 description 2
- 229940027941 immunoglobulin g Drugs 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 210000004705 lumbosacral region Anatomy 0.000 description 2
- RLSSMJSEOOYNOY-UHFFFAOYSA-N m-cresol Chemical compound CC1=CC=CC(O)=C1 RLSSMJSEOOYNOY-UHFFFAOYSA-N 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 230000010534 mechanism of action Effects 0.000 description 2
- 230000009401 metastasis Effects 0.000 description 2
- 230000001394 metastastic effect Effects 0.000 description 2
- 206010061289 metastatic neoplasm Diseases 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 229940055729 papain Drugs 0.000 description 2
- 235000019834 papain Nutrition 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- AQIXEPGDORPWBJ-UHFFFAOYSA-N pentan-3-ol Chemical compound CCC(O)CC AQIXEPGDORPWBJ-UHFFFAOYSA-N 0.000 description 2
- 229940111202 pepsin Drugs 0.000 description 2
- 229920000570 polyether Polymers 0.000 description 2
- 229920005862 polyol Polymers 0.000 description 2
- 150000003077 polyols Chemical class 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 239000012460 protein solution Substances 0.000 description 2
- 230000006432 protein unfolding Effects 0.000 description 2
- 238000001959 radiotherapy Methods 0.000 description 2
- 230000000306 recurrent effect Effects 0.000 description 2
- GHMLBKRAJCXXBS-UHFFFAOYSA-N resorcinol Chemical compound OC1=CC=CC(O)=C1 GHMLBKRAJCXXBS-UHFFFAOYSA-N 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 235000009566 rice Nutrition 0.000 description 2
- 238000004088 simulation Methods 0.000 description 2
- 206010041569 spinal fracture Diseases 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 238000004448 titration Methods 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 229960004441 tyrosine Drugs 0.000 description 2
- 230000002485 urinary effect Effects 0.000 description 2
- 239000008215 water for injection Substances 0.000 description 2
- 229940014556 xgeva Drugs 0.000 description 2
- ZAIZDXVMSSDZFA-QRPNPIFTSA-N (2s)-2-amino-3-phenylpropanoic acid;hydrochloride Chemical compound Cl.OC(=O)[C@@H](N)CC1=CC=CC=C1 ZAIZDXVMSSDZFA-QRPNPIFTSA-N 0.000 description 1
- CLUOUYPICNCYNF-IPIKRLCPSA-N (2s)-2-amino-5-(diaminomethylideneamino)pentanoic acid;(2s)-2-amino-3-phenylpropanoic acid Chemical compound OC(=O)[C@@H](N)CCCNC(N)=N.OC(=O)[C@@H](N)CC1=CC=CC=C1 CLUOUYPICNCYNF-IPIKRLCPSA-N 0.000 description 1
- CYDQOEWLBCCFJZ-UHFFFAOYSA-N 4-(4-fluorophenyl)oxane-4-carboxylic acid Chemical compound C=1C=C(F)C=CC=1C1(C(=O)O)CCOCC1 CYDQOEWLBCCFJZ-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 208000008884 Aneurysmal Bone Cysts Diseases 0.000 description 1
- OMLWNBVRVJYMBQ-YUMQZZPRSA-N Arg-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O OMLWNBVRVJYMBQ-YUMQZZPRSA-N 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 241000699802 Cricetulus griseus Species 0.000 description 1
- 150000008574 D-amino acids Chemical class 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 206010061819 Disease recurrence Diseases 0.000 description 1
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical class [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 239000004386 Erythritol Substances 0.000 description 1
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-M Glycolate Chemical compound OCC([O-])=O AEMRFAOFKBGASW-UHFFFAOYSA-M 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 206010062904 Hormone-refractory prostate cancer Diseases 0.000 description 1
- 201000002980 Hyperparathyroidism Diseases 0.000 description 1
- 208000013038 Hypocalcemia Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 102000037982 Immune checkpoint proteins Human genes 0.000 description 1
- 108091008036 Immune checkpoint proteins Proteins 0.000 description 1
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 1
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 1
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 206010023862 Laryngeal stenosis Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 208000029725 Metabolic bone disease Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 1
- 125000003047 N-acetyl group Chemical group 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- 208000010191 Osteitis Deformans Diseases 0.000 description 1
- 206010031252 Osteomyelitis Diseases 0.000 description 1
- 206010031264 Osteonecrosis Diseases 0.000 description 1
- 206010049088 Osteopenia Diseases 0.000 description 1
- 239000012270 PD-1 inhibitor Substances 0.000 description 1
- 239000012668 PD-1-inhibitor Substances 0.000 description 1
- 239000012271 PD-L1 inhibitor Substances 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 208000027868 Paget disease Diseases 0.000 description 1
- 229920002562 Polyethylene Glycol 3350 Polymers 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- 125000000218 acetic acid group Chemical group C(C)(=O)* 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 238000012443 analytical study Methods 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 229940041181 antineoplastic drug Drugs 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 150000001483 arginine derivatives Chemical class 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- UREZNYTWGJKWBI-UHFFFAOYSA-M benzethonium chloride Chemical compound [Cl-].C1=CC(C(C)(C)CC(C)(C)C)=CC=C1OCCOCC[N+](C)(C)CC1=CC=CC=C1 UREZNYTWGJKWBI-UHFFFAOYSA-M 0.000 description 1
- 229960001950 benzethonium chloride Drugs 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- HUTDDBSSHVOYJR-UHFFFAOYSA-H bis[(2-oxo-1,3,2$l^{5},4$l^{2}-dioxaphosphaplumbetan-2-yl)oxy]lead Chemical compound [Pb+2].[Pb+2].[Pb+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O HUTDDBSSHVOYJR-UHFFFAOYSA-H 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 239000008366 buffered solution Substances 0.000 description 1
- 239000004067 bulking agent Substances 0.000 description 1
- LRHPLDYGYMQRHN-UHFFFAOYSA-N butyl alcohol Substances CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 235000011148 calcium chloride Nutrition 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 210000003679 cervix uteri Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 230000007012 clinical effect Effects 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 239000000599 controlled substance Substances 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 230000001687 destabilization Effects 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 150000002016 disaccharides Chemical class 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000010889 donnan-equilibrium Methods 0.000 description 1
- 229940056913 eftilagimod alfa Drugs 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 description 1
- 235000019414 erythritol Nutrition 0.000 description 1
- 229940009714 erythritol Drugs 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 229940085942 formulation r Drugs 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-L glutamate group Chemical group N[C@@H](CCC(=O)[O-])C(=O)[O-] WHUUTDBJXJRKMK-VKHMYHEASA-L 0.000 description 1
- 229960002989 glutamic acid Drugs 0.000 description 1
- 229960002449 glycine Drugs 0.000 description 1
- 230000008642 heat stress Effects 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 230000005661 hydrophobic surface Effects 0.000 description 1
- 230000000705 hypocalcaemia Effects 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000036737 immune function Effects 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 239000005414 inactive ingredient Substances 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000004968 inflammatory condition Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000009878 intermolecular interaction Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 239000012931 lyophilized formulation Substances 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229940049920 malate Drugs 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N malic acid Chemical compound OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 208000027202 mammary Paget disease Diseases 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- IZXGZAJMDLJLMF-UHFFFAOYSA-N methylaminomethanol Chemical compound CNCO IZXGZAJMDLJLMF-UHFFFAOYSA-N 0.000 description 1
- 229960002216 methylparaben Drugs 0.000 description 1
- 230000000116 mitigating effect Effects 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 238000000329 molecular dynamics simulation Methods 0.000 description 1
- 238000000302 molecular modelling Methods 0.000 description 1
- 238000009099 neoadjuvant therapy Methods 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 230000006911 nucleation Effects 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-M octanoate Chemical compound CCCCCCCC([O-])=O WWZKQHOCKIZLMA-UHFFFAOYSA-M 0.000 description 1
- 229920002113 octoxynol Polymers 0.000 description 1
- 238000006384 oligomerization reaction Methods 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 210000002997 osteoclast Anatomy 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- LXCFILQKKLGQFO-UHFFFAOYSA-N p-hydroxybenzoic acid methyl ester Natural products COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 1
- 239000003002 pH adjusting agent Substances 0.000 description 1
- 239000006179 pH buffering agent Substances 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 229940121655 pd-1 inhibitor Drugs 0.000 description 1
- 229940121656 pd-l1 inhibitor Drugs 0.000 description 1
- 230000002572 peristaltic effect Effects 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 238000011518 platinum-based chemotherapy Methods 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 239000001103 potassium chloride Substances 0.000 description 1
- 235000011164 potassium chloride Nutrition 0.000 description 1
- 229910000160 potassium phosphate Inorganic materials 0.000 description 1
- 235000011009 potassium phosphates Nutrition 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000006916 protein interaction Effects 0.000 description 1
- 230000029983 protein stabilization Effects 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- IFGCUJZIWBUILZ-UHFFFAOYSA-N sodium 2-[[2-[[hydroxy-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyphosphoryl]amino]-4-methylpentanoyl]amino]-3-(1H-indol-3-yl)propanoic acid Chemical compound [Na+].C=1NC2=CC=CC=C2C=1CC(C(O)=O)NC(=O)C(CC(C)C)NP(O)(=O)OC1OC(C)C(O)C(O)C1O IFGCUJZIWBUILZ-UHFFFAOYSA-N 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000001540 sodium lactate Substances 0.000 description 1
- 235000011088 sodium lactate Nutrition 0.000 description 1
- 229940005581 sodium lactate Drugs 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 235000011008 sodium phosphates Nutrition 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 238000012421 spiking Methods 0.000 description 1
- 238000013097 stability assessment Methods 0.000 description 1
- SFVFIFLLYFPGHH-UHFFFAOYSA-M stearalkonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CC1=CC=CC=C1 SFVFIFLLYFPGHH-UHFFFAOYSA-M 0.000 description 1
- 238000011146 sterile filtration Methods 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 150000005846 sugar alcohols Chemical class 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 229950007217 tremelimumab Drugs 0.000 description 1
- 239000013638 trimer Substances 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-M tryptophanate Chemical compound C1=CC=C2C(CC(N)C([O-])=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-M 0.000 description 1
- 208000025421 tumor of uterus Diseases 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 206010046766 uterine cancer Diseases 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39591—Stabilisation, fragmentation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/12—Carboxylic acids; Salts or anhydrides thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/16—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing nitrogen, e.g. nitro-, nitroso-, azo-compounds, nitriles, cyanates
- A61K47/18—Amines; Amides; Ureas; Quaternary ammonium compounds; Amino acids; Oligopeptides having up to five amino acids
- A61K47/183—Amino acids, e.g. glycine, EDTA or aspartame
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/22—Heterocyclic compounds, e.g. ascorbic acid, tocopherol or pyrrolidones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/26—Carbohydrates, e.g. sugar alcohols, amino sugars, nucleic acids, mono-, di- or oligo-saccharides; Derivatives thereof, e.g. polysorbates, sorbitan fatty acid esters or glycyrrhizin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/08—Solutions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/14—Drugs for genital or sexual disorders; Contraceptives for lactation disorders, e.g. galactorrhoea
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
- A61P19/10—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease for osteoporosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/12—Drugs for disorders of the metabolism for electrolyte homeostasis
- A61P3/14—Drugs for disorders of the metabolism for electrolyte homeostasis for calcium homeostasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2875—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF/TNF superfamily, e.g. CD70, CD95L, CD153, CD154
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/21—Immunoglobulins specific features characterized by taxonomic origin from primates, e.g. man
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/94—Stability, e.g. half-life, pH, temperature or enzyme-resistance
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Physical Education & Sports Medicine (AREA)
- Rheumatology (AREA)
- Epidemiology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Endocrinology (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Urology & Nephrology (AREA)
- Obesity (AREA)
- Oncology (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Gynecology & Obstetrics (AREA)
- Pregnancy & Childbirth (AREA)
- Reproductive Health (AREA)
- Pulmonology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicinal Preparation (AREA)
Description
2017年4月28日に出願された米国仮特許出願第62/492,056号の35 U.S.C.§119(e)による利益が、ここに主張され、その開示は参照により本明細書に援用される。
本明細書と同時に提出された、コンピュータ読み取り可能なヌクレオチド/アミノ酸配列リストは、参照によりその全体が本明細書に取り込まれており、以下と特定される:2018年4月20日に作成された、49キロバイトのASCII(テキスト)ファイル「51689A_Seqlisting.txt」。
開示の分野
本発明は、デノスマブ及びそのバイオシミラーの高濃度水性製剤などのヒト抗RANKLモノクローナル抗体に関する。
デノスマブは、60mg/mL及び70mg/mLの濃度にて溶液形態で市販されている。
1.70mg/mLを超える濃度及び約5.0から5.2未満の範囲のpHを有するヒト抗ヒト核因子カッパ-B受容体活性化剤リガンド(抗RANKL)モノクローナル抗体またはその抗原結合部分を含む水性医薬製剤。
ヒト抗ヒト核因子カッパ-B受容体活性化剤リガンド(抗RANKL)モノクローナル抗体またはその抗原結合部分を含む前記水性医薬製剤を約5.0から5.2未満の範囲のpHで調製することを含み、
前記水性医薬製剤が、約5.0から5.2未満の範囲のpHにない同等の水性医薬製剤と比較して、約5.0から5.2未満の範囲のpHで改善された安定性を示す、前記方法。
アミノ酸凝集阻害剤と混合した、ヒト抗ヒト核因子カッパ-B受容体活性化剤リガンド(抗RANKL)モノクローナル抗体またはその抗原結合部分を含む前記水性医薬製剤を調製することを含み、
前記水性医薬製剤は、前記アミノ酸凝集阻害剤を含まない同等の水性医薬製剤と比較して、前記アミノ酸凝集阻害剤による改善された安定性を示す、前記方法。
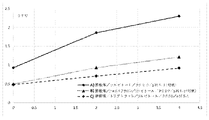
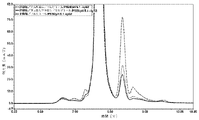
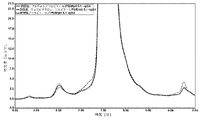
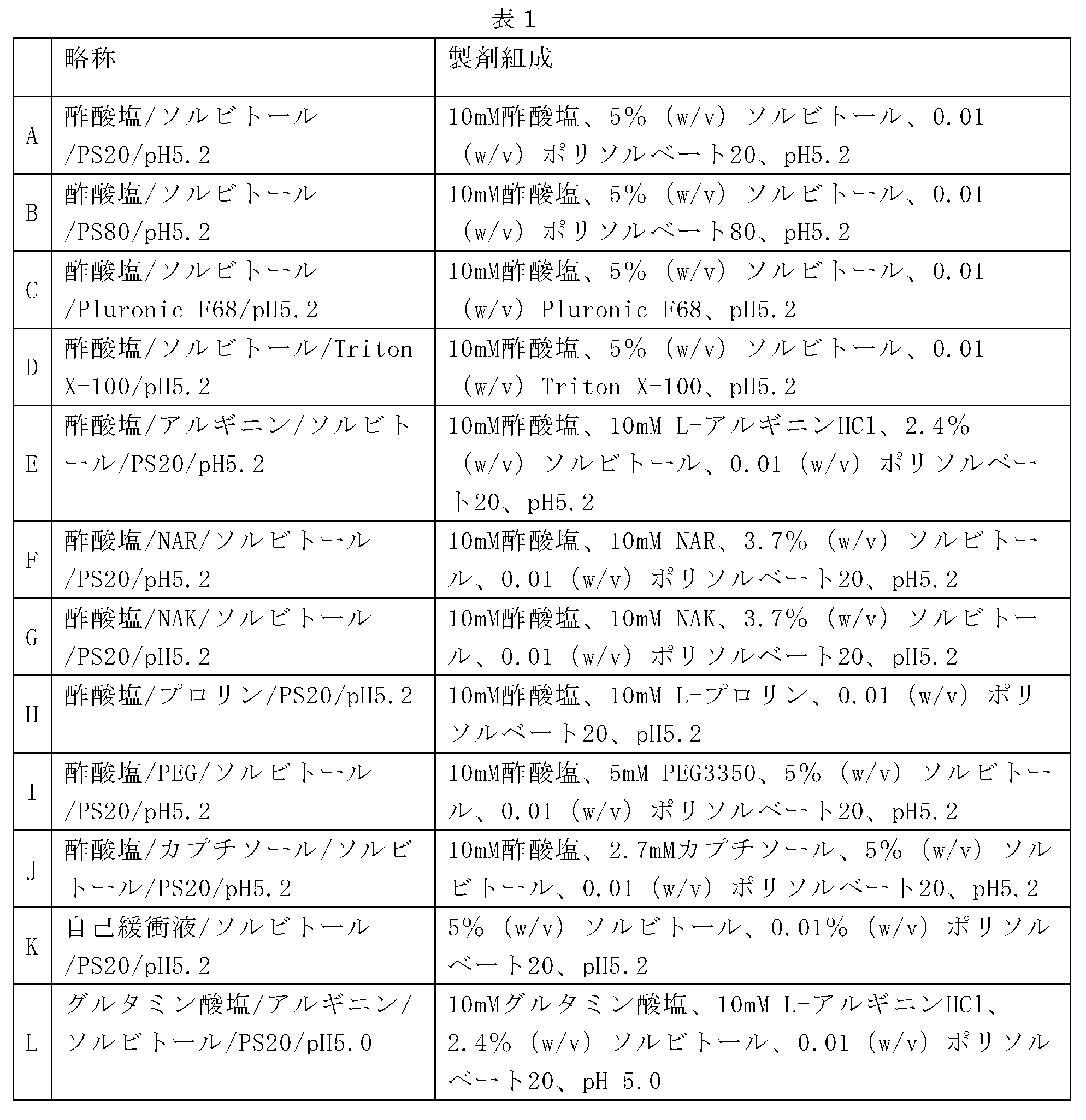
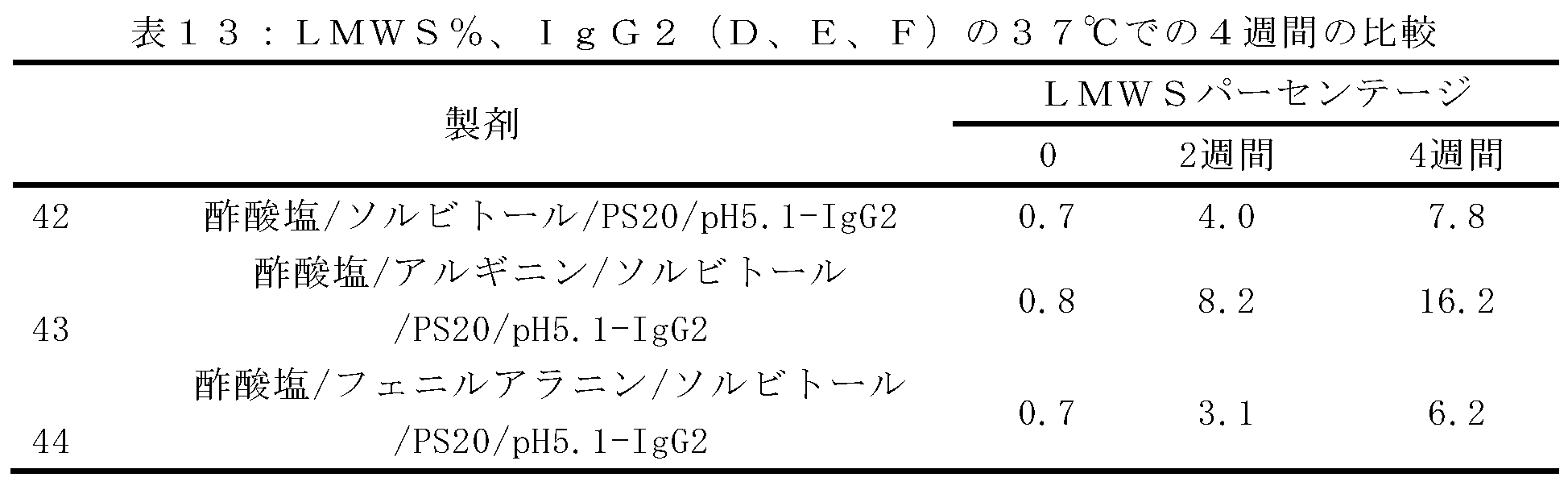
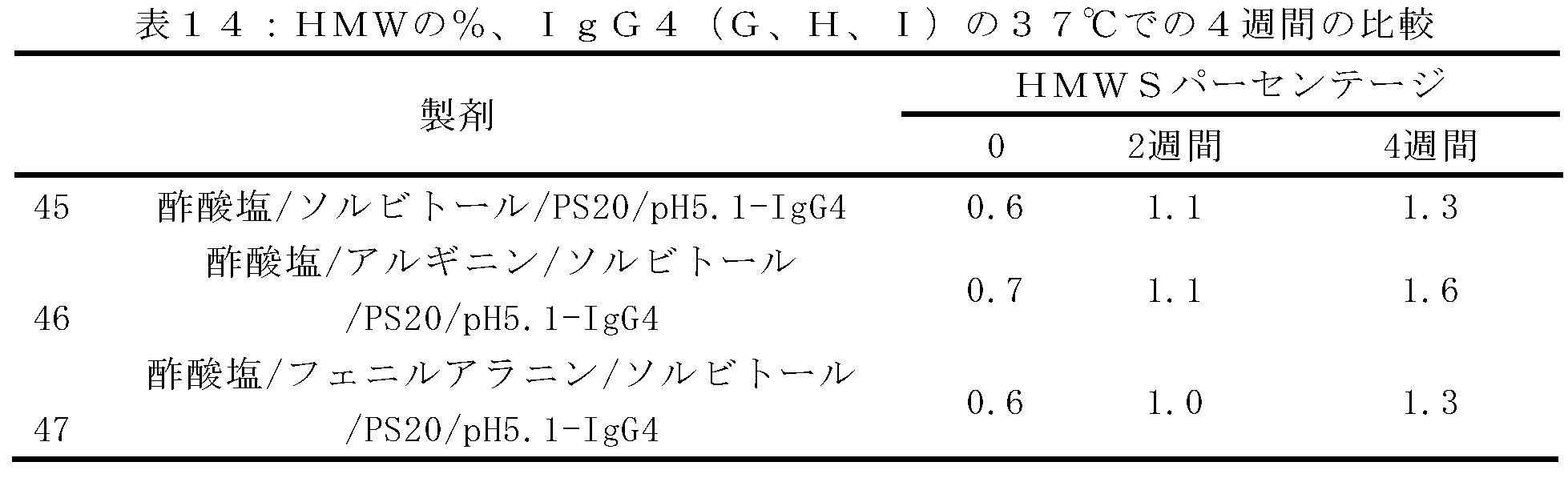
12の製剤の初期評価を、高濃度液体デノスマブ製剤(120mg/mL)中のHMWSの量(%)及びその経時的形成を最小化する効果について行った。製剤の代替物には、緩衝液の種類、安定剤及び溶液のpHの変更が含まれた。試験した製剤、A~Lを以下の表1に記載する。引用された全ての緩衝液値は、抗体が透析ろ過される緩衝液濃度に関するものである。各添加剤及び界面活性剤を、表に示すレベルまで緩衝液交換後の溶液に添加した。本製剤中の酢酸塩濃度は測定されなかったが、10mMの酢酸塩に対して透析ろ過されたソルビトールを有する120mg/mLデノスマブ製剤は、25mM~35mMの酢酸塩の近似最終酢酸塩値を有していた。
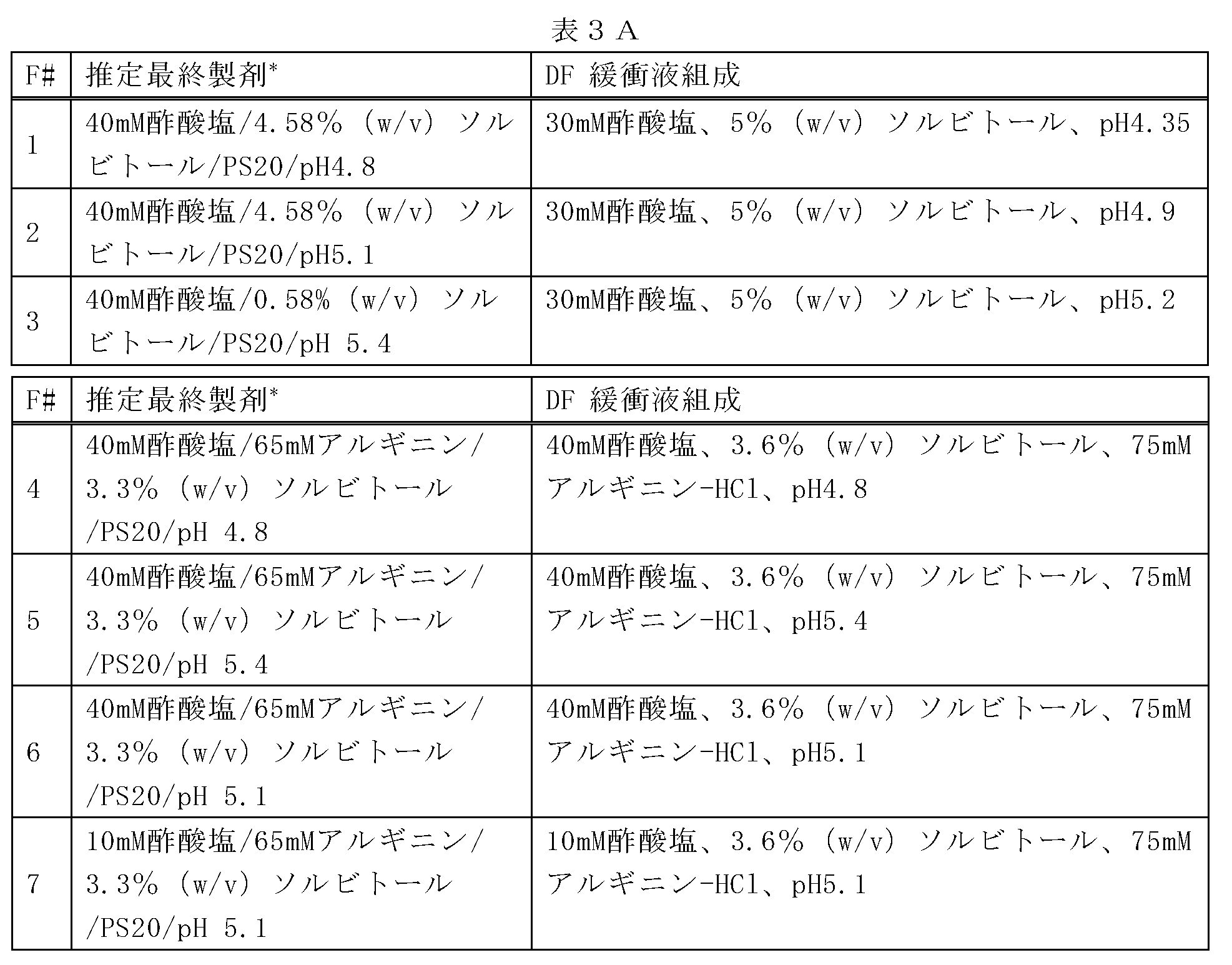
10mMの酢酸塩、75mMのL-アルギニン、2.4%(w/v)のソルビトール、0.01%(w/v)のポリソルベート20の添加剤の製剤、及び10mMの酢酸塩、5%(w/v)のソルビトール、0.01%(w/v)のポリソルベート20の添加剤の製剤で、それぞれは高濃度(120mg/mL)のデノスマブを有する製剤を、37℃において温度にて最大1ヶ月で評価し、HMWS形成の速度及び程度に対するpH及びアミノ酸凝集阻害剤の効果を明らかにした。試験された製剤は、以下の表2に記載されている。引用された全ての緩衝液及び添加剤の値は、抗体が透析ろ過される緩衝液及び添加剤の濃度に関するものである。
この実施例は、高濃度デノスマブ製剤についてのpHの効果を実証する。
この実施例は、種々のデノスマブ濃度を含む異なるデノスマブ製剤についてのpHとHMWS形成との間の関係を実証する。
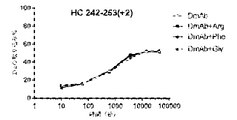
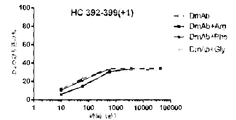
アルギニン、NAR、及びアルギニン-アルギニン(Arg-Arg)及びアルギニン-フェニルアラニン(Arg-Phe)からなる2種のジペプチドの種々の濃度の製剤を、デノスマブ濃度が120mg/mLの溶液に対する安定化効果について評価した。
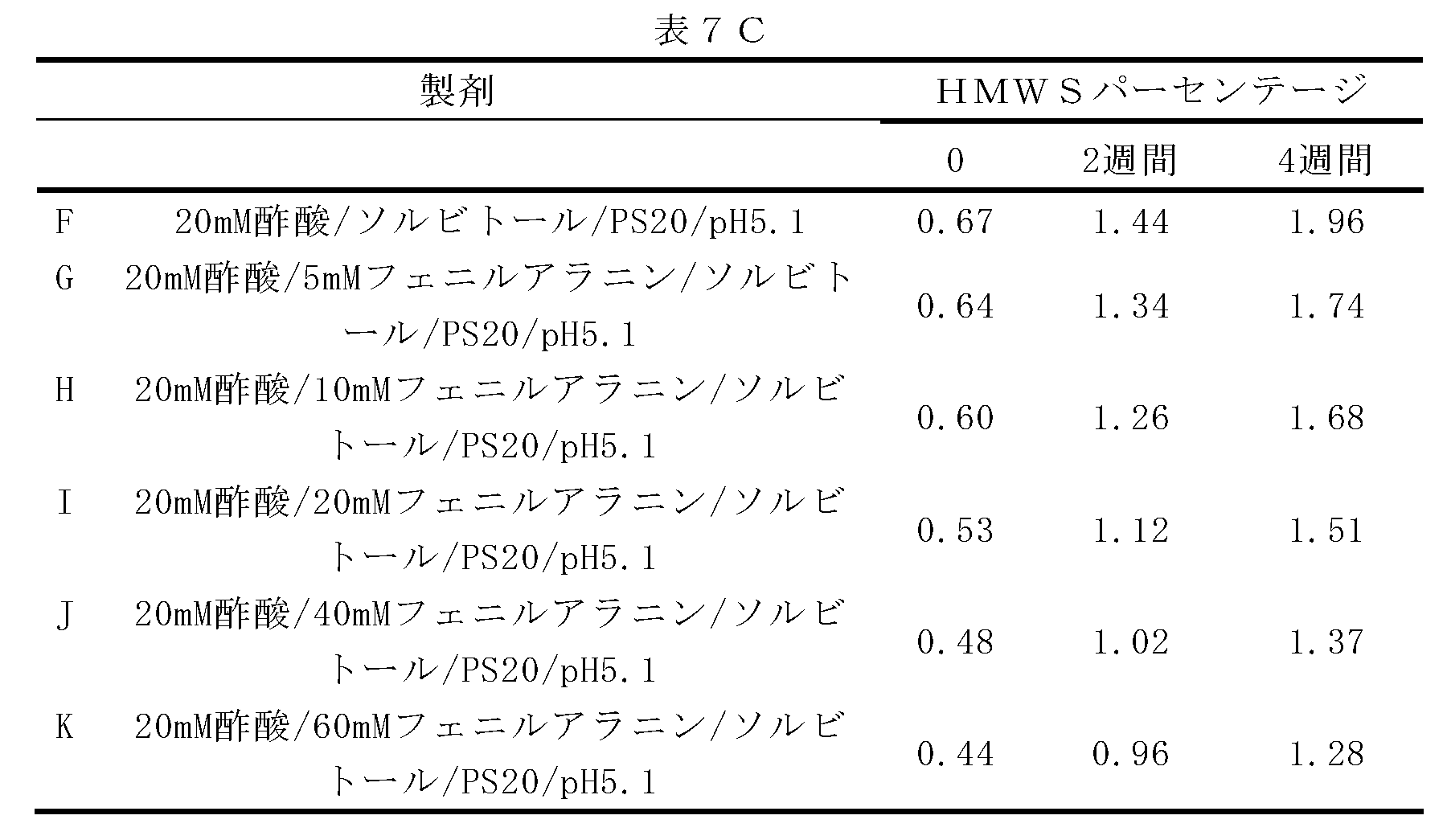
この実施例は、異なる濃度のアルギニンとフェニルアラニン、ならびにアルギニンとフェニルアラニンの比較混合物の関数としてのデノスマブの凝集阻害及び安定性を実証する。
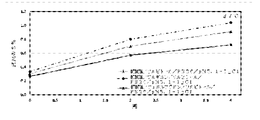
この実施例は、フェニルアラニンの異なる濃度の関数としてのデノスマブの凝集阻害及び安定性を実証する。
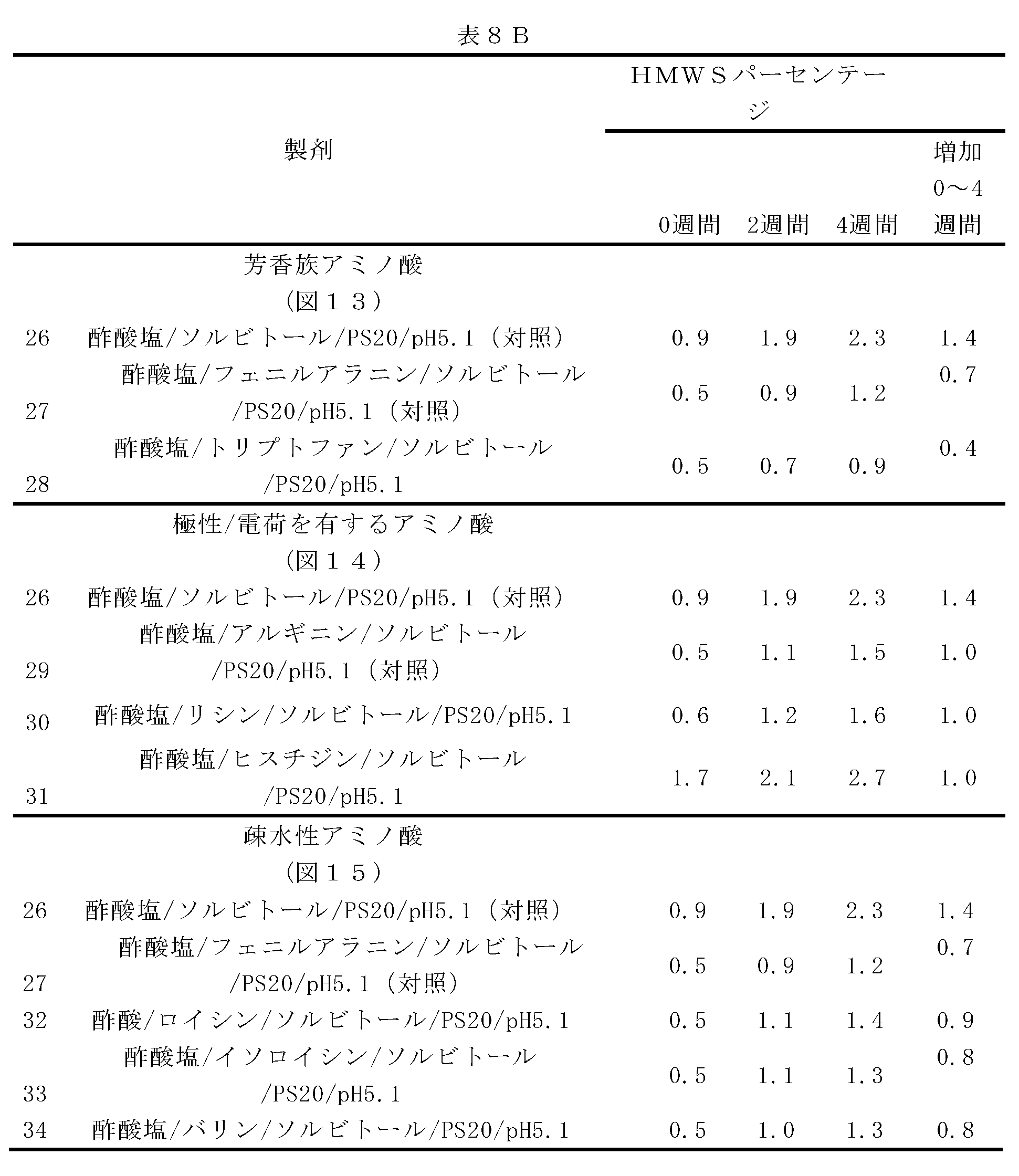
この実施例は、種々のアミノ酸凝集阻害剤の評価を実証する。
I.芳香族アミノ酸:
(a)38mMのフェニルアラニン(製剤27)、
(b)38mMのトリプトファン(製剤28)、
II.極性/電荷を有するアミノ酸:
(a)75mMのアルギニンHCl(製剤29)、
(b)75mMのリシン(製剤30)、
(c)75mMのヒスチジン(製剤31)、
III.疎水性アミノ酸:
(a)38mMのロイシン(製剤32)、
(b)38mMのイソロイシン(製剤33)、
(c)38mMのバリン(製剤34)。
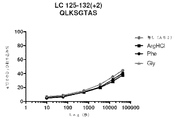
この実施例は、デノスマブの安定化におけるアルギニン及びフェニルアラニンの可能性のある作用メカニズムを実証する。重水素交換質量分析法(HDX-MS)は、タンパク質-タンパク質/リガンド/添加剤の相互作用を特徴付けるための感度が高く堅固な技術である。この方法は、添加剤との相互作用による骨格アミド水素結合の変化を検出する。
この実施例は、フェニルアラニン安定化デノスマブの作用の可能性のあるメカニズムを実証する。
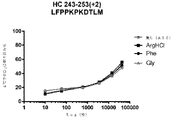
(アイソタイプIgG1、IgG2、及びIgG4の)抗RANKL抗体の複数の構築物の安定性評価を行った。上記のように、アルギニンHCl及びフェニルアラニンの両方は、デノスマブ(IgG2免疫グロブリンである)の酢酸塩/ソルビトール対照製剤と比較して、HMWSの開始及びHWMSの経時的レベルを最小にする。この評価は、異なる抗RANKL抗体構築物を含有する製剤中のHMWSを低下させるArg-HCl及びPheの可能性を比較するために行った。この研究で試験したIgG1及びIgG4構築物は、デノスマブと比較して同じ相補性決定領域(CDR)を含有していたが、異なる定常ドメイン足場も含んでいた。この研究において試験された異なるIgG2構築物は、デノスマブとは異なるCDRを有していたが、同じ定常ドメイン足場を含んでいた。
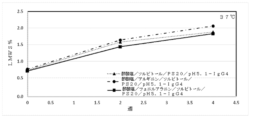
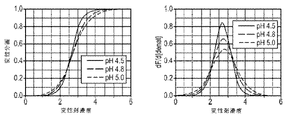
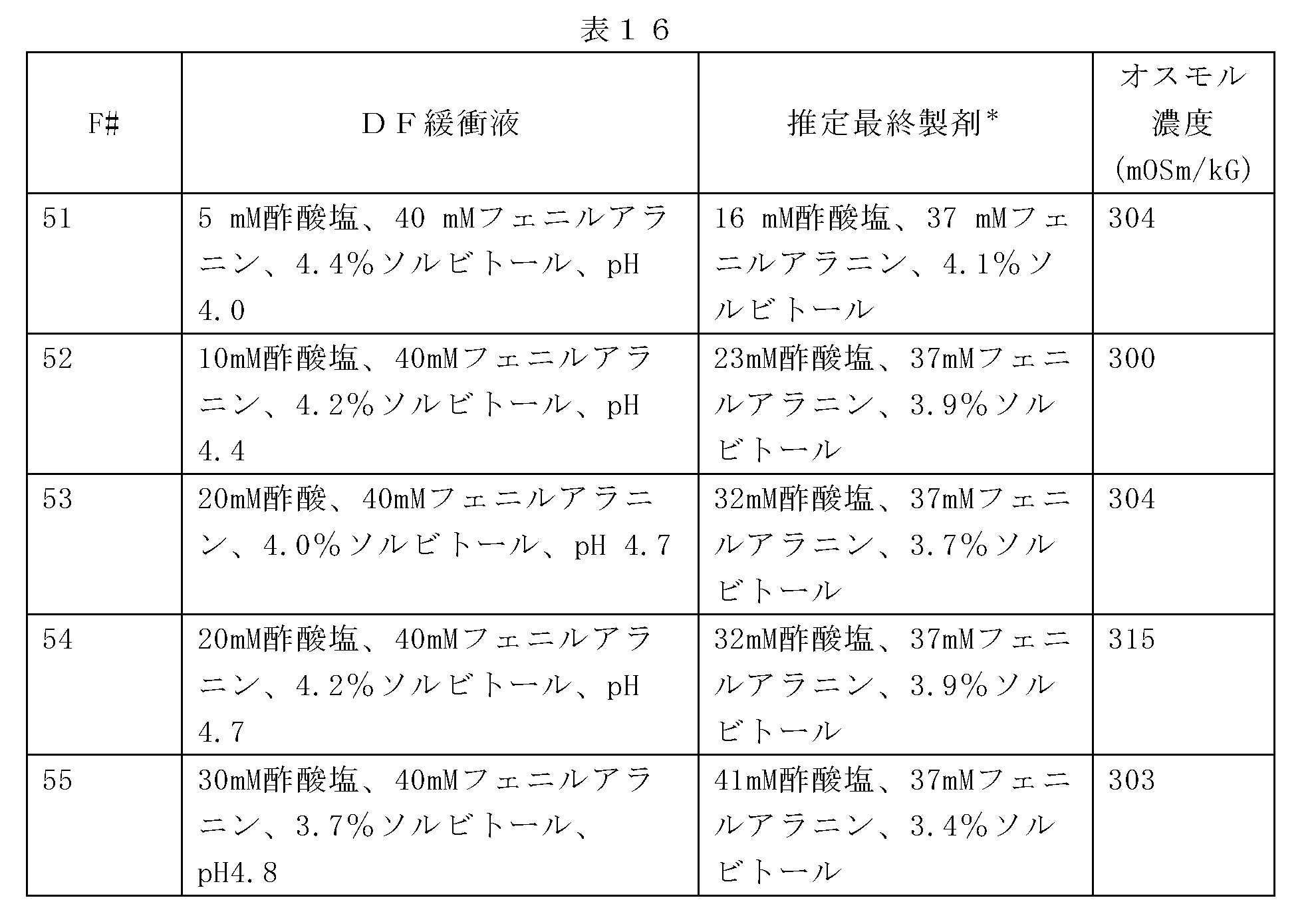
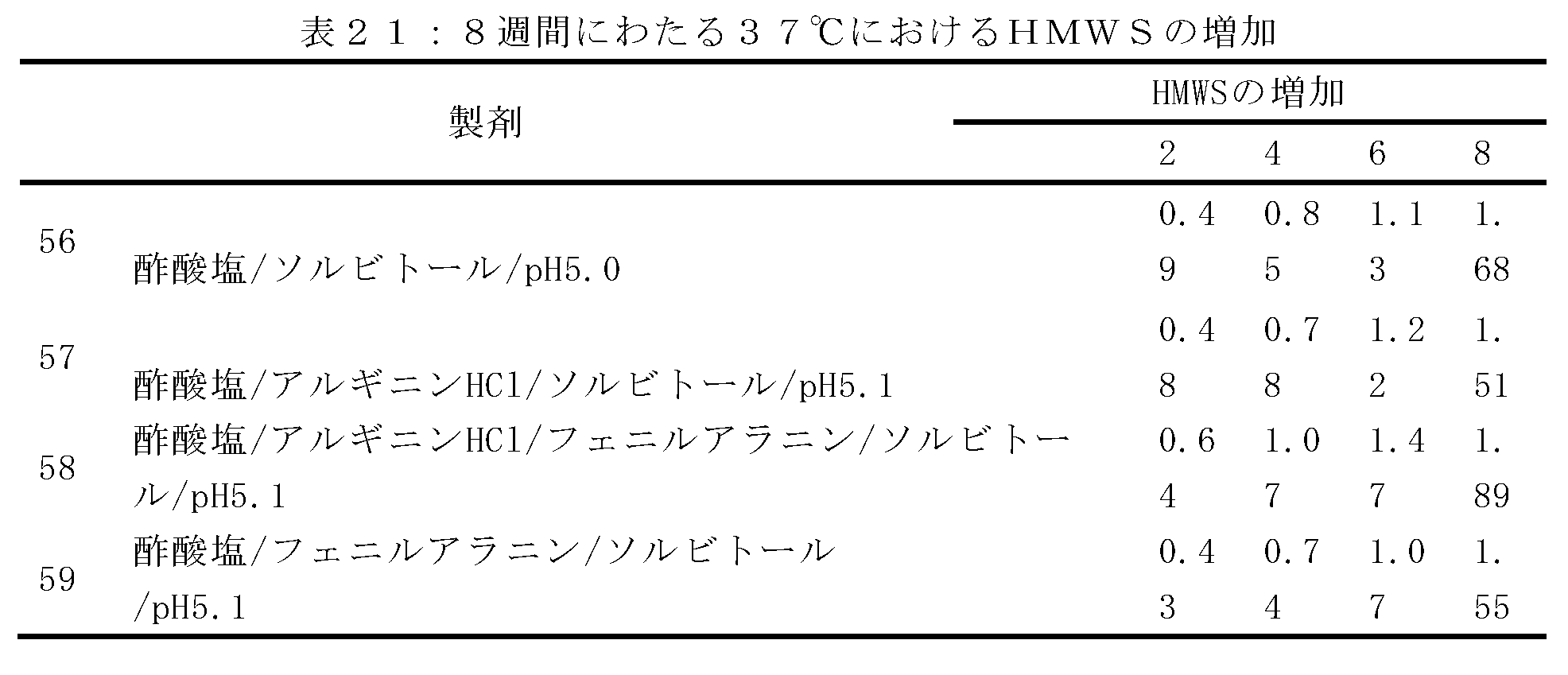
以下に記載のように、表16に関連して製剤化されたデノスマブ(製剤51~55)の安定性をモニターするために研究が行われる。透析ろ過緩衝液は、酢酸塩濃度及び開始pHが異なり、120mg/mlのデノスマブ濃度でpH5.1を有する最終製剤を産生する。さらに、ソルビトールレベルは、最終生成物の等張性を維持するように調節される(約300mOsm/Kg)。70mg/mLのデノスマブを、7倍を超えた透析体積で各緩衝液に対して透析ろ過し、次いで、限外ろ過して約180gm/mLとし、透析ろ過緩衝液及びポリソルベートで希釈して、120mg/mLのデノスマブ濃度及び0.01%のポリソルベート20とする。安定性が、37℃で保存後、SE-UHPLCを用いて評価され、これらの製剤におけるデノスマブ安定性は非常に類似していることを示す。初期HMW種は、最初のアセテート濃度が増加するにつれてわずかに減少する。対照的に、凝集速度は、酢酸塩のレベルが低い製剤でわずかに改善する。
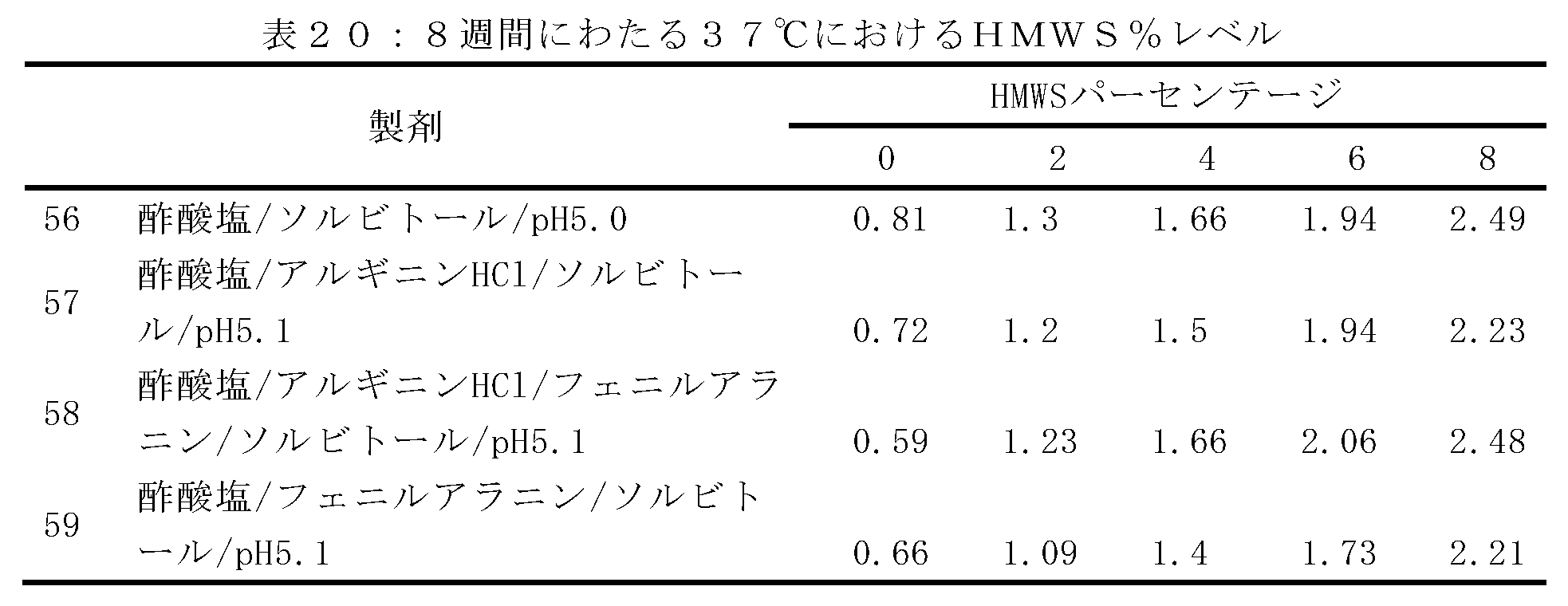
以下の実施例は、3つの異なるpH値:4.5、4.8及び5(または5.2)におけるデノスマブの化学的変性安定性に対するアルギニンの影響に関する研究の結果を報告する。
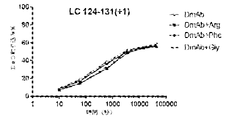
以下の実施例は、シリンジ中の高濃度デノスマブ製剤の経時安定性に対するアルギニン及びフェニルアラニンの効果に関する研究の結果を提供する。
Claims (44)
- (i)ヒト抗ヒト核因子カッパ-B受容体活性化剤リガンド(抗RANKL)モノクローナル抗体又はその抗原結合部分、及び(ii)フェニルアラニン又はトリプトファンを含む水性医薬製剤。
- L-フェニルアラニン又はL-トリプトファンを含む、請求項1に記載の水性医薬製剤。
- 5mM~300mMの前記フェニルアラニン又はトリプトファンを含む、請求項1に記載の水性医薬製剤。
- 5mM~180mMのフェニルアラニン又はトリプトファンを含む、請求項3に記載の水性医薬製剤。
- 5mM~100mMのフェニルアラニン又はトリプトファンを含む、請求項4に記載の水性医薬製剤。
- (i)フェニルアラニン又はトリプトファンのただ1つを含む、(ii)前記フェニルアラニン又はトリプトファンの前記抗RANKL抗体に対するモル比が10~200である、及び/又は(iii)さらに、張性調節剤を含む、請求項1に記載の水性医薬製剤。
- 張性調節剤が、ソルビトール、マンニトール、スクロース、トレハロース、グリセロール、及びこれらの組み合わせからなる群から選択される、請求項6に記載の水性医薬製剤。
- 5.0~5.4の範囲のpHを有する、請求項1に記載の水性医薬製剤。
- (i)5℃で6cP以下の粘度を有する、(ii)500μS/cm~2000μS/cmの導電率を有する、(iii)200mOsm/kg~500mOsm/kgの範囲のオスモル濃度を有する、及び/又は(iv)2℃~8℃で、少なくとも12ヶ月間、24ヶ月間、又は36ヶ月間保存した後、SE-UHPLCによって測定して、2%未満の高分子量種(HMWS)及び/又は98%超の抗体主ピークを含む、請求項1に記載の水性医薬製剤。
- 前記粘度が4.5cP~5.5cPである、請求項9に記載の水性医薬製剤。
- 25mM~90mMのフェニルアラニン又はトリプトファンを含む、請求項5に記載の水性医薬製剤。
- フェニルアラニンを含む、請求項4、5又は11に記載の水性医薬製剤。
- 20mM~50mMのフェニルアラニン又はトリプトファンを含む、請求項11に記載の水性医薬製剤。
- pHが5.0から5.2である、請求項8に記載の水性医薬製剤。
- オスモル濃度が225mOsm/kg~400mOsm/kgである、請求項9に記載の水性医薬製剤。
- オスモル濃度が250mOsm/kg~350mOsm/kgである、請求項15に記載の水性医薬製剤。
- 20℃~30℃で、1ヶ月間保存した後、SE-UHPLCによって測定して、2%未満の高分子量種(HMWS)及び/又は98%超の抗体主ピークを含む、請求項1に記載の水性医薬製剤。
- 2℃~8℃での、少なくとも12ヶ月間、24ヶ月間、又は36ヶ月間の保存及び、20℃~30℃での1ヶ月間の第2の保存の後、SE-UHPLCによって測定して、2%未満の高分子量種(HMWS)及び/又は98%超の抗体主ピークを含む、請求項1に記載の水性医薬製剤。
- 37℃で1ヶ月、又は30℃で3ヶ月保存した後、SE-UHPLCによって測定して、2%未満の高分子量種(HMWS)及び/又は98%超の抗体主ピークを含む、請求項1に記載の水性医薬製剤。
- 前記抗体又はその抗原結合部分の濃度が、70mg/mLより大きい、請求項1に記載の水性医薬製剤。
- 前記抗体又はその抗原結合部分の濃度が、300mg/mLより小さい、請求項20に記載の水性医薬製剤。
- 前記抗体又はその抗原結合部分の濃度が、200mg/mLより小さい、請求項21に記載の水性医薬製剤。
- 前記抗体又はその抗原結合部分の濃度が、75mg/mL~200mg/mLである、請求項22に記載の水性医薬製剤。
- 前記抗体又はその抗原結合部分の濃度が、100mg/mL~140mg/mLである、請求項23に記載の水性医薬製剤。
- さらに緩衝剤及び/又は界面活性剤を含む、請求項1に記載の水性医薬製剤。
- 前記緩衝剤が酢酸塩又はグルタミン酸塩である、請求項25に記載の水性医薬製剤。
- 5mM~60mMの緩衝剤を含む、請求項26に記載の水性医薬製剤。
- 16mM~41mMの酢酸塩緩衝剤を含む、請求項27に記載の水性医薬製剤。
- 前記界面活性剤がポリソルベート20である、請求項25に記載の水性医薬製剤。
- 少なくとも0.004(w/v)%の界面活性剤を含み、そして0.15(w/v)%未満の界面活性剤を含む、請求項25に記載の水性医薬製剤。
- 2.0(w/w)%~5.0(w/w)%のソルビトールを含む、請求項6に記載の水性医薬製剤。
- 前記抗RANKL抗体がIgGである、請求項1に記載の水性医薬製剤。
- 前記抗RANKL抗体がIgG2である、請求項32に記載の水性医薬製剤。
- 前記抗RANKL抗体又はその抗原結合部分が、(A)配列番号5のアミノ酸配列を含む軽鎖CDR1を含む軽鎖可変ドメイン、配列番号6のアミノ酸配列を含む軽鎖CDR2を含む軽鎖可変ドメイン、及び配列番号7のアミノ酸配列を含む軽鎖CDR3を含む軽鎖可変ドメイン、及び(B)配列番号8のアミノ酸配列を含む重鎖CDR1を含む重鎖可変ドメイン、配列番号9のアミノ酸配列を含む重鎖CDR2を含む重鎖可変ドメイン、及び配列番号10のアミノ酸配列を含む重鎖CDR3を含む重鎖可変ドメイン、を含む、請求項1に記載の水性医薬製剤。
- 前記抗RANKL抗体又はその抗原結合部分が、配列番号1の軽鎖可変領域及び配列番号2の重鎖可変領域を含む、請求項34に記載の水性医薬製剤。
- 前記抗RANKL抗体又はその抗原結合部分が、配列番号13の軽鎖及び配列番号14の重鎖を含む、請求項35に記載の水性医薬製剤。
- 20mM~50mMのL-フェニルアラニン又はL-トリプトファンを含む、請求項13に記載の水性医薬製剤。
- 請求項1に記載の水性医薬製剤を含有する容器。
- バイアル、プレフィルドシリンジ(PFS)、又はガラス容器である、請求項38に記載の容器。
- 対象における、骨関連事象(SRE)、骨の巨細胞腫、高カルシウム血症、若しくは骨粗鬆症の治療、又は骨量の増加のための、請求項1に記載の水性医薬製剤。
- (a)固形腫瘍からの骨転移を有する対象におけるSREの治療、
(b)切除不能であるか、又は外科的切除が重度の罹患率を生じる可能性がある、骨の巨細胞腫を有する成人又は骨格の成熟した青年である対象におけるSREの治療、
(c)対象におけるビスホスホネート治療に難治性である悪性腫瘍の高カルシウム血症の治療、
(d)多発性骨髄腫、又は固形腫瘍由来の骨転移を有する対象におけるSREの治療、
(e)骨折の危険性が高い閉経後女性の骨粗鬆症の治療、
(f)乳癌のアジュバントアロマターゼ阻害剤療法を受けている骨折の危険性が高い女性の骨量を増加させる治療、
(g)非転移性前立腺癌のアンドロゲン除去療法を受けている骨折のリスクが高い男性の骨量を増加させる治療、
(h)骨折の危険性が高い骨粗鬆症を有する男性の骨量を増加させるための治療、及び/又は
(i)カルシウム又はビタミンDによる療法、
のための、請求項40に記載の水性医薬製剤。 - ヒト抗ヒト核因子カッパ-B受容体活性化剤リガンド(抗RANKL)モノクローナル抗体又はその抗原結合部分を含む安定な水性医薬製剤を作製する方法であって、前記抗RANKLモノクローナル抗体、又はその抗原結合部分を70mg/mLを超える濃度で、フェニルアラニン又はトリプトファン、緩衝剤、及び界面活性剤と混合することを含む、方法。
- 前記抗RANKLモノクローナル抗体、又はその抗原結合部分を70mg/mLを超える濃度で、フェニルアラニン又はトリプトファン、緩衝剤、界面活性剤、及び張性調節剤と混合することを含む、請求項42に記載の方法。
- ヒト抗ヒト核因子カッパ-B受容体活性化剤リガンド(抗RANKL)モノクローナル抗体又はその抗原結合部分を70mg/mLを超える濃度で含む水性医薬製剤の安定性を改善する方法であって、
ヒト抗ヒト核因子カッパ-B受容体活性化剤リガンド(抗RANKL)モノクローナル抗体又はその抗原結合部分を含む前記水性医薬製剤を5.0から5.2未満の範囲のpHで調製すること、又はフェニルアラニン又はトリプトファンと混合した、ヒト抗ヒト核因子カッパ-B受容体活性化剤リガンド(抗RANKL)モノクローナル抗体又はその抗原結合部分を含む前記水性医薬製剤を調製することを含み、
前記水性医薬製剤が、5.0から5.2未満の範囲のpHでない同等の水性医薬製剤と比較して、5.0から5.2未満の範囲のpHで改善された安定性を示すか、又は、前記水性医薬製剤が、フェニルアラニン又はトリプトファンを含まない同等の水性医薬製剤と比較して、フェニルアラニン又はトリプトファンによる改善された安定性を示す、前記方法。
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2022004150A JP7356525B2 (ja) | 2017-04-28 | 2022-01-14 | ヒト抗rankl抗体の製剤及びその使用方法 |
| JP2023130715A JP2023166396A (ja) | 2017-04-28 | 2023-08-10 | ヒト抗rankl抗体の製剤及びその使用方法 |
| JP2025023142A JP2025093923A (ja) | 2017-04-28 | 2025-02-17 | ヒト抗rankl抗体の製剤及びその使用方法 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762492056P | 2017-04-28 | 2017-04-28 | |
| US62/492,056 | 2017-04-28 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2022004150A Division JP7356525B2 (ja) | 2017-04-28 | 2022-01-14 | ヒト抗rankl抗体の製剤及びその使用方法 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2018188430A JP2018188430A (ja) | 2018-11-29 |
| JP2018188430A5 JP2018188430A5 (ja) | 2022-07-01 |
| JP7190822B2 true JP7190822B2 (ja) | 2022-12-16 |
Family
ID=62165668
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018086261A Active JP7190822B2 (ja) | 2017-04-28 | 2018-04-27 | ヒト抗rankl抗体の製剤及びその使用方法 |
| JP2022004150A Active JP7356525B2 (ja) | 2017-04-28 | 2022-01-14 | ヒト抗rankl抗体の製剤及びその使用方法 |
| JP2023130715A Pending JP2023166396A (ja) | 2017-04-28 | 2023-08-10 | ヒト抗rankl抗体の製剤及びその使用方法 |
| JP2025023142A Pending JP2025093923A (ja) | 2017-04-28 | 2025-02-17 | ヒト抗rankl抗体の製剤及びその使用方法 |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2022004150A Active JP7356525B2 (ja) | 2017-04-28 | 2022-01-14 | ヒト抗rankl抗体の製剤及びその使用方法 |
| JP2023130715A Pending JP2023166396A (ja) | 2017-04-28 | 2023-08-10 | ヒト抗rankl抗体の製剤及びその使用方法 |
| JP2025023142A Pending JP2025093923A (ja) | 2017-04-28 | 2025-02-17 | ヒト抗rankl抗体の製剤及びその使用方法 |
Country Status (37)
| Country | Link |
|---|---|
| US (3) | US11192952B2 (ja) |
| EP (3) | EP4477667A3 (ja) |
| JP (4) | JP7190822B2 (ja) |
| KR (2) | KR20240110083A (ja) |
| CN (2) | CN118987200A (ja) |
| AR (1) | AR111497A1 (ja) |
| AU (2) | AU2018260750B2 (ja) |
| BR (1) | BR112019022188A2 (ja) |
| CA (1) | CA3054165A1 (ja) |
| CL (3) | CL2019003032A1 (ja) |
| CO (1) | CO2019011463A2 (ja) |
| CR (2) | CR20190538A (ja) |
| DK (2) | DK4190355T3 (ja) |
| EA (1) | EA201992570A1 (ja) |
| ES (2) | ES2997833T3 (ja) |
| FI (2) | FI3615066T3 (ja) |
| HR (2) | HRP20241604T1 (ja) |
| HU (2) | HUE069382T2 (ja) |
| IL (2) | IL293127B2 (ja) |
| JO (1) | JOP20190255A1 (ja) |
| LT (2) | LT3615066T (ja) |
| MA (2) | MA62173B1 (ja) |
| MX (1) | MX2023013460A (ja) |
| MY (1) | MY202479A (ja) |
| PE (2) | PE20251534A1 (ja) |
| PH (1) | PH12019502444A1 (ja) |
| PL (2) | PL4190355T3 (ja) |
| PT (2) | PT4190355T (ja) |
| RS (2) | RS66284B1 (ja) |
| SG (1) | SG11201909998TA (ja) |
| SI (2) | SI4190355T1 (ja) |
| SM (2) | SMT202400095T1 (ja) |
| TN (2) | TN2021000055A1 (ja) |
| TW (1) | TWI839331B (ja) |
| UY (2) | UY37707A (ja) |
| WO (1) | WO2018200918A1 (ja) |
| ZA (1) | ZA202205141B (ja) |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JOP20190255A1 (ar) | 2017-04-28 | 2019-10-27 | Amgen Inc | صيغ أجسام مضادة لـ rankl بشري، وطرق لاستخدامها |
| US12257343B2 (en) | 2017-04-28 | 2025-03-25 | Amgen Inc. | Excipients to reduce the viscosity of antibody formulations and formulation compositions |
| KR20210079373A (ko) * | 2018-11-16 | 2021-06-29 | 삼성바이오에피스 주식회사 | 단백질을 포함하는 안정한 액상 조성물 |
| CN109374779A (zh) * | 2018-12-17 | 2019-02-22 | 杭州奕安济世生物药业有限公司 | 一种蔗糖含量的快速检测方法 |
| CN120241997A (zh) | 2019-02-18 | 2025-07-04 | 伊莱利利公司 | 治疗性抗体制剂 |
| JP2022548950A (ja) * | 2019-09-19 | 2022-11-22 | イノベント バイオロジクス(スーチョウ)カンパニー,リミティド | 抗pcsk9抗体を利用したコレステロール関連疾患の予防または治療方法 |
| CN111704670B (zh) * | 2020-08-18 | 2020-11-03 | 迈威(上海)生物科技股份有限公司 | 重组抗RANKL抗体IgG2型的二硫键异构体及其纯化方法 |
| KR20230118573A (ko) * | 2020-11-11 | 2023-08-11 | 에이치큐 한 | 액티빈/tgf-베타 및 rankl의 이작용성 길항제 및 이의 용도 |
| BR112023022707A2 (pt) * | 2021-05-12 | 2024-01-16 | Jiangsu Hengrui Pharmaceuticals Co Ltd | Molécula de ligação ao antígeno a qual se liga especificamente a rankl e ngf, e seu uso médico |
| UY39896A (es) | 2021-08-12 | 2023-02-28 | Amgen Inc | Formulaciones de anticuerpos |
| US20250297020A1 (en) * | 2021-11-23 | 2025-09-25 | Adalta Limited | Rank-l binding molecules |
| WO2023179791A1 (en) * | 2022-03-24 | 2023-09-28 | Angitia Biomedicines Limited | Treatment of musculoskeletal disorders |
| WO2024064878A1 (en) | 2022-09-23 | 2024-03-28 | The University Of North Carolina At Chapel Hill | Methods and compositions for the treatment of melanoma |
| WO2024104409A1 (zh) * | 2022-11-16 | 2024-05-23 | 苏州盛迪亚生物医药有限公司 | 一种含抗rankl-ngf双特异性抗体的药物组合物 |
| WO2024184280A1 (en) * | 2023-03-03 | 2024-09-12 | Alvotech Hf | Denosumab formulation |
| WO2025036493A1 (en) * | 2023-08-17 | 2025-02-20 | Sinomab Bioscience Limited | Antibodies targeting rankl and sclerostin and uses thereof |
| WO2025116648A1 (ko) * | 2023-12-01 | 2025-06-05 | 삼성바이오에피스 주식회사 | 항-IL-4Rα 항체의 안정한 완충제-프리 액상 제형 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010531306A (ja) | 2007-06-25 | 2010-09-24 | アムゲン インコーポレイティッド | 肝細胞増殖因子に対する特異的結合剤の組成物 |
| JP2011519347A (ja) | 2008-02-07 | 2011-07-07 | アムジエン・インコーポレーテツド | 安定化されたタンパク質組成物 |
| JP2014510152A (ja) | 2011-04-07 | 2014-04-24 | グラクソスミスクライン・リミテッド・ライアビリティ・カンパニー | 粘度が低減された処方物 |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DK2270052T3 (en) | 2001-06-26 | 2018-07-02 | Amgen Inc | Antibodies to OPGL |
| PE20070684A1 (es) | 2005-11-14 | 2007-08-06 | Amgen Inc | MOLECULAS QUIMERICAS DE ANTICUERPO RANKL-PTH/PTHrP |
| JP5577098B2 (ja) | 2006-12-21 | 2014-08-20 | アムジエン・インコーポレーテツド | ポリペプチドを含有する安定な緩衝化された製剤 |
| TW201019959A (en) | 2008-08-19 | 2010-06-01 | Regeneron Pharma | Human antibodies to human RANKL |
| WO2010030670A2 (en) | 2008-09-10 | 2010-03-18 | Genentech, Inc. | Compositions and methods for the prevention of oxidative degradation of proteins |
| US9265834B2 (en) | 2009-03-05 | 2016-02-23 | Ablynx N.V. | Stable formulations of polypeptides and uses thereof |
| TWI440470B (zh) | 2009-03-19 | 2014-06-11 | Chugai Pharmaceutical Co Ltd | 含改良抗體分子之醫藥配方 |
| RU2600847C2 (ru) | 2010-05-10 | 2016-10-27 | Интас Биофармасьютикалс Лимитед | Жидкий состав полипептидов, содержащих fc-домен иммуноглобулина |
| EP2433644A1 (en) | 2010-09-22 | 2012-03-28 | IMBA-Institut für Molekulare Biotechnologie GmbH | Breast cancer therapeutics |
| WO2013012022A1 (ja) | 2011-07-19 | 2013-01-24 | 中外製薬株式会社 | アルギニンアミドまたはその類似化合物を含む安定なタンパク質含有製剤 |
| AU2012328524B2 (en) | 2011-10-28 | 2017-05-18 | Excelse Bio, Inc. | Protein formulations containing amino acids |
| TWI679019B (zh) | 2013-04-29 | 2019-12-11 | 法商賽諾菲公司 | 抗il-4/抗il-13之雙特異性抗體調配物 |
| CN103965357B (zh) | 2013-12-31 | 2016-08-17 | 嘉和生物药业有限公司 | 一种抗人rankl抗体 |
| US20150225479A1 (en) | 2014-02-12 | 2015-08-13 | Sanofi | Anti-IL-4/Anti-IL-13 Bispecific Antibody/Polyglutamate Formulations |
| WO2015134406A1 (en) | 2014-03-03 | 2015-09-11 | La Jolla Biologics, Inc. | Stable aqueous recombinant protein formulations |
| EP3139960B1 (en) | 2014-05-07 | 2024-01-17 | Takeda Pharmaceutical Company Limited | Liquid formulation comprising gm-csf neutralizing compound |
| US20180000932A1 (en) | 2014-12-31 | 2018-01-04 | Novelmed Therapeutics, Inc. | Formulation of aglycosylated therapeutic antibodies |
| WO2016162819A1 (en) | 2015-04-07 | 2016-10-13 | Lupin Limited | Stable aqueous pharmaceutical composition of anti-tnf alpha antibody |
| JOP20190255A1 (ar) | 2017-04-28 | 2019-10-27 | Amgen Inc | صيغ أجسام مضادة لـ rankl بشري، وطرق لاستخدامها |
-
2017
- 2017-06-16 JO JOP/2019/0255A patent/JOP20190255A1/ar unknown
-
2018
- 2018-04-27 SM SM20240095T patent/SMT202400095T1/it unknown
- 2018-04-27 CA CA3054165A patent/CA3054165A1/en not_active Abandoned
- 2018-04-27 PT PT222067092T patent/PT4190355T/pt unknown
- 2018-04-27 AU AU2018260750A patent/AU2018260750B2/en active Active
- 2018-04-27 PT PT187246905T patent/PT3615066T/pt unknown
- 2018-04-27 UY UY0001037707A patent/UY37707A/es active IP Right Grant
- 2018-04-27 EA EA201992570A patent/EA201992570A1/ru unknown
- 2018-04-27 ES ES22206709T patent/ES2997833T3/es active Active
- 2018-04-27 MY MYPI2019006326A patent/MY202479A/en unknown
- 2018-04-27 HU HUE22206709A patent/HUE069382T2/hu unknown
- 2018-04-27 SM SM20240491T patent/SMT202400491T1/it unknown
- 2018-04-27 RS RS20241343A patent/RS66284B1/sr unknown
- 2018-04-27 HR HRP20241604TT patent/HRP20241604T1/hr unknown
- 2018-04-27 EP EP24194699.5A patent/EP4477667A3/en active Pending
- 2018-04-27 KR KR1020247021825A patent/KR20240110083A/ko active Pending
- 2018-04-27 HU HUE18724690A patent/HUE065544T2/hu unknown
- 2018-04-27 EP EP18724690.5A patent/EP3615066B1/en active Active
- 2018-04-27 TW TW107114370A patent/TWI839331B/zh active
- 2018-04-27 HR HRP20240167TT patent/HRP20240167T1/hr unknown
- 2018-04-27 CR CR20190538A patent/CR20190538A/es unknown
- 2018-04-27 PE PE2024002513A patent/PE20251534A1/es unknown
- 2018-04-27 TN TNP/2021/000055A patent/TN2021000055A1/en unknown
- 2018-04-27 JP JP2018086261A patent/JP7190822B2/ja active Active
- 2018-04-27 KR KR1020197034225A patent/KR102681171B1/ko active Active
- 2018-04-27 EP EP22206709.2A patent/EP4190355B1/en active Active
- 2018-04-27 MA MA62173A patent/MA62173B1/fr unknown
- 2018-04-27 AR ARP180101105A patent/AR111497A1/es unknown
- 2018-04-27 SG SG11201909998T patent/SG11201909998TA/en unknown
- 2018-04-27 FI FIEP18724690.5T patent/FI3615066T3/fi active
- 2018-04-27 UY UY0001040974A patent/UY40974A/es unknown
- 2018-04-27 PE PE2019002120A patent/PE20200343A1/es unknown
- 2018-04-27 SI SI201831178T patent/SI4190355T1/sl unknown
- 2018-04-27 TN TNP/2019/000297A patent/TN2019000297A1/en unknown
- 2018-04-27 FI FIEP22206709.2T patent/FI4190355T3/fi active
- 2018-04-27 CN CN202411089892.7A patent/CN118987200A/zh active Pending
- 2018-04-27 MA MA48462A patent/MA48462B1/fr unknown
- 2018-04-27 PL PL22206709.2T patent/PL4190355T3/pl unknown
- 2018-04-27 WO PCT/US2018/029728 patent/WO2018200918A1/en not_active Ceased
- 2018-04-27 LT LTEPPCT/US2018/029728T patent/LT3615066T/lt unknown
- 2018-04-27 CR CR20250034A patent/CR20250034A/es unknown
- 2018-04-27 IL IL293127A patent/IL293127B2/en unknown
- 2018-04-27 SI SI201831067T patent/SI3615066T1/sl unknown
- 2018-04-27 LT LTEP22206709.2T patent/LT4190355T/lt unknown
- 2018-04-27 US US16/608,375 patent/US11192952B2/en active Active
- 2018-04-27 PL PL18724690.5T patent/PL3615066T3/pl unknown
- 2018-04-27 DK DK22206709.2T patent/DK4190355T3/da active
- 2018-04-27 RS RS20240313A patent/RS65299B1/sr unknown
- 2018-04-27 CN CN201880028012.1A patent/CN110621342B/zh active Active
- 2018-04-27 BR BR112019022188-3A patent/BR112019022188A2/pt unknown
- 2018-04-27 ES ES18724690T patent/ES2973572T3/es active Active
- 2018-04-27 DK DK18724690.5T patent/DK3615066T5/da active
-
2019
- 2019-08-14 IL IL268704A patent/IL268704B/en unknown
- 2019-10-16 CO CO2019011463A patent/CO2019011463A2/es unknown
- 2019-10-21 MX MX2023013460A patent/MX2023013460A/es unknown
- 2019-10-23 CL CL2019003032A patent/CL2019003032A1/es unknown
- 2019-10-28 PH PH12019502444A patent/PH12019502444A1/en unknown
-
2020
- 2020-07-31 CL CL2020002012A patent/CL2020002012A1/es unknown
-
2021
- 2021-11-18 US US17/529,794 patent/US11873343B2/en active Active
-
2022
- 2022-01-14 JP JP2022004150A patent/JP7356525B2/ja active Active
- 2022-05-10 ZA ZA2022/05141A patent/ZA202205141B/en unknown
-
2023
- 2023-08-10 JP JP2023130715A patent/JP2023166396A/ja active Pending
- 2023-08-24 CL CL2023002505A patent/CL2023002505A1/es unknown
- 2023-12-11 US US18/535,068 patent/US20250326853A1/en active Pending
-
2025
- 2025-02-17 JP JP2025023142A patent/JP2025093923A/ja active Pending
- 2025-07-15 AU AU2025205470A patent/AU2025205470A1/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010531306A (ja) | 2007-06-25 | 2010-09-24 | アムゲン インコーポレイティッド | 肝細胞増殖因子に対する特異的結合剤の組成物 |
| JP2011519347A (ja) | 2008-02-07 | 2011-07-07 | アムジエン・インコーポレーテツド | 安定化されたタンパク質組成物 |
| JP2014510152A (ja) | 2011-04-07 | 2014-04-24 | グラクソスミスクライン・リミテッド・ライアビリティ・カンパニー | 粘度が低減された処方物 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7190822B2 (ja) | ヒト抗rankl抗体の製剤及びその使用方法 | |
| US11612659B2 (en) | Anti-CD40 antibody formulation delivery device | |
| US12065502B2 (en) | Bispecific antibody formulation | |
| WO2018179138A1 (ja) | 抗体含有液体製剤 | |
| HK40119362A (en) | Formulations of human anti-rankl antibodies, and methods of using the same | |
| HK40092695B (en) | Formulations of human anti-rankl antibodies, and methods of using the same | |
| HK40092695A (en) | Formulations of human anti-rankl antibodies, and methods of using the same | |
| EA050520B1 (ru) | Составы на основе человеческих антител к rankl, а также способы их применения | |
| HK40017180B (zh) | 人类抗rankl抗体的配制品及其使用方法 | |
| HK40017180A (en) | Formulations of human anti-rankl antibodies, and methods of using the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210419 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20210419 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20220114 |
|
| A871 | Explanation of circumstances concerning accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A871 Effective date: 20220114 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20220329 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20220623 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20220705 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20221102 |
|
| C60 | Trial request (containing other claim documents, opposition documents) |
Free format text: JAPANESE INTERMEDIATE CODE: C60 Effective date: 20221102 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20221111 |
|
| C21 | Notice of transfer of a case for reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C21 Effective date: 20221115 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20221129 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20221206 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7190822 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |