JP7161237B2 - 丹参(Salvia miltiorrhiza Bunge)抽出物を有効成分として含む前立腺肥大症又は脱毛症の治療又は予防用組成物 - Google Patents
丹参(Salvia miltiorrhiza Bunge)抽出物を有効成分として含む前立腺肥大症又は脱毛症の治療又は予防用組成物 Download PDFInfo
- Publication number
- JP7161237B2 JP7161237B2 JP2020567509A JP2020567509A JP7161237B2 JP 7161237 B2 JP7161237 B2 JP 7161237B2 JP 2020567509 A JP2020567509 A JP 2020567509A JP 2020567509 A JP2020567509 A JP 2020567509A JP 7161237 B2 JP7161237 B2 JP 7161237B2
- Authority
- JP
- Japan
- Prior art keywords
- extract
- danshen
- prostatic hyperplasia
- benign prostatic
- alopecia
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q7/00—Preparations for affecting hair growth
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/53—Lamiaceae or Labiatae (Mint family), e.g. thyme, rosemary or lavender
- A61K36/537—Salvia (sage)
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/105—Plant extracts, their artificial duplicates or their derivatives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/40—Complete food formulations for specific consumer groups or specific purposes, e.g. infant formula
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/34—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having five-membered rings with one oxygen as the only ring hetero atom, e.g. isosorbide
- A61K31/343—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having five-membered rings with one oxygen as the only ring hetero atom, e.g. isosorbide condensed with a carbocyclic ring, e.g. coumaran, bufuralol, befunolol, clobenfurol, amiodarone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/58—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids containing heterocyclic rings, e.g. danazol, stanozolol, pancuronium or digitogenin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
- A61K8/9783—Angiosperms [Magnoliophyta]
- A61K8/9789—Magnoliopsida [dicotyledons]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
- A61K9/0056—Mouth soluble or dispersible forms; Suckable, eatable, chewable coherent forms; Forms rapidly disintegrating in the mouth; Lozenges; Lollipops; Bite capsules; Baked products; Baits or other oral forms for animals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/14—Drugs for dermatological disorders for baldness or alopecia
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2200/00—Function of food ingredients
- A23V2200/30—Foods, ingredients or supplements having a functional effect on health
- A23V2200/318—Foods, ingredients or supplements having a functional effect on health having an effect on skin health and hair or coat
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2236/00—Isolation or extraction methods of medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2236/00—Isolation or extraction methods of medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicine
- A61K2236/10—Preparation or pretreatment of starting material
- A61K2236/15—Preparation or pretreatment of starting material involving mechanical treatment, e.g. chopping up, cutting or grinding
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2236/00—Isolation or extraction methods of medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicine
- A61K2236/30—Extraction of the material
- A61K2236/33—Extraction of the material involving extraction with hydrophilic solvents, e.g. lower alcohols, esters or ketones
- A61K2236/331—Extraction of the material involving extraction with hydrophilic solvents, e.g. lower alcohols, esters or ketones using water, e.g. cold water, infusion, tea, steam distillation or decoction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2236/00—Isolation or extraction methods of medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicine
- A61K2236/30—Extraction of the material
- A61K2236/33—Extraction of the material involving extraction with hydrophilic solvents, e.g. lower alcohols, esters or ketones
- A61K2236/333—Extraction of the material involving extraction with hydrophilic solvents, e.g. lower alcohols, esters or ketones using mixed solvents, e.g. 70% EtOH
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2236/00—Isolation or extraction methods of medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicine
- A61K2236/50—Methods involving additional extraction steps
- A61K2236/51—Concentration or drying of the extract, e.g. Lyophilisation, freeze-drying or spray-drying
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Mycology (AREA)
- Botany (AREA)
- Nutrition Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Polymers & Plastics (AREA)
- Food Science & Technology (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Urology & Nephrology (AREA)
- Alternative & Traditional Medicine (AREA)
- Medical Informatics (AREA)
- Dermatology (AREA)
- Pediatric Medicine (AREA)
- Zoology (AREA)
- Physiology (AREA)
- Birds (AREA)
- Medicines Containing Plant Substances (AREA)
- Inorganic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
- Cosmetics (AREA)
Description
前記丹参(Salvia miltiorrhiza Bunge)抽出物は、40~99%エタノール抽出物であり得る。
前記で丹参抽出物は、メチレンクロリドを含む有機溶媒で分別抽出でき、カラムを用いて特定成分を濃縮することができる。
前記丹参(Salvia miltiorrhiza Bunge)抽出物は、タンシノン類が全重量の2%~70%で含まれたものであり得る。
本発明は、前記丹参(Salvia miltiorrhiza Bunge)抽出物が、
不純物を除去した丹参を破砕させる段階(1);
前記(1)段階の破砕した丹参を50~80℃で40~99%の酒精(EtOH)に浸漬させ、2~24時間放置する段階(2);
前記(2)~(4)段階で分離しておいた酒精を集めて45~75℃で蒸発、乾燥させる段階(5);
1.1.丹参抽出物Sの製造
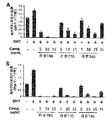
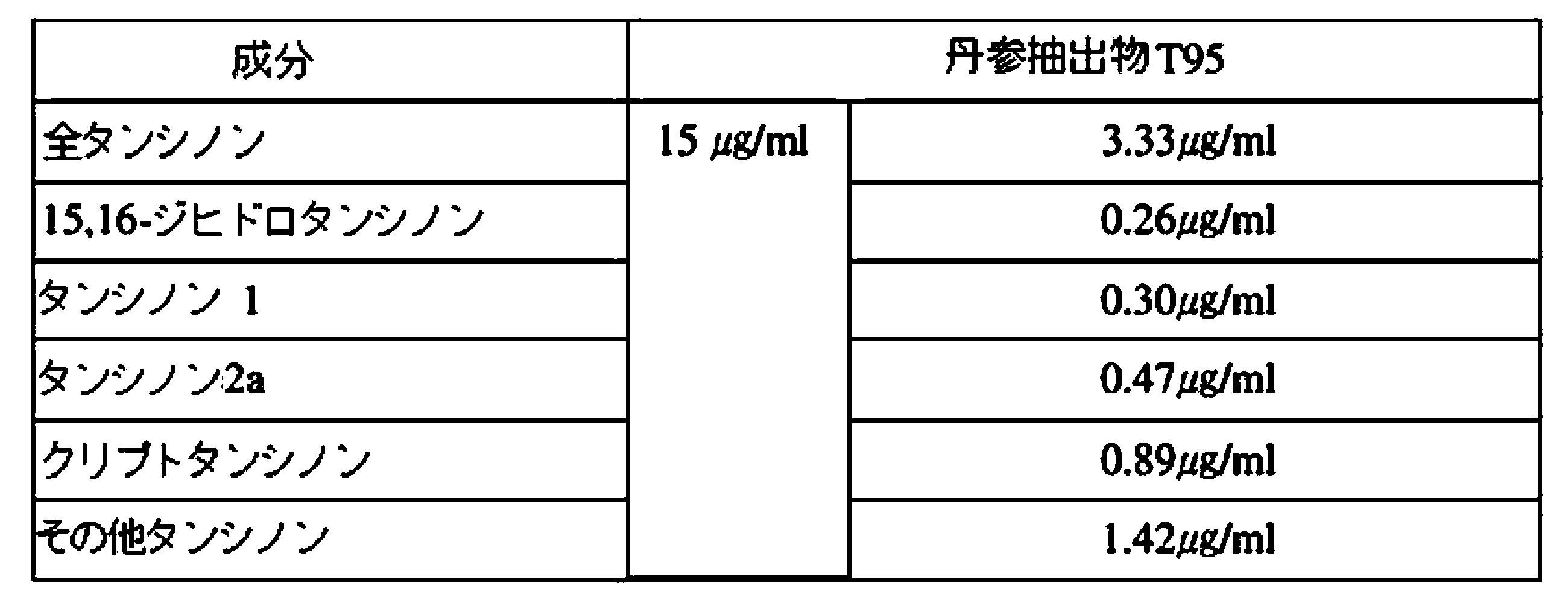
丹参抽出物Tは、破砕させた丹参根を50~80℃で酒精(EtOH)で抽出した(丹参抽出物T)。また、抽出酒精の濃度による効果を確認するために、酒精の濃度を異にして丹参抽出物T10(EtOH10%)、T40(EtOH40%)、T70(EtOH70%)、T95(EtOH95%)、T99(EtOH99%)をそれぞれ得た。前記酒精で2~24時間抽出し、必要によって新しい酒精でさらに2~12時間抽出した後、必要によって新しい酒精で2~5時間さらに抽出して遠心分離し、上澄液を得た。その後、得られた上澄液を集めて45~75℃で蒸発、乾燥させた後に粉砕し、80mesh以上でフィルタリングして準備した。
前記実施例1.1及び1.2で抽出した丹参抽出物S及び丹参抽出物Tの成分及び含有量を測定した。特に、丹参に含まれている主要タンシノン4種成分を設定するために、既存研究者によって明らかにされた様々な化合物のうち、標準品が販売されているタンシノン1、タンシノン2a、クリプトタンシノン、ジヒドロタンシノン及びサルビアノール酸Bなどの成分が丹参抽出物に存在するか否かをHPLC分析で確認した。HPLC条件は、次の通りである。カラム(Column);cadeza cd-C18 3.0μm、3.0mm×250mm、検出器(Detector);DAD(254nm)、温度(Temp.);40℃、試料量(Injection Volume);10μL、流速(Flow rate);0.2mL/min、移動相(Mobile phase);80% MeOHである。
3.1.細胞の培養
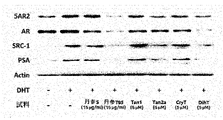
ヤギ抗ウサギ免疫グロブリンG(IgG,7074)及び抗マウスIgG(7076)に対する抗体は、Cell Signaling(Danvers,MA)から購入した。AR(SC-816)、SRC1(SC-32789)、PSA(SC-7638)及びβ-アクチン(SC-1616)に対する抗体は、Santa Cruz Biotechnology(米国テキサス州ダラス)から、5AR2に対する抗体(ab124877)はAbcam Inc.(米国マサチューセッツ州ケンブリッジ市)から購入した。RPMI(Roswell Park Memorial Institute medium)培地、胎児ウシ血清(FBS)及びペニシリン/ストレプトマイシンはGibco(ビックキャビン、OK、米国)から購入した。その他、フィナステリド、Fi(≧97%純度)及びDHT(≧99%純度)を含む試薬は、Sigma-Aldrich Inc.(米国ミズーリ州セントルイス市)から購買した。
3.3 DHT処理LNCaP細胞に対する丹参抽出物の効果
エタノール濃度による丹参抽出物のアンドロゲン関連タンパク質の抑制効果を確認するために、エタノール95%、70%、40%でそれぞれ抽出した丹参抽出物T95、T70、T40を、DHT処理LNCaP細胞に濃度別に処理し、その効果を確認した。
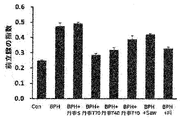
4.1.テストステロンで誘導した前立腺肥大症動物モデル構築
9週齢(体重350g以下)のSprague-Dawleyラットを無作為に(1)正常群、(2)前立腺肥大症誘導及びビークル投与群(陰性対照群)、(3)前立腺肥大症誘導及び丹参抽出物S投与群、(4)前立腺肥大症誘導及び丹参抽出物T70投与群、(5)前立腺肥大症誘導及び丹参抽出物T40投与群、(6)前立腺肥大症誘導及び丹参抽出物T10投与群、(7)前立腺肥大症誘導及びソーパルメットの実抽出物投与群(陽性対照群、告示型原料)、(8)前立腺肥大症誘導及びフィナステリド(Finasteride)投与群(陽性対照群、治療剤)に分離した。
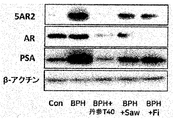
図9では、丹参抽出物T40の前立腺肥大症動物モデルにおいて前立腺肥大関連蛋白である5α-還元酵素(5α-reductase,5AR2)、アンドロゲン受容体(Androgen receptor,AR)、前立腺特異抗原(PSA)の発現に及ぼす効果を確認した。
Claims (7)
- 丹参(Salvia miltiorrhiza Bunge)抽出物を有効成分として含み、
前記丹参(Salvia miltiorrhiza Bunge)抽出物は、40% ~99%エタノール抽出物であり、タンシノン類を前記丹参(Salvia milti orrhiza Bunge)抽出物全重量の2%~70%含有し、
前記タンシノン類は、タンシノン1、タンシノン2a、クリプトタンシノン、及び15 ,16-ジヒドロタンシノンを含む
ことを特徴とする前立腺肥大症又は脱毛症の治療又は予防用組成物。 - 前記丹参(Salvia miltiorrhiza Bunge)抽出物は、5α還元酵素タイプ2(5AR2)、前立腺特異的抗原(PSA)、ステロイド受容共活性因子-1(SRC-1)及びアンドロゲン受容体(AR)からなる群から選ばれる一つ以上のタンパク質発現を減少させる
請求項1に記載の前立腺肥大症又は脱毛症の治療又は予防用組成物。 - 請求項1または2に記載の前立腺肥大症又は脱毛症の治療又は予防用の組成物の製造方 法であって、
前記組成物は、丹参(Salvia miltiorrhiza Bunge)抽出物を含み、
前記丹参(Salvia miltiorrhiza Bunge)抽出物を、
不純物を除去した丹参を破砕させる段階(1);
前記(1)段階の破砕した丹参を50℃~80℃で40%~99%の酒精(EtOH)に浸漬させ、2~24時間放置する段階(2);
前記(2)段階の丹参が抽出された酒精を分離し、残った残渣に新しい酒精を入れ、50℃~80℃で40%~99%の酒精(EtOH)に浸漬させ、2~12時間放置する段階(3);
前記(3)段階の丹参が抽出された酒精を分離し、残った残渣に新しい酒精を入れ、50℃~80℃で40%~99%の酒精(EtOH)に浸漬させ、2~5時間放置する段階(4);
前記(2)~(4)段階で分離しておいた酒精を集めて45℃~75℃で蒸発、乾燥させる段階(5);
前記(5)段階で蒸発、乾燥させた固形抽出物を粉砕し、80mesh以上でフィルタリングして丹参抽出物を得る段階(6);を含む製造方法によって製造する
ことを特徴とする前立腺肥大症又は脱毛症の治療又は防用組成物の製造方法。 - 前記不純物を除去した丹参を破砕させる段階(1)は、丹参を-196℃~-80℃の極低温で短時間に凍結し、5mm以下に粉砕する超微細粉砕工程(Cryogenic Micro Grinding Technology,CMGT)で粉砕する
請求項3に記載の前立腺肥大症又は脱毛症の治療又は予防用組成物の製造方法 。 - 丹参(Salvia miltiorrhiza Bunge)抽出物を有効成分として含み、
前記丹参(Salvia miltiorrhiza Bunge)抽出物は、40% ~99%エタノール抽出物であり、タンシノン類を前記丹参(Salvia milti orrhiza Bunge)抽出物全重量の2%~70%含有し、
前記タンシノン類は、タンシノン1、タンシノン2a、クリプトタンシノン、及び15 ,16-ジヒドロタンシノンを含む
ことを特徴とする立腺肥大症又は脱毛症の予防又は改善用健康機能食品。 - 15,16-ジヒドロタンシノンを有効成分として含む
ことを特徴とする前立腺肥大症又は脱毛症の治療又は予防用組成物。 - 15,16-ジヒドロタンシノンを有効成分として含む
ことを特徴とする前立腺肥大症又は脱毛症の予防又は改善用健康機能食品。
Applications Claiming Priority (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR20190137533 | 2019-10-31 | ||
| KR10-2019-0137533 | 2019-10-31 | ||
| KR10-2019-0157928 | 2019-12-02 | ||
| KR20190157928 | 2019-12-02 | ||
| KR20200049163 | 2020-04-23 | ||
| KR10-2020-0049163 | 2020-04-23 | ||
| KR10-2020-0142540 | 2020-10-29 | ||
| KR1020200142540A KR102462458B1 (ko) | 2019-10-31 | 2020-10-29 | 단삼(Salvia miltiorrhiza Bunge) 추출물을 유효성분으로 포함하는 전립선비대증 또는 탈모증의 치료 또는 예방용 조성물 |
| PCT/KR2020/015058 WO2021086120A1 (ko) | 2019-10-31 | 2020-10-30 | 단삼(Salvia miltiorrhiza Bunge) 추출물을 유효성분으로 포함하는 전립선비대증 또는 탈모증의 치료 또는 예방용 조성물 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2022510743A JP2022510743A (ja) | 2022-01-28 |
| JP7161237B2 true JP7161237B2 (ja) | 2022-10-26 |
Family
ID=75918033
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020567509A Active JP7161237B2 (ja) | 2019-10-31 | 2020-10-30 | 丹参(Salvia miltiorrhiza Bunge)抽出物を有効成分として含む前立腺肥大症又は脱毛症の治療又は予防用組成物 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US20210369803A1 (ja) |
| EP (1) | EP3838284A4 (ja) |
| JP (1) | JP7161237B2 (ja) |
| KR (2) | KR102462458B1 (ja) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102800570B1 (ko) * | 2021-12-01 | 2025-04-30 | 대한민국 | 간접열풍방식 청초법을 이용한 단삼 추출물에서 생리활성물질의 함량 및 항산화능을 증가시키는 방법 |
| KR102637204B1 (ko) * | 2022-11-15 | 2024-02-16 | (주) 옵트바이오 | 살비아놀산 b가 증강된 단삼 추출물 및 이를 함유하는 여드름 개선용 화장료 조성물 |
| KR20240122046A (ko) * | 2023-02-03 | 2024-08-12 | 주식회사 건강중심주의 | 단삼추출물, 금속 미네랄 디아미네이트 및 우리딘을 유효성분으로 포함하는 전립선비대증의 예방 또는 치료용 조성물 |
| CN116999427A (zh) * | 2023-08-30 | 2023-11-07 | 复旦大学附属华山医院 | 一种丹酚酸b在制备治疗脱发药物中的应用 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007517025A (ja) | 2003-12-30 | 2007-06-28 | エムディー バイオアルファ カンパニー リミテッド | 代謝活性を上昇させるタンシノン誘導体を用いる、肥満およびメタボリックシンドロームの治療 |
| JP2011500557A (ja) | 2007-10-11 | 2011-01-06 | マゼンス インコーポレイテッド | ナフトキノン系化合物の微粒化粒子を含有する医薬組成物 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5234698A (en) * | 1988-07-05 | 1993-08-10 | Fahim Mostafa S | Intraprostatic injection of zinc ions for treatment of inflammatory conditions and benign and malignant tumors of the prostate |
| KR100259037B1 (ko) | 1998-04-01 | 2000-07-01 | 박효석 | 발모촉진용 외용액제 |
| KR20010076810A (ko) | 2000-01-28 | 2001-08-16 | 문성수 | 전립선 질환 및 치질 치료용 조성물의 제조방법 및 그제조방법에 의한 조성물 |
| CN100477990C (zh) * | 2006-03-07 | 2009-04-15 | 博仲盛景医药技术(北京)有限公司 | 丹参酮滴丸及其制备方法 |
| CN102233084B (zh) * | 2010-05-08 | 2014-02-19 | 北京六盛合医药科技有限公司 | 一种用于治疗前列腺炎的中药组合物及其制备方法 |
| KR20120020639A (ko) | 2010-08-30 | 2012-03-08 | 주식회사한국전통의학연구소 | 단삼을 포함하는 전립선암 치료용 조성물 |
| KR20120053248A (ko) * | 2010-11-17 | 2012-05-25 | 경희대학교 산학협력단 | 탄시논 ⅱa의 신규 용도 |
| CN102127037A (zh) * | 2011-01-11 | 2011-07-20 | 上海交通大学 | 丹参酮类化合物及其应用 |
| KR101903745B1 (ko) * | 2011-11-29 | 2018-10-05 | (주)아모레퍼시픽 | 탈모방지 또는 모발 성장 촉진용 조성물 |
| CN102526305A (zh) * | 2012-02-14 | 2012-07-04 | 史志辉 | 一种治疗前列腺炎及前列腺增生的中药胶囊剂及其制备方法 |
| CN103041042B (zh) * | 2013-01-25 | 2015-01-21 | 西藏福田藏医药研究开发有限公司 | 一种藏药组合物及其在防脱生发产品中的应用 |
| CN103751466B (zh) * | 2013-12-31 | 2016-06-08 | 赵玉妹 | 一种治疗脂溢性脱发的中药制剂及其制备方法 |
| KR101842577B1 (ko) * | 2016-09-29 | 2018-05-14 | 제이씨나노텍(주) | 극저온 초미세분쇄를 이용한 한약재 혼합물의 제조방법 |
| KR20180055945A (ko) * | 2016-11-16 | 2018-05-28 | 영진약품 주식회사 | 간기능 개선용 단삼 추출물을 포함하는 조성물 및 그 제조방법 |
| CN106617053A (zh) * | 2016-12-01 | 2017-05-10 | 西安交通大学医学院第附属医院 | 一种防治前列腺炎及前列腺增生的保健食品及制备方法 |
| KR102005423B1 (ko) * | 2017-10-31 | 2019-07-30 | 주식회사 휴엔 | 단삼 추출물을 함유하는 내장지방형 비만 예방, 개선 또는 치료 조성물 |
| CN109045156A (zh) * | 2018-10-19 | 2018-12-21 | 山东宏济堂制药集团股份有限公司 | 中药组合物、贴膏剂及制备方法 |
-
2020
- 2020-10-29 KR KR1020200142540A patent/KR102462458B1/ko active Active
- 2020-10-30 JP JP2020567509A patent/JP7161237B2/ja active Active
- 2020-10-30 US US17/059,984 patent/US20210369803A1/en active Pending
- 2020-10-30 EP EP20810850.6A patent/EP3838284A4/en active Pending
-
2022
- 2022-06-22 KR KR1020220076468A patent/KR102583293B1/ko active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007517025A (ja) | 2003-12-30 | 2007-06-28 | エムディー バイオアルファ カンパニー リミテッド | 代謝活性を上昇させるタンシノン誘導体を用いる、肥満およびメタボリックシンドロームの治療 |
| JP2011500557A (ja) | 2007-10-11 | 2011-01-06 | マゼンス インコーポレイテッド | ナフトキノン系化合物の微粒化粒子を含有する医薬組成物 |
Non-Patent Citations (1)
| Title |
|---|
| C. Wang et al.,Journal of Steroid Biochemistry & Molecular Biology,2015年,vol. 145,p. 28-37 |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20220100809A (ko) | 2022-07-18 |
| US20210369803A1 (en) | 2021-12-02 |
| KR20210052341A (ko) | 2021-05-10 |
| EP3838284A1 (en) | 2021-06-23 |
| KR102583293B1 (ko) | 2023-09-26 |
| EP3838284A4 (en) | 2022-11-23 |
| JP2022510743A (ja) | 2022-01-28 |
| KR102462458B1 (ko) | 2022-11-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7161237B2 (ja) | 丹参(Salvia miltiorrhiza Bunge)抽出物を有効成分として含む前立腺肥大症又は脱毛症の治療又は予防用組成物 | |
| EP3113784B1 (en) | Compositions based on plant extracts for inhibition of the 5-alpha reductase | |
| KR102861212B1 (ko) | 황기 및 참당귀를 유효성분으로 포함하는 전립선 질환 또는 탈모의 예방, 치료 및 개선용 조성물 | |
| JP2000095649A (ja) | テストステロン−5α−レダクターゼ阻害剤 | |
| KR20190115925A (ko) | 필발 추출물을 유효성분으로 포함하는 탈모 방지 또는 발모 촉진용 조성물 | |
| JP7117030B2 (ja) | ゴカヒ、ロコン、及びアカマツ抽出物を有効成分として含む前立腺疾患の予防、治療、又は改善用組成物 | |
| WO2021086120A1 (ko) | 단삼(Salvia miltiorrhiza Bunge) 추출물을 유효성분으로 포함하는 전립선비대증 또는 탈모증의 치료 또는 예방용 조성물 | |
| KR101876201B1 (ko) | 자근 추출물을 유효성분으로 포함하는 안드로겐 수용체 관련 질환의 예방, 개선 또는 치료용 조성물 | |
| CA3200081C (en) | COMPOSITION INTENDED TO PREVENT, ALLEVIATE OR TREAT ANDROGEN-DEPENDENT DISEASES, CONTAINING BAMBOO LEAF EXTRACT AS AN ACTIVE INGREDIENT | |
| KR20230117828A (ko) | 향청란 추출물을 유효성분으로 포함하는 불안장애 또는 우울증의 예방 또는 치료용 조성물 | |
| KR20240122046A (ko) | 단삼추출물, 금속 미네랄 디아미네이트 및 우리딘을 유효성분으로 포함하는 전립선비대증의 예방 또는 치료용 조성물 | |
| KR102536101B1 (ko) | 선학초 추출물을 유효성분으로 포함하는 전립선 질환의 예방, 개선 또는 치료용 조성물 | |
| JP5419259B2 (ja) | 化粧料 | |
| KR102563879B1 (ko) | 청호 추출물을 유효성분으로 포함하는 전립선 질환의 예방, 개선 또는 치료용 조성물 | |
| JP2010184873A (ja) | 化粧料及び飲食品 | |
| KR102129409B1 (ko) | 탈모 치료 및 발모 촉진용 조성물 | |
| WO2018088272A1 (en) | Endothelin-converting enzyme inhibitor | |
| KR20250163437A (ko) | 비파엽 추출물을 유효성분으로 포함하는 전립선 비대증 또는 전립선염 예방, 개선 또는 치료용 조성물 | |
| CN121240868A (zh) | 富含总状芦笋根组合物及其制备方法 | |
| WO2020234770A1 (en) | Composition for use in the prevention and/or treatment of pathologies associated to the prostate | |
| KR20240033981A (ko) | 회화나무 추출물을 유효성분으로 포함하는 비만의 예방 및 개선용 조성물 | |
| KR20250178244A (ko) | 해당화 꽃봉오리 추출물을 유효성분으로 포함하는 탈모증 예방, 개선 또는 치료용 조성물 | |
| KR20150111793A (ko) | 펄첼라민 g을 유효성분으로 포함하는 항염증제 | |
| CN118043336A (zh) | 具有脱发预防或毛发生长促进活性的肽及其用途 | |
| JPH11199503A (ja) | テストステロン−5α−レダクターゼ阻害剤および飲食品 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20201210 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20220208 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20220506 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20221004 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20221006 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7161237 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |