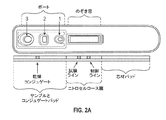
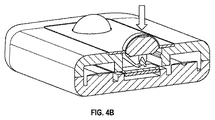
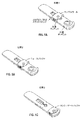
JP6075799B2 - 血小板第4因子(pf4)/ヘパリン抗体を検出する診断デバイス、方法及びシステム - Google Patents
血小板第4因子(pf4)/ヘパリン抗体を検出する診断デバイス、方法及びシステム Download PDFInfo
- Publication number
- JP6075799B2 JP6075799B2 JP2014514560A JP2014514560A JP6075799B2 JP 6075799 B2 JP6075799 B2 JP 6075799B2 JP 2014514560 A JP2014514560 A JP 2014514560A JP 2014514560 A JP2014514560 A JP 2014514560A JP 6075799 B2 JP6075799 B2 JP 6075799B2
- Authority
- JP
- Japan
- Prior art keywords
- zone
- sample
- conjugate
- heparin
- antibody
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6854—Immunoglobulins
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54366—Apparatus specially adapted for solid-phase testing
- G01N33/54386—Analytical elements
- G01N33/54387—Immunochromatographic test strips
- G01N33/54388—Immunochromatographic test strips based on lateral flow
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6863—Cytokines, i.e. immune system proteins modifying a biological response such as cell growth proliferation or differentiation, e.g. TNF, CNF, GM-CSF, lymphotoxin, MIF or their receptors
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/86—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving blood coagulating time or factors, or their receptors
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/32—Cardiovascular disorders
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Hematology (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Cell Biology (AREA)
- Food Science & Technology (AREA)
- General Health & Medical Sciences (AREA)
- Biotechnology (AREA)
- Medicinal Chemistry (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Investigating, Analyzing Materials By Fluorescence Or Luminescence (AREA)
- Investigating Or Analysing Materials By The Use Of Chemical Reactions (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161495171P | 2011-06-09 | 2011-06-09 | |
| US61/495,171 | 2011-06-09 | ||
| PCT/US2012/040938 WO2012170435A2 (en) | 2011-06-09 | 2012-06-05 | Diagnostic devices, methods and systems for detecting platelet factor 4 (pf4)/heparin antibodies |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2014517311A JP2014517311A (ja) | 2014-07-17 |
| JP2014517311A5 JP2014517311A5 (enExample) | 2015-07-23 |
| JP6075799B2 true JP6075799B2 (ja) | 2017-02-08 |
Family
ID=46317508
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014514560A Active JP6075799B2 (ja) | 2011-06-09 | 2012-06-05 | 血小板第4因子(pf4)/ヘパリン抗体を検出する診断デバイス、方法及びシステム |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US9599623B2 (enExample) |
| EP (1) | EP2718724B1 (enExample) |
| JP (1) | JP6075799B2 (enExample) |
| AU (1) | AU2012268393B2 (enExample) |
| CA (1) | CA2838708C (enExample) |
| WO (1) | WO2012170435A2 (enExample) |
Families Citing this family (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI498166B (zh) * | 2013-07-02 | 2015-09-01 | Univ Nat Taiwan | 以表面電漿共振定量分析之自動操作檢測程序的多孔性薄膜微流體裝置 |
| EP3063544B1 (en) * | 2013-10-29 | 2021-05-26 | Versiti Blood Research Institute Foundation, Inc. | Method of detecting platelet activating antibodies that cause heparin-induced thrombocytopenia/thrombosis |
| CN105980842B (zh) * | 2013-12-06 | 2019-12-17 | 奥索临床诊断有限公司 | 具有清洗口的测定装置 |
| GB2523135A (en) * | 2014-02-13 | 2015-08-19 | Molecular Vision Ltd | Assay device |
| US20150044707A1 (en) * | 2014-02-18 | 2015-02-12 | Melinda Sanders | At-Home Blood Pregnancy Test Kit |
| CN104076014A (zh) * | 2014-06-30 | 2014-10-01 | 安徽师范大学 | 一种荧光传感器,其制备方法,用途以及检测Heparin分子的方法 |
| GB2528657B (en) * | 2014-07-24 | 2019-03-13 | Intelligent Fingerprinting Ltd | Sample analysing device |
| BR112017005121A2 (pt) * | 2014-09-23 | 2018-07-31 | Tearlab Res Inc | sistemas e métodos para integração de coleta de lágrima microfluídica e análise de fluxo lateral de analitos de interesse. |
| RU2702645C2 (ru) * | 2014-12-11 | 2019-10-09 | Критикал Кэа Дайэгностикс, Инк. | Тестовый аппарат и способы для сердечного биологического маркера st2 |
| US10324089B2 (en) | 2014-12-11 | 2019-06-18 | Critical Care Diagnostics, Inc. | Test apparatus and methods for ST2 cardiac biomarker |
| GB2535998A (en) | 2015-02-27 | 2016-09-07 | Intelligent Fingerprinting Ltd | A device for receiving and analysing a sample |
| KR101635956B1 (ko) * | 2015-03-16 | 2016-07-06 | (주)큐브바이오 | 천공 부재를 구비하는 진단 키트 |
| EP3392655B1 (en) * | 2015-12-18 | 2020-08-19 | FUJIFILM Corporation | Immunochromatographic kit |
| CN108780076A (zh) | 2016-01-27 | 2018-11-09 | 隐蔽色彩股份有限公司 | 用于检测液体中目标物质的可穿戴装置 |
| US10073092B2 (en) * | 2016-02-19 | 2018-09-11 | Andrew Wang | Apparatus for assay strip(s) with specimen loading to multiple zones and related methods |
| WO2017151642A1 (en) * | 2016-02-29 | 2017-09-08 | Flora Bioscience, Inc. | Detection apparatus |
| WO2018128749A1 (en) * | 2017-01-06 | 2018-07-12 | Dnt Scientific Research, Llc | Rapid diagnostic test device by driven flow technology |
| EP3413051B1 (de) * | 2017-06-09 | 2020-07-29 | Siemens Healthcare Diagnostics Products GmbH | Aktivierungstest zur diagnose einer heparin-induzierten thrombozytopenie |
| US10458974B2 (en) * | 2017-06-09 | 2019-10-29 | Optimum Imaging Diagnostics LLC | Universal testing system for quantitative analysis |
| EP3438667B1 (de) * | 2017-08-02 | 2020-12-30 | Siemens Healthcare Diagnostics Products GmbH | Bindungstest zur diagnose einer heparin-induzierten thrombozytopenie |
| CN107727851A (zh) * | 2017-11-15 | 2018-02-23 | 北京舜雷科技有限公司 | 一种免疫凝集和胶体金层析试纸条相结合的用于检测hit抗体的系统 |
| WO2019202395A1 (en) * | 2018-04-20 | 2019-10-24 | Biokit Research & Developments, S.L.U. | Heparin-induced thrombocytopenia assay |
| USD959015S1 (en) * | 2020-06-05 | 2022-07-26 | Zhejiang Orient Gene Biotech Co., LTD | COVID immunoassay test device |
| USD954982S1 (en) * | 2020-06-30 | 2022-06-14 | Hangzhou Biotest Biotech Co., Ltd. | COVID immunoassay test device |
| USD954983S1 (en) * | 2020-09-04 | 2022-06-14 | Hangzhou Biotest Biotech Co., Ltd. | COVID immunoassay test device |
| CN112842334A (zh) * | 2021-02-08 | 2021-05-28 | 德莫德(苏州)机械科技有限公司 | 一种翻转式加样及按压式加液的一体化胶体金卡盒 |
| USD977673S1 (en) * | 2021-03-26 | 2023-02-07 | Zhejiang Orient Gene Biotech Co., LTD | Test card |
| USD1069617S1 (en) * | 2021-03-29 | 2025-04-08 | Owen Mumford Limited | Testing device |
| KR102281805B1 (ko) * | 2021-04-22 | 2021-07-26 | 주식회사 제트바이오텍 | 형광염료를 이용한 건식 타입 형광 정량분석 체외 진단키트 및 그 제조방법 |
| USD1025392S1 (en) * | 2022-01-25 | 2024-04-30 | Acon Biotech (Hangzhou) Co.Ltd. | Test device |
| USD990704S1 (en) * | 2022-07-14 | 2023-06-27 | Andon Health Co., Ltd. | Test strip for medical or laboratory purposes |
| USD1054585S1 (en) * | 2023-01-14 | 2024-12-17 | Zhejiang Orient Gene Biotech Co., LTD | Test card |
| CA219722S (en) * | 2023-01-16 | 2024-06-27 | Zhejiang Orient Gene Biotech Co Ltd | Test card |
| USD1053379S1 (en) * | 2023-01-19 | 2024-12-03 | Zhejiang Orient Gene Biotech Co., LTD | United test card |
| USD1101961S1 (en) * | 2023-04-28 | 2025-11-11 | Charles River Laboratories International, Inc. | Cartridge |
| CA221817S (en) * | 2023-05-24 | 2024-09-25 | Zhejiang Orient Gene Biotech Co Ltd | Test card |
| CA221818S (en) * | 2023-05-24 | 2024-10-22 | Zhejiang Orient Gene Biotech Co Ltd | Test card |
| USD1086484S1 (en) * | 2023-06-26 | 2025-07-29 | Hangzhou Biotest Biotech Co., Ltd | Liquid sample test device |
| CN117471105B (zh) * | 2023-12-27 | 2024-03-26 | 北京芯迈微生物技术有限公司 | 双向双驱序贯式血小板hla抗体检测的微流控芯片及应用 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4981786A (en) * | 1987-09-04 | 1991-01-01 | Syntex (U.S.A.) Inc. | Multiple port assay device |
| US5466582A (en) | 1990-08-09 | 1995-11-14 | Serbio | Thrombocytopenia determination |
| US5972717A (en) | 1995-05-10 | 1999-10-26 | The Blood Center Research Foundation, Inc. | Method and kit for detecting heparin induced thrombocytopenia |
| US5972718A (en) * | 1996-02-28 | 1999-10-26 | The Blood Center Research Foundation | Method of detecting heparin-induced thrombocytopenia |
| DE19927783A1 (de) * | 1999-06-18 | 2000-12-21 | Roche Diagnostics Gmbh | Element zur Bestimmung eines Analyts in einer Flüssigkeit, entsprechendes Bestimmungsverfahren unter Verwendung des Elementes sowie Kit zur Bestimmugn eines Analyts |
| US6964854B1 (en) | 1999-07-13 | 2005-11-15 | Science & Technology Corporation | Compositions and methods useful for the diagnosis and treatment of heparin induced thrombocytopenia/thrombosis |
| US6713309B1 (en) * | 1999-07-30 | 2004-03-30 | Large Scale Proteomics Corporation | Microarrays and their manufacture |
| DE60031576D1 (en) | 1999-08-30 | 2006-12-07 | Wesagen | Diagnostisches assay für typ-2-heparin induzierte thrombocytopenie |
| EP2144064A3 (en) | 2004-10-04 | 2010-05-26 | Akers Biosciences, Inc. | Methods and kits for detecting heparin/platelet factor 4 antibodies |
| US7629127B2 (en) * | 2005-01-21 | 2009-12-08 | Dexall Biomedical Labs, Inc. | Method for the visual detection of specific antibodies by the use of lateral flow assays |
| US7871781B2 (en) * | 2005-05-23 | 2011-01-18 | Phadia Ab | Two step lateral flow assay methods and devices |
| FR2902884B1 (fr) | 2006-06-23 | 2008-12-26 | Hyphen Biomed Soc Par Actions | Procede pour la detection des anticorps heparine-dependants et le diagnostic des pathologies immunes ou auto-immnues potentialisees par l'heparine telle que la thrombopenie induite par l'heparine |
| JP4198182B1 (ja) * | 2007-12-28 | 2008-12-17 | 有限会社バイオデバイステクノロジー | イムノクロマト分析法及びイムノクロマト分析キット |
| US8940495B2 (en) * | 2008-02-29 | 2015-01-27 | BioMedomics, Inc | Rapid and sensitive method for quantitative determination of the level of heparin—PF4 complex induced immunoglobulin antibodies |
| JP5642361B2 (ja) * | 2008-07-03 | 2014-12-17 | ジョンソン・アンド・ジョンソン・アーベー | 循環抗体の分析のための方法 |
| WO2010024271A1 (ja) * | 2008-08-27 | 2010-03-04 | 三菱化学メディエンス株式会社 | 修飾抗ヘパリン/pf4複合体抗体及びhit抗体標準品 |
| US10114012B2 (en) * | 2008-10-31 | 2018-10-30 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and assays for detecting and quantifying pure subpopulations of white blood cells in immune system disorders |
-
2012
- 2012-06-05 CA CA2838708A patent/CA2838708C/en active Active
- 2012-06-05 EP EP12727977.6A patent/EP2718724B1/en active Active
- 2012-06-05 JP JP2014514560A patent/JP6075799B2/ja active Active
- 2012-06-05 WO PCT/US2012/040938 patent/WO2012170435A2/en not_active Ceased
- 2012-06-05 US US14/124,533 patent/US9599623B2/en active Active
- 2012-06-05 AU AU2012268393A patent/AU2012268393B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| US20150064800A1 (en) | 2015-03-05 |
| EP2718724A2 (en) | 2014-04-16 |
| WO2012170435A2 (en) | 2012-12-13 |
| US9599623B2 (en) | 2017-03-21 |
| CA2838708C (en) | 2020-07-21 |
| JP2014517311A (ja) | 2014-07-17 |
| CA2838708A1 (en) | 2012-12-13 |
| AU2012268393B2 (en) | 2016-09-15 |
| WO2012170435A3 (en) | 2013-06-13 |
| EP2718724B1 (en) | 2017-08-09 |
| AU2012268393A1 (en) | 2014-01-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6075799B2 (ja) | 血小板第4因子(pf4)/ヘパリン抗体を検出する診断デバイス、方法及びシステム | |
| US9250236B2 (en) | Method to increase specificity and/or accuracy of lateral flow immunoassays | |
| US12385925B2 (en) | Anti-VLA-4 related assays | |
| US20100129841A1 (en) | Detection of a blood coagulation activity marker in a body fluid sample | |
| EP3775896B1 (en) | Lateral flow immunoassay strip device | |
| KR20150125002A (ko) | 바이러스 및 박테리아 감염의 복합 검출을 위한 방법 및 장치 | |
| CN115184620B (zh) | 一种pla2r抗体的量子点荧光检测试纸条、试剂盒及其应用 | |
| CN115097129A (zh) | 一种用于胎盘生长因子和可溶性fms样酪氨酸激酶-1的检测试剂组合 | |
| US9201065B2 (en) | Agglutination assay | |
| EP3816626A1 (en) | Lateral flow test arrangement suitable for detection of an analyte in saliva | |
| WO2019026870A1 (ja) | sFlt-1(可溶型血管内皮増殖因子受容体-1)の新規測定法 | |
| HK1256154B (en) | Anti-vla-4 related assays | |
| HK1173967A (en) | Anti-vla-4 related assays | |
| HK1173967B (en) | Anti-vla-4 related assays |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150605 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20150605 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20160328 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20160405 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160705 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20161206 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20170105 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6075799 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |